Abstract
Human papillomavirus type 16 (HPV-16) infects the genital tract and is closely associated with the development of cervical cancer. HPV-16 initiates infection at the genital mucosal surface; thus, mucosal immune responses are likely to contribute to defense against HPV-16 infection. However, little information is available regarding the induction of immune responses in the genital tract mucosa. In this study, we evaluated the potential of intranasally administered papillomavirus vaccines to elicit both systemic and vaginal immune responses. HPV-16 virus-like particles (VLPs) produced by self-assembly of L1 protein and the HPV-16 L1 gene cloned into a mammalian expression vector were used as vaccines. Intranasally administered VLPs induced serum immunoglobulin G (IgG) and vaginal IgA secretory antibodies. Very weak serum IgG and vaginal IgA responses were found after DNA immunization. Both splenic and vaginal lymphocytes could be activated by intranasal immunization with VLPs and the HPV-16 L1 gene. Activated CD4+ Th1-like T cells were shown to synthesize gamma interferon, and activated CD8+ T cells were demonstrated to be cytotoxic.
Papillomaviruses infect and cause proliferative lesions in cutaneous and mucosal squamous epithelia. Over 100 types of human papillomavirus (HPV) have been identified (8), and 35 of them infect the genital tract. Among the genital HPVs, low-risk types induce benign lesions or genital warts, while high-risk or oncogenic types are the main cause of cervical cancer (39), the second most common cancer in women worldwide (48). High-risk HPVs are also associated with cancers of the anogenital tract and possibly also with cancers of the upper respiratory tract and skin cancers (4, 7, 54).
Since there is no effective treatment for HPV-induced lesions and since the control of cervical cancer through screening programs has largely failed in developing countries, it is essential to develop HPV vaccines to prevent and treat HPV infections and the associated diseases.
Two types of HPV vaccine are currently under development: therapeutic and prophylactic. Therapeutic vaccines are based on the induction of cellular immunity directed against cells expressing viral antigens to effect the regression of HPV-associated lesions. The E6 and E7 proteins are the natural targets for these vaccines because they are consistently expressed in cervical cancer cells. Prophylactic vaccines are based on the induction of neutralizing antibodies able to prevent HPV infection. The antigens used for the latter are the capsid proteins, L1 and L2. The generation of virus-like particles (VLPs) for the most common HPV types (51) has greatly accelerated the development of these vaccines. Animal studies have indicated that neutralizing antibodies against conformational epitopes of the L1 major capsid protein are necessary to prevent HPV infection (6, 9, 22, 53).
The principal aim of prophylactic vaccines is to prevent genital HPV infections and HPV-associated genital tumors; thus, an effective vaccine ideally should stimulate immune responses in mucosal tissues and associated lymph nodes (LNs). Such responses include the secretion of immunoglobulin A (IgA), which mediates virus neutralization to induce complete inactivation of the virus before any cells become infected. The induction of mucosal IgA responses strongly depends on help provided by CD4+ T cells, and the costimulatory molecules and cytokines that they express may play a major role in mucosal immune responses (20, 49). These T cells residing in mucosal response-inducing sites may provide a mechanism to eliminate cells undergoing productive viral infection and to prevent virus dissemination if the mucosal barrier is breached. Recent studies have provided evidence that the female reproductive tract has the characteristics of mucosal effector tissues, with IgA-producing cells, T-lymphocyte subpopulations, and secretory components (15, 27, 38, 44), indicating that a mucosal immunization strategy should elicit the secretion of both antibodies and cytotoxic T lymphocytes (CTL) in the genital tract.
Based on the concept of a common mucosal immune system (37), intranasal immunization with particular antigens has already been proven to generate specific cellular and humoral responses to numerous immunogens and to confer protection (10, 18, 55, 62). The mucosal response was shown to be enhanced by cholera toxin (CT), which has great potential as an adjuvant in intranasal vaccinations (5, 10, 55). Another candidate approach to induce mucosal immunity consists of the administration of plasmid DNA containing a viral gene (43). DNA administered intranasally is effective at inducing mucosal antibody responses (25, 61) and at conferring partial protection against genital tract pathogens (61).
Only limited information is available concerning HPV-specific mucosal immunity in the female reproductive tract. Mucosal immunity is considered essential for protection against invading pathogens, and it is therefore important to find optimal administration routes that elicit protection in the genital mucosa. Experiments with mice have shown that systemic immunization with HPV type 1 (HPV-1) VLPs does not induce cervical IgA (17), whereas i.n. administration of HPV-6b and bovine papillomavirus type 1 VLPs induces both IgG and IgA in vaginal secretions (30). In addition, anti-HPV-11 VLP cervicovaginal IgG elicited after intramuscular immunization of monkeys is sufficient to neutralize HPV-11 in the athymic mouse xenograft model (33). These results illustrate that immunization with papillomavirus VLPs via mucosal and systemic routes triggers both a systemic T-cell immune response and neutralizing antibodies at mucosal surfaces. However, none of these models is adequate to address the question of whether vaginal immunoglobulins are sufficient to protect the genital mucosa and whether VLPs elicit T-cell responses at the mucosal level.
The abilities of HPV-16 VLPs to activate systemic humoral and cellular immunity in mice when injected subcutaneously and to induce a vaginal IgA response when administered intranasally have already been described (1, 14, 41). Neutralizing antibodies have also been obtained after intramuscular immunization of rabbits with DNA coding for the L1 protein of cottontail rabbit papillomavirus (13).
We report a study to establish whether the intranasal administration of either HPV-16 L1 protein VLPs or the HPV-16 L1 gene in combination with CT elicits both systemic and mucosal humoral and cellular immune responses.
MATERIALS AND METHODS
HPV VLP vaccine preparation.
Recombinant HPV-16 L1 VLPs were prepared as previously described (28, 56). Briefly, the HPV-16 L1 coding sequence from HPV strain Sen32 (57) was cloned into the pBlueBacIII vector (Invitrogen, San Diego, Calif.). The expression vector obtained was cotransfected into Sf-21 cells together with Autographa californica multiple nuclear polyhedrosis virus genomic DNA. Sf-21 cells were infected with a recombinant baculovirus selected by end-point dilution. Nuclei of infected insect cells were sonicated, the lysate was ultracentrifuged through a 40% sucrose cushion, and VLPs were purified by isopycnic centrifugation in a CsCl gradient. After ultracentrifugation, fractions were collected and tested for density and the presence of VLPs by electron microscopy and an enzyme-linked immunosorbent assay (ELISA). The positive fractions were pooled and concentrated by ultracentrifugation (3 h at 28,000 rpm in a Beckman SW28 rotor). HPV-16 L1 VLPs were resuspended in phosphate-buffered saline (PBS) and inactivated with formaldehyde. Hepatitis B core (HBc) VLPs were prepared as previously described (58) and used as controls.
HPV DNA vaccine preparation.
The L1 coding sequence of HPV-16 from strain Fra63 (57) was cloned by PCR amplification with primers designed to introduce BglII restriction enzyme sites at the 5′ and 3′ ends. The amino acid sequences of the L1 proteins from strains Sen32 and Fra63 are identical (57). Following amplification, the PCR product was cloned into the pCRII vector (TA cloning kit; Invitrogen). After digestion with restriction enzymes HindIII and NotI, the L1 gene was cloned into pcDNA3, a mammalian expression vector (Invitrogen), under the control of the cytomegalovirus immediate-early promoter. This construct was designated HPV-16 L1 DNA. Plasmid DNA was grown in Escherichia coli DH5α and purified with Nucleobond AX 2000 (Macherey-Nalgen, Hoerdt, France). DNA concentrations were determined by spectrophotometry (A260) and confirmed by quantitative electrophoresis. HPV-16 L1 DNA was stored in 1 mM Tris (pH 7.8)–0.1 mM EDTA. DNA was diluted in 0.15 M NaCl prior to injections.
The HBc DNA plasmid contained an insertion of the HBc gene coding for the first 144 amino acids of the HBc antigen into the pcDNA3 vector under the control of the cytomegalovirus promoter (58). For this purpose, the HBc gene was excised from the pCRII-HBc vector (58) with restriction enzyme EcoRI and cloned into the pcDNA3 EcoRI restriction site. This construct was designated HBc DNA.
Mice.
Seven groups of 8- to 10-week-old female BALB/c mice (IFFA Credo, St. Germain l’Arbresle, France) were used in the immunization studies. Each experimental group was composed of five to seven mice. Experimental groups for the purification of iliac LNs and vaginal T-lymphocyte subsets were composed of 20 mice.
Immunization.
Mice received three doses of 10 μg of HPV-16 L1 VLPs combined with 2.5 μg of CT (Sigma, Saint-Quentin Fallavier, France) or 100 μg of HPV-16 L1 DNA alone or combined with 2.5 μg of CT at 15-day intervals. Each vaccine dose was diluted to 20 μl in 0.15 M NaCl and was instilled into the nostrils with a micropipette. Mice were either anesthetized with diethyl ether (Prolabo, Fontenay sous Bois, France) or conscious. Control mice received 10 μg of either HBc VLPs or HBc DNA combined with 2.5 μg of CT under anesthesia. Splenocytes and vaginal lymphocytes were harvested 15 days after the last vaccine dose. Each experiment was repeated twice.
Biological fluids.
Serum blood samples were obtained by retroorbital puncture just before vaccination and 1 week after each injection. Vaginal secretions were obtained by washing the vaginal lumen with 50 μl of PBS containing 0.1% phenylmethylsulfonyl fluoride (Sigma).
Purification of splenocytes and iliac LN cells.
Mice were sacrificed 8 days after the last immunization. Spleens and iliac LNs were harvested. Lymphocytes were isolated from both organs, and contaminating erythrocytes were lysed by hypotonic shock with a 0.83% ammonium chloride solution. Cells were resuspended in RPMI medium containing 1% HEPES (Life Technologies, Cergy Pointoise, France), 5% fetal calf serum (FCS) (Life Technologies), 2 mM l-glutamine (Gibco), 100 IU of penicillin per ml, and 100 μg of streptomycin (Gibco). For the lymphoproliferation assay, 2 × 105 cells were seeded in triplicate in wells of flat-bottom microplates (Nunc, Cergy Pontoise, France) in the presence of various dilutions of purified HPV-16 L1 VLPs (0.8 to 100 μg/ml), 100 μg of bovine serum albumin, and 10 or 25 μg of concanavalin A and incubated for 4 days (37°C, 5% CO2). Then, [3H]thymidine (18.5 kBq/mmol; NEN, Zaventem, Belgium was added and incubated for 18 h, and radioactive incorporation was measured by liquid scintillation counting. Results (means for three wells) were expressed as the change in counts per minute (counts per minute in immunized mice minus counts per minute in control mice).
Vaginal lymphocyte purification.
Vaginas were excised, cut longitudinally, and minced with a sterile scalpel in Hanks buffer without calcium and magnesium (Life Technologies). After four washes with Hanks balanced salt solution (HBSS) medium (Life Technologies) containing 1 mM EDTA, minced tissues were digested in RPMI medium-FCS containing 1 mg of collagenase type IV (Sigma) per ml and 1 mg of Dispase (Boehringer, Meylan, France) per ml. Digestion was performed under magnetic stirring (1 h, 37°C). Cells were filtered through a sterile gauze mesh and washed with RPMI medium-FCS. Additional tissue debris was excluded by low-speed centrifugation (200 × g, 10 min). Cells were collected by an additional centrifugation (400 × g, 10 min), resuspended in RPMI-FCS, and purified with a Ficoll-Hystopaque (Sigma) gradient. Approximately 3 × 105 cells were collected from seven mice.
Purification of iliac LN cells and vaginal T-lymphocyte subsets.
Iliac LN cells and vaginal cells were purified from 20 mice and were restimulated for 4 days with 15 μg of HPV-16 VLPs per ml. After 4 days, cells were resuspended in HBSS–10% FCS and washed twice before separation. Thirty million cells were incubated with a rat anti–mouse Thy-1.2 monoclonal antibody conjugated with superparamagnetic microbeads developed for a magnetic cell sorter MACS; Miltenyi, Berisch Gladbach, Germany) for 15 min at 4°C or with a rat anti-mouse CD8β monoclonal antibody (53-5.8; Pharmingen, San Diego, Calif.) for 30 min at 4°C, followed by 15 min of incubation at 4°C with goat anti-rat IgG-conjugated magnetic microbeads (Miltenyi). These complexes were applied to a column prewashed with PBS–10% FCS (PBS-FCS) on a mini-MACS system (Miltenyi). Nonadherent Thy-1-negative and CD8β-negative (CD8β−) cells were collected by passage through PBS-FCS and then were reapplied three times to the column. The separation column was then removed from the mini-MACS system, and Thy-1.2+ or CD8β+ cells were eluted by washing with PBS-FCS. Both positive and negative cells were analyzed by flow cytometry with a FACSsort (Becton Dickinson, Le Pont de Claix, France). The mini-MACS separation yielded a purity of up to 90% for each cell fraction.
Immunofluorescence labeling and flow cytometry.
Splenic and vaginal lymphocytes were phenotyped with anti-CD22 (B lymphocytes), anti-CD4+, anti-CD8+, and anti-Thy-1.2 (predominantly T lymphocytes) antibodies conjugated to fluorescein (Pharmingen). Lymphocytes were incubated (30 min at room temperature) with sheep sera to eliminate nonspecific reactions. Cells were stained with the appropriate antibodies (diluted 1:1,000) for 1 h at 4°C. After washing, phenotype analysis was performed by flow cytometry.
Cytotoxicity assay.
Thioglycolate-elicited macrophages were used as target cells. Peritoneal macrophages from three mice were collected 4 to 7 days after thioglycolate administration. Macrophages were washed three times in HBSS and dispensed in culture medium at a concentration of 3 × 104 cells/well into round-bottom tissue culture plates (Falcon, Lincoln Park, N.J.). They were incubated for at least 4 h at 37°C in 5% CO2 and then radiolabeled with 51Cr (1 μCi/well; specific activity, 469 mCi/mg; NEN) for 3 h. After washing, radiolabeled target cells were sensitized with 15 μg of HPV-16 VLPs per ml and incubated overnight. Macrophages were washed just before contact with effector cells, which were purified iliac LN or vaginal cells. After purification, the effector cells (106/ml) were activated over 4 days with 15 μg of HPV-16 VLPs per ml in plates containing nonradiolabeled macrophages (104/ml). The activated cells were collected, washed, and enumerated just before the cytotoxicity assay.
Target cells were incubated with iliac LN cells or vaginal lymphocytes at an effector/target ratio of 10:1 in a final volume of 200 μl of culture medium. After centrifugation at 200 × g for 2 min, culture plates were incubated at 37°C for 4 h. Thereafter, plates were centrifuged at 200 × g, 100 μl of supernatant was harvested from each well, and 51Cr release was assayed by liquid scintillation counting (Packard 1600 TR; Meuden). The percentage of specific 51Cr release was calculated as the mean counts per minute of the tested sample minus the mean counts per minute of spontaneous release divided by the mean counts per minute of maximal release minus the mean counts per minute of spontaneous release, multiplied by 100.
Analysis of IFN-γ in culture supernatants.
Splenic lymphocytes (2 × 106) or vaginal lymphocytes (2 × 104) were seeded in duplicate in 24-well flat-bottom culture plates (Life Technologies) in the presence or absence of 15 μg of purified HPV-16 L1 VLPs per ml. Gamma interferon (IFN-γ) production was assessed in 72-h culture supernatants by a sandwich ELISA according to the manufacturer’s recommendations with an anti–mouse IFN-γ antibody. Cytokine concentration was determined by comparison to standard curves constructed with fixed amounts of mouse recombinant IFN-γ (Genzyme, Boston, Mass.).
IgG and IgA antibody responses.
Wells of flat-bottom microplates (96 wells; Nunc) were coated overnight at 4°C with HPV-16 L1 VLPs in PBS (pH 7.4). After washing with PBS–0.1% Tween 20, PBS containing 1% newborn bovine serum (Sigma) was added (30 min at 37°C). The blocking solution was replaced with 100 μl of sera diluted from 1:50 to 1:100,000 in 5× PBS–10% newborn bovine serum–2% Tween 20, and the plates were incubated at 45°C for 90 min. Bound antibodies were detected with goat anti-mouse IgG (diluted 1:1,000) conjugated to horseradish peroxidase (Sigma). The experimental procedure for anti-IgA determination was identical to that for the detection of anti-HPV-16 L1 VLP IgG, except that the vaginal secretions were diluted from 1:2 to 1:800. Bound antibodies were detected with goat anti-mouse IgA (diluted 1:1,000) conjugated to horseradish peroxidase (Sigma). Results were expressed as geometric mean titers (GMT). An IgG GMT of less than 50 and an IgA GMT of less than 4 were considered negative.
RESULTS
Intranasal instillation of HPV-16 L1 VLPs without anesthesia induced serological antibody responses when associated with CT.
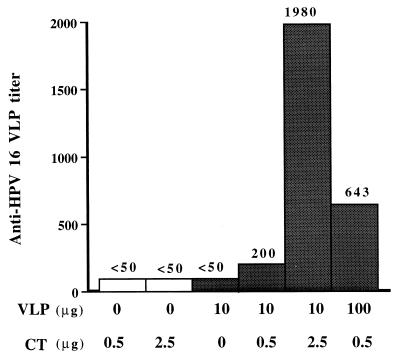
A dose-effect experiment was carried out with HPV-16 L1 VLPs and CT (Fig. 1). HPV-16 L1 VLPs alone failed to induce detectable antibodies against VLPs, but a serum IgG response to HPV-16 L1 VLPs was found in all groups of mice intranasally instilled with HPV-16 L1 VLPs combined with CT. Antibody titers increased with VLP doses and CT concentrations. The highest titer (1,980) was observed when mice received 10 μg of VLPs combined with 2.5 μg of CT. Antibody responses after intranasal instillation remained significantly lower than those obtained after subcutaneous immunization, whatever the CT or HPV-16 L1 VLP concentration (data not shown). The dose chosen for all subsequent intranasal immunizations was 10 μg of HPV-16 L1 VLPs combined with 2.5 μg of CT. As anesthesia has been shown (1, 41) to increase the immune response after intranasal immunization, both anesthetized and conscious groups were included in the experimental schedule.
FIG. 1.
IgG antibody response elicited by HPV-16 L1 VLPs in BALB/c mice immunized intranasally with VLPs combined with CT. Serum samples were tested by an ELISA with HPV-16 L1 VLP-coated plates.
VLPs administered intranasally with CT triggered serum IgG and vaginal IgA antibody responses.
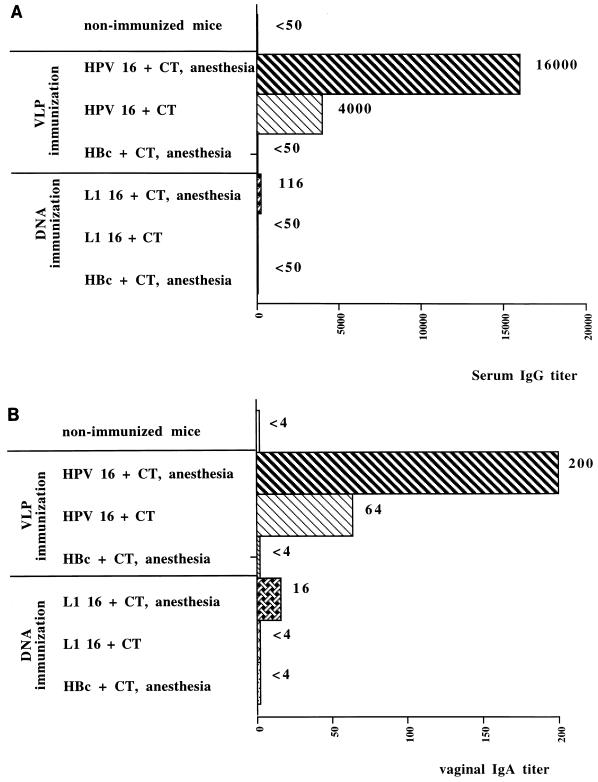
Serum anti–HPV-16 L1 VLP IgG antibodies were detected in anesthetized mice 2 weeks after the first immunization. No anti–HPV-16 L1 VLP antibodies were detected in the sera of conscious mice at that time. Serum IgG antibodies were first detected in conscious mice 15 days after the second immunization at a titer of 500; the value in anesthetized mice was 5,000 (data not shown). After the third immunization, the titer of anti-VLP antibodies remained lower in the conscious group (4,000) than in the anesthetized group (16,000) (Fig. 2A). Anti-HPV antibodies were not detected in the sera of mice immunized with HBc VLPs (control group).
FIG. 2.
IgG and IgA antibody responses in BALB/c mice immunized intranasally with HPV-16 L1 VLPs, HBc VLPs, HPV-16 L1 DNA, or HBc DNA. (A) The IgG antibody response in serum samples was assayed 7 days after the third immunization by an ELISA with HPV-16 L1 VLP-coated plates. (B) The IgA antibody response in vaginal secretions of mice was tested 7 days after the third immunization.
Among mice receiving HPV-16 L1 DNA, low levels of anti–HPV-16 IgG antibodies (titer, 116) were detected in the sera of anesthetized mice immunized with HPV-16 L1 DNA together with CT. Anti-HPV antibodies were not detected in the sera of mice receiving HBc DNA (control group).
IgA antibody responses were induced in the vaginal secretions of mice immunized with VLPs under anesthesia soon after the second immunization (data not shown). No anti–HPV-16 antibodies were detected in conscious mice at that time. The highest titer of IgA antibodies (200) was observed after the third immunization in the group of mice receiving HPV-16 L1 VLPs under anesthesia (Fig. 2B). Three immunizations were necessary to induce vaginal IgA antibody responses in the conscious group; nevertheless, titers remained lower than those in the anesthetized group. Anti–HPV-16 IgA antibodies were not detected in the HBc VLP control group. A very low IgA antibody response (titer, 16) was observed in vaginal secretions from the anesthetized mice immunized with HPV-16 L1 DNA combined with CT, but the titer obtained was higher than those obtained in the conscious mice and the mice receiving HBc DNA. Vaginal anti–HPV-16 IgG antibodies could not be detected in any of the immunization groups.
L1 VLPs and L1 DNA induced systemic cellular immune responses.
The development of cellular immune responses after immunization was monitored by enumeration of splenocytes and by phenotype characterization (Table 1). The number of splenocytes in immunized mice was higher than the number in nonimmunized mice (7 × 108 versus 1 × 108 cells/ml). The variations in splenic lymphocyte phenotypes were similar in the anesthetized and conscious HPV-16 L1 VLP-treated groups. Compared to that in nonimmunized mice, the number of B cells in mice immunized with VLPs was greatly increased (6.7 × 107 versus 28 × 107). The number of T cells was higher in mice immunized with VLPs, but to a lesser extent (7 × 107 in the nonimmunized group versus 22 × 107 in the immunized group). The increase in the number of T cells was mainly due to an increase in the number of CD4+ T cells.
TABLE 1.
Splenic and vaginal lymphocyte subsets in mice immunized intranasally with HPV-16 L1 VLPs or HPV-16 L1 DNA
| Immunization groupa | Anesthesia | Mean ± SD no. (105) of the following lymphocytes of the indicated subset:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Splenic
|
Vaginal
|
||||||||
| B | T | CD4+ | CD8+ | B | T | CD4+ | CD8+ | ||
| Nonimmunized | No | 700 ± 141 | 700 ± 99 | 300 ± 13 | 100 ± 9 | 2 ± 0.4 | 2 ± 0.07 | 0.5 ± 0.03 | 2 ± 0.4 |
| VLPs | Yes | 3,000 ± 75 | 2,000 ± 128 | 2,000 ± 104 | 700 ± 54 | 7 ± 0.2 | 7 ± 0.1 | 5 ± 0.5 | 4 ± 0.08 |
| No | 4,000 ± 158 | 2,000 ± 112 | 1,400 ± 34 | 500 ± 26 | 5 ± 0.2 | 5 ± 0.05 | 3 ± 0.06 | 4 ± 0.7 | |
| DNA | Yes | 900 ± 51 | 1,500 ± 42 | 800 ± 35 | 400 ± 15 | 6 ± 0.1 | 6 ± 0.09 | 4 ± 0.1 | 10 ± 0.06 |
| No | 900 ± 78 | 200 ± 22 | 800 ± 47 | 400 ± 29 | 5 ± 0.06 | 1 ± 0.02 | 2 ± 0.02 | 4 ± 0.07 | |
CT was included in all immunized groups.
Anesthesia and CT were essential for lymphocyte stimulation in the group receiving HPV-16 L1 DNA. The number of lymphocytes was higher in mice receiving L1 DNA combined with CT under anesthesia, whatever the lymphocyte population considered. The greatest increase was in T cells (7 × 107 in the nonimmunized group versus 2 × 108 in the anesthetized immunized group) due to increases in CD4+ and CD8+ T cells.
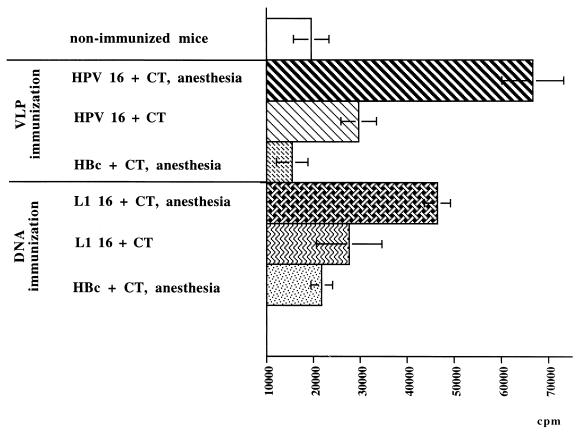
Splenocytes from mice immunized with VLPs under anesthesia displayed a specific proliferative response (65,000 cpm) following in vitro incubation with 25 μg of HPV-16 L1 VLPs per ml (Fig. 3). Proliferative responses occurred in both groups of mice immunized with HPV-16 L1 VLPs and were higher than those in the HBc VLP control group (17,000 cpm). Splenocytes from mice immunized with HPV-16 L1 DNA displayed a specific proliferative response. The proliferative response was enhanced by anesthesia, with 46,000 cpm observed in the anesthetized group and 28,000 cpm observed in the conscious group. Splenocytes from HBc DNA-immunized mice did not proliferate.
FIG. 3.
In vitro proliferation of splenic lymphocytes from mice immunized intranasally with HPV-16 L1 VLPs, HBc VLPs, HPV 16 L1 DNA, or HBc DNA and restimulated with 25 μg of HPV-16 L1 VLPs per ml. Error bars show standard deviations.
IFN-γ production by activated splenocytes was monitored after 72 h of in vitro stimulation of 2 × 106 splenocytes with 15 μg of HPV-16 L1 VLPs per ml. IFN-γ was released into culture supernatants by splenocytes purified from mice immunized with HPV-16 L1 VLPs under anesthesia (418 pg/ml) (Table 2) but was not detected in the HBc VLP control group. Splenic lymphocytes from mice immunized intranasally with HPV-16 L1 DNA released IFN-γ whether the DNA was given with anesthesia (655 pg/ml) or without anesthesia (506 pg/ml).
TABLE 2.
Quantification of IFN-γ in culture supernatants of splenic and vaginal lymphocytes from mice immunized with HPV-16 L1 VLPs, HBc VLPs, HPV-16 L1 DNA, or HBc DNAa
| Immunization groupb | Anesthesia | IFN-γ release (pg/ml) from the following lymphocytes:
|
|
|---|---|---|---|
| Splenic (2 × 106 cells) | Vaginal (2 × 104 cells) | ||
| Nonimmunized | No | <20 | <20 |
| VLPs | Yes | 418 | 60 |
| No | <20 | <20 | |
| Control (HBc) | Yes | <20 | <20 |
| DNA | Yes | 655 | <20 |
| No | 506 | <20 | |
| Control (HBc) | Yes | <20 | <20 |
The limit of detection of the assay was 20 pg/ml.
CT was included in all immunized groups.
L1 VLPs and L1 DNA induced a vaginal cellular immune response.
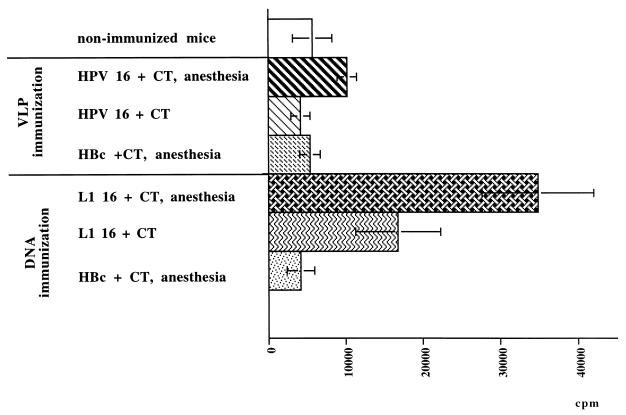
The number of vaginal lymphocytes was increased (7 × 105 cells/ml) in all of the immunized groups compared to the nonimmunized group (2 × 105 to 7 × 105 cells/ml), with an increase in the numbers of B and T cells in the group receiving HPV-16 L1 VLPs under anesthesia and in the group immunized with HPV-16 L1 DNA, whatever the immunization protocol. The difference between the L1 VLP-immunized group and the L1 DNA-immunized group depended on the cell subset stimulated. After L1 VLP immunization, the increase in the number of CD4+ T cells might have explained the change in the number of T cells, whereas after L1 DNA immunization, the change in the number of T cells was explained by the increase in the number of CD8+ T cells. Vaginal lymphocytes stimulated in vivo in the VLP-immunized group displayed a specific proliferative response (12,000 cpm) about two times higher than that of vaginal lymphocytes from the nonimmunized and HBc VLP control groups (7,000 cpm) (Fig. 4). Anesthesia enhanced vaginal lymphocyte stimulation, since a twofold increase in proliferation was obtained in anesthetized mice (6,000 and 12,000 cpm, respectively). The proliferative response was greatly increased in vaginal lymphocytes from mice immunized with HPV-16 L1 DNA and CT under anesthesia (33,000 cpm). This response was greater than that obtained in conscious mice (18,000 cpm). Proliferation was antigen specific, since lymphocytes from mice immunized with HBc DNA did not proliferate.
FIG. 4.
In vitro proliferation of vaginal lymphocytes from mice immunized intranasally with HPV-16 L1 VLPs, HBc VLPs, HPV-16 L1 DNA, or HBc DNA and restimulated with 15 μg of HPV-16 L1 VLPs per ml. Error bars show standard deviations.
IFN-γ production (Table 2) was monitored with 2 × 104 vaginal cells. IFN-γ production was detected in mice immunized with HPV-16 L1 VLPs combined with CT under anesthesia (60 pg/ml). No IFN-γ was detected in stimulated vaginal lymphocytes from the other mice.
HPV-16 L1 VLPs and HPV-16 L1 DNA induced local cytotoxicity.
Iliac LN, vaginal, and splenic lymphocytes obtained from mice immunized intranasally under anesthesia with HPV-16 L1 VLPs or with HPV-16 L1 DNA were restimulated for 4 days in vitro with HPV-16 L1 VLPs and then assayed for cytotoxicity. The effector/target ratio was 10:1 due to the low number of cells obtained from mouse vaginas. Lymphocytes purified from iliac LNs exhibited specific cytotoxicity when mice were immunized with HPV-16 L1 VLPs (14%) or with HPV-16 L1 DNA (17%) (Table 3). No cytotoxic activity was detected with vaginal and splenic lymphocytes. CD8+ T cells from iliac LNs were cytotoxic (Table 4) in mice immunized with L1 VLPs (9%) or with L1 DNA (19%). CD4+ T cells did not show cytotoxic activity.
TABLE 3.
Cytotoxic activities of splenic, iliac LN, and vaginal lymphocytes purified from 20 mice immunized intranasally with HPV-16 L1 VLPs or HPV-16 L1 DNA
| Vaccine group | % of the following lymphocytes with cytotoxic activity:
|
||
|---|---|---|---|
| Splenic | Iliac LN | Vaginal | |
| Nonimmunized | 5 | 2 | 0 |
| HPV-16 L1 VLPs | 4 | 14 | 5 |
| HPV-16 L1 DNA | 3 | 17 | 3 |
TABLE 4.
Cytotoxic activities of iliac LN CD8+ and CD8− cells from mice immunized with HPV-16 L1 VLPs or HPV-16 L1 DNA
| Iliac LN lymphocytes | % of cytotoxic iliac LN cells from mice immunized with HPV-16 L1:
|
|
|---|---|---|
| VLPs | DNA | |
| Total | 14 | 17 |
| CD8+ | 9 | 19 |
| CD8− | 0 | 5 |
DISCUSSION
Genital papillomavirus infections occur when basal cells of the genital squamous epithelium are exposed to viruses. A prophylactic vaccine against HPV infections and associated diseases should therefore provide protective immunity at the site of pathogen entry. One important component in the efforts to develop a vaccine against genital papillomaviruses is the definition of routes and conditions of immunization that stimulate mucosal immune responses as well as systemic immune responses. Detection of cell-mediated immunity and antibody-secreting cells in genital tissues following virus infections in humans and monkeys (32) has provided evidence that an appropriate vaccine strategy might be able to elicit both antibodies and CTL in the genital tract (38) and could provide protection. Such lymphocytes have already been detected in the vaginal mucosa and draining LNs after mucosal immunization (16, 23, 35).
The concept of the common mucosal immune system (37) implies that an immune response may occur in mucosal sites other than the site of vaccine administration. Indeed, some intranasally administered antigens have already been shown to induce a specific IgA antibody response in the genital tract (12, 16, 31, 36, 60). Some reports have also demonstrated that intranasal administration of a DNA vaccine leads to vaginal secretion of IgA antibodies and to major histocompatibility complex (MHC)-restricted CTL in genital LNs (6, 24, 25, 31, 50). Virus-neutralizing IgA antibodies are important in preventing local infection and disease, since binding of IgA to the virus in the mucosa can prevent attachment to epithelial cells. Systemic immunization of African green monkeys with HPV-11 VLPs resulted in transuded cervicovaginal IgG antibodies that only partially neutralized HPV-11 infection in vitro (33), suggesting that IgG alone may be insufficient for long-lasting protection.
In the present study, we observed that intranasally administered HPV-16 L1 VLPs combined with CT induced anti-VLP IgG antibodies, in agreement with the results obtained for other viral antigens (19, 29, 34, 40, 42, 52). The IgG response observed was CT dose dependent, with a 10-fold increase in IgG immune response corresponding to a 5-fold increase in the CT concentration in the vaccine. In contrast, an increase in the dose of HPV-16 L1 VLPs did not enhance the immune response proportionally. In contrast to the results of Liu et al. (30), we failed to detect any intestinal or vaginal IgA after parenteral vaccination (data not shown) as well as after intranasal immunization, regardless of the antigen and CT doses used. In our study, the highest serum reactivity was obtained after immunization with 10 μg of HPV-16 L1 VLPs combined with 2.5 μg of CT. Like Balmelli et al. (1), we found that anesthesia combined with CT increased the potential of HPV-16 L1 VLPs to induce serum IgG and vaginal IgA production. Anesthesia has also been demonstrated to promote antibody responses after intranasal administration of VLPs without an adjuvant (1). Anesthesia may increase antigen retention in the respiratory tract and consequently lead to a better interaction with lymphoid cells in nose-associated lymphoid tissues (1). Intranasal inoculation is assumed to allow the antigen to cross the nose-associated lymphoid tissues to generate a stronger secretory IgA response (26, 61). It has been demonstrated that mucosal secretions containing both anti-HPV-16 L1 VLP IgA and IgG are neutralizing (1). However, differential efficacies of neutralization by IgG and IgA have not been ruled out. Nevertheless, as demonstrated in the herpes simplex virus (HSV) type 2 and human immunodeficiency virus type 1 models (25, 45), immunity to HPV in the genital tract may also depend on the T-cell defense system in mucosal tissues, as suggested by McDermott et al. (35).
In accordance with these findings, we showed that intranasally administered HPV-16 L1 VLPs enhanced specific CD4+ T-cell populations in both the spleen and the vagina. These CD4+ T cells appeared to produce IFN-γ, known to inhibit viral infection. The CD8+ T-cell population remained stable. In vitro proliferation and IFN-γ synthesis by mucosal cells in response to antigenic stimulation provide strong evidence for putative local immune reactivity. In the HSV type 2 model, MHC class II expression was up-regulated more rapidly when immune mice were challenged with virus (34), due to the rapidly increased synthesis of IFN-γ by memory T cells. Variations in the proportions of T- and B-cell subpopulations in the vaginal mucosa add support for the concept of local cell-mediated immunity as a potential host defense mechanism in the female reproductive tract (21, 35). Moreover, we provided evidence that specific cytotoxic CD8+ cells were activated in iliac LNs by intranasally administered VLPs. This result adds support for the potential of VLPs to induce MHC class I CD8-restricted responses (11, 14), although CTL responses are usually induced by endogenously presented antigens.
Another approach to vaccination relies on the use of a DNA vaccine to stimulate humoral and cellular immunity. MHC class I CD8-restricted T cells could be continually generated after DNA vaccination due to DNA persistence in transfected cells (59). Recent reports have demonstrated the potential of DNA vaccination to induce a mucosal immune response after intranasal immunization (2, 31, 61). In our study, the adjuvant CT given with the intranasally administered DNA initiated a weak mucosal IgA response compared to that observed in an HSV model (25). We also demonstrated that intranasal immunization with HPV-16 L1 DNA provided systemic cell-mediated immune responses as well as cellular immunity at the vaginal mucosal site. The cellular immune response obtained with the vaginal lymphocytes when we used HPV-16 L1 DNA was enhanced compared to the response obtained when we used HPV-16 L1 VLPs. The low IgA level and the increase in the number of CD8+ T cells suggested that the immune response was skewed toward cytotoxicity.
In agreement with this conclusion, we demonstrated that the CD8+ T lymphocytes purified from vaginal draining LNs obtained from mice immunized with HPV-16 L1 VLPs or with HPV-16 L1 DNA displayed cytotoxicity despite the small number of effector cells used. The lack of detection of cytotoxic activity with the splenic lymphocytes might have been due to the small number of effector cells used (25). As demonstrated by Klavinskis et al. (24), we found that intranasally administered plasmid DNA can prime mucosal CTL that recirculate and localize in the iliac LNs. The mechanism for the appearance of lymphocytes in the genital tract after intranasal immunization is not clear, and little information is available regarding cell-mediated immunity in the vaginal mucosa. Recent studies have shown that vaginal lymphocytes express systemic homing receptors, αLβ2 and α4β1, suggesting that these lymphocytes are recruited at the periphery (46). Nevertheless, as suggested by Mitchell et al. (38), primed CD8+ T cells from iliac LNs should migrate to the vaginal mucosa following local antigen stimulation, thus explaining why virus-specific CTL localized in genital LNs are effective in the clearance of virus from the vagina (21, 35, 46).
In conclusion, the HPV-16 L1 DNA vaccine strongly promotes the stimulation of vaginal CD8+ T cells, which are essential for the elimination of virus-infected cells. However, the HPV-16 L1 DNA vaccine does not seem to be a good candidate for a prophylactic vaccine when given intranasally, since it induces weak humoral immunity. However, such a vaccine could be given in addition to an HPV-16 L1 VLP vaccine to increase its ability to eliminate infected cells. On the other hand, an intranasally administered E6 or E7 DNA vaccine should be considered for therapeutic use, since the L1 DNA vaccine induces a very strong T-cell immune response in the vagina. Our results also provide evidence that an intranasal HPV-16 L1 VLP vaccine induces a vaginal IgA response and activates vaginal Th1-like CD8+ T-cell-mediated cytotoxicity. Thus, intranasally administered HPV VLPs probably constitute the vaccine formulation and route of administration of choice to obtain maximum protection at the site of virus entry.
Anesthesia and CT are, of course, not suitable for human vaccination against HPV. However, an aerosol with VLPs could be administered by inhalation to target the BALT, and CT could be replaced by its nontoxic B subunit, which elicits both IgG and IgA production in the genital tract when given intranasally to humans (3). On the other hand, protective efficacy against vaginal infection of immunized mice with Chlamydia trachomatis is correlated with the production of specific vaginal IgG and IgA antibodies and a T-cell immune response (47). Thus, if VLP vaccines currently being investigated for intramuscular immunization of humans are not completely efficient against natural HPV infections, an HPV vaccine containing nontoxic CT as an adjuvant and administered via an aerosol might be an alternative.
ACKNOWLEDGMENTS
This work was supported by grants from MGEN/INSERM, the Ligue Contre le Cancer, and the Association pour la Recherche sur le Cancer (grant 5064). Catherine Dupuy was supported by a fellowship from the Conseil Régional de la Région Centre.
We thank Doreen Raine for revision of the manuscript and Isabelle Dimier, Florence Velge, Christine Bonnenfant, and Alba-Lucia Combita for technical assistance.
REFERENCES
- 1.Balmelli C, Roden R, Potts A, Schiller J, De Grandi P, Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J Virol. 1998;72:8220–8229. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ban E M, Van Gimkel F W, Simecka J W, Kiyona H, Robinson H L, McGhee J R. Mucosal immunization with DNA encoding influenza hemagglutinin. Vaccine. 1997;15:811–813. doi: 10.1016/s0264-410x(96)00263-0. [DOI] [PubMed] [Google Scholar]
- 3.Bergquist C, Johanson E-L, Lagergard T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch F X, Manos M M, Muñoz N, Sherman M, Jansen H, Peto J, Schiffman M H, Moreno V, Kurman R, Shah K V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 5.Bourguin I, Chardes T, Mevelec M-N, Woodman J P, Bout D. Amplification of the secretory IgA response to Toxoplasma gondii using cholera toxin. FEMS Microbiol Lett. 1991;81:265–272. doi: 10.1016/0378-1097(91)90225-y. [DOI] [PubMed] [Google Scholar]
- 6.Breitburd F, Kirnbauer R, Hubbert N L, Nonnenlacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller J T, Lowy D R. Immunization with virus-like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campion M J. Clinical manifestations and natural history of genital papillomavirus infections. Obstet Gynecol Clin North Am. 1987;14:363–388. [PubMed] [Google Scholar]
- 8.Chan S-H, Bernard H-U, Ratterree M, Birkebak T A, Faras A J, Ostrow R. Genomic diversity and evolution of papillomaviruses in rhesus monkeys. J Virol. 1997;71:4938–4943. doi: 10.1128/jvi.71.7.4938-4943.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen N D, Kirnbauer R, Schiller J T, Ghim S J, Schlegel R, Jenson A B, Kreider J W. Human papillomavirus types 6 and 11 have antigenically distinct strongly immunogenic conformationally dependent neutralizing epitopes. Virology. 1994;205:329–335. doi: 10.1006/viro.1994.1649. [DOI] [PubMed] [Google Scholar]
- 10.Debard N, Buzoni-Gatel D, Bout D. Intranasal immunization with SAG1 protein of Toxoplasma gondii in association with cholera toxin dramatically reduces development of cerebral cysts after oral infection. Infect Immun. 1996;64:2158–2166. doi: 10.1128/iai.64.6.2158-2166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bruijn M L H, Greenstone H L, Vermeulen H, Melief C J M, Lowy D R, Schiller J T, Kast W M. L1-specific protection from tumour challenge elicited by HPV 16 virus-like particles. Virology. 1998;250:371–376. doi: 10.1006/viro.1998.9372. [DOI] [PubMed] [Google Scholar]
- 12.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris M T. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxin mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly J J, Martinez D, Jansen K U, Ellis R W, Montgomery D L, Liu M A. Protection against papillomavirus with a polynucleotide vaccine. J Infect Dis. 1995;713:314–320. doi: 10.1093/infdis/173.2.314. [DOI] [PubMed] [Google Scholar]
- 14.Dupuy C, Buzoni-Gatel D, Touzé A, Le Cann P, Bout D, Coursaget P. Cell mediated immunity induced in mice by HPV 16 L1 virus-like particles. Microb Pathog. 1997;22:219–225. doi: 10.1006/mpat.1996.0113. [DOI] [PubMed] [Google Scholar]
- 15.Fidel P L, Wolf N A, Kukuruga M A. T lymphocytes in the murine vaginal mucosa are phenotypically distinct from those in the periphery. Infect Immun. 1996;64:3793–3799. doi: 10.1128/iai.64.9.3793-3799.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallichan W S, Rosenthal K L. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 17.Hagensee M E, Carter J J, Wipf G C, Galloway D A. Immunization of mice with HPV vaccinia virus recombinants generates serum IgG, IgM, and mucosal IgA antibodies. Virology. 1995;206:174–182. doi: 10.1016/s0042-6822(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 18.Hazama M, Mayumi-Aono A, Miyazaki T, Hinuma S, Fujisawa Y. Intranasal immunization against herpes simplex virus infection by using a recombinant glycoprotein D fused with immunomodulating proteins, the B subunit of Escherichia coli heat-labile enterotoxin and interleukin-2. Immunology. 1993;78:643–649. [PMC free article] [PubMed] [Google Scholar]
- 19.Hurwitz J L, Kenneth F S, Sangster M Y, Portner A, Sealy R E, Dawson D H, Coleclough C. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine. 1997;15:533–540. doi: 10.1016/s0264-410x(97)00217-x. [DOI] [PubMed] [Google Scholar]
- 20.Kawanishi H, Saltzman L, Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. I. T cells derived from Peyer’s patches that switch sIgM B cells to sIgA B cells in vitro. J Exp Med. 1983;157:433–450. doi: 10.1084/jem.157.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King N J, Parr E L, Parr M B. Migration of lymphoid cells from vaginal epithelium to iliac lymph nodes in relation to vaginal infection by herpes simplex virus type 2. J Immunol. 1998;160:1173–1180. [PubMed] [Google Scholar]
- 22.Kirnbauer R, Booy F, Cheng N, Lowy D R. Papillomavirus L1 major capsid protein self assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klavinskis L S, Bergmeier L A, Gao L, Mitchell E, Ward R, Layton G, Brookes R, Meyers N J, Lehner T. Mucosal or targeted lymph node immunization of macaques with a particulate SIVp27 protein elicits virus-specific CTL in the genito-rectal mucosa and draining lymph nodes. J Immunol. 1996;157:2521–2527. [PubMed] [Google Scholar]
- 24.Klavinskis L S, Barnfield C, Gao L, Parker S. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J Immunol. 1999;162:254–262. [PubMed] [Google Scholar]
- 25.Kuklin N, Daheshia M, Karem K, Manickan E, Rouse B T. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J Virol. 1997;71:3138–3145. doi: 10.1128/jvi.71.4.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuper C F, Koornstra P J, Hameleers M H, Biewenga B J, Split A M, Duijvestijn P J C, Van Breda Vriesman P, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 27.Kutteh W H, Mesteskey J. Secretory immunity in the female reproductive tract. Am J Reprod Immunol. 1994;31:40–45. doi: 10.1111/j.1600-0897.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 28.Le Cann P, Coursaget P, Iochman S, Touzé A. Self-assembly of human papillomavirus type 16 capsids by expression of the L1 protein in insect cells. FEMS Microbiol Lett. 1994;117:269–274. doi: 10.1111/j.1574-6968.1994.tb06778.x. [DOI] [PubMed] [Google Scholar]
- 29.Lehner T, Bergmeier L A, Panagiotidi C, Tao L, Brookes R, Klavinskis L S, Walker J, Ward R G, Hussain L, Gearing A J H, Adams S E. Induction of mucosal and systemic immunity to a recombinant simian immunodeficiency viral protein. Science. 1992;258:1365–1369. doi: 10.1126/science.1360702. [DOI] [PubMed] [Google Scholar]
- 30.Liu X S, Abdul-Jabbar I, Mei Qi Y, Frazer I H, Zhou J. Mucosal immunization with papillomavirus virus-like particles elicits systemic and mucosal immunity. Virology. 1999;252:39–45. doi: 10.1006/viro.1998.9442. [DOI] [PubMed] [Google Scholar]
- 31.Livingston J B, Lu S, Robinson H, Anderson D J. Immunization of the female genital tract with a DNA-based vaccine. Infect Immun. 1998;66:322–329. doi: 10.1128/iai.66.1.322-329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohman B L, Miller C J, McChesney M B. Antiviral cytotoxic T lymphocytes in vaginal mucosa of simian immunodeficiency virus-infected rhesus macaques. J Immunol. 1995;155:5855–5860. [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe R S, Brown D R, Bryan J T, Cook J C, George H A, Hofmann K J, Hurni W M, Joyce J G, Lehman E D, Markus H Z, Neeper M P, Shultz L D, Shaw A R, Jansen K U. Human papillomavirus type 11 (HPV-11) neutralizing antibodies in the serum and genital mucosal secretions of African green monkeys immunized with HPV-11 virus-like particles expressed in yeast. J Infect Dis. 1997;176:1141–1145. doi: 10.1086/514105. [DOI] [PubMed] [Google Scholar]
- 34.Marx P A, Compans R W, Gettie A, Staas J K, Gilley R M, Mulligan M J, Yamschikov G V, Chen D, Eldridge J H. Protection against vaginal SIV transmission with microencapsulated vaccine. Science. 1993;260:1323–1327. doi: 10.1126/science.8493576. [DOI] [PubMed] [Google Scholar]
- 35.McDermott M R, Brais L J, Evelegh M J. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. J Gen Virol. 1990;71:1497–1504. doi: 10.1099/0022-1317-71-7-1497. [DOI] [PubMed] [Google Scholar]
- 36.Menge A C, Michalek S M, Russell M W, Mestecky J. Immune response of the female genital tract after oral and local immunization with keyhole limpet hemocyanin conjugated to the cholera toxin B subunit. Infect Immun. 1993;61:2162–2171. doi: 10.1128/iai.61.5.2162-2171.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell E A, Bergmeier L A, Doyle C, Brookes R, Wang Y, Lehner T. Homing of mononuclear cells from iliac lymph nodes to the genital and rectal mucosa in non-human primates. Eur J Immunol. 1998;28:3066–3072. doi: 10.1002/(SICI)1521-4141(199810)28:10<3066::AID-IMMU3066>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Muñoz N, Bosch F X, De Sanjosé S, Tafur L, Izarzugaza I, Gili M, Viladiu P, Navarro C, Martos C, Ascunce N, Gonzales L C, Kaldot J M, Guerreoro E, Lorincz A, Santamaria M, Alonzo de Ruiz P, Arisizabal N, Shah K. The causal link between human papillomavirus and invasive cervical cancer, a population-based case-control study in Colombia and Spain. Int J Cancer. 1992;52:743–749. doi: 10.1002/ijc.2910520513. [DOI] [PubMed] [Google Scholar]
- 40.Muster T, Ferko B, Klima A, Purtscher M, Trkola A, Schulz P, Grassauer A, Engelhardt O G, Garcia-Sastre A, Palese P, Katinger H. Mucosal model of immunization against human immunodeficiency virus type 1 with a chimeric influenza virus. J Virol. 1995;69:6678–6686. doi: 10.1128/jvi.69.11.6678-6686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nardelly-Haefliger D, Roden R B S, Benyacoub J, Sahli R, Kraehenbuhl J-P, Schiller J T, Lachat P, Potts A, De Grandi P. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect Immun. 1997;65:3328–3336. doi: 10.1128/iai.65.8.3328-3336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Neal C M, Crawford S E, Estes M K, Conner M E. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pardoll D M, Beckering A M. Exposing the immunology of naked DNA vaccines. Immunity. 1995;3:165–169. doi: 10.1016/1074-7613(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 44.Parr M B, Parr E L. Mucosal immunity in the female and male reproductive tract. In: Ogra P L, Mestecky J, Lamm M E, Strober W, McGhee J R, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1994. pp. 677–689. [Google Scholar]
- 45.Parr M B, Parr E L. Mucosal immunity to herpes simplex virus type 2 in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J Virol. 1998;72:2677–2685. doi: 10.1128/jvi.72.4.2677-2685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry L L, Feilzer K, Portis J L, Caldwell H D. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J Immunol. 1998;160:2905–2914. [PubMed] [Google Scholar]
- 47.Peterson E M, You J Z, Motin V, de la Maza L M. Intranasal immunization with Chlamydia trachomatis, serovar E, protects from a subsequent vaginal challenge with the homologous serovar. Vaccine. 1999;17:2901–2907. doi: 10.1016/s0264-410x(99)00131-0. [DOI] [PubMed] [Google Scholar]
- 48.Pisani P, Parkin D M, Munoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev. 1997;6:387–400. [PubMed] [Google Scholar]
- 49.Ramsay A J. Genetic approaches to the study of cytokine regulation of mucosal immunity. Immunol Cell Biol. 1995;73:484–488. doi: 10.1038/icb.1995.78. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki S, Hamajima K, Fukushima J, Ahata A, Ishii N, Gorai I, Hirahara F, Mohri H, Okuda K. Comparison of intranasal and intramuscular immunization against human immunodeficiency virus type 1 with a DNA-monophosphoryl lipid A adjuvant. Infect Immun. 1998;66:823–826. doi: 10.1128/iai.66.2.823-826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiller J T, Roden R B S. Papillomavirus-like particles. Papillomavirus Rep. 1995;6:121–128. [Google Scholar]
- 52.Staats H F, Nichols W G, Palkers T J. Mucosal immunity to HIV 1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A) J Immunol. 1996;157:462–472. [PubMed] [Google Scholar]
- 53.Suzich J A, Ghim S J, Palmer-Hill F J, White W T, Tamura J K, Bell J A, Newsome J A, Jenson A B, Schlegel R S. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Syrjanen K J. Biology of human papillomavirus infections and their role in squamous cell carcinogenesis. Med Biol. 1987;65:21–39. [PubMed] [Google Scholar]
- 55.Tamura S I, Samegai Y, Kurata H, Nagamine T, Aizawa C, Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988;6:409–413. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 56.Touzé A, Dupuy C, Chabaud M, Le Cann P, Coursaget P. Production of human papillomavirus type 45 virus-like particles in insect cells using recombinant baculovirus. FEMS Microbiol Lett. 1995;141:111–116. doi: 10.1111/j.1574-6968.1996.tb08371.x. [DOI] [PubMed] [Google Scholar]
- 57.Touzé A, El Mehdaoui S, Sizaret P Y, Mougin C, Munoz N, Coursaget P. The L1 major capsid protein of human papillomavirus type 16 variants affects yield of virus-like particles produced in an insect cell expression system. J Clin Microbiol. 1998;36:2046–2051. doi: 10.1128/jcm.36.7.2046-2051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Touzé A, Enogat N, Buisson Y, Coursaget P. Baculovirus expression of chimeric hepatitis B core particles with hepatitis E epitopes and their use in a hepatitis E immunoassay. J Clin Microbiol. 1999;37:438–441. doi: 10.1128/jcm.37.2.438-441.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 60.VanCott T C, Kaminski R W, Mascola J R, Kalyanaraman V S, Wassef N M, Alving C R, Ulrich J T, Lowell G H, Birx D L. HIV-1 neutralizing antibodies in the genital and respiratory tracts of mice intranasally immunized with oligomeric gp 160. J Immunol. 1998;160:2000–2012. [PubMed] [Google Scholar]
- 61.Wang B, Dang K, Agadjannyan M G, Srikantan V, Li F, Uen K E, Boyer J, Merva M, Williams W V, Weiner D B. Mucosal immunization with a DNA vaccine induces immune responses against HIV-1 at a mucosal site. Vaccine. 1997;15:821–825. doi: 10.1016/s0264-410x(96)00259-9. [DOI] [PubMed] [Google Scholar]
- 62.Wu H Y, Russell M W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]