Abstract
Backround
Level one evidence reported poorer outcomes among patients taking dietary supplements after breast cancer (BC) diagnosis.
Methods
We evaluated dietary supplement behaviours among adult BC patients via questionnaire. Sociodemographic data, supplement use, attitudes, and healthcare provider (HCP) advice were analysed.
Results
Of 185 participants, 45% were regular supplement users following diagnosis. Regular supplement use was associated with higher education level (p = 0.05). The majority perceived supplements to be safe. Over half reported not receiving advice from HCPs.
Conclusion
In summary, supplement use is prevalent among BC patients. Development of guidelines in relation to safe use of dietary supplements after cancer diagnosis is crucial.
Keywords: Breast cancer, Dietary supplements, Nutrition, Survivorship
Dietary supplement use has become increasingly popular among breast cancer patients [1]. Recent research has raised safety concerns suggesting the use of antioxidants such as vitamins A, C, and E; carotenoids and coenzyme Q10; and vitamin B12 supplements may be associated with increased recurrence and mortality rates in patients with early breast cancer [2]. It has been postulated that the antioxidant properties of certain supplements may interfere with the oxidising free radicals of chemotherapy and radiotherapy, reducing their ability to target cancer cells [3]. Conversely, several publications indicate that dietary supplement use may play a role in optimising cancer treatments and improving breast cancer outcomes [4]. The contradictory findings to date, in combination with data largely drawn from observational cohort studies, limit their validity. Consequently, this study aimed to characterise dietary supplement behaviours, perceptions, and attitudes among breast cancer patients and to assess the prevalence of supplement use both pre- and post-breast cancer diagnosis.
This was a multicentre study conducted between August, 2021, and November, 2022, in adult patients diagnosed with early stage breast cancer at Cork University Hospital. An anonymous patient administered questionnaire was devised by the authors and circulated to those attending clinics. The study was approved by the institutional ethics committee and all participants provided informed consent. Behaviours, attitudes, and perceptions regarding supplements, as well as sociodemographic and lifestyle data were recorded. Supplement use patterns both pre- and post-breast cancer diagnosis were collected. Regular use was defined as greater than 3 different dietary supplements taken at least once weekly, generating two distinct groups. Data analysis was conducted using IBM SPSS V28.0.0.0. Demographic characteristics associated with regular use were analysed via logistic regression. Multiple chi-squared tests were performed to determine the association between post-diagnosis regular use and non-regular use and survivors’ attitudes and perceptions, and healthcare provider advice. Statistical significance was assumed by a p-value < 0.05.
A total of 185 questionnaires were completed, with baseline demographics shown in Table 1. Regular supplement use following breast cancer diagnosis was reported by 45% of participants, with 16% being new regular users. Regular use prior to diagnosis was associated with increased age, higher education levels, and lower BMI (p < 0.05), while regular use following diagnosis was associated with higher education level (p = 0.05). The most commonly used dietary supplements were multivitamins, vitamin C, vitamin D, calcium, and omega 3 (Fig. 1). The use of calcium and vitamin D was considerably increased following diagnosis.
Table 1.
Summary of participant characteristics
| Socioeconomic and lifestyle demographic characteristics | No. of participants | % | Mean | Range | |
|---|---|---|---|---|---|
| Age | 57 | 53 | |||
| Sex | Male | 2 | 1.1% | ||
| Female | 183 | 98.9% | |||
| BMI | 27.3 | 25.6 | |||
| Education level | Primary school | 8 | 4.3% | ||
| Secondary school | 103 | 56.0% | |||
| Third level education—bachelor | 46 | 25.0% | |||
| Third level education—master | 27 | 14.7% | |||
| Exercise/physical activity | More than 4 times a week | 73 | 39.5% | ||
| 3–4 times a week | 68 | 36.8% | |||
| 1–2 times a week | 33 | 17.8% | |||
| Less than once a week | 11 | 5.9% | |||
| Daily fruit and vegetable | > 5 | 24 | 13.0% | ||
| intake | 4–5 | 73 | 39.5% | ||
| 2–3 | 77 | 41.6% | |||
| 1 | 11 | 5.9% | |||
| None | 0 | 0.0% | |||
| Weekly alcohol intake | > 14 units | 3 | 1.6% | ||
| 10–14 units | 13 | 7.0% | |||
| 5–9 units | 35 | 18.9% | |||
| 1–4 units | 57 | 30.8% | |||
| None | 77 | 41.6% | |||
| Smoking status | Current smoker | 14 | 7.6% | ||
| Ex-smoker | 67 | 36.2% | |||
| Never smoker | 104 | 56.2% | |||
| Hormone therapy | 128 | 69.6% | |||
| Chemotherapy | 130 | 70.7% | |||
| Surgery | 149 | 81.0% | |||
| Radiation therapy | 143 | 77.7% | |||
Fig. 1.
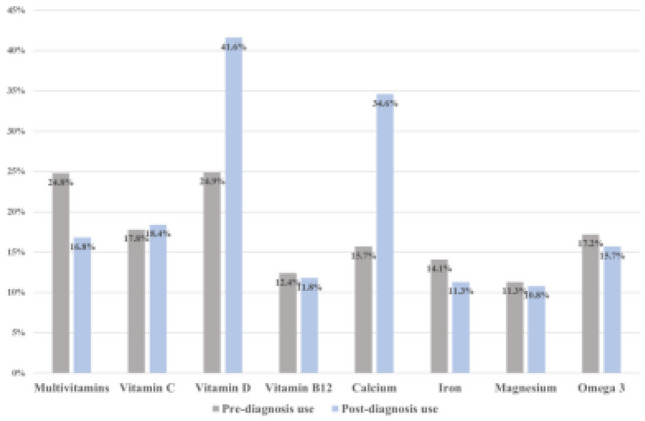
Most commonly used dietary supplements and proportion of use by breast cancer patients pre- and post-breast cancer diagnosis
The majority of patients (77%) desired more information regarding safe dietary supplement use in survivorship. Many were uncertain as to whether dietary supplement intake affected their risk of breast cancer development, recurrence and mortality, and about the safety of supplements when used concurrently with cancer treatments (Fig. 2). These participants were more likely to be non-regular users. A significant proportion believed dietary supplement use had no effect on breast cancer outcomes, a belief evenly distributed between groups. Those who perceived supplement use carried a reduced risk of poorer outcomes were more likely to be regular users. Very few participants felt supplement use increased the risk of poor outcomes (Table 2).
Fig. 2.
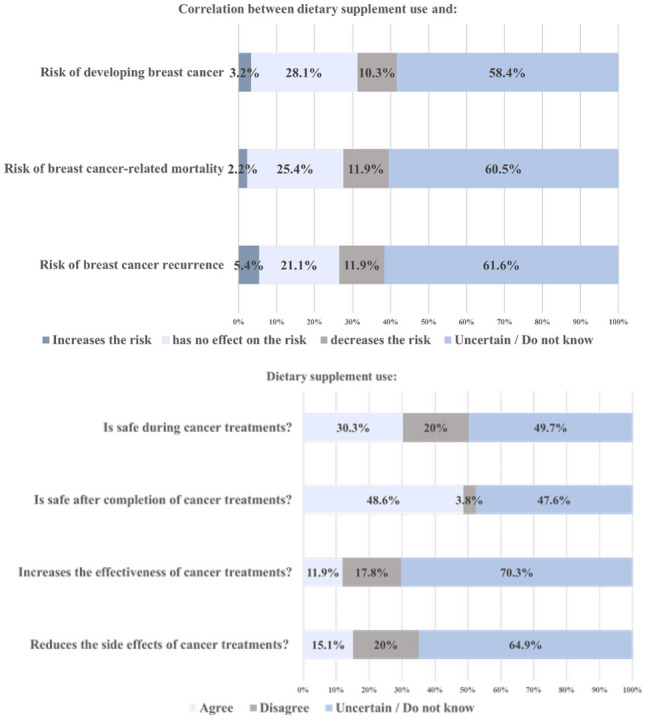
Attitudes toward and perceptions of dietary supplement use in breast cancer patients
Table 2.
Association of post-diagnosis regular and non-regular supplement use with attitudes and perceptions
| Attitudes & Perceptions | Post diagnosis regular users N (%) |
Post diagnosis non-regular users N (%) |
p-value | 95% CI | |
|---|---|---|---|---|---|
| Dietary supplement use and risk of developing breast cancer | Increases risk | 1 (0.5) | 5 (2.7) | 0.016 | 0.013-0.018 |
| No effect on risk | 26 (14.1) | 26 (14.1) | |||
| Decreases risk | 14 (7.6) | 5 (2.7) | |||
| Uncertain/Do not know | 43 (23.2) | 65 (35.1) | |||
| Dietary supplement use and risk of breast cancer recurrence | Increases risk | 1 (0.5) | 9 (4.9) | 0.000 | 0.000<0.001 |
| No effect on risk | 20 (10.8) | 19 (10.3) | |||
| Decreases risk | 18 (9.7) | 4 (2.2) | |||
| Uncertain/Do not know | 45 (24.3) | 69 (37.3) | |||
| Dietary supplement use and risk of breast cancer-related mortality | Increases risk | 0 (0) | 4 (2.2) | 0.014 | 0.012-0.017 |
| No effect on risk | 26 (14.1) | 21 (11.4) | |||
| Decreases risk | 14 (7.6) | 8 (4.3) | |||
| Uncertain/Do not know | 44 (23.8) | 68 (36.8) | |||
| Dietary supplement use is safe during cancer treatments | Agree | 33 (17.8) | 23 (12.4) | 0.033 | 0.029-0.037 |
| Disagree | 12 (6.5) | 25 (13.5) | |||
| Uncertain/Do not know | 39 (21.1) | 53 (28.6) | |||
| Dietary supplement use is safe after the completion of cancer treatments | Agree | 47 (25.4) | 43 (23.2) | 0.186 | 0.178-0.193 |
| Disagree | 3 (1.6) | 4 (2.2) | |||
| Uncertain/Do not know | 34 (18.4) | 54 (29.2) | |||
| Dietary supplements increase the effectiveness of cancer treatments | Agree | 15 (8.1) | 7 (3.8) | 0.052 | 0.047-0.056 |
| Disagree | 12 (6.5) | 21 (11.4) | |||
| Uncertain/Do not know | 57 (30.8) | 73 (39.5) | |||
| Dietary supplements reduce the side effects of cancer treatments | Agree | 16 (8.6) | 12 (6.5) | 0.314 | 0.305-0.323 |
| Disagree | 18 (9.7) | 19 (10.3) | |||
| Uncertain/Do not know | 50 (27) | 70 (37.8) | |||
| It is important to inform healthcare providers about the dietary supplements I am taking | Agree | 68 (36.8) | 87 (47) | 0.421 | 0.411-0.430 |
| Disagree | 1 (0.5) | 0 (0) | |||
| Uncertain/Do not know | 15 (8.1) | 14 (7.6) | |||
| I would like more information on dietary supplements | Agree | 68 (36.8) | 72 (38.9) | 0.176 | - |
| Disagree | 15 (8.1) | 26 (14.1) | |||
CI confidence interval
Over half of the study participants (54%) received no recommendations or advice regarding dietary supplements from healthcare providers, 21% were recommended to use dietary supplements, while the remainder were recommended to avoid supplement use.
Our findings highlight that while many patients believe dietary supplements are worthwhile, the majority of study participants did not receive advice regarding supplement use from healthcare providers. Dangerous misconceptions regarding ‘cancer-beating diets’ are widespread on social media and potentially harmful for patients with cancer especially those with lower health literacy, reinforcing the need for robust evidence-based information [5].
The most commonly used supplements in this study were multivitamins, vitamin C, vitamin D, calcium, and omega 3. The increase in vitamin D and calcium use may reflect guidelines supporting adjuvant bisphosphonates with calcium and vitamin D to improve outcomes and alleviate the side effects of treatments [6]. Other supplements commonly used by breast cancer patients to mitigate side effects of therapy include vitamin E for taxane-induced peripheral neuropathy, glutamine for mucositis, and selenium for post-operative upper limb lymphoedema [7]. Large randomised trials have previously demonstrated increased risk of lung and gastric cancer with beta-carotene use, as well as an association between vitamin E and prostate cancer, with mixed effects of antioxidants on chemotherapy toxicity [8]. Unmonitored use of herbal medicines is becoming a more frequent phenomenon [9]. Additionally in the era of immunotherapy, the evolving role of the gut microbiome and interactions between what we ingest and treatment outcomes will be a major research area [10]. Taking all of the above into consideration, open communication between patient and physician regarding dietary supplement use is critical.
The strengths of this study include a substantial data collection window,, as well as broad selection criterion, with no exclusion of particular demographic characteristics, current disease activity, or co-morbidities, generating a study sample more representative of the ‘real-world’. Limitations include the self-reporting of supplement use, particularly pre-diagnosis use, creating the potential for recall bias. Our initial ambition was to perform a survey of healthcare professional attitudes in parallel with this study, in order to assess healthcare provider advice concerning dietary supplements in breast cancer survivorship; however, unfortunately we did not have adequate response rates for this to be feasible.
In conclusion, dietary supplement use is prevalent among breast cancer patients; however, attitudes and perceptions suggest a lack of awareness of potential consequences of dietary supplements in breast cancer survivorship. Patients also report a lack of sufficient guidance from healthcare providers regarding dietary supplement use. Future steps include exploration of healthcare provider attitudes and knowledge of dietary supplements and recommendations. Large-scale randomized controlled trials (RCTs) to compile safety data on individual dietary supplements in order to provide robust evidence would be optimal to guide clinical practice. Recommendations for nutritional supplements and dietary advice should be integrated into oncology patient care plans and survivorship guidelines.
Acknowledgements
MH received salary support from Breakthrough Cancer Research.
Funding
Open Access funding provided by the IReL Consortium
Data availability
Data from the study available via corresponding author.
Declarations
Conflict of interest
MH—Travel: Roche, AstraZeneca, MSD. SOR - Travel Roche, Novartis, Nordic Pharma. Consultancy: AstraZeneca
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hauer M et al (2023) Dietary supplement use in women diagnosed with breast cancer. J Nutr 153(1):301–311. 10.1016/j.tjnut.2022.12.007 10.1016/j.tjnut.2022.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosone CB, Zirpoli GR, Hutson AD et al (2020) Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J Clin Oncol 38(8):804–814. 10.1200/JCO.19.01203 10.1200/JCO.19.01203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Andrea GM (2005) Use of antioxidants during chemotherapy and radiotherapy should be avoided. CA: Cancer J Clin 55(5):319–321. 10.3322/canjclin.55.5.319 [DOI] [PubMed] [Google Scholar]
- 4.Wassertheil-Smoller S, McGinn AP, Budges N et al (2013) Multivitamin and mineral use and breast cancer mortality in older women with invasive breast cancer in the Women’s Health initiative. Breast Cancer Res Treat 141(3):495–505. 10.1007/s10549-013-2712-x 10.1007/s10549-013-2712-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimes DR, O’Riordan E (2023) Starving cancer and other dangerous dietary misconceptions. Lancet Oncol 24(11):1177–1178. 10.1016/S1470-2045(23)00483-7. PMID: 37922928 10.1016/S1470-2045(23)00483-7 [DOI] [PubMed] [Google Scholar]
- 6.Madden JM, Murphy L, Zgaga L et al (2018) De novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res Treat 172(1):179–190. 10.1007/s10549-018-4896-6 10.1007/s10549-018-4896-6 [DOI] [PubMed] [Google Scholar]
- 7.Samuels N, Schiff E, Ben-Arye E (2014) Non-herbal nutritional supplements for symptom relief in adjuvant breast cancer: creating a doctor–patient dialogue. BMJ Support Palliat Care 4(3):e1. 10.1136/bmjspcare-2013-000463 10.1136/bmjspcare-2013-000463 [DOI] [PubMed] [Google Scholar]
- 8.Harvie M (2014) Nutritional supplements and cancer: potential benefits and proven harms. Am Soc Clin Oncol Educ Book 34(1):e478-86. 10.14694/EdBook_AM.2014.34.e478. PMID: 24857143 10.14694/EdBook_AM.2014.34.e478 [DOI] [PubMed] [Google Scholar]
- 9.Samuels N, Ben-Arye E, Maimon Y et al (2017) Unmonitored use of herbal medicine by patients with breast cancer: reframing expectations. J Cancer Res Clin Oncol 143(11):2267–2273. 10.1007/s00432-017-2471-x. Epub 2017 Jun 30 PMID: 28667389 10.1007/s00432-017-2471-x [DOI] [PubMed] [Google Scholar]
- 10.Lee KA, Luong MK, Shaw H et al (2021) The gut microbiome: what the oncologist ought to know. Br J Cancer 125(9):1197–1209. 10.1038/s41416-021-01467-x. Epub 2021 Jul 14. PMID: 34262150 10.1038/s41416-021-01467-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the study available via corresponding author.


