Abstract
The paper investigated the possibility of extractive separation of palladium from platinum and rhodium with ionic liquid Cyphos IL 101. A technological solution obtained by dissolving waste materials was used as the test material. Based on the experiments performed, it was found that a 10% (v/v) solution of the Cyphos IL 101 ionic liquid in toluene allows the extraction of both Pd and Pt with an efficiency of 99% from the initial solution when extraction is carried out at the pH 0.5, vorg:vaq phase ratio 1:1 and contact time of 15 min. Moreover, the research proved that it is possible to separate Pd from Pt at the stripping stage using a 0.1 mol/dm3 thiourea solution while maintaining a high selectivity coefficient.
Keywords: Platinum group metals, Solvent extraction, Separation of PGMs, Palladium recovery
Subject terms: Chemical engineering, Environmental chemistry, Chemical engineering
Introduction
Platinum group metals (PGMs) constitute a group of metals with low abundance in the Earth’s crust1–3 and high market value4,5. Due to their exceptional properties such as corrosion and oxidation resistance6, good thermal and electrical conductivity, as well as high chemical and temperature resistance, this group has found applications in various industries, with the automotive7–9, chemical10–12, and petroleum industries13–15 being their main recipients. The similarity in properties and high chemical resistance of PGMs means that they can be obtained in their pure state only using hydrometallurgical methods16,17. Among the available methods, solvent extraction has gained particular importance over the years and is currently the main worldwide method for PGM separation in refinery processes, such as Anglo Platinum Corporation, Rand Refinery in South Africa, and Johnson Matthey in England7,18,19. Furthermore, there are many effective PGM extractants20,21, among which ionic liquids are gaining particular popularity. The growing trend towards environmental protection has led to an increasing use of ionic liquids, which are considered to be “green solvents”22,23. Their favourable properties, such as non-flammability24, low volatility25,26, ability to dissolve many substances27, and possibility of regeneration22,23,28, have allowed their application in various processes, including organic synthesis, drug production, catalytic reactions, liquid chromatography, and electrochemistry. The popularity and versatility of such chemical compounds results from their diverse structures and availability on the market29,30. One particularly popular and highly effective extractant in liquid–liquid extraction processes is the ionic liquid trihexyl(tetradecyl)phosphonium chloride, commercially known as Cyphos IL 101 (Fig. 1).
Figure 1.
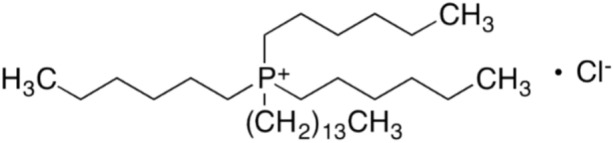
The structure of trihexyl(tetradecyl)phosphonium chloride (Cyphos IL 101).
Numerous scientific studies indicate that this compound can be a highly efficient extractant for Pd and Pt from chloride solutions. For example, in studies conducted by Nguyen et al., Pd and Pt were extracted from an acidic chloride solution with the organic phase of the ionic liquid Cyphos IL 101 diluted with xylene. The obtained extraction efficiencies of both these metals indicated the quantitative recovery of the tested platinum group metals in relation to Rh, with the initial concentrations of Pt, Pd and Rh in the solution being 100 mg/dm3, 55 mg/dm3 and 25 mg/dm3, respectively31.
Svecova et al. obtained 89.7–99.9% Pd extraction efficiency from acidic solutions with various hydrochloric acid contents. The authors also described the dependence of extraction efficiency and selectivity on the type of anion associated with the trihexyl(tetradecyl)phosphonium cation27.
In other studies, conducted by Cieszyńska et al., Pd was extracted with an efficiency of 97% from a synthetic 0.1 mol/dm3 HCl solution. Additionally, the authors proved that the extraction of palladium(II) from a chloride environment with Cyphos IL101 depends on the content of hydrochloric acid and the concentration of added sodium chloride24.
The above-mentioned data indicate the high effectiveness of the Cyphos IL 101 ionic liquid. An example of the extraction mechanism is shown in Fig. 2.
Figure 2.
The proposal for the extraction reaction of platinum and palladium chlorocomplexes using Cyphos IL 101.
Moreover, Cyphos IL 101 is widely available on both the European and global markets. Most current scientific researches using this extractant focuses on the extraction of PGMs from synthetic solutions, which, due to their simple composition and lack of other impurities, are unable to fully replicate the conditions present in technological solutions, which are multi-component systems characterized by high concentrations of other base metals. Additionally, continuous economic development and increasingly stringent environmental protection regulations necessitate the recovery of PGMs from secondary sources12,27,32,33. This study aimed to evaluate the suitability of using the ionic liquid Cyphos IL 101 for the extraction of Pd and Pt from a technological solution. This solution has low concentrations of PGMs and other accompanying metals, such as iron and nickel, and high concentrations of copper.
While the separation of precious metals from base metals does not pose significant difficulties, the selective separation of individual platinum group metals is one of the most demanding challenges in chemical engineering, which results from their high similarity in physicochemical properties and exceptional chemical passivity34. Processing of materials rich in platinum group metals typically begins with a leaching step, which uses acidic solutions with an addition of strong oxidants to effectively transfer all the valuable metals into solution32,33,35. Selective separation of individual metals is carried out using solvent extraction, ion exchange or selective precipitation36. In the case of PGMs, hydrochloric acid turns out to be the most effective and economical leaching agent. Its popularity is due not only to its availability and low cost but also the fact that the chemistry of platinum group chlorocomplexes is well researched, which significantly facilitates the later stages of refining (Table 1)19. Factors such as solution pH, redox potential, chloride ion concentration and the phenomenon known as “aging” of solutions are of key importance for the behaviour and properties of PGMs chlorocomplexes6,19,26. The latter is particularly important in solutions with low acidity and low chloride concentration, where aquacomplexes may be formed. Scientific research has shown that the type of formed PGM chlorocomplex has a significant impact on the efficiency of selective extraction and ion exchange processes19. This is due to differences in the molecular geometry and electric charge distribution of these complexes, which translates into their different reactivity and kinetics in an aqueous environment. These factors therefore have a significant impact on the efficiency and selectivity of PGM extraction6,19.
Table 1.
The PGMs’ chlorocomplexes.
| Ru3+ | [RuCl6]3− | Rh3+ | [RhCl6]3− | Pd2+ | [PdCl4]2− |
| [RuCl5(H2O)]2− | [RhCl5(H2O)]2− | ||||
| [RuCl4(H2O)2]− | Pd4+ | [PdCl6]2− | |||
| [RuCl3(H2O)3] | Ir3+ | [IrCl6]3− | |||
| Ru4+ | [RuCl6]2− | [IrCl5(H2O)]2− | Pt2+ | [PtCl4]2− | |
| [Ru2OCl10]4− | |||||
| [Ru2OCl8(H2O)2]2− | Ir4+ | [IrCl4(H2O)2] | Pt4+ | [PtCl6]2− | |
| Os4+ | [OsCl6]2− | [IrCl6]2− |
Source compiled from Ref.19.
The use of the technological solution, obtained from the processing of waste materials rather than a synthetic solution in the research, allowed for the simulation of the industrial-like conditions and complemented the previously available knowledge in the field of Pd and Pt extraction with the Cyphos IL 101 ionic liquid.
Experimental section
Reagents and materials
The research used a technological solution belonging to the Łukasiewicz Research Network—Institute of Non-Ferrous Metals (Gliwice, Poland), which was obtained as a result of oxidative leaching of waste materials, such as concentrates, used electronic parts, PGM filtration filters, etc. Concentrated hydrochloric acid, with the addition of nitric acid as an oxidizing agent, was used as the leaching medium. The composition of the tested solution is presented in Table 2.
Table 2.
Composition of the technological solution obtained by the quantitative analysis.
| Analyte | Pd | Pt | Rh | Au | Ir | Ag | Cu | Fe |
|---|---|---|---|---|---|---|---|---|
| Concentration [g/dm3] | 1.80 | 2.26 | 0.30 | 9.39 × 10–2 | 8.46 × 10–2 | 5.33 × 10–2 | 16.8 | 0.53 |
| Analyte | Ni | Te | Si | Cr | Sn | Al | NO3− | Cl− |
|---|---|---|---|---|---|---|---|---|
| Concentration [g/dm3] | 0.13 | 0.26 | 0.01 | 0.11 | 0.05 | 0.01 | 1.72 | 73.4 |
Mixtures of pure ionic liquid Cyphos IL 101 (abcr GmbH, Karlsruhe, Germany) in toluene (Chempur, Piekary Śląskie, Poland) were used as the extraction solution. Stripping was carried out using thiourea solutions (Avantor, Gliwice, Poland) of various concentrations.
Analytical techniques
The concentrations of precious metals in the tested solution and raffinates were measured using the inductively coupled plasma optical emission spectrometry (ICP-OES, Agilent 5110 SVDV ICP-OES spectrometer, Agilent Technologies) and the atomic absorption spectrometry techniques (AAS, iCE 3300 AAS spectrometer, Thermo Scientific). The measures were performed at the Łukasiewicz Research Network—Institute of Non-Ferrous Metals, Center of Analytical Chemistry (Gliwice, Poland).
Possible analytical errors and deviations in the calculated extraction and stripping efficiencies may result from the small volumes of samples used in the tests and from the inaccuracies of the laboratory glassware used to measure the volumes of samples before and after extraction or stripping. The observed slight changes in volumes resulted from the inability to quantitatively transfer the liquid to the separatory funnel and then to the measuring cylinder due to the surface tensions of both phases, their viscosity and the adhesion of the liquid to the surface of the laboratory glass, affecting, among others, on the precision and accuracy of the presented analysis results.
Extraction and stripping procedure
The extraction of the platinum group metals was carried out on a laboratory scale at room temperature by contacting specific volumes of the technological solution with a solution of the extractant diluted in toluene. Then the phases were separated using a glass separatory funnel, and the composition of the obtained raffinates was analysed for the content of Pd, Pt and Rh. The obtained results were used to calculate the Pd, Pt and Rh extraction efficiencies, E [%], extraction coefficients, D, and selectivity coefficients, based on the following formulas:
| 1 |
where C0—initial concentration of the platinum group element X in the aqueous phase [g/dm3], V0—initial volume of the aqueous phase [dm3], C1—concentration of the platinum group element X in the aqueous phase after extraction [g/dm3], V1—volume of the aqueous phase after extraction [dm3],
| 2 |
where CX org—concentration of the platinum group element X in the organic phase after extraction [g/dm3], CX aq—concentration of the platinum group element X in the aqueous phase after extraction [g/dm3],
| 3 |
where DI—distribution ratio of the platinum element I, DII—distribution ratio of the platinum element II.
In the first stage of the study, the effect of the pH on the efficiency of PGM extraction was examined. For this purpose, 50% (v/v) solutions of the extractant Cyphos IL 101 were contacted with the equal volumes of the aqueous phase (20 cm3) at the selected pH values and vigorously mixed using a magnetic stirrer for 30 min. The pH of the initial solution, which was 0, was adjusted by adding an appropriate amount of solid NaOH until the pH was in the range of 0–1.5.
The study of the effect of the concentration of the extractant in the organic phase included the preparation of a series of extraction solutions with different volume concentrations of the extracting agent 1–50% (v/v) (Cyphos IL 101) in toluene. Equal volumes of the aqueous and organic phases (20 cm3 each) were stirred intensively using a magnetic stirrer for 30 min.
The influence of the phase ratio on the extraction efficiency was tested by contacting different volumes of the aqueous and organic phases, maintaining the ratio of organic to aqueous phases of 1:1–1:5. As in previous studies, the contact time was 30 min and the pH of aqueous phase 0.5.
The minimum contact time allowing to achieve the highest possible Pd extraction efficiencies was tested by mixing the aqueous phase with the organic phase in time intervals ranging from 1 to 30 min, maintaining the ratio of organic to aqueous phases of 1:1 and the pH of aqueous phase 0.5.
A similar research procedure was adopted for stripping tests, where the stripping effectiveness of the platinum group metals from the organic phase was checked using aqueous thiourea solutions. The stripping agent was selected based on a review of the scientific literature and after preliminary tests, which confirmed the high effectiveness of this solution in the process of stripping of Pd and Pt.
The influence of thiourea concentration on the stripping efficiency was investigated in the concentrations range from 0.1 to 2.0 mol/dm3, by contacting equal volumes (20 cm3) of both phases. In the case of studying the effect of phase ratio on stripping efficiency, the phase ratios of vaq:vorg ranging from 1:1 to 1:5 were examined (t = 30 min). Similarly to the extraction studies, the impact of contact time was conducted in the range of 1–30 min, maintaining the ratio of organic to aqueous phases of 1:1.
The possibility of Pt recovery from the organic phase was tested in accordance with the adopted methodology by contacting 25 cm3 of the organic phase (after stripping of Pd) twice with the same volume of fresh 2.0 mol/dm3 thiourea solution for 30 min. After each stripping step, the organic phase was washed with demineralized water. In the obtained aqueous solutions, the concentrations of Pt and Pd were analysed.
Results and discussion
The extraction studies
The effect of pH
The obtained results, presented in Fig. 3, indicate that high extraction efficiency of Pd (99.97–99.99%) and Pt (94.82–99.95%) is maintained throughout the investigated pH range (0–1.5). Unsatisfactory extraction efficiency results were obtained for Rh, where the highest extraction efficiency reached 49.11%, and at a pH of 0.5, it dropped below 15%. Taking into account the typically low efficiency of rhodium extraction, it was decided to adjust the process parameters in order to achieve quantitative extraction of palladium and platinum, while leaving rhodium in the mother solution. This approach would be the first stage of the separation of the three tested platinum group metals. Due to the reduced degree of Rh co-extraction at a pH of 0.5, the subsequent experiments utilized a test solution alkalized with solid NaOH to achieve such an acid–base pH value. Minor concentration changes due to aqueous phase volume alterations, resulting from solid NaOH additions, were consistently accounted for in all calculations. The chosen boundary pH value was associated with the risk of precipitation of solution components at higher pH values.
Figure 3.
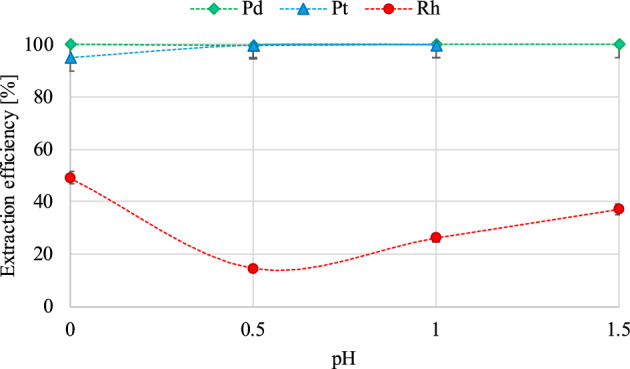
The influence of pH variation on PGMs extraction efficiency (vorg:vaq phase ratio—1:1; contact time—30 min).
The effect of extractant concentration
The obtained data (Table 3) indicate that even a 5% (v/v) content of ionic liquid in the extraction phase allows the separation of both tested metals, i.e. platinum and palladium, with very high efficiencies, amounting to 99.80% for Pt and 97.91% for Pd, respectively. Above the volume concentration of the Cyphos IL 101 extractant equal to 10% (v/v) for both of the above metals, separation efficiency above 99% was obtained each time. It is worth noting that changes in the concentration of rhodium in the raffinate were observed only in the case of samples extracted with a solution containing at least 20% (v/v) of ionic liquid. Despite this, the selectivity coefficients of Pd and Pt towards Rh are very high in the entire tested range.
Table 3.
The results of testing the influence of extractant concentration on PGM extraction efficiency.
| Concentration of the extractant [% (v/v)] | PGM concentration in raffinate [g/dm3] | Extraction efficiency [%] | Extraction coefficient (D) | Selectivity coefficient (α) | |
|---|---|---|---|---|---|
| 1 | Pd | 1.54 | 4.7 | < 0.1 | αPd/Pt = 0.14 |
| Pt | 1.575 | 27.1 | 0.4 | ||
| Rh | 0.300 | – | – | ||
| 5 | Pd | 0.0338 | 97.9 | 46.9 | αPd/Pt = 0.09 |
| Pt | 0.00425 | 99.8 | 507.2 | ||
| Rh | 0.300 | – | – | ||
| 10 | Pd | 0.00906 | 99.4 | 177.8 | αPd/Pt = 0.29 |
| Pt | 0.00354 | 99.8 | 609.2 | ||
| Rh | 0.300 | – | – | ||
| 20 | Pd | 0.00853 | 99.5 | 188.9 |
αPd/Pt = 0.17 αPd/Rh = 18,892 |
| Pt | 0.00193 | > 99.9 | 1118.2 | ||
| Rh | 0.296 | 1.3 | < 0.1 | ||
| 30 | Pd | 0.00716 | 99.6 | 225.3 |
αPd/Pt = 0.30 αPd/Rh = 5631 |
| Pt | 0.00286 | > 99.9 | 754.2 | ||
| Rh | 0.2893 | 3.6 | < 0.1 | ||
| 40 | Pd | 0.0054 | 99.7 | 2993.0 |
αPd/Pt = 1.91 αPd/Rh = 14,252 |
| Pt | 0.00138 | > 99.9 | 1564.2 | ||
| Rh | 0.247 | 17.7 | 0.2 | ||
| 50 | Pd | 0.0005 | > 99.9 | 3539.0 |
αPd/Pt = 7.62 αPd/Rh = 20,818 |
| Pt | 0.0047 | 99.8 | 464.1 | ||
| Rh | 0.240 | 14.3 | 0.2 | ||
“–”No change in metal concentration after extraction; volume of aqueous and organic phase—20 cm3.
The effect of the phase ratio of organic to aqueous phase
According to the data provided (Fig. 4), extraction conducted at volume ratios vorg:vaq of 1:1 and 1:2 allows for almost quantitative recovery of palladium and platinum from the test solution (efficiency of 99.4–96.8%) in a single-stage extraction using 10% (v/v) Cyphos IL 101 in toluene. For platinum, very high values were also achieved when using a vorg:vaq ratio of 1:3, reaching an efficiency of approximately 96%.
Figure 4.
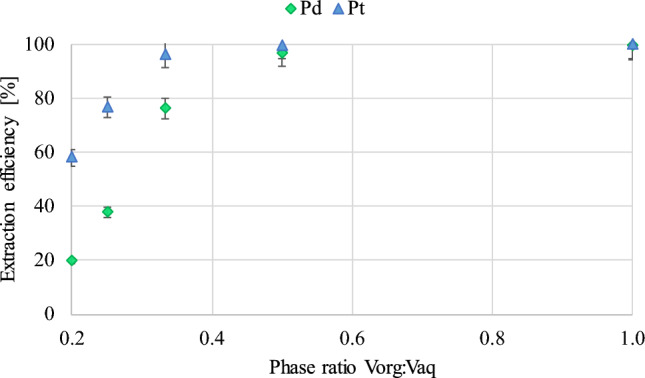
The influence of the vorg:vaq ratio on Pt and Pd extraction efficiency (pH of the aqueous phase—0,5; contact time—30 min).
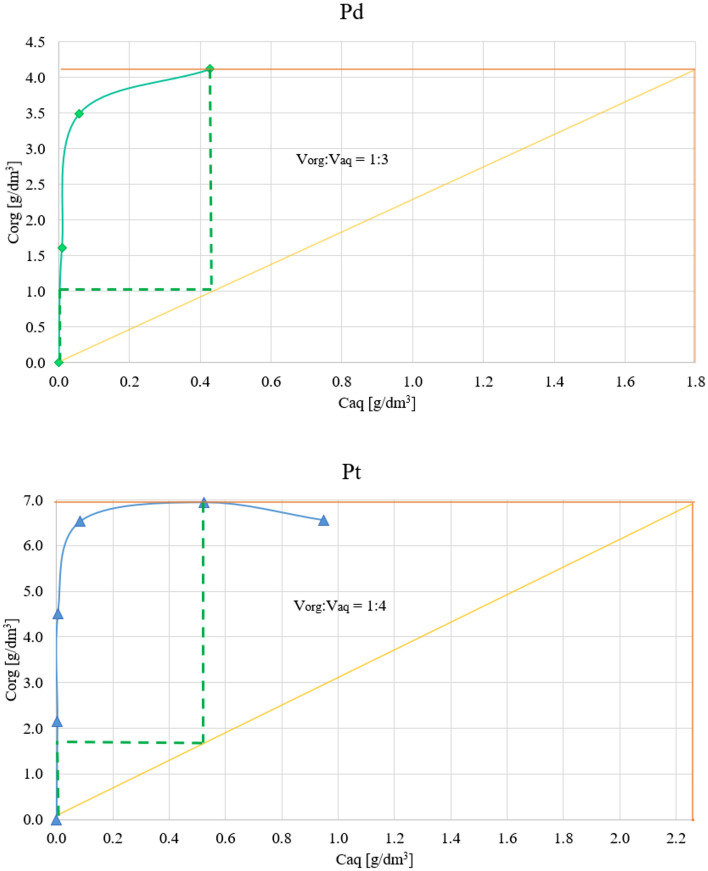
The obtained results were used to plot the extraction isotherms (Fig. 5), which demonstrate that the highest saturation level of Pd in the organic phase occurs at a 1:3 ratio, while for Pt it is at a 1:4 ratio. The number of extraction stages, determined using the graphical McCabe–Thiele method, was 2 in both cases.
Figure 5.
The extraction isotherms.
The effect of extraction time
The extraction time study showed that the 10% (v/v) Cyphos IL 101 ionic liquid in toluene is an extremely effective extractant, allowing to achieve high extraction efficiencies of both platinum metals after just 1 min of the process—the lowest extraction degrees achieved were 95.4% for Pd and 91.6% for Pt (Fig. 6). The equilibrium state was obtained each time after exceeding 3 min of contact time, and in the case of Pt, a small, gradual increase in the secretion efficiency was observed in the range of 97.4–99.8%. The minimum extraction time allowing for the isolation of both Pd and Pt with an efficiency exceeding 99% was 15 min in the tests performed, however, high extraction yields were obtained in the entire tested range, which indicates a high migration rate of the tested metals from one phase to another.
Figure 6.
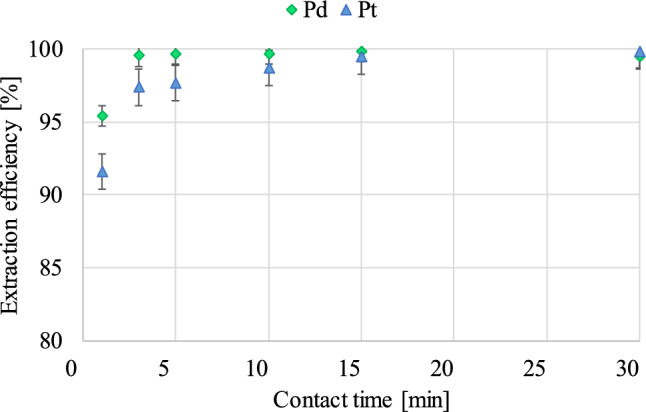
The influence of contact time variation on Pt and Pd extraction efficiency (vorg:vaq phase ratio -1:1; pH of the aqueous phase—0.5).
The stripping studies
The effect of stripping agent concentration
The conducted research showed that a high efficiency of palladium stripping can be achieved using a thiourea solution with a concentration of 0.1 mol/dm3 (Table 4). It additionally presented a simultaneous low co-stripping of platinum. The calculated Pd/Pt selectivity coefficient assumed a favourable, very high value. As the concentration of the stripping agent increased, the efficiency of Pt separation increased, with the highest extraction efficiency—26.7%—obtained in the case of the solution with the concentration of 2.0 mol/dm3. For three out of four tested samples, high Pd stripping yields exceeding 90% were obtained.
Table 4.
The results of testing the influence of thiourea concentration on PGM stripping efficiency.
| Thiourea concentration [mol/dm3] | Concentration in the stripping solution [g/dm3] | Stripping efficiency [%] | Stripping coefficient (D) | Selectivity coefficient (α) | |
|---|---|---|---|---|---|
| 0.1 | Pd | 2.38 | > 99.9 | 6842.0 | αPd/Pt = 1,183,574 |
| Pt | 0.018 | 0.6 | < 0.1 | ||
| 0.5 | Pd | 1.48 | 62.2 | 1.6 | αPd/Pt = 16.7 |
| Pt | 0.28 | 8.9 | 0.1 | ||
| 1.0 | Pd | 2.25 | 94.5 | 17.1 | αPd/Pt = 127.9 |
| Pt | 0.37 | 11.8 | 0.1 | ||
| 2.0 | Pd | 2.17 | 91.5 | 10.7 | αPd/Pt = 29.3 |
| Pt | 0.84 | 26.7 | 0.4 | ||
Volume of aqueous and organic phase—20 cm3.
The effect of the volume ratio
Similarly to the previous extraction tests, the influence of changing the phase ratio on the efficiency of Pd separation from the extract was determined. The study was carried out using a 0.1 M thiourea solution, with which Pd was recovered almost quantitatively, with a high Pd/Pt selectivity coefficient (α = 10,225.04; vaq:vorg = 1:1). Contacting specific volumes of the aqueous phase and the extract took place for 30 min using a magnetic stirrer. The results of the conducted research are summarized in chart (Fig. 7).
Figure 7.
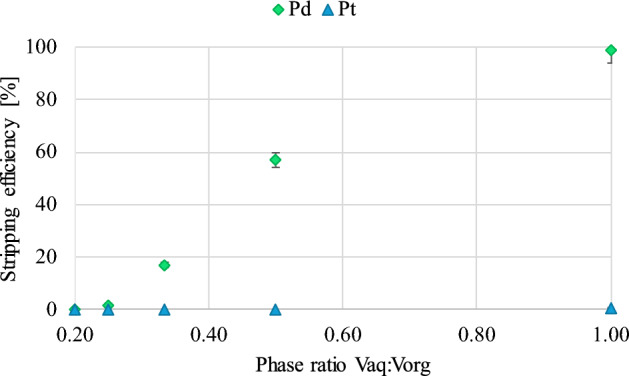
The influence of the vaq:vorg ratio on Pd and Pt stripping efficiency (contact time—30 min).
The cited data indicate that only for volume vaq:vorg ratios equal to 1:1 and 1:2 can satisfactory Pd stripping results be obtained—99.0 and 57.1%, respectively. The analysis of the remaining results indicates a sharp decrease in the stripping efficiency of Pd as the difference in the volume of both phases increases. Moreover, for the ratios 1:3–1:5, precipitation was observed, which additionally hindered the process and subsequent analysis of the samples. As expected, no significant decrease in Pt concentration was observed in any of the tested samples, which suggests that the selectivity of Pd stripping with respect to Pt with thiourea solution is maintained regardless of the phase ratio adopted.
The effect of stripping time
The above data (Fig. 8) indicate that the leaching of Pd from the Cyphos IL 101 ionic liquid solution, similarly to the extraction itself, occurs very quickly—after just 1 min of the process, 53.17% of the stripping efficiency was obtained, and the equilibrium state was reached. within the next 2 min of the process.
Figure 8.

The influence of contact time variation on Pd and Pt stripping efficiency (vaq:vorg phase ratio—1:1).
The Pt extraction efficiency in the entire tested time range was very low, resulting in very high Pd/Pt selectivity coefficients. Each time, platinum losses were less than 1%.
Pt stripping tests
Since the highest Pt stripping efficiencies were obtained for a 2 mol/dm3 thiourea solution (Table 4) and because some literature sources also indicate thiourea as an effective stripping agent, additional stripping tests were performed to check the possibility of Pt recovery from the organic phase after Pd removing. The experiments were performed according to the methodology described in “Extraction and stripping procedure” section.
The obtained results were collected and presented in the form of a column chart (Fig. 9).
Figure 9.

The efficiency of Pt stripping with various thiourea solutions (vaq:vorg phase ratio—1:1, contact time—30 min).
The presented test results show that a 2 mol/dm3 thiourea solution can be used to recover Pt, but the stripping efficiency results obtained are lower than in the case of Pd. In the first stage, the stripping efficiency was 86.1%, in the second stage it was 17.3%. The overall Pt recovery efficiency was 88.5% in 2 stages of stripping with 2 mol/dm3 thiourea. To obtain satisfactory results, it is necessary to use at least several stages of stripping. Furthermore, a decrease in stripping efficiency was observed in the second stripping stage, suggesting that quantitative recovery of platinum from the organic phase may be difficult to achieve. For this reason, it is recommended to consider alternative approaches, i.e. using an additional stripping medium to recover the remaining small amounts of Pt from the organic phase, or recycling the organic phase to the next extraction processes.
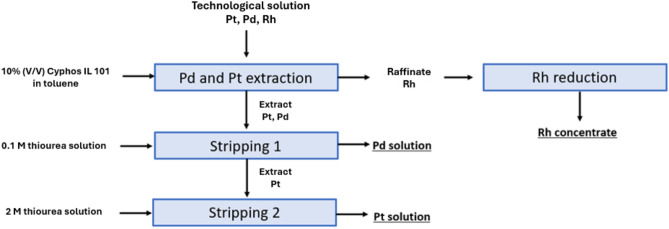
Nevertheless, the data obtained during all tests allowed the development of a concept of a technological scheme for the separation of Pd, Pt and Rh (Fig. 10).
Figure 10.
The proposed scheme for the separation of Pd from Pt and Rh using the Cyphos IL 101 ionic liquid.
Comparison with other research
The obtained extraction results confirmed the usefulness of the tested extractant in the selective recovery of Pd and Pt with respect to Rh from multi-component solutions with high concentrations of other base metals. Moreover, the obtained data are consistent with the results of the work of other authors, but it should be noted that many such works focus on tests carried out on pure synthetic solutions (Table 5).
Table 5.
The results of PGM extraction using Cyphos IL 101 ionic liquid in scientific literature.
| Test solution | Extraction efficiency | Stripping with thiourea solution | Stripping efficiency | References |
|---|---|---|---|---|
| Synthetic chloride solution containing Pt, Pd, Ru and Rh |
> 95% Pt/Pd ~ 60% Ru |
0.1 M TU in 0.5 M HCl | > 90% Pd | 32 |
| Chloride solution obtained after spent catalyst leaching | ~ 100% | – | – | 33 |
| Chloride solution obtained after spent catalyst leaching | ~ 100% Pd, Pt | 0.1 M TU in 0.5 M HCl | ~ 100% Pd | 37 |
| Chloride solution obtained after spent catalyst leaching | 99–100% Pd, Pt | 0.1 M TU in 5% HCl | 34% Pd | 38 |
| Synthetic chloride solution containing Pt, Pd, Rh |
99.9% Pt 98.1% Pd |
– | – | 31 |
| Synthetic chloride solution containing Rh and Pd | 99.9–89.7% Pd | – | – | 27 |
| Pt and Rh salts obtained by leaching of waste materials and dissolved in ethylene glycol | ~ 100% Pt | 1.0 M TU | ~ 100% Pt | 12 |
| Technological solution containing Pt, Pd, Rh and significant amounts of other metals |
99.8% Pt 99.4% Pd |
0.1 M TU |
~ 100% Pd 0.9% Pt |
This study |
“–”No data.
Our tests carried out on the industrial solution with a complex composition, containing significant amounts of copper and other metals such as nickel and iron, showed high extraction efficiency comparable to literature data regarding simpler synthetic solutions.
In the literature, both in the case of pure synthetic solutions and solutions after leaching of catalysts with low PGMs concentrations and some impurity content, Pd and Pt extraction efficiencies of approximately 95–100% have been reported27,31–33,37. Although most studies indicate thiourea solutions as the most effective stripping agents, some discrepancies in the yields obtained for Pd and Pt are observed in stripping studies12,32,37,38. This may result from the properties of the starting solutions used in the tests, such as differences in the concentrations of individual metals, used leaching solution, the concentration of chloride ions, pH and other physicochemical parameters.
In the case of this research, despite the use of a technological solution with relatively high Pt and Pd contents (i.e. 1.8 g/dm3 and 2.26 g/dm3) compared to other published works, equally high extraction efficiencies were obtained—99.4% for Pd and 99.8% for Pt. Moreover, the stripping step managed to almost quantitatively recover Pd from the organic phase, while limiting Pt co-stripping to below 1%. It should be emphasized that this is a great achievement.
This result suggests that the tested extractant, Cyphos IL 101, is equally effective in the recovery of platinum group metals from both synthetic solutions and potentially from industrial solutions. Moreover, in the experiments performed, all three tested platinum metals were successfully separated: first, platinum and palladium from rhodium at the extraction stage, and then palladium from platinum during stripping. It is worth emphasizing that the proposed solution using Cyphos IL 101 has not been previously described in the literature in relation to this type of complex and concentrated solutions. Based on the results obtained, a general outline of the potential technology for the separation of Pd, Pt and Rh was developed. An important aspect of this research is the fact that it was conducted on a technological solution, which additionally highlights the effectiveness of the proposed method and allows for a better assessment of its potential in real industrial applications.
Analysis of the obtained results in the context of available scientific literature allows us to conclude that ionic liquids demonstrate high effectiveness as extraction media, both on a laboratory and industrial scale. Although the studies focused on Cyphos IL 101, it is worth noting that researchers’ interest also encompasses a range of other types of ionic liquids, which are compared in Table 6 for reference.
Table 6.
The results of PGM extraction using other ionic liquids in scientific literature.
| Ionic liquid | Test solution | Extraction efficiency | Stripping solution | Stripping efficiency | References |
|---|---|---|---|---|---|
|
Trihexyl(tetradecyl)phosphonium bromide C32H68BrP Trade name: Cyphos IL 102 |
Synthetic chloride solution containing Pd | > 80–100% Pd | 0.5 M NH4OH | 84–90% | 39 |
|
Trihexyl(tetradecyl)phosphonium bis(2,4,4-trimethylpentyl)phosphinate C48H102O2P2 Trade name: Cyphos IL 104 |
Synthetic chloride solution containing Pd | 52–96% Pd | 0.5 M NH4OH | ~ 90% | 40 |
|
Mixture of tricaprylylmethylammonium chloride and trioctylmethylammonium chloride C25H54ClN Trade name: Aliquat 336 |
Synthetic chloride solution containing Pt, Rh, Al, Mg, Fe | 99.97% Pt |
0.1–0.5 M TU in 0.1–0.5 M HCl |
97–100% | 41 |
|
Trioctyl(dodecyl) phosphonium chloride (P88812Cl) C36H74ClP |
Synthetic chloride solution containing Pt, Pd and Rh |
Pt, Pd: 99.9% Rh: 10.0–90.0% |
(1) 5.0 M HNO3 (2) 1.0 M TU (3) 5.0 M HCl |
(1) 74.9% Pt (2) 91.2% Pd (3) 73.7% Rh |
30 |
As can be seen, ionic liquids with the trade names Cyphos IL 102, Cyphos IL 104 and Aliquat 336 show equally high efficiency in the extraction of Pd and Pt, achieving efficiencies in the range of 52–100%39–41. In the case of stripping, other authors again indicated solutions of thiourea and additionally aqueous ammonia as the most effective stripping solutions.
Conclusions
In this study, the possibility of selectively separating Pd from Pt using solvent extraction was investigated. A technologic solution containing low concentrations of Pt, Pd, and Rh, as well as significant amounts of impurities such as Cu, Fe, Cr, and Ni, was used as the research subject.
Experiments conducted with the commercial extractant Cyphos IL 101 showed that the use of this ionic liquid allowed for very effective extraction of both elements (Pt and Pd) even at low concentrations of ionic liquid solution. The results confirmed that a 10% (v/v) content of Cyphos IL 101 in toluene was sufficient to extract both elements with an efficiency exceeding 99%. The high yield combined with the small amount of extractant required for the process is an important economic factor, especially for extraction on a larger scale than laboratory-scale. However, since Cyphos IL 101 extracts both elements to the same extent, the selectivity of the process is low.
Nevertheless, the study demonstrated that selective separation of Pd from Pt is possible during stripping stage using thiourea solutions. For the tested solution, extraction at pH 0.5 is recommended to reduce the co-extraction of Rh. Phase ratios of vorg:vaq equal to 1:1 and 1:2 were found to be the most favourable, allowing for high efficiencies in the separation of platinum and palladium. Despite the high efficiencies achieved through a single extraction, at least two extraction stages are necessary for quantitative separation of the investigated platinum group metals from the original solution.
It is worth noting that the extraction of PGM using Cyphos IL 101 occurs very quickly throughout the investigated range (1–30 min), which may have significant technological importance.
Based on the obtained results, the following conclusions can be formulated:
The ionic liquid Cyphos IL 101 allows for the selective separation of Pd and Pt from Rh.
A 0.1 mol/dm3 thiourea solution is a selective stripping agent for Pd over Pt when stripping from a 10% (v/v) Cyphos IL 101 solution.
Author contributions
Conceptualization, K.P.; methodology, K.P. and G.B.; investigation, K.P., K.G. and J.M.; writing—original draft preparation, K.P.; writing—review and editing, K.P., J.K. and G.B.; visualization, K.P.; supervision, G.B., J.K. and K.L.-S. All authors have read and agreed to the published version of the manuscript.
Funding
The funding was provided by Łukasiewicz Research Network—Institute of Non-Ferrous Metals, Poland (0334111002).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith, W. et al. Element mapping the Merensky reef of the bushveld complex. Geosci. Front.12, 101101 (2021). 10.1016/j.gsf.2020.11.001 [DOI] [Google Scholar]
- 2.Liang, Q. L., Song, X. Y., Long, T. M., Wirth, R. & Dai, Z. H. The effect of platinum-group minerals on differentiation of platinum-group elements in magmatic sulfide deposits: Evidence from the Cu-Ni-PGE deposits in the Yangliuping area of the Emeishan large igneous province, SW China. Chem. Geol.636, 121645 (2023). 10.1016/j.chemgeo.2023.121645 [DOI] [Google Scholar]
- 3.Bielański, A. Podstawy Chemii Nieorganicznej tom III 930–939 (PWN, 1994). [Google Scholar]
- 4.https://matthey.com/documents/161599/509428/PGM-market-report-May-2022.pdf/542bcada-f4ac-a673-5f95-ad1bbfca5106?t=1655877358676 (Accessed 15 May 2023).
- 5.Seetharaman, S. Treatise on Process Metallurgy Volume 3: Industrial Processes. Treatise on Process Metallurgy (2014).
- 6.Paiva, A. P. Recycling of palladium from spent catalysts using solvent extraction—Some critical points. Metals7, 505 (2017). 10.3390/met7110505 [DOI] [Google Scholar]
- 7.Panda, R., Jha, M. K. & Pathak, D. D. Commercial processes for the extraction of platinum group metals (PGMs). In Minerals, Metals and Materials Series Vol. 5 (eds Panda, R. et al.) 119–130 (Springer, 2018). [Google Scholar]
- 8.Saguru, C., Ndlovu, S. & Moropeng, D. A review of recent studies into hydrometallurgical methods for recovering PGMs from used catalytic converters. Hydrometallurgy182, 44–56 (2018). 10.1016/j.hydromet.2018.10.012 [DOI] [Google Scholar]
- 9.Yakoumis, I., Panou, M., Moschovi, A. M. & Panias, D. Recovery of platinum group metals from spent automotive catalysts: A review. Clean. Eng. Technol.3, 100112 (2021). 10.1016/j.clet.2021.100112 [DOI] [Google Scholar]
- 10.Zheng, H., Ding, Y., Wen, Q., Liu, B. & Zhang, S. Separation and purification of platinum group metals from aqueous solution: Recent developments and industrial applications. Resour. Conserv. Recycl.167, 105417 (2021). 10.1016/j.resconrec.2021.105417 [DOI] [Google Scholar]
- 11.Cornish, L. A., Süss, R., Douglas, A., Chown, L. H. & Glaner, L. The platinum development initiative: Platinum-based alloys for high temperature and special applications: Part I. Platin. Met. Rev.53, 2–10 (2009). 10.1595/147106709X393299 [DOI] [Google Scholar]
- 12.Nguyen, V. T. et al. Solvometallurgical recovery of platinum group metals from spent automotive catalysts. ACS Sustain. Chem. Eng.9, 337–350 (2021). 10.1021/acssuschemeng.0c07355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinisalo, P. & Lundström, M. Refining approaches in the platinum group metal processing value chain—A review. Metals8, 203 (2018). 10.3390/met8040203 [DOI] [Google Scholar]
- 14.Gervilla, F. & Kojonen, K. The platinum-group minerals in the upper section of the Keivitsansarvi Ni-Cu-PGE deposit, Northern Finland. Can. Mineral.40, 337–394 (2002). 10.2113/gscanmin.40.2.377 [DOI] [Google Scholar]
- 15.Mohamed, N. et al. Chemical and mineralogical mapping of platinum-group element ore samples using laser-induced breakdown spectroscopy and micro-X-ray fluorescence. Geostand. Geoanal. Res.45, 539 (2021). 10.1111/ggr.12385 [DOI] [Google Scholar]
- 16.Pianowska, K. et al. Solvent extraction as a method of recovery and separation of platinum group metals. Materials16, 4681 (2023). 10.3390/ma16134681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goc, K. et al. Application of ion exchange for recovery of noble metals. Minerals11, 1188 (2021). 10.3390/min11111188 [DOI] [Google Scholar]
- 18.Rydberg, J., Cox, M., Musikas, C. & Choppin, G. R. Solvent Extraction: Principles and Practice, Second Edition, Revised and Expanded Edited Vol. 1 (Marcel Dekker, 2004). [Google Scholar]
- 19.Bernardis, F. L., Grant, R. A. & Sherrington, D. C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym.65, 205–217 (2005). 10.1016/j.reactfunctpolym.2005.05.011 [DOI] [Google Scholar]
- 20.Rane, M. V. PGM ore processing: LIX reagents for palladium extraction & platinum stripping from Alamine 336 using NaOH-NaCl. Miner. Eng.138, 1 (2019). 10.1016/j.mineng.2019.04.044 [DOI] [Google Scholar]
- 21.Wiecka, Z., Rzelewska-Piekut, M., Wojciechowska, I., Wieszczycka, K. & Regel-Rosocka, M. Recovery of palladium(II) and platinum(IV) in novel extraction systems. Materials14, 285 (2021). 10.3390/ma14020285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makanyire, T., Sanchez-Segado, S. & Jha, A. Separation and recovery of critical metal ions using ionic liquids. Adv. Manuf.4, 33–46 (2016). 10.1007/s40436-015-0132-3 [DOI] [Google Scholar]
- 23.Ilyas, S., Kim, H. & Srivastava, R. R. Separation of platinum group metals from model chloride solution using phosphonium-based ionic liquid. Sep. Purif. Technol.278, 119577 (2022). 10.1016/j.seppur.2021.119577 [DOI] [Google Scholar]
- 24.Cieszyńska, A. & Regel-Rosocka, M. Extraction of Palladium(II) ions from chloride solutions with Phospho-niumIonicLiquidCyphos®IL101. Polish J. Chem. Technol.9, 99–101 (2007). 10.2478/v10026-007-0037-4 [DOI] [Google Scholar]
- 25.Firmansyah, M. L., Kubota, F., Yoshida, W. & Goto, M. Application of a novel phosphonium-based ionic liquid to the separation of platinum group metals from automobile catalyst leach liquor. Ind. Eng. Chem. Res.58, 3845–3852 (2019). 10.1021/acs.iecr.8b05848 [DOI] [Google Scholar]
- 26.Lee, J. C., Kurniawan, K., Kim, S., Nguyen, V. T. & Pandey, B. D. Ionic liquids-assisted solvent extraction of precious metals from chloride solutions. Sep. Purif. Rev.52, 1–20 (2022).
- 27.Svecova, L., Papaiconomou, N. & Billard, I. Quantitative extraction of Rh(III) using ionic liquids and its simple separation from Pd(II). Dalt. Trans.45, 15162–15169 (2016). 10.1039/C6DT02384C [DOI] [PubMed] [Google Scholar]
- 28.Regel-Rosocka, M., Cieszyńska, A. & Wiśniewski, M. Extraction of palladium (II) ions from chloride solutions with phosphonium ionic liquid CyphosŽIL101. Polish J. Chem. Technol.9, 99–101 (2007). 10.2478/v10026-007-0037-4 [DOI] [Google Scholar]
- 29.Wiecka, Z. et al. Pd(II) and Pt(IV) dispersive or non-dispersive extraction from model and real leach solutions with alkoxyimine-1-propylpyridinium derivatives. Sep. Purif. Technol.317, 123800 (2023). 10.1016/j.seppur.2023.123800 [DOI] [Google Scholar]
- 30.Firmansyah, M. L., Kubota, F. & Goto, M. Solvent extraction of Pt(IV), Pd(II), and Rh(III) with the ionic liquid trioctyl(dodecyl) phosphonium chloride. J. Chem. Technol. Biotechnol.93, 1714–1721 (2018). 10.1002/jctb.5544 [DOI] [Google Scholar]
- 31.Nguyen, V. T. et al. Highly selective separation of individual platinum group metals (Pd, Pt, Rh) from acidic chloride media using phosphonium-based ionic liquid in aromatic diluent. RSC Adv.6, 62717–62728 (2016). 10.1039/C6RA09328K [DOI] [Google Scholar]
- 32.Rzelewska-Piekut, M. & Regel-Rosocka, M. Separation of Pt(IV), Pd(II), Ru(III) and Rh(III) from model chloride solutions by liquid–liquid extraction with phosphonium ionic liquids. Sep. Purif. Technol.212, 791–801 (2019). 10.1016/j.seppur.2018.11.091 [DOI] [Google Scholar]
- 33.Rzelewska-Piekut, M., Paukszta, D. & Regel-Rosocka, M. Hydrometallurgical recovery of platinum group metals from spent automotive converters. Physicochem. Probl. Miner. Process.57, 83–94 (2021). 10.37190/ppmp/132779 [DOI] [Google Scholar]
- 34.Nguyen, T. H., Sonu, C. H. & Lee, M. S. Separation of Pt(IV), Pd(II), Rh(III) and Ir(IV) from concentrated hydrochloric acid solutions by solvent extraction. Hydrometallurgy164, 71–77 (2016). 10.1016/j.hydromet.2016.05.014 [DOI] [Google Scholar]
- 35.Marinho, R. S., Afonso, J. C. & da Cunha, J. W. S. D. Recovery of platinum from spent catalysts by liquid–liquid extraction in chloride medium. J. Hazard. Mater.179, 488–494 (2010). 10.1016/j.jhazmat.2010.03.029 [DOI] [PubMed] [Google Scholar]
- 36.Nguyen, T. H., Sonu, C. H. & Lee, M. S. Separation of platinum(IV) and palladium(II) from concentrated hydrochloric acid solutions by mixtures of amines with neutral extractants. J. Ind. Eng. Chem.32, 238–245 (2015). 10.1016/j.jiec.2015.08.022 [DOI] [Google Scholar]
- 37.Wiecka, Z., Rzelewska-Piekut, M. & Regel-Rosocka, M. Recovery of platinum group metals from spent automotive converters by leaching with organic and inorganic acids and extraction with quaternary phosphonium salts. Sep. Purif. Technol.280, 119933 (2022). 10.1016/j.seppur.2021.119933 [DOI] [Google Scholar]
- 38.Paiva, A. P., Piedras, F. V., Rodrigues, P. G. & Nogueira, C. A. Hydrometallurgical recovery of platinum-group metals from spent auto-catalysts—Focus on leaching and solvent extraction. Sep. Purif. Technol.286, 120474 (2022). 10.1016/j.seppur.2022.120474 [DOI] [Google Scholar]
- 39.Regel-Rosocka, M., Rzelewska, M., Baczynska, M., Janus, M. & Wisniewski, M. Removal of palladium(II) from aqueous chloride solutions with cyphos phosphonium ionic liquids as metal ion carriers for liquid-liquid extraction and transport across polymer inclusion membranes. Physicochem. Probl. Miner. Process.51, 621–631 (2015). [Google Scholar]
- 40.Cieszyńska, A. & Wiśniewski, M. Extractive recovery of palladium(II) from hydrochloric acid solutions with Cyphos®IL 104. Hydrometallurgy113–114, 79–85 (2012). 10.1016/j.hydromet.2011.12.006 [DOI] [Google Scholar]
- 41.Raju, B. et al. Separation of platinum and rhodium from chloride solutions containing aluminum, magnesium and iron using solvent extraction and precipitation methods. J. Hazard. Mater.227–228, 142–147 (2012). 10.1016/j.jhazmat.2012.05.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.