Summary
Background
Coronavirus disease 2019 (COVID-19) frequently leads to neurological complications after recovery from acute infection, with higher prevalence in women. However, mechanisms by which SARS-CoV-2 disrupts brain function remain unclear and treatment strategies are lacking. We previously demonstrated neuroinflammation in the olfactory bulb of intranasally infected hamsters, followed by alpha-synuclein and tau accumulation in cortex, thus mirroring pathogenesis of neurodegenerative diseases such as Parkinson's or Alzheimer's disease.
Methods
To uncover the sex-specific spatiotemporal profiles of neuroinflammation and neuronal dysfunction following intranasal SARS-CoV-2 infection, we quantified microglia cell density, alpha-synuclein immunoreactivity and inhibitory interneurons in cortical regions, limbic system and basal ganglia at acute and late post-recovery time points.
Findings
Unexpectedly, microglia cell density and alpha-synuclein immunoreactivity decreased at 6 days post-infection, then rebounded to overt accumulation at 21 days post-infection. This biphasic response was most pronounced in amygdala and striatum, regions affected early in Parkinson's disease. Several brain regions showed altered densities of parvalbumin and calretinin interneurons which are involved in cognition and motor control. Of note, females appeared more affected.
Interpretation
Our results demonstrate that SARS-CoV-2 profoundly disrupts brain homeostasis without neuroinvasion, via neuroinflammatory and protein regulation mechanisms that persist beyond viral clearance. The regional patterns and sex differences are in line with neurological deficits observed after SARS-CoV-2 infection.
Funding
Federal Ministry of Health, Germany (BMG; ZMV I 1-2520COR501 to G.G.), Federal Ministry of Education and Research, Germany (BMBF; 03COV06B to G.G.), Ministry of Science and Culture of Lower Saxony in Germany (14-76403-184, to G.G. and F.R.).
Keywords: Neurodegenerative disease, Neuroinflammation, Neurological symptoms, Post COVID-19 condition
Research in context.
Evidence before this study
Neurological complications during and after COVID-19 are highly prevalent and exhibit sex-specific differences in their prevalence and severity. Previous studies have demonstrated that both severe and mild forms of COVID-19 can lead to persistent neurological and psychiatric symptoms, including cognitive impairments, mood disorders, and motor dysfunctions. Despite the multitude of clinical studies on these long-term CNS-related symptoms, which point to a wide range of changes in the brain including neuroinflammation and degenerative processes, the underlying mechanisms remain poorly understood and animal studies are needed to gain further insights.
Added value of this study
We performed immunohistological analyses of brains from male and female Syrian golden hamsters six and 21 days post SARS-CoV-2 infection. We observed a broad range of sex- and brain region-dependent processes during and after recovery from COVID19: Our findings reveal a biphasic response in alpha-synuclein levels post-infection, with initial reductions followed by significant increases at three weeks post-infection, with female animals exhibiting a more pronounced response. In females, alpha-synuclein response correlated with changes of myeloid cell density. Furthermore, we observed altered densities of specific interneurons with pronounced differences between male and female subjects.
Implications of all the available evidence
Together with findings in patients with Post COVID-19 condition, our results suggest the potential presence of neuroinflammatory and neurodegenerative processes. The pronounced effect of sex on the outcomes of this study calls for further in-depth analysis into the mechanisms underlying sex-specific differences on CNS-related outcomes of COVID-19 in this model.
Introduction
There is clear evidence that, besides the common respiratory symptoms of coronavirus disease 2019 (COVID-19), infections with the pandemic severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) affect the central nervous system (CNS).1,2 At acute infection, COVID-19 may present with headache, disturbed sense of smell and taste, dizziness and encephalopathy.3, 4, 5 A significant subgroup of patients with mild to severe COVID-19 experience persisting or newly developing neurological and psychiatric symptoms such as anxiety, depression and cognitive impairment weeks to months after recovery, often referred to as Post COVID-19 condition,6, 7, 8, 9, 10, 11 with higher prevalence in women.12,13 The lack of rational therapeutic strategies for this severe and debilitating condition represents an urgent unmet medical need.
Among the potential mechanisms, in the majority of cases, injury via hyperinflammatory responses and dysregulation of critical neuronal proteins appear as the most likely scenarios.14, 15, 16, 17, 18 Microglia, the immune cells of the brain, respond as resident macrophages to infection and injury.19 However, this so called neuroinflammation may, if prolonged or excessive, even contribute to neuronal damage.20, 21, 22 We previously reported microglia reactivity and signs of cortical accumulation of neuronal alpha-synuclein (aSyn) in brains of Syrian golden hamsters 14 days post-infection (dpi), i.e., after COVID-19 remission.23,24 This animal model is well-established for COVID-19 research due to its high susceptibility to the original virus strain and resemblance to human infection, especially regarding pathogenesis, clinical aspects and sex differences.25, 26, 27 ASyn is a highly abundant, soluble and intrinsically disordered presynaptic protein with a role in synaptic vesicle exocytosis.28 Recently, an immune-modulatory role of aSyn was discovered.18 However, if aSyn protein levels increase, for example in response to injury, this protein can aggregate into oligomers and insoluble fibrils with potentially neurotoxic capacities.29,30 Its deposits can be found in several neurodegenerative diseases, together termed synucleinopathies, including Parkinson's Disease (PD).31,32 Interestingly, SARS-CoV-2 proteins were shown to interact with aSyn and moreover increase its expression in vitro.33 In mild to moderately infected non-human primates persisting aSyn accumulations were found in the midbrain several weeks after recovery.34
In order to develop rational therapy for the diverse range of neurological symptoms reported in patients with COVID-19, there is a need to understand which neuronal networks could be affected. Imaging and post-mortem studies of COVID-19 patients and our previous work in the hamster model argue against overt acute neuronal loss in response to the non-neuroinvasive SARS-CoV-2.23,35 Of note, alterations in small but functionally-indispensable interneuron populations, such as Parvalbumin- or Calretinin-positive inhibitory interneurons, could lead to the observed cognitive impairments and neuropsychiatric symptoms,36,37 and even contribute to neurodegenerative diseases such as Alzheimer's disease (AD) and PD.38, 39, 40, 41 Yet, to our knowledge, the impact of SARS-CoV-2 infection on these interneurons is unknown.
With regard to disease modifiers, emerging evidence suggests that sex may influence the severity and manifestations of COVID-19, including its neurological effects.42 Male sex is a risk factor for poor disease outcome probably related to sex hormones,27,43, 44, 45 while Post COVID-19 condition is more prevalent in female patients.12,46, 47, 48 The mechanisms for the latter remain elusive, but could serve as rational therapeutic targets.
Previously, we presented first evidence for microglia reactivity and aSyn accumulation in the COVID-19 hamster model.23 Here, we deciphered sex-specific temporal dynamics and regional specificity, as well as the impact on neurons up to 3 weeks post SARS-CoV-2 infection in this model. We studied the first 3 weeks after SARS-CoV-2 infection to provide insights how brain homeostasis is altered from acute infection (in hamsters this is only up to day 6 post-infection) to the period of viral clearance (from day 6 to 21 post-infection for most animals). We expect that during this late recovery period alterations are initiated and most prominent. If present they could be expected to persist and constitute Post COVID-19 condition symptoms in some patients.
Methods
Virus
Virus was isolated as described previously.49 The SARS-CoV-2/Germany/Hamburg/01/2020 (ENA study 378 PRJEB41216 and sample ERS5312751) was isolated from a nasopharyngeal swab of a PCR positive male patient with COVID-19 hospitalized at the intensive care unit (ICU) of the University Medical Center Hamburg-Eppendorf (UKE). Isolated virus was inoculated for three serial passages in VeroE6 cells cultivated in Dulbecco's Modified Eagle's Medium (DMEM; Sigma–Aldrich GmbH) with 2% fetal bovine serum, 1% penicillin-streptomycin and 1% l-glutamine at 37 °C for virus propagation. Infection experiments were accomplished under biosafety level 3 (BSL-3) conditions in the Department for Viral Zoonoses-One Health, Leibniz Institute of Virology, Hamburg, Germany.
Ethics
All experiments were accomplished in accordance with the EU directive 688 2010/63/EU and approved by the relevant German authority (Behörde für Gesundheit und Verbraucherschutz; protocols N 32/2020 and N 103/2020). All animal experiments were performed in compliance with the ARRIVE guidelines.
Animal experiments
Animal experiments were performed as previously described.23,27,49 110 Syrian golden hamsters (Mesocricetus auratus), purchased from Janvier, were housed under BSL-3 conditions at the Department for Viral Zoonoses-One Health, Leibniz Institute of Virology, Hamburg, Germany, using standardized housing conditions (12:12 light–dark cycle, 21 ± 2 °C, 40–50% humidity, food and water ad libitum). 8–10 weeks old male and female hamsters were administered with 10ˆ5 plaque forming units (PFU)/ml SARS-CoV-2 or phosphate-buffered saline (PBS, vehicle control) intranasally under anaesthesia with 150 mg/kg ketamine and 10 mg/kg xylazine intraperitoneally (i.p.). Clinical signs and pathology of other organs from these animals were published previously.27 Hamsters developed mild signs of COVID-19 including respiratory dysfunction and weight loss up to day 6 pi and recovered up to day 14 pi.27 Up to day 6 pi virus was cleared from most organs except of the lung of some animals.27,50 Male animals showed more severe and longer-lasting respiratory dysfunction and slower recovery of weight during the recovery phase compared to females.27 Animals were euthanized via pentobarbital injection (800 mg/kg i.p.) 6 and 21 days post-infection (dpi) and blood withdrawal was performed via cardiac puncture. During skull opening to enable penetration of formalin into brain tissue for virus inactivation and tissue fixation, some brain regions were damaged by the procedure and could not be evaluated, or staining quality was not appropriate for quantification (a priori quality control). These regions were not included into analyses for the respective animal resulting in different number of replicates across experiments as stated in the figure legends. For information on sample size determination, randomization, blinding, inclusion/exclusion criteria of the animals please refer to Stanelle-Bertram et al., 2023.27
Immunohistochemistry
Brains were removed from skulls and cryoprotected by placing in 10%, 20%, then 30% sucrose solutions for 24 h each. Afterwards, brains were cut into coronal sections of 40 μm thickness using a cryostat. Brain sections were treated as published previously.41 Briefly, sections were blocked in serum and incubated at 4 °C overnight in primary antibody as indicated below.
Then, sections were incubated with secondary antibody solution (Invitrogen donkey or goat anti-guinea pig, goat anti-mouse, conjugated with Alexa Fluor (AF) 488, AF555, AF647) and cover slipped with ProLongTM Gold Antifade Mountant with DAPI (Thermo Fisher).
Slices were digitized using a Zeiss AxioObserver 7 microscope, provided with a Colibri 7 LED light source. Images were taken at 10× or 20× magnification using the Zeiss Zen Pro Software (Carl Zeiss AG, Oberkochen, Germany). For confocal laser scanning microscopy a Zeiss LSM 980 with Airyscan 2 at the Research Core Unit for Laser Microscopy at the Medical School Hannover was used using a 40× objective in the airyscan confocal mode with a 2× confocal zoom factor. Z-Stacks were sampled using a z-step of 0.18 μm. All images were analysed using the Fiji package of ImageJ.51 Analysis was performed blinded and randomized.
Myeloid cell infiltration and reactivity was evaluated for both time points 6 and 21 days dpi via immunostaining of Iba1 (guinea pig anti-Iba1, 1:500, Synaptic Systems Cat# 234004, RRID:AB_2493179, Göttingen, Germany). Iba1-positive cells were manually counted in regions of interest (ROIs) as illustrated in Fig. S5.
To assess the amount of aSyn, immunostaining of aSyn was performed for 6 and 21 dpi (mouse anti-a-Synuclein, 1:1000, BD Biosciences Cat# 610787, RRID:AB_398108, Heidelberg, Germany) and the mean fluorescent intensity of aSyn staining was measured (aSyn-IR) in ROIs as illustrated in Fig. S1.
Interneuron staining was performed for 21 dpi hamster brain sections using Parvalbumin (guinea pig anti-Parvalbumin, 1:1000, Synaptic Systems Cat# 195004, RRID:AB_2156476, Göttingen, Germany) and Calretinin (mouse anti-Calretinin, 1:500, abcam Cat#277631-1001, Cambridge, Great Britain). Parvalbumin and Calretinin positive cells were counted manually in ROIs as illustrated in Fig. S7a, j. For all antibodies negative and positive controls were used to validate specificity, in addition to validation provided by the supplier.
Statistics
For data comprising more than two experimental groups 2 way or mixed effects model ANOVA followed by Sidak's or Dunns post hoc tests for multiple comparisons was used for statistical analyses as indicated. Normality was tested using the Shapiro–Wilk test. To understand the relationship between aSyn and Iba1, linear regression analyses were performed and Pearson and Spearman rank correlation coefficients were computed. For two-group comparison for the interneuron data, Mann–Whitney U tests were used. Statistical analyses were performed using the software GraphPad Prism 9.1.2 (GraphPad Software, Inc., San Diego, CA, USA, 2003) and a significant differences was defined as p < 0.05 and a trend defined as p < 0.1.
Role of funders
The funders had no role in study design, analysis, or writing of the study.
Results
Biphasic response of alpha-synuclein immunoreactivity following SARS-CoV-2 infection
We previously observed aSyn accumulation in cortical neurons 14 days after SARS-CoV-2 infection.23 To investigate the time course of this signature of proteinopathy across the entire brain, we quantified aSyn-immunoreactivity (aSyn-IR) in hamster brains at 6 and 21 dpi. At 6 dpi, all peripheral organs had already cleared the virus while lungs of few hamsters remained virus positive.27 Hence the 21 dpi timepoint represents a late recovery phase, as the hamsters had cleared the virus and no virus could be detected by 14 dpi.24 Notably, significant differences emerged in relation to both the post-infection time point and sex (Fig. 1, Figs. S1–S4).
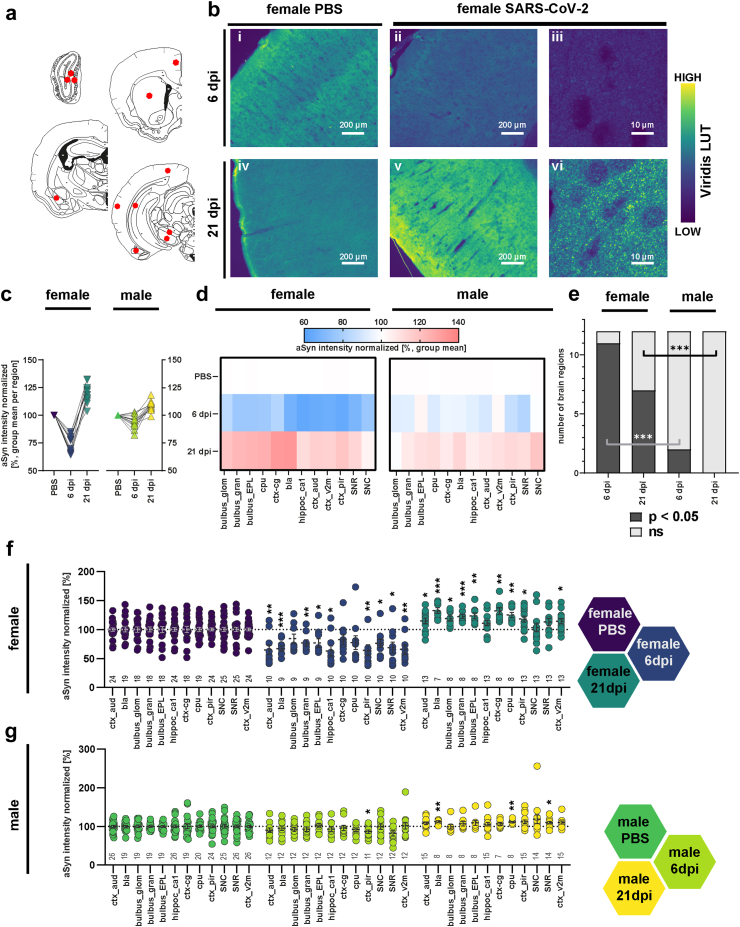
Fig. 1.
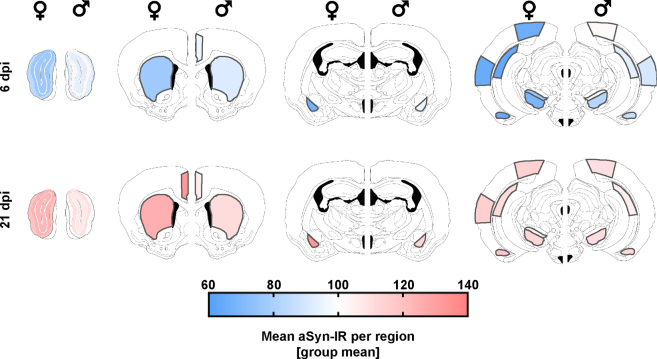
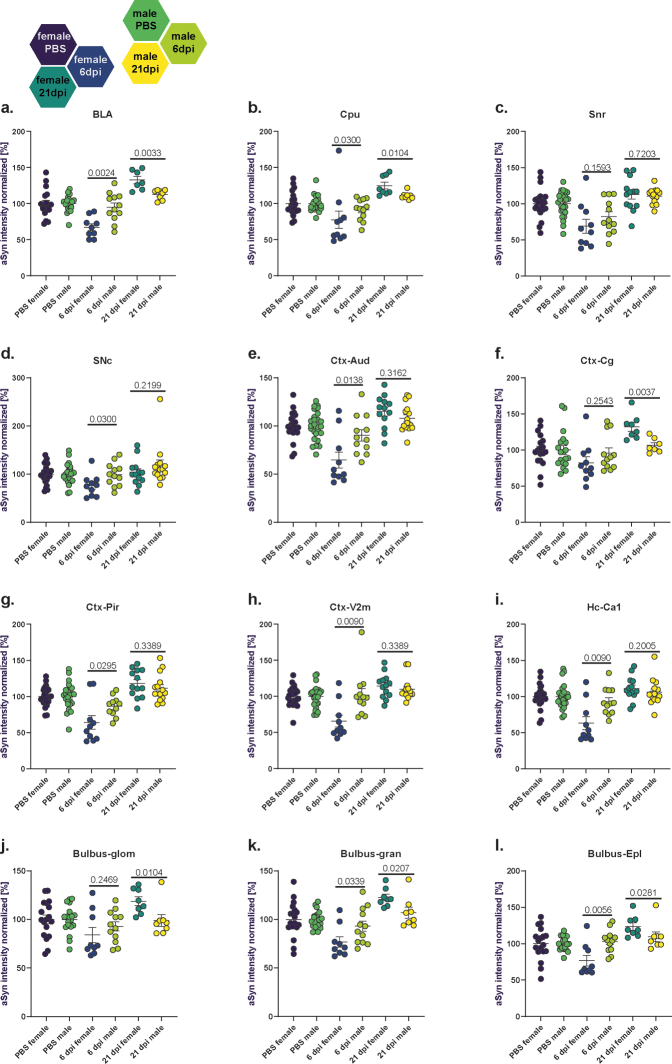
Biphasic response of alpha-synuclein immunoreactivity following SARS-CoV-2 infection. (a). Schematic representation of the coronal hamster brain sections that were stained, red dots highlighting the regions of interest examined (Olfactory bulb glomerular, granular and external plexiform layers (bulbus_glom, gran, EPL), caudate putamen (cpu), cingulate cortex (ctx_cg), basolateral amygdala (bla), ca1 region of hippocampus (hippoc-ca1), and aud, v2m and pir regions of cortex, substantia nigra partes reticulata and compacta (SNR, SNC)). Modification of coronal brain illustrations of Wood et al., 2001.121 b. Representative histological stainings of aSyn recorded at a 10× magnification (i-ii, iv-v) and confocal laser scanning macroscopy at 160× (iii, vi) in the cortex from female animals with PBS or SARS-CoV-2 infection at 6 and 21 dpi. Scale bar in widefield images = 200 μm, in confocal images = 10 μm. The viridis lookup table was used for the histological images to increase the visibility of expression changes. (c) Group mean of the normalized aSyn-IR per region in sex/infection groups demonstrating the general biphasic tendency of the aSyn response to infection. (d) Heatmaps of group mean normalized aSyn-IR per brain region in female and male animals in the three groups PBS, 6 dpi and 21 dpi. (e) Distribution of brain regions where PBS and SARS-CoV-2 groups differ significantly. Statistics: fishers exact test between male and female groups at 6 and 21 dpi. (f–g) Quantification of mean aSyn-IR in relevant regions for female (f) and male (g) hamsters. Data normalized to female and male PBS control group. Circles depict individual animals. The mean ± SEM is additionally shown. Mark the different scales on the y-axes. Mixed-effect analysis of aSyn-IR across brain regions in female (f) and male (g) hamsters evaluating effects of region and infection followed by a Dunnett's multiple comparisons test to evaluate infection effects within brain regions. Further information on Mixed-effect statistics can be found in Supplementary data file 1. Differences are depicted as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Sample sizes are shown at the bottom of the groups in the graphs. No experimental outliers were excluded, group differences originate from apriori quality control.
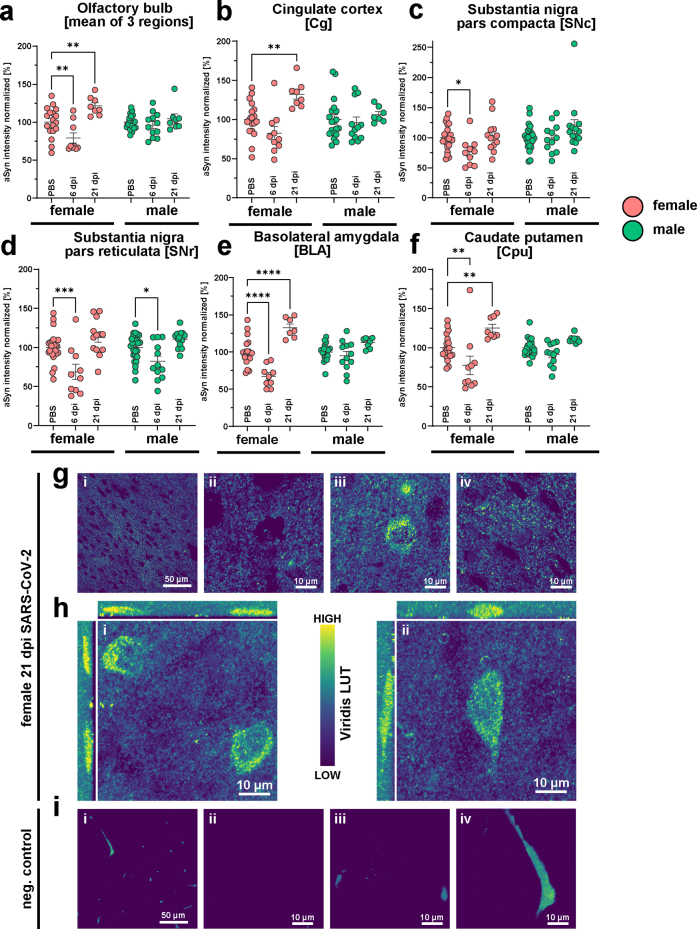
We analysed a variety of mid- and forebrain regions (n = 12, Fig. 1a, Fig. S1) involved in functions such as memory and cognition, emotion, sensory processing and motor control, which could potentially be involved in Post COVID-19 condition symptoms and/or be related to PD. Firstly, we analysed time point and sex effects across all regions. Unexpectedly, at 6 dpi, SARS-CoV-2 hamsters presented with a marked reduction of aSyn-IR compared to controls in brain (Fig. 1b–d, Fig. S2a–f, Fig. S4; Mixed-effects model test comparing all regions vs sex separated infection/PBS groups: female infection factor p < 0.0001, male p = 0.018; female region factor p = 0.0024, male ns; regions range for females = 63–84%; for males = 82.2–103.1%). While 2 out of 12 analysed brain regions showed reduced aSyn-IR in male animals, the effect was more pronounced and widespread in females in 11 out of 12 analysed regions (Fig. 1e; fishers exact test female vs male 6 dpi p = 0.0006). Conversely, at 3 weeks post-infection — a time-point well after remission and recovery from the infection — the aSyn-IR was significantly elevated relative to both PBS controls and 6 dpi in females and in males (Fig. 1b–d; Fig. S2a–f, Fig. S4; Mixed-effects model test comparing all regions vs sex separated infection/PBS groups: female p < 0.0001, male p = 0.0001; female mean = 119%; male mean 108%). The staining pattern resembled aSyn accumulation in transgenic overexpressing mice at young age, prior to the formation of protein aggregates.52,53 Again, females were more affected than males by aSyn-IR accumulation at 21 dpi (Fig. 1e, Fig. S4; 7 of 12 region in females, 0 of 12 region in males with significant differences to PBS controls, fishers exact test female vs male 21 dpi p = 0.0046).
This unexpected biphasic response, with a sharp initial decrease followed by an increase in aSyn-IR, prompted us to proceed with a detailed analysis into the different brain regions across sex and time points.
As SARS-CoV-2 was introduced intranasally, and we previously observed a microglial response in hamster olfactory bulbs (OB) at 14 dpi,23 we undertook a detailed examination of this region (Fig. 1f and g, Figs. S2a and S4). Three subregions, the glomerular layer (GL), the external plexiform layer (EPL) and the granule cell layer (GCL) were measured (Fig. 1f and g, Figs. S1 and S4). Two of three regions (GCL and EPL) showed a significant decrease in aSyn-IR in females at 6 dpi while in males differences did not reach significance. In female hamsters at 21 dpi, the aSyn-IR of all three regions developed increased aSyn-IR. In contrast, male animals showed no significant differences from controls at 21 dpi (Fig. 1f and g).
Next, four cortical regions—associated with different physiological functions—were analysed (Fig. 1f and g, Figs. S1, S2b, S4e–h; cingulate cortex (Cg), piriform cortex (Pir), visual cortex (V2m) and auditory cortex (Aud)). In female hamsters at 6 dpi, cortex regions presented with decreased aSyn-IR compared to controls (Fig. 1e and f, Aud, Pir, V2m), while at 21 dpi aSyn-IR was elevated (Fig. 1f, Fig. S2b, Aud, Cg, Pir, V2m). Due to less magnitude of change, infection of male hamsters only led to a minor decrease of aSyn-IR in Pir and did not cause further significant differences in aSyn at 6 or 21 dpi in cortex, albeit the tendencies were similar to the females (Fig. 1f and g). Both the hippocampus (Fig. 1f and g, Fig. S4i; HC, specifically the cornu ammonis 1 (CA1) region), a region involved in cognitive functions, and the substantia nigra partes compacta and reticulata (Fig. 1f–g, Figs. S2c and d and S4c and d; SNc, SNr) — the region of dopamine neuron loss in PD — exhibited a decrease in aSyn-IR at 6 dpi in females with a return to PBS levels at 21 dpi. The SNr showed a reduced aSyn-IR in males at 6 dpi (Fig. S2d), and, in direct region wide analysis, exceeded PBS levels at 21 dpi (Fig. 1g). Notably, two regions which are hubs with regard to their connectivity to other brain regions, the basolateral amygdala (Fig. 1f and g, Figs. S2e, S4a; BLA), a limbic region associated with emotional processing, and the caudate putamen (Fig. 1f and g, Figs. S2f, S4b; Cpu), a region involved in motor control and the site of dopamine loss in PD, developed a robust biphasic aSyn response in females reaching the highest levels at 21 dpi (32% increased aSyn-IR in BLA, 25% increase in Cpu).
In summary, following SARS-CoV-2 infection, aSyn-IR assessment revealed a notable biphasic response: a dip in reactivity levels at 6 dpi, followed by a rise above control levels at 21 dpi in most of the regions including OB, cortical regions, BLA and Cpu. Female hamsters exhibited notably more pronounced infection-induced changes in aSyn-IR than males.
Sex-specific microglial response following SARS-CoV-2 infection
Given the observed changes in aSyn detected in the brain, we undertook an investigation into neuroimmune homeostasis in different brain regions post SARS-CoV-2 infection. We hypothesized that alterations in aSyn-IR could correspond to variations in neuroinflammatory response to the viral infection, typically manifested as microgliosis. A precedent for this was our prior detection of microglial changes (i.e., increased numbers of Iba1+ cells at 3 and 14 dpi) in the OB of hamsters post-infection.23 We therefore evaluated if myeloid cell counts deviated at 6 and 21 dpi and if there were sex differences.
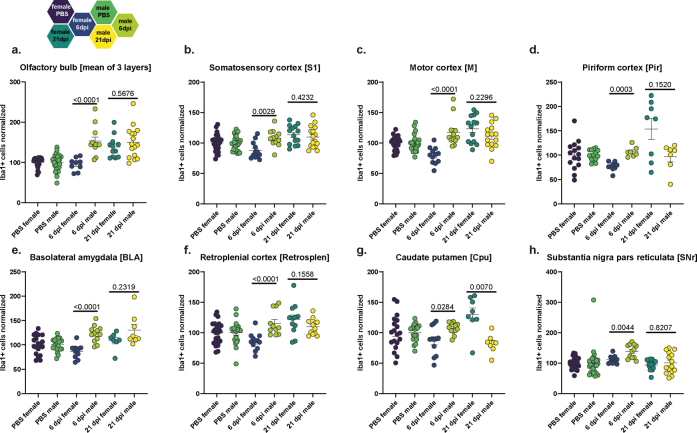
Data derived from cell counting of Iba1+ myeloid cells in various brain regions is shown in Figs. 2 and 3, Figs. S5 and S6. Similar to the aSyn investigations, we analysed a broad range of regions and subregions (n = 17) for myeloid cell density changes upon infection (Fig. 2b, Fig. S5a). Analysis across all regions revealed sex differences in the inflammatory response to SARS-CoV-2: in female animals we observed a reduced number of Iba1+ cells compared to controls at 6 dpi followed by a steep increase at 21 dpi (all regions overview 2way ANOVA with post hoc test p < 0.001; mean for females at 6 dpi = 86.2%, 21 dpi = 116%). In contrast, male animals showed a different pattern again, responding to the infection with an overall increase in Iba1 cell counts at 6 and 21 dpi (2way ANOVA p < 0.0001; mean for males at 6 dpi = 122% and at 21 dpi = 114%). Next, we will present the detailed examination of the different brain (sub)regions.
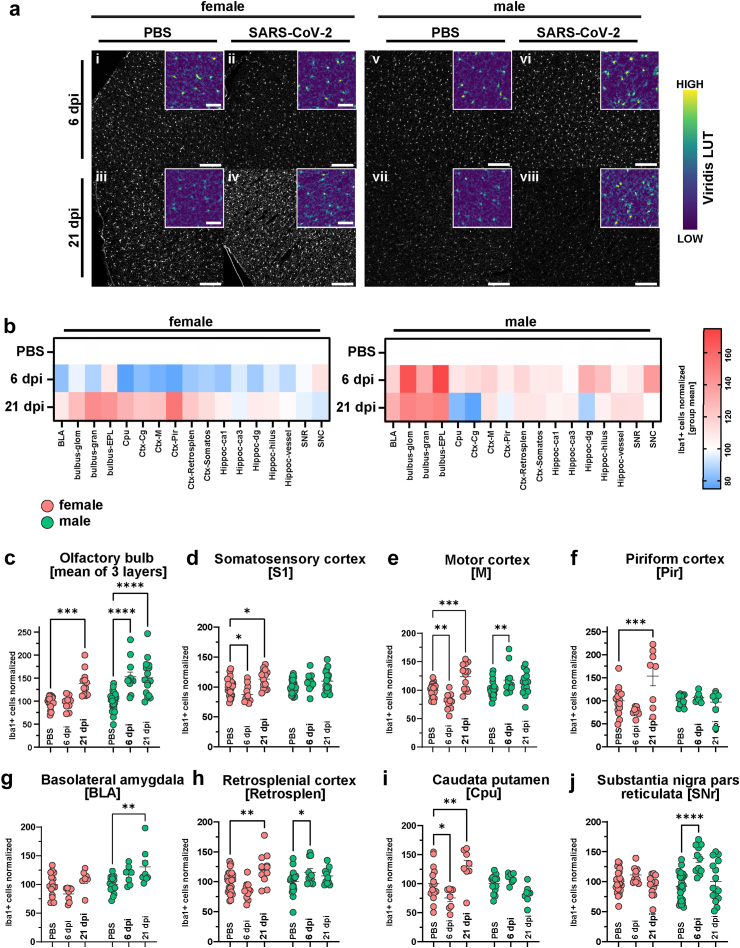
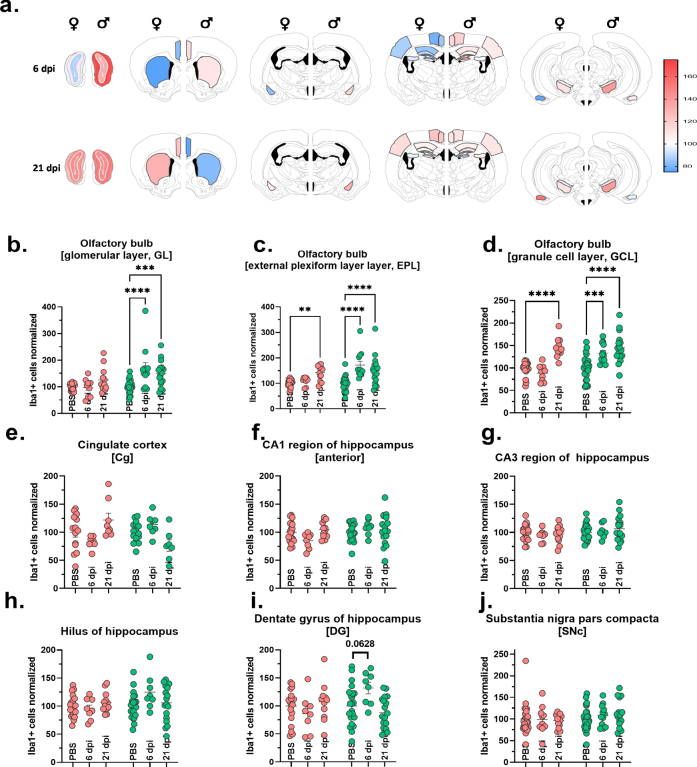
Fig. 2.
Sex-specific microglial response following SARS-CoV-2 infection. (a) Representative histological stainings of Iba1+ myeloid cells at a 10× widefield magnification from cinglulate cortex of female (i-iv) and male (v-viii) animals with PBS or SARS-CoV-2 infection at 6 and 21 dpi. Right images are zoomed in versions of the left images. Scale bar = 200 μm for overview images and 50 μm for inset close-up images. The viridis lookup table was used for the histological images in the inset to increase the visibility of intensity differences. The overview images are depicted using a gray LUT to increase visibility of cell morphology. (b) Heatmaps of group mean normalized Iba1+ cell densities per brain region in female and male animals in the three groups (PBS, 6 dpi and 21 dpi). (c–j) Quantification of mean Iba1+ cell densities (normalized to female and male PBS mean) in relevant regions. Bars represent mean ± SEM, circles depict individual animals. Red: female, green: male. Mark the different scales on the y-axes. Statistics: Two-way ANOVA analysis of Iba1+ cells across sex and brain regions followed by Šídák's multiple comparisons test (PBS vs 6 dpi and PBS vs 21 dpi for each sex). Further information on 2way ANOVA statistics and sample sizes can be found in Supplementary data file 1. (p < 0.05, ∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. No outliers excluded.
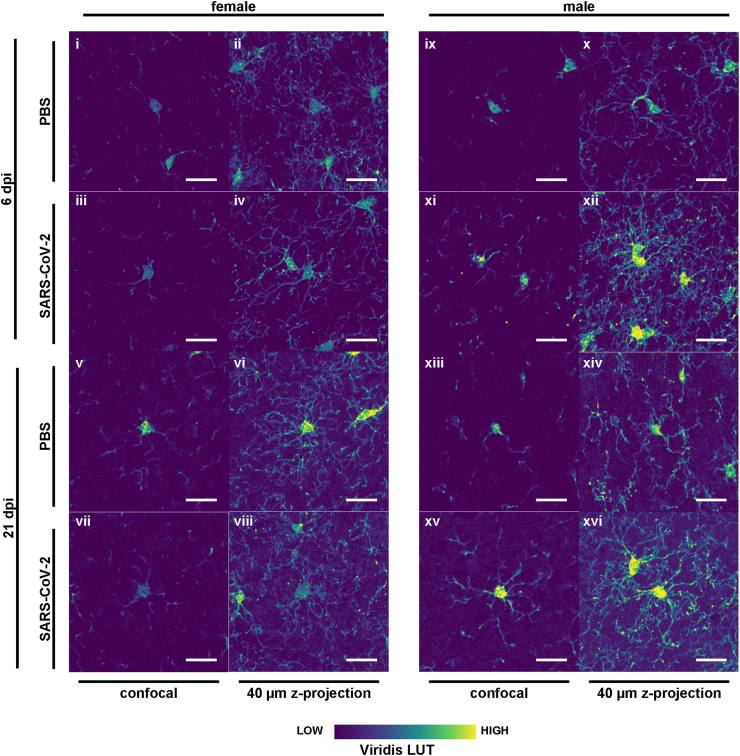
Fig. 3.
Heterogenous morphology of Iba1+ myeloid cells from female and male hamsters with SARS-CoV-2 infection. Confocal laser scanning microscopy was used to capture highly detailed Iba1+ cells for direct comparison between groups. One field is shown for females (i-viii) and males (ix-xvi) at both timepoints (6 and 21 dpi) following PBS or SARS-CoV-2 infection. Left images are confocal slices and right images are z-projections through a 40 μm brain slice. The viridis lookup table was used for the histological images to increase the visibility of instensity differences. Scale bars = 20 μm.
In female hamsters, Iba1+ cell counts in the OB, the brain region closest to the nasal site of infection, increased at 21 dpi, but not yet at 6 dpi compared to controls (Fig. 2b and c, Fig. S5a–d and S6a). Conversely, in male hamsters an early microglial response is apparent at 6 dpi and persisting until 21 dpi (Fig. 2b and c, Fig. S5a), replicating our previous observations at 14 dpi in the OB.23 We further examined the subregions of the OB, quantifying cell densities in the GL, EPL and GCL (Fig. S5a–d, Fig. S6a). These regions mirrored the mean OB results, except for the GL in female hamsters, which did not differ from controls (Fig. S5a–d).
Fig. 5.
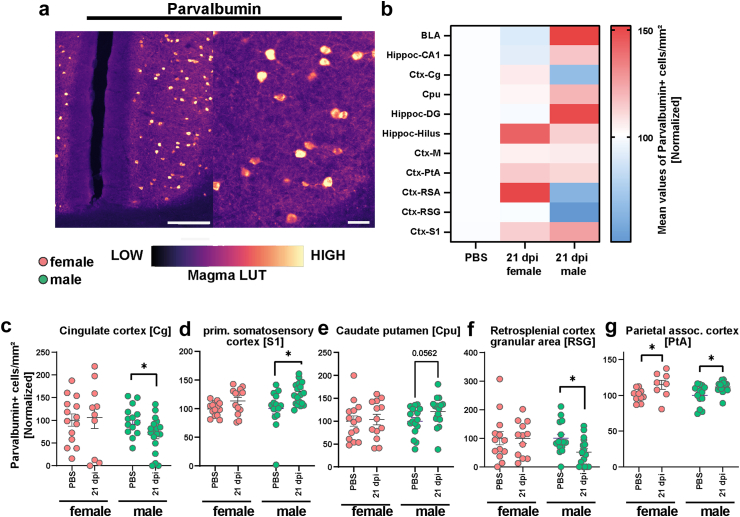
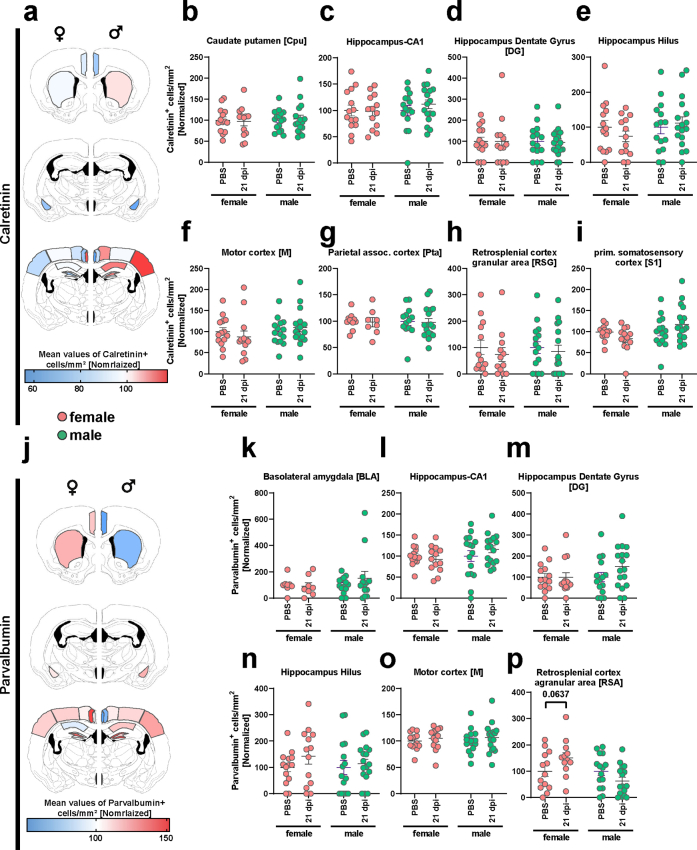
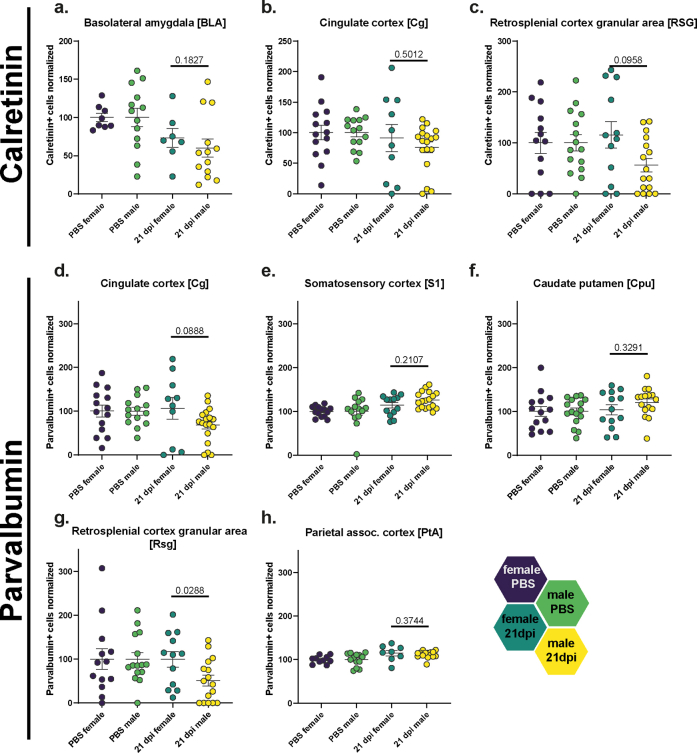
Derailing of parvalbumin immunoreactivity following SARS-CoV-2 infection. (a) Representative staining of Parvalbumin+ cells in the hamster cortex. Scale bar left 200 μm, right 50 μm. The magma lookup table was used for the histological images to increase the visibility of instensity differences. (b) Heatmaps of mean normalized Parvalbumin+ cell densities per brain region in PBS (=100%) and female and male animals at 21 dpi (region abbreviations: BLA: basolateral amygdala, CA1: hippocampus CA1 region, Cg: cingulate cortex, Cpu: caudate putamen, DG: hippocampus dentate gyrus, Hilus: hippocampus hilus region, M: motor cortex, PtA: parietal association cortex, RSA and RSG: retrosplenial cortex agranular and granular areas, S1: primary somatosensory cortex). (c–g) Parvalbumin+ cell density in the (c) Cg, (d) S1, (e) Cpu, (f) RSG and (g) PtA of female and male hamsters at 21 dpi. Data normalized to PBS control group. Bars represent mean ± SEM, circles depict individual animals. Mark the different scales on the y-axes. Statistics: Mann–Whitney U test was used to compare SARS-CoV-2 21 dpi vs PBS. Decimal numbers above columns are p values of trends. Significance levels above the bars: ∗p < 0.05. Sample sizes: Cg: 14, 10, 14, 18; S1: 14, 13, 14, 18; Cpu: 14, 13, 16, 16; RSG: 13, 12, 15, 16; PtA: 10, 8, 13, 17. No outliers were excluded.
To correlate to the observed aSyn-related changes, we inspected myeloid cell densities in similar brain regions. In female hamsters, cortical regions showed significant variations, with a dip in Iba1+ cell numbers at 6 dpi in several regions (somatosensory cortex (S1), motor cortex (M), Fig. 2d and e, Fig. S6b and c), followed by a late-onset increase that exceeded controls at 21 dpi (S1, M, piriform cortex (Pir), retrosplenial cortex (RS), Fig. 2d–f, h, Fig. S6b–d, f). Male hamsters exhibited only a subtle but significant Iba1+ cell number increase at 6 dpi (M, RS; Fig. 2e, h, Fig. S6c, f), which receded by 21 dpi to control numbers.
Iba1+ cell counts in the HC and its subregions (CA1 anterior, CA1 posterior, CA3, Hilus, dentate gyrus (DG)) did not significantly differ for both sexes (Fig. S5f–i), apart from a trend for increased numbers of cells in the DG of males at 6 dpi (Fig. S5i).
No significant differences were observed within the SNc (Fig. S5j). In the SNr, the Iba1+ cell count in female hamsters remained at control levels, while it temporarily increased at 6 dpi in male hamsters (Fig. 2j, Fig. S6h). Despite the overt biphasic changes in aSyn-IR, females did not show changes in Iba1+ cell counts in the BLA (Fig. 2g, Fig. S6e). Conversely, in infected male hamsters Iba1+ cell numbers rose to significance at 21 dpi compared to controls (Fig. 2g, Fig. S6e). Interestingly, in the Cpu, aSyn- and Iba1-related changes are aligned (Fig. 2i, Fig. S2f); females but not males exhibited the biphasic response of reduction at 6 dpi followed by an increase over control numbers at 21 dpi.
We proceeded with describing microglia cell morphology using high-resolution confocal imaging (Fig. 3). In male hamsters post SARS-CoV-2 infection, Iba1+ microglia appear reactive, characterized by numerous strongly labeled processes and an intensively labeled cell body at 6 days post-infection (dpi), with similar observations at 21 dpi (Fig. 3ix–xvi). In contrast, female microglia at 6 dpi display fewer processes and lack a reactive phenotype, presenting with small cell bodies, low staining intensity, and short, weakly labeled processes (Fig. 3i–iv). By 21 dpi, while the density of Iba1+ microglia in infected females increases, the cells remain weakly labeled and do not exhibit the ramified reactive phenotype observed in the PBS control group (Fig. 3v–viii). The microglial response is heterogeneous; some cells exhibit numerous but faintly labeled processes, whereas others have a single, thick but comparably weakly labeled process. The cell bodies vary in shape and size, more closely resembling those observed in SARS-CoV-2 infected female hamsters at 6 dpi rather than the PBS control group. In summary, male microglia exhibit pronounced reactivity post-SARS-CoV-2 infection, particularly at 6 dpi, whereas female microglia do not show the classical reactive or ramified phenotype, albeit increasing in cell number.
Our quantification of Iba1+ myeloid cells in various brain regions post SARS-CoV-2 infection unveiled infection effects that varied according to region, time post-infection, and sex. Notably, we observed a late-onset myeloid cell response in the OB of female hamsters and a rapid-onset response in the OB of males. While overall changes in the HC were marginal, we did detect specific alterations in its subregions. In the cortex, we noticed a biphasic response in females, whereas male hamsters exhibited subtle effects, displaying only a transient increase in cells during the acute phase of infection. Lastly, the SNr, Cpu and BLA regions demonstrated pronounced Iba1+ cell number shifts depending on sex, whereby only in the Cpu, the region of dopamine loss in PD, aSyn and Iba1+ alterations align in magnitude and direction.
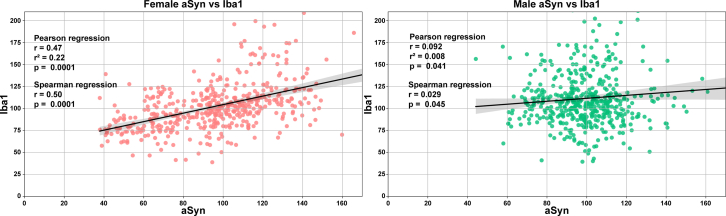
To examine potential relationships between the observed changes in aSyn-IR and microglial marker Iba1, regression analyses were performed (Fig. S9). Data across brain regions for each animal were analysed to identify correlations between aSyn and Iba1 levels based on sex. In female hamsters, a moderate positive linear relationship was uncovered between aSyn and Iba1 (p < 0.0001). Male hamsters exhibited a weaker positive linear association that reached marginal significance (p < 0.05). These findings indicate that levels of aSyn and the microglial response are closely associated, especially in female animals.
Sex differences could at least in parts be explained by altered sex hormone levels in the male hamsters, that we published recently.27 Male hamsters showed a drop in testosterone levels at 3 dpi, which fully recovered from 6 up to 14 dpi and was associated with more severe signs compared to females.27
Alterations of interneuron-marker immunoreactivity following SARS-CoV-2 infection
Based on the observed loss of aSyn homeostasis and a substantial microglial response, persisting up to three weeks after intranasal SARS-CoV-2 infection, we hypothesized that certain neuronal populations are altered post COVID-19. Our previous study examined the densities of NeuN+ neurons in the cortex and HC of infected hamsters at 14 dpi, yielding no significant differences in cell numbers.23 However, alterations in small neuronal sub-populations, especially in inhibitory interneurons, could severely alter brain function. Therefore, we performed quantification of inhibitory Parvalbumin (Parv)- and Calretinin-positive (Calret) interneurons in several brain regions of infected hamsters at 21 dpi compared to controls (Figs. 4 and 5, Fig. S7). Parv and Calret are Calcium-binding proteins that are specific markers but also relevant to neuronal function.41,54,55
Fig. 4.
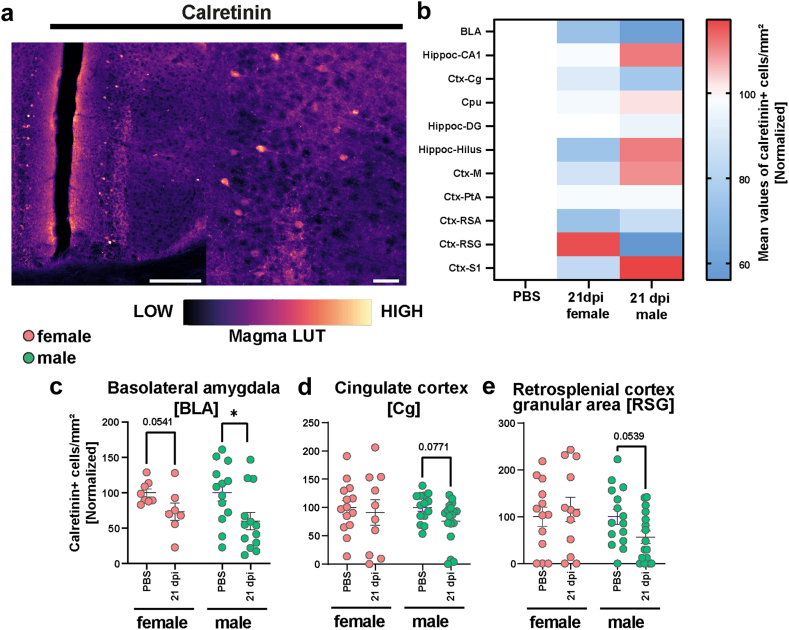
Derailing of calretinin immunoreactivity following SARS-CoV-2 infection. (a) Representative staining of Calretinin+ cells in the hamster cortex. Scale bar left 200 μm, right 50 μm. The magma lookup table was used for the histological images to increase the visibility of instensity differences. (b) Heatmaps of mean normalized Calretinin+ cell densities per brain region in PBS (=100%) and female and male animals at 21 dpi (region abbreviations: BLA: basolateral amygdala, CA1: hippocampus CA1 region, Cg: cingulate cortex, Cpu: caudate putamen, DG: hippocampus dentate gyrus, Hilus: hippocampus hilus region, M: motor cortex, PtA: parietal association cortex, RSA and RSG: retrosplenial cortex agranular and granular areas, S1: primary somatosensory cortex). (c–e) Calretinin+ cell density in the (c) BLA, (d) Cg and (e) RSG of female and male hamsters at 21 dpi. Data normalized to PBS control group. Bars represent mean ± SEM, circles depict individual animals. Mark the different scales on the y-axes. Statistics: Mann–Whitney U test was used to compare SARS-CoV-2 21 dpi vs PBS. Decimal numbers above columns are p values of trends. Significance levels above the bars: ∗p < 0.05. Sample sizes (from left to right): BLA: 8, 7, 13, 13; Cg: 14, 10, 14, 18; RSG: 13, 12, 15, 16. No outliers were excluded.
For Calret+ interneurons (Fig. 4a), male hamsters were more affected than females compared to respective controls (Fig. 4b–e, Figs. S7 and S8a–i). In males, we observed decreased numbers of Calret+ cells in the BLA (Fig. 4c, Fig. S8a), as well as a trend in the Cg (Fig. 4d) and retrosplenial cortex granular area (RSG, Fig. 4e, Fig. S8c), compared to controls. In female hamsters, a trend towards decrease was only observed within the BLA, a region highly relevant for neuropsychiatric symptoms (Fig. 4c).
In Parv+ neuron numbers, again, male hamsters were more affected by the virus infection than females compared to respective controls (Fig. 5a–g, Fig. S7j–p). While the Cg and RSG regions of males displayed a significant decrease in cell numbers (Fig. 5c, f), the parietal association (PtA) and S1 cortical areas showed significant increases (Fig. 5d, g). The Cpu showed a trend towards increased cell densities (Fig. 5e). Conversely, females only exhibited differences in the PtA, with infection associated with an increased number of cells in this area (Fig. 5g, Fig. S8h). Other regions were analysed but did not reveal significant effects (Fig. S5j–p). Correlation analyses between interneuron changes and Iba1+ cell count or aSyn-IR did not show any significant correlations (data not shown).
In summary, we identified five brain regions with altered densities of interneurons marked by Parv and Calret in hamsters post SARS-CoV-2 infection, with changes strongly depending on sex. Each region with significant Calret+ cell changes displayed decreased densities. In contrast, regions with significant changes in Parv+ cells exhibited a mix of increased and decreased densities when compared to controls.
Discussion
We discovered changes in brain homeostasis one and three weeks post-infection, including alterations in aSyn-IR, microglia densities and inhibitory interneurons, depending on sex, brain region and time post-infection.
Importantly, we previously reported absence of viral protein in brain of SARS-CoV-2 infected hamsters, which reflects observations in humans. Hence, SARS-CoV-2 infection mediates a chain of responses in brain tissue without neuroinvasion. Our previous work already suggested early microgliosis at 3 dpi restricted to the OB, and beginning accumulation of aSyn at 14 dpi in the cortex. The current study confirms microgliosis in the OB, but also demonstrates neuroinflammation across several brain regions including cortex, nigrostriatal nuclei and limbic regions at 3 weeks pi, representing a timepoint 1–2 weeks after viral clearance. Unexpectedly, in female hamsters these microglia-related changes appeared biphasic especially in the most severely affected regions, with an initial decrease in microglia cells at 6 dpi followed by microgliosis at 21 dpi. In male hamsters, microgliosis rose early and steadily in the OB, but receded at 21 dpi in most brain regions. Together with our previous observations, we conclude that the appearance of microgliosis in brain regions distant to the OB occurs after remission of the acute COVID-19 signs, and thus most likely represents a CNS resident pathomechanism. Microgliosis in the OB is likely mediated by inflammatory signalling from the nasal epithelium and invading immune cells or their mediators, and it is conceivable that such inflammatory molecules spread throughout the brain with time post-infection, involving highly complex regulatory mechanisms.19 Importantly, such processes may, if prolonged or excessive, contribute to neuronal damage.20, 21, 22 Alternatively or additionally, there could be communication of the peripheral immune response across the blood brain barrier,56,57 which would require time to develop and thus appear only at the Post COVID-19 condition phase. However, microglia reactivity could also be a protective response to SARS-CoV-2 infection and the resulting alterations in brain. To distinguish more protective from detrimental microglia reactivity, their morphology, receptor expression and transcriptome needs to be characterized.58 Even with a fully characterized microglia, statements regarding their impact on neurons requires measuring neuronal markers, such as done in this study. To further dissect these mechanisms, future studies need to define the molecular makeshift of these microglia cells and the blood brain barrier in response to SARS-CoV-2 infection.
In our previous work we detected aSyn accumulation in cortical cells at 14dpi. Apparently this accumulation progressed further and now includes several brain regions and presynaptic protein at 21dpi. Unexpectedly, aSyn-IR also responded in a biphasic fashion to the infection in female, but not in male hamsters. In females, aSyn-IR decreased in most brain regions at 6 dpi. Thereafter, confirming our earlier findings, aSyn-IR accumulates overtly at the time point 21 dpi. Interestingly, the brain regions mostly affected by this biphasic response were the BLA and the Cpu, both highly connected brain regions related to early PD pathogenesis.59, 60, 61 The Cpu represents the main input region from the dopamine neurons of the SNc, and dopamine loss in response to dopamine neurodegeneration is causing the hallmark motor symptoms of PD.62 It has long been suggested that pathology may, at least in some cases, start at the dopaminergic terminals followed by cell loss.63 Similarly, the amygdala is suggested as being affected early in PD by aSyn pathology.60,64 The recent compelling hypothesis of a dichotomy in PD pathogenesis in brain first vs gut first may well be supported by our results.65 If virus indirectly causes aSyn accumulation in highly connected hub regions, such regions may develop aSyn aggregation which could spread across the brain to connected regions in a prion like fashion, as has been demonstrated in animal models of aSyn preformed fibril inoculation.66 Further studies at later time points after SARS-CoV-2 infection are required to test this hypothesis.
But what could be the purpose or driver of neuronal aSyn accumulation upon peripheral virus infection? Many factors, such as aging, oxidative stress, genetic mutations or environmental changes have been connected to increases in aSyn67, 68, 69 and reports confirm that inflammation and infection are a possible cause for increased aSyn levels in humans and animal models.18,70,71 Therefore, aSyn increase could be mediated by microgliosis in the SARS-CoV-2 infected hamster, and in females there is significant overlap between the biphasic response across brain regions. However, in several regions microgliosis and aSyn-IR response are disconnected, and in male hamsters aSyn-IR remains elevated even after microgliosis receded. Of particular note, regression analyses uncovered a significant positive correlation between aSyn and Iba1. This relationship was stronger in female compared to male hamsters, indicating closer links between neuroinflammation and alterations in aSyn homeostasis in a sex-specific manner. The regional microgliosis observed in female animals could therefore be an important driver of the biphasic aSyn response and its accumulation by 3 weeks post-infection. These results support neuroinflammatory mechanisms as contributing to pathological protein changes relevant to neurodegeneration after SARS-CoV-2 exposure, especially in the female brain.
Additionally or alternatively, aSyn directly responds to viral induced mediators to combat a potential viral invasion.72, 73, 74 In western equine encephalitis virus (WEEV) infection of mice, a temporary aSyn pathology at 8 weeks post-infection and loss of dopaminergic neurons were demonstrated,75 likely mediated by gliosis.76 Similarly, in West Nile Virus (WNV) encephalitis increased expression of aSyn was demonstrated post mortem. Of note, aSyn expression is protective against WNV: infected aSyn knockout mice have higher viral titers, mortality and clinical severity including neurodegeneration compared to mice with aSyn expression.71 WNV was also speculated to have accelerated a prodromal case of DLB in a patient post WNV encephalitis.77 Influenza virus strains have long been connected to brain damage, e.g. invasive H5N1 or H1N1 strains can cause neurodegeneration and aSyn aggregation.78,79 Importantly, aSyn increased in non-human primates post SARS-CoV-2 infection in different brain regions, which is in line with our findings of increased levels of this protein in various regions of the brain.34 These results suggest that SARS-CoV-2 infection primes microglia enhancing responses to aSyn aggregates. Of note, while SARS-CoV-2 increases expression of AD risk genes in myeloid cells and brain tissue,80, 81, 82 plasma or CSF aSyn levels do not change in neuro COVID-19, at least around the time of neurological symptom onset.83 According to previous work, it appears unlikely that the direct interplay of SARS-CoV-2 and aSyn is the main pathogenic mechanism: while in-vitro and computer-modelling studies have shown that aSyn directly interacts with different parts of SARS-CoV-2 proteins, there is evidence for both, facilitating or inhibiting effects on aSyn pathology.33,84, 85, 86, 87.
Altogether, it is conceivable that aSyn protein levels and protein folding are altered by viral infections with or without neuroinvasion. Recently, knock-out studies revealed that aSyn is crucial for interferon responses of neurons—an essential protective mechanism against viral infections of the brain.88 Additionally, a recent study demonstrated that type I interferons (IFN-I) promote the aggregation of tau, another protein implicated in neurodegenerative diseases.89 Thus the defence mechanisms of aSyn against viral infection could employ immune regulation together with amyloid misfolding as antimicrobial strategy. In that case, viral infection-induced loss of aSyn-IR and decrease in microglia cells at 6 dpi in female hamsters would render the CNS more vulnerable to infection. Apart from this, the loss of soluble aSyn can lead to impaired dopaminergic neurotransmission.90 There is growing interest in a “toxic loss of aSyn function” theory, stating that physiological monomeric aSyn is decreased or trapped in a sink of aggregated aSyn.91, 92, 93 We did not find evidence in the staining pattern that aSyn has been redistributed or is aggregated inside the tissue or intracellularly. Thus, loss of aSyn-IR likely results from decreased expression or increased degradation through mechanisms such as the Autophagy-Lysosome Pathway, Ubiquitin-Proteasome System, proteases or other extracellular processes.94 Given that we only observed this aSyn loss at 6 dpi but not at 14 or 21 dpi, it is unlikely to mediate Post COVID-19 condition symptoms, but could be involved in acute neurological symptoms of COVID-19. Decreased measurements of aSyn in the brain or blood have already been associated with CNS disease such as bipolar disorder or schizophrenia.95,96 Interestingly, a recent study by Limanaqi and colleagues investigated the dynamics of aSyn in peripheral cells during acute SARS-CoV-2 infection in vitro.97 They found that at later time points post-infection (48 h and beyond), SARS-CoV-2 led to a decrease in SNCA mRNA expression and α-syn immunostaining in lung epithelial cells permissive to productive viral replication. Apart from loss of the protein there is the possibility that antibody binding sites were masked by an unknown folding mechanism of aSyn; however, we used the Syn1 antibody which is well characterized for its broad detection of aSyn species.98,99 Interestingly, symptoms observed in patients with COVID-19 and patients with PD overlap significantly. For example, loss of taste and smell is a common symptom of COVID-192 and a preclinical symptom of PD.100,101 While such symptoms in COVID-19 could be due to local olfactory epithelium or neuronal damage, the neural substrate of Post COVID-19 condition symptoms remains elusive. In order to derive functional consequences of microgliosis and aSyn derailing across the brain at late recovery phase in the hamster model,24 we quantified inhibitory interneurons which are integral to the excitatory/inhibitory balance of the neurocircuitry, but also especially sensitive to loss of CNS homeostasis.102, 103, 104, 105, 106, 107, 108 As they account for less than 5% of neurons in respective brain regions, their loss will not lead to apparent tissue damage, which would be in line with observations in many patients with Post COVID-19 condition.35,109,110 Thus, they are excellent candidates to illicit Post COVID-19 condition symptoms, and we studied these neurons in brain regions that could be linked to sensorimotor function, executive processing and memory formation.111,112 Parv-positive interneurons constitute the majority of cortex interneurons and are involved in the modulation of cognition and synaptic plasticity.102,113 Out of the eleven brain regions analysed, seven manifested density changes of these neurons. Once again, these differences where highly influenced by sex, which could explain sex-specific Post COVID-19 condition symptoms. Interestingly, the decrease of GABAergic interneurons in the BLA could result in disinhibition of the fear circuitry and increased fear and anxiety, as observed in patients with PD and respective animal models.41,60
The observed increase or decrease of Parv- or Calret-positive neurons in different brain regions are unlikely to represent loss or gain of neurons, but rather result from down- or upregulation of the respective calcium binding protein used to identify inhibitory interneurons. These proteins are highly regulated which can be impacted by disease processes as shown by us and others.54,114,115 Further recent studies corroborate this notion, for instance Frere et al. (2022) reported region-specific alterations in metabolism, synaptic signaling, and plasticity in hamster brains after SARS-CoV-2 infection, suggesting a disrupted brain homeostasis.26 Additionally, Radke et al. (2024) detected an interferon response and alterations in the transcriptional states of neurons and glia in brain samples from COVID-19 patients using single-cell RNAseq.116 They also found persistent changes in neuronal/synaptic gene expression even in the late disease phase. Furthermore, Yang et al. (2021) evaluated single nucleus sequencing data from brain tissue of patients who died following SARS-CoV-2 infection and observed downregulation of synaptic vesicle component genes like SNAP25 and VAMP2 in Parv+ inhibitory interneurons, suggesting impaired synaptic transmission in this neuronal population.117 These findings align with our observations of altered expression patterns in interneurons. In summary, such alterations of calcium-binding protein expression could impact interneurons and thereby brain function, but may be reversible with time, as the neurons remain intact. This is in line with the observation that the majority of Post COVID-19 condition patients experiences symptom remission. Further research is required to better understand underlying mechanisms, which could provide potential novel therapeutic targets.
Notably, men suffer from more severe symptoms and higher mortality,118 while females have a higher risk to develop Post COVID-19 condition consequences.47 One compelling explanation for sex-derived differences are hormone imbalances, as we recently showed for the hamsters used in this study. Male hamsters showed a drop in testosterone levels at 3 dpi, which fully recovered from 6 up to 14 dpi and was associated with more severe signs compared to females.27 This is in line with observations in human patients.27,45 Further mechanisms are differences in immune response, exposure to lifestyle risk factors and higher expression levels of angiotensin-converting enzyme-2 (ACE2), the receptor SARS-CoV-2 binds to.119 Females show a higher susceptibility to develop persistent immune dysregulation, autoimmune responses and persistent virus particles in reservoirs.120 Our results point out that sex-specific impacts on aSyn homeostasis, neuroinflammation, and interneuron populations following SARS-CoV-2 infection require attention. As discussed above, alterations of interneuron homeostasis is likely a specific response to the acute viral infection, and thus more prominent in males, while increased inflammatory and proteinopathy signature in females could potentially mediate persistence of altered brain homeostasis beyond the acute disease, thereby explaining the higher prevalence of Post COVID-19 condition in women.
We acknowledge that this study is limited to data from an animal model. While the COVID-19 hamster model has been extensively validated and is generally regarded as suitable to replicate the human condition, it remains an animal model and all data derived thereof need to be interpreted in light of comparable human data. This is especially challenging for brain pathology, which will require further studies in the future. Utilization of hamster tissue results in a limited number of antibodies suitable for immunohistochemistry compared to stainings of mouse tissue. However, the hamster model closely mirrors most of the main aspects of SARS-CoV-2 infection in human patients, leading to a higher transferability compared to mouse models outweighing the possibility for a broader spectrum of stainings by using mouse tissue.
Conclusion
Our findings provide insight into spatiotemporal profiles of SARS-CoV-2 effects on the brain. We demonstrate robust microglial and pathological protein responses weeks after recovery from transient infection. The regional patterns and female preponderance of pathology align with diverse neurological deficits reported in patients with Post COVID-19 condition. Of concern, the biphasic responses in highly connected limbic and motor regions could accelerate pathological processes underlying neurodegenerative disorders and Post COVID-19 condition neurological symptoms. Whether the overt aSyn protein accumulation leads to formation of early insoluble protein seeds which could serve as starting point for prion-like pathology requires future in depth comprehensive analyses. The decrease of inhibitory interneurons is a plausible substrate for imbalanced neural activity underlying cognitive and psychiatric symptoms.
These results shift focus to a time frame a time frame 7–14 days after viral clearance as a critical phase for understanding and preventing lasting neurological damage. They highlight microglial reactivity, aSyn aggregation, and neuronal homeostasis as promising therapeutic targets to mitigate both acute and delayed effects of SARS-CoV-2 on the central nervous system. Our study elucidates pathological mechanisms by which coronaviruses broadly alter brain health, and underscores the need for strategies promoting neuroprotection after exposure. Future studies will determine which of these changes in brain homeostasis persist or progress into the Post COVID-19 condition phase, and determine therapeutic interventions based on underlying mechanisms.
Contributors
Conceptualization: CK, FR; Funding acquisition: FR, GG; Supervision: CK, FR, GG; Investigation: CK, CSS; Animal experiments, necropsy: SS, SB, NM, BS; tissue processing and histological evaluation: CSS, IW, CK; Statistical Analysis and visualization: CSS, CK, FR; Resources: GG, FR; Manuscript preparation: CSS, CK, FR; Writing-Reviewing and editing: GG, SS, SB. CK and FR directly accessed and verified the underlying data reported in the manuscript. All authors read and approved the final version of the manuscript.
Data sharing statement
Data supporting the figures of this manuscript are available from the corresponding author upon reasonable request.
Declaration of interests
The authors declare that they have no conflict of interest.
Acknowledgements
We thank Anna–Sophia Hartke, Mara Lissek, Larsen Kirchoff, Scotty Käufer, Pia Hollasch, Lena-Marie Sölter, and Karolin Fockenbrock for excellent technical assistance. Supported by Federal Ministry of Health, Germany (BMG; ZMV I 1-2520COR501 to G.G.), Federal Ministry of Education and Research, Germany (BMBF; project no. 03COV06B to G.G.), the COVID-19-Research Network Lower Saxony (COFONI) through funding from the Ministry of Science and Culture of Lower Saxony in Germany (14-76403-184, project 5LZF23 to G.G. and F.R.).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105191.
Contributor Information
Franziska Richter, Email: franziska.richter@tiho-hannover.de.
Christopher Käufer, Email: Christopher.kaeufer@tiho-hannover.de.
Appendix A. Supplementary data
Fig. S1.
Fig. S2.
Fig. S3.
Fig. S4.
Fig. S5.
Fig. S6.
Fig. S7.
Fig. S8.
Fig. S9.
References
- 1.Khatoon F., Prasad K., Kumar V. Neurological manifestations of COVID-19: available evidences and a new paradigm. J Neurovirol. 2020;26(5):619–630. doi: 10.1007/s13365-020-00895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu E., Xie Y., Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022;28(11):2406–2415. doi: 10.1038/s41591-022-02001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdullahi A., Candan S.A., Abba M.A., et al. Neurological and musculoskeletal features of COVID-19: a systematic review and meta-analysis. Front Neurol. 2020;11:687. doi: 10.3389/fneur.2020.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brola W., Wilski M. Neurological consequences of COVID-19. Pharmacol Rep. 2022;74(6):1208–1222. doi: 10.1007/s43440-022-00424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanou M.I., Palaiodimou L., Bakola E., et al. Neurological manifestations of long-COVID syndrome: a narrative review. Ther Adv Chronic Dis. 2022;13 doi: 10.1177/20406223221076890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Méndez R., Balanzá-Martínez V., Luperdi S.C., et al. Long-term neuropsychiatric outcomes in COVID-19 survivors: a 1-year longitudinal study. J Intern Med. 2022;291(2):247–251. doi: 10.1111/joim.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taquet M., Dercon Q., Luciano S., Geddes J.R., Husain M., Harrison P.J. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9) doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glynne P., Tahmasebi N., Gant V., Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2022;70(1):61–67. doi: 10.1136/jim-2021-002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q., Dang C., Wang L.-H. Neuroinflammation in mild respiratory COVID-19: insights into cognitive impairment in milder cases. Mil Med Res. 2022;9(1):72. doi: 10.1186/s40779-022-00431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai F., Tomasoni D., Falcinella C., et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2022;28(4):611.e9. doi: 10.1016/j.cmi.2021.11.002. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans R.A., McAuley H., Harrison E.M., et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9(11):1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song E., Zhang C., Israelow B., et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218(3) doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seehusen F., Clark J.J., Sharma P., et al. Neuroinvasion and neurotropism by SARS-CoV-2 variants in the K18-hACE2 mouse. Viruses. 2022;14(5):1020. doi: 10.3390/v14051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce J.D., Shen Q., Cintron S.A., Hiebert J.B. Post-COVID-19 syndrome. Nurs Res. 2022;71(2):164–174. doi: 10.1097/NNR.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 17.Pratt J., Lester E., Parker R. Could SARS-CoV-2 cause tauopathy? Lancet Neurol. 2021;20(7):506. doi: 10.1016/S1474-4422(21)00168-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasen A., Houck C., Burmeister A.R., Sha Q., Brundin L., Brundin P. Upregulation of α-synuclein following immune activation: possible trigger of Parkinson's disease. Neurobiol Dis. 2022;166 doi: 10.1016/j.nbd.2022.105654. [DOI] [PubMed] [Google Scholar]
- 19.Waltl I., Kalinke U. Beneficial and detrimental functions of microglia during viral encephalitis. Trends Neurosci. 2022;45(2):158–170. doi: 10.1016/j.tins.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Phetsouphanh C., Darley D.R., Wilson D.B., et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23(2):210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 21.Fong S.-W., Goh Y.S., Torres-Ruesta A., et al. Prolonged inflammation in patients hospitalized for coronavirus disease 2019 (COVID-19) resolves 2 years after infection. J Med Virol. 2023;95(5) doi: 10.1002/jmv.28774. [DOI] [PubMed] [Google Scholar]
- 22.Käufer C., Chhatbar C., Bröer S., et al. Chemokine receptors CCR2 and CX3CR1 regulate viral encephalitis-induced hippocampal damage but not seizures. Proc Natl Acad Sci U S A. 2018;115(38):E8929–E8938. doi: 10.1073/pnas.1806754115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Käufer C., Schreiber C.S., Hartke A.-S., et al. Microgliosis and neuronal proteinopathy in brain persist beyond viral clearance in SARS-CoV-2 hamster model. eBioMedicine. 2022;79 doi: 10.1016/j.ebiom.2022.103999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usai C., Mateu L., Brander C., Vergara-Alert J., Segalés J. Animal models to study the neurological manifestations of the post-COVID-19 condition. Lab Anim. 2023;52(9):202–210. doi: 10.1038/s41684-023-01231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braxton A.M., Creisher P.S., Ruiz-Bedoya C.A., et al. Hamsters as a model of severe acute respiratory syndrome coronavirus-2. Comp Med. 2021;71(5):398–410. doi: 10.30802/AALAS-CM-21-000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frere J.J., Serafini R.A., Pryce K.D., et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations after recovery. Sci Transl Med. 2022;14(664) doi: 10.1126/scitranslmed.abq3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanelle-Bertram S., Beck S., Mounogou N.K., et al. CYP19A1 mediates severe SARS-CoV-2 disease outcome in males. Cell Rep Med. 2023;4(9):101152. doi: 10.1016/j.xcrm.2023.101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bendor J.T., Logan T.P., Edwards R.H. The function of α-synuclein. Neuron. 2013;79(6):1044–1066. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giasson B.I., Forman M.S., Higuchi M., et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300(5619):636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 30.Siegert A., Rankovic M., Favretto F., et al. Interplay between tau and alpha-synuclein liquid-liquid phase separation. Protein Sci. 2021;30(7):1326–1336. doi: 10.1002/pro.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postuma R.B., Aarsland D., Barone P., et al. Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Mov Disord. 2012;27(5):617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- 32.Visanji N.P., Lang A.E., Kovacs G.G. Beyond the synucleinopathies: alpha synuclein as a driving force in neurodegenerative comorbidities. Transl Neurodegener. 2019;8:28. doi: 10.1186/s40035-019-0172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Z., Zhang X., Huang Z., Ma K. SARS-CoV-2 proteins interact with alpha synuclein and induce lewy body-like pathology in vitro. Int J Mol Sci. 2022;23(6):3394. doi: 10.3390/ijms23063394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philippens I., Böszörményi K.P., Wubben J.A.M., et al. Brain inflammation and intracellular α-synuclein aggregates in macaques after SARS-CoV-2 infection. Viruses. 2022;14(4):776. doi: 10.3390/v14040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Douaud G., Lee S., Alfaro-Almagro F., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson B.R., Gao W.-J. PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circ. 2018;12:37. doi: 10.3389/fncir.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brisch R., Bielau H., Saniotis A., et al. Calretinin and parvalbumin in schizophrenia and affective disorders: a mini-review, a perspective on the evolutionary role of calretinin in schizophrenia, and a preliminary post-mortem study of calretinin in the septal nuclei. Front Cell Neurosci. 2015;9:393. doi: 10.3389/fncel.2015.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zallo F., Gardenal E., Verkhratsky A., Rodríguez J.J. Loss of calretinin and parvalbumin positive interneurones in the hippocampal CA1 of aged Alzheimer's disease mice. Neurosci Lett. 2018;681:19–25. doi: 10.1016/j.neulet.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Leuba G., Kraftsik R., Saini K. Quantitative distribution of parvalbumin, calretinin, and calbindin D-28k immunoreactive neurons in the visual cortex of normal and alzheimer cases. Exp Neurol. 1998;152(2):278–291. doi: 10.1006/exnr.1998.6838. [DOI] [PubMed] [Google Scholar]
- 40.Giesers N.K., Wirths O. Loss of hippocampal calretinin and parvalbumin interneurons in the 5XFAD mouse model of Alzheimer's disease. ASN Neuro. 2020;12 doi: 10.1177/1759091420925356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torres E.R.S., Stanojlovic M., Zelikowsky M., et al. Alpha-synuclein pathology, microgliosis, and parvalbumin neuron loss in the amygdala associated with enhanced fear in the Thy1-aSyn model of Parkinson's disease. Neurobiol Dis. 2021;158 doi: 10.1016/j.nbd.2021.105478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haitao T., Vermunt J.V., Abeykoon J., et al. COVID-19 and sex differences: mechanisms and biomarkers. Mayo Clin Proc. 2020;95(10):2189–2203. doi: 10.1016/j.mayocp.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J.H., Kim Y.C., Cho S.H., et al. Effect of sex hormones on coronavirus disease 2019: an analysis of 5,061 laboratory-confirmed cases in South Korea. Menopause. 2020;27(12):1376–1381. doi: 10.1097/GME.0000000000001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stasi V.D., Rastrelli G. The role of sex hormones in the disparity of COVID-19 outcomes based on gender. J Sex Med. 2021;18(12):1950–1954. doi: 10.1016/j.jsxm.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroeder M., Schaumburg B., Mueller Z., et al. High estradiol and low testosterone levels are associated with critical illness in male but not in female COVID-19 patients: a retrospective cohort study. Emerg Microbes Infect. 2021;10(1):1807–1818. doi: 10.1080/22221751.2021.1969869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelà G., Goldoni M., Solinas E., et al. Sex-related differences in long-COVID-19 syndrome. J Womens Health. 2022;31(5):620–630. doi: 10.1089/jwh.2021.0411. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian A., Nirantharakumar K., Hughes S., et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706–1714. doi: 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hastie C.E., Lowe D.J., McAuley A., et al. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in scotland study. Nat Commun. 2022;13(1):5663. doi: 10.1038/s41467-022-33415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becker K., Beythien G., de Buhr N., et al. Vasculitis and neutrophil extracellular traps in lungs of golden syrian hamsters with SARS-CoV-2. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.640842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zickler M., Stanelle-Bertram S., Ehret S., et al. Replication of SARS-CoV-2 in adipose tissue determines organ and systemic lipid metabolism in hamsters and humans. Cell Metab. 2022;34(1):1–2. doi: 10.1016/j.cmet.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schindelin J., Arganda-Carreras I., Frise E., et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chesselet M.F., Richter F., Zhu C., Magen I., Watson M.B., Subramaniam S.R. A progressive mouse model of Parkinson's disease: the Thy1-aSyn ("Line 61") mice. Neurotherapeutics. 2012;9(2):297–314. doi: 10.1007/s13311-012-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richter F., Stanojlovic M., Käufer C., Gericke B., Feja M. A mouse model to test novel therapeutics for Parkinson's disease: an update on the thy1-aSyn ("line 61") mice. Neurotherapeutics. 2023;20(1):97–116. doi: 10.1007/s13311-022-01338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulz A., Richter F., Richter A. In vivo optogenetic inhibition of striatal parvalbumin-reactive interneurons induced genotype-specific changes in neuronal activity without dystonic signs in male DYT1 knock-in mice. J Neurosci Res. 2023;101(4):448–463. doi: 10.1002/jnr.25157. [DOI] [PubMed] [Google Scholar]
- 55.Cauli B., Zhou X., Tricoire L., Toussay X., Staiger J.F. Revisiting enigmatic cortical calretinin-expressing interneurons. Front Neuroanat. 2014;8:52. doi: 10.3389/fnana.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Low R.N., Low R.J., Akrami A. A review of cytokine-based pathophysiology of long COVID symptoms. Front Med. 2023;10 doi: 10.3389/fmed.2023.1011936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elizalde-Díaz J.P., Miranda-Narváez C.L., Martínez-Lazcano J.C., Martínez-Martínez E. The relationship between chronic immune response and neurodegenerative damage in long COVID-19. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1039427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paolicelli R.C., Sierra A., Stevens B., et al. Microglia states and nomenclature: a field at its crossroads. Neuron. 2022;110(21):3458–3483. doi: 10.1016/j.neuron.2022.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alexander G.E. Biology of Parkinson's disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin Neurosci. 2004;6(3):259–280. doi: 10.31887/DCNS.2004.6.3/galexander. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai T.T., Gericke B., Feja M., Conoscenti M., Zelikowsky M., Richter F. Anxiety in synucleinopathies: neuronal circuitry, underlying pathomechanisms and current therapeutic strategies. NPJ Parkinsons Dis. 2023;9(1):97. doi: 10.1038/s41531-023-00547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu A., Lin S.-J., Mi T., et al. Decreased subregional specificity of the putamen in Parkinson's Disease revealed by dynamic connectivity-derived parcellation. Neuroimage Clin. 2018;20:1163–1175. doi: 10.1016/j.nicl.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kandel E.R. 5th ed. McGraw Hill Professional; 2013. Principles of neural science; p. 1761. [Google Scholar]
- 63.Kordower J.H., Olanow C.W., Dodiya H.B., et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136(8):2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flores-Cuadrado A., Ubeda-Bañon I., Saiz-Sanchez D., de la Rosa-Prieto C., Martinez-Marcos A. α-Synuclein staging in the amygdala of a Parkinson's disease model: cell types involved. Eur J Neurosci. 2015;41(1):137–146. doi: 10.1111/ejn.12763. [DOI] [PubMed] [Google Scholar]
- 65.Borghammer P., Horsager J., Andersen K., et al. Neuropathological evidence of body-first vs. brain-first Lewy body disease. Neurobiol Dis. 2021;161 doi: 10.1016/j.nbd.2021.105557. [DOI] [PubMed] [Google Scholar]
- 66.Vargas J.Y., Grudina C., Zurzolo C. The prion-like spreading of α-synuclein: from in vitro to in vivo models of Parkinson's disease. Ageing Res Rev. 2019;50:89–101. doi: 10.1016/j.arr.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 67.Villar-Piqué A., Lopes da Fonseca T., Sant'Anna R., et al. Environmental and genetic factors support the dissociation between α-synuclein aggregation and toxicity. Proc Natl Acad Sci U S A. 2016;113(42):E6506–E6515. doi: 10.1073/pnas.1606791113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chu Y., Kordower J.H. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is this the target for Parkinson's disease? Neurobiol Dis. 2007;25(1):134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 69.Delaidelli A., Richner M., Jiang L., et al. α-Synuclein pathology in Parkinson disease activates homeostatic NRF2 anti-oxidant response. Acta Neuropathol Commun. 2021;9(1):105. doi: 10.1186/s40478-021-01209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Linard M., Ravier A., Mougué L., et al. Infectious agents as potential drivers of α-synucleinopathies. Mov Disord. 2022;37(3):464–477. doi: 10.1002/mds.28925. [DOI] [PubMed] [Google Scholar]
- 71.Beatman E.L., Massey A., Shives K.D., et al. Alpha-synuclein expression restricts RNA viral infections in the brain. J Virol. 2016;90(6):2767–2782. doi: 10.1128/JVI.02949-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DiSabato D.J., Quan N., Godbout J.P. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139 Suppl 2(Suppl 2):136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ariño H., Heartshorne R., Michael B.D., et al. Neuroimmune disorders in COVID-19. J Neurol. 2022;269(6):2827–2839. doi: 10.1007/s00415-022-11050-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bower J.E., Radin A., Kuhlman K.R. Psychoneuroimmunology in the time of COVID-19: why neuro-immune interactions matter for mental and physical health. Behav Res Ther. 2022;154 doi: 10.1016/j.brat.2022.104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bantle C.M., Phillips A.T., Smeyne R.J., Rocha S.M., Olson K.E., Tjalkens R.B. Infection with mosquito-borne alphavirus induces selective loss of dopaminergic neurons, neuroinflammation and widespread protein aggregation. NPJ Parkinsons Dis. 2019;5(1):1–15. doi: 10.1038/s41531-019-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bantle C.M., Rocha S.M., French C.T., et al. Astrocyte inflammatory signaling mediates α-synuclein aggregation and dopaminergic neuronal loss following viral encephalitis. Exp Neurol. 2021;346 doi: 10.1016/j.expneurol.2021.113845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Segers K., Van Ranst A., Bostan A., et al. West nile virus neuroinvasive disease accelerating probable dementia with lewy bodies. Alzheimer Dis Assoc Disord. 2021;35(3):269. doi: 10.1097/WAD.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 78.Jang H., Boltz D., Sturm-Ramirez K., et al. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A. 2009;106(33):14063–14068. doi: 10.1073/pnas.0900096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marreiros R., Müller-Schiffmann A., Trossbach S.V., et al. Disruption of cellular proteostasis by H1N1 influenza A virus causes α-synuclein aggregation. Proc Natl Acad Sci U S A. 2020;117(12):6741–6751. doi: 10.1073/pnas.1906466117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Green R., Mayilsamy K., McGill A.R., et al. SARS-CoV-2 infection increases the gene expression profile for Alzheimer's disease risk. Mol Ther Methods Clin Dev. 2022;27:217–229. doi: 10.1016/j.omtm.2022.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Magusali N., Graham A.C., Piers T.M., et al. A genetic link between risk for Alzheimer's disease and severe COVID-19 outcomes via the OAS1 gene. Brain. 2021;144(12):3727–3741. doi: 10.1093/brain/awab337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou Y., Xu J., Hou Y., et al. Network medicine links SARS-CoV-2/COVID-19 infection to brain microvascular injury and neuroinflammation in dementia-like cognitive impairment. Alzheimer's Res Ther. 2021;13(1):110. doi: 10.1186/s13195-021-00850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blanco-Palmero V.A., Azcárate-Díaz F.J., Ruiz-Ortiz M., et al. Serum and CSF alpha-synuclein levels do not change in COVID-19 patients with neurological symptoms. J Neurol. 2021;268(9):3116–3124. doi: 10.1007/s00415-021-10444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Semerdzhiev S.A., Fakhree M.A.A., Segers-Nolten I., Blum C., Claessens M. Interactions between SARS-CoV-2 N-protein and α-synuclein accelerate amyloid formation. ACS Chem Neurosci. 2022;13(1):143–150. doi: 10.1021/acschemneuro.1c00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhardwaj T., Gadhave K., Kapuganti S.K., et al. Amyloidogenic proteins in the SARS-CoV and SARS-CoV-2 proteomes. Nat Commun. 2023;14(1):945. doi: 10.1038/s41467-023-36234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mesias V.S.D., Zhu H., Tang X., et al. Moderate binding between two SARS-CoV-2 protein segments and α-synuclein alters its toxic oligomerization propensity differently. J Phys Chem Lett. 2022;13(45):10642–10648. doi: 10.1021/acs.jpclett.2c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stroylova Y., Konstantinova A., Stroylov V., Katrukha I., Rozov F., Muronetz V. Does the SARS-CoV-2 spike receptor-binding domain hamper the amyloid transformation of alpha-synuclein after all? Biomedicines. 2023;11(2):498. doi: 10.3390/biomedicines11020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monogue B., Chen Y., Sparks H., et al. Alpha-synuclein supports type 1 interferon signalling in neurons and brain tissue. Brain. 2022;145(10):3622–3636. doi: 10.1093/brain/awac192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanford S.A.I., Miller L.V.C., Vaysburd M., et al. The type-I interferon response potentiates seeded tau aggregation and exacerbates tau pathology. Alzheimers Dement. 2023;20(2):1013–1025. doi: 10.1002/alz.13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abeliovich A., Schmitz Y., Fariñas I., et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 91.Kanaan N.M., Manfredsson F.P. Loss of functional alpha-synuclein: a toxic event in Parkinson's disease? J Parkinsons Dis. 2012;2(4):249–267. doi: 10.3233/JPD-012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ezzat K., Sturchio A., Espay A.J. The shift to a proteinopenia paradigm in neurodegeneration. Handb Clin Neurol. 2023;193:23–32. doi: 10.1016/B978-0-323-85555-6.00001-1. [DOI] [PubMed] [Google Scholar]
- 93.Collier T.J., Redmond D.E., Steece-Collier K., Lipton J.W., Manfredsson F.P. Is alpha-synuclein loss-of-function a contributor to parkinsonian pathology? Evidence from non-human primates. Front Neurosci. 2016;10:12. doi: 10.3389/fnins.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xilouri M., Brekk O.R., Stefanis L. α-Synuclein and protein degradation systems: a reciprocal relationship. Mol Neurobiol. 2013;47(2):537–551. doi: 10.1007/s12035-012-8341-2. [DOI] [PubMed] [Google Scholar]
- 95.Jellinger K.A. Lewy body/α-synucleinopathy in schizophrenia and depression: a preliminary neuropathological study. Acta Neuropathol. 2009;117(4):423–427. doi: 10.1007/s00401-009-0492-5. [DOI] [PubMed] [Google Scholar]
- 96.Demirel Ö.F., Cetin İ., Turan Ş., Sağlam T., Yıldız N., Duran A. Decreased expression of α-synuclein, nogo-A and UCH-L1 in patients with schizophrenia: a preliminary serum study. Psychiatry Investig. 2017;14(3):344–349. doi: 10.4306/pi.2017.14.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Limanaqi F., Zecchini S., Saulle I., et al. Alpha-synuclein dynamics bridge Type-I Interferon response and SARS-CoV-2 replication in peripheral cells. Biol Res. 2024;57(1):2. doi: 10.1186/s40659-023-00482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar S.T., Jagannath S., Francois C., Vanderstichele H., Stoops E., Lashuel H.A. How specific are the conformation-specific α-synuclein antibodies? Characterization and validation of 16 α-synuclein conformation-specific antibodies using well-characterized preparations of α-synuclein monomers, fibrils and oligomers with distinct structures and morphology. Neurobiol Dis. 2020;146 doi: 10.1016/j.nbd.2020.105086. [DOI] [PubMed] [Google Scholar]
- 99.Lashuel H.A., Mahul-Mellier A.L., Novello S., et al. Revisiting the specificity and ability of phospho-S129 antibodies to capture alpha-synuclein biochemical and pathological diversity. NPJ Parkinsons Dis. 2022;8(1):136. doi: 10.1038/s41531-022-00388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doty R.L., Deems D.A., Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38(8):1237. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- 101.Ponsen M.M., Stoffers D., Booij J., van Eck-Smit B.L.F., Wolters E.C., Berendse H.W. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol. 2004;56(2):173–181. doi: 10.1002/ana.20160. [DOI] [PubMed] [Google Scholar]
- 102.Nahar L., Delacroix B.M., Nam H.W. The role of parvalbumin interneurons in neurotransmitter balance and neurological disease. Front Psychiatry. 2021;12:679960. doi: 10.3389/fpsyt.2021.679960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tremblay R., Lee S., Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron. 2016;91(2):260–292. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moser E.I. Interneurons take charge. Nature. 2003;421(6925):797–799. doi: 10.1038/421797a. [DOI] [PubMed] [Google Scholar]
- 105.Calcagnotto M.E. Interneurons: role in maintaining and restoring synaptic plasticity. Front Psychiatry. 2016;7:86. doi: 10.3389/fpsyt.2016.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dehorter N., Marichal N., Marín O., Berninger B. Tuning neural circuits by turning the interneuron knob. Curr Opin Neurobiol. 2017;42:144–151. doi: 10.1016/j.conb.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 107.Aksenov D.P., Gascoigne D.A., Duan J., Drobyshevsky A. Function and development of interneurons involved in brain tissue oxygen regulation. Front Mol Neurosci. 2022;15 doi: 10.3389/fnmol.2022.1069496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mathys H., Peng Z., Boix C.A., et al. Single-cell atlas reveals correlates of high cognitive function, dementia, and resilience to Alzheimer's disease pathology. Cell. 2023;186(20):4365–4385.e27. doi: 10.1016/j.cell.2023.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian T., Wu J., Chen T., et al. Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight. 2022;7(4) doi: 10.1172/jci.insight.155827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Díez-Cirarda M., Yus M., Gómez-Ruiz N., et al. Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain. 2023;146(5):2142–2152. doi: 10.1093/brain/awac384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hugon J., Msika E.-F., Queneau M., Farid K., Paquet C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol. 2022;269(1):44–46. doi: 10.1007/s00415-021-10655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baillie J.K., Lone N.I., Jones S., et al. Multiorgan MRI findings after hospitalisation with COVID-19 in the UK (C-MORE): a prospective, multicentre, observational cohort study. Lancet Respir Med. 2023;11(11):1003–1019. doi: 10.1016/S2213-2600(23)00262-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Permyakov E.A., Uversky V.N. What is parvalbumin for? Biomolecules. 2022;12(5):656. doi: 10.3390/biom12050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwaller B. In: Handbook of neurochemistry and molecular neurobiology: neural protein metabolism and function. Lajtha A., Banik N., editors. Springer US; Boston, MA: 2007. Emerging functions of the “Ca2+ buffers” parvalbumin, calbindin D-28k and calretinin in the brain; pp. 197–221. [Google Scholar]
- 115.Bode C., Richter F., Spröte C., et al. Altered postnatal maturation of striatal GABAergic interneurons in a phenotypic animal model of dystonia. Exp Neurol. 2017;287(Pt 1):44–53. doi: 10.1016/j.expneurol.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 116.Radke J., Meinhardt J., Aschman T., et al. Proteomic and transcriptomic profiling of brainstem, cerebellum and olfactory tissues in early- and late-phase COVID-19. Nat Neurosci. 2024;27(3):409–420. doi: 10.1038/s41593-024-01573-y. [DOI] [PubMed] [Google Scholar]
- 117.Yang A.C., Kern F., Losada P.M., et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595(7868):565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bwire G.M. Coronavirus: why men are more vulnerable to covid-19 than women? SN Compr Clin Med. 2020;2(7):874–876. doi: 10.1007/s42399-020-00341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spudich S., Nath A. Nervous system consequences of COVID-19. Science. 2022;375(6578):267–269. doi: 10.1126/science.abm2052. [DOI] [PubMed] [Google Scholar]
- 121.Wood R.I., Morin L.P. Elsevier Science; 2001. A stereotaxic atlas of the golden hamster brain; p. 146. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.