Abstract
Henipaviruses, highly fatal zoonotic viruses with mortality rates up to 100%, pose a significant threat to humans. Despite sporadic cases, including infections from Cedar, Langya, and Nipah Viruses, there are no established drugs or vaccines for treatment. This lack of specific medication led us to explore 57 non-toxic compounds from Indian Medicinal Plants, selected from 232 compounds, aiming to combat these viruses. Through in silico ADMET analyses, Three compounds—andrographolide, pterygospermin and Salidroside—stood out for their exceptional non-toxic properties. These compounds underwent in silico target prediction, molecular docking and dynamics with Cedar, Langya, and Nipah Virus proteins from the Protein Data Bank. Among them, Andrographolide displayed the most promising negative free energy scores and stability in Cedar Virus-Attachment G-Protein binding pockets. Pterygospermin and Salidroside showed efficacy against Langya and Nipah Virus target proteins throughout the simulation. These compounds not only exhibited antiviral properties but also demonstrated immunomodulatory, anti-inflammatory, and hepatoprotective effects by our in-silico studies. Their potential as treatments or preventive measures against henipaviral infections makes them promising candidates for further research and development.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40203-024-00236-x.
Keywords: Henipaviruses, Indian medicinal plants, Nipah virus, Langya virus, Hendra virus
Introduction
Since the 1990s, a group of zoonotic viruses that have crossed into humans has caused severe respiratory and encephalitic symptoms. These viruses were later identified as henipaviruses. Henipaviruses have a high mortality rate of about 90% and can cause seasonal epidemics in tropical regions around the world. There are four known henipavirus species that infect humans: Nipah henipavirus, Hendra henipavirus, Cedar henipavirus, and Langya henipavirus. Henipaviruses are classified under the family Paramyxoviridae and belong to the genus Henipavirus. The deadliest strains, with fatality rates of 60% and 90% in humans, are the Hendra and Nipah henipaviruses, respectively (Quarleri et al. 2022; Broder & Wong et al., 2016).
Outbreaks of henipavirus infections have caused hundreds of infections and deaths over the past two decades. Sporadic infections have since arisen in countries such as India, Bangladesh, Malaysia, Singapore, and Australia, occurring at least once a decade. Each outbreak is deadly, spreading through contact with bodily fluids from infected individuals, which can lead to pathogen mutation (Conroy 2023; Halpin and Rota 2014). Henipaviruses enter human cells via glycoprotein G and membrane-mediated fusion by the F protein. The viruses primarily use Ephrin-B2 cells, which are expressed in major organs like the lungs, kidneys, heart, and central nervous system (CNS). The virus’s genetic material is single-stranded RNA. After fusion, the RNA is inserted into the host cell, translated by ribosomes, assembled intracellularly, and the newly assembled viral particles then exit the cell.
Henipavirus infections do not have any approved therapeutics or vaccines, despite two proposed compounds: Ribavirin and Chloroquine (Bonaparte et al. 2005; Quarleri et al. 2022). Both proposed therapeutics have been found to cause severe side effects. Ribavirin is not advised for pregnant women as it may cause birth defects, fetal death, and anemia in some cardiac patients (Russmann et al. 2006; Sinclair et al. 2017). Chloroquine can cause the elongation of heart rhythm with long exposure to the QT wave and may pass into breast milk (Mubagwa, 2020; Drugs and Lactation Database 2021). The mechanism by which Ribavirin affects the henipavirus life cycle is unknown, but it is believed to inhibit RNA and produce immunomodulatory effects (Graci and Cameron 2006). Chloroquine is found to inhibit DNA and RNA synthesis and degrade ribosomes, including ribosomal RNA (Ciak and Hahn 1966). Drugs like remdesivir, favipiravir, and Griffithsin have been studied in animal models, but their mechanisms of action and efficacy remain unclear. Some synthetic compounds have been examined in vitro, but their effects are inconclusive (Johnson et al. 2021). Due to these adverse effects and the need for effective therapeutics, researchers are turning to classical molecular searches from medicinal plants of the Indian subcontinent.
Medicinal plants and herbs offer diverse health benefits and show potential as antiviral agents, continuing to be utilized by traditional healers. For centuries, plant-based remedies have been employed to treat a range of ailments, with several exhibiting antiviral properties. The antiviral efficacy of these plants is often linked to their bioactive constituents (Sharma et al. 2023). Chaachouay and Zidane listed the compounds that originate from various plants and exhibit a wide array of medicinal properties. Atropine, sourced from Atropa belladonna L., functions as an anticholinergic agent. Berberine, derived from Berberis vulgaris L., is utilized in treating bacillary dysentery. Caffeine, found in Camellia sinensis (L.) Kuntze, offers neuroprotection. Camptothecin, extracted from Camptotheca acuminata Decne., shows promise in anticancer therapy. Cocaine, obtained from Erythroxylum coca Lam., serves as an anesthetic. Colchicine, sourced from Colchicum autumnale L., aids in the treatment of gout and tumors. Convallatoxin, from Convallaria majalis L., acts as a cardiotonic. Various cardiac medications like Digitoxin and Digoxin are sourced from Digitalis species. Ephedrine, from Ephedra sinica Stapf, acts as a sympathomimetic. Glaucine, found in Glaucium flavum Crantz, functions as an antitussive. Glycyrrhizin, derived from Glycyrrhiza glabra L., is used in treating Addison’s disease. Morphine, sourced from Papaver somniferum L., offers analgesic effects. Ouabain, obtained from Strophanthus gratus (Wall. & Hook.) Baill., is another cardiotonic. Quinine, extracted from Cinchona officinalis L., is effective against malaria. Reserpine, found in Rauvolfia serpentina (L.) Benth. ex Kurz, acts as an antihypertensive. Salicin, derived from Salix alba L., provides analgesic effects. Scopolamine, sourced from Datura metel L., acts as a sedative. Silymarin, from Silybum marianum (L.) Gaertn., functions as an antihepatotoxic. Taxol, obtained from Taxus brevifolia Nutt., shows promise in anticancer treatment. Theophylline, found in Theobroma cacao L., acts as a diuretic. Thymol, extracted from Thymus vulgaris L., provides topical antifungal effects. Vinblastine and Vincristine, sourced from Catharanthus roseus (L.) G. Don, are both effective against cancer. Yuanhuacine, obtained from Daphne genkwa Siebold & Zucc., acts as an abortifacient (Chaachouay and Zidane 2024).
In this study, we thoroughly examined the broad-spectrum antiviral properties of Indian medicinal plants commonly used in the Indian Systems of Medicine and Homeopathy. We aimed to identify the safest compounds with no predicted toxicity through in silico toxicity testing, which can affect, inhibit, or destroy the viral replication life cycle. This study was conducted because approximately 25% of commonly used medical drugs are derived from plants (Mukhtar et al. 2008). The molecular targets chosen in this study are essential proteins required for the life cycle of henipaviruses. We investigated the potential role of these compounds in impeding the activity of lethal strains such as Nipah, Cedar, and Langya henipaviruses.
Materials and methods
Antiviral Indian medicinal plants
The search for potential broad-spectrum antiviral properties in the main compounds of Indian medicinal plants was conducted extensively through a literature review (Khare 2008; Rao and Ramakrishna 2020; Dhawan 2012). Only small molecules with such properties were chosen for the study. Each medicinal plant was examined for its main compounds, which had been investigated previously through various literature sources. Then, each main compound was studied for its broad-spectrum antiviral properties.
Identification of the best ligands
The best antiviral small molecular ligands were chosen by examining the main compounds that possess broad antiviral activities. Further characteristics of these compounds were studied using in silico tools as follows:
In silico ADMET and target prediction
The main compounds of each medicinal plant were subjected to SWISS-ADME (https://www.swissadme.ch) by importing the SMILES from PubChem (https://pubchem.ncbi.nlm.nih.gov). The compounds were then screened and filtered based on the results produced by the software. The SMILES of the filtered compounds were submitted to ADMETLab v.2.0 (https://admetmesh.scbdd.com) for a complete investigation of toxicological properties through in silico study. Similarly, in silico target predictions were done by submitting them via the Swiss Target Prediction website (https://www.swisstargetprediction.ch). Selected ligands were then analyzed in the ligand pathway of specific organs using PathwayMap (https://www.playmolecule.com/PathwayMap) in KEGG Database mode. These compounds were finalized and shortlisted based on the results retrieved from the website. The selected small compounds were fixed as ligands for molecular docking.
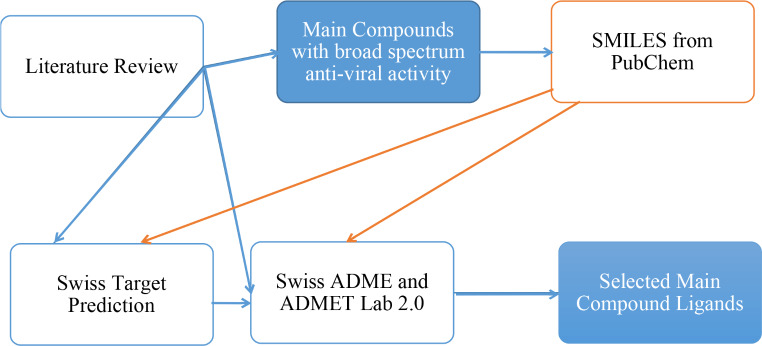
The entire process of selecting the main compound ligands is schematically represented in Fig. 1.
Fig. 1.
Schematic of Process Involved in Selection of Main Compound Ligands
Molecular docking of best ligands with respective receptors
Receptor preparation
As mentioned earlier, we have selected readily available protein formats from the Protein Data Bank (PDB) as targets for the viruses of interest. The viruses chosen are Cedar Virus, Langya Virus, and Nipah Virus. The target protein for Cedar Virus was taken from PDB ID: 6P72, which is the attachment G glycoprotein through which the viral load enters the cell. Similarly, the target protein for Langya Virus was retrieved from PDB ID: 8FEJ, which contains molecular data for the pre-fusion conformation of its fusion protein. Additionally, the target protein for Nipah Virus was obtained from PDB ID: 6EB9, which includes the structural phosphoprotein essential for its structural arrangement.
Cofactors, ligands, and water molecules were eliminated using Molegro Molecular Viewer, and the results were exported in PDB format (Receptor.pdb). Additionally, all other protein chains were removed, except for the one containing the active site. The Autodock 4 tool was used to prepare the target structure by adding polar hydrogens, merging non-polar hydrogens, and assigning Kollman charges.
Ligand preparation
The selected ligands were used for docking against the receptors. Using Molegro Molecular Viewer, the ligand downloaded from the PDB database in SDF format was converted to MOL2 format. Then, the ligand structures were prepared using the Autodock 4 tool by adding polar hydrogens, as well as non-polar hydrogens, and assigning Gasteiger charges. Finally, they were saved in PDBQT format.
Molecular docking
After adding hydrogens and charges to both the receptor and ligand, the search space was defined by creating a grid box around the target active site using AutoGrid and saved in GPF format (Grid_1.gpf). To find the best docking pose, the Genetic algorithm was selected from the search parameters, with 50 runs of the Genetic Algorithm and a population size of 300. This setup allows for the maximum number of binding poses inside the active site. Outputs for docking were set as Lamarkian GA algorithm and saved in DPF format (Dock_1.dpi). After running AutoGrid4.exe and Autodock4.exe in the command prompt, the outputs were saved in the file location, and the docking results were analyzed to identify the best binding pose and binding energy.
Molecular dynamics
Gromacs v.2021 was utilized to simulate the docked structures using CHARMM force fields and the TIP3P water model. The ligand topology was obtained using Swissparam. The system underwent vacuum energy minimization, consisting of 50,000 steps of steepest descent followed by 5000 steps of conjugate gradient. To neutralize the system, physiological concentrations of Na + and Cl- ions were added. The docked structure was then placed in a 10Å unit cell dodecahedral box, and 50,000 steps of energy minimization in solvent were conducted to stabilize the docked structure. The dynamics of the solvated system involved positional constraint, unconstrained simulations, and equilibration (NVT and NPT) at 300 K using a Nose-Hoover thermostat and 1 atmospheric pressure for 1 ns. LINCS was applied to constrain all the bonds, and the particle mesh Ewald method was used to calculate the electrostatics with a 1.4 nm cut-off for Coulomb and Van der Waals interactions. Finally, a 15 ns production run was performed.
The trajectories obtained from simulations were analyzed using the tools provided by GROMACS. PyMOL was employed for visualization and analysis of the stimulation. Built-in programs of GROMACS were utilized for calculating RMSD and RMSF. Commands for Radius of Gyration (RoG) and Solvent Accessible Surface (SAS) were simultaneously executed within GROMACS. The built-in h-bond function was employed to analyze the hydrogen bonds formed throughout the 100 ns trajectory. Plots were generated using Xmgrace, MS Excel, and GraphPad Prism V.9.0.
Results
Antiviral Indian medicinal plants
A word search was conducted for “anti-viral” properties of Indian medicinal plants in the sources of literature review mentioned earlier (Khare 2008; Rao and Ramakrishna 2020; Dhawan 2012). Forty-seven plants with anti-viral properties were identified, resulting in a total of 232 main compounds that were manually searched and referenced from these plants through numerous individual studies. Tabulations of the individual plants, main compounds, and the corresponding references can be found in Supplemental Material Sects. 1 and 2.
Identification of best ligands
Initially, based on the literature review, only the broad-spectrum antiviral compounds were selected for in silico ADME analyses, while others were discarded from the list. A total of 57 main compounds with broad-spectrum antiviral properties were identified.
In silico ADME
The in silico ADME analyses of the main compounds of interest yielded remarkable results, which are subdivided into the following categories:
Cytochrome inhibitors
Out of the 57 main compounds, six compounds were found to inhibit essential cytochromes such as CYP1A2, CYP2D6, and CYP3A4. These compounds are Apigenin, Quercetin, Luteolin, Kaempferol, Lignochalone-A, and Formonoetin, so they have been excluded from the study. The remaining compounds total 51.
Drug likeness and medicinal properties
Among the 51 compounds, 43 do not adhere to drug likeness rules such as Lipinski, Ghose, and Muegge, rendering them non-druggable. These compounds are Ellagitannin, Procyanidin, Azardirachtin, Limonene, Himachalol, Himachalene, Eugenol, β-Sitosterol, γ-Terpinene, α-pinene, p-cymene, Chlorogenic Acid, Dicaffeoylquinic Acid, Caffeic Acid, Cyanroside, Psoralen, β-Caryophyllene, α-Terpinyl acetate, E-Anethole, Eucalyptol, Gallic Acid, Ascorbic Acid, Ellagic Acid, Rutin, β-Mycrene, Lupeol, β-Amyrin, Glucotropaeolin, Spathulenol, Geranial, Neral, Citronellal, Geraniol, Menthol, Pulegone, Menthofuran, Vulgaxanthin, Peganine, Phyllanthin, Shikimic Acid, Salicin, and Borneol. Therefore, these compounds have been eliminated from the study.
The same compounds that exhibited violations in drug likeness properties also showed violations in medicinal chemical properties, particularly in PAINS and Brenk’s law. However, among these compounds, Curzerene, Baicalein, Coriandrin, Camphor, Andrographolide, Pterygospermin, Roseoside, and Salidroside demonstrated good drug likeness and medicinal properties.
In silico toxicology
During the analysis, the compound Curezerene showed respiratory toxicity and carcinogenicity. Baicalein exhibited almost all Tox21 pathway alerts, along with alerts for drug-induced liver injury (DILI), skin sensitization, and eye irritation, as well as breaking toxicophore rules with 11 alerts. Coriandrin triggered positive alerts for DILI, eye irritation, Tox21 pathway, and 5 alerts in toxicophore rules. Camphor gave alerts for carcinogenicity, eye corrosion, eye irritation, and respiratory toxicity, with 3 alerts in toxicophore rules. In contrast, compounds like Andrographolide, Pterygospermin, Roseoside, and Salidroside showed no toxicology alerts and have been proven not to elicit harmful responses in pre-clinical and clinical studies (Benjaponpitak et al. 2023; Ogbu et al., 2019; Aggarwal et al. 2011; Qi et al. 2016). Therefore, they were selected for further study.
Andrographolide’s predicted toxicity falls under Class-4, with an LD50 value of 1890 mg/kg. Pterygospermin, also classified under Class-4, has a predicted LD50 value of 898 mg/kg. Roseoside, categorized in Class-4, shows a predicted LD50 value of 1750 mg/kg. Salidroside’s predicted toxicity is classified as Class-5, with an LD50 value of 5000 mg/kg.
In silico target prediction and pathway map
The Target Prediction results for the selected compounds reveal distinct affinities towards various protein targets. Andrographolide demonstrates 20% affinity towards kinase enzymes, 12% towards proteases, 8% towards other enzymes and oxidoreductases, and 4% towards other protein targets. Pterygospermin exhibits 64% affinity towards G protein-coupled receptors (GPCRs), 16% towards proteases, and 4% towards other protein targets. Roseoside displays 24% affinity towards other enzymes, 20% towards proteases, 16% towards electrochemical transporters, and varying affinity towards other significant proteins ranging from 4 to 12%. Salidroside shows 40% affinity towards lyases, 30% towards hydrolases, 8% towards G protein-coupled receptors (Family A), and 4% towards other significant proteins. Pie charts depicting the affinity distributions are provided in Fig. 2a, b and c, and 2d, while the complete predicted protein lists and their corresponding affinity scores are available in the supplementary file section-c.
Fig. 2.
Ligand Target Prediction Pie Chart, A) Andrographolide, B) Pterygospermin C) Roseoside and D) Salidroside
In Pathway Map with KEGG database mode, the Andrographolide KEGG heat-map highlights its high interaction in the endocrine system, cell growth and death, and signaling and molecular interaction pathways. Pterygospermin’s KEGG heat-map demonstrates interactions with the endocrine system, cell growth, motility, membrane transport, specific types of cancers, cardiovascular diseases, and parasitic infectious diseases. Roseoside shows interactions with cell growth, death, the endocrine system, and cardiovascular disease pathways. Salidroside exhibits very high interaction with cancer pathways, membrane transport, the endocrine system, protein folding, sorting and degradation, cardiovascular diseases, and parasitic infectious diseases. The ligand-pathway interaction heat-maps are provided in Fig. 3a, b and c, and 3d.
Fig. 3.
KEGG Pathway Heat-maps of Ligands, A) Andrographolide, B) Pterygospermin, C) Roseoside and D) Salidroside
Molecular Docking
The docking analyses of the Cedar Virus, Langya Virus, and Nipah Virus targets are as follows:
6P72- Cedar Virus- attachment G- glycoprotein
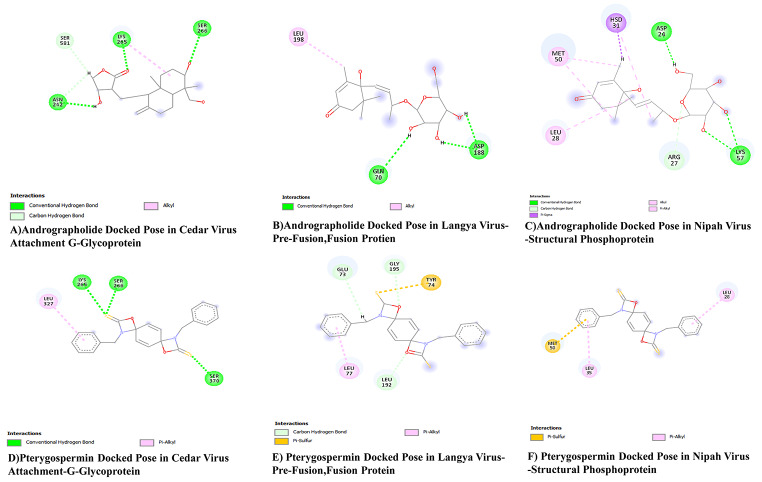
The docking analyses of compounds Andrographolide, Pterygospermin, Roseoside, along with controls Ribavirin and Chloroquine with G Glycoprotein produced negative values of free energies, such as -10.18 kJ/mol, -7.7 kJ/mol, -6.27 kJ/mol, -5.8 kJ/mol, and − 5.13 kJ/mol in their respective grid boxes.
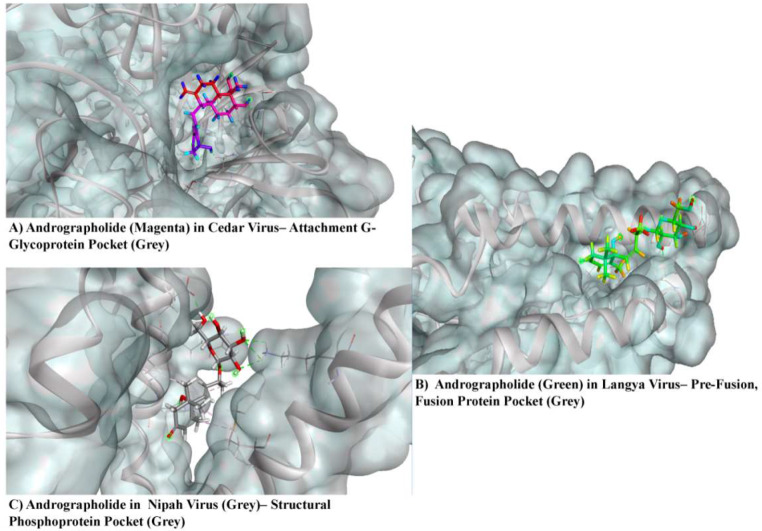
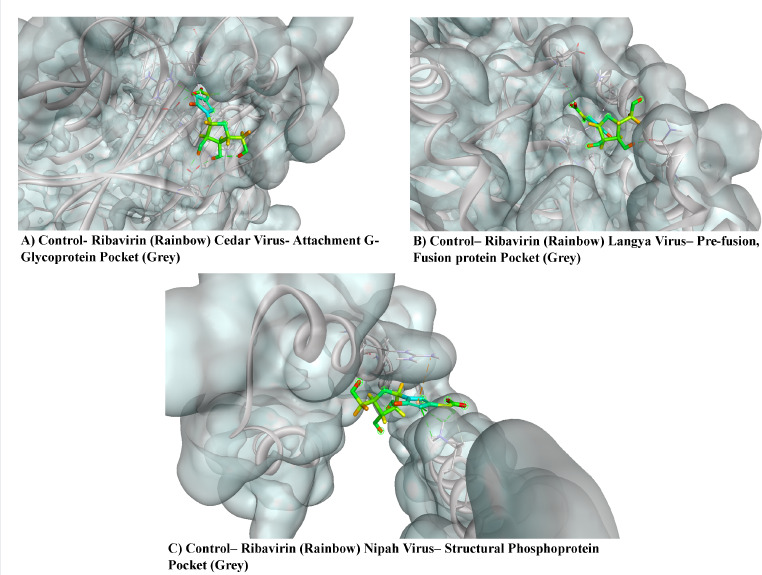
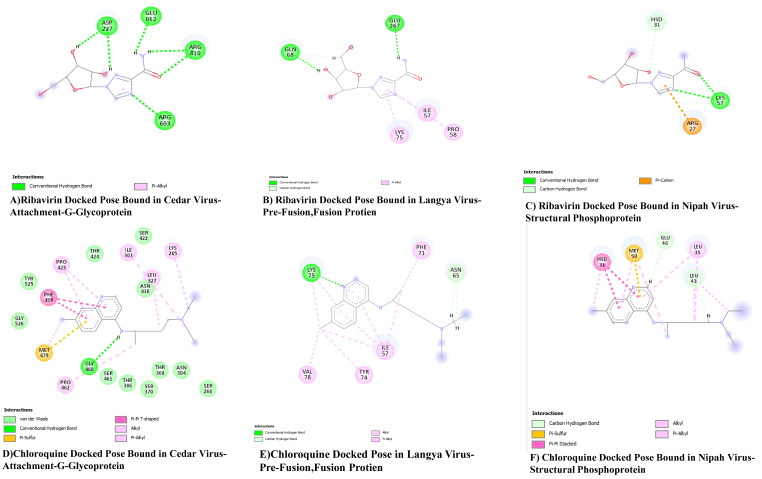
Andrographolide binds to the G glycoprotein of Cedar Virus via three hydrogen bonds with amino acid residues such as Asn 242, Lys 265, and Ser 266. Pterygospermin links to the G glycoprotein of Cedar Virus through three hydrogen bonds and a pi-alkyl bond, involving amino acid residues like Lys 265, Ser 266, Ser 370, and Leu 327. Roseoside binds to the G glycoprotein of Cedar Virus through four hydrogen bonds with amino acid residues Asn 304, Gly 460, Thr 324, and Asp 328. The control ligands Ribavirin and Chloroquine attach to the G glycoprotein of Cedar Virus through six hydrogen bonds with amino acid residues such as Asp 227, Gly 612, Arg 610, and Arg 603. Chloroquine binds to the G glycoprotein of Cedar Virus via one hydrogen bond with amino acid residue Gly 460.
8FEJ- Langya Virus- Pre-fusion Fusion protein
The docking of compounds such as Andrographolide, Pterygospermin, Salidroside, along with controls Ribavirin and Chloroquine to the Pre-Fusion and Fusion protein of Langya Virus resulted in negative values of free energies, such as -9.82 kJ/mol, -6.97 kJ/mol, -5.22 kJ/mol, -5.8 kJ/mol, and − 5.13 kJ/mol in their respective grid boxes.
Langya Pre-fusion and Fusion protein bind to Andrographolide via three hydrogen bonds with amino acid residues Gln 70 and Asp 188. The same targets bind to Pterygospermin with three Carbon-Hydrogen bonds with amino acid residues Leu 192, Gly 195, and Glu 73, 1 Pi-Alkyl bond with amino acid residue Leu 77, and 1 Pi-Sulphur bond with amino acid residue Tyr 74. Salidroside binds to the same targets via three hydrogen bonds between the ligand and the amino acid residues Glu 73 and Asp 188, along with a pi-alkyl bond formed between the ligand and the residue Asp 188.
The control ligand Ribavirin binds to amino acid residues Gln 68 and Glu 267 via two hydrogen bonds, and three pi-Alkyl linkages between amino acid residues Lys 75, Ile 57, and Pro 58 with the ligand. Chloroquine attaches to the target protein via two hydrogen bonds with Lys 75 and Asn 65, as well as through 8 pi-alkyl and alkyl linkages of amino acid residues Val 78, Tyr 74, Ile 57, and Phe 71.
6EB9- Nipah Virus- Structural Phosphoprotein
The docking of compounds Andrographolide, Pterygospermin, along with the controls Ribavirin and Chloroquine to the structural phosphoprotein of the Nipah Virus resulted in negative values of free energies, such as -8.17 kJ/mol, -6.38 kJ/mol, -5.94 kJ/mol, and − 5.36 kJ/mol in their respective grid boxes.
The structural phosphoprotein of the Nipah virus bound to Andrographolide via four hydrogen bonds, one pi-sigma linkage, two alkyl linkages, and one pi-alkyl linkage. The hydrogen bonds were linked to amino acid residues such as Asp24, Arg27, and Lys57, with a pi-sigma linkage formed from amino acid residue Hsd31, alkyl linkages from Met50, Leu28, and a pi-alkyl linkage from Hsd31. Pterygospermin binds to the structural phosphoprotein of the Nipah virus through one pi-sulphur and two pi-alkyl linkages, with the pi-sulphur linkage formed through amino acid residue Met50 and the pi-alkyl linkages through amino acid residues Leu28 and Leu35.
The control Ribavirin binds to the structural phosphoprotein through three hydrogen bonds and one pi-cation linkage, with hydrogen bonds linked to the ligand via amino acid residues Lys57, Hsd31, and a pi-cation linkage formed from the amino acid residue Arg27. Chloroquine is bound to the target via two hydrogen bonds, one pi-sulphur linkage, two pi-pi linkages, five alkyl linkages, and one pi-alkyl linkage, with the bonds and linkages formed by the amino acid residues Glu46, Leu43, Hsd31, Met50, and Leu35.
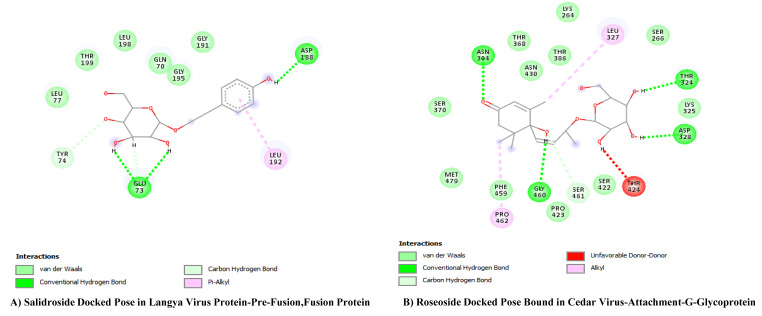
In the final docked compounds, some ligands were left out from the picture due to their equal or very low negative Gibbs energy compared to the control ligands Ribavirin and Chloroquine. In the case of the Cedar virus G Glycoprotein, Salidroside was left out due to its negative energy of -5.44 kJ/mol. Similarly, in the Langya Pre-fusion and Fusion protein, Roseoside was left out because of its low negative energy of -6.6 kJ/mol. Additionally, in the Nipah virus structural phosphoprotein, both Roseoside and Salidroside were excluded for the same reason mentioned earlier, with negative energies of -4.54 kJ/mol and − 4.18 kJ/mol respectively.
Fig. 4.
(A) Salidroside (Rainbow) bound to Langya Virus- Pre-Fusion, Fusion Protein Pocket (Grey). (B) Roseoside (Rainbow) bound to Cedar Virus G- Glycoprotein Pocket (Grey)
Fig. 5.
Andrographolide Bound Target Proteins
Fig. 6.
Pterygospermin Bound with Target Proteins
Fig. 7.
Ribavirin Bound with Target Proteins
Fig. 8.
Chroroquine Bound with Target Proteins
Fig. 9.
Docked Poses of Andrographolide and Pterygospermin with Target Proteins
Fig. 10.
Docked Poses of Controls- Ribavirin and Chloroquine with Target Proteins
Fig. 11.
Salidroside and Roseoside Docked Poses with Their Respective Targets
Molecular Dynamics
The docking analyses of the Cedar Virus, Langya Virus, and Nipah Virus targets are as follows:
6P72- Cedar Virus- attachment G- glycoprotein
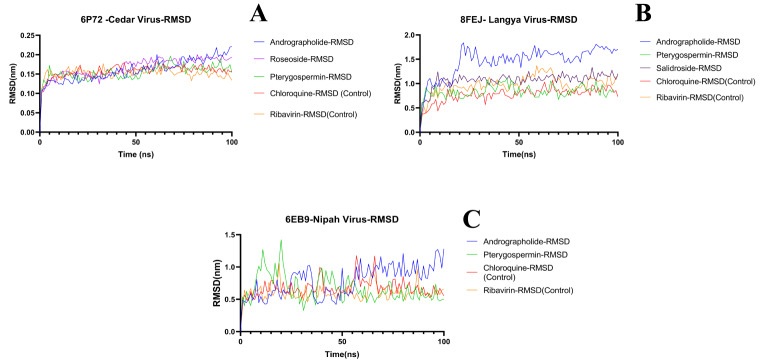
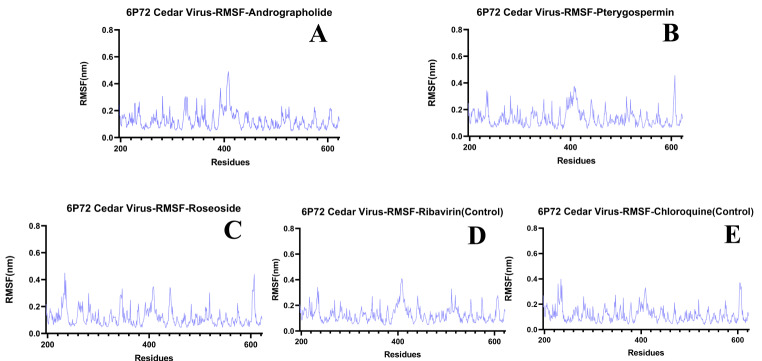
The RMSD values for Andrographolide, Pterygospermin, Roseoside, and the controls Ribavirin and Chloroquine are 0.20 nm, 0.15 nm, 0.10 nm, 0.15 nm, and 0.15 nm respectively. In terms of RMSF values, these ligands docked to the target protein exhibited values of 0.40 nm, 0.43 nm, 0.43 nm, 0.42 nm, and 0.40 nm respectively. Hydrogen bonds (HBs) formed during the 100ns duration between the ligands and the protein target varied, with Andrographolide forming 1–6 HBs, no HB for Pterygospermin, 0–5 HBs for Roseoside, 0–6 HBs for Ribavirin, and 0–2 HBs for Chloroquine.
In the Radius of Gyration (RoG) and Solvent Accessible Surface (SAS) analyses, Andrographolide exhibited RoG ranging from 2.12 nm to 2.18 nm and SAS ranging from 189 nm to 205 nm. Pterygospermin showed RoG with a range of 2.12 nm to 2.14 nm and SAS ranging from 189 nm to 195 nm. Roseoside displayed a RoG range of 2.12 nm to 2.14 nm and SAS ranging from 189 nm to 195 nm. Chloroquine had a RoG range of 2.12 nm to 2.14 nm and SAS values ranging from 189 nm to 195 nm. Ribavirin expressed RoG in the range of 2.12 nm to 2.14 nm and SAS values within the range of 189 nm to 190 nm.
8FEJ- Langya Virus- Pre-fusion and Fusion protein
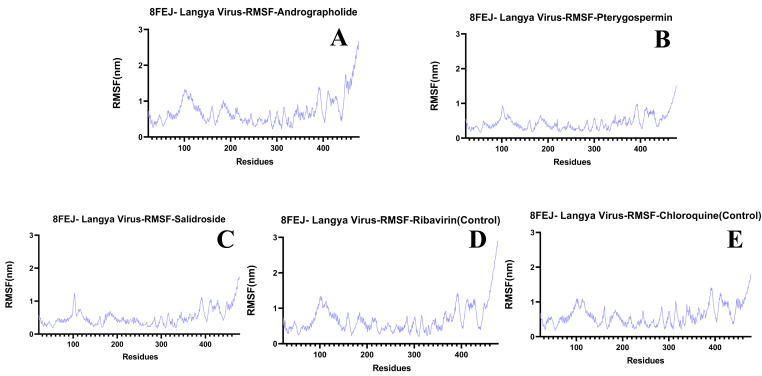
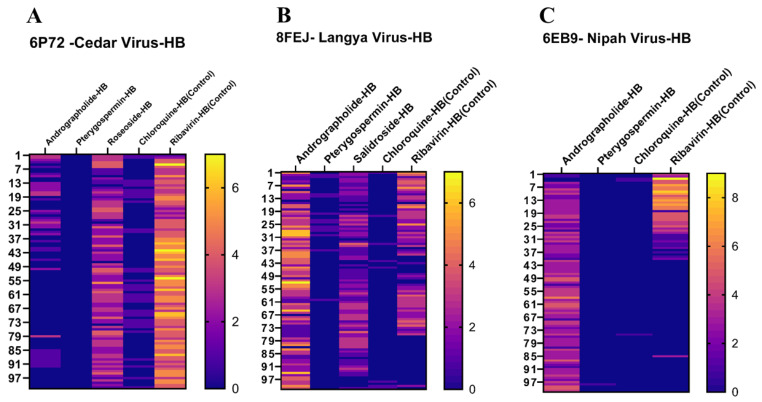
The RMSD values for the selected ligands Andrographolide, Pterygospermin, Salidroside, and controls Ribavirin and Chloroquine are 1.55 nm, 1.0 nm, 1.0 nm, and for controls 0.8 nm, 1.0 nm respectively, indicating stability over the 100ns simulation run (Fig. 12B). Regarding RMSF values when bound to the target protein of Langya virus, these ligands exhibited values of 1.4 nm, 1.0 nm, 1.4 nm, and for controls 1.4 nm and 1.4 nm (Fig. 14A, B, C and D, and 14E) respectively. The Hydrogen bond formations of these ligands ranged from 0 to 6, 0–2, 0–4, 0–2, and 0–5 respectively (Fig. 16B).
Fig. 12.
RMSD Values of Ligands Docked with Target Proteins. A) Cedar Virus (PDB: 6P72), B) Langya Virus (PDB: 8FEJ) and C) Nipah Virus (PDB: 6EB9)
Fig. 14.
RMSF Graphs of Ligands Bound to the Langya Virus Receptor- 8FEJ. A) Andrographolide, B) Pterygospermin, C) Salidroside D) Ribavirin (Control) and E) Chloroquine (Control)
Fig. 16.
Hydrogen Bond Formation throughout the 100 ns simulation Between Ligands and Target Proteins represented by heat map. A) Hydrogen bonds formed between Cedar Viral Target 6P72 with specific Ligands, B) Hydrogen bonds formed between Langya Virus Target 8FEJ and specific Ligands and C) Hydrogen bonds formed between Nipah Virus Target 6E89 and specific Ligands
Regarding RoG and SAS in the protein-ligand complexes, the results are as follows for each ligand: Andrographolide ranged from 3.3 nm to 3.6 nm in RoG, and in SAS they ranged from 275 nm to 260 nm. Pterygospermin ranged from 3.2 nm to 3.4 nm, with SAS ranging from 275 nm to 264 nm. Salidroside exhibited a range from 3.4 nm to 3.1 nm in RoG analysis, and in SAS, they were within the range of 275 nm to 255 nm. Chloroquine expressed RoG activity within the range of 3.3 nm to 3.1 nm, with SAS ranging from 276 nm to 269 nm. Ribavirin showed RoG activity within the range of 3.3 nm to 3.0 nm, with SAS activity ranging from 275 nm to 255 nm.
6EB9- Nipah Virus- Structural Phosphoprotein
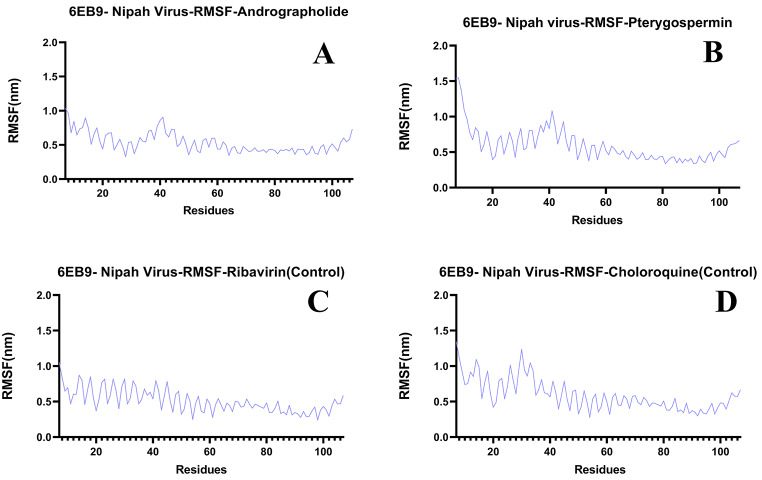
The selected ligands Andrographolide, Pterygospermin, and controls Ribavirin, Chloroquine, docked with the target protein of the Nipah virus, exhibited RMSD values of 1.3 nm, 1.4 nm, 1.2 nm, and 1.0 nm, respectively, and remained stable throughout the 100 ns simulation run (Fig. 12C). Individually, these ligands showed RMSF values of 0.8 nm, 1.1 nm, 0.9 nm, and 1.3 nm, respectively (Fig. 15A, B and C, and 15D). The Hydrogen bond formations of these ligands ranged from 0 to 7, 0–1, 0–1, and 0–8, respectively (Fig. 16C).
Fig. 15.
RSMF Graphs of Ligands Bound to Nipah Virus Target Proteins. A) Andrographolide, B) Pterygospermin, C) Salidroside D) Ribavirin (Control) and E) Chloroquine (Control)
When subjected to RoG analysis with the docked protein, Andrographolide remained within the range of 3.0 nm to 2.6 nm, while SAS analysis of the docked protein remained within the range of 100 nm to 94 nm. Pterygospermin exhibited RoG analysis within the range of 3.4 nm to 2.6 nm, and SAS analysis ranged from 105 nm to 96 nm. Chloroquine displayed RoG analysis ranging from 3.0 nm to 2.5 nm, while SAS analysis remained within the range of 104 nm to 94 nm. Ribavirin, when subjected to RoG analysis, ranged from 3.0 nm to 2.6 nm, and in SAS analysis, it ranged from 104 nm to 89 nm. These results are illustrated in Figs. 17C and 18C.
Fig. 17.
Radius of Gyration (RoG) observed between Protein Targets and their respective ligands. A) Cedar Virus- RoG, B) Langya Virus- RoG and C) Nipah Virus- RoG
Fig. 18.
Solvent Accessible Surface (SAS) of the protein docked with ligands of interest. A) Cedar Virus- SAS, B) Langya Virus- SAS and C) Nipah Virus- SAS.
Fig. 13.
RMSF Graphs of Ligands Docked with Cedar Virus Glycoprotein. A) Andrographolide, B) Pterygospermin, C) Roseoside, D) Ribavirin- Control and E) Chloroquine- Control
Discussion
Since the COVID-19 pandemic in 2020 brought the world to a standstill, concerns about emerging diseases caused by viruses have escalated, affecting many individuals mentally (Mahmood et al., 2023; Porcelli, 2020; Bakioğlu et al. 2021). Most zoonotic viruses, particularly RNA viruses, pose a significant challenge as they can develop antiviral resistance more rapidly than DNA viruses, potentially leading to global pandemics (Villa et al. 2021). The absence of approved therapeutics or vaccines for Henipaviruses adds to the urgency, especially considering the increasing risk of mutations. Notably, viruses like the Nipah Virus, due to mutations in their viral attachment and fusion proteins, are emerging threats alongside similar henipa-like viruses due to genetic similarities (Yuan et al. 2011; Li et al. 2023). With mortality rates ranging from 50 to 100% and transmission primarily from animals such as pigs, horses, and bats to humans, Henipaviruses are classified as biosafety level 4 organisms, posing a significant global threat (Marsh and Wang 2012; Esposito et al. 2023; Meadows et al. 2023; Kummer and Kranz 2022). They remain a priority on the WHO’s primary diseases list for research and development, aligning with the goals of the Coalition for Epidemic Preparedness and Innovations (CEPI). Given these reasons, there is a critical need for investigations into therapeutics and vaccine development strategies (source). Although drugs like ribavirin and chloroquine have been proposed for Henipaviruses, their side effects underscore the necessity for research into safer alternatives.
While analyzing the results of the study, it became evident that the compounds Andrographolide, Pterygospermin, Roseoside, and Salidroside exhibited exceptional binding quality with the target proteins. These compounds possess excellent small-molecular structures and show varying degrees of solubility. While Andrographolide and Pterygospermin demonstrate moderate solubility, they are soluble in organic solvents. On the other hand, Roseoside and Salidroside exhibit water-soluble properties.
In terms of target prediction, these compounds show high affinity for binding with specific proteins. Andrographolide, Pterygospermin, Roseoside, and Salidroside exhibit the highest affinity for binding with the Cedar Virus Attachment G Glycoprotein, particularly targeting Family A-G protein-coupled receptors. Similarly, these compounds also show affinity for other proteins such as the Langya Virus Pre-Fusion and Fusion proteins, as well as the structural phosphoprotein of the Nipah virus. Specifically, Andrographolide shows an affinity of 16% for the Nipah virus target, while Pterygospermin, Roseoside, and Salidroside show affinities of 8%, 4%, and 4%, respectively. The KEGG Pathway analysis interpretation indicates that Andrographolide can interact with the endocrine system, Pterygospermin with membrane transport, and other essential pathways. Similarly, Roseoside shows interaction with pathways related to cell growth and death, the endocrine system, and cardiovascular diseases, while Salidroside interacts with pathways related to cancer cells, membrane transport, and signaling molecules.
In the protein target of Cedar Virus (PDB: 6P72), Andrographolide stands out with superior properties compared to other ligands of interest. In comparison to the controls Ribavirin and Chloroquine, Pterygospermin exhibits similar properties in most analyses, except for hydrogen bond formation. Andrographolide shows better performance in terms of hydrogen bonding and stability. Conversely, Roseoside and Pterygospermin were excluded from consideration due to their lack of hydrogen bond formation to support the complex. Andrographolide emerges as a potential candidate for treating diseases caused by Cedar Virus. Moving to the protein target of Langya Virus (PDB: 8FEJ), Salidroside demonstrates excellent properties comparable to the controls. From RMSD to SAS analyses, Salidroside outperforms others, making it a promising option for treating Langya viral infections. For the protein target of Nipah Virus (PDB: 6EB9), Pterygospermin emerges as a suitable candidate due to its excellent properties across all analyses and stability throughout the simulation.
Regarding toxicity, Andrographolide shows no toxicity even at the highest doses, with no deaths or symptoms of clinical-grade toxicity observed, even in non-immune patients during the COVID-19 pandemic (Al Batran et al. 2013; Benjaponpitak et al. 2023). Pterygospermin exhibits low and non-lethal toxic effects in animal studies against bacterial infections and has shown no toxicity in studies with plant extracts containing pterygospermin (Luqman et al. 2012; Rao and Natarajan 1949). Salidroside has been found to have low toxicity with consistent efficacy in its respective mechanisms of action. These compounds may inhibit integral proteins vital for the life cycles of henipaviruses, suggesting their potential as therapeutic agents (Xie et al. 2020; Zhang et al. 2021).
These molecules not only demonstrate prophylactic properties but can also be utilized as preventive medicinal drugs. For example, Andrographolide, Pterygospermin, and Salidroside have been studied for their immunostimulatory effects (Mishra et al. 2021; Pareek et al. 2023; Recio et al. 2016). Considering their solubility properties, formulations combining these compounds or individual ones can be developed to address various stages of henipavirus infection, regardless of whether they are in primitive or advanced stages. Andrographolide and Pterygospermin, being partially soluble in water and organic solvents, can be formulated into inhalable therapeutics to target the initial viral load in the lungs. Salidroside, on the other hand, can be administered orally to aid in treating the viral infection.
While these results appear promising and may prove to be effective against henipaviruses, further investigation through in vitro studies is necessary. However, conducting such studies poses challenges due to the need for biosafety level four labs and the scarcity of viral stocks in culture. Nevertheless, conducting in vitro studies could potentially reduce the infection rate and transmission of henipaviruses, especially in tropical regions like Southeast Asia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to express their sincere gratitude to the dearest best friend of the first author JC for his inspiration and timely inputs on this subject matter. And special gratitude to the Gilberts, Sharon and Derek as well as the author’s parents Mr. Enmozhi and Mrs. Vasugi Enmozhi.
Author contributions
S.K.E- Study Plan, Images Processing and Manuscript WritingI.X, T.R and S.R- In silico Toxicity Analysis, Manuscript WritingJ.B- Molecular DockingY.K- Manuscript ProofreadingR.V- Molecular DynamicsK.R and S.A- Manuscript Proofreading, Supplementary PreparationJ.J and A.R- Study Counsellors and Manuscript Proofreaders.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aggarwal BB, Prasad S, Reuter S, Kannappan R, Yadev VR, Park B, Kim JH, Gupta SC, Phromnoi K, Sundaram C, Prasad S, Chaturvedi MM, Sung B (2011) Identification of novel anti-inflammatory agents from ayurvedic medicine for prevention of chronic diseases: reverse pharmacology and bedside to bench approach. Curr Drug Targets 12(11):1595–1653. 10.2174/138945011798109464 10.2174/138945011798109464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Batran R, Al-Bayaty F, Al-Obaidi MM, Abdulla MA (2013) Acute toxicity and the effect of andrographolide on Porphyromonas gingivalis-induced hyperlipidemia in rats. Biomed Res Int 2013:594012. 10.1155/2013/594012 10.1155/2013/594012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakioğlu F, Korkmaz O, Ercan H (2021) Fear of COVID-19 and positivity: mediating role of intolerance of uncertainty, Depression, anxiety, and stress. Int J Mental Health Addict 19(6):2369–2382. 10.1007/s11469-020-00331-y 10.1007/s11469-020-00331-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjaponpitak A, Sawaengtham T, Thaneerat T, Wanaratna K (2023) Effect of Andrographis paniculata treatment for Nonimmune patients with early-stage COVID-19 on the prevention of pneumonia: a retrospective cohort study. Archives Intern Med Res 06(02). 10.26502/aimr.0146
- Bonaparte MI, Dimitrov AS, Bossart KN, Crameri G, Mungall BA, Bishop KA, Choudhry V, Dimitrov DS, Wang LF, Eaton BT, Broder CC (2005) Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc Natl Acad Sci USA 102(30):10652–10657. 10.1073/pnas.0504887102 10.1073/pnas.0504887102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder CC, Wong KT (2016) Henipaviruses. Neurotropic Viral Infections: Volume 1: Neurotropic RNA Viruses, 45–83. 10.1007/978-3-319-33133-1_3
- Chaachouay N, Zidane L (2024) Plant-derived natural products: a source for drug discovery and development. Drugs Drug Candidates 3(1):184–207. 10.3390/ddc301001 10.3390/ddc301001 [DOI] [Google Scholar]
- Ciak J, Hahn FE (1966) Chloroquine: mode of action, vol 151. Science (New York, pp 347–349. 370810.1126/science.151.3708.347 [DOI] [PubMed]
- Conroy G (2023) Nipah virus outbreak: what scientists know so far. Nature. 10.1038/d41586-023-02967-x 10.1038/d41586-023-02967-x [DOI] [PubMed] [Google Scholar]
- Dhawan BN (2012) Anti-viral activity of Indian plants. Proc Natl Acad Sci India Sect B 82(1):209–224. 10.1007/s40011-011-0016-7 10.1007/s40011-011-0016-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-. Chloroquine. [Updated 2021 Aug 16]. https://www.ncbi.nlm.nih.gov/books/NBK501146/
- Esposito MM, Turku S, Lehrfield L, Shoman A (2023) The impact of human activities on zoonotic infection transmissions. Animals: Open Access J MDPI 13(10):1646. 10.3390/ani13101646 10.3390/ani13101646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graci JD, Cameron CE (2006) Mechanisms of action of Ribavirin against distinct viruses. Rev Med Virol 16(1):37–48. 10.1002/rmv.483 10.1002/rmv.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin K, Rota P (2014) A review of Hendra Virus and Nipah Virus infections in Man and other animals. Zoonoses - Infections Affecting Hum Animals: Focus Public Health Aspects 997–1012. 10.1007/978-94-017-9457-2_40
- Johnson K, Vu M, Freiberg AN (2021) Recent advances in combating Nipah virus. Fac Reviews 10:74. 10.12703/r/10-74 10.12703/r/10-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare C (2008) Indian Medicinal plants: an Illustrated Dictionary. Springer Science & Business Media
- Kummer S, Kranz DC (2022) Henipaviruses-A constant threat to livestock and humans. PLoS Negl Trop Dis 16(2):e0010157. 10.1371/journal.pntd.0010157 10.1371/journal.pntd.0010157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kim JV, Pickering BS (2023) Henipavirus zoonosis: outbreaks, animal hosts and potential new emergence. Front Microbiol 14:1167085. 10.3389/fmicb.2023.1167085 10.3389/fmicb.2023.1167085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqman S, Srivastava S, Kumar R, Maurya AK, Chanda D (2012) Experimental Assessment of Moringa oleifera Leaf and Fruit for Its Antistress, Antioxidant, and Scavenging Potential Using In Vitro and In Vivo Assays. Evidence-based complementary and alternative medicine: eCAM, 2012, 519084. 10.1155/2012/519084 [DOI] [PMC free article] [PubMed]
- Marsh GA, Wang LF (2012) Hendra and Nipah viruses: why are they so deadly? Curr Opin Virol 2(3):242–247. 10.1016/j.coviro.2012.03.006 10.1016/j.coviro.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Meadows AJ, Stephenson N, Madhav NK, Oppenheim B (2023) Historical trends demonstrate a pattern of increasingly frequent and severe spillover events of high-consequence zoonotic viruses. BMJ Global Health 8(11):e012026. 10.1136/bmjgh-2023-012026 10.1136/bmjgh-2023-012026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Shaik HA, Sinha RK, Shah BR (2021) Andrographolide: A Herbal-Chemosynthetic Approach for enhancing immunity, combating viral infections, and its implication on Human Health. Molecules 26(22):7036. 10.3390/molecules26227036 10.3390/molecules26227036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar M, Arshad M, Ahmad M, Pomerantz RJ, Wigdahl B, Parveen Z (2008) Antiviral potentials of medicinal plants. Virus Res 131(2):111–120. 10.1016/j.virusres.2007.09.008 10.1016/j.virusres.2007.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbu OS, Zigabelbari ZV (2019) The immuno-modulatory and thrombocytopaenic effects of the varying concentrations of the aqueous leaf extract of moringa Oleifera in male albino Wistar rats. J Adv Med Pharm Sci 1–8. 10.9734/jamps/2019/v21i430141
- Pareek A, Pant M, Gupta MM, Kashania P, Ratan Y, Jain V, Pareek A, Chuturgoon AA (2023) Moringa oleifera: an updated Comprehensive Review of its pharmacological activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological aspects. Int J Mol Sci 24(3):2098. 10.3390/ijms24032098 10.3390/ijms24032098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Qi S, Ling L, Lv J, Feng Z (2016) Salidroside attenuates inflammatory response via suppressing JAK2-STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int Immunopharmacol 35:265–271. 10.1016/j.intimp.2016.04.004 10.1016/j.intimp.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Quarleri J, Galvan V, Delpino MV (2022) Henipaviruses: an expanding global public health concern? GeroScience 44(5):2447–2459. 10.1007/s11357-022-00670-9 10.1007/s11357-022-00670-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Natarajan S (1949) Toxicity of pterygospermin and allicin. Proc / Indian Acad Sci 29(4):148–154. 10.1007/bf03049967 10.1007/bf03049967 [DOI] [Google Scholar]
- Rao S, Ramakrishna A (2020) Indian Medicinal plants: uses and propagation aspects. CRC
- Recio MC, Giner RM, Máñez S (2016) Immunmodulatory and Antiproliferative Properties of Rhodiola Species. Planta Med 82(11–12):952–960. 10.1055/s-0042-107254 10.1055/s-0042-107254 [DOI] [PubMed] [Google Scholar]
- Russmann S, Grattagliano I, Portincasa P, Palmieri VO, Palasciano G (2006) Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr Med Chem 13(27):3351–3357. 10.2174/092986706778773059 10.2174/092986706778773059 [DOI] [PubMed] [Google Scholar]
- Sharma R, Bhattu M, Tripathi A, Verma M, Acevedo R, Kumar P, Rajput VD, Singh J (2023) Potential medicinal plants to combat viral infections: a way forward to environmental biotechnology. Environ Res 227:115725. 10.1016/j.envres.2023.115725 10.1016/j.envres.2023.115725 [DOI] [PubMed] [Google Scholar]
- Sinclair SM, Jones JK, Miller RK, Greene MF, Kwo PY, Maddrey WC (2017) The ribavirin pregnancy Registry: an interim analysis of potential teratogenicity at the mid-point of enrollment. Drug Saf 40(12):1205–1218. 10.1007/s40264-017-0566-6 10.1007/s40264-017-0566-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa TG, Abril AG, Sánchez S, de Miguel T, Sánchez-Pérez A (2021) Animal and human RNA viruses: genetic variability and ability to overcome vaccines. Arch Microbiol 203(2):443–464. 10.1007/s00203-020-02040-5 10.1007/s00203-020-02040-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Shen C, Jiang J (2020) The sources of salidroside and its targeting for multiple chronic diseases. J Funct Foods 64:103648. 10.1016/j.jff.2019.103648 10.1016/j.jff.2019.103648 [DOI] [Google Scholar]
- Yuan J, Marsh G, Khetawat D, Broder CC, Wang LF, Shi Z (2011) Mutations in the G-H loop region of ephrin-B2 can enhance Nipah virus binding and infection. J Gen Virol 92(Pt 92142–2152. 10.1099/vir.0.033787-0 [DOI] [PMC free article] [PubMed]
- Zhang X, Xie L, Long J, Xie Q, Zheng Y, Liu K, Li X (2021) Salidroside: a review of its recent advances in synthetic pathways and pharmacological properties. Chemico-Biol Interact 339:109268. 10.1016/j.cbi.2020.109268 10.1016/j.cbi.2020.109268 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.