Abstract
The mechanism of entry of vaccinia virus (VV) into cells is still a poorly understood process. A 14-kDa protein (encoded by the A27L gene) in the envelope of intracellular mature virus (IMV) has been implicated in virus-cell attachment, virus-cell fusion, and virus release from cells. We have previously described the structural organization of the VV 14-kDa protein, consisting of a triple-stranded coiled-coil region responsible for oligomer formation and a predicted Leu zipper-like third alpha helix with an important role in the interaction with a 21-kDa membrane protein (encoded by the A17L gene) thought to anchor the 14-kDa protein to the envelope of IMV (M.-I. Vázquez, G. Rivas, D. Cregut, L. Serrano, and M. Esteban, J. Virol. 72:10126–10137, 1998). To identify the functional domains important for virus entry and release, we have generated VV recombinants containing a copy of the A27L gene regulated by the lacI operator-repressor system of Escherichia coli (VVIndA27L) in the thymidine kinase locus and a mutant form of the A27L gene in the hemagglutinin locus but expressed constitutively under the control of an early-late VV promoter. Cells infected with a VV recombinant that expresses a mutant 14-kDa form lacking the first 29 amino acids at the N terminus failed to form extracellular enveloped virus (EEV). Fusion-from-without assays with purified virus confirmed that the fusion process was mediated by the 14-kDa protein and the fusion domain to be contained within amino acids 29 to 43 of the N-terminal region. Competitive inhibition of the infection process with soluble heparin and synthetic peptides and in vitro experiments with purified mutant proteins identified the heparin binding domain within amino acids 21 to 33, suggesting that this domain is involved in virus-cell binding via heparan sulfate. Thus, the N terminus of the 14-kDa protein contains a heparin binding domain, a fusion domain, and a domain responsible for interacting with proteins or lipids in the Golgi stacks for EEV formation and virus spread.
The entry of enveloped viruses into cells requires the fusion of viral and cellular membranes in a process catalyzed by specific viral fusion proteins. This event involves a series of steps that are initiated with the binding of the fusion protein itself or an associated protein to a cellular receptor, exposure of the fusion peptide in an active conformation, and insertion of the fusion peptide into the target cell membrane. As a result of the fusion process, the virus genome is released into the cytoplasm of the host cell (for reviews, see references 50 and 51). The first event, binding of the viral particle to the surface of the host cell, can be mediated by several types of molecules, specific or not for each virus. For human immunodeficiency virus type 1 (HIV-1), entry is necessary for the interaction with the CD4 receptor (39) and one of the several coreceptors that are members of the chemokine receptor family (10, 12). Other viruses that infect a wide variety of cell lines do not require specific receptors, and the interaction can be mediated by surface molecules that are ubiquitously expressed on cells. This is the case for heparan sulfate (HS), a glycosaminoglycan known to mediate the attachment of herpes simplex virus (41, 53), dengue virus (7), adeno-associated virus type 2 (44), respiratory syncitial virus (28), Sindbis virus (1), and foot-and-mouth disease virus (24).
The second step, the action of viral and cellular fusion itself, is mediated by fusion peptides. The peptides that are present in the well-characterized influenza virus hemagglutinin (HA) protein as well as in the gp41 membrane protein of HIV-1 or in the transmembrane subunit of Moloney murine leukemia virus have similar structures: a short alpha helix which is inserted into the cellular membrane and a triple-stranded coiled-coil region (3, 6, 14, 49). In the best-studied system, the HA of influenza virus, the fusion mechanism can include partial insertion of the coiled-coil region into the target membrane, bringing the cellular and viral membranes closer together (4, 54).
In vaccinia virus (VV), the prototype member of the poxvirus family, the study of the entry process, attachment, and fusion and the proteins and receptors involved is complicated by the existence of two different infectious forms: the intracellular mature virus (IMV) with a membrane surrounding the viral core (38, 40, 42) and the extracellular enveloped virus (EEV) with an additional outer membrane acquired from the trans-Golgi network cisternae and containing proteins that are absent from IMV (19, 22, 32, 38, 40). The different morphologies of IMV and EEV suggest the occurrence of different mechanisms for the entry of these VV forms into the host cell. It has been recently proposed that while the entry of EEV might occur by endocytosis and posterior fusion in a pH-dependent pathway, IMV might fuse directly with the cellular membrane in a low-pH-independent manner (47).
The fusion process, a common feature during EEV and IMV infections, has been attributed to the action of the 14-kDa protein (encoded by the A27L gene). This attribution is based on several observations. First, the 14-kDa protein is located on the surface of IMV (43) and has a trimeric coiled-coil structure characteristic of fusion proteins (48). Second, syncytium formation, the most dramatic visual manifestation of viral fusion activity, observed in cells infected with several fusogenic VV mutants or after a brief treatment at an acid pH, is inhibited in the continuous presence of a monoclonal antibody (MAb) specific for the 14-kDa protein (11, 17, 35, 45). The initial attachment of VV to the cell surface has also been related to the 14-kDa protein based on binding experiments with purified protein (29) and recent observations which described the interaction of the 14-kDa protein with cell surface HS (8).
The exit of VV from cells has also been attributed to the 14-kDa protein. This attribution is based on the inability of a conditional lethal VV mutant to exit from cells when the 14-kDa protein is repressed (36, 37). Under those conditions, IMV is produced but the formation of EEV is restricted (37).
Due to the biological importance of the 14-kDa protein in virus entry and exit from cells, in this study we have carried out an analysis of its functional domains. We have constructed recombinant viruses expressing constitutively different mutant forms of the 14-kDa protein and the wild-type 14-kDa protein under the regulation of the Escherichia coli operator-repressor system. Through in vivo and in vitro experiments, we have mapped the domains of the 14-kDa protein with roles in binding to heparin and hence to the cell surface via HS and in the fusion process during VV infection. Both of these domains are located adjacent to the N terminus of the 14-kDa protein. Deletion of the N terminus of the protein blocked the release of the virus to the extracellular medium. Thus, we have found three functional domains of the 14-kDa protein required for binding to heparin, for virus-cell fusion, and for the release of the virus from cells. These domains are clustered within the first 43 amino acids of the N terminus of this VV envelope protein.
(This work, which was presented at the XIIth International Poxvirus-Iridovirus Meeting, St. Thomas, U.S. Virgin Islands, in June 1998, was carried out by M.-I.V. in partial fulfillment of the requirements for a Ph.D. degree from the School of Pharmacy, University of Santiago de Compostela, Santiago de Compostela, Spain.)
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney cells (BSC40) and HeLa cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated newborn calf serum (NCS). VV strain WR was propagated and titrated in BSC40 cells by a plaque assay. Inducible virus VVIndA27L (36, 37) was grown in the absence or presence of 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and purified by banding after sucrose gradient centrifugation (13, 25). Inducible virus was titrated in the continuous presence of IPTG.
Antisera.
A polyclonal antibody against the 14-kDa protein (Rα14K) was produced by immunization of rabbits with purified 14-kDa protein (9). MAb C3, specific for the 14-kDa protein, has been previously described (34). Rhodamine-labeled goat anti-rabbit antibody was obtained from Organon Teknika. Horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit antibodies were provided by Cappel.
Peptides and purified proteins.
The peptide corresponding to the amino-terminal region of the 14-kDa protein, amino acids 13 to 33 (14K-13/33) (AIPDTEFFSTKAAKKPEAKRE), was synthesized acetylated at the N terminus and amidated at the C terminus. Peptide homogeneity and identity were determined by analytical high-performance liquid chromatography. The synthetic peptides corresponding to amino acids 1 to 21 (R7) (33) and 75 to 106 (14K-75/106) (48) in the sequence of the VV 14-kDa protein were analyzed by mass spectrometry. Purified proteins 14K-A, 14K-A-Δ29, 14K-A-Δ43, and 14K-29/74 were obtained as previously described by Vázquez et al. (48).
Generation of recombinant VV.
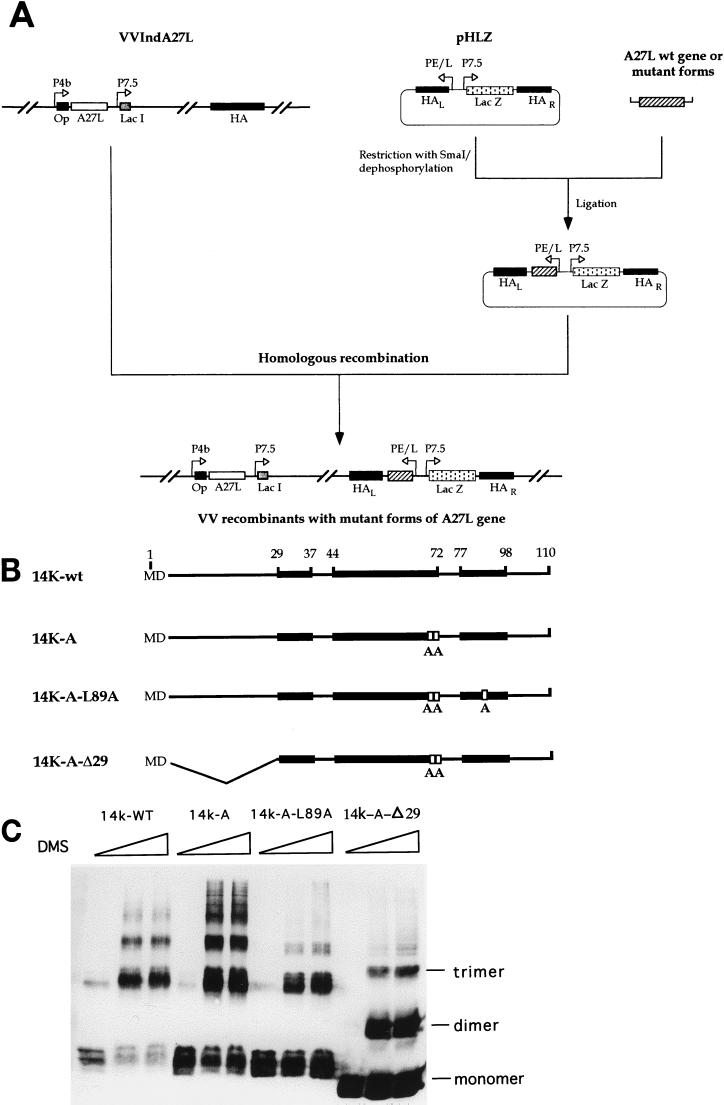
Mutant forms of the 14-kDa protein were obtained by PCR and cloned into the SmaI site of pHLZ (48). Recombinant viruses were generated by transfecting VVIndA27L-infected BSC40 cells with plasmids containing the mutant forms of the 14-kDa protein under the control of the synthetic early-late viral promoter. Plasmid insertion vectors pHLZ-14K-wt, pHLZ-14K-A, pHLZ-14K-A-L89A, and pHLZ-14K-A-Δ29 contained the A27L gene and mutant forms flanked by the VV HA sequence to provide sites for homologous recombination; they also contained the lacZ gene for blue-plaque-phenotype selection of recombinant viruses after the addition of 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) to the infected monolayers (48). A scheme for recombinant virus construction and the proteins constitutively expressed through the HA locus are shown in Fig. 1A and B, respectively. To confirm that the virus mutants maintained the mutated copies of the A27L gene during virus growth, we sequenced the constitutively expressed genes to see if they all retained the mutations. Thus, each mutant virus was grown in BSC40 cells and purified on sucrose gradients, cells were infected with 5 PFU/cell, and total DNA was extracted and used for PCR amplification with the following primers: 5′-TATTTTTTTTTTTTGGGATATAAATAAGCTCGAAGTCGACAGATCTAGGCCTG-3′ (for the early-late promoter) and 5′-CCTGCCGCGGGTATACTCATTGGGTTCGAACCC-3′ (for the A27L gene). The DNA was sequenced at Centro Nacional de Biotecnología with an automated DNA sequencer apparatus. The particle/PFU ratios of the purified viruses grown in the absence of IPTG and titrated in the presence of IPTG were as follows: VVInd14K-wt, 130; VVInd14K-A-L89A, 229; and VVInd14K-A-Δ29, 158. When VVInd14K-wt was grown in the presence of IPTG, the particle/PFU ratio was 79.
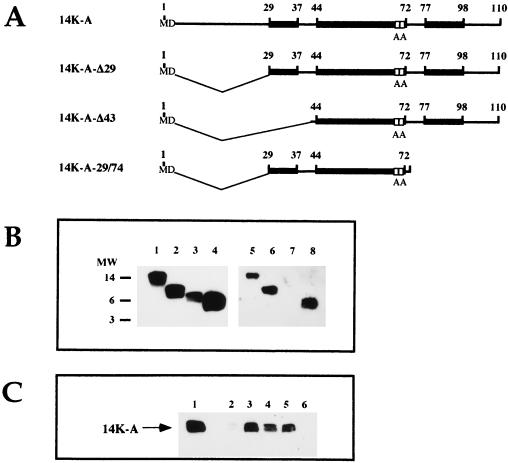
FIG. 1.
(A) Strategy for the construction of recombinant viruses that express mutant forms of the 14-kDa protein. We used VVIndA27L, which contains an IPTG-inducible copy of the A27L gene in the TK locus downstream of a hybrid-inducible promoter consisting of the VV 4b promoter (P4b) fused to the lacI operator (Op) unit. The lacI repressor gene is under the control of VV promoter 7.5 (P7.5). The DNA fragments corresponding to the wild-type (wt) or mutant forms of the 14-kDa protein were cloned into pHLZ under the control of the synthetic early-late viral promoter (PE/L). The plasmid contains the E. coli lacZ gene controlled by viral promoter 7.5 and is used for blue-plaque-phenotype selection after the addition of X-Gal. Both the A27L and lacZ genes are placed between the left (L) and right (R) VV HA sequences to provide sites for homologous recombination within the viral genome of VVIndA27L. (B) The recombinant viruses generated were called VVInd14K-wt, VVInd14K-A, VVInd14K-A-L89A, and VVInd14K-A-Δ29, according to the constitutively expressed proteins: 14K-wt has the same sequence as the wild-type protein in VV; 14K-A has two point mutations in two contiguous Cys residues (replaced by two Ala residues); 14K-A-L89A is like 14K-A but also contains a point mutation in the Leu residue at position 89 (replaced by Ala); and 14K-A-Δ29 has a deletion of 28 amino acids in the N-terminal region and two Cys mutations (replaced by Ala). Black boxes show the three predicted alpha helixes in the sequence (48), and white boxes show the amino acids that have been mutated. The amino acid positions in the sequence of the 14-kDa protein are indicated by numbers at the top. (C) Oligomerization of the mutant proteins. Cross-linking experiments were carried out with full-length E. coli-expressed 14-kDa protein and mutant forms. Bacterial lysates obtained from 10-ml cultures of an E. coli expression system at 5 h postinduction were concentrated and resuspended in 100 μl of PBS. Different amounts of dimethyl suberimidate (0, 2, and 5 μl) in dimethyl sulfoxide at a stock concentration of 50 mg/ml (freshly prepared) were added to the protein sample, and the mixtures were incubated on ice for 90 min. The reactions were quenched by the addition of Tris-HCl (pH 8.0) to a final concentration of 20 mM, and the mixtures were incubated at 4°C for 15 min. After the addition of sample buffer, proteins were analyzed on sodium dodecyl sulfate–13% polyacrylamide gels, and profiles were revealed by Western blotting after reaction with MAb C3. Monomer, dimer, and trimer forms are indicated, and no differences were observed with increasing amounts of dimethyl suberimidate.
Plaque assays.
Confluent monolayers of BSC40 cells seeded in a six-well plate were infected with 150 PFU of VVIndA27L or recombinant viruses expressing mutant proteins constitutively per well. The inoculum was removed after 1 h of virus adsorption, and the infected cells were overlaid with DMEM supplemented with 2% NCS and containing or lacking 2 mM IPTG. At 48 h postinfection (p.i.), the medium was removed and the monolayers were stained with 1% crystal violet in 20% methanol.
Indirect immunofluorescence staining of transfected cells.
HeLa cells grown on coverslips were infected with VVIndA27L at a multiplicity of infection of 2 PFU/cell. At 1 h p.i., cells were transfected with plasmid vectors expressing mutant proteins by use of Lipofectamine reagent (Gibco BRL) in accordance with the manufacturer’s instructions. At 8 h p.i., cells were fixed in phosphate-buffered saline (PBS) containing 4% (wt/vol) paraformaldehyde for 30 min at 20°C and permeabilized for 5 min with 2% Triton X-100 in PBS. Blockage and incubation with primary (Rα14K) and secondary (rhodamine-labeled goat anti-rabbit) antibodies as well as Hoechst staining (0.5 μg/ml) were performed with PBS containing 20% (vol/vol) fetal calf serum and 0.1% Tween 20. Samples were mounted in Mowiol mounting medium and photographed with an Axiophot microscope (Zeiss).
Virus-induced fusion from without.
Fusion from without was performed as described by Gong et al. (17). Briefly, monolayers of BSC40 cells were kept on ice for 15 min and then infected with sucrose gradient-purified virus (2,000 virions per cell) in DMEM in the continuous presence of cycloheximide (300 μg/ml). After 1 h, unbound virus was removed with the medium and fresh DMEM-cycloheximide supplemented with 2% NCS was added. After 30 min at 0°C, the medium was replaced with low-pH fusion buffer [PBS with 10 mM 2-(N-morpholino) ethanosulfonic acid (MES) and 10 mM HEPES at pH 5.5] or with neutral-pH nonfusion buffer containing the same components at pH 7.4 for 3 min on ice. Incubation with medium-cycloheximide supplemented with 2% NCS was continued for 3 h at 37°C. Cells were fixed, stained with crystal violet in methanol, and photographed.
Inhibition of viral infection.
Soluble heparin (Sigma) at 0, 5, and 10 μg/ml or peptides (see above) at 0, 25, 50, 100, and 500 μg/ml were incubated at 4°C for 30 min with purified VVIndA27L (800 PFU/ml) grown in the absence or presence of 2 mM IPTG. The mixture was added to BSC40 cells at 37°C for 1 h. After the inoculum was removed, cells were overlaid with DMEM–1.9% agar supplemented with 2% NCS and 2 mM IPTG, fixed, and stained with crystal violet in methanol 3 days later. The number of plaques was counted, and the number obtained in the absence of heparin or peptides was considered 100%. Inhibition of virus plaque formation by MAb C3 was carried out by incubation of cell supernatants or cell extracts with a 1/100 dilution of the neutralizing antibody at 37°C for 1 h, followed by plating on BSC40 cells with an agar overlay. The number of plaques was counted after staining.
Heparin binding assays.
Heparin–Sepharose CL-6B beads (Pharmacia) were swollen in 0.5% Nonidet P-40 (NP-40)–0.5% sodium deoxycholate (DOC) in PBS at 4°C overnight and washed in PBS. The washed beads were blocked for 1 h at 4°C with 1% bovine serum albumin in PBS. Binding assays were performed with 50 μg of purified 14-kDa mutant proteins in 100 μl of PBS–NP-40–DOC and 100 μl of heparin beads (50% [vol/vol]) for 1 h at 4°C. The unbound protein was collected from the supernatant after a brief centrifugation, and the pellet was washed with the same buffer three times. The heparin bead-bound protein was eluted with 150 μl of PBS–NP-40–DOC containing soluble heparin at 5 mg/ml as described by Herold et al. (18). Proteins in the supernatant were analyzed by Western blotting after trichloroacetic acid precipitation. For a peptide competition assay of binding of purified proteins to heparin beads, peptides were added instead of soluble heparin at 2.5 and 5 mg/ml.
RESULTS
Generation and characterization of recombinant VV expressing two copies of the A27L gene, the wild type under regulation and mutant forms constitutively.
In order to investigate the consequences of mutations in the 14-kDa protein of VV on virus infection, we constructed recombinant viruses by transfecting VVIndA27L-infected cells with plasmid vectors encoding mutant forms of the protein. The recombinant viruses could be distinguished due to the formation of blue plaques when X-Gal was added to the medium. The genomes of these viruses contain the wild-type A27L gene in the thymidine kinase (TK) locus under the control of the E. coli lacI operator-repressor system and mutant forms of the A27L gene in the HA locus, which is constitutively expressed (Fig. 1A). When the viruses are grown in the absence of IPTG, only the mutant forms are produced, since the genes are under the control of a constitutive early-late synthetic viral promoter. When IPTG is added to the infected cells, the recombinant viruses produce the inducible wild-type 14-kDa protein and constitutive mutant forms.
A scheme of the mutant proteins generated is shown in Fig. 1B and are designated as follows: 14K-wt protein, the constitutively expressed wild-type protein which is used as a control and which contains the same amino acid sequence as that present in strain WR; 14K-A protein, in which two contiguous cysteine residues have been replaced by two alanines; 14K-A-L89A protein, which contains a point mutation, Leu at position 89 replaced by Ala, in addition to the contiguous alanine substitutions; and 14K-A-Δ29 protein, which has an N-terminal deletion of 29 amino acids and the cysteines at positions 71 and 72 replaced by alanines. As determined by chemical cross-linking, all mutant proteins form oligomers, dimers, and trimers (Fig. 1C), a characteristic property of the VV 14-kDa protein. This is so because the coiled-coil structure of the mutant proteins is maintained in all constructs (48). The corresponding recombinant viruses are designated VVInd14K-wt, VVInd14K-A, VVInd14K-A-L89A, and VVInd14K-A-Δ29, respectively.
Because of the recombinogenic property of VV, the fact that the mutant viruses contain both a wild-type copy and a mutant copy of the A27L gene may pose a problem in the maintenance of mutated copies during passage, and mixtures of viruses might emerge in the virus population. Thus, to ensure the purity and stability of the mutant forms in the VV recombinants, we performed PCR amplification and carried out DNA sequence analysis of the inserted mutations from viruses grown in cells in cultures and purified after more than 10 passages. We confirmed the nature of the mutations described in Fig. 1B; the point mutations and deletions in the A27L gene were stably maintained in the VV mutants (data not shown).
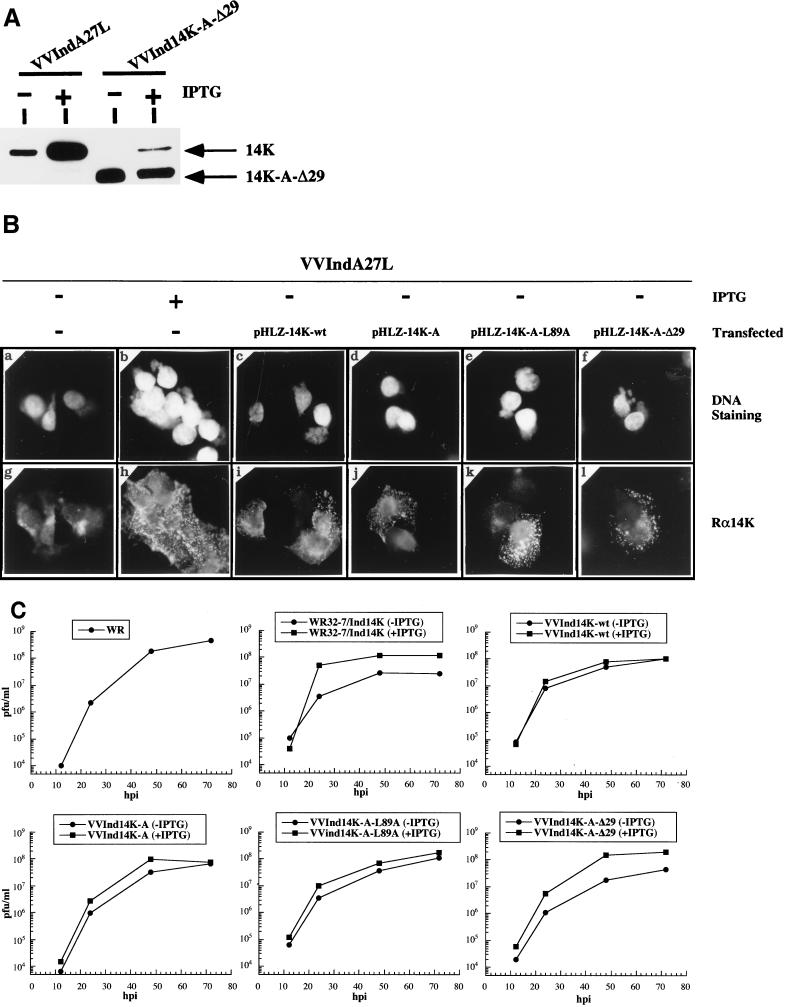
As determined by Western blot analysis with extracts of cells infected in the absence of IPTG and MAb C3, all of the recombinant viruses produced proteins with the expected molecular weights in similar amounts. The amount of mutant proteins produced in cells infected in the absence of IPTG was higher than that produced in cells infected with VVIndA27L in the presence of IPTG due to differences in promoter strength (data not shown). Furthermore, the mutant proteins were incorporated in the virus particle, as shown by Western blotting of purified virions (Fig. 2A). However, with sodium dodecyl sulfate-polyacrylamide gel electrophoresis we could only differentiate the full-length form from that with the N-terminal deletion. Using the mutant lacking the first 29 amino acids, we found that in the absence of IPTG, the constitutively expressed protein was incorporated in the virion, while the incorporation of the wild-type 14-kDa protein was minimal. When the virus was grown in the presence of IPTG and purified, the wild-type 14-kDa protein was incorporated in the virion but, as determined by densitometric analysis, the ratio of wild-type to mutant forms was 1:3 (Fig. 2A). The reduced incorporation of full-length 14-kDa protein in the virion can be explained by differences in expression from the two viral promoters. The optimal design of a strong synthetic early-late viral promoter that regulates the mutant form (as opposed to the late 4b promoter, which regulates the wild-type form in the TK region) favored the accumulation of the mutant protein on the virus particle (5). In addition, expression from an early promoter might favor the incorporation of mutant proteins in the virion due to early protein synthesis. We have also noted by densitometric analysis that VVIndA27L grown in the absence of IPTG incorporates into the IMV surface about 28% of the 14-kDa protein with respect to the total protein present when the inducer is added, due to leakiness of the inducible system (36).
FIG. 2.
Incorporation of wild-type or mutant 14-kDa proteins into virus particles and influence on virus growth. (A) Western blot of sucrose gradient-purified virus. Viral particles were measured as the absorbance at 260 nm (25), and the amount of each virus preparation was adjusted to 109 particles/lane. The reactivity obtained with MAb C3 after incubation with secondary antibody coupled to horseradish peroxidase and proteins revealed by ECL kit Western blotting reagents (Amersham) are shown. VVIndA27L and VVInd14K-A-Δ29 were grown in the absence (−) or in the continuous presence (+) of IPTG. The 14-kDa wild-type protein (14K) and the protein with the N-terminal deletion (14K-A-Δ29) are indicated. Densitometric analyses revealed that the amount of the 14-kDa protein present on IMV grown in the absence of IPTG was 28% that in VVIndA27L grown in the presence of IPTG. (B) Localization of wild-type or mutant protein forms by immunofluorescence. HeLa cells grown on coverslips were infected with VVIndA27L in the absence (panels a, c, d, e, f, g, i, j, k, and l) or presence (panels b and h) of IPTG (2 mM). At 1 h p.i., cells were transfected with plasmids expressing 14K-wt (c and i), 14K-A (d and j), 14K-A-L89A (e and k), and 14K-A-Δ29 (panel f and l). At 8 h p.i., cells were fixed and incubated with anti–14-kDa protein polyclonal antibody, followed by rhodamine-conjugated goat anti-rabbit antibody and Hoechst DNA dye. The results of DNA staining (panels a to f) and rhodamine labeling (panels g to l) are shown. In VVIndA27L-infected cells plus IPTG and infected cells that had been transfected, labeling with antibody Rα14K showed an accumulation of 14-kDa protein in strongly labeled small dots that are localized all over the cells. This pattern corresponds to mature virions (43, 46) and indicates that mutant proteins are incorporated into the virions. In the absence of IPTG, the 14-kDa protein was not observed in VVIndA27L-infected cells (panel g). (C) Virus growth curves. BSC40 cells in 12-well plates were infected (0.005 PFU/cell) with the various VV recombinants in the absence or presence of IPTG. At various hours p.i., (hpi), media and cells were removed and pelleted by centrifugation at 13,000 rpm (Biofuge; Heraeus) to recover the EEV in the supernatant, the pellet was resuspended, and virus was titrated in BSC40 cells in the presence of IPTG by plaque formation.
Immunofluorescence analysis with an antibody to the 14-kDa protein (Rα14K) of cells infected with VVIndA27L or mutants and transfected with plasmid vectors revealed small dots on cells which corresponded to individual IMVs, as previously reported (43) (Fig. 2B, panels i, j, k, and l). The specificity of the antibody was confirmed after infection of cells with VVIndA27L in the absence (Fig. 2B, panel g) or presence (Fig. 2B, panel h) of IPTG. DNA staining of the same fields (Fig. 2B, panels c, d, e, and f) revealed that not all cells showed a reaction with the anti–14-kDa protein antibody, due to a transfection efficiency of about 40% after infection with VVIndA27L. When the immunofluorescence analysis was carried out with cells infected with recombinant viruses expressing mutant forms of the protein, we obtained results similar to those obtained in the transient transfection analysis (data not shown). However, since in the absence of IPTG the amount of wild-type or mutant protein constitutively expressed under the control of the synthetic early-late viral promoter is higher than in transient transfection assays (see above), the specific antibody labeled not only virus particles but also the 14-kDa protein dispersed throughout the cytoplasm. These results clearly show the nonlinear relationship between the synthesis and the incorporation into the virion of the 14-kDa protein, probably due to saturation of anchoring domains on the IMV surface. The location of wild-type or mutant forms of the 14-kDa protein on the virion surface was demonstrated by immunofluorescence of purified particles fixed on fibronectin-coated coverslips (47) with polyclonal antibody Rα14K, which recognizes only the envelope protein. No such labeling occurred in fixed virions with an antibody against the 39-kDa core protein, which is encoded by the A4L gene (data not shown).
Since the mutant proteins were incorporated on the surface of virions, it was of interest to determine the influence of these envelope proteins on virus growth. Thus, we next determined the ability of VV mutants to replicate in cultured cells by using virus growth curves. BSC40 cells were infected at a low multiplicity with the recombinant viruses in the absence or presence of IPTG, media and cells were collected at various times p.i., and the total virus produced was titrated in BSC40 cells in the presence of IPTG. As shown in Fig. 2C, the kinetics of virus growth were the same in the absence or presence of IPTG for VVInd14K-wt, VVInd14K-A, and VVInd14K-A-L89A. However, there was a reduction of about one logarithm in the absence of IPTG for parental VVIndA27L (previously named WR32-7/Ind14K [36]) and mutant VVInd14K-A-Δ29. Since VVInd14K-wt does not produce EEV in the absence of IPTG (36), the results suggest that VVInd14K-A-Δ29 is also unable to produce EEV. These findings also indicate that VVInd14K-A-Δ29 grown in the absence of IPTG and hence containing in the virion mostly the mutant protein has a reduced ability to infect cells, indicative of a defect in virus entry.
With the data obtained from the above experiments, we established that the mutant proteins are expressed constitutively, form oligomers, are incorporated on the virion surface, and do not alter virus growth, with the exception of the 14K-A-Δ29 protein.
VVInd14K-A-Δ29 does not form EEV.
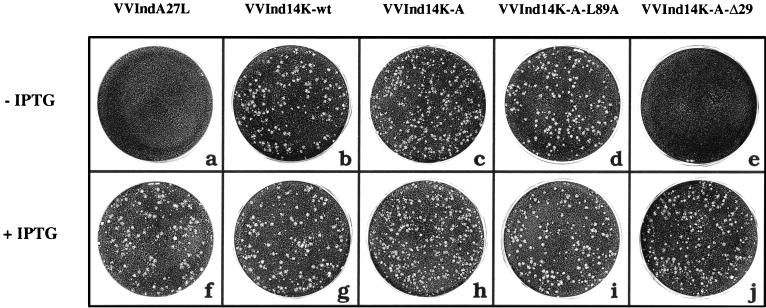
Previous studies have shown the inability of the IMV form lacking the 14-kDa protein to interact with Golgi apparatus-derived membranes and to release EEV (37). As a result, in VVIndA27L-infected cells, in the absence of IPTG, a small-plaque-size phenotype was observed (36, 37). Thus, we investigated if mutant proteins incorporated into virus particles would acquire the two membranes of the trans-Golgi network needed for EEV formation. This investigation was carried out by measuring the ability of mutant viruses to form plaques. Cells infected in the absence of IPTG with viruses that constitutively express 14K-wt (Fig. 3b), 14K-A (Fig. 3c), and 14K-A-L89A (Fig. 3d) formed a large-plaque-size phenotype indicative of EEV formation. Significantly, the virus expressing 14K-A-Δ29 in the absence of IPTG did not form plaques (Fig. 3e) and, hence, extracellular virus. This phenotype is the same as that observed for cells infected with VVIndA27L, which do not produce the full-length wild type protein (Fig. 3a). When IPTG was added to cells infected with VVInd14K-A-Δ29 (Fig. 3j), the incorporation of wild-type 14-kDa protein on the surface of IMV rescued the large-plaque-size phenotype. That EEV was not produced in cells infected with VVInd14K-A-Δ29 in the absence of IPTG was confirmed by virus titration of cell supernatants treated with the IMV-neutralizing antibody MAb C3 and by evaluation of IMV and EEV by CsCl density centrifugation (data not shown).
FIG. 3.
Characterization of the plaque-size phenotype obtained with VV recombinants that constitutively express mutant forms of the 14-kDa protein. The experiment was carried out with BSC40 cells infected in the absence (a, b, c, d, and e) or presence (f, g, h, i, and j) of IPTG (2 mM). For recombinant viruses constitutively expressing 14K-wt, 14K-A, and 14K-A-L89A, we rescued the large-plaque-size phenotype in the absence of IPTG (b, c, and d, respectively). Under the same conditions, VVInd14K-A-Δ29 expressing the 14K-A-Δ29 protein (e) did not recover virus plaque size, like VVIndA27L grown in the absence of IPTG (a). As expected, when IPTG was added, all recombinant viruses produced a large-plaque-size phenotype (f to j).
From the results of Fig. 3, we conclude that the first 29 amino acids of the 14-kDa protein are essential for interactions with lipids or proteins of the Golgi stacks for the envelopment of the virus. The substitution of two contiguous Cys residues with two Ala residues does not affect the biological function of the 14-kDa protein, at least for anchoring to the IMV surface or for EEV formation.
The N terminus (amino acids 29 to 43) of the 14-kDa protein mediates virus-cell fusion.
Different mechanisms and different receptors for the EEV or IMV entry process have been proposed (21, 46). The most recent data suggest fusion of both infectious forms with the plasma membrane, directly for IMV at a neutral pH and after endosome internalization and exposure at an acid pH for EEV (47). Both of these mechanisms imply a fusion process as an important feature for releasing the virus core into the cytoplasm of the host cell. The 14-kDa protein has been considered the VV protein involved in the fusion process at neutral and acid pHs (17, 35). Supporting this notion is our previous finding that the 14-kDa protein is stable over a wide range of pHs (48) and therefore could mediate fusion at neutral and acid pHs.
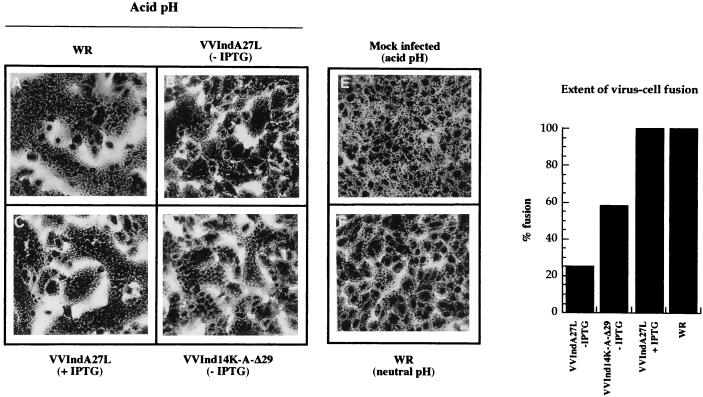
An approach to demonstrating if viruses containing the 14-kDa protein can induce cell fusion is to bind purified virus particles to cells, to use a short treatment with an acid pH, and to evaluate the extent of cell-cell fusion by phase-contrast microscopy, a valid criterion used previously for the virus-cell fusion effect (11, 17). Thus, purified virus was bound to cells (2,000 particles/cell) on ice, cells were subjected to a brief exposure to an acid pH on ice and then incubated at 37°C, and cell-cell fusion was evaluated by phase-contrast microscopy 3 h later under conditions of inhibition of protein synthesis, as described in Materials and Methods. Figure 4 shows phase-contrast micrographs and quantitation of the extent of virus-cell fusion. Similar levels of virus-cell fusion were induced by wild-type strain WR and by inducible VVIndA27L grown in the presence of IPTG. These levels were considered to represent 100% of virus-cell fusion activity and were calculated as previously described by Gong et al. (17). VVIndA27L grown in the absence of IPTG produced at an acid pH a low level of fusion activity (25%) due to the presence of a small amount of wild-type protein synthesized as a result of some leakiness of the inducible system (Fig. 2A). The fusion process was inhibited by the 14-kDa protein-reactive MAb C3 (17 and data not shown).
FIG. 4.
Fusion-from-without experiments at an acid pH with sucrose gradient-purified viruses. Monolayers of BSC40 cells were infected with WR (A), VVIndA27L grown in the absence (B) or presence (C) of IPTG, and VVInd14K-A-Δ29 grown without IPTG (D) under the conditions described in Materials and Methods. No cell fusion was observed in mock-infected cells at an acid pH (E) or in WR-infected cells at a neutral pH (F). The extent of virus-cell fusion was quantified by phase-contrast microscopy after counting about 103 cells from various fields (graph at right).
Since we have previously demonstrated that the domain recognized by MAb C3 is located between amino acids 29 and 43 (48), the fusion domain should still be present at the N terminus of the 14K-A-Δ29 mutant protein. To test this possibility, we carried out fusion-from-without experiments at an acid pH with the purified recombinant virus that contains the 14K-A-Δ29 protein on the IMV surface instead of the wild-type protein. We observed that this virus induces syncytium formation at an acid pH, although to a lesser extent than wild-type virus (Fig. 4). The fusion activity of purified VVInd14K-A-Δ29 was calculated to be 58%. Because this virus does not incorporate wild-type protein in detectable amounts, the fusion-from-without effect must be due to the presence of the 14K-A-Δ29 protein in the virion.
The results shown in Fig. 4 indicate that the 14-kDa protein is directly involved in syncytium formation and that the region comprising the second putative alpha helix (amino acids 29 to 43) is implicated in virus-cell fusion.
A cluster of amino acids between positions 21 and 33 in the 14-kDa protein are involved in binding to heparin.
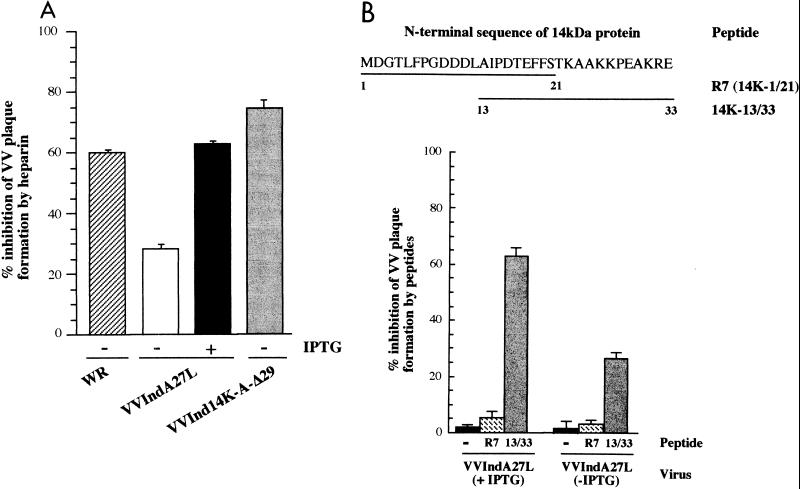
Chung et al. (8) have shown an interaction of VV with HS on the cell surface, with the entry of VV being inhibited to a maximum level of 60% at a concentration of 10 μg of soluble heparin/ml. The same work identified the 14-kDa protein as the protein mediating virus attachment to HS on cells. In view of the role of HS in entry for many viruses (see above), we carried out two types of assays to confirm the contribution of the 14-kDa protein to attachment mediated by HS and to attempt to identify the binding domain of the 14-kDa protein. The first assay is based on the inhibition of virus entry by heparin with purified VVIndA27L (grown in the absence or presence of IPTG). The second assay is based on a competitive binding assay with synthetic peptides as competitors. As shown in Fig. 5A, the extent of inhibition of virus plaque formation by 10 μg of soluble heparin per ml was the same with purified VVIndA27L grown in the presence of IPTG or with wild-type virus (60% inhibition). There was no increase in the inhibition of virus plaque formation when saturating amounts of heparin were added (10 mg/ml). When VVIndA27L was grown in the absence of IPTG, heparin still inhibited virus plaque formation by 30%. With these data, we confirmed the contribution of the 14-kDa protein to an HS interaction, but we could not distinguish if the 30% inhibition of VVIndA27L (in the absence of IPTG) was due to the small amount of the 14-kDa protein present in the virion or to the existence of another protein or proteins with the same function. This question was investigated with specific peptides spanning the N terminus of the 14-kDa protein. Several concentrations of soluble peptides were mixed with VVIndA27L grown in the absence or presence of IPTG, and their ability to inhibit virus plaque formation was analyzed. Figure 5B shows a maximum of 62% inhibition of virus entry by 500 μg of the peptide corresponding to amino acids 13 to 33 of the 14-kDa protein per ml. This inhibition was not observed with the R7 peptide (amino acids 1 to 21). The extent of inhibition of virus plaque formation by peptides correlated with the presence in the virus envelope of the 14-kDa protein. Purified VVIndA27L (grown in the absence of IPTG) with about 28% of the 14-kDa protein in the envelope was inhibited by 25%, while virus with 100% of the 14-kDa protein was inhibited by about 60%. These data demonstrate that the inhibition of VV entry by the 14K-13/33 peptide is due specifically to competition with the 14-kDa protein for attachment to the cell surface.
FIG. 5.
Inhibition of VV plaque formation by soluble heparin and peptides of the 14-kDa protein. (A) Inhibition of VV plaque formation by soluble heparin. Purified VVIndA27L grown in the absence or presence of IPTG (2 mM) and wild-type virus (WR) were incubated with or without soluble heparin at a final concentration of 10 μg/ml. The mixture was then added to monolayers of BSC40 cells for 1 h, and the inoculum was removed; after 3 days of incubation in DMEM containing 2% NCS, 2 mM IPTG, and 1.9% agar, the number of plaques was counted. The plaques obtained in the absence of heparin were used as the 100% value for each virus. Error bars show standard deviations. (B) Inhibition of VV plaque formation by peptides. The experiments was carried out as for panel A but with specific peptides corresponding to different domains of the 14-kDa protein. The sequences of R7 (amino acids 1 to 21) and 14K-13/33 (amino acids 13 to 33) peptides are shown in the top part of the panel. Values represent the inhibition of virus plaque formation obtained with 500 μg of peptides per ml and with purified VVIndA27L grown in the presence (+) or absence (−) of IPTG. The number of plaques obtained by infection in the absence of peptides was used as the 100% value. Error bars show standard deviations.
To confirm the specificity of the N terminus of the 14-kDa protein for binding to the cell surface via HS, we carried out in vitro experiments measuring the binding of purified mutant proteins to heparin-Sepharose beads. Figure 6A shows a diagram of the mutant proteins with deletions at either the N- or the C-terminal region or in both termini. We observed binding to heparin-Sepharose beads of the following purified proteins: the full-length protein, which was used as a positive control (Fig. 6B, lane 5); the protein with the first 29 amino acids deleted at the N terminus (Fig. 6B, lane 6); and the protein containing both a deletion of 29 amino acids at the N terminus and a deletion of 36 amino acids at the C terminus (Fig. 6B, lane 8). However, the protein with the deletion of amino acids 1 to 43 at the N terminus was unable to bind heparin (Fig. 6B, lane 7). Since the protein with a deletion of 29 amino acids at the N terminus still conserves two positively charged amino acids at positions 31 and 32, these data indicate that these two residues could be sufficient to mediate the interaction of the 14-kDa protein with HS. These results are in line with the results obtained with VVInd14K-A-Δ29, which was inhibited by heparin to the same extent as wild-type strain WR (Fig. 5A).
FIG. 6.
Identification of the domain of the 14-kDa protein involved in binding to heparin and in cell attachment. (A) Schematic presentation of E. coli-purified proteins used for in vitro binding experiments. Black boxes are the three predicted alpha helixes (48), and white boxes represent the mutations in the 14-kDa wild-type protein sequence: two Cys residues at positions 71 and 72 replaced by two Ala residues. (B) E. coli-purified mutant proteins 14K-A (lanes 1 and 5), 14K-A-Δ29 (lanes 2 and 6), 14K-A-Δ43 (lanes 3 and 7), and 14K-A-29/74 (lanes 4 and 8) were incubated with heparin-Sepharose beads at 4°C for 60 min. The supernatants were collected, and unbound fractions were analyzed by Western blotting with a polyclonal rabbit antibody against the 14-kDa protein (lanes 1, 2, 3, and 4). The heparin-Sepharose-retained proteins were incubated with soluble heparin (5 mg/ml), which liberates the bound proteins into supernatants, and these were analyzed by Western blotting with antibody Rα14K (lanes 5 to 8). MW, molecular weight (in thousands). (C) 14K-A mutant protein bound to heparin-Sepharose beads was incubated with 5 mg of heparin/ml (lane 3) or peptides corresponding to amino acids 13 to 33 (5 mg/ml, lane 4; 2.5 mg/ml, lane 5) and 75 to 106 (5 mg/ml, lane 6). The supernatants were collected and analyzed by Western blotting with antibody Rα14K. The protein was liberated from heparin-Sepharose when heparin (lane 3) or peptide 14K-13/33 (lanes 4 and 5) was added. No protein appeared in supernatants from samples incubated with buffer alone (lane 2) or with peptide 14K-75/106 (lane 6). Lane 1, unbound fraction of protein 14K-A.
The ability of peptide 14K-13/33 to compete specifically with the 14-kDa protein for binding to heparin was also confirmed by in vitro experiments (Fig. 6C). In this case, the purified 14-kDa protein was bound to heparin-Sepharose, and the protein was released from the matrix either by the addition of soluble heparin at a concentration of 5 mg/ml (Fig. 6C, lane 3) or by the addition of peptide 14K-13/33 at 5 and 2.5 mg/ml (lanes 4 and 5, respectively). Controls without peptide (Fig. 6C, lane 2) and with an unrelated peptide (14K-75/106, corresponding to the C terminus of the 14-kDa protein) (lane 6) did not elute the protein.
From the results of Fig. 6, we conclude that the cluster of basic amino acids present between positions 21 and 33 in the sequence of the 14-kDa protein is involved in binding to heparin, implying that the 14-kDa protein in the virion could mediate the attachment of the virus to the cell surface via HS.
DISCUSSION
The entry of enveloped viruses into cells constitutes the initial step in the infection process. It is known that this event begins with the attachment of the virus to the cell, followed by membrane fusion to liberate the viral DNA into the cytoplasm of the host cell. To understand virus entry, it is necessary to characterize the proteins that mediate the attachment and fusion processes.
One of the proteins that has been implicated in the entry of VV is the 14-kDa envelope protein encoded by the A27L gene. Several observations support the role of this protein in virus entry: (i) its location on the surface of IMV, which is the most abundant infectious form of VV (43), and the ease with which the membrane of EEV is disrupted, exposing the 14-kDa protein (47); (ii) the predicted structure, which is similar to those of other fusion proteins with a characteristic coiled-coil region for trimer formation (48); (iii) the inhibition of the fusion process and the neutralization of virus infection by specific MAbs against the 14-kDa protein (11, 31, 34, 35, 45); and (iv) the recent observation that the 14-kDa protein interacts with cell surface HS as a step in virus infection (8). This protein also plays an important role in EEV formation, acting in virion envelopment by Golgi apparatus-derived membranes (37).
Since in a previous study we identified the domains of the 14-kDa protein required for membrane anchoring to the IMV surface and for oligomer formation (48), in this investigation we defined the functional domains involved in virus-cell fusion, in binding to heparin, and in EEV formation. We used VVIndA27L (36, 37) which contains, under the control of the lacI operator-repressor system of E. coli, the A27L gene in the TK locus. We introduced into the HA locus a second A27L gene, which is constitutively expressed and encodes the 14-kDa wild-type or mutant proteins under the control of the strong synthetic early-late viral promoter. The recombinant viruses generated maintained the mutated A27L genes in the genome, as confirmed by direct DNA sequencing.
Western blots and immunofluorescence analyses provided evidence that both the wild-type and the mutant forms are produced during infection and that they are incorporated on the surface of the virion. By chemical cross-linking, we showed that the mutations did not alter the characteristic property of the 14-kDa protein to form oligomers. This is because the coiled-coil structure of the 14-kDa protein which is needed for oligomerization is present in all of the mutant proteins (48). Because the mutant forms were expressed constitutively under the control of a virus promoter (synthetic early-late) stronger than the inducible wild-type promoter (late p4b), when the virus was grown in the presence of IPTG, the amount of wild-type protein in IMV was reduced by one third, probably because of differences in promoter strength and in the kinetics of protein synthesis. This finding was established with the recombinant virus lacking the first 29 amino acids. Since other mutant forms of the 14-kDa protein could not be distinguished by size from the wild-type form, it was assumed that their synthesis and incorporation into virions were the same as those of the N-terminally deleted 29-amino-acid form. This assumption was supported by immunofluorescence microscopy, since cells infected with VVIndA27L in the absence of IPTG and transfected with plasmids expressing mutant forms showed small dots known to represent virus particles after labeling with an antibody to the 14-kDa protein (Fig. 2B).
While the 14-kDa protein exists in the virus as a trimer (35, 48), the mutant proteins also formed trimers (Fig. 1C) and should be incorporated in virions grown in the absence of IPTG as homotrimers because the mutant forms were more abundant than the leaky wild-type form. When the virus was grown in the presence of IPTG, it is likely that heterotrimers were formed by mixing, as previously described (16).
The influence of the mutant proteins on virus replication was studied by use of virus growth curves (Fig. 2C). The kinetics of virus growth were the same for most of the viruses, except for virus recombinants lacking the 14-kDa protein or having a 14-kDa protein with a deletion of the first 29 amino acids. Such viruses failed to produce EEV, leading to a one-log reduction in virus yields.
In view of the above characterization of the recombinant viruses, we were in a position to define critical domains in the 14-kDa protein. Since the 14-kDa protein is essential for EEV formation and a large-plaque-size phenotype (37), we used these parameters to show the rescue in functionally when the constitutively expressed mutant proteins were produced in cells infected with recombinant viruses. As expected, replacement of the two contiguous Cys residues in the sequence of the 14-kDa protein (amino acids 71 and 72) had no effect on EEV formation, confirming our previous hypothesis of the nonessential role of Cys residues (48). VVInd14K-A grown in the absence of IPTG produced a large-plaque-size phenotype, like VVInd14K-wt. In agreement with previous observations, the amino-terminal region is essential for the envelopment of IMV by Golgi membranes and the release of EEV from cells (16). Clearly, replacement of the wild-type 14-kDa protein by an N-terminally deleted mutant form on the surface of IMV results in the failure to produce EEV. When IPTG was added to the incubation medium, the smaller amount of the 14-kDa wild-type protein than of 14K-A-Δ29 incorporated into the virion (1:3 ratio) was enough to rescue the plaque size phenotype.
The most surprising result was obtained from the analysis of the 14K-A-L89A mutant protein, because we previously observed that this protein failed to interact with the membrane-anchoring 21-kDa protein in binding assays in vitro and in immunoprecipitation experiments with transfected cells (48) or with cells infected with recombinant viruses (data not shown). This result could be interpreted in two ways: (i) the interaction between the 14- and 21-kDa proteins is not strong enough because of the point mutation in the Leu residue and because the conditions used in the binding experiments cannot maintain this interaction; (ii) alternatively, there is an additional mechanism, such as palmitoylation or myristoylation, for anchoring the 14-kDa protein to the membrane in the virus particle, although repeated attempts failed to reveal labeling of 14-kDa protein with 3H-palmitic or 3H-myristic acid (unpublished observations). We believe that a weak interaction between 14K-A-L89A and the 21-kDa protein explains the rescue of plaque formation in cultured cells. Western blotting of purified virus, fusion-from-without experiments, and neutralization data obtained with MAb C3 for VVInd-14K-A-L89A grown in the absence of IPTG support this hypothesis. Our data indicate that only about 50% of the mutant protein was present in purified VVInd14K-A-L89A virions with respect to the amount of the 14-kDa protein present in the other viruses; for this reason, VVInd14K-A-L89A virions were partially resistant to neutralization by MAb C3 and to soluble heparin and could not induce fusion from without to the same extent as wild-type strain WR (unpublished observations).
By evaluating virus-cell fusion induced by the different recombinant viruses, we showed that VVIndA27L grown in the presence of IPTG produced levels of fusion activity similar to those obtained with the wild-type virus. However, fusion by VVIndA27L grown in the absence of IPTG was reduced to about 25%, the percentage expected if we consider that the wild-type 14-kDa protein is present in virions in a relative abundance of about 28% due to leakiness of the inducible system. These results clearly implicate the 14-kDa protein in the fusion-from-without process. The recombinant virus expressing the N-terminally deleted protein 14K-A-Δ29 induces fusion from without at an acid pH to about 58% that observed with the wild-type virus. The explanation for this value (58%) is that the mutant protein still contains the fusion domain but lacks most of the heparin binding domain. Thus, the reduction in fusion is probably mediated by a lower binding affinity of the mutant virus for cells due to a reduction in the positive charges of the 14-kDa mutant protein. This conclusion is supported by the virus-growth curves (Fig. 2C). Since the mutant protein 14K-A-Δ29 contains the domain that is recognized by MAb C3 (amino acids 29 to 43) and that inhibits the fusion process (48), we would expect this mutant protein to exhibit the same behavior as the wild-type (WR) protein. Because fusion was reduced, we suggest that the deletion might interfere with the attachment of the virus to the cell (see below), reducing the speed or the extent of the fusion process.
Based on the above observations and on the structural similarities found with other fusion proteins, we propose that the stretch of amino acids corresponding to the predicted short alpha helix adjacent to the coiled-coil region comprises the fusion domain (Fig. 7). This fusion domain (short alpha helix) would be similar to that reported for influenza virus HA, the transmembrane region of Moloney murine leukemia virus, and gp41 of HIV-1 next to the oligomerization domain. It is significant that while a fusion peptide results from the specific cleavage of HA and gp160, in the case of the 14-kDa protein we have not seen such cleavage. This result could be due to the clustering of the attachment and fusion domain so that only as a result of the interaction process would the fusion peptide be exposed for its biological activity. The VVInd14K-A-Δ29 recombinant virus provided additional information. Considering that VVInd14K-A-Δ29 only forms IMV, the ability of VV to produce fusion from without does not require EEV.
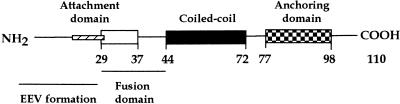
FIG. 7.
Schematic representation of functional domains in the 14-kDa protein. The fusion domain (white box) is located between amino acids 29 and 43, the attachment domain (hatched box) comprises amino acids 21 to 33, and the amino acids necessary for EEV formation are located between positions 1 and 29. The localizations of coiled-coil and anchoring domains (black and checkerboard boxes, respectively) were previously determined (48).
In this investigation, we have also characterized the domain responsible for the interaction of the 14-kDa protein with heparin. Using VVIndA27L grown in the presence of IPTG and specific peptides for amino acids 1 to 21 and 13 to 33 in the 14-kDa protein sequence, we demonstrated that the region between residues 21 and 32 is implicated in virus-cell attachment, in agreement with the recent observations of Hsiao et al. (20). We showed that the 14K-13/33 peptide can inhibit selectively the entry of the virus (Fig. 5B). This effect of the peptide on the inhibition of virus plaque formation correlated with the presence of the 14-kDa protein on the IMV surface, demonstrating the specificity of the inhibition. Moreover, this peptide can interact specifically with heparin-Sepharose beads, removing the full-length protein previously bound to the matrix (Fig. 6C).
The results obtained for the inhibition of plaque formation by heparin are in concordance with those described above and with the observations reported by Chung et al. (8). When we used VVIndA27L grown in the presence of IPTG and heparin at 10 μg/ml or higher concentrations, we obtained 60% inhibition of plaque formation, the same value obtained with the wild-type virus (8). When the virus was grown in the absence of IPTG, this percentage was reduced to about 30% inhibition, presumably due to residual 14-kDa protein. The limited effect on VVIndA27L grown in the absence of IPTG could be attributed to the small amount of the 14-kDa protein present or to the limited HS binding activity of other VV proteins, as has been reported for other viruses (18, 30). Since the existence of negative charges in HS was found to be essential for 14-kDa protein–GAG binding (8), the region of the 14-kDa protein implicated should contain a cluster of basic amino acids in a three-dimensional conformation to provide an interaction between positive and negative groups (26). The N terminus of the 14-kDa protein (amino acids 21 to 32) contains a total of five Arg and Lys residues in a predicted structureless region (48). The prediction of the nonexistence of defined structural elements in this region can explain the fact that the distribution of charged residues does not fit with previously established patterns for exposing the amino acids implicated in the interaction (2, 15, 27). We found that the interaction of the 14-kDa protein with heparin does not require the participation of all of the positively charged residues within amino acids 21 to 33. In vitro experiments with purified mutant proteins revealed that the 14K-A-Δ29 protein, containing only two basic amino acids, interacts with heparin-Sepharose beads, while a deletion of 43 amino acids in the N terminus resulted in the lack of binding to heparin-Sepharose beads (Fig. 6B). In cell cultures, the virus that incorporates this mutant form is inhibited by soluble heparin to the same extent as wild-type virus. Previous findings by others (8, 20) that cells deficient in HS are infected less effectively by VV and that cell surface proteoglycans are necessary for A27L protein-mediated cell fusion, together with our results, strongly suggest that the heparin binding domain that we have identified in the 14-kDa protein is involved in virus-cell binding.
A proposed organization of the different functional domains in the 14-kDa protein is shown in Fig. 7. The fusion domain and the attachment domain in the 14-kDa protein would be located adjacent to each other, near the oligomerization region (triple-stranded coiled-coil formation) (48). The domain in the 14-kDa protein responsible for the interaction with lipids or membranes in the Golgi stacks to form EEV would be located within the first 29 amino acids. The short stretch of hydrophobic amino acids at the N terminus could play an important role in this interaction.
Even with the data provided here and with the well-established evidence that the entry process of VV is mediated by the 14-kDa protein, we cannot rule out the existence in VV of other proteins that interact with HS in the absence of the 14-kDa protein, because VVIndA27L, lacking the 14-kDa protein, still enters cells (37). The complexity of VV and the large number of proteins incorporated into the virion indicate the existence of more than a single protein in the entry process. Indeed, the protein encoded by the L1R gene could mediate entry, since specific MAbs to L1R are potent inhibitors of viral entry (23, 52). Since IMV and EEV enter cells by apparently different mechanisms, proteins present in the corresponding membranes and with roles in the entry process should be different. Vanderplasschen et al. (47) and Ichihashi (21) suggested the entry of EEV by endocytosis (the rupture of the outer membrane in acidic endosomal medium and fusion of IMV) but the entry of IMV by direct fusion with the cell plasma membrane in the extracellular medium. The 14-kDa protein could mediate fusion at a neutral pH (directly from the extracellular medium) or an acidic pH (after internalization of EEV in the endosome), since the protein maintains its native conformation in a pH range of 4.5 to 10.5 (48).
ACKNOWLEDGMENTS
We thank J. F. Rodriguez for the gift of WR32-7/Ind14K (VVIndA27L) and Victoria Jiménez for expert technical assistance. We are grateful to Carmen E. Gomez for PCR amplification and the core facility of the Centro Nacional de Biotecnología for automated DNA sequencing of mutant forms of the A27L gene.
M.-I. Vázquez was a recipient of a fellowship from Comunidad Autónoma de Madrid. This investigation was supported by grants BIO95-0022 and SAF95-0072 from CICYT of Spain, and by EU project FMRX-CT98-0225.
REFERENCES
- 1.Byrnes A P, Griffin D E. Binding of Sindbis virus to cell surface heparan sulfate. J Virol. 1998;72:7349–7356. doi: 10.1128/jvi.72.9.7349-7356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardin A D, Weintraub H J R. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1998;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Carr C M, Kim P S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- 4.Carr C M, Kim P S. Flu virus invasion: halfway there. Science. 1994;266:234–236. doi: 10.1126/science.7939658. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti S, Sisler J R, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 6.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Maguire R E, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 8.Chung C-S, Hsiao J-C, Chang Y-S, Chang W. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J Virol. 1998;72:1577–1585. doi: 10.1128/jvi.72.2.1577-1585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demkowicz W E, Maa J S, Esteban M. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J Virol. 1992;66:386–398. doi: 10.1128/jvi.66.1.386-398.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 11.Doms R W, Blumenthal R, Moss B. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J Virol. 1990;64:4884–4892. doi: 10.1128/jvi.64.10.4884-4892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Souza M P, Harden V A. Chemokines and HIV-1 second receptors. Nat Med. 1996;2:1293–1300. doi: 10.1038/nm1296-1293. [DOI] [PubMed] [Google Scholar]
- 13.Esteban M. Defective vaccinia virus particles in interferon-treated infected cells. Virology. 1984;133:220–227. doi: 10.1016/0042-6822(84)90443-4. [DOI] [PubMed] [Google Scholar]
- 14.Fass D, Harrison S C, Kim P S. Structure of Moloney murine virus envelope domain at 1.7Å resolution. Nat Struct Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 15.Flynn S J, Ryan P. The receptor-binding domain of pseudorabies virus glycoprotein gC is composed of multiple discrete units that are functionally redundant. J Virol. 1996;70:1355–1364. doi: 10.1128/jvi.70.3.1355-1364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong S, Lai C, Dallo S, Esteban M. A single point mutation of Ala-25 to Asp in the 14,000-Mr envelope protein of vaccinia virus induces a size change that leads to the small plaque size phenotype of the virus. J Virol. 1989;63:4507–4514. doi: 10.1128/jvi.63.11.4507-4514.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong S C, Lai C, Esteban M. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology. 1990;178:81–91. doi: 10.1016/0042-6822(90)90381-z. [DOI] [PubMed] [Google Scholar]
- 18.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpex simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiller G, Weber K. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J Virol. 1985;55:651–659. doi: 10.1128/jvi.55.3.651-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsiao J-C, Chung C-S, Chang W. Cell surface proteoglycans are necessary for A27L protein-mediated cell fusion: identification of the N-terminal region of A27L protein as the glycosaminoglycan-binding protein. J Virol. 1998;72:8374–8379. doi: 10.1128/jvi.72.10.8374-8379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichihashi Y. Extracellular enveloped vaccinia virus escapes neutralization. Virology. 1996;217:478–485. doi: 10.1006/viro.1996.0142. [DOI] [PubMed] [Google Scholar]
- 22.Ichihashi Y, Matsumoto S, Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971;46:507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- 23.Ichihashi Y, Takahashi T, Oie M. Identification of a vaccinia virus penetration protein. Virology. 1994;202:834–843. doi: 10.1006/viro.1994.1405. [DOI] [PubMed] [Google Scholar]
- 24.Jackson T, Ellard F M, Ghazaleh R A, Brookes S M, Blakemore W E, Corteyn A H, Stuart D I, Newman J W, King A M. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J Virol. 1996;70:5282–5287. doi: 10.1128/jvi.70.8.5282-5287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joklik W K. The purification of four strains of poxvirus. Virology. 1962;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- 26.Kjellén L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 27.Klimstra W B, Ryman K D, Johnston R E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol. 1998;72:7357–7366. doi: 10.1128/jvi.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krusat T, Streckert H-J. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch Virol. 1997;142:1247–1254. doi: 10.1007/s007050050156. [DOI] [PubMed] [Google Scholar]
- 29.Lai C, Gong S, Esteban M. Structural and functional properties of 14kDa envelope protein of vaccinia virus synthesized in Escherichia coli. J Biol Chem. 1990;265:22174–22180. [PubMed] [Google Scholar]
- 30.Laquerre S, Argnani R, Anderson D B, Zucchini S, Manservigi R, Glorioso J C. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J Virol. 1998;72:6119–6130. doi: 10.1128/jvi.72.7.6119-6130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer H, Osterrieder N, Czerny C-P. Identification of binding sites for neutralizing monoclonal antibodies on the 14-kDa fusion protein of orthopox viruses. Virology. 1994;200:778–783. doi: 10.1006/viro.1994.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976;73:43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez D, Rodriguez J-R, Esteban M. The vaccinia virus 14-kilodalton fusion protein forms a stable complex with the processed protein encoded by the vaccinia virus A17L gene. J Virol. 1993;67:3435–3440. doi: 10.1128/jvi.67.6.3435-3440.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez J F, Janezcko R, Esteban M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J Virol. 1985;56:482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez J F, Paez E, Esteban M. A 14,000-Mr envelope protein of vaccinia virus is involved in cell fusion and forms covalently linked trimers. J Virol. 1987;61:395–404. doi: 10.1128/jvi.61.2.395-404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez J F, Smith G L. Inducible gene expression from vaccinia virus vectors. Virology. 1990;177:239–250. doi: 10.1016/0042-6822(90)90477-9. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez J F, Smith G L. IPTG-dependent vaccinia virus: identification of a virus protein enabling virion envelopment by Golgi membrane and egress. Nucleic Acids Res. 1990;18:5347–5351. doi: 10.1093/nar/18.18.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roos N, Cyrklaff M, Cudmore S, Blasco R, Krijnse Locker J, Griffiths G. A novel immunogold cryo EM method to investigate the structure of IMV/EEV. EMBO J. 1996;15:2343–2355. [PMC free article] [PubMed] [Google Scholar]
- 39.Sattentau Q J, Weiss R A. The CD4 antigen: physiological ligand and HIV receptor. Cell. 1991;52:631–633. doi: 10.1016/0092-8674(88)90397-2. [DOI] [PubMed] [Google Scholar]
- 40.Schmelz M, Sodeik B, Ericsson M, Wolffe E J, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheih M, Montgomery R I, Esko J D, Spear P. Cell surface receptors for herpex simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sodeik B, Doms R W, Ericsson M, Hiller G, Machamer C E, van’t Hof W, van Meer G, Moss B, Griffiths G. Assembly of vaccinia virus: role of intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993;121:521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sodeik B, Cudmore S, Ericsson M, Esteban M, Niles E G, Griffiths G. Assembly of vaccinia virus: incorporation of p14 and p32 into the membrane of the intracellular mature virus. J Virol. 1995;69:3560–3574. doi: 10.1128/jvi.69.6.3560-3574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner P C, Moyer R W. An orthopoxvirus serpinlike gene controls the ability of infected cells to fuse. J Virol. 1992;66:2076–2085. doi: 10.1128/jvi.66.4.2076-2085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanderplasschen A, Smith G L. A novel virus binding assay using confocal microscopy: demonstration that the intracellular and extracellular vaccinia virions bind to different cellular receptors. J Virol. 1997;71:4032–4041. doi: 10.1128/jvi.71.5.4032-4041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanderplasschen A, Hollinshead M, Smith G L. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J Gen Virol. 1998;79:877–887. doi: 10.1099/0022-1317-79-4-877. [DOI] [PubMed] [Google Scholar]
- 48.Vázquez M-I, Rivas G, Cregut D, Serrano L, Esteban M. The vaccinia virus 14-kilodalton (A27L) fusion protein forms a triple coiled-coil structure and interacts with the 21-kilodalton (A17L) virus membrane protein through a C-terminal α-helix. J Virol. 1998;72:10126–10137. doi: 10.1128/jvi.72.12.10126-10137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 50.White J M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- 51.White J M. Membrane fusion. Science. 1992;258:917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- 52.Wolffe E J, Vijaya S, Moss B. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology. 1995;211:53–63. doi: 10.1006/viro.1995.1378. [DOI] [PubMed] [Google Scholar]
- 53.WuDunn D, Spear D. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu Y G, King D S, Shin Y-K. Insertion of a coiled-coil peptide from influenza virus hemagglutinin into membranes. Science. 1994;266:274–276. doi: 10.1126/science.7939662. [DOI] [PubMed] [Google Scholar]