Objectives
To propose the grounds for “diabetic sarcopenia” as a new comorbidity of diabetes, and to establish a muscle screening algorithm proposal to facilitate its diagnosis and staging in clinical practice. Method: A qualitative expert opinion study was carried out using the nominal technique. A literature search was performed with the terms “screening” or “diagnostic criteria” and “muscle loss” or “sarcopenia” and “diabetes” that was sent to a multidisciplinary group of 7 experts who, in a face-to-face meeting, discussed various aspects of the screening algorithm. Results: The hallmark of diabetic sarcopenia (DS) is muscle mass atrophy characteristic of people with diabetes mellitus (DM) in contrast to the histological and physiological normality of muscle mass. The target population to be screened was defined as patients with DM with a SARC-F questionnaire > 4, glycosylated haemoglobin (HbA1C) ≥ 8.0%, more than 5 years since onset of DM, taking sulfonylureas, glinides and sodium/glucose cotransporter inhibitors (SGLT2), as well as presence of chronic complications of diabetes or clinical suspicion of sarcopenia. Diagnosis was based on the presence of criteria of low muscle strength (probable sarcopenia) and low muscle mass (confirmed sarcopenia) using methods available in any clinical consultation room, such as dynamometry, the chair stand test, and Body Mass Index (BMI)-adjusted calf circumference. DS was classified into 4 stages: Stage I corresponds to sarcopenic patients with no other diabetes complication, and Stage II corresponds to patients with some type of involvement. Within Stage II are three sublevels (a, b and c). Stage IIa refers to individuals with sarcopenic diabetes and some diabetes-specific impairment, IIb to sarcopenia with functional impairment, and IIc to sarcopenia with diabetes complications and changes in function measured using standard tests Conclusion: Diabetic sarcopenia has a significant impact on function and quality of life in people with type 2 diabetes mellitus (T2DM), and it is important to give it the same attention as all other traditionally described complications of T2DM. This document aims to establish the foundation for protocolising the screening and diagnosis of diabetic sarcopenia in a manner that is simple and accessible for all levels of healthcare.
Keywords: Muscle mass, Type 2 diabetes, Screening, Diagnosis, Sarcopenia, Malnutrition, HMB
Introduction
Sarcopenia is a progressive and widespread disorder associated with a reduction in the quantity and quality of muscle, as well as of its function [1, 2]. This reduction causes an increase in falls, fractures and physical disability, reduced function, loss of quality of life and increased mortality [1, 2].
The hallmark of diabetic sarcopenia is muscle mass atrophy characteristic of people with diabetes mellitus in contrast to the histological and physiological normality of muscle mass (MM) [3, 4]. Diabetes behaves as an independent factor for the major loss of skeletal muscle mass [5]. In people with type 2 diabetes mellitus (T2DM), various changes occur in body composition, particularly in the form of increased visceral fat and decreased muscle and bone mass [6, 7]. The basal inflammatory state generates an increase in oxidative stress, an increased prevalence of malnutrition, and different energy imbalances [8–11].
The loss of skeletal muscle mass (SMM) occurs when the rate of protein degradation exceeds that of synthesis, i.e., due to the imbalance between the anabolic and catabolic processes of proteins [12]. In people with T2DM, the loss of SMM is excessive and independent of changes in body weight, particularly in the presence of poor glycaemic control, insulin resistance [12], and is greater in women than in men [13]. The fact that in people with T2DM a greater deterioration of appendicular muscle mass is detected at the time of diagnosis shows that these changes occur as soon as the early stages, even before the disease is diagnosed [13].
Poor glycaemic control is directly related to loss of MM, strength, and decreased general physical performance. Four possible mechanisms of this anomalous link have been described: First of all, insulin plays a key role in muscle function by increasing glucose uptake and promoting intracellular glucose metabolism [14]. Therefore, insulin resistance can affect muscle strength [12]. Second, insulin resistance downregulates the mammalian Target of Rapamycin (mTOR) metabolic pathway, which is the major anabolic activation pathway in mammals [15]. This reduction in protein synthesis reduces the amount of protein available for protein anabolism. Third, cytokines associated to chronic inflammation present in T2DM contribute to insulin resistance, lipolysis, muscle protein degradation, and nitrogen loss [14, 16]. Finally, sustained hyperglycaemia promotes the accumulation of advanced glycation end-products (AGES) that contribute to decrease muscle strength [16].
In patients with poor glycaemic control, improved glycaemic control (assessed by HbA1C) was associated to an increased skeletal mass index and gait speed [17, 18]. HbA1C ≥ 8.0% (64 mmol/mol) has been reported as a risk factor for muscle mass quality decline regardless of diabetes duration [10, 19], and there is a positive linear relationship between glycosylated haemoglobin levels, lower muscle mass levels, and the frequency of sarcopenia in non-obese adult patients with T2DM [18, 20, 21].
Another factor to be considered is the pharmacological treatments received by a person with diabetes, since certain therapeutic classes appear to have a negative impact on body composition [22–25].
Lastly, we should not forget the role of malnutrition in muscle mass loss and the onset of sarcopenia in people with DM [26–28]. In Spain, 21.2% of all patients with T2DM seen in the hospital suffer malnutrition, and 15% suffer obesity [29–32]. The association between T2DM and sarcopenic obesity in patients is usually common [22, 33, 34].
There are multiple comorbidities associated with T2DM (retinopathy, renal, vascular involvement, etc.), duly reflected in the diabetes management guides in order to ensure adequate screening, prevention and treatment [35], which have even been directly related to the presence of sarcopenia in diabetic patients [36]. However, despite the clear impact of sarcopenia or altered muscle states on function and on the control of blood glucose levels, there are scarce references in the national and international guidelines to the measurement, diagnosis and control of what this group of professionals called “diabetic sarcopenia” (DS), defined as a disorder characterized by the presence of type 1 or 2 diabetes mellitus and sarcopenia [3].
A previous study assessed the reality of muscle mass loss in people with diabetes and emphasized the need for clinical management of this comorbidity associated to T2DM in Spain [3]. The purpose of this article is to comprehensively review the literature to propose the grounds to allow for the definition of another comorbidity associated to diabetes, “diabetic sarcopenia”, and to develop a screening algorithm that facilitates diagnosis and staging in our standard clinical practice.
Materials and methods
Design
A qualitative expert opinion study was carried out using the nominal technique. A literature search was performed in the PubMed Medline medical database of articles published in the past 5 years in English or Spanish related to the terms “screening” or “diagnostic criteria” and “muscle loss” or “sarcopenia” and “diabetes”. The selected articles were sent to the expert group for review and consultation. The expert group was then convened to a meeting to discuss a possible screening algorithm in diabetic patients.
The structure of other sarcopenia screening algorithms was followed, such as that of the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO) [2] in people with obesity or of the European Working Group on Sarcopenia in Older People (EWGSOP) in elderly people [1], first defining the target population on which to establish screening, the criteria for establishing the diagnosis, and finally the stage of the resulting disorder. Once the nominal meeting was completed, a medical writer prepared the aspects agreed on by the experts, which were reviewed by the group for final approval of this manuscript.
Selection of experts
The group of multidisciplinary experts (endocrinology, clinical nutrition, internal medicine and geriatrics), consisting of 7 experts, was the same as that established in 2022 for the publication of the paper “La masa muscular disminuida en la diabetes de tipo 2. Una comorbilidad oculta” [Decreased muscle mass in type-2 diabetes. A hidden comorbidity to consider] [3]. The experts were selected based on the following criteria: 1) scientific publications in PubMed indexed journals; 2) studies presented at national and international congresses of each specialty; 3) proven clinical experience in type 2 diabetes mellitus, clinical nutrition, and body composition of patients; and 4) geographic representativeness.
Results
Study population
Since it is not feasible for efficiency reasons to screen all people with diabetes, the profiles with the highest risk of sarcopenia were defined.
In line with the European Consensus on the Management of Sarcopenia in the Elderly [1], patients with a positive SARC-F questionnaire (score ≥ 4 points, range of 0–6 points) were screened [37, 38]. The SARC-F consists of 5 questions that the patient responds to based on his/her ability or difficulty to perform the following activities: strength activity, walking, rising from a chair, climbing stairs, and falls in the past year. Since it has reduced sensitivity and high specificity for detecting muscle strength, the SARC-F will enable the detection of the most serious and evident cases of sarcopenia [39] in elderly diabetic patients, since its use in a young population has not been validated. Studies have suggested that sensitivity may be improved by adding calf circumference (CC) [40]. However, very little data are currently available on the use of CC in the assessment of diabetic patients.
Similarly, consideration will be given to all patients with diabetes mellitus in whom a suspected factor is present (e.g., history of falls, limited mobility, weakness or fatigue), the presence of polypharmacy (more than 5 drugs), frailty criteria (defined as a score higher than 5 in the Clinical Frailty Scale [41, 42], risk of malnutrition detected by a Mini Nutritional Assessment Short-Form (MNA®-SF) [43] ≤ 11 points or a CONtrolling NUTritional status (CONUT) [44] > 5 points, nursing home placement or the detection of any clinical symptom (Table 1). These include the presence of several chronic diseases that increase the risk of loss of muscle mass and function (e.g., inflammatory disease, organ or transplant failure, as well as recent catabolic events or nutritional disorders that can cause muscle involvement [45, 46] such as hospital admissions, surgery, immobilization due to fractures, or rapid changes in body weight.
Table 1.
Clinical symptoms or suspected factors in diabetic sarcopenia
| 1. Diagnosis of chronic disease (e.g. inflammatory disease and organ failure or chronic disease) including, but not limited to: |
|---|
| • Chronic heart failure |
| • Chronic kidney disease (renal transplant in particular) |
| • Chronic intestinal failure or dysfunction |
| • Chronic liver disease (metabolic-associated fatty liver disease MAFLD and cirrhosis of the liver in particular) |
| • Chronic respiratory disease |
| • Chronic neurologic and neurodegenerative diseases |
| • Chronic cognitive impairment |
| • Depression |
| • Organ transplantation |
| • Endocrine diseases (e.g. metabolic syndrome, hypercortisolism, hypogonadism, and corticosteroid therapy) |
| • Osteoarthritis |
| • Cancer (especially but not limited to chemotherapy for breast or prostate cancer) |
| 2. Acute diseases/recent nutritional events: |
|---|
| • Recent hospitalization (in particular but not limited to COVID-19, ICU stay, surgery) |
| • Recent major surgery or trauma with/without complications |
| • Recent sustained immobilization or reduced mobility (e.g., trauma, fracture, orthopaedic disease) |
| • Recent history of reduced food intake (e.g., <50% for >2 weeks) |
| • Recent weight loss (voluntary diet-induced and weight cycling syndrome) |
| • Long-term restrictive diets and bariatric surgery |
| 3. Diabetes-specific involvement: |
|---|
| - Macrovascular: |
| • Ischemic heart disease (angina, infarction, dyspnoea) |
| • Cerebrovascular disease (acute cerebrovascular accident, ACVA, or transient ischaemic attack, TIA) |
| • Peripheral vascular disease (PVD) |
| - Microvascular: |
| • Retinopathy |
| • Nephropathy and diabetic foot |
| • Neuropathy |
| 4. History – reason for visit: |
|---|
| • Repeated falls |
| • Weakness, exhaustion |
| • Fatigue |
| • Perceived progressive movement limitations |
Specific risk factors include the following: HbA1C (HbA1C ≥ 8%), more than 5 years since T2DM onset, presence of chronic complications, and use of blood glucose-lowering drugs.
Whether or not glycosylated haemoglobin > 8 should be considered a patient screening criterion was a controversial point. Good control is considered to have been achieved with an HbA1C < 7%, and an HbA1C ≥ 8% is related to frailty or cognitive impairment in the elderly [47, 48], in addition to being a risk factor for the decline in quality of muscle mass regardless of the duration of diabetes [10, 49]. It is important to review the patient’s history of blood glucose control over the past 5 years since sustained poor metabolic control is associated with greater impairment in the quantity and quality of muscle mass [10, 47–49].
The time since T2DM diagnosis is also key. A time since diagnosis of longer than 5 years is considered a screening criterion because of the potential continued effect of T2DM on muscle status. Furthermore, at the time of diagnosis, diabetes is characterized by a variable period of progression, with hyperglycaemia and cumulative macro- and microvascular damage [50]. The presence of chronic microvascular complications (retinopathy, nephropathy and diabetic neuropathy) or macrovascular complications (coronary disease, cerebrovascular disease, heart failure, acute transient ischemia or peripheral vascular disease) caused by diabetes evidences systemic damage [51–54] and must be taken into account to identify the patient at risk (Table 1).
Finally, it is important to consider the current or prior antihyperglycemic therapy the patient has received. The role of glucose-lowering drugs in muscle physiology is known to favour or adversely affect muscle mass and function [22].
Sulfonylureas, particularly glibenclamide, and glinides (repaglinide and nateglinide) would have a potential atrophic effect, and should therefore be used with caution in diabetic patients [22, 23].
Special mention must be made of sodium/glucose cotransporter inhibitors (SGLT2i), due to their mechanism of action by reducing insulin levels and increasing glucagon levels. This hormonal situation results in decreased muscle absorption of glucose and amino acids, and facilitates proteolysis - thereby favouring the presence of diabetic sarcopenia [55]. Two recent meta-analyses showed a reduction in weight, total body, subcutaneous and visceral fat, with the use of SGLT2i in patients with type 2 diabetes and obesity, though as an adverse effect, they also recorded a decrease in muscle mass (indicate the % loss of muscle mass if referred to in the article because the statement suggests that it is important and the % is not reflected) [23, 24].
New incretin-based glucose-lowering treatments such as glucagon-like peptide-1 agonists (GLP-1 RAs) may cause marked weight loss that must be controlled and taken into account to prevent inadequate muscle mass loss. Although there are controversial data from this therapeutic group on a possible protective role on muscle [56–63], significant weight loss (> 10% of body weight) in a short period of time should be a warning sign for assessing patient body mass [22, 64, 65].
Other treatments like insulin [66] or metformin [67–69] have shown positive effects. DPP4i have a neutral or at least non-deleterious effect on muscle mass [56, 57, 70, 71], since they do not cause substantial weight changes. Diabetic patients receiving any of these drugs would not initially receive special care except in the event of associated significant weight loss.
Screening
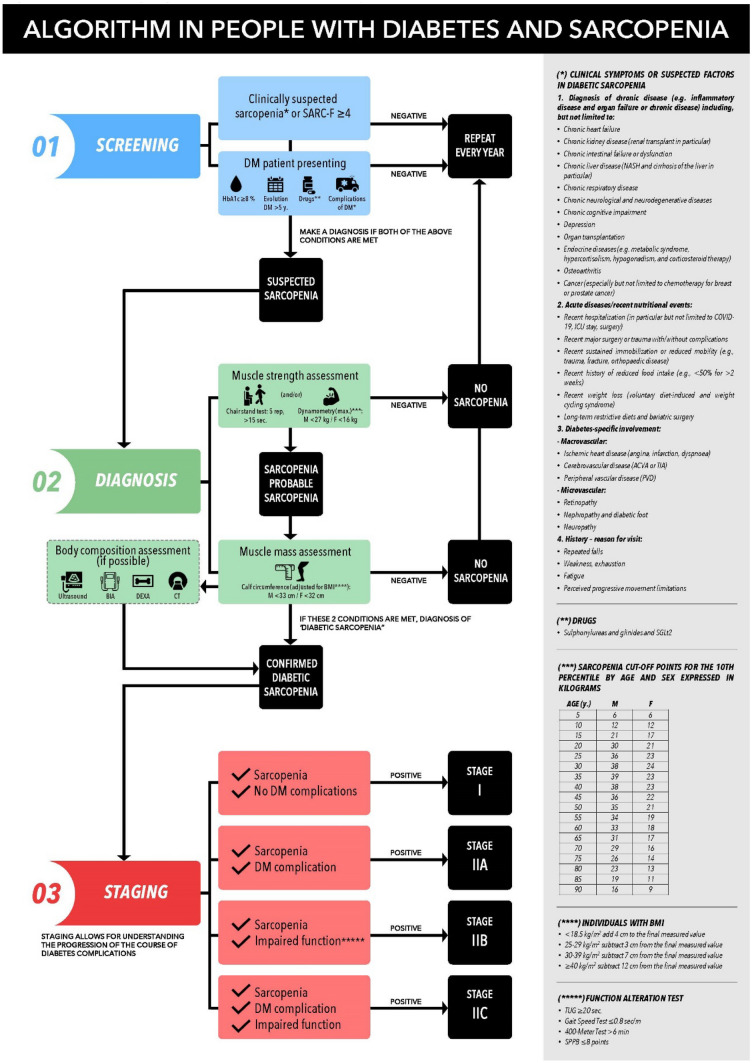
If any of the above characteristics were present in the screening of a person with diabetes, the patient would progress to the next phase and be diagnosed. In the event of a negative result, it is advisable to repeat screening on an annual basis in the same way as in all other complications of T2DM [72].
Diagnosis
Diagnosis of diabetic sarcopenia was based on the presence of criteria of low muscle strength (probable sarcopenia) and low muscle mass (confirmed sarcopenia) using methods available to any clinical visit such as dynamometry, the chair stand test, and BMI-adjusted calf circumference.
Muscle strength
Dynamometry
Grip strength moderately correlates with strength in other body compartments, so it is used instead of more complicated measures of arm and leg strength. Due to its ease of use, grip strength is recommended for routine use in hospital practice [73–75]. Low grip strength is correlated to poor patient outcome, such as longer hospital stays, greater functional limitations, poor quality of life, and death [76–80]. The JammarR dynamometer is validated and widely used [81], though other types of dynamometers have also been used in population studies such as Druck [82], Collin [83] or the Martin vigorimeter [84]. To compare our results in the clinic, we recommend using the cut-off points of reference tables of the geographical area in which we are located. Studies should be performed using a proven Southampton methodology [85]. For example, in the case of Spanish patients, we would use as cut-off points the 10th percentile of the tables of the Teruel study [82], since the age groups are broader than those of the Pizarra study [83]. If our own data is not available, we will use the general age- and sex-adjusted tables of the Dodds study [86]. The Dodds tables are obtained after aggregating data from 20 studies conducted in the British Isles with nearly 50,000 subjects evaluated and provide cut-off points for percentiles ranging from 5 to 90 years. Table 2 shows the cut-off points of the 10th percentile according to age and sex expressed in kilograms in that study.
Table 2.
Sarcopenia cut-off points for the 10th percentile according to age and sex expressed in kilograms. From Dodds 2014 [86]
| Age (years) | Men | Women |
|---|---|---|
| 5 | 6 | 6 |
| 10 | 12 | 12 |
| 15 | 21 | 17 |
| 20 | 30 | 21 |
| 25 | 36 | 23 |
| 30 | 38 | 24 |
| 35 | 39 | 23 |
| 40 | 38 | 23 |
| 45 | 36 | 22 |
| 50 | 35 | 21 |
| 55 | 34 | 19 |
| 60 | 33 | 18 |
| 65 | 31 | 17 |
| 70 | 29 | 16 |
| 75 | 26 | 14 |
| 80 | 23 | 13 |
| 85 | 19 | 11 |
| 90 | 16 | 9 |
Chair stand test
The chair stand test measures the amount of time required for a person to rise five times from a seated position without using their arms. It is considered abnormal if more than 15 s are needed to perform the 5 elevations [1, 87]. Since it requires both strength and endurance and some coordination of other muscle groups, it is a qualitative strength test. The chair stand test was selected because it is an easily implemented test in standard clinical practice. Other tests that measure physical changes commonly used are the Timed-Up and Go test (TUG) with a value greater than or equal to 20 s, a Gait Speed (GS) test [88, 89] less than or equal to 0.8 s/meter, a 400-Meter Walk Test [90] performed in 6 min or more or that cannot be completed or a Short Physical Performance Battery (SPPB) [91, 92] with a score less than or equal to 8 points; these last 3 tests are used to grade the severity of sarcopenia.
If muscle strength changes occur with dynamometry and/or the Timed-Up and Go test, the patient will be considered to have probable sarcopenia, and muscle mass will be assessed.
Muscle mass assessment
Calf circumference
Calf circumference has been shown to be a good indicator for predicting functionality and survival in elderly patients [93]. The BMI-adjusted calf circumference measurement is easy to use and a good indicator of body mass status prior to the use of other body measurement techniques. Calf values < 33 cm in males and < 32 cm in females are the cut-off points to be used [94]. In subjects with BMI values < 18.5 kg/m2, 4 cm should be added to the final measured value; with BMI values 25–29 kg/m2, subtract 3 cm from the value; with BMI values 30–39 kg/m2, subtract 7 cm from the value; and with BMI values ≥ 40 kg/m2 subtract 12 cm from the value [94].
An age-adjusted calf circumference below the cut-off points, along with the previous decrease in functional capacity, will indicate the presence of diabetic sarcopenia. If other tools are available to assess body composition such as impedancemetry (BIA), DEXA, muscle ultrasound or CT, the use of these tools in the patient is recommended to confirm the diagnosis.
Severity staging
Depending on the sarcopenia and the patient’s involvement, four resulting stages have been established. Stage I corresponds to sarcopenic patients with no other diabetes complication, and Stage II corresponds to patients with some type of involvement. Within Stage II are three sublevels (a, b and c). Stage IIa refers to individuals with sarcopenic diabetes and some diabetes-specific impairment, IIb to sarcopenia with functional impairment, and IIc to sarcopenia with diabetes complications and changes in function measured using standard tests. Figure 1 shows general recommendations for the patient in line with the latest expert report presented [3]. The generation of recommendations adapted to the characteristics of the patient and to the stage of his/her diabetic sarcopenia is the subject of a future document, given the multiple nutritional and physical activity considerations that should be taken into account.
Fig. 1.
Screening algorithm for diabetic sarcopenia
Conclusions
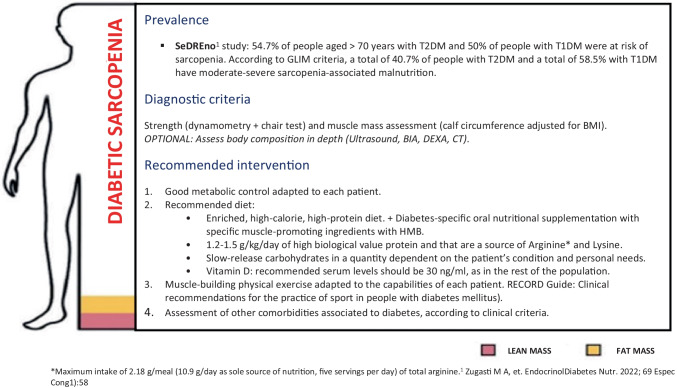
This document aims to establish the foundation for protocolising the screening and diagnosis of diabetic sarcopenia in a manner that is simple and accessible for all levels of healthcare. Figure 2 shows the management algorithm for diabetic sarcopenia in its aspects of screening, diagnosis, and staging.
Fig. 2.
Proposed treatment algorithm in people with diabetes
An effort has been made to simplify processes and make them accessible to both specialized healthcare and primary care settings by establishing accessible tests with defined cut-off points.
Diabetic sarcopenia has a significant impact on function and quality of life in people with T2DM, and it is important to give it the same attention as all other traditionally described complications of T2DM. Studies in real clinical practice, with the aim of validating this protocol and determining the true magnitude of diabetic sarcopenia in our clinics and hospitals, are undoubtedly needed.
Author contributions
D.L.R.D.: conceptualization, writing original draft, reviewing and editing. C.G.J.: conceptualization, writing original draft, reviewing and editing. G.A.J.: conceptualization, writing original draft, reviewing and editing. G.V.F.: conceptualization, writing original draft, reviewing and editing. G.R.G.: supervision. L.G.J.J.: conceptualization, writing original draft, reviewing and editing. D.S.: reviewing and editing. T.S.F.J.: reviewing and editing, supervision. S.P.A.: reviewing and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The expert meeting was funded by Abbott laboratories.
Data availability statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Declarations
Informed consent statement
Not applicable.
Competing interest
The authors declare no competing interests.
Institutional review board statement
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing [Internet]. 2019 Jan 1 [cited 2022 Jul 26];48(1):16–31. Available from: https://pubmed.ncbi.nlm.nih.gov/30312372/. [DOI] [PMC free article] [PubMed]
- 2.Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO Consensus Statement. Obes Facts. 2022;15(3):321–35. 10.1159/000521241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Luis Román D, Vallo FG, Gómez JC, Gómez JJL, Santabalbina FJT, Rolo GG, et al. Decreased muscle mass in type-2 diabetes. A hidden comorbidity to consider. Nutr Hosp [Internet]. 2023 Jan 1 [cited 2023 Jun 13];40(1):59–66. Available from: https://pubmed.ncbi.nlm.nih.gov/36633517/. [DOI] [PubMed]
- 4.Giha HA, Alamin OAO, Sater MS. Diabetic sarcopenia: metabolic and molecular appraisal. Acta Diabetol [Internet]. 2022 Aug 1 [cited 2023 Jul 27;59(8):989–1000. Available from: https://pubmed.ncbi.nlm.nih.gov/35429264/. [DOI] [PubMed]
- 5.Seok WP, Goodpaster BH, Jung SL, Kuller LH, Boudreau R, De Rekeneire N, et al. Excessive loss of skeletal muscle mass in older adults with type 2 Diabetes. Diabetes Care. 2009;32(11):1993–7. 10.2337/dc09-0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jungert A, Eichner G, Neuhäuser-Berthold M. Trajectories of body composition during advanced aging in consideration of diet and physical activity: A 20-year longitudinal study. Nutrients [Internet]. 2020 Nov 25 [cited 2022 Jun 12];12(12):3626. Available from: https://www.mdpi.com/2072-6643/12/12/3626/htm. [DOI] [PMC free article] [PubMed]
- 7.JafariNasabian P, Inglis JE, Reilly W, Kelly OJ, Ilich JZ. Aging human body: Changes in bone, muscle and body fat with consequent changes in nutrient intake. J Endocrinol [Internet]. 2017 Jul 1 [cited 2022 Jun 12;234(1):R37-51. Available from: https://joe.bioscientifica.com/view/journals/joe/234/1/R37.xml. [DOI] [PubMed]
- 8.Viña J, Borras C, Gomez-Cabrera MC. A free radical theory of frailty. Free Radic Biol Med. 2018;124:358–63. 10.1016/j.freeradbiomed.2018.06.028 [DOI] [PubMed] [Google Scholar]
- 9.Al-Sofiani ME, Ganji SS, Kalyani RR. Body composition changes in Diabetes and aging. J Diabetes Complications. 2019;33(6):451–9. 10.1016/j.jdiacomp.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SW, Goodpaster BH, Strotmeyer ES, De Rekeneire N, Harris TB, Schwartz AV, et al. Decreased muscle strength and quality in older adults with type 2 Diabetes: the health, aging, and body composition study. Diabetes. 2006;55(6):1813–8. 10.2337/db05-1183 [DOI] [PubMed] [Google Scholar]
- 11.Alabadi B, Civera M, De la Rosa A, Martinez-Hervas S, Gomez-Cabrera MC, Real JT. Frailty is associated with oxidative stress in older patients with type 2 Diabetes. Nutrients. 2021;13(11):1–13. 10.3390/nu13113983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campins L, Camps M, Riera A, Pleguezuelos E, Yebenes JC, Serra-Prat M. Oral drugs related with muscle wasting and sarcopenia. A Review Pharmacology [Internet]. 2017 Jan 25 [cited 2023 Jun 15;99(1–2):1–8. 10.1159/000448247. [DOI] [PubMed]
- 13.Seok WP, Goodpaster BH, Jung SL, Kuller LH, Boudreau R, De Rekeneire N, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care [Internet]. 2009 Nov [cited 2022 Jun 11];32(11):1993–7. Available from: https://pubmed.ncbi.nlm.nih.gov/19549734/. [DOI] [PMC free article] [PubMed]
- 14.Abbatecola AM, Ferrucci L, Ceda GP, Russo CR, Lauretani F, Bandinelli S, et al. Insulin resistance and muscle strength in older persons. J Gerontol Ser A [Internet]. 2005 Oct 1 [cited 2023 Jun 15];60(10):1278–82. 10.1093/gerona/60.10.1278. [DOI] [PubMed]
- 15.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol [Internet]. 2001 [cited 2023 Jun 15;3(11):1014–9. Available from https://pubmed.ncbi.nlm.nih.gov/11715023/. [DOI] [PubMed]
- 16.Mori H, Kuroda A, Ishizu M, Ohishi M, Takashi Y, Otsuka Y, et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in patients with type 2 diabetes. J Diabetes Investig [Internet]. 2019 Sep 1 [cited 2023 Jun 15;10(5):1332–40. Available from 10.1111/jdi.13014. [DOI] [PMC free article] [PubMed]
- 17.Sugimoto K, Ikegami H, Takata Y, Katsuya T, Fukuda M, Akasaka H, et al. Glycemic control and insulin improve muscle mass and gait speed in type 2 diabetes: the MUSCLES-DM Study. J Am Med Dir Assoc [Internet]. 2021;22(4):834–838.e1. 10.1016/j.jamda.2020.11.003. [DOI] [PubMed]
- 18.Hiromine Y, Noso S, Rakugi H, Sugimoto K, Takata Y, Katsuya T, et al. Poor glycemic control rather than types of diabetes is a risk factor for sarcopenia in diabetes mellitus: The MUSCLES-DM study. J Diabetes Investig [Internet]. 2022 Nov 1 [cited 2023 May 29;13(11):1881–8. Available from: https://pubmed.ncbi.nlm.nih.gov/35796583/. [DOI] [PMC free article] [PubMed]
- 19.de Luis D, Primo D, Izaola O, Gómez Hoyos E, López Gómez JJ. Relationship of circulating resistin levels with muscle mass determined by bioelectrical impedance in females with obesity. Endocrinol Diabetes Nutr [Internet]. 2023 Aug [cited 2023 Aug 30;70(7):468–75. Available from: https://pubmed.ncbi.nlm.nih.gov/37516610/. [DOI] [PubMed]
- 20.Sugimoto K, Tabara Y, Ikegami H, Takata Y, Kamide K, Ikezoe T, et al. Hyperglycemia in non-obese patients with type 2 diabetes is associated with low muscle mass: The multicenter study for clarifying evidence for sarcopenia in patients with diabetes mellitus. J Diabetes Investig [Internet]. 2019 Nov 1 [cited 2023 Jun 13;10(6):1471–9. Available from: https://pubmed.ncbi.nlm.nih.gov/31074209/. [DOI] [PMC free article] [PubMed]
- 21.Primo D, Izaola O, López Gómez JJ, De Luis D. Correlation of the phase angle with muscle ultrasound and quality of life in obese females. Dis Markers [Internet]. 2022 [cited 2023 Aug 30];2022. Available from: https://pubmed.ncbi.nlm.nih.gov/35983408/. [DOI] [PMC free article] [PubMed]
- 22.Wu CN, Tien KJ. The impact of antidiabetic agents on sarcopenia in type 2 diabetes: a literature review. J Diabetes Res. 2020;2020. [DOI] [PMC free article] [PubMed]
- 23.Pan R, Zhang Y, Wang R, Xu Y, Ji H, Zhao Y. Effect of SGLT-2 inhibitors on body composition in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. PLoS One [Internet]. 2022 Dec 1 [cited 2023 May 30];17(12):e0279889. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0279889. [DOI] [PMC free article] [PubMed]
- 24.Zhang S, Qi Z, Wang Y, Song D, Zhu D. Effect of sodium-glucose transporter 2 inhibitors on sarcopenia in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Front Endocrinol (Lausanne) [Internet]. 2023 [cited 2023 Jul 27];14. Available from: https://pubmed.ncbi.nlm.nih.gov/37465122/. [DOI] [PMC free article] [PubMed]
- 25.Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017;5(5):377–90. 10.1016/S2213-8587(17)30014-1 [DOI] [PubMed] [Google Scholar]
- 26.Carretero Gómez J, Galeano Fernández TF, Vidal Ríos AS, Pérez Palacios MR, García García GM, García Carrasco C, et al. Malnutrition and sarcopenia worsen short- and long-term outcomes in internal medicine inpatients. Postgrad Med J [Internet]. 2023 Mar 31 [cited 2023 Aug 30;99(1168):56–62. Available from: https://pubmed.ncbi.nlm.nih.gov/36828395/. [DOI] [PubMed]
- 27.Serrano Valles C, López Gómez JJ, García Calvo S, Jiménez Sahagún R, Torres Torres B, Gómez Hoyos E, et al. Influence of nutritional status on hospital length of stay in patients with type 2 diabetes. Endocrinol Diabetes Nutr [Internet]. 2020;67(10):617–24. 10.1016/j.endinu.2020.05.004. [DOI] [PubMed]
- 28.Zugasti A, Riestra M, Petrina E. Sarcopenia otra complicación crónica a considerar en personas con diabetes. Endocrinol Diabetes Nutr. 2022;69:32–32. (Espec Cong 1).
- 29.París AS, García JM, Gómez-Candela C, Burgos R, Martín Á, Matía P, et al. Malnutrition prevalence in hospitalized elderly diabetic patients. Nutr Hosp. 2013;28(3):592–9. [DOI] [PubMed] [Google Scholar]
- 30.Sanz-París A, Martín-Palmero A, Gomez-Candela C, García-Almeida JM, Burgos-Pelaez R, Sanz-Arque A, et al. GLIM criteria at hospital admission predict 8-year all-cause mortality in elderly patients with type 2 diabetes mellitus: results from VIDA Study. JPEN J Parenter Enteral Nutr [Internet]. 2020 Nov 1 [cited 2023 Aug 30;44(8):1492–500. Available from: https://pubmed.ncbi.nlm.nih.gov/32026501/. [DOI] [PubMed]
- 31.Lardiés-Sánchez B, Sanz-París A, Pérez-Nogueras J, Serrano-Oliver A, Torres-Anoro ME, Cruz-Jentoft AJ. Influence of nutritional status in the diagnosis of sarcopenia in nursing home residents. Nutrition [Internet]. 2017 Sep 1 [cited 2023 Aug 30];41:51–7. Available from: https://pubmed.ncbi.nlm.nih.gov/28760428/. [DOI] [PubMed]
- 32.Sanz-Paris A, González-Fernandez M, Hueso-Del Río LE, Ferrer-Lahuerta E, Monge-Vazquez A, Losfablos-Callau F, et al. Muscle thickness and echogenicity measured by ultrasound could detect local sarcopenia and malnutrition in older patients hospitalized for hip fracture. Nutrients [Internet]. 2021 Jul 1 [cited 2023 Aug 30];13(7). Available from: https://pubmed.ncbi.nlm.nih.gov/34371911/. [DOI] [PMC free article] [PubMed]
- 33.Ahmed N, Choe Y, Mustad VA, Chakraborty S, Goates S, Luo M, et al. Impact of malnutrition on survival and healthcare utilization in Medicare beneficiaries with diabetes: A retrospective cohort analysis. BMJ Open Diabetes Res Care. 2018;6(1):1–9. 10.1136/bmjdrc-2017-000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Luis D, Primo D, Izaola O, Martinez A, Lopez Gomez JJ. Relationship between fat-free mass and metabolic syndrome in obese females. Eur Rev Med Pharmacol Sci [Internet]. 2023 [cited 2023 Aug 30];27(10):4648–55. Available from: https://pubmed.ncbi.nlm.nih.gov/37259765/. [DOI] [PubMed]
- 35.Committee ADAPP. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care [Internet]. 2022 Jan 1 [cited 2022 Jul 19];45(Supplement_1):S17–38. Available from: https://diabetesjournals.org/care/article/45/Supplement_1/S17/138925/2-Classification-and-Diagnosis-of-Diabetes. [DOI] [PubMed]
- 36.Fukuda T, Bouchi R, Takeuchi T, Nakano Y, Murakami M, Minami, I et al. Association of diabetic retinopathy with both sarcopenia and muscle quality in patients with type 2 diabetes: A cross-sectional study. BMJ Open Diabetes Res Care [Internet]. 2017 May 1 [cited 2023 May 30];5(1):e000404. Available from: https://drc.bmj.com/content/5/1/e000404. [DOI] [PMC free article] [PubMed]
- 37.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle [Internet]. 2016 Mar 1 [cited 2023 Jun 14];7(1):28–36. Available from: https://pubmed.ncbi.nlm.nih.gov/27066316/. [DOI] [PMC free article] [PubMed]
- 38.Parra-Rodríguez L, Szlejf C, García-González AI, Malmstrom TK, Cruz-Arenas E, Rosas-Carrasco O. Cross-cultural adaptation and validation of the spanish-language version of the SARC-F to assess sarcopenia in mexican community-dwelling older adults. J Am Med Dir Assoc [Internet]. 2016 Dec 1 [cited 2022 Jul 22;17(12):1142–6. Available from: http://www.jamda.com/article/S1525861016304236/fulltext. [DOI] [PubMed]
- 39.Bahat G, Yilmaz O, Kiliç C, Oren MM, Karan MA. Performance of SARC-F in regard to sarcopenia definitions, muscle mass and functional measures. J Nutr Health Aging [Internet]. 2018 Oct 1 [cited 2023 Jun 14;22(8):898–903. Available from: https://pubmed.ncbi.nlm.nih.gov/30272090/. [DOI] [PubMed]
- 40.Yang M, Hu X, Xie L, Zhang L, Zhou J, Lin J, et al. Screening sarcopenia in community-dwelling older adults: SARC-F vs SARC-F combined with calf circumference (SARC-CalF). J Am Med Dir Assoc [Internet]. 2018 Mar 1 [cited 2023 Jun 15];19(3):277.e1–277.e8. Available from: http://www.jamda.com/article/S1525861017307016/fulltext. [DOI] [PubMed]
- 41.Ruiz JG, Dent E, Morley JE, Merchant RA, Beilby J, Beard J, et al. Screening for and managing the person with frailty in primary care: ICFSR consensus guidelines. J Nutr Health Aging 2020 249 [Internet]. 2020 Oct 17 [cited 2023 Jul 20];24(9):920–7. Available from: https://link.springer.com/article/10.1007/s12603-020-1498-x. [DOI] [PMC free article] [PubMed]
- 42.Oviedo-Briones M, Laso ÁR, Carnicero JA, Cesari M, Grodzicki T, Gryglewska B, et al. A comparison of frailty assessment instruments in different clinical and social care settings: The frailtools project. J Am Med Dir Assoc [Internet]. 2021 Mar 1 [cited 2023 Jul 20;22(3):607.e7–607.e12. Available from: http://www.jamda.com/article/S1525861020308215/fulltext. [DOI] [PubMed]
- 43.Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J Nutr Health Aging [Internet]. 2009[cited 2023 Jul 28;13(9):782–8. Available from: https://pubmed.ncbi.nlm.nih.gov/19812868/. [DOI] [PubMed]
- 44.De Ulíbarri JI, González-Madroño A, De Villar NG, González P, González B, Mancha A, et al. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45. [PubMed]
- 45.Dhillon RJS, Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med [Internet]. 2017 Feb 1 [cited 2023 Jun 15];33(1):17–26. Available from: http://www.geriatric.theclinics.com/article/S0749069016300714/fulltext. [DOI] [PMC free article] [PubMed]
- 46.Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res [Internet]. 2017 Feb 1 [cited 2023 Jun 15];29(1):43–8. Available from: https://link.springer.com/article/10.1007/s40520-016-0709-0. [DOI] [PubMed]
- 47.Elsayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 6. Glycemic targets: Standards of care in diabetes—2023. Diabetes Care [Internet]. 2023 Jan 1 [cited 2023 May 29];46(Supplement_1):S97–110. Available from: https://diabetesjournals.org/care/article/46/Supplement_1/S97/148053/6-Glycemic-Targets-Standards-of-Care-in-Diabetes. [DOI] [PMC free article] [PubMed]
- 48.Elsayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 13. Older adults: Standards of care in diabetes—2023. Diabetes Care [Internet]. 2023 Jan 1 [cited 2023 May 29];46(Supplement_1):S216–29. Available from: https://diabetesjournals.org/care/article/46/Supplement_1/S216/148044/13-Older-Adults-Standards-of-Care-in-Diabetes-2023. [DOI] [PMC free article] [PubMed]
- 49.Yoon JW, Ha YC, Kim KM, Moon JH, Choi SH, Lim S, et al. Hyperglycemia is associated with impaired muscle quality in older men with diabetes: The Korean longitudinal study on health and aging. Diabetes Metab J [Internet]. 2016 Apr 1 [cited 2023 May 29];40(2):140–6. Available from: http://www.e-dmj.org/journal/view.php?doi=10.4093/dmj.2016.40.2.0. [DOI] [PMC free article] [PubMed]
- 50.Porta M, Curletto G, Cipullo D, de la Longrais RR, Trento M, Passera P, et al. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care [Internet]. 2014 Jun 1 [cited 2023 Jun 15];37(6):1668–74. 10.2337/dc13-2101. [DOI] [PubMed]
- 51.Fukuda T, Bouchi R, Takeuchi T, Nakano Y, Murakami M, Minami I, et al. Association of diabetic retinopathy with both sarcopenia and muscle quality in patients with type 2 diabetes: A cross-sectional study. BMJ open diabetes Res care [Internet]. 2017 May 1 [cited 2023 May 29];5(1). Available from: https://pubmed.ncbi.nlm.nih.gov/28761661/. [DOI] [PMC free article] [PubMed]
- 52.Andersen H, Nielsen S, Mogensen CE, Jakobsen J. Muscle strength in type 2 diabetes. Diabetes [Internet]. 2004 Jun [cited 2023 Jun 15];53(6):1543–8. Available from: https://pubmed.ncbi.nlm.nih.gov/15161759/. [DOI] [PubMed]
- 53.Andreassen CS, Jakobsen J, Ringgaard S, Ejskjaer N, Andersen H. Accelerated atrophy of lower leg and foot muscles–a follow-up study of long-term diabetic polyneuropathy using magnetic resonance imaging (MRI). Diabetologia [Internet]. 2009[cited 2023 Jun 15;52(6):1182–91. Available from: https://pubmed.ncbi.nlm.nih.gov/19280173/. [DOI] [PubMed]
- 54.Sundar VV, Ong SH, Easaw MEPM, Chee WSS. Sarcopenia with co-existent type 2 diabetes mellitus is associated with worse clinical outcomes among hospitalised cardiac patients. Clin Nutr ESPEN [Internet]. 2021 Dec 1 [cited 2023 May 29];46:380–5. Available from: https://pubmed.ncbi.nlm.nih.gov/34857224/. [DOI] [PubMed]
- 55.Yabe D, Nishikino R, Kaneko M, Iwasaki M, Seino Y. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: considerations for their appropriate use to avoid serious adverse events. Expert Opin Drug Saf [Internet]. 2015 Jun 1 [cited 2023 Jun 15;14(6):795–800. Available from: https://pubmed.ncbi.nlm.nih.gov/25851664/. [DOI] [PubMed]
- 56.Rizzo MR, Barbieri M, Fava I, Desiderio M, Coppola C, Marfella R, et al. Sarcopenia in elderly diabetic patients: Role of dipeptidyl peptidase 4 inhibitors. J Am Med Dir Assoc [Internet]. 2016 Oct 1 [cited 2023 Jun 15];17(10):896–901. Available from: http://www.jamda.com/article/S1525861016301104/fulltext. [DOI] [PubMed]
- 57.Perna S, Guido D, Bologna C, Solerte SB, Guerriero F, Isu A, et al. Liraglutide and obesity in elderly: Efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin Exp Res [Internet]. 2016 Dec 1 [cited 2023 Jun 15;28(6):1251–7. Available from: https://pubmed.ncbi.nlm.nih.gov/26749118/. [DOI] [PubMed]
- 58.Yajima T, Yajima K, Takahashi H, Yasuda K. The effect of dulaglutide on body composition in type 2 diabetes mellitus patients on hemodialysis. J Diabetes Complications [Internet]. 2018 Aug 1 [cited 2023 Jul 27,;32(8):759–63. Available from: https://pubmed.ncbi.nlm.nih.gov/29937137/. [DOI] [PubMed]
- 59.Ishii S, Nagai Y, Kato H, Fukuda H, Tanaka Y. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin on muscle mass and the muscle/fat ratio in patients with type 2 diabetes. J Clin Med Res [Internet]. 2020 [cited 2023 Jul 27;12(2):122–6. Available from: https://pubmed.ncbi.nlm.nih.gov/32095182/. [DOI] [PMC free article] [PubMed]
- 60.Rondanelli M, Perna S, Astrone P, Grugnetti A, Solerte SB, Guido D. Twenty-four-week effects of liraglutide on body composition, adherence to appetite, and lipid profile in overweight and obese patients with type 2 diabetes mellitus. Patient Prefer Adherence [Internet]. 2016 Mar 24 [cited 2023 Jul 27];10:407–13. Available from: https://pubmed.ncbi.nlm.nih.gov/27069358/. [DOI] [PMC free article] [PubMed]
- 61.Hong JY, Park KY, Kim BJ, Hwang WM, Kim DH, Lim DM. Effects of short-term exenatide treatment on regional fat distribution, glycated hemoglobin levels, and aortic pulse wave velocity of obese type 2 diabetes mellitus patients. Endocrinol Metab [Internet]. 2016 Mar 1 [cited 2023 Jul 27];31(1):80–5. Available from: http://www.e-enm.org/journal/view.php?doi=10.3803/EnM.2016.31.1.80. [DOI] [PMC free article] [PubMed]
- 62.Ozeki Y, Masaki T, Kamata A, Miyamoto S, Yoshida Y, Okamoto M, et al. The effectiveness of GLP-1 receptor agonist semaglutide on body composition in elderly obese diabetic patients: A pilot study. Med (Basel, Switzerland) [Internet]. 2022 Sep 16 [cited 2023 Jul 27];9(9):47. Available from: https://pubmed.ncbi.nlm.nih.gov/36135828/. [DOI] [PMC free article] [PubMed]
- 63.Osaka T, Hamaguchi M, Fukui M. Favorable appendicular skeletal muscle mass changes in older patients with type 2 diabetes receiving GLP-1 receptor agonist and basal insulin co-therapy. Clin Med Insights Endocrinol Diabetes [Internet]. 2023 Jan 1 [cited 2023 Jul 27];16. Available from: https://pubmed.ncbi.nlm.nih.gov/37025567/. [DOI] [PMC free article] [PubMed]
- 64.Zhang X, Zhao Y, Chen S, Shao H. Anti-diabetic drugs and sarcopenia: Emerging links, mechanistic insights, and clinical implications. J Cachexia Sarcopenia Muscle [Internet]. 2021 Dec 1 [cited 2023 Jul 20;12(6):1368–79. Available from: https://pubmed.ncbi.nlm.nih.gov/34676695/. [DOI] [PMC free article] [PubMed]
- 65.Massimino E, Izzo A, Riccardi G, Della Pepa G. The impact of glucose-lowering drugs on sarcopenia in type 2 diabetes: Current evidence and underlying mechanisms. Cells. 2021;10(8). [DOI] [PMC free article] [PubMed]
- 66.Bouchi R, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, et al. Insulin treatment attenuates decline of muscle mass in Japanese patients with type 2 diabetes. Calcif Tissue Int [Internet]. 2017 Jul 1 [cited 2023 Jun 15;101(1):1–8. Available from: https://kyushu-u.elsevierpure.com/ja/publications/insulin-treatment-attenuates-decline-of-muscle-mass-in-japanese-p. [DOI] [PMC free article] [PubMed]
- 67.Lee CG, Boyko EJ, Barrett-Connor E, Miljkovic I, Hoffman AR, Everson-Rose SA, et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care [Internet]. 2011 Nov 1 [cited 2023 Jun 15;34(11):2381–6. 10.2337/dc11-1032. [DOI] [PMC free article] [PubMed]
- 68.Laksmi PW, Setiati S, Tamin TZ, Soewondo P, Rochmah W, Nafrialdi N, et al. Effect of metformin on handgrip strength, gait speed, myostatin serum level, and health-related quality of life: A double blind randomized controlled trial among non-diabetic pre-frail elderly patients. Acta Med Indones [Internet]. 2017 Aug 8 [cited 2023 Jun 15];49(2):118. Available from: https://www.actamedindones.org/index.php/ijim/article/view/330. [PubMed]
- 69.Lee CG, Schwartz AV, Yaffe K, Hillier TA, Leblanc ES, Cawthon PM. Changes in physical performance in older women according to presence and treatment of diabetes mellitus. J Am Geriatr Soc [Internet]. 2013 Nov 1 [cited 2023 Jun 15;61(11):1872–8. Available from: 10.1111/jgs.12502. [DOI] [PMC free article] [PubMed]
- 70.Rajaobelina K, Helmer C, Vélayoudom-Céphise FL, Nov S, Farges B, Pupier E, et al. Progression of skin autofluorescence of AGEs over 4 years in patients with type 1 diabetes. Diabetes Metab Res Rev [Internet]. 2017 Oct 1 [cited 2023 Jun 15];33(7). Available from: https://pubmed.ncbi.nlm.nih.gov/28719154/. [DOI] [PubMed]
- 71.Sencan C, Dost FS, Ates Bulut E, Isik AT. DPP4 inhibitors as a potential therapeutic option for sarcopenia: A 6-month follow-up study in diabetic older patients. Exp Gerontol [Internet]. 2022 Jul 1 [cited 2023 Jul 27];164. Available from: https://pubmed.ncbi.nlm.nih.gov/35526704/. [DOI] [PubMed]
- 72.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, et al. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–8. 10.2337/dc16-2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rossi AP, Fantin F, Micciolo R, Bertocchi M, Bertassello P, Zanandrea V, et al. Identifying sarcopenia in acute care setting patients. J Am Med Dir Assoc [Internet]. 2014 [cited 2023 Jun 16];15(4):303.e7–303.e12 . Available from: https://pubmed.ncbi.nlm.nih.gov/24508329/. [DOI] [PubMed]
- 74.Steiber N. Strong or Weak Handgrip? Normative reference values for the german population across the life course stratified by sex, age, and body height. PLoS One [Internet]. 2016 Oct 1 [cited 2023 Jun 16];11(10). Available from: https://pubmed.ncbi.nlm.nih.gov/27701433/. [DOI] [PMC free article] [PubMed]
- 75.Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr [Internet]. 2016 [cited 2023 Jun 16];16(1):1–10. Available from: https://pubmed.ncbi.nlm.nih.gov/27716195/. [DOI] [PMC free article] [PubMed]
- 76.Ibrahim K, May C, Patel HP, Baxter M, Sayer AA, Roberts H. A feasibility study of implementing grip strength measurement into routine hospital practice (GRImP): Study protocol. Pilot Feasibility Stud [Internet]. 2016 Sep 23 [cited 2023 Jun 16];2(1). Available from: https://pubmed.ncbi.nlm.nih.gov/27965846/. [DOI] [PMC free article] [PubMed]
- 77.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet (London, England) [Internet]. 2015 Jul 18 [cited 2023 Jun 16];386(9990):266–73. Available from: https://pubmed.ncbi.nlm.nih.gov/25982160/. [DOI] [PubMed]
- 78.Koo BK, Moon S, Moon MK. Muscle strength, an independent determinant of glycemic control in older adults with long-standing type 2 diabetes: A prospective cohort study. BMC Geriatr [Internet]. 2021 Dec 1 [cited 2023 May 29];21(1). Available from: https://pubmed.ncbi.nlm.nih.gov/34876063/. [DOI] [PMC free article] [PubMed]
- 79.Chen CN, Chen TC, Tsai SC, Hwu CM. Factors associated with relative muscle strength in patients with type 2 diabetes mellitus. Arch Gerontol Geriatr [Internet]. 2021 Jul 1 [cited 2023 May 29];95. Available from: https://pubmed.ncbi.nlm.nih.gov/33740478/. [DOI] [PubMed]
- 80.Chen S, Yan S, Aiheti N, Kuribanjiang K, Yao X, Wang Q, et al. A bi-directional Mendelian randomization study of sarcopenia-related traits and type 2 diabetes mellitus. Front Endocrinol (Lausanne) [Internet]. 2023 Mar 8 [cited 2023 May 29];14. Available from: https://pubmed.ncbi.nlm.nih.gov/36967750/. [DOI] [PMC free article] [PubMed]
- 81.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing [Internet]. 2011 Jul [cited 2023 Aug 30;40(4):423–9. Available from: https://pubmed.ncbi.nlm.nih.gov/21624928/. [DOI] [PubMed]
- 82.Mateo Lázaro ML, Penacho Lázaro MA, Berisa Losantos F, Plaza Bayo A. Nuevas tablas de fuerza de la mano para población adulta de Teruel. Nutr Hosp. 2008;23(1):35–40. [PubMed] [Google Scholar]
- 83.Sánchez Torralvo FJ, Porras N, Abuín Fernández J, García Torres F, Tapia MJ, Lima F, et al. Valores de normalidad de dinamometría de mano en España. Relación con la masa magra. Nutr Hosp. 2018;35(1):98–103. [DOI] [PubMed] [Google Scholar]
- 84.Sipers WMWH, Verdijk LB, Sipers SJE, Schols JMGA, van Loon LJC. The Martin vigorimeter represents a reliable and more practical tool than the jamar dynamometer to assess handgrip strength in the geriatric patient. J Am Med Dir Assoc [Internet]. 2016 May 1 [cited 2023 Jun 16];17(5):466.e1–466.e7. Available from: https://pubmed.ncbi.nlm.nih.gov/27107163/. [DOI] [PubMed]
- 85.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJP, Cooper C. Cohort profile: The Southampton women’s survey. Int J Epidemiol [Internet]. 2006 Feb [cited 2023 Jul 20;35(1):42–8. Available from: https://pubmed.ncbi.nlm.nih.gov/16195252/. [DOI] [PMC free article] [PubMed]
- 86.Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, et al. Grip strength across the life course: Normative data from twelve British studies. PLoS One [Internet]. 2014 Dec 4 [cited 2023 Jun 16];9(12):e113637. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed]
- 87.Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, et al. Added value of physical performance measures in predicting adverse health-related events: Results from the Health, Aging, and Body Composition Study. J Am Geriatr Soc [Internet]. 2009 Feb [cited 2023 Jun 16];57(2):251. Available from: https://pubmed.ncbi.nlm.nih.gov/19207142/. [DOI] [PMC free article] [PubMed]
- 88.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing [Internet]. 2010 Apr 13 [cited 2023 Jul 28;39(4):412–23. Available from: https://pubmed.ncbi.nlm.nih.gov/20392703/. [DOI] [PMC free article] [PubMed]
- 89.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA [Internet]. 2011 Jan 5 [cited 2023 Jul 28];305(1):50–8. Available from: https://pubmed.ncbi.nlm.nih.gov/21205966/. [DOI] [PMC free article] [PubMed]
- 90.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA [Internet]. 2006[cited 2023 Jul 28,;295(17):2018–26. Available from: https://pubmed.ncbi.nlm.nih.gov/16670410/. [DOI] [PubMed]
- 91.Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, et al (1995) Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med [Internet]. 2016 Dec 22 [cited 2023 Jul 28];14(1). Available from:https://pubmed.ncbi.nlm.nih.gov/28003033/. [DOI] [PMC free article] [PubMed]
- 92.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med [Internet]. 1995 Mar 2 [cited 2023 Jul 28];332(9):556–62. Available from: https://pubmed.ncbi.nlm.nih.gov/7838189/. [DOI] [PMC free article] [PubMed]
- 93.Tosato M, Marzetti E, Cesari M, Savera G, Miller RR, Bernabei R, et al. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res [Internet]. 2017 Feb 1 [cited 2023 Jun 16];29(1):19–27. Available from: https://pubmed.ncbi.nlm.nih.gov/28176249/. [DOI] [PubMed]
- 94.Gonzalez MC, Mehrnezhad A, Razaviarab N, Barbosa-Silva TG, Heymsfield SB. Calf circumference: cutoff values from the NHANES 1999–2006. Am J Clin Nutr [Internet]. 2021 Jun 1 [cited 2023 May 30];113(6):1679–87. Available from: https://pubmed.ncbi.nlm.nih.gov/33742191/. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.