Abstract
All living organisms sense and respond to harmful changes in their intracellular and extracellular environment through complex signaling pathways that lead to changes in gene expression and cellular function in order to maintain homeostasis. Long non-coding RNAs (lncRNAs), a large and heterogeneous group of functional RNAs, play important roles in cellular response to stressful conditions. lncRNAs constitute a significant fraction of the genes differentially expressed in response to diverse stressful stimuli, and once induced, contribute to regulation of downstream cellular processes, including feedback regulation of key stress response proteins. While many lncRNAs seem to be induced in response to a specific stress, there is significant overlap between lncRNAs induced in response to different stressful stimuli. In addition to stress-induced RNAs, several constitutively expressed lncRNAs also exert a strong regulatory impact on the stress response. Although our understanding of the contribution of lncRNAs to the cellular stress response is still highly rudimentary, the existing data point to the presence of a complex network of lncRNAs, miRNAs and proteins in regulation of the cellular response to stress.
1. Introduction
Living systems have evolved highly sophisticated molecular networks to monitor changes in intra- and extra-cellular environment and launch complex responses against harmful disturbances to maintain cellular homeostasis and viability. These cellular responses, collectively termed the cellular stress response, are induced upon exposure to a wide variety of stimuli including changes in temperature, altered oxidative state, ischemia, harmful radiations and changes in availability of nutrients. The stressful stimuli can originate from the environment, or result from organismal responses to diseases such as cancer or infection, which result in stressful conditions including hypoxia, acidosis and oxidative stress among others (Dandekar et al. 2015). Further, some stressful conditions such as hypoxia occur during normal development (Saunders 1966; Caniggia et al. 2000). The cellular response to each stressful condition is tailored to mitigate the damage induced by the specific stressor and reprioritize appropriate aspects of cellular function in order to maintain homeostasis under suboptimal conditions. As a final safeguard, failure to curtail the stress-induced damage will activate programmed cell death pathways to eliminate the irreversibly impaired cells in order to ensure organismal viability.
As mentioned above, the cellular response to stress involves the activation of complex signaling pathways, many of which lead to transcriptional cascades aiming to alter the cellular transcriptome and proteome to optimally combat the damage induced by the stressor. This response also frequently includes reprioritization of cellular gene expression away from normal housekeeping expression pattern toward optimal expression of stress response factors (Johnson and Barton 2007; Velichko et al. 2013; Liu and Qian 2014; Espinosa-Diez et al. 2015). The result is profound changes in the composition of cellular RNAs and proteins, many aspects of which has been studied and shown to play critical roles in alleviating the impact of stress. While the study of the function of many proteins and miRNAs in stress response has provided us with a basic understanding of the regulation of this critical aspect of cellular homeostasis (Sunkar 2010; Leung and Sharp 2010; Majmundar et al. 2010; Richter et al. 2010; Cannizzo et al. 2011; Arrigo and Pulliero 2015), the role of a large class of functional cellular transcripts, the long non-coding RNAs (lncRNAs), has remained largely unstudied (Amaral et al. 2013).
1.1. lncRNAs are an Abundant and Functionally Diverse Class of Cellular Transcripts
The discovery of the extent of non-coding transcription from higher eukaryotic genomes is perhaps the most significant outcome of the high throughput transcriptome analysis efforts (Rinn and Chang 2012; Morris and Mattick 2014). Indeed, while less than 2% of the genome in mammalians codes for protein-coding transcripts, a much larger fraction is transcribed into tens of thousands of long RNAs that do not seem to have protein-coding capacity (ENCODE Project Consortium et al. 2012; Clark et al. 2013). lncRNAs are a highly heterogeneous class of transcripts and some can be tens of thousands of nucleotides long and include a large number of unspliced and non-polyadenylated transcripts (Djebali et al. 2012; ENCODE Project Consortium et al. 2012; Engelhardt and Stadler 2015). Although originally an arbitrary lower length limit of 200 nucleotides was proposed, it should not be applied too strictly as it mainly serves to distinguish lncRNAs from the small non-protein-coding cellular RNAs such as snRNAs, snoRNAs, miRNAs etc. which have distinct functions and modes of action (Clark and Mattick 2011; Rinn and Chang 2012; Mattick and Rinn 2015). While the recent availability of high throughput transcriptome analysis technologies has revealed the existence of tens of thousands of lncRNAs, it is likely that many more such transcripts remain to be discovered. This is partly due to the fact that a large fraction of lncRNAs, unlike their protein-coding counterparts, are expressed in a strongly cell type- and state-specific manner (Rinn and Chang 2012; Amaral et al. 2013; Gloss and Dinger 2015). In fact, almost every RNA-seq experiment of sufficient depth can potentially yield novel lncRNAs that are highly specific to the cell type and experimental conditions of the study. Further, a significant fraction of protein-coding loci yield alternatively transcribed or processed RNAs that lack protein-coding capacity and thus are categorized as lncRNAs (Carninci et al. 2005; Djebali et al. 2012; ENCODE Project Consortium et al. 2012).
Due to the large number and relatively recent discovery of the vast majority of lncRNAs, most of them remain unstudied. However, emerging evidence from functional analysis of a small number of lncRNAs indicate the involvement of this class of transcripts in virtually every aspect of cellular function (Wapinski and Chang 2011; Rinn and Chang 2012; Moran et al. 2012; Clark et al. 2013; Young and Ponting 2013; Amaral et al. 2013; Ulitsky and Bartel 2013; Dey et al. 2014; Yang et al. 2014b; Kapusta and Feschotte 2014). Many lncRNAs seem to be predominantly or even exclusively nuclear-localized, and consistently, their expression mostly impacts various aspects of nuclear events including regulation of the epigenetic state of chromatin and transcription (Rinn and Chang 2012; Amaral et al. 2013; Rinn 2014; Quinodoz and Guttman 2014). Indeed, many lncRNAs associate with chromatin modifying complexes and likely affect the genomic localization or other aspects of function of these complexes (Khalil et al. 2009; Hung and Chang 2010; Rinn and Chang 2012). In addition to the identity of the proteins with which a lncRNA interacts, the genomic locus of transcription of lncRNAs can yield crucial mechanistic insights into their potential function (Fig. 1). Many lncRNAs fall into the “intergenic” category of lncRNAs, as they arise from genomic loci that are located far from other annotated genes. In contrast, other lncRNAs either overlap with other genes, or are located in the vicinity of other genes without overlapping with them or with their promoter or 3’ processing sequences. In many studied cases, the expression of such “vicinal” intergenic lncRNAs affects the expression of their neighboring genes through transcriptional interference or epigenetic regulation (Valadkhan and Nilsen 2010; Rinn and Chang 2012; Mattick and Rinn 2015). Other lncRNA genes can overlap the genic region or promoter/3’ processing sequences of protein-coding genes or other non-coding RNA genes in the sense or antisense orientation (Fig. 1). Such RNAs can originate from a promoter within an exon or intron of the overlapped gene, or from promoters located within the 3’ processing sequences of the overlapped gene or even further downstream. The expression of studied overlapping genes frequently affects the biogenesis or function of the other genes in the locus through several mechanisms, including epigenetic regulation of the activity of the entire locus, transcriptional interference, or masking of functionally critical elements through basepairing to the other transcripts originating from the overlapped locus (Valadkhan and Nilsen 2010). Transcription from the so-called bidirectional promoters is another frequently observed conformation of lncRNA loci (Fig. 1) (Adachi and Lieber, 2002; Uesaka et al., 2014; Wakano et al., 2012) and in a number of studied examples, one member of the promoter-sharing pair regulates the expression of the other RNA (Wei et al. 2011; Uesaka et al. 2014). Finally, many lncRNAs are transcribed from promoters in enhancer loci and emerging evidence suggest that these transcripts play important roles in the function of the enhancers from which they originate (Fig. 1) (Lam et al. 2014; Gardini and Shiekhattar 2015). Thus, analysis of the locus of a lncRNA may help guide the study of its potential cellular function. As will be discussed in the following sections, a number of studied lncRNAs are involved in regulation of diverse aspects of stress response in all kingdoms of life, including in bacteria, unicellular eukaryotes, plants and animals (Amaral et al. 2013). In many of the examples discussed below, the genomic location of the lncRNA shows a clear correlation with its function, indicating the importance of the analysis of lncRNA loci in the study of their function.
Fig. 1. Diverse genomic origins of lncRNAs.

The genomic position of lncRNAs relative to other genes is shown. The broken arrows mark the location of transcription start sites and direction of transcription.
1.3. The Importance of Being RNA
Expansion of the non-coding transcriptome in higher eukaryotes has led to the evolution of RNA-mediated regulatory networks in nearly every aspect of cellular function. Indeed, it has been noted that the rise in RNA-mediated regulation is concomitant with evolution of complexity (Taft et al. 2007). As a biological macromolecule, RNA has many unique properties which complement those of proteins and makes it particularly suitable for many cellular functions. For example, RNAs can easily recognize and bind to another nucleic acid molecule. Through formation of RNA-RNA basepairing interactions, a regulatory RNA can sequester its target RNA, or stabilize it, or mask its cognate sequence from other regulatory elements such as proteins or other RNAs (e.g. miRNAs). Through base triplex formation with DNA and simultaneous interaction with proteins, RNAs can help increase the local concentration of proteins in a certain genomic locus (Rinn and Chang 2012; Rinn 2014).
These RNA-mediated regulatory and targeting strategies are particularly beneficial considering the much lower energetic requirement for RNA synthesis, compared to the synthesis of a protein which also involves the energetic cost of translation and folding. This can be especially helpful in cases of starvation stress or other metabolic stresses, when lowering the energetic cost of induction of stress response is highly desirable. Further, some regulatory RNAs, such as intronic lncRNAs, originate from otherwise degraded products of gene expression (Ayupe et al. 2015). Further, it has been shown that mammalian promoters are inherently bidirectional, however, the RNAs transcribed in one of the two possible directions are frequently degraded immediately after being made (Almada et al. 2013; Ntini et al. 2013; Marquardt et al. 2014; Grzechnik et al. 2014). Interestingly, some cellular lncRNAs are the result of stabilization of such “waste” RNAs generated in bidirectional promoters (Adachi and Lieber, 2002; Uesaka et al., 2014; Wakano et al., 2012), further reducing the energetic cost of lncRNA-mediated regulation. Such RNAs also constitute an ideal means for regulation of their promoter sharing transcript, since they can easily function as a homing scaffold for recruitment of regulatory protein factors, eliminating the need for protein-mediated recognition of genomic loci (Rinn and Chang 2012; Rinn 2014). In addition to the energetic cost of the extra steps involved in protein synthesis, generating an RNA regulator is much less time-consuming than making a protein. Thus, in cases such as stress response when launching a rapid cellular protective response is of paramount importance, use of RNA confers a significant advantage.
Further, RNAs can evolve much faster than proteins, as point mutations or small deletions/insertions in the sequence of a functional RNA will much less frequently lead to a catastrophic negative impact compared to a protein. Further, the modular nature of RNAs results in very different evolutionary constraints on functional RNA genes, such that despite lack of sequence conservation, the function of the RNA may remain conserved (Pang et al. 2006; Ulitsky et al. 2011). Finally, the majority of cellular proteins are over 100 amino acids long, requiring an open reading frame of 300 nucleotides or more, plus additional regulatory sequences at their 3’ and 5’ UTRs. Functional RNAs can be much smaller than this size, thus, in organisms with compact genomes such as bacteria, or wherever parsimony of size is important, RNAs can be very attractive regulatory options. In the sections that follow, study of the lncRNAs involved in response to various stresses provides examples in which evolution has taken advantage of the unique properties of RNAs for generating highly efficient and specific stress response networks.
2. Role of Non-coding RNAs in Bacterial Stress Response
The impact of non-coding RNAs on stress in bacteria has been known for over four decades with a wealth of studies uncovering the mechanism of action of many bacterial regulatory RNAs (Ikemura and Dahlberg 1973; Storz et al. 2011). The non-coding bacterial RNAs, referred to as small RNAs (sRNAs), are often conserved, range between 50–300 nucleotides in length and are ubiquitous, even to the point of outnumbering protein regulators (Storz et al. 2011). Many of these RNAs play important roles in bacterial stress response. The majority of studied sRNAs act in trans via forming short, imperfect basepairing interactions to their target RNAs and often mask ribosome binding sites to block translation (Waters and Storz 2009; Storz et al. 2011). In gram negative bacteria, this class of regulatory RNAs often associate with the Hfq RNA-binding protein (Storz et al. 2011).
Trans-acting sRNAs play important roles in bacterial response to a diverse variety of stresses, for example, two abundant non-coding RNAs in E. coli, MicA and RybB, are critical factors in the σE stress response which monitors and repairs the outer membrane of the bacteria. When envelope homeostasis is perturbed, MicA and RybB act as post-transcriptional repressors with both distinct and shared targets including several abundant porins, thus complementing the transcriptional activation function of the σE protein (Gogol et al. 2011). Another RNA, OxyS RNA, is a stable abundant transcript induced in E. coli in response to oxidative stress. It regulates the expression of several genes, including transcriptional regulators and helps protect the cells against oxidative damage (Altuvia et al. 1997). In Staphylococcus aureaus, another sRNA named RsaE accumulates in late exponential growth and interacts with the 5’ region of opp3A mRNA, which encodes an ABC transporter component. RsaE prevents the formation of the ribosomal initiation complex on opp3A and help in downregulation of metabolism when carbon sources become scarce (Bohn et al. 2010).
The translation of two key bacterial stress response proteins, the stress response sigma factor σS and H-NS, a histone-like nucleoid protein, are also regulated by an sRNA named DsrA. This RNA is induced at low temperatures in E. coli and forms a complex with Hfq. DsrA forms basepairing interactions with its target genes which in the case of the mRNA coding for σS, relieves an intramolecular secondary structure that blocks ribosome access to the ribosome binding site of the mRNA, thus allowing translation (Sledjeski et al. 1996; Lease et al. 2004; Večerek et al. 2010). Additional examples of the function of trans-acting sRNAs in stress response include roles in iron deficiency stress (RyhB) (Massé et al. 2005), elevated glycine (GcvB), glucose level changes (Spot42 and CyaR), and high glucose-phosphate levels (SgrS) (reviewed in Waters and Storz 2009). Last but not least, trans-acting bacterial RNAs also play a pivotal role in response to viral infections and the presence of foreign DNA. A well-studied class of such RNAs are the CRISPR RNAs, which target bacteriophages and plasmids and likely also play a role in silencing genes from other mobile elements (Barrangou et al. 2007; Marraffini and Sontheimer 2008).
Another class of sRNAs originate from the opposite strand of their target genes and unlike the trans-acting RNAs, which are often only induced under certain conditions, are constitutively expressed. Antisense RNAs are transcribed from the loci of a significant number of bacterial genes and regulate their overlapping RNAs through diverse mechanisms including changing the translation efficiency or stability of their target genes, or by interfering with its transcription (Thomason and Storz 2010; Georg and Hess 2011). A number of studied antisense sRNAs regulate target mRNAs that code for proteins that are toxic in higher levels. It has been proposed that such RNAs, by regulating the level of the toxic protein, help adjust the rate of growth of bacteria under stress conditions to allow the cells to repair or otherwise adapt to their new environment (Kawano et al. 2007; Unoson and Wagner 2008). In addition to regulation of the rate of bacterial growth, antisense RNAs can play direct roles in cellular response to diverse stressful stimuli. For example, an antisense sRNA, IsrR (iron stress-repressed RNA), is an antisense RNA transcribed from the opposite strand of the IsiA (iron stress-induced protein A) locus in Cyanobacteria and negatively regulates this gene. During iron deficiency, IsrR is repressed, allowing for induction of IsiA, which forms a giant ring structure around photosystem I, thus regulating photosynthesis (Dühring et al. 2006).
A third group of sRNAs, including the CsrB and 6S RNAs of E. coli, act via binding to protein targets and modifying their RNA-binding or enzymatic activity (Babitzke and Romeo 2007; Wassarman 2007). In addition to the regulatory RNAs discussed above, bacteria also take advantage of RNA motifs often located at the 5’ end of their mRNAs to regulate the expression of the gene containing the motif. Such motifs, named riboswitches, have been extensively reviewed elsewhere (Breaker 2011).
3. lncRNAs in Eukaryotic Stress Response
3.1. lncRNAs in Hypoxic Stress: lnc-ing Stress to Cancer
In addition to physiological hypoxia occurring during development (Saunders 1966; Caniggia et al. 2000) and at high altitudes, hypoxia plays an important role in a number of human pathologies, including ischemic stroke and many solid tumors (Beasley et al. 2002; Kaidi et al. 2006; Semenza 2012a; Oh et al. 2012; Luo et al. 2014; Erickson et al. 2015; Chang et al. 2015). The hypoxia-inducible factors 1 and 2 (HIF-1 and HIF-2, Fig. 2) are transcription factors which act as key mediators of the hypoxic response. HIF-1 and HIF-2 differ in one of their subunits, HIF-1α and HIF-2α, which have similar DNA binding and dimerization domains but distinct transactivation domains. Consequently, while they share many of their target genes, each of them also induces several unique targets. During hypoxia, HIF-1α and HIF-2α form dimers with HIF-1β to form the active HIF-1 and HIF-2 complexes, which will translocate to the nucleus and bind to hypoxia response elements (HREs,5′-RCGTG-3′) in the promoters or enhancers of hypoxia-responsive genes (Fig. 2) (Kaelin and Ratcliffe 2008; Majmundar et al. 2010). Hypoxia-induced genes, in turn, play important roles in regulation of cellular metabolism, survival/apoptosis pathways, proliferation, angiogenesis, and several aspects of tumorigenesis including migration, invasion and metastasis (Majmundar et al. 2010; Semenza 2012b). Indeed, activation of HIF1 and HIF2 is associated with a more aggressive tumor phenotype and poor prognosis in many cancers (Yang et al. 2011; Yang et al. 2013b).
Fig. 2. Simplified schematic summary of the role of lncRNAs in the hypoxia response pathway.

The hypoxia-induced and repressed lncRNAs are shown in red and blue font, respectively.
3.1.1. HIF-Induced lncRNAs and Their Role in Hypoxic Response
The transcriptional cascade induced by HIF proteins involve the induction of a large number of both protein-coding RNAs and lncRNAs (Choudhry et al. 2014), including a number of studied RNAs such as NEAT1, UCA1, linc-ROR and H19 among others (Choudhry et al. 2014; Yu et al. 2015; Yang et al. 2015; Chang et al. 2015). In a high throughput study of the transcriptome of hypoxic and normoxic MCF-7 human cells, Choudhry and colleagues (2014) identified a large number of lncRNAs, including many previously unannotated ones, that were induced in response to hypoxia. These RNAs included many antisense lncRNAs, which showed coordinated regulation with the genes they overlapped. Many hypoxia-induced lncRNAs showed proximity to previously-identified HIF-1 and HIF-2 binding sites (Choudhry et al. 2014), suggesting that they were directly regulated by these transcription factors. Another high throughput study using microarrays targeting the transcribed ultraconserved regions of the human genome identified ~ 60 putative lncRNAs that were differentially expressed between hypoxic and normoxic cells in a HIF-dependent manner (Ferdin et al. 2013). One of the identified RNAs, which originated from an intron of the O-linked N-acetylglucosamine (GlcNAc) transferase (OGT) gene, seems to correspond to a hypoxia-stabilized intronic RNA of ~400 nucleotide length. This RNA, named HINCUT-1/uc.475, may play a role in regulation of the function of its host gene, which is also overexpressed in response to hypoxia and in epithelial cancers, and plays an important role in tumor formation (Ferdin et al. 2013). A third high throughput study used lncRNA microarrays to analyze the changes in the pattern of gene expression in cortex of rats subjected to an hour of cerebral ischemia. Similar to the two previous studies, the results indicated significant changes in the non-coding transcriptome, with over three hundred and fifty RNAs upregulated and over eighty showing significantly reduced expression (Dharap et al. 2012). It will be interesting to compare the three high throughput datasets obtained by the above studies to determine the degree of overlap of the hypoxic response in different tissues/cell lines and under slightly different conditions.
In addition to the global studies of the lncRNA expression patterns, several functional studies have analyzed the role of individual lncRNAs in the hypoxic response. Study of the function of NEAT1, a nuclear-localized lncRNA which participate in formation of paraspeckles, has shown that it is directly induced by HIF-2 (Choudhry et al. 2015). In a number of breast cancer cell lines and solid tumors, hypoxia-mediated induction of NEAT1 led to an increase in the number of paraspeckles. This, in turn, led to changes in subcellular localization of some RNAs, increased cellular proliferation and survival and a decrease in apoptosis, thus providing a mechanistic explanation for the observed correlation between the expression level of NEAT1 in breast cancer and poor survival (Choudhry et al. 2015) (Fig. 2).
Another hypoxia-induced lncRNA, lincRNA-p21, is induced by HIF-1 and interacts with both HIF-1α subunit of HIF-1 and the von Hippel-Lindau (VHL) protein, which acts as a ubiquitin E3 ligase (Yang et al. 2014a). In the absence of hypoxia, HIF-1α is hydroxylated followed by ubiquitination by VHL, leading to its rapid degradation. On the other hand, during hypoxia, hydroxylation of HIF-1α is inhibited, leading to its accumulation and formation of the active HIF-1 complex. Through interaction with both HIF-1α and VHL, lincRNA-p21 disrupts the interaction of the two proteins, leading to accumulation of HIF-1α even in the presence of oxygen (Yang et al. 2014a). Through induction of expression of glucose transporters, glycolytic enzymes and additional metabolic enzymes, activation of HIF-1 leads to upregulation of glycolysis and downregulation of oxidative phosphorylation during normoxia, which is observed in many tumor cells (Bartrons and Caro 2007). This metabolic adaptation results in easy availability of precursors for de novo nucleotide and lipid synthesis and minimizes the reactive oxygen species generated in the mitochondria, leading to a growth advantage for cancer cells. As expected, the level of lincRNA-p21 was found to be proportional to tumor growth level in mouse xenograft models (Yang et al. 2014a). In addition to its HIF-1-mediated effect on cancer cells, lincRNA-p21 has been shown to be induced by p53 and play an important role in repression of p53-dependent transcriptional response in human cells (Huarte et al. 2010; Zhang et al. 2014a), thus acting as a bifunctional pro-survival factor in tumor cells. Interestingly, another non-coding RNA, miR-210, is also involved in repression of mitochondrial respiration during hypoxia (Chan et al. 2009), pointing to the presence of an RNA regulatory network in metabolic changes occurring during the hypoxic response (also see below). As will be discussed below, lincRNA-p21 is also induced in response to genotoxic stress in human cancer cells (Özgür et al. 2013). Although the mechanism of this induction has not been studied, it is likely resulting from the activation of p53 transcriptional response, indicating a significant degree of lncRNA-mediated crosstalk between different stress response pathways.
Studies on a number of other hypoxia-induced lncRNAs similarly indicate the exploitation of lncRNA-mediated aspects of the hypoxic response by tumor cells. Urothelial Cancer-Associated 1 (UCA1), also named Cancer Up-regulated Drug Resistant (CUDR), which was discovered as a marker for bladder cancer (Wang et al. 2006), is another HIF-1 induced lncRNA (Xue et al. 2014). Studies in human cancer cell lines have indicated the contribution of UCA1 to proliferation, migration, invasiveness and enhanced survival of bladder cancer cells under hypoxia (Wang et al. 2006; Wang et al. 2008a; Yang et al. 2012a; Xue et al. 2014). Similarly, H19 lncRNA, an imprinted lncRNA and miRNA precursor which is known to play an important role in regulation of cancer-related pathways, is induced during hypoxic stress (Hao et al. 1993; Matouk et al. 2007; Yoshimizu et al. 2008; Keniry et al. 2012; Keniry et al. 2012; Yang et al. 2012b; Guo et al. 2014; Matouk et al. 2014). Interestingly, the induction of H19 by HIF-1 during hypoxia is strongly inhibited by p53 (Matouk et al. 2010).
As mentioned above, many protein-coding RNAs have non-coding isoforms that originate from alternative promoters, alternative 3’ end processing signals, or alternative splicing events (Djebali et al. 2012; ENCODE Project Consortium 2012). An example of such lncRNAs in hypoxia has been recently reported in the Ephrin-A3 (EFNA3) locus, which encodes for a cell surface protein involved in modulating cellular adhesion and repulsion and cancer metastasis. Several non-coding isoforms of EFNA3 arise from alternative, HIF-regulated promoters and alternative 3’ processing sites during hypoxia, leading to accumulation of the Ephrin-A3 protein (Gómez-Maldonado et al. 2015). These lncRNAs act through sequestration of miR-210, which is induced by hypoxia and negatively regulates the translation of Ephrin-A3 mRNA (Kulshreshtha et al. 2007; Fasanaro et al. 2008; Huang et al. 2009) (also see above), thus relieving the post-transcriptional inhibition and resulting in an increase in the level of Ephrin-A3 protein. As expected, overexpression of the EFNA3 lncRNAs led to enhanced metastatic ability in breast cancer cells (Gómez-Maldonado et al. 2015).
3.1.2. Hypoxia-induced Epigenetic Changes Regulate the Expression of Functional lncRNAs
In addition to direct induction by HIF-1 or HIF-2 transcription factors, many lncRNAs are expressed during hypoxia as a result of hypoxia-mediated epigenetic changes or secondary to activation of other pathways and play critical roles in regulation of the hypoxic response. An interesting example of such RNAs is linc-RoR (regulator of Reprogramming), which is upregulated in response to hypoxia and shows increased expression in hypoxic regions in tumors (Takahashi et al. 2014). Linc-RoR functions at least partially through sequestering miR-145, which negative regulates the translation of HIF-1α by targeting p70S6K1 (RPS6KB1) (Xu et al. 2012; Wang et al. 2013). Thus, expression of linc-RoR leads to an increase in HIF-1α protein level (Takahashi et al. 2014), leading to potentiation of the hypoxic response. In another study, lincRNA-ROR was found to exert a strong negative regulation on p53 translation in a manner that requires hnRNP I, thus enhancing cellular survival (Zhang et al. 2013a). Interestingly, linc-RoR was abundantly found in extracellular vesicles released by hepatocellular cancer cells during hypoxia, likely acting as an intercellular signal to promote cellular survival during hypoxia (Takahashi et al. 2014).
In addition to linc-RoR, lncRNA-LET (lncRNA Low Expression in Tumor) (Yang et al. 2011) also plays an important role in regulation of HIF-1α. Unlike the previously discussed examples of hypoxia-regulated lncRNAs, lncRNA-LET is downregulated during hypoxia via repressive epigenetic changes at its promoter through the action of hypoxia-induced histone deacetylase 3 (HDAC3), which itself is a HIF-1α-regulated gene (Yang et al. 2013a). When expressed, lncRNA-LET associates with nuclear factor 90 (NF90), enhancing its degradation by inducing its ubiquitination through an unknown mechanism. As NF90, a double-stranded RNA-binding protein, is involved in biogenesis of many target mRNAs including HIF-1α (Kuwano et al. 2010), an increase in NF90 degradation leads to post-transcriptional downregulation of HIF-1α protein level. Thus, lncRNA-LET, together with NF90, HIF-1α and HDAC3 constitute a positive feedback loop that likely plays an important role in initiation and resolution of the hypoxic response. As expected, reducing the level of lncRNA-LET led to increased metastatic activity in human tumor cells (Yang et al. 2013a). In addition to regulation of expression of HIF-1α by trans-acting lncRNAs, two antisense lncRNAs, one overlapping the first (5’aHIF-1α) and the other overlapping the last exon of HIF-1α (3’aHIF-1α), are expressed from the locus of HIF-1α gene (Bertozzi et al. 2011). 5’aHIF-1α was induced in response to camptothecin and its upregulation was associated with a decrease in the level of HIF-1α, suggesting the possibility of a regulatory function for the antisense RNA (Bertozzi et al. 2011). The expression of 3’aHIF-1α is induced in response to hypoxia, likely mediated by binding sites for HIF-1/HIF-2 complexes in its promoter (Thrash-Bingham and Tartof 1999; Uchida et al. 2004). Knockdown of 3’aHIF-1α prevented the hypoxia-induced decrease in HIF-1α mRNA, likely through loss of a destabilizing effect exerted by this antisense RNA (Uchida et al. 2004).
In addition to global regulation of the hypoxic stress response, two examples of local, cis-regulation by hypoxia-induced lncRNAs have been reported. One study focused on WT1-AS lncRNAs, which originate from the first intron or the promoter region of the WT1 protein-coding gene in an antisense orientation and are alternatively spliced, generating a number of distinct transcripts. At least some of these transcripts are exported to the cytoplasm, where they form basepairing interactions with their complementary region on the first exon of WT1 protein-coding mRNA (Dallosso et al. 2007). The expression of WT1-AS lncRNAs is partially regulated by changes in methylation of a CpG island in intron 1 of WT1 gene, which occurs during hypoxia (Malik et al. 2000; Dallosso et al. 2007; McCarty and Loeb 2015). This leads to an increase in the expression of both WT1 and WT1-AS transcripts. Interestingly, shRNA-mediated down-regulation of WT1-AS leads to reduced WT1 level, suggesting cis-regulation of the expression of the protein-coding gene by its non-coding antisense overlapping transcripts, although shRNA-mediated silencing of the locus must be ruled out. Since WT1 is overexpressed in a number of cancers including leukemias, this lncRNA-mediated regulation may contribute to the development or progression of these malignancies (McCarty and Loeb 2015). Another report pointed to the potential cis-regulation of the metastasis-related γ-synuclein (SNCG) gene by the hypoxia-induced lncRNA AK058003 originating about 10 Kb away in an antisense divergent conformation (Wang et al. 2014b). The expression of the two genes showed a strong correlation in gastric cancer clinical samples and cell lines, and knockdown of the lncRNA led to reduced SNCG expression and increased methylation of the CpG island near its promoter (Wang et al. 2014b) (Fig. 2).
3.2. lncRNAs in Oxidative Stress
In mammalian cells, oxidative stress leads to a transcriptional cascade targeting many protein-coding genes involved in the regulation of the cellular redox state (Ma 2010). In addition, the expression of a large number of genes is altered in response to oxidative stress via post-transcriptional mechanisms including regulation of RNA stability and translation (Abdelmohsen et al. 2008). Until recently, the contribution of the non-coding transcriptome to the oxidative stress response had remained largely unexplored.
The first study of the impact of oxidative stress on global gene expression pattern in mammalians was recently reported by Giannakakis and colleagues (2015). High throughput RNA-seq analysis of human fibroblasts treated with hydrogen peroxide indicated that except the known oxidative stress response genes that were upregulated, the vast majority of the protein-coding genes had reduced cellular levels (Giannakakis et al. 2015). In contrast, the non-coding transcriptome was strongly upregulated, in addition to a large number of novel transcripts. Over a thousand intergenic and antisense RNAs and RNAs arising from bidirectional promoters were detected, with the majority of stress-induced RNAs belonging to the latter group (Giannakakis et al. 2015). Many of the induced novel RNAs were predominantly nuclear and did not show significant protein-coding capacity, however, among those with higher predicted protein-coding capacity, many associated with polysomes to a significant extent (Giannakakis et al. 2015). The predominance of transcripts originating from bidirectional promoters suggest a large degree of gene-specific fine-tuning during the response to oxidative stress, as many such transcripts affect the expression of their promoter-sharing neighboring transcript.
In addition to the above study, the upregulation of a number of novel transcripts that are likely to be lncRNAs in response to induction of oxidative stress by hydrogen peroxide treatment has been reported (Tani and Torimura 2013; Tani et al. 2014). However, very few oxidative stress-induced lncRNAs have been subjected to functional analysis. One studied RNA is lncRNA gadd7 (Growth arrested DNA-damage inducible gene 7), which was originally discovered as a non-coding DNA damage response gene with a central role in regulation of the G1/S checkpoint following DNA damage (Hollander et al. 1996; Liu et al. 2012). Liu and coworkers (2012) could show that after UV irradiation, gadd7 binds to TAR DNA‐binding protein (TDP‐43) and induces its dissociation from cyclin‐dependent kinase 6 (Cdk6) mRNA, leading to degradation of Cdk6 mRNA. As Cdk6 is a key factor in regulation of G1/S transition of the cell cycle, gadd7-mediated regulation of this key cell cycle step after genotoxic stress contributes to the maintenance of genomic fidelity. Interestingly, in addition to genotoxic stress, gadd7 is induced by lipotoxicity in a manner that depends on the reactive oxygen species (ROS) and is required for lipotoxicity-mediated and oxidative stress-mediated cell death (Brookheart et al. 2009). Indeed, depletion of gadd7 resulted in reduction in lipid-induced ROS and ROS-induced endoplasmic reticulum stress (Brookheart et al. 2009). While the mechanism of action of gadd7 in the context of lipid-mediated oxidative stress is not known, it is possible that by interacting with TDP-43, which regulates the biogenesis of a large subset of cellular mRNAs, gadd7 controls the stability of key proteins in multiple stress response pathways.
Many human diseases are associated with oxidative stress, and a causal relationship is proven or strongly suspected in a large subset of human diseases (Galea et al. 2012; Dandekar et al. 2015). A study of the exfoliation syndrome (XFS), a systemic fibrillinopathy, found a lncRNA transcribed antisense to the XFS-associated LOXL1 gene (Hauser et al. 2015). Interestingly, the genomic region with strongest disease association lies upstream of this antisense lncRNA, LOXL1-AS1, and mutations in this region affect its expression. LOXL1-AS1 is strongly induced in response to oxidative stress, and although the impact of its expression on cellular homeostasis after stress is not known, it is possible that dysregulation of LOXL1-AS1 expression by disease-causing mutations plays an important role in XFS pathogenesis (Hauser et al. 2015).
3.3. The role of lncRNAs in Genotoxic Stress and the DNA Damage Response
To identify the lncRNAs that are differentially expressed in response to DNA damage, Mizutani and colleagues computationally screened a library of human cDNAs for novel potentially non-coding transcripts (Mizutani et al. 2012). Among the identified putative lncRNAs, twenty five were nuclear localized, and several showed expression in multiple human tissues. After treatment of HeLa cells with mitomycin C or doxorubicin, two completely distinct subsets of the putative lncRNAs showed differential expression in response to the two genotoxic agents (Mizutani et al. 2012). While the function of these RNAs have not yet been studied, the lack of overlap between lncRNAs induced in response to the two genotoxic agents is intriguing and point to the specificity of the response of the non-coding transcriptome to each DNA damage mechanism. Further evidence for this high level of specificity was provided by another report, in which the expression of a number of well-studied lncRNAs was analyzed in two human cell lines after the induction of DNA damage using bleomycin and γ-radiation (Özgür et al. 2013). Interestingly, similar to the results of Mizutani et al. (2012), differentially expressed lncRNAs showed a high level of cell type- and genotoxic agent-specificity (Özgür et al. 2013). For example, ANRIL and GAS5 were mainly induced in irradiated cells, while HOTAIR, MALAT1, lincRNA-p21, ncRNA-CCND1 and MEG3 seemed to mostly respond to bleomycin treatment (Özgür et al. 2013, see also Chaudhry 2013). A third study, similarly, showed a high level of specificity in the pattern of induction of lncRNAs in response to genotoxic agents. Analysis of the expression of a set of candidate lncRNAs in two human glioma cell lines after treatment with resveratrol or two concentrations of doxorubicin indicated that not only the pattern of induction of lncRNAs was genotoxic agent-specific, but it was also dose-dependent (Liu et al. 2015b). While the results of the above studies are certainly thought-provoking, neither study involved an unbiased, comprehensive study of the changes in global gene expression pattern in response to the DNA damage inducing agents. Comparison of the results of high throughput studies using unbiased techniques such as RNA-seq or whole genome tiling arrays (e.g., Silva et al. 2010) can shed light on the extent and physiological significance of specificity and overlap between the transcriptomic changes induced in response to the different genotoxic agents.
While based on the above data, there seems to be a significant degree of specificity in the response of the non-coding transcriptome to each genotoxic agent, there is nevertheless some degree of overlap. For example, one of the differentially-expressed RNAs described in the above studies, ncRNA-CCND1, was originally identified as a heterogeneous group of single-stranded, low copy number RNAs that were expressed in response to ionizing irradiation from the regulatory regions upstream of the promoter of the CCND1 locus (Wang et al. 2008b). These RNAs seemed to recruit the RNA-binding protein FUS/TLS to the CCND1 promoter, resulting in transcriptional repression through inhibition of the CREB-binding protein (CBP) and p300 histone acetyltransferase activities by FUS/TLS (Wang et al. 2008b). Another radiation-induced lncRNA, PARTICLE (promoter of MAT2A-antisense radiation-induced circulating lncRNA), is transcribed from a bidirectional promoter that also gives rise to the MAT2A gene, which encodes the catalytic subunit of methionine adenosyltrasferase (O’Leary et al. 2015). PARTICLE forms a triple helix with the CpG island at the promoter of MAT2A and binds and recruits the repressive chromatin modifying complexes G9a and PRC2, resulting in downregulation of MAT2A expression (O’Leary et al. 2015). Such cis-acting mechanisms observed at the CCND1 and MAT2A loci provide an elegant and efficient means for fine tuning of expression of individual genes in response to stressful stimuli. Similar mechanisms are likely to be involved in regulation of many other critical genes during the cellular response to genotoxic agents and other stressful stimuli.
A third example of regulation by neighboring RNAs is provided by lncRNA-JADE, which was shown to be induced in an ATM (ataxia‐telangiectasia mutated)-dependent manner in response to a radiomimetic drug, neocarzinostatin, that generates double stranded breaks in DNA (Wan et al. 2013a). The induction of expression of this lncRNA leads to transcriptional induction of its neighboring gene, Jade1, which is a component of the HBO1 (human acetylase binding to ORC1) histone acetylation complex (Wan et al. 2013a). Mechanistic analyses have indicated that lncRNA-JADE physically binds Brca1, which in turn stimulates the interaction of Brca1 with the p300/CBP complex, leading to the induction of expression of Jade1 gene (Wan et al. 2013a). Another lncRNA, ANRIL/CDKN2B-AS1, is also induced after DNA damage in an ATM/E2F1-dependent manner from a locus in chromosome 9p21 which overlaps the CDKN2B/p15/INK4b gene in an antisense orientation (Wan et al. 2013b). Further, the transcription start site of ANRIL is less than 500 nucleotides away from that of the CDKN2A/p16/INK4a/ARF and some isoforms of CDKN2A may even overlap the first exon of ANRIL in antisense orientation. Transcriptional induction of ANRIL results in suppression of expression of CDKN2A and CDKN2B at the later stages of DNA damage response, contributing to the termination of the DNA damage response (Wan et al. 2013b).
As mentioned above, lincRNA-p21 plays an important role in hypoxia (Yang et al. 2014a). However, in an interesting example of crosstalk between different stress response pathways, this lncRNA is also induced in response to the DNA damaging agent doxorubicin and through its inhibitory effect on p53 function blocks doxorubicin-induced apoptosis (Huarte et al. 2010). A more recent study has also indicated a role for lincRNA-p21 in induction of ER stress (Ning et al. 2015). Another lncRNA, which similar to lincRNA-p21 originates from the vicinity of p21/CDKN1A locus, is also induced after doxorubicin treatment in a p53-dependent manner (Hung et al. 2011). Functional analysis of this lncRNA, named PANDA, indicated that it interacts with the alpha subunit of nuclear transcription factor Y (NF-YA), which is involved in inducing the expression of apoptotic genes. Association with PANDA prevents NF-YA from binding its target genes, leading to impaired apoptosis (Hung et al. 2011). Thus, both lincRNA-p21 and PANDA act in RNA-mediated negative feedback loops to regulate p53 activity.
3.4. lncRNA in Heat Stress
The heat shock response involves global adjustments to diverse cellular processes in order to improve survival under hyperthermia, including general repression of transcription, RNA processing and translation, and the selective expression of heat shock proteins (HSP) and other chaperones (Yost and Lindquist 1986; Calderwood 2005; Lakhotia 2012; Audas and Lee 2015). In addition, it is also a part of cellular defense mechanisms against other stresses, including ischemia (Arya et al. 2007; Brown 2007; Brown 2007; Richter et al. 2010; Vabulas et al. 2010; Lakhotia 2012). In eukaryotes, the transcription factor HSF1 (heat shock factor 1) plays a key role in activation of the heat shock response. During hyperthermia, monomeric HSF1 is released from its interaction with HSPs and other chaperone proteins and forms a homotrimer which translocates to the nucleus (Fig. 3). Once in the nucleus, the trimeric HSF1 binds specific sequence motifs, named the heat shock elements (HSEs) in the promoters of its targets genes including HSPs. The induced HSP proteins, in turn, re-sequester HSF1 in a negative regulatory feedback loop. In addition to transcriptional induction of the HSP genes, the stability of these transcripts is also improved through decreased deadenylation via interactions involving AU-rich domains (AREs) in their 3’ UTRs (Moseley et al. 1993; Dellavalle et al. 1994). In contrast, the cellular level of most non-HSP transcripts is reduced during heat stress through destabilization or sequestration in stress granules or p-bodies (Buchan and Parker 2009). Although our understanding of the role of lncRNAs in the heat shock response remains highly rudimentary, several studies have highlighted the importance of this class of RNAs in regulation of the heat shock response (Place and Noonan 2014; Audas and Lee 2015).
Fig. 3.
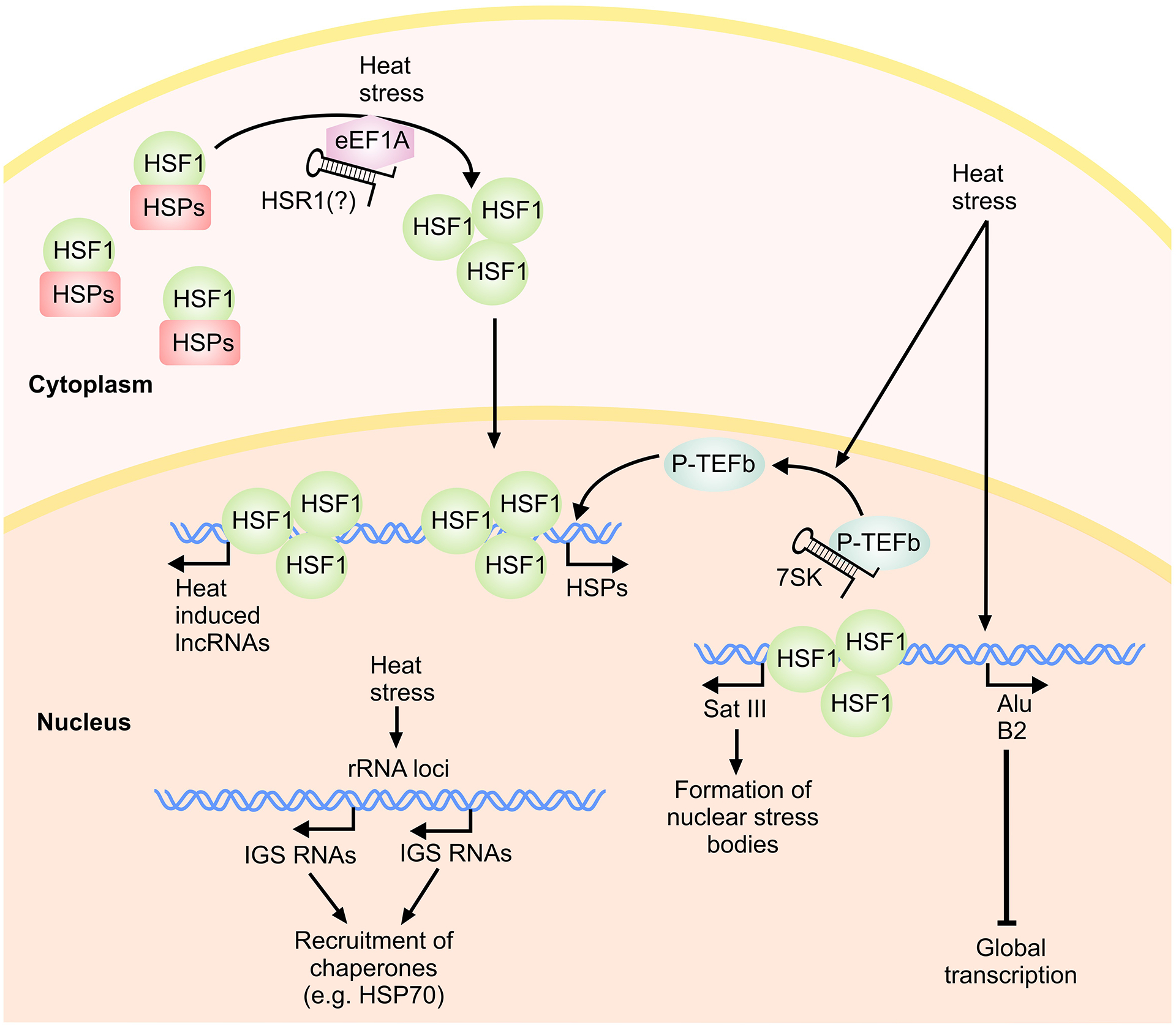
lncRNAs in the heat shock pathways.
One of the earliest reports on the involvement of lncRNAs in the heat shock response centered on an RNA named heat shock RNA-1 (HSR1) which interacted with the translation elongation factor eEF1A and regulated the activation of HSF1 by heat shock (Shamovsky et al. 2006). HSR1 was found to be required for HSF1 activation in vitro and in cultured cells after heat stress. Although Shamovsky and colleagues (2006) reported that HSR1 is expressed in both human and hamster cells (Shamovsky et al. 2006; Shamovsky and Nudler 2009), efforts at defining the locus of the RNA on the reference human and rodent genomes were unsuccessful (Kim et al. 2010). Analysis of the sequence of HSR1 indicated strong similarities to the bacterial genomes (Kim et al. 2010; Choi et al. 2015), indicating its bacterial origin. Despite the current confusion about the origin of HSR1, the fact that bacterial sequences can strongly affect the regulation of the eukaryotic heat shock response can point to a hitherto unknown layer of lncRNA-mediated host-pathogen interactions during bacterial infections.
As mentioned above, an important aspect of heat shock response is a general transcriptional repression affecting nearly all cellular transcripts except the heat shock response-regulated genes. Several studies suggest that this repression is at least partially lncRNA-mediated. It has been known that the expression of RNA polymerase III transcripts Alu RNA (in humans) and B2 RNA (in mice), which are derived from short interspersed elements (SINEs), are strongly upregulated following certain cellular stresses, including heat shock (Liu et al. 1995; Li et al. 1999). Both B2 and Alu RNAs directly associate with RNA polymerase II (Pol II) and transcriptional complexes formed at promoters both in vitro and in cultured cells. This association leads to a transcriptional block by preventing Pol II from contacting the promoter during closed complex formation (Allen et al. 2004; Mariner et al. 2008; Yakovchuk et al. 2009). In these structurally altered transcriptional complexes, Pol II is held on DNA through contacts with DNA-binding transcriptional proteins in an inactive conformation (Mariner et al. 2008; Yakovchuk et al. 2009). More recent studies indicate that B2 RNA can also specifically block the phosphorylation of the Pol II C-terminal domain (CTD) by TFIIH, providing an additional mechanism by which B2 RNA regulates transcription in response to heat shock (Yakovchuk et al. 2011). Interestingly, B2 and Alu RNAs don’t show any sequence homology, but their induction and function in response to heat shock are highly similar. This transcriptional repression function seems to be specific to only a subset of SINE-derived RNAs, as human scAlu RNA and mouse B1 RNA also associate with Pol II but do not repress its activity in vitro (Mariner et al. 2008).
An additional aspect of the involvement of Alu elements in heat shock response stems from the presence of HSF-binding sequences within Alu elements. Indeed, a significant fraction of the transcripts that show differential expression in response to heat shock contain Alu elements that harbor HSF binding sites (Pandey et al. 2011). The Alu elements integrated within the host transcript can be in the sense or antisense orientation relative to the direction of transcription of the host gene. The presence of sense Alu elements harboring HSF binding sites was associated with upregulation of the host gene in response to heat shock (Pandey et al. 2011), likely through acting at the DNA level as a landing pad for recruitment of HSF1. A subset of the antisense Alu elements that contained HSF binding sites, similarly, resulted in heat shock-induced transcription of RNAs, however, the transcriptional activity triggered by these elements occurred in the antisense orientation relative to the direction of transcription of the host gene. These antisense transcripts, in turn, resulted in reduced cellular level of the sense host gene (Pandey et al. 2011), most likely via direct basepairing to the sense RNA or induction of epigenetic changes. The above results provide an elegant example of mass regulation of gene expression through the use of repeat elements.
While B2 and Alu RNAs mediate transcriptional repression by blocking the initiation step, another Pol III-derived non-coding RNA, 7SK RNA, seems to participate in the induction of stress response genes under heat shock and other stressful conditions including genotoxic stress (Chen et al. 2008). In the absence of heat shock, the elongation phase of transcription is stimulated by the kinase activity of P-TEFb (positive transcription elongation factor b) leading to hyperphosphorylation of Pol II CTD (Lis et al. 2000). 7SK RNA and its associated proteins act as a repressor of the P-TEFb complex by binding and sequestering over half of all cellular P-TEFb complexes under normal conditions (Nguyen et al. 2001; Peterlin et al. 2012). During hyperthermia, almost all cellular P-TEFb complexes are released from 7SK due to a conformational change in the RNA (Chen et al. 2008), making them available to the active transcriptional complexes forming on the heat shock response genes, thus promoting highly efficient transcriptional activation of these critical loci (Lis et al. 2000; Peterlin et al. 2012) (Fig. 3).
3.4.1. Functional Role of Heat Shock-Induced lncRNAs
The drosophila hsr-omega (hsrω) non-coding RNA is perhaps the most extensively studied heat shock-induced non-coding transcript (Prasanth et al. 2000; Jolly and Lakhotia 2006; Lakhotia et al. 2012; Lakhotia 2012; Audas and Lee 2015). The hsrω nuclear transcripts and several cellular proteins including the heterogeneous nuclear RNA binding proteins (hnRNPs) colocalize in nuclear structures named the omega speckles, which coalesce during heat shock into speckles overlapping the genomic locus of hsromega (Prasanth et al. 2000; Jolly and Lakhotia 2006). The hsrω nuclear RNAs regulate the localization, trafficking and availability of hnRNPs and other proteins found in the omega speckles and play a critical role in thermo-tolerance and recovery from heat shock in drosophila (Prasanth et al. 2000; Jolly and Lakhotia 2006; Lakhotia et al. 2012).
In mammals, the satellite III repeat sequences found in the pericentromeric region of a number of chromosomes are transcribed into non-coding RNAs which accumulate in response to several stressful stimuli including heat stress (Jolly et al. 2004; Rizzi et al. 2004; Valgardsdottir et al. 2005; Jolly and Lakhotia 2006; Valgardsdottir et al. 2008; Eymery et al. 2010). It it thought that in the absence of heat stress, the Pol II-transcribed satellite III RNAs are rapidly degraded by cellular RNA interference mechanisms in a dicer-dependent fashion (Jolly and Lakhotia 2006). After induction of their transcription by HSF1 during heat shock, the sat III transcripts remain associated with their loci and form the nuclear stress bodies, which recruit HSF1 and several hnRNPs and splicing factors during heat exposure (Denegri et al. 2001; Denegri et al. 2002; Metz et al. 2004; Jolly and Lakhotia 2006). The sat III RNAs may play a role in maintenance of the chromatin structure of the repeat-rich satellite III loci, or similar to hsrω RNAs, may nucleate the formation of a regulatory protein repository during stress (Jolly and Lakhotia 2006; Lakhotia 2012). Transcription of non-coding RNAs from another repeat-rich locus, the pericentromeric satellite 2 loci, is also strongly induced during heat shock response in an HSF1-dependent manner (Tilman et al. 2012). While the impact of this transcriptional activity on cellular homeostasis during heat shock is not known, it may play a role in tumor progression in cancers. The heat shock response is upregulated in many tumors, and the HSF1-mediated activation of transcription from satellite 2 loci results in demethylation of these loci (Tilman et al. 2012). Demethylation of the satellite 2 regions, in turn, has been shown to favor chromosomal rearrangements and progression of the cancerous phenotype (Tilman et al. 2012). Interestingly, heat shock regulatory elements have been found in the telomeric repeats of fly species leading to telomeric puffing and transcription under heat shock conditions (Martinez et al. 2001), however, the physiological significance of this transcriptional activity is currently unknown. In addition to inducing changes in methylation marks on repeat-containing genomic regions, heat stress also results in changes in epigenetic marks elsewhere in the genome, including methylation marks associated with imprinting. It has been shown that the methylation marks in a subset of imprinted lncRNAs such as the paternally-imprinted H19 and Igf-2r is altered in heat-stressed blastocyst stage mouse embryos compared to controls (Zhu et al. 2008).
Another lncRNA-mediated aspect of the heat shock response is nucleolar remodeling, which involves the recruitment of several regulatory and chaperone proteins including HSP70 to the nucleoli (Kotoglou et al. 2009; Boulon et al. 2010; Bański et al. 2010a; Bański et al. 2010b). It is thought that the recruitment of chaperones, co-chaperones and other regulatory proteins to the nucleoli provides a protective mechanism for the nucleoli during stress (Kotoglou et al. 2009; Boulon et al. 2010). Interestingly, heat stress results in induction of expression of a number of lncRNAs from specific intergenic spacer (IGS) loci positioned between ribosomal genes (Audas et al. 2012; Jacob et al. 2013). These lncRNAs, which are transcribed by Pol I from the same strand as the rRNA at IGS 22 and 16 loci, mediate the recruitment of HSP70 to the nucleoli during stress. Surprisingly, other types of stress, such as acidosis, also lead to expression of similar lncRNAs from other IGS loci and recruitment of HSP70 and several additional proteins including VHL (Audas et al. 2012; Jacob et al. 2012; Jacob et al. 2013), suggesting the extensive use of lncRNAs as protein recruitment scaffolds in the nucleoli during the stress response. As mentioned above, VHL plays an important role in restriction of HIF-1 activity and thus, its sequestration by IGS RNA loci points to a physiological crosstalk between acidosis and hypoxic responses. In addition to the ISG RNAs, a number of lncRNAs antisense to the ribosomal RNA promoter or pre-rRNA itself are transcribed from the ribosomal RNA loci in response to serum starvation and growth arrest (Bierhoff et al. 2010; Bierhoff et al. 2014). These RNAs induce chromatin compaction via trimethylation of histone H4 lysine 20 at these loci in a manner that seems to involve the formation of a DNA:RNA triplex in order to downregulate the synthesis of ribosomal RNAs during starvation and quiescence (Bierhoff et al. 2010; Bierhoff et al. 2014).
3.5. Role of lncRNAs in Other Stress Response Pathways
The role of lncRNAs in a number of important cellular stress response pathways including osmotic stress, ER stress and starvation has remained almost entirely unknown. To our knowledge, no high throughput analysis of the global changes in the non-coding transcriptome in response to these three stressful stimuli has been reported in mammalians, despite their critical physiological and clinical importance. However a number of reports have described the outcome of functional study of individual lncRNAs involved in these three stress pathways. A study in yeast has revealed a role for antisense non-coding transcription during osmotic stress (Nadal-Ribelles et al. 2014; Solé et al. 2015). The stress-activated protein kinase (SAPK) p38/Hog1 induces the expression of tens of stress response genes after osmotic stress, including a number of lncRNAs (Nadal-Ribelles et al. 2014). One of the Hog1-induced lncRNAs is transcribed from the antisense strand of CDC28, which has an important role in regulation of the cell cycle in yeast. This antisense RNA, named Cdc28 lncRNA, originates from the 3’ UTR of CDC28 gene near a Hog1 binding site in this region. Interestingly, induction of transcription of the Cdc28 lncRNA promoted the formation of a DNA loop between the transcriptional start site of CDC28 and its 3’ UTR, allowing the 3’ UTR-bound Hog1 to induce the expression of CDC28 gene. This action of Cdc28 lncRNA was mediated in cis, likely through nascent or tethered Cdc28 lncRNA transcripts, and resulted in accumulation of CDC28, which in turn primes the cells to re-enter the cell cycle after resolution of the stress (Nadal-Ribelles et al. 2014).
BACE1 (β-site amyloid-β precursor protein cleaving enzyme 1) is a transmembrane enzyme that participates in amyloid-β generation. BACE1 mRNA and protein expression is regulated by an antisense lncRNA, BACE1-AS, which overlaps the sixth exon of BACE1 mRNA in human (Faghihi et al. 2008). BACE1-AS is upregulated in response to a variety of cellular stressors, including ER stress, heat shock and oxidative stress; resulting in increased BACE1 mRNA stability through formation of an RNA duplex (Faghihi et al. 2008; Nogalska et al. 2010). In addition, BACE1-AS masks the binding site of miR-485–5p on BACE1 mRNA, thus further increasing the cellular level of BACE1 protein (Faghihi et al. 2010). Thus, BACE1-AS acts as an RNA-mediated link through which cellular stresses can affect the formation of amyloid-β and ultimately the progression of neurodegenerative disorders.
Finally, the expression of the growth arrest–specific 5 (Gas5) noncoding RNA is induced in response to starvation and has been shown to suppress the glucocorticoid-mediated induction of a number of key target genes, including those inhibiting apoptosis. Mechanistic studies have suggested that Gas5 RNA forms a structure that mimics that of the glucocorticoid response elements in the genome. Through this molecular mimicry, Gas5 binds to the DNA-binding domain of the glucocorticoid receptor as a competitive inhibitor, thus blocking the transcriptional activity of the glucocorticoid receptor (Kino et al. 2010).
3.6. lncRNAs and stress in plants
The plant genomes, similar to that of mammalians, harbor a large number of lncRNAs that perform a wide range of functions in response to different stressful stimuli (Boerner and McGinnis 2012; Li et al. 2014; Wang et al. 2014a; Bai et al. 2015; Liu et al. 2015a; Liu et al. 2015a; Ariel et al. 2015; Xuan et al. 2015). Several studies have defined the extent of lncRNA transcriptional response following stressful stimuli in plants, and have studied the role of individual lncRNAs in the context of stress (Amor et al. 2009; Xin et al. 2011; Wu et al. 2012; Lembke et al. 2012; Qi et al. 2013; Zhang et al. 2013b; Zhang et al. 2014b; Zhu et al. 2014; Wunderlich et al. 2014; Csorba et al. 2014; Di et al. 2014; Bazin and Bailey-Serres 2015; Wang et al. 2015; Aversano et al. 2015). Since a number of recent reviews have provided excellent summaries and discussion of our current state of knowledge of the role of plant lncRNAs in stress (Liu et al. 2015c; Liu et al. 2015c; Shafiq et al. 2015; Chekanova 2015), in the interest of space, a similar discussion is not included in this review.
4. Concluding remarks
Although our knowledge of the role of lncRNAs in stress response is still in its infancy, existing studies have pointed to a critical and ubiquitous role for lncRNAs in stress response in all kingdoms of life. As discussed above, unique properties of RNAs make them highly suitable for function during the stress response. While the vast majority of currently identified stress-responsive lncRNAs have not been functionally studied, it is very likely that the list of stress-responsive lncRNAs and the processes which they regulate will significantly grow in near future. Due to technical shortcomings, many bona-fide lncRNAs which originate in introns, are short-lived and expressed at very low copy numbers, and unspliced RNAs are frequently filtered during high throughput studies as noise. However, recent improvements in sequencing depth is likely to at least partially address this issue (Mercer et al. 2014). Further, transcribed pseudogenes, which fall under the category of lncRNAs, can play critical roles in cellular function as exemplified by a number of reports (Poliseno et al. 2010; Johnsson et al. 2013), however as a group they remain highly understudied. Improving our understanding of the role of all classes of lncRNAs in stress response will be highly fruitful, as the stress response pathways are known to be involved in pathogenesis of a wide range of human diseases from cancer to neurodegeneration (Romano et al. 2010; Facecchia et al. 2011; Ramalingam and Kim 2012; Gabr and Al-Ghadir 2012; Luca et al. 2015; Saito et al. 2015; Ng et al. 2015). Further, considering the cell type- and state-specific nature of lncRNA expression, it is likely that many members of this class of RNAs can be used as diagnostic or prognostic markers in human diseases (Di Gesualdo et al. 2014).
Acknowledgments
Funding for this work was provided by CFAR grant number P30-AI036219 to S.V. and a postdoctoral scholarship from the National Board of Science and Technology of Mexico to A.V.H. The authors declare no conflict of interest.
References
- Abdelmohsen K, Kuwano Y, Kim HH, Gorospe M (2008) Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence. Biol Chem 389:243–255. doi: 10.1515/BC.2008.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi N, Lieber MR (2002) Bidirectional gene organization: a common architectural feature of the human genome. Cell 109:807–809. [DOI] [PubMed] [Google Scholar]
- Allen TA, Von Kaenel S, Goodrich JA, Kugel JF (2004) The SINE-encoded mouse B2 RNA represses mRNA transcription in response to heat shock. Nat Struct Mol Biol 11:816–821. doi: 10.1038/nsmb813 [DOI] [PubMed] [Google Scholar]
- Almada AE, Wu X, Kriz AJ, et al. (2013) Promoter directionality is controlled by U1 snRNP and polyadenylation signals. Nature 499:360–363. doi: 10.1038/nature12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altuvia S, Weinstein-Fischer D, Zhang A, et al. (1997) A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell 90:43–53. [DOI] [PubMed] [Google Scholar]
- Amaral PP, Dinger ME, Mattick JS (2013) Non-coding RNAs in homeostasis, disease and stress responses: an evolutionary perspective. Brief Funct Genomics 12:254–278. doi: 10.1093/bfgp/elt016 [DOI] [PubMed] [Google Scholar]
- Amor BB, Wirth S, Merchan F, et al. (2009) Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res 19:57–69. doi: 10.1101/gr.080275.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel F, Romero-Barrios N, Jégu T, et al. (2015) Battles and hijacks: noncoding transcription in plants. Trends Plant Sci 20:362–371. doi: 10.1016/j.tplants.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Arrigo P, Pulliero A (2015) Effect of Environmental Chemical Stress on Nuclear Noncoding RNA Involved in Epigenetic Control. Biomed Res Int 2015:761703. doi: 10.1155/2015/761703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya R, Mallik M, Lakhotia SC (2007) Heat shock genes - integrating cell survival and death. J Biosci 32:595–610. [DOI] [PubMed] [Google Scholar]
- Audas TE, Jacob MD, Lee S (2012) Immobilization of Proteins in the Nucleolus by Ribosomal Intergenic Spacer Noncoding RNA. Molecular Cell 45:147–157. doi: 10.1016/j.molcel.2011.12.012 [DOI] [PubMed] [Google Scholar]
- Audas TE, Lee S (2015) Stressing out over long noncoding RNA. Biochim Biophys Acta. doi: 10.1016/j.bbagrm.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aversano R, Contaldi F, Ercolano MR, et al. (2015) The Solanum commersonii Genome Sequence Provides Insights into Adaptation to Stress Conditions and Genome Evolution of Wild Potato Relatives. Plant Cell 27:954–968. doi: 10.1105/tpc.114.135954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayupe AC, Tahira AC, Camargo L, et al. (2015) Global analysis of biogenesis, stability and sub-cellular localization of lncRNAs mapping to intragenic regions of the human genome. RNA Biol 12:877–892. doi: 10.1080/15476286.2015.1062960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babitzke P, Romeo T (2007) CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Current Opinion in Microbiology 10:156–163. doi: 10.1016/j.mib.2007.03.007 [DOI] [PubMed] [Google Scholar]
- Bai Y, Dai X, Harrison AP, Chen M (2015) RNA regulatory networks in animals and plants: a long noncoding RNA perspective. Brief Funct Genomics 14:91–101. doi: 10.1093/bfgp/elu017 [DOI] [PubMed] [Google Scholar]
- Bański P, Kodiha M, Stochaj U (2010a) Chaperones and multitasking proteins in the nucleolus: networking together for survival? Trends Biochem Sci 35:361–367. doi: 10.1016/j.tibs.2010.02.010 [DOI] [PubMed] [Google Scholar]
- Bański P, Mahboubi H, Kodiha M, et al. (2010b) Nucleolar targeting of the chaperone hsc70 is regulated by stress, cell signaling, and a composite targeting signal which is controlled by autoinhibition. J Biol Chem 285:21858–21867. doi: 10.1074/jbc.M110.117291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrangou R, Fremaux C, Deveau H, et al. (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712. doi: 10.1126/science.1138140 [DOI] [PubMed] [Google Scholar]
- Bartrons R, Caro J (2007) Hypoxia, glucose metabolism and the Warburg’s effect. J Bioenerg Biomembr 39:223–229. doi: 10.1007/s10863-007-9080-3 [DOI] [PubMed] [Google Scholar]
- Bazin J, Bailey-Serres J (2015) Emerging roles of long non-coding RNA in root developmental plasticity and regulation of phosphate homeostasis. Front Plant Sci 6:400. doi: 10.3389/fpls.2015.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley NJP, Leek R, Alam M, et al. (2002) Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res 62:2493–2497. [PubMed] [Google Scholar]
- Bertozzi D, Iurlaro R, Sordet O, et al. (2011) Characterization of novel antisense HIF-1α transcripts in human cancers. Cell Cycle 10:3189–3197. [DOI] [PubMed] [Google Scholar]
- Bierhoff H, Dammert MA, Brocks D, et al. (2014) Quiescence-induced LncRNAs trigger H4K20 trimethylation and transcriptional silencing. Mol Cell 54:675–682. doi: 10.1016/j.molcel.2014.03.032 [DOI] [PubMed] [Google Scholar]
- Bierhoff H, Schmitz K, Maass F, et al. (2010) Noncoding transcripts in sense and antisense orientation regulate the epigenetic state of ribosomal RNA genes. Cold Spring Harb Symp Quant Biol 75:357–364. doi: 10.1101/sqb.2010.75.060 [DOI] [PubMed] [Google Scholar]
- Boerner S, McGinnis KM (2012) Computational identification and functional predictions of long noncoding RNA in Zea mays. PLoS ONE 7:e43047. doi: 10.1371/journal.pone.0043047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn C, Rigoulay C, Chabelskaya S, et al. (2010) Experimental discovery of small RNAs in Staphylococcus aureus reveals a riboregulator of central metabolism. Nucl Acids Res 38:6620–6636. doi: 10.1093/nar/gkq462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulon S, Ahmad Y, Trinkle-Mulcahy L, et al. (2010) Establishment of a Protein Frequency Library and Its Application in the Reliable Identification of Specific Protein Interaction Partners. Mol Cell Proteomics 9:861–879. doi: 10.1074/mcp.M900517-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breaker RR (2011) Prospects for Riboswitch Discovery and Analysis. Molecular Cell 43:867–879. doi: 10.1016/j.molcel.2011.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookheart RT, Michel CI, Listenberger LL, et al. (2009) The Non-coding RNA gadd7 Is a Regulator of Lipid-induced Oxidative and Endoplasmic Reticulum Stress. J Biol Chem 284:7446–7454. doi: 10.1074/jbc.M806209200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IR (2007) Heat shock proteins and protection of the nervous system. Ann N Y Acad Sci 1113:147–158. doi: 10.1196/annals.1391.032 [DOI] [PubMed] [Google Scholar]
- Buchan JR, Parker R (2009) Eukaryotic stress granules: the ins and outs of translation. Mol Cell 36:932–941. doi: 10.1016/j.molcel.2009.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK (2005) Regulatory interfaces between the stress protein response and other gene expression programs in the cell. Methods 35:139–148. doi: 10.1016/j.ymeth.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, et al. (2000) Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest 105:577–587. doi: 10.1172/JCI8316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzo ES, Clement CC, Sahu R, et al. (2011) Oxidative stress, inflamm-aging and immunosenescence. J Proteomics 74:2313–2323. doi: 10.1016/j.jprot.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Carninci P, Kasukawa T, Katayama S, et al. (2005) The transcriptional landscape of the mammalian genome. Science 309:1559–1563. doi: 10.1126/science.1112014 [DOI] [PubMed] [Google Scholar]
- Chang Y-N, Zhang K, Hu Z-M, et al. (2015) Hypoxia-regulated lncRNAs in Cancer. Gene. doi: 10.1016/j.gene.2015.08.049 [DOI] [PubMed] [Google Scholar]
- Chan SY, Zhang Y-Y, Hemann C, et al. (2009) MicroRNA-210 Controls Mitochondrial Metabolism during Hypoxia by Repressing the Iron-Sulfur Cluster Assembly Proteins ISCU1/2. Cell Metabolism 10:273–284. doi: 10.1016/j.cmet.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry MA (2013) Expression Pattern of Small Nucleolar RNA Host Genes and Long Non-Coding RNA in X-rays-Treated Lymphoblastoid Cells. Int J Mol Sci 14:9099–9110. doi: 10.3390/ijms14059099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekanova JA (2015) Long non-coding RNAs and their functions in plants. Curr Opin Plant Biol 27:207–216. doi: 10.1016/j.pbi.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Chen R, Liu M, Li H, et al. (2008) PP2B and PP1alpha cooperatively disrupt 7SK snRNP to release P-TEFb for transcription in response to Ca2+ signaling. Genes Dev 22:1356–1368. doi: 10.1101/gad.1636008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Oh HJ, Goh CJ, et al. (2015) Heat Shock RNA 1, Known as a Eukaryotic Temperature-Sensing Noncoding RNA, Is of Bacterial Origin. J Microbiol Biotechnol 25:1234–1240. doi: 10.4014/jmb.1505.05014 [DOI] [PubMed] [Google Scholar]
- Choudhry H, Albukhari A, Morotti M, et al. (2015) Tumor hypoxia induces nuclear paraspeckle formation through HIF-2α dependent transcriptional activation of NEAT1 leading to cancer cell survival. Oncogene 34:4482–4490. doi: 10.1038/onc.2014.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry H, Schödel J, Oikonomopoulos S, et al. (2014) Extensive regulation of the non-coding transcriptome by hypoxia: role of HIF in releasing paused RNApol2. EMBO Rep 15:70–76. doi: 10.1002/embr.201337642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MB, Choudhary A, Smith MA, et al. (2013) The dark matter rises: the expanding world of regulatory RNAs. Essays Biochem 54:1–16. doi: 10.1042/bse0540001 [DOI] [PubMed] [Google Scholar]
- Clark MB, Mattick JS (2011) Long noncoding RNAs in cell biology. Semin Cell Dev Biol 22:366–376. doi: 10.1016/j.semcdb.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Csorba T, Questa JI, Sun Q, Dean C (2014) Antisense COOLAIR mediates the coordinated switching of chromatin states at FLC during vernalization. Proc Natl Acad Sci USA 111:16160–16165. doi: 10.1073/pnas.1419030111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallosso AR, Hancock AL, Malik S, et al. (2007) Alternately spliced WT1 antisense transcripts interact with WT1 sense RNA and show epigenetic and splicing defects in cancer. RNA 13:2287–2299. doi: 10.1261/rna.562907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar A, Mendez R, Zhang K (2015) Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol Biol 1292:205–214. doi: 10.1007/978-1-4939-2522-3_15 [DOI] [PubMed] [Google Scholar]
- Dellavalle RP, Petersen R, Lindquist S (1994) Preferential deadenylation of Hsp70 mRNA plays a key role in regulating Hsp70 expression in Drosophila melanogaster. Mol Cell Biol 14:3646–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denegri M, Chiodi I, Corioni M, et al. (2001) Stress-induced nuclear bodies are sites of accumulation of pre-mRNA processing factors. Mol Biol Cell 12:3502–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denegri M, Moralli D, Rocchi M, et al. (2002) Human Chromosomes 9, 12, and 15 Contain the Nucleation Sites of Stress-Induced Nuclear Bodies. Mol Biol Cell 13:2069–2079. doi: 10.1091/mbc.01-12-0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey BK, Mueller AC, Dutta A (2014) Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription 5:e944014. doi: 10.4161/21541272.2014.944014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Nakka VP, Vemuganti R (2012) Effect of Focal Ischemia on Long Noncoding RNAs. Stroke 43:2800–2802. doi: 10.1161/STROKEAHA.112.669465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di C, Yuan J, Wu Y, et al. (2014) Characterization of stress-responsive lncRNAs in Arabidopsis thaliana by integrating expression, epigenetic and structural features. Plant J 80:848–861. doi: 10.1111/tpj.12679 [DOI] [PubMed] [Google Scholar]
- Di Gesualdo F, Capaccioli S, Lulli M (2014) A pathophysiological view of the long non-coding RNA world. Oncotarget 5:10976–10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebali S, Davis CA, Merkel A, et al. (2012) Landscape of transcription in human cells. Nature 489:101–108. doi: 10.1038/nature11233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dühring U, Axmann IM, Hess WR, Wilde A (2006) An internal antisense RNA regulates expression of the photosynthesis gene isiA. PNAS 103:7054–7058. doi: 10.1073/pnas.0600927103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489:57–74. doi: 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium, Bernstein BE, Birney E, et al. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489:57–74. doi: 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt J, Stadler PF (2015) Evolution of the unspliced transcriptome. BMC Evol Biol 15:166. doi: 10.1186/s12862-015-0437-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson L, Highsmith E Jr., Fei P, Zhang J (2015) Targeting the hypoxia pathway to treat pancreatic cancer. Drug Design, Development and Therapy 2029. doi: 10.2147/DDDT.S80888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Diez C, Miguel V, Mennerich D, et al. (2015) Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 6:183–197. doi: 10.1016/j.redox.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eymery A, Souchier C, Vourc’h C, Jolly C (2010) Heat shock factor 1 binds to and transcribes satellite II and III sequences at several pericentromeric regions in heat-shocked cells. Exp Cell Res 316:1845–1855. doi: 10.1016/j.yexcr.2010.02.002 [DOI] [PubMed] [Google Scholar]
- Facecchia K, Fochesato L-A, Ray SD, et al. (2011) Oxidative toxicity in neurodegenerative diseases: role of mitochondrial dysfunction and therapeutic strategies. J Toxicol 2011:683728. doi: 10.1155/2011/683728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihi MA, Modarresi F, Khalil AM, et al. (2008) Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med 14:723–730. doi: 10.1038/nm1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihi MA, Zhang M, Huang J, et al. (2010) Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biology 11:R56. doi: 10.1186/gb-2010-11-5-r56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanaro P, D’Alessandra Y, Di Stefano V, et al. (2008) MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 283:15878–15883. doi: 10.1074/jbc.M800731200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdin J, Nishida N, Wu X, et al. (2013) HINCUTs in cancer: hypoxia-induced noncoding ultraconserved transcripts. Cell Death Differ 20:1675–1687. doi: 10.1038/cdd.2013.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabr SA, Al-Ghadir AH (2012) Role of cellular oxidative stress and cytochrome c in the pathogenesis of psoriasis. Archives of dermatological research. doi: 10.1007/s00403-012-1230-8 [DOI] [PubMed] [Google Scholar]
- Galea E, Launay N, Portero-Otin M, et al. (2012) Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: A paradigm for multifactorial neurodegenerative diseases? Biochimica Et Biophysica Acta. doi: 10.1016/j.bbadis.2012.02.005 [DOI] [PubMed] [Google Scholar]
- Gardini A, Shiekhattar R (2015) The many faces of long noncoding RNAs. FEBS J 282:1647–1657. doi: 10.1111/febs.13101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georg J, Hess WR (2011) cis-Antisense RNA, Another Level of Gene Regulation in Bacteria. Microbiol Mol Biol Rev 75:286–300. doi: 10.1128/MMBR.00032-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakakis A, Zhang J, Jenjaroenpun P, et al. (2015) Contrasting expression patterns of coding and noncoding parts of the human genome upon oxidative stress. Sci Rep 5:9737. doi: 10.1038/srep09737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss BS, Dinger ME (2015) The specificity of long noncoding RNA expression. Biochim Biophys Acta. doi: 10.1016/j.bbagrm.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Gogol EB, Rhodius VA, Papenfort K, et al. (2011) Small RNAs endow a transcriptional activator with essential repressor functions for single-tier control of a global stress regulon. PNAS 108:12875–12880. doi: 10.1073/pnas.1109379108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Maldonado L, Tiana M, Roche O, et al. (2015) EFNA3 long noncoding RNAs induced by hypoxia promote metastatic dissemination. Oncogene 34:2609–2620. doi: 10.1038/onc.2014.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzechnik P, Tan-Wong SM, Proudfoot NJ (2014) Terminate and make a loop: regulation of transcriptional directionality. Trends Biochem Sci 39:319–327. doi: 10.1016/j.tibs.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Kang Q, Chen Q, et al. (2014) High expression of long non-coding RNA H19 is required for efficient tumorigenesis induced by Bcr-Abl oncogene. FEBS Lett 588:1780–1786. doi: 10.1016/j.febslet.2014.03.038 [DOI] [PubMed] [Google Scholar]
- Hao Y, Crenshaw T, Moulton T, et al. (1993) Tumour-suppressor activity of H19 RNA. Nature 365:764–767. doi: 10.1038/365764a0 [DOI] [PubMed] [Google Scholar]
- Hauser MA, Aboobakar IF, Liu Y, et al. (2015) Genetic Variants and Cellular Stressors Associated with Exfoliation Syndrome Modulate Promoter Activity of a lncRNA within the LOXL1 Locus. Hum Mol Genet. doi: 10.1093/hmg/ddv347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander MC, Alamo I, Fornace AJ (1996) A Novel DNA Damage-Inducible Transcript, gadd7, Inhibits Cell Growth, but Lacks a Protein Product. Nucl Acids Res 24:1589–1593. doi: 10.1093/nar/24.9.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ding L, Bennewith KL, et al. (2009) Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell 35:856–867. doi: 10.1016/j.molcel.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte M, Guttman M, Feldser D, et al. (2010) A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142:409–419. doi: 10.1016/j.cell.2010.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T, Chang HY (2010) Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol 7:582–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T, Wang Y, Lin MF, et al. (2011) Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet 43:621–629. doi: 10.1038/ng.848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T, Dahlberg JE (1973) Small Ribonucleic Acids of Escherichia coli II. NONCOORDINATE ACCUMULATION DURING STRINGENT CONTROL. J Biol Chem 248:5033–5041. [PubMed] [Google Scholar]
- Jacob MD, Audas TE, Mullineux S-T, Lee S (2012) Where no RNA polymerase has gone before: novel functional transcripts derived from the ribosomal intergenic spacer. Nucleus 3:315–319. doi: 10.4161/nucl.20585 [DOI] [PubMed] [Google Scholar]
- Jacob MD, Audas TE, Uniacke J, et al. (2013) Environmental cues induce a long noncoding RNA-dependent remodeling of the nucleolus. Mol Biol Cell 24:2943–2953. doi: 10.1091/mbc.E13-04-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AB, Barton MC (2007) Hypoxia-induced and stress-specific changes in chromatin structure and function. Mutat Res 618:149–162. doi: 10.1016/j.mrfmmm.2006.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson P, Ackley A, Vidarsdottir L, et al. (2013) A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat Struct Mol Biol 20:440–446. doi: 10.1038/nsmb.2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Lakhotia SC (2006) Human sat III and Drosophila hsrω transcripts: a common paradigm for regulation of nuclear RNA processing in stressed cells. Nucl Acids Res 34:5508–5514. doi: 10.1093/nar/gkl711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Metz A, Govin J, et al. (2004) Stress-induced transcription of satellite III repeats. J Cell Biol 164:25–33. doi: 10.1083/jcb.200306104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30:393–402. doi: 10.1016/j.molcel.2008.04.009 [DOI] [PubMed] [Google Scholar]
- Kaidi A, Qualtrough D, Williams AC, Paraskeva C (2006) Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res 66:6683–6691. doi: 10.1158/0008-5472.CAN-06-0425 [DOI] [PubMed] [Google Scholar]
- Kapusta A, Feschotte C (2014) Volatile evolution of long noncoding RNA repertoires: mechanisms and biological implications. Trends Genet 30:439–452. doi: 10.1016/j.tig.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M, Aravind L, Storz G (2007) An antisense RNA controls synthesis of an SOS-induced toxin evolved from an antitoxin. Mol Microbiol 64:738–754. doi: 10.1111/j.1365-2958.2007.05688.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keniry A, Oxley D, Monnier P, et al. (2012) The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol 14:659–665. doi: 10.1038/ncb2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AM, Guttman M, Huarte M, et al. (2009) Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA 106:11667–11672. doi: 10.1073/pnas.0904715106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Lee Y, Hahn Y (2010) Evidence for bacterial origin of heat shock RNA-1. RNA 16:274–279. doi: 10.1261/rna.1879610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Hurt DE, Ichijo T, et al. (2010) Noncoding RNA Gas5 Is a Growth Arrest– and Starvation-Associated Repressor of the Glucocorticoid Receptor. Sci Signal 3:ra8–ra8. doi: 10.1126/scisignal.2000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoglou P, Kalaitzakis A, Vezyraki P, et al. (2009) Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress Chaperones 14:391–406. doi: 10.1007/s12192-008-0093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshreshtha R, Ferracin M, Wojcik SE, et al. (2007) A microRNA signature of hypoxia. Mol Cell Biol 27:1859–1867. doi: 10.1128/MCB.01395-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano Y, Pullmann R, Marasa BS, et al. (2010) NF90 selectively represses the translation of target mRNAs bearing an AU-rich signature motif. Nucleic Acids Res 38:225–238. doi: 10.1093/nar/gkp861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhotia SC (2012) Long non-coding RNAs coordinate cellular responses to stress. Wiley Interdiscip Rev RNA 3:779–796. doi: 10.1002/wrna.1135 [DOI] [PubMed] [Google Scholar]
- Lakhotia SC, Mallik M, Singh AK, Ray M (2012) The large noncoding hsrω-n transcripts are essential for thermotolerance and remobilization of hnRNPs, HP1 and RNA polymerase II during recovery from heat shock in Drosophila. Chromosoma 121:49–70. doi: 10.1007/s00412-011-0341-x [DOI] [PubMed] [Google Scholar]
- Lam MTY, Li W, Rosenfeld MG, Glass CK (2014) Enhancer RNAs and regulated transcriptional programs. Trends Biochem Sci 39:170–182. doi: 10.1016/j.tibs.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lease RA, Smith D, McDonough K, Belfort M (2004) The small noncoding DsrA RNA is an acid resistance regulator in Escherichia coli. J Bacteriol 186:6179–6185. doi: 10.1128/JB.186.18.6179-6185.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembke CG, Nishiyama MY, Sato PM, et al. (2012) Identification of sense and antisense transcripts regulated by drought in sugarcane. Plant Mol Biol 79:461–477. doi: 10.1007/s11103-012-9922-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AKL, Sharp PA (2010) MicroRNA functions in stress responses. Mol Cell 40:205–215. doi: 10.1016/j.molcel.2010.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Eichten SR, Shimizu R, et al. (2014) Genome-wide discovery and characterization of maize long non-coding RNAs. Genome Biol 15:R40. doi: 10.1186/gb-2014-15-2-r40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis JT, Mason P, Peng J, et al. (2000) P-TEFb kinase recruitment and function at heat shock loci. Genes Dev 14:792–803. [PMC free article] [PubMed] [Google Scholar]
- Li T, Spearow J, Rubin CM, Schmid CW (1999) Physiological stresses increase mouse short interspersed element (SINE) RNA expression in vivo. Gene 239:367–372. [DOI] [PubMed] [Google Scholar]
- Liu B, Qian S-B (2014) Translational reprogramming in cellular stress response. Wiley Interdiscip Rev RNA 5:301–315. doi: 10.1002/wrna.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang H, Chua N-H (2015a) Long noncoding RNA transcriptome of plants. Plant Biotechnol J 13:319–328. doi: 10.1111/pbi.12336 [DOI] [PubMed] [Google Scholar]
- Liu Q, Sun S, Yu W, et al. (2015b) Altered expression of long non-coding RNAs during genotoxic stress-induced cell death in human glioma cells. J Neurooncol 122:283–292. doi: 10.1007/s11060-015-1718-0 [DOI] [PubMed] [Google Scholar]
- Liu WM, Chu WM, Choudary PV, Schmid CW (1995) Cell stress and translational inhibitors transiently increase the abundance of mammalian SINE transcripts. Nucleic Acids Res 23:1758–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hao L, Li D, et al. (2015c) Long Non-coding RNAs and Their Biological Roles in Plants. Genomics Proteomics Bioinformatics 13:137–147. doi: 10.1016/j.gpb.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li D, Zhang W, et al. (2012) Long non-coding RNA gadd7 interacts with TDP-43 and regulates Cdk6 mRNA decay. EMBO J 31:4415–4427. doi: 10.1038/emboj.2012.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca M, Luca A, Calandra C (2015) The Role of Oxidative Damage in the Pathogenesis and Progression of Alzheimer’s Disease and Vascular Dementia. Oxid Med Cell Longev 2015:504678. doi: 10.1155/2015/504678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Wang Z, Wu J, et al. (2014) The Role of Hypoxia Inducible Factor-1 in Hepatocellular Carcinoma, The Role of Hypoxia Inducible Factor-1 in Hepatocellular Carcinoma. BioMed Research International, BioMed Research International 2014, 2014:e409272. doi: 10.1155/2014/409272, 10.1155/2014/409272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC (2010) Hypoxia inducible factors and the response to hypoxic stress. Mol Cell 40:294–309. doi: 10.1016/j.molcel.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik K, Salpekar A, Hancock A, et al. (2000) Identification of differential methylation of the WT1 antisense regulatory region and relaxation of imprinting in Wilms’ tumor. Cancer Res 60:2356–2360. [PubMed] [Google Scholar]
- Ma Q (2010) Transcriptional responses to oxidative stress: pathological and toxicological implications. Pharmacol Ther 125:376–393. doi: 10.1016/j.pharmthera.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Mariner PD, Walters RD, Espinoza CA, et al. (2008) Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol Cell 29:499–509. doi: 10.1016/j.molcel.2007.12.013 [DOI] [PubMed] [Google Scholar]
- Marquardt S, Escalante-Chong R, Pho N, et al. (2014) A chromatin-based mechanism for limiting divergent noncoding transcription. Cell 157:1712–1723. doi: 10.1016/j.cell.2014.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini LA, Sontheimer EJ (2008) CRISPR Interference Limits Horizontal Gene Transfer in Staphylococci by Targeting DNA. Science 322:1843–1845. doi: 10.1126/science.1165771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JL, Sanchez-Elsner T, Morcillo G, Diez JL (2001) Heat shock regulatory elements are present in telomeric repeats of Chironomus thummi. Nucl Acids Res 29:4760–4766. doi: 10.1093/nar/29.22.4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé E, Vanderpool CK, Gottesman S (2005) Effect of RyhB small RNA on global iron use in Escherichia coli. J Bacteriol 187:6962–6971. doi: 10.1128/JB.187.20.6962-6971.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matouk IJ, DeGroot N, Mezan S, et al. (2007) The H19 Non-Coding RNA Is Essential for Human Tumor Growth. PLoS ONE 2:e845. doi: 10.1371/journal.pone.0000845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matouk IJ, Mezan S, Mizrahi A, et al. (2010) The oncofetal H19 RNA connection: hypoxia, p53 and cancer. Biochim Biophys Acta 1803:443–451. doi: 10.1016/j.bbamcr.2010.01.010 [DOI] [PubMed] [Google Scholar]
- Matouk IJ, Raveh E, Abu-lail R, et al. (2014) Oncofetal H19 RNA promotes tumor metastasis. Biochim Biophys Acta 1843:1414–1426. doi: 10.1016/j.bbamcr.2014.03.023 [DOI] [PubMed] [Google Scholar]
- Mattick JS, Rinn JL (2015) Discovery and annotation of long noncoding RNAs. Nat Struct Mol Biol 22:5–7. doi: 10.1038/nsmb.2942 [DOI] [PubMed] [Google Scholar]
- McCarty G, Loeb DM (2015) Hypoxia-sensitive epigenetic regulation of an antisense-oriented lncRNA controls WT1 expression in myeloid leukemia cells. PLoS ONE 10:e0119837. doi: 10.1371/journal.pone.0119837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer TR, Clark MB, Crawford J, et al. (2014) Targeted sequencing for gene discovery and quantification using RNA CaptureSeq. Nat Protocols 9:989–1009. doi: 10.1038/nprot.2014.058 [DOI] [PubMed] [Google Scholar]
- Metz A, Soret J, Vourc’h C, et al. (2004) A key role for stress-induced satellite III transcripts in the relocalization of splicing factors into nuclear stress granules. J Cell Sci 117:4551–4558. doi: 10.1242/jcs.01329 [DOI] [PubMed] [Google Scholar]
- Mizutani R, Wakamatsu A, Tanaka N, et al. (2012) Identification and characterization of novel genotoxic stress-inducible nuclear long noncoding RNAs in mammalian cells. PLoS ONE 7:e34949. doi: 10.1371/journal.pone.0034949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran VA, Perera RJ, Khalil AM (2012) Emerging functional and mechanistic paradigms of mammalian long non-coding RNAs. Nucl Acids Res 40:6391–6400. doi: 10.1093/nar/gks296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KV, Mattick JS (2014) The rise of regulatory RNA. Nat Rev Genet 15:423–437. doi: 10.1038/nrg3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley PL, Wallen ES, McCafferty JD, et al. (1993) Heat stress regulates the human 70-kDa heat-shock gene through the 3’-untranslated region. Am J Physiol 264:L533–537. [DOI] [PubMed] [Google Scholar]
- Nadal-Ribelles M, Solé C, Xu Z, et al. (2014) Control of Cdc28 CDK1 by a Stress-Induced lncRNA. Mol Cell 53:549–561. doi: 10.1016/j.molcel.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S-Y, Soh BS, Rodriguez-Muela N, et al. (2015) Genome-wide RNA-Seq of Human Motor Neurons Implicates Selective ER Stress Activation in Spinal Muscular Atrophy. Cell Stem Cell. doi: 10.1016/j.stem.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Kiss T, Michels AA, Bensaude O (2001) 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414:322–325. doi: 10.1038/35104581 [DOI] [PubMed] [Google Scholar]
- Ning Y, Yong F, Haibin Z, et al. (2015) LincRNA-p21 activates endoplasmic reticulum stress and inhibits hepatocellular carcinoma. [DOI] [PMC free article] [PubMed]
- Nogalska A, Engel WK, Askanas V (2010) Increased BACE1 mRNA and noncoding BACE1-antisense transcript in sporadic inclusion-body myositis muscle fibers--possibly caused by endoplasmic reticulum stress. Neurosci Lett 474:140–143. doi: 10.1016/j.neulet.2010.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntini E, Järvelin AI, Bornholdt J, et al. (2013) Polyadenylation site-induced decay of upstream transcripts enforces promoter directionality. Nat Struct Mol Biol 20:923–928. doi: 10.1038/nsmb.2640 [DOI] [PubMed] [Google Scholar]
- Oh YS, Kim HY, Song I-C, et al. (2012) Hypoxia induces CXCR4 expression and biological activity in gastric cancer cells through activation of hypoxia-inducible factor-1α. Oncol Rep 28:2239–2246. doi: 10.3892/or.2012.2063 [DOI] [PubMed] [Google Scholar]
- O’Leary VB, Ovsepian SV, Carrascosa LG, et al. (2015) PARTICLE, a Triplex-Forming Long ncRNA, Regulates Locus-Specific Methylation in Response to Low-Dose Irradiation. Cell Rep 11:474–485. doi: 10.1016/j.celrep.2015.03.043 [DOI] [PubMed] [Google Scholar]
- Özgür E, Mert U, Isin M, et al. (2013) Differential expression of long non-coding RNAs during genotoxic stress-induced apoptosis in HeLa and MCF-7 cells. Clin Exp Med 13:119–126. doi: 10.1007/s10238-012-0181-x [DOI] [PubMed] [Google Scholar]
- Pandey R, Mandal AK, Jha V, Mukerji M (2011) Heat shock factor binding in Alu repeats expands its involvement in stress through an antisense mechanism. Genome Biol 12:R117. doi: 10.1186/gb-2011-12-11-r117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang KC, Frith MC, Mattick JS (2006) Rapid evolution of noncoding RNAs: lack of conservation does not mean lack of function. Trends Genet 22:1–5. doi: 10.1016/j.tig.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Peterlin BM, Brogie JE, Price DH (2012) 7SK snRNA: a noncoding RNA that plays a major role in regulating eukaryotic transcription. Wiley Interdiscip Rev RNA 3:92–103. doi: 10.1002/wrna.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place RF, Noonan EJ (2014) Non-coding RNAs turn up the heat: an emerging layer of novel regulators in the mammalian heat shock response. Cell Stress Chaperones 19:159–172. doi: 10.1007/s12192-013-0456-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliseno L, Salmena L, Zhang J, et al. (2010) A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465:1033–1038. doi: 10.1038/nature09144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanth KV, Rajendra TK, Lal AK, Lakhotia SC (2000) Omega speckles - a novel class of nuclear speckles containing hnRNPs associated with noncoding hsr-omega RNA in Drosophila. J Cell Sci 113 Pt 19:3485–3497. [DOI] [PubMed] [Google Scholar]
- Qi X, Xie S, Liu Y, et al. (2013) Genome-wide annotation of genes and noncoding RNAs of foxtail millet in response to simulated drought stress by deep sequencing. Plant Mol Biol 83:459–473. doi: 10.1007/s11103-013-0104-6 [DOI] [PubMed] [Google Scholar]
- Quinodoz S, Guttman M (2014) Long noncoding RNAs: an emerging link between gene regulation and nuclear organization. Trends Cell Biol 24:651–663. doi: 10.1016/j.tcb.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam M, Kim S-J (2012) Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. Journal of Neural Transmission (Vienna, Austria: 1996). doi: 10.1007/s00702-011-0758-7 [DOI] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J (2010) The Heat Shock Response: Life on the Verge of Death. Molecular Cell 40:253–266. doi: 10.1016/j.molcel.2010.10.006 [DOI] [PubMed] [Google Scholar]
- Rinn JL (2014) lncRNAs: Linking RNA to Chromatin. Cold Spring Harb Perspect Biol. doi: 10.1101/cshperspect.a018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Chang HY (2012) Genome Regulation by Long Noncoding RNAs. Annu Rev Biochem 81:145–166. doi: 10.1146/annurev-biochem-051410-092902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi N, Denegri M, Chiodi I, et al. (2004) Transcriptional activation of a constitutive heterochromatic domain of the human genome in response to heat shock. Mol Biol Cell 15:543–551. doi: 10.1091/mbc.E03-07-0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano AD, Serviddio G, de Matthaeis A, et al. (2010) Oxidative stress and aging. J Nephrol 23 Suppl 15:S29–36. [PubMed] [Google Scholar]
- Saito S, Lin Y-C, Tsai M-H, et al. (2015) Emerging roles of hypoxia-inducible factors and reactive oxygen species in cancer and pluripotent stem cells. Kaohsiung J Med Sci 31:279–286. doi: 10.1016/j.kjms.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Saunders JW (1966) Death in Embryonic Systems. Science 154:604–612. doi: 10.1126/science.154.3749.604 [DOI] [PubMed] [Google Scholar]
- Semenza GL (2012a) Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med 18:534–543. doi: 10.1016/j.molmed.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL (2012b) Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408. doi: 10.1016/j.cell.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiq S, Li J, Sun Q (2015) Functions of plants long non-coding RNAs. Biochim Biophys Acta. doi: 10.1016/j.bbagrm.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Shamovsky I, Ivannikov M, Kandel ES, et al. (2006) RNA-mediated response to heat shock in mammalian cells. Nature 440:556–560. doi: 10.1038/nature04518 [DOI] [PubMed] [Google Scholar]
- Shamovsky I, Nudler E (2009) Isolation and characterization of the heat shock RNA 1. Methods Mol Biol 540:265–279. doi: 10.1007/978-1-59745-558-9_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JM, Perez DS, Pritchett JR, et al. (2010) Identification of Long stress-induced non-coding transcripts that have altered expression in cancer. Genomics 95:355–362. doi: 10.1016/j.ygeno.2010.02.009 [DOI] [PubMed] [Google Scholar]
- Sledjeski DD, Gupta A, Gottesman S (1996) The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. EMBO J 15:3993–4000. [PMC free article] [PubMed] [Google Scholar]
- Solé C, Nadal-Ribelles M, de Nadal E, Posas F (2015) A novel role for lncRNAs in cell cycle control during stress adaptation. Curr Genet 61:299–308. doi: 10.1007/s00294-014-0453-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G, Vogel J, Wassarman KM (2011) Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 43:880–891. doi: 10.1016/j.molcel.2011.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R (2010) MicroRNAs with macro-effects on plant stress responses. Semin Cell Dev Biol 21:805–811. doi: 10.1016/j.semcdb.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Taft RJ, Pheasant M, Mattick JS (2007) The relationship between non-protein-coding DNA and eukaryotic complexity. Bioessays 29:288–299. doi: 10.1002/bies.20544 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yan IK, Haga H, Patel T (2014) Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci 127:1585–1594. doi: 10.1242/jcs.141069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Onuma Y, Ito Y, Torimura M (2014) Long Non-Coding RNAs as Surrogate Indicators for Chemical Stress Responses in Human-Induced Pluripotent Stem Cells. PLoS ONE 9:e106282. doi: 10.1371/journal.pone.0106282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Torimura M (2013) Identification of short-lived long non-coding RNAs as surrogate indicators for chemical stress response. Biochem Biophys Res Commun 439:547–551. doi: 10.1016/j.bbrc.2013.09.006 [DOI] [PubMed] [Google Scholar]
- Thomason MK, Storz G (2010) Bacterial Antisense RNAs: How Many Are There, and What Are They Doing? Annual Review of Genetics 44:167–188. doi: 10.1146/annurev-genet-102209-163523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrash-Bingham CA, Tartof KD (1999) aHIF: a natural antisense transcript overexpressed in human renal cancer and during hypoxia. J Natl Cancer Inst 91:143–151. [DOI] [PubMed] [Google Scholar]
- Tilman G, Arnoult N, Lenglez S, et al. (2012) Cancer-linked satellite 2 DNA hypomethylation does not regulate Sat2 non-coding RNA expression and is initiated by heat shock pathway activation. Epigenetics 7:903–913. doi: 10.4161/epi.21107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Rossignol F, Matthay MA, et al. (2004) Prolonged Hypoxia Differentially Regulates Hypoxia-inducible Factor (HIF)-1α and HIF-2α Expression in Lung Epithelial Cells IMPLICATION OF NATURAL ANTISENSE HIF-1α. J Biol Chem 279:14871–14878. doi: 10.1074/jbc.M400461200 [DOI] [PubMed] [Google Scholar]
- Uesaka M, Nishimura O, Go Y, et al. (2014) Bidirectional promoters are the major source of gene activation-associated non-coding RNAs in mammals. BMC Genomics 15:35. doi: 10.1186/1471-2164-15-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky I, Bartel DP (2013) lincRNAs: genomics, evolution, and mechanisms. Cell 154:26–46. doi: 10.1016/j.cell.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky I, Shkumatava A, Jan CH, et al. (2011) Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell 147:1537–1550. doi: 10.1016/j.cell.2011.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoson C, Wagner EGH (2008) A small SOS-induced toxin is targeted against the inner membrane in Escherichia coli. Mol Microbiol 70:258–270. doi: 10.1111/j.1365-2958.2008.06416.x [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Raychaudhuri S, Hayer-Hartl M, Hartl FU (2010) Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb Perspect Biol 2:a004390. doi: 10.1101/cshperspect.a004390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadkhan S, Nilsen TW (2010) Reprogramming of the non-coding transcriptome during brain development. J Biol 9:5. doi: 10.1186/jbiol197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valgardsdottir R, Chiodi I, Giordano M, et al. (2005) Structural and Functional Characterization of Noncoding Repetitive RNAs Transcribed in Stressed Human Cells. Mol Biol Cell 16:2597–2604. doi: 10.1091/mbc.E04-12-1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valgardsdottir R, Chiodi I, Giordano M, et al. (2008) Transcription of Satellite III non-coding RNAs is a general stress response in human cells. Nucl Acids Res 36:423–434. doi: 10.1093/nar/gkm1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Večerek B, Beich-Frandsen M, Resch A, Bläsi U (2010) Translational activation of rpoS mRNA by the non-coding RNA DsrA and Hfq does not require ribosome binding. Nucl Acids Res 38:1284–1293. doi: 10.1093/nar/gkp1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velichko AK, Markova EN, Petrova NV, et al. (2013) Mechanisms of heat shock response in mammals. Cell Mol Life Sci 70:4229–4241. doi: 10.1007/s00018-013-1348-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakano C, Byun JS, Di L-J, Gardner K (2012) The dual lives of bidirectional promoters. Biochim Biophys Acta 1819:688–693. doi: 10.1016/j.bbagrm.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Li X, Xie X, et al. (2008a) UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Letters 582:1919–1927. doi: 10.1016/j.febslet.2008.05.012 [DOI] [PubMed] [Google Scholar]
- Wang H, Chung PJ, Liu J, et al. (2014a) Genome-wide identification of long noncoding natural antisense transcripts and their responses to light in Arabidopsis. Genome Res 24:444–453. doi: 10.1101/gr.165555.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G, Hu X, Liu Y, et al. (2013a) A novel non-coding RNA lncRNA-JADE connects DNA damage signalling to histone H4 acetylation. EMBO J 32:2833–2847. doi: 10.1038/emboj.2013.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G, Mathur R, Hu X, et al. (2013b) Long non-coding RNA ANRIL (CDKN2B-AS) is induced by the ATM-E2F1 signaling pathway. Cell Signal 25:1086–1095. doi: 10.1016/j.cellsig.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T-Z, Liu M, Zhao M-G, et al. (2015) Identification and characterization of long non-coding RNAs involved in osmotic and salt stress in Medicago truncatula using genome-wide high-throughput sequencing. BMC Plant Biol 15:131. doi: 10.1186/s12870-015-0530-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Arai S, Song X, et al. (2008b) Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 454:126–130. doi: 10.1038/nature06992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-S, Zhang Z, Wang H-C, et al. (2006) Rapid Identification of UCA1 as a Very Sensitive and Specific Unique Marker for Human Bladder Carcinoma. Clin Cancer Res 12:4851–4858. doi: 10.1158/1078-0432.CCR-06-0134 [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu X, Zhang H, et al. (2014b) Hypoxia-inducible lncRNA-AK058003 promotes gastric cancer metastasis by targeting γ-synuclein. Neoplasia 16:1094–1106. doi: 10.1016/j.neo.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu Z, Jiang J, et al. (2013) Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell 25:69–80. doi: 10.1016/j.devcel.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Wapinski O, Chang HY (2011) Long noncoding RNAs and human disease. Trends Cell Biol 21:354–361. doi: 10.1016/j.tcb.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Wassarman KM (2007) 6S RNA: a regulator of transcription. Molecular Microbiology 65:1425–1431. doi: 10.1111/j.1365-2958.2007.05894.x [DOI] [PubMed] [Google Scholar]
- Waters LS, Storz G (2009) Regulatory RNAs in Bacteria. Cell 136:615–628. doi: 10.1016/j.cell.2009.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Pelechano V, Järvelin AI, Steinmetz LM (2011) Functional consequences of bidirectional promoters. Trends Genet 27:267–276. doi: 10.1016/j.tig.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Okada T, Fukushima T, et al. (2012) A novel hypoxic stress-responsive long non-coding RNA transcribed by RNA polymerase III in Arabidopsis. RNA Biol 9:302–313. doi: 10.4161/rna.19101 [DOI] [PubMed] [Google Scholar]
- Wunderlich M, Gross-Hardt R, Schöffl F (2014) Heat shock factor HSFB2a involved in gametophyte development of Arabidopsis thaliana and its expression is controlled by a heat-inducible long non-coding antisense RNA. Plant Mol Biol 85:541–550. doi: 10.1007/s11103-014-0202-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Wang Y, Yao Y, et al. (2011) Identification and characterization of wheat long non-protein coding RNAs responsive to powdery mildew infection and heat stress by using microarray analysis and SBS sequencing. BMC plant biology 11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan H, Zhang L, Liu X, et al. (2015) PLNlncRbase: A resource for experimentally identified lncRNAs in plants. Gene. doi: 10.1016/j.gene.2015.07.069 [DOI] [PubMed] [Google Scholar]
- Xue M, Li X, Li Z, Chen W (2014) Urothelial carcinoma associated 1 is a hypoxia-inducible factor-1α-targeted long noncoding RNA that enhances hypoxic bladder cancer cell proliferation, migration, and invasion. Tumour Biol 35:6901–6912. doi: 10.1007/s13277-014-1925-x [DOI] [PubMed] [Google Scholar]
- Xu Q, Liu L-Z, Qian X, et al. (2012) MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucl Acids Res 40:761–774. doi: 10.1093/nar/gkr730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovchuk P, Goodrich JA, Kugel JF (2009) B2 RNA and Alu RNA repress transcription by disrupting contacts between RNA polymerase II and promoter DNA within assembled complexes. Proc Natl Acad Sci USA 106:5569–5574. doi: 10.1073/pnas.0810738106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovchuk P, Goodrich JA, Kugel JF (2011) B2 RNA represses TFIIH phosphorylation of RNA polymerase II. Transcription 2:45–49. doi: 10.4161/trns.2.1.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Li X, Wang Y, et al. (2012a) Long non-coding RNA UCA1 regulated cell cycle distribution via CREB through PI3-K dependent pathway in bladder carcinoma cells. Gene 496:8–16. doi: 10.1016/j.gene.2012.01.012 [DOI] [PubMed] [Google Scholar]
- Yang F, Bi J, Xue X, et al. (2012b) Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells: H19 regulates gastric cancer. FEBS Journal 279:3159–3165. doi: 10.1111/j.1742-4658.2012.08694.x [DOI] [PubMed] [Google Scholar]
- Yang F, Huo X, Yuan S, et al. (2013a) Repression of the Long Noncoding RNA-LET by Histone Deacetylase 3 Contributes to Hypoxia-Mediated Metastasis. Molecular Cell 49:1083–1096. doi: 10.1016/j.molcel.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Yang F, Zhang H, Mei Y, Wu M (2014a) Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the Warburg effect. Mol Cell 53:88–100. doi: 10.1016/j.molcel.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Yang F, Zhang L, Huo X, et al. (2011) Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology 54:1679–1689. doi: 10.1002/hep.24563 [DOI] [PubMed] [Google Scholar]
- Yang L, Froberg JE, Lee JT (2014b) Long noncoding RNAs: fresh perspectives into the RNA world. Trends Biochem Sci 39:35–43. doi: 10.1016/j.tibs.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sun M, Wang L, Jiao B (2013b) HIFs, angiogenesis, and cancer. J Cell Biochem 114:967–974. doi: 10.1002/jcb.24438 [DOI] [PubMed] [Google Scholar]
- Yang Z-G, Gao L, Guo X-B, Shi Y-L (2015) Roles of long non-coding RNAs in gastric cancer metastasis. World J Gastroenterol 21:5220–5230. doi: 10.3748/wjg.v21.i17.5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimizu T, Miroglio A, Ripoche M-A, et al. (2008) The H19 locus acts in vivo as a tumor suppressor. Proc Natl Acad Sci U S A 105:12417–12422. doi: 10.1073/pnas.0801540105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost HJ, Lindquist S (1986) RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell 45:185–193. doi: 10.1016/0092-8674(86)90382-X [DOI] [PubMed] [Google Scholar]
- Young RS, Ponting CP (2013) Identification and function of long non-coding RNAs. Essays Biochem 54:113–126. doi: 10.1042/bse0540113 [DOI] [PubMed] [Google Scholar]
- Yu C, Xue J, Zhu W, et al. (2015) Warburg meets non-coding RNAs: the emerging role of ncRNA in regulating the glucose metabolism of cancer cells. Tumour Biol 36:81–94. doi: 10.1007/s13277-014-2875-z [DOI] [PubMed] [Google Scholar]
- Zhang A, Xu M, Mo Y-Y (2014a) Role of the lncRNA–p53 regulatory network in cancer. J Mol Cell Biol 6:181–191. doi: 10.1093/jmcb/mju013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Zhou N, Huang J, et al. (2013a) The human long non-coding RNA-RoR is a p53 repressor in response to DNA damage. Cell Res 23:340–350. doi: 10.1038/cr.2012.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chen X, Wang C, et al. (2013b) Long non-coding genes implicated in response to stripe rust pathogen stress in wheat (Triticum aestivum L.). Mol Biol Rep 40:6245–6253. doi: 10.1007/s11033-013-2736-7 [DOI] [PubMed] [Google Scholar]
- Zhang W, Han Z, Guo Q, et al. (2014b) Identification of maize long non-coding RNAs responsive to drought stress. PLoS ONE 9:e98958. doi: 10.1371/journal.pone.0098958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-Q, Liu J-H, Liang X-W, et al. (2008) Heat stress causes aberrant DNA methylation of H19 and Igf-2r in mouse blastocysts. Mol Cells 25:211–215. [PubMed] [Google Scholar]
- Zhu Q-H, Stephen S, Taylor J, et al. (2014) Long noncoding RNAs responsive to Fusarium oxysporum infection in Arabidopsis thaliana. New Phytol 201:574–584. doi: 10.1111/nph.12537 [DOI] [PubMed] [Google Scholar]


