Abstract
Among clinically highly efficient antiseizure medications (ASMs) there are modifiers of the presynaptic release machinery. Of them, levetiracetam and brivaracetam show a high affinity to the synaptic vesicle protein type 2 A (SV2A), whereas pregabalin and gabapentin are selective ligands for the α2δ1 subunits of the voltage-gated calcium channels. In this paper, we present recent progress in understanding the significance of presynaptic release machinery in the neurochemical mechanisms of epilepsy and ASMs. Furthermore, we discuss whether the knowledge of the basic mechanisms of the presynaptically acting ASMs might help establish a rational polytherapy for drug-resistant epilepsy.
Keywords: Epilepsy, Antiseizure medications, Neurochemical mechanisms, Presynaptic machinery, Clinical efficacy, Rational polytherapy
Introduction
Epilepsy is a common and multifactorial neurological disorder characterized by a recurrent occurrence of unprovoked convulsive or non-convulsive seizures, which are clinical manifestations of abnormal, transient, and synchronous hyperactivity of specific neuronal circuits in the brain. Depending on the brain region involved in the generation and spreading of seizures, distinct autonomic, motor, and sensory symptoms can be observed [1, 2]. Despite the introduction of new antiseizure medications (ASMs; formerly referred to as antiepileptic drugs), nearly 30% of patients with epilepsy are still resistant to pharmacological treatment [3]. Therefore, both designing new antiepileptic compounds, as well as developing a rational polytherapy using the already marketed ASMs for treating drug-resistant forms of epilepsy seem entirely justified. The maximal electroshock (MES) test, the subcutaneous pentylenetetrazol (scPTZ) seizure test, and the kindling model are animal models routinely used for screening potential ASMs. However, a predictive value of MES and scPTZ is limited to generalized tonic-clonic seizures, while kindling is predictive of human focal epilepsy. Furthermore, the genetic absence epileptic rat of Strasbourg and the lethargic (lh/lh) mouse are predictive of human generalized spike-wave seizures [4]. It is emphasized that no single model has a predictive value for drug-resistant seizures, but rather a battery of such models should be used [5]. Recently, more etiologically relevant models in the preclinical evaluation of new investigational drugs for the treatment of drug-resistant epilepsy have been comprehensively reviewed [6].
Neurochemical and electrophysiological studies show that seizures result from brain region-specific excessive excitatory processes or insufficiency of inhibitory neurotransmission. Thus, the hyperactivity of the glutamatergic neurons leads to excessive activation of the ionic excitatory amino acid receptors, e.g. the N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate receptors, resulting in depolarization of neurons and facilitation of epileptic phenomena [7, 8]. Furthermore, alterations in expression, loss- or gain-of-function mutations of protein subunits, polymorphisms, and cellular energetic deficits can all contribute to dysfunction of the voltage- and ligand-dependent sodium, calcium, potassium, and chloride channels, eventually promoting seizure discharges [9]. The γ-aminobutyric acid (GABA) is regarded as the principal inhibitory neurotransmitter in the mammalian brain. It has been well evidenced that even a moderate inhibition of the chloride ion-gated GABAA receptors can provoke seizures. The deficit in the inhibitory processes is predominantly linked with insufficiency in GABAA receptor-mediated neurotransmission [10].
Accordingly, pharmacological strategies in the treatment of epilepsy include stabilization of neuronal membranes and preventing depolarization by acting on ion channels, increasing and decreasing the GABAergic and excitatory amino acid (EAA) transmission, respectively. In this paper, we review the recent progress in understanding the neurochemical and molecular mechanism of presynaptic release machinery as targets for ASMs. Furthermore, we discuss whether the knowledge of the neurochemical mechanisms of the ASMs - with a special emphasis on racetams and gabapentinoids - might help establish a rational polytherapy for drug-resistant epilepsy.
Mechanisms of action of current ASMs
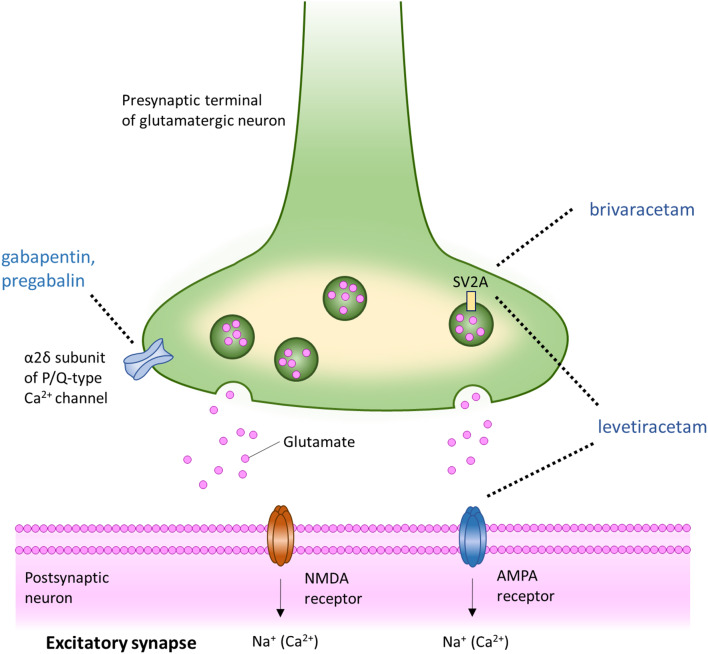
Current ASMs have diverse mechanisms of action. Some of them block voltage-gated Na+ channels (VGSCs) or voltage-gated Ca2+ channels (VGCCs), increase GABA concentrations, block glutamate receptors, inhibit carbonic anhydrase, activate GABAA receptors (GABAAR) r or modulate synaptic vesicles [11]. Most of the ASMs are multitargeted drugs, although in each drug one of the neurochemical mechanisms appears to predominate. Of the marketed ASMs, phenytoin, primidone, carbamazepine, oxcarbazepine, eslicarbazepine, lamotrigine, rufinamide, and zonisamide bind to VGSCs in their state of fast inactivation (within milliseconds) and induce transient, voltage- and frequency-dependent reduction in the channel conductance [12]. Lacosamide was reported to block slow inactivation (within seconds and beyond) of VGSCs through binding to sodium channel in its state of slow inactivation and inducing its long-lasting, voltage- and frequency-dependent reduction in the channel conductance. Alternatively, its mode of action can be linked to its slow dissociation from the target molecule [13]. Reduction of low-threshold calcium current in thalamic neurons by ethosuximide or sodium valproate prevents synchronized depolarization, particularly in thalamic-cortical circuits [14]. Among ASMs that recover the balance between the excitatory glutamatergic and inhibitory GABAergic transmission on the receptor levels are phenobarbital (a positive allosteric modulator of the GABAA receptor and an antagonist of the glutamatergic AMPA receptors), felbamate (an antagonist of the NMDA receptors and an allosteric positive modulator of the GABAAR), perampanel (a selective non-competitive antagonist of the glutamatergic AMPA receptors) and clobazam (a positive allosteric modulator of the GABAAR. Cenobamate is a positive allosteric modulator of the high-affinity GABAAR, and it blocks persistent rather than transient sodium currents. ASMs that enhance the synaptic GABA level, include vigabatrin and tiagabine. Vigabatrin irreversibly inhibits GABA-aminotransferase (transaminase) - the enzyme responsible for the metabolic degradation of GABA - leading to a global increase in brain GABA concentration. Tiagabine inhibits the re-uptake of GABA from the synaptic cleft into nerve terminals and glial cells by selective action on the GAT-1 transporter, resulting in a transient increase in synaptic GABA levels. ASMs with complex and only partially recognized mechanisms of action comprise valproate (a voltage-gated sodium channel blocker, an inhibitor of the GABA degradative enzymes and an inhibitor of GABA re-uptake), topiramate (a modulator of the voltage-dependent sodium channels, an enhancer of GABA inhibition, an inhibitor of excitatory neurotransmission, an inhibitor of carbonic anhydrase and possibly a modulator of the voltage- and receptor-gated calcium ion channels) and stiripentol (a positive allosteric modulator of the GABAAR which also interferes with GABA reuptake and metabolism) [15, 16]. Carbonic anhydrase inhibitors, such as acetazolamide, topiramate, and zonisamide, restore the equilibrium among CO2, H+, and HCO3−, reduce the NMDA-mediated excitation, potentiate the GABAAR- mediated inhibition, and affect the activity of ligand-gated Ca2+ channels and voltage-gated Ca2+ channels (VGCC) [17, 18]. There are also several ASMs with predominant mechanisms of action that are not related to modulation of the voltage- and ligand-gated sodium and calcium channels or to enhancement of the GABA brain concentration, such as everolimus (an inhibitor of the mTORC1), fenfluramine (a serotonergic 5-HT2 receptor agonist and a σ1 receptor antagonist) and cannabidiol. Cannabidiol has a complex and partly unknown mechanism of antiseizure effects. It probably reduces neuronal hyper-excitability through modulation of intracellular calcium via G protein-coupled receptor 55 (GPR55), transient receptor potential vanilloid 1 (TRPV-1) channels, and upregulation of the peroxisome proliferator-activated receptor gamma (PPAR-γ) [19, 20]. ASMs inhibit or prevent seizures, but they neither cure epilepsy nor prevent epileptogenesis. Since epilepsy and epileptogenesis seem to be connected with the pathological transformation of neuronal circuits [21], drugs that interfere with molecular mechanisms of synaptic plasticity merit special attention [22]. Two classes of ASMs with unique mechanisms of action are racetams (levetiracetam, brivaracetam) and gabapentinoids (gabapentin, pregabalin), which modulate the neurotransmitter release via binding to glycoprotein 2 A (SV2A) in synaptic secretory vesicles and α2δ subunits of presynaptic voltage-gated calcium channels, respectively [15]. Figure 1.
Fig. 1.
The main neuronal targets of racetams and gabapentinoids (inspired by the figure in the paper by Bialer and White [166])
Presynaptic release machinery as targets for ASMs
Since an imbalance in excitatory versus inhibitory neurotransmission is thought to be the key player in the pathophysiology of seizures, it implies that the presynaptic release machinery of these neurons could serve as an important target for ASMs and possibly for designing epilepsy-modifying drugs. The molecular presynaptic events include the neurotransmitter cycle (biosynthesis, storage, reuptake, and degradation of neurotransmitters) and the synaptic vesicle cycle (targeting synaptic vesicles (SVs) to the nerve terminal and their docking, fusion, endocytosis, and recycling) [23]. Each of these processes in the overlapping presynaptic cycles may affect the mode and the amount of neurotransmitter released. Both GABA and glutamate accumulate in SVs, which undergo exocytosis within the active zone of the synapse. High rates of exocytosis depend on the efficient packaging of neurotransmitter into SV and the fast recycling of SV from the presynaptic plasma membrane or via an endosomal intermediate. The SV recycling comprises sorting of its proteins from proteins of the plasma membrane, clathrin-regulated endocytosis, and docking of SV at the plasma membrane. Activation of presynaptically localized voltage-gated calcium channels leads to a local elevation of Ca2+ in the nerve terminal which regulates vesicle fusion [24]. Several proteins that are constituents of the SV cycle have been identified and their role partially unraveled [25]. Among SV proteins involved in targeting and docking are synapsins which link SVs to the actin cytoskeleton, piccolo and bassoon engaged in active zone assembly, and the Sect. 6/8 complex which defines the site of the active zone. Several synaptic proteins such as synaptobrevin, vesicle-associated SNARE (v-SNARE), and two target or plasma membrane SNAREs (t-SNAREs), synaptosomal associated protein 25 (SNAP25) and syntaxin form a stable complex that is probably engaged in both SV docking and fusion [26]. Proteins that are involved in modulation and Ca2+ dependence of the synaptic vesicle cycle are represented by C1 and C2 domain protein (Munc13) required for neurotransmitter release, N, and P/Q-type Ca2+ channels, Ca2+ sensor (synaptotagmin) C2 domain SV protein; and SV2 transporter which inhibits SV fusion. The synaptic vesicle glycoprotein 2 A is selectively expressed in the vesicular membranes of neuronal terminals and - via the modulation of the calcium sensor - synaptotagmin-1 activity - it regulates the Ca2+−dependent synaptic exocytosis. It may be engaged in the stabilization of vesicular loading of neurotransmitters, vesicular transporting, vesicular proteins anchoring, and vesicle trafficking [27, 28]. To better understand the role of SV2A in neurotransmitter release, Bradberry and Chapman (2022) used a new chemogenetic approach for all-optical monitoring of excitation-secretion coupling in knockout (KO) phenotype in cultured hippocampal neurons. This method allowed for the detection of the presynaptic Ca2+ influx and glutamate release at the same axonal boutons. The authors showed that a loss of SV2A decreased glutamate release without reducing the Ca2+ influx at the hippocampal nerve terminals. These data indicate that SV2A, which is trafficked to synaptic vesicles, supports vesicle fusion by increasing the efficiency of the Ca2+−regulated membrane fusion machinery [29]. Accumulating data indicate that malfunctioning of the presynaptic release machinery plays an important role in the pathomechanisms of seizures [30]. Hence, disturbances of mechanisms regulating the physiological synergy among presynaptic and postsynaptic components due to mutations of genes encoding some synaptic proteins lead to developmental and epileptic encephalopathies, known as “synaptopathies” [31]. Of them, mutation of the CPLX1 gene encoding the complexin 1 which interacts with the SNARE complex, the STXBP1 gene which encodes the crucial for synaptic exocytosis Syntaxin1a binding protein, and the PRRT2 gene which encodes the presynaptic proline-rich transmembrane protein 2 (Prrt2) are associated with epileptic symptoms [31]. As far as the preclinical research is concerned, convincing evidence for the role of the presynaptic neurotransmitter release machinery in seizures comes from studying Tetanus neurotoxin. Thus, an intracortical injection of Tetanus neurotoxin which cleaves the synaptic protein VAMP/synaptobrevin induces pharmacoresistant and refractory focal cortical hyperexcitability and electrographic seizures in rodents [32, 33]. Furthermore, Vannini et al. (2020) showed in the mouse model of neocortical epilepsy induced by Tetanus neurotoxin an early-onset lengthening of active zones at inhibitory synapses and a delayed spatial reorganization of recycled vesicles at excitatory synapses [34]. Regarding other presynaptically localized proteins, the role of dynamin in clathrin-mediated endocytosis and synaptic vesicle recycling in epilepsy has been postulated [35]. Dynamin 1 (DNM1) is a guanosine triphosphatase engaged in clathrin-mediated endocytosis, and de novo mutations in synaptic transmission genes including DNM1 were reported to be a causative factor in epileptic encephalopathies [36]. Other investigators found that the dynamin 1 expression pattern was altered in the pilocarpine rat model of epilepsy and patients with temporal lobe epilepsy. Moreover, in the above study, inhibition of dynamin diminished seizures [37]. More recently, a new class of GTP-competitive dynamin inhibitors which inhibit dynamin I GTPase and clathrin-mediated endocytosis showed a significant anti-seizure activity in a 6 Hz mouse psychomotor seizure test [38]. The role of Syntaxin 7 (STX7) - a member of the SNARE superfamily involved in membrane fusion - was investigated in the models of kainate-induced seizures and PTZ-induced kindling. In both models of epilepsy, the STX7 expression was decreased in the brain tissue. It was also observed that overexpression of STX7 alleviated seizures, while the downregulation of this protein evoked opposite effects [39]. The above-cited reports suggest that some proteins involved in presynaptic release machinery, such as dynamin and STX7, may be considered targets for novel ASMs, but future studies should show which of these proteins may be druggable. Unsurprisingly, more extensive studies were devoted to the engagement of SV2A in the pathomechanism of seizures, since this protein is an established target for the already marketed ASMs. It has been shown that the dysfunction of SV2A which regulates the action potential-dependent neurotransmitter release may be involved in epileptogenesis since SV2A expression in the brain was elevated specifically in the dentate hilus in PTZ kindling and down-regulated in the anterior temporal neocortex in patients with intractable temporal lobe epilepsy and focal cortical dysplasia [40]. On the other hand, the physiological level of SV2A prevents seizures, because the homozygous sv2a knockout leads to a lethal seizure phenotype in mice, probably resulting from an imbalance between the inhibitory and excitatory neurotransmission [41]. This hypothesis has been supported by electrophysiological studies which demonstrated that sv2a deletion reduces the action of the potential-dependent release of GABA in the CA3 region of the hippocampus [41], while the cultured hippocampal neurons from sv2a/2b double knockouts display a sustained increase in the excitatory calcium-dependent synaptic neurotransmission [42]. Also, another body of evidence points to a possible role of SV2A in epileptogenesis. To this end, the microarray study on ipsilateral hippocampal CA3 and entorhinal cortex showed that sv2a was transiently downregulated in the entorhinal cortex during pilocarpine-induced epileptogenesis in rats [43]. Furthermore, van Vliet et al. (2009) conducted a comparative study on the expression of SV2A protein in resected temporal lobe specimens from patients with refractory epilepsy and in the hippocampi of rats subjected to experimental epileptogenesis by tetanic stimulation of the angular bundle. They found that the SV2A immunoreactivity was decreased in all specimens from patients with confirmed hippocampal sclerosis, whereas the SV2A protein expression in the hippocampus of rats in the chronic epileptic phase was also reduced, but only in those animals with a progressive form of epilepsy [44]. The authors suggested that a decreased expression of SV2A did not cause immediate seizures but could contribute to a state of heightened epileptogenicity. This assumption has been supported by the study performed by Kaminski et al. (2009), who showed that SV2A (+/−) heterozygous mice had a high sensitivity to seizures evoked by kainate, pilocarpine, PTZ, and 6-Hz electrical stimulation, but no such sensitivity was seen in the model of MES. The aforementioned investigators also observed that SV2A (+/−) heterozygous mice showed accelerated epileptogenesis in the amygdala and corneal kindling models, suggesting that even a partial SV2A insufficiency may accelerate epileptogenesis and increase the vulnerability to seizures [45]. Interestingly, studies using animals with the Sv2a missense mutation revealed that the dysfunction of SV2A preferentially disrupts the action potential-induced GABA, but not glutamate, released in the hippocampus and amygdala and facilitates kindling, which points to the important role of SV2A in GABA neurotransmission [40]. Loss of SV2 affects the calcium-stimulated exocytosis and lack of SV2 proteins, presynaptic calcium accumulation during consecutive action potentials produces abnormal enhancement in neurotransmitter release that destabilizes the synaptic circuits and evokes seizures [42]. As pointed out by Ciruelas et al. (2019), since SV2A is the primary SV2 paralog in the majority of GABAergic neurons, reductions in its expression could potentially contribute to the etiology of epilepsy and that precise control of SV2A expression is needed to prevent aberrant excitability [46]. The intriguing hypothesis addressing a possible relationship between a lowered SV2A expression and the progressive nature of the resultant epileptic state has been questioned and needs further evidence [47].
Kaminski et al. (2008) demonstrated that there is a strong correlation between the SV2A binding affinity of structurally diverse ligands and their anticonvulsant potency in vivo [48]. This hypothesis might contradict the fact that the absence of SV2A in mice led to an epileptic phenotype [41], but the elevated expression of SV2 was also reported to result in a neurotransmission phenotype that resembled that seen in SV2 KO neurons [49] suggesting that either too little or too much SV2A activity may promote neurological abnormality [27]. Furthermore, although SV2A is localized at both inhibitory and excitatory synapses, and the expression of SV2A is ubiquitous, stronger associations between SV2A and GABAergic rather than glutamatergic synapses were observed in some brain structures [50]. SV2A regulates the size of the readily releasable pool of vesicles in the majority of GABAergic neurons, and it has been suggested that reductions in its expression could potentially contribute to the etiology of epilepsy [46]. It has been postulated that reduced expression of SV2A in the hippocampus does not cause immediate seizures but it could contribute to the increased epileptogenicity [44]. On the other hand, pilocarpine-induced seizures– a well-validated model of temporal lobe epilepsy– lead to overexpression of SV2A, which can be normalized by brivaracetam. Bivaracetam treatment ameliorated the over-expression of SV2A in the hippocampus and rescued the synaptic dysfunction in epileptic rats [51]. This drug has been found to modulate short-term synaptic potentiation and abnormal low-frequency brain activities during the interictal phase of epileptic seizures by slowing down the mobilization of synaptic vesicles [52]. Overall, it seems safe to assume that racetams „normalize” SV2A functions which is in line with the view that both SV2A overexpression and SV2A deficits seem to have negative effects on neurotransmission and excitability [53].
Neurochemical mechanisms of levetiracetam and brivaracetam
The presynaptic inhibitory effect of levetiracetam on the activity-dependent glutamate and GABA release from rat brain slices, the said effect occurring in a use-dependent manner, was reported [54]. Lynch et al. (2004) first provided several lines of evidence that the synaptic vesicle protein SV2A was the binding site for levetiracetam ((S)-alpha-ethyl-2-oxo-pyrrolidine acetamide). They demonstrated that the levetiracetam binding site was enriched in synaptic vesicles, established its apparent molecular weight, and found that synaptic vesicles from homozygous SV2A knockout mice did not bind labeled levetiracetam. They also observed that levetiracetam bound to SV2A, but not to SV2B and SV2C isoforms expressed in the fibroblasts. Moreover, a strong correlation was found between the affinities of the above compound for SV2A and its antiseizure efficacy in the audiogenic animal model of epilepsy [55]. The anticonvulsant efficacy of levetiracetam was also reduced in heterozygous SV2A+/- mice (the expression of the SV2A protein was decreased by 50%), which lends further support to the notion that SV2A is the primary target for seizure protection [45]. Brivaracetam was identified as a result of screening of 12,000 compounds for binding affinity to the synaptic vesicle protein 2 A (SV2A). Brivaracetam binds selectively in a reversible, saturable, and stereospecific fashion - and with a 20-fold higher affinity than levetiracetam - to SV2A in rat and human brains, and no specific binding was detected in the brain of SV2A(-/-) knock-out mice. The effects of brivaracetam on the synaptic plasticity in experimental models of epilepsy have been demonstrated. Thus, chronic administration of brivaracetam ameliorated the over-expression of SV2A and the synaptic dysfunction in the pilocarpine model of temporal lobe epilepsy in rats. These effects were accompanied by normalization of fast phosphorylation of the synaptosomal-associated protein 25 (SNAP-25) during long-term potentiation in the epileptic rats, which may be relevant to the modulation of SV exocytosis and activity of voltage-gated calcium channels [51]. Based on its affinity and pharmacokinetic parameters, it is predicted that at therapeutic concentrations, brivaracetam should occupy over 80% of SV2A in the human brain [56]. Brivaracetam decreased the short-term synaptic potentiation and abnormal low-frequency brain activities during the interictal phase of epileptic seizures by slowing down the mobilization of synaptic vesicles in two rodent models of epilepsy evoked by pilocarpine or high potassium concentrations [52]. Repeated administration of brivaracetam reduced the over-expression of SV2A. It alleviated the abnormal SNAP-25 phosphorylation at Ser187 during long-term potentiation (LTP) induction in epileptic rats, which is relevant to the modulation of synaptic vesicle exocytosis and voltage-gated calcium channels. The exact mechanism of anti-seizure effects of SV2A ligands has not been elucidated [15]. It has been presumed that levetiracetam reduces the excitatory neurotransmitter release during epileptic high-frequency activity by accessing its binding site through vesicular endocytosis into excitatory synaptic terminals. However, as shown by patch-clamp recording, levetiracetam evoked a similar, frequency-dependent effect on both inhibitory postsynaptic currents (IPSCs) and excitatory postsynaptic currents (EPSCs) in the hippocampal neurons; thus, its effects on pathological discharges remain yet to be elucidated [54]. Contreras-García et al. (2022) discussed the main hypothesis referring to the effect of levetiracetam on SV2A. Thus, blocking of SV2A by levetiracetam may prevent the SV2A-induced vesicular priming, decrease the size of the readily releasable pool of neurotransmitter and decrease the synaptic transmission. Alternatively, levetiracetam could stabilize the best functional conformation of SV2A and improve synaptic vesicle exocytosis [57]. Both SV2A overexpression and SV2A deficits exert negative effects on neurotransmission. Treatment with levetiracetam was shown to reestablish normal neurotransmission and restore the normal levels of SV2 and synaptotagmin in the overexpressed SV2A synapses [49]. Of note, levetiracetam is regarded as a multitargeted drug because - apart from interacting with SV2A protein - it triggers several other pre- and postsynaptic effects. At the presynaptic level, it reduces the potassium currents, and calcium transients of ryanodine and inositol 1,4,5-trisphosphate receptor (IP3R), modulates the level of glutamic acid decarboxylase, and increases the activity of GABA transaminase (GABA-T). At the post-synaptic level, it modulates the AMPA receptors [57]. A radioligand binding study revealed that levetiracetam and brivaracetam may interact differently with the SV2A protein binding sites or interact with different conformational states of this protein [58]. Furthermore, a recent electrophysiological patch-clamp study showed that brivaracetam dose-dependently inhibited the depolarization-induced M-type K+ current (IK(M)), decreased the delayed-rectifier K+ current (IK(DR)), decreased the hyperpolarization-activated cation current and had a concentration-dependent inhibitory effect on the voltage-gated Na+ current (INa) in the GH3 neurons. It is suggested that brivaracetam may have a multiple ionic mechanism of action in disorders linked to neuronal hyperexcitability [59]. However, in contrast to carbamazepine, brivaracetam did not affect sustained repetitive firing in cultured cortical neurons and in CA1 neurons, which indicates that its effect on the voltage-gated sodium channels does not contribute to its antiepileptic activity [60]. Using the SV2A knockout model in zebrafish, Zhang et al. (2022) observed that homozygous SV2A -/- mutant zebrafish larvae, but not SV2A +/- and SV2A +/+ larvae, showed a spontaneous epileptiform activity without brain malformations. Both valproate and, surprisingly, levetiracetam partially reduced the epileptiform activity, which suggests that besides SV2A, other mechanisms may be involved in the antiepileptic effects of levetiracetam [61]. As far as the tripartite synaptic transmission is concerned, it has been reported that levetiracetam and brivaracetam - through the inhibitory effect on SV2A - suppress the glutamate release from astrocytes during high-frequency oscillation bursts. The authors suggest that the suppression of SV2A function and the subsequent inhibition of turnover prolongation of the activated astroglia hemichannels may contribute to the mechanism of anti-seizure effects of levetiracetam and brivaracetam [62, 63]. No data have been found in PubMed that would indicate that ASMs other than racetams have been tested regarding their affinities to SV2A. However, padsevonil, an antiepileptic drug candidate with a high affinity for SV2A, SV2B, and SV2C isoforms and low-to-moderate affinity for the benzodiazepine binding site on GABAA receptors, has been recently designed [64].
Pharmacological profiles of levetiracetam and brivaracetam in rodent models of seizures and epilepsy
Levetiracetam shows a high anti-seizure activity in electrically and PTZinduced kindling in mice, as well as in pilocarpine- and kainic acid-induced secondary generalized seizures in mice and rats. In contrast, levetiracetam was ineffective in the acute MES test, in the maximal PTZ seizure test in mice, and in several maximal chemoconvulsive seizure tests (Table 1). Furthermore, levetiracetam displayed a very high safety margin in animal models of seizures [65]. Matagne et al. (2008) conducted a comparative in vitro and in vivo study of the anti-seizure activity of brivaracetam and levetiracetam. They reported that brivaracetam was more potent than levetiracetam in reducing epileptiform responses in rat hippocampal slices. As opposed to levetiracetam, brivaracetam protected mice against MES or maximal doses of PTZ-induced seizures. Furthermore, brivaracetam showed a higher than levetiracetam activity against secondarily generalized motor seizures in corneally kindled mice and hippocampal-kindled rats. Brivaracetam was also more effective than levetiracetam in suppressing clonic convulsions in audiogenic seizure-susceptible mice and spontaneous spike-and-wave discharges in genetic absence epilepsy rats [66]. Brivaracetam differs markedly from levetiracetam by its distinct interaction with SV2A, higher lipophilicity, faster brain penetration, and a more profound anti-seizure activity in animal models including amygdalar kindling in mice [67]. Racetams have been also tested in models of genetic epilepsy such as genetically sound-sensitive mice derived from a DBA strain and in the Genetic Absence Epilepsy Rats from the Strasbourg (GAERS) strain. Padsevonil provided potent, dose-dependent protection against seizures induced in sound-sensitive mice, a genetic model of generalized epilepsy. Brivaracetam was also active in this model, while levetiracetam showed a lower potency, correlating with their SV2A binding affinity [48, 66] In the GAERS model which is considered predictive of human absence epilepsy, padsevonil showed a higher potency than brivaracetam, and levetiracetam had a weak effect in this model. It is noteworthy that these drugs suppressed spontaneous spike-wave discharges (SWDs) in doses correlating with their affinity for SV2A [48, 66, 68]. Of new genetic models, recent data showed that levetiracetam significantly reduced ECoG spike frequency medication in a mouse model of potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 1 (HCN1) developmental and epileptic encephalopathy [69].
Table 1.
Anticonvulsant potencies of levetiracetam, brivaracetam, gabapentin and pregabalin in the commonly used acute experimental seizure models in mice
| ASM \ Model | MES | PTZ | 6 Hz (32 mA) | 6 Hz (44 mA) |
|---|---|---|---|---|
| Levetiracetam | N.A. | N.A. | 14.42a | 345.4b |
| Brivaracetam | 113c | 30c | 4.4d | N.D. |
| Gabapentin | > 400f | 199.3e | 72.11a | N.D. |
| Pregabalin | 130.3f | N.D. | 31.66a | N.D. |
Data are presented as median effective doses (ED50 values in mg/kg) of the ASMs. MES– maximal electroshock-induced generalized tonic seizures; PTZ– pentylenetetrazole-induced generalized clonic seizures; 6 Hz–6 Hz corneal stimulation-induced secondary generalized motor seizures; N.A.– not active; N.D.– not determined. a– results from [77]; b– results from [167]; c– results from [66]; d– results from [168]; e– results from [133]; f– results from [130]
Interaction profile of levetiracetam when combined with other ASMs– a preclinical perspective
Levetiracetam is considered to be “virtually ineffective” in the acute mouse generalized tonic-clonic (MES) and clonic (PTZ-induced) seizure models (Table 1). However, based on type II isobolographic analysis, it was found that levetiracetam produced either synergistic or additive interactions with carbamazepine, oxcarbazepine, topiramate, felbamate, and retigabine in the mouse MES model [70–72]. Levetiracetam produced also additive interactions with phenobarbital, valproate, phenytoin, lamotrigine, and pregabalin in the mouse MES model [70, 72]. In the mouse PTZ-induced seizure model, levetiracetam combined with gabapentin, clonazepam, ethosuximide, phenobarbital, and valproate produced synergistic or additive interactions, depending on the proportions of the ASMs used in the mixtures. The additive interactions were observed for the combinations of levetiracetam with tiagabine and vigabatrin in the mouse PTZ-induced seizure model [73, 74]. On the other hand, experimental evidence indicates that levetiracetam is fully effective in the mouse 6 Hz corneal stimulation-induced secondarily generalized motor seizure model (Table 1).
In this seizure model, the type I isobolographic analysis revealed that levetiracetam combined with lacosamide [75], phenobarbital [76], gabapentin, pregabalin, and retigabine [77] evoked supra-additive (synergistic) interactions. Levetiracetam combined with clonazepam, oxcarbazepine, tiagabine, and valproate triggered additive interactions in the mouse 6 Hz corneal stimulation-induced (32 mA) seizure model [76]. Additionally, levetiracetam in combination with the galanin agonist (810-2) produced supra-additive (synergistic) interaction in the mouse 6 Hz corneal stimulation-induced (32 mA) seizure model [78]. Levetiracetam in combination with JNJ-46,356,479, JNJ-42,153,605, JNJ-40,411,813, and LY-404,039 (four positive allosteric modulators of the metabotropic glutamate receptor subtype 2) resulted in supra-additive interactions in the mouse 6 Hz corneal stimulation-induced (44 mA) seizure model [79, 80]. Although neither the galanin agonist nor the positive allosteric modulators of the metabotropic glutamate receptor subtype 2 are ASMs, the interactions of levetiracetam with these agents were assessed using the isobolographic analysis of interaction. This was the reason for including the results of these experiments in the present review to attract attention to novel potentially effective drug candidates with unique molecular mechanisms of action, especially if these combinations evoked synergistic interactions in preclinical studies.
Considering the interaction profile of levetiracetam, one can ascertain that when combined with various ASMs, the drug triggered either synergistic or additive interactions in various acute models of experimentally evoked seizures in rodents. No antagonistic interactions between levetiracetam and ASMs were detected while using the isobolographic analysis of interactions.
The isobolographic analysis of interactions is the best method employed in pharmacological and toxicological studies to classify the pharmacodynamic interactions between ASMs [81, 82]. More detailed information on type I and II isobolographic analyses used in experimental epileptology can be found elsewhere [83].
In this review, we assessed and described only the interactions evoked by levetiracetam, gabapentin, and pregabalin with the currently available ASMs, based on the aforementioned type I and II isobolographic methods. Preclinical experiments assessing the effect of other drugs or compounds on the anticonvulsant potencies of the three ASMs (levetiracetam, gabapentin, and pregabalin) evaluated with a subthreshold method or other methods, which neglected the effects triggered by the tested compounds, were not considered herein.
Clinical use of racetams in the treatment of epilepsy
Racetams are a class of drugs that share a pyrrolidone nucleus but possess diverse pharmacological activities and no well-defined mechanism of action. Of them, piracetam, aniracetam, oxiracetam, pramiracetam and phenylpiracetam show procognitive properties via positive allosteric modulation of the AMPA receptors, while levetiracetam, brivaracetam, and seletracetam belong to ASMs [84] [Löscher and Richter, 2000]. Levetiracetam is an ASM approved for the treatment of focal and generalized epilepsy, both in adults and children. The recommended dose range in adults is 1000–3000 mg/day. The advantages of levetiracetam are non-hepatic metabolism and lack of major interactions with other drugs which makes it a suitable medication for the elderly [85]. Along with lamotrigine and oxcarbazepine, it is also one of the safest ASMs used in the treatment of women at the childbearing age, with the potential teratogenic effect within the range of the population risk of major congenital defects [86–88]. Levetiracetam is also recommended in the treatment of status epilepticus due to its availability in the intravenous formula [5–7]. The limitations of therapy with levetiracetam are serious psychiatric side effects which can occur in about 13–30% of patients treated with this drug. The side effect spectrum consists of irritability and aggression and can be aggravated by psychosis, depression, and suicidal ideation [92]. The mechanism that can underlie these effects might be related to the levetiracetam impact on the AMPA receptor [93]. It has also been proven that the effectiveness of levetiracetam is lower than that of valproate in the treatment of idiopathic generalized epilepsies [94] and lower than lamotrigine in the treatment of focal epilepsies [95], which puts into question its use as a first-line drug in the treatment of epilepsy [96]. However, levetiracetam is currently widely used both as the first-choice drug or add-on therapy for the treatment of focal seizures, myoclonias, and generalized tonic-clonic seizures [97].
Brivaracetam has been approved for the treatment of focal seizures in adults and children ≥ 4 years of age, with an effective dose of 50–200 mg/day in the treatment of adults [98]. According to the US Food and Drug Administration (FDA), this indication includes monotherapy and adjunctive therapy, although brivaracetam has not been specifically evaluated in clinical trials for initial monotherapy and is only registered in add-on therapy in Europe [97]. Brivaracetam also proves to be effective in generalized epilepsy; however, it is used off-label in keeping with this indication [99]. It is metabolized in the liver and therefore its maximal dose has to be reduced by 1/3 in patients with liver impairment. It has a low potential for clinically relevant drug-drug interactions and can be used in the elderly [98]. Brivaracetam is available in an intravenous formula; which is why it can be a promising new option for the treatment of status epilepticus in the future [100]. The post-hoc results of clinical studies suggest that brivaracetam may be effective in the treatment of epilepsy in patients in whom treatment with levetiracetam has failed [101]. However, brivaracetam does not seem to be as effective in the treatment of epilepsy as it was previously thought, taking into consideration its superiority over levetiracetam in experimental studies [102, 103], where it was 15–30 times more potently bound to the SV2A molecule than levetiracetam and it entered the brain much faster due to its lipophilic properties [104]. Nevertheless, brivaracetam seems to have a better safety profile allowing for the use of the medication in the treatment of patients in which levetiracetam had to be discontinued due to psychiatric side effects [92, 105]. The optimal scheme in which the drugs should be replaced is not clear; however, the most frequently used ratio is 10:1 (for example, 1000 mg of levetiracetam is the equivalent of 100 mg of brivaracetam) or 15:1 [103, 106]. The results of observational studies suggest that this change can be done overnight without fearing new side effects development or deterioration of the previously experienced side effects [102, 103, 107]. Treatment with brivaracetam can, however, result in adverse events similar to these observed while administering levetiracetam, among them are somnolence, fatigue, irritability, dizziness, insomnia, anxiety, and depression [98]. Despite close structural similarities, various racetams have different pharmacological and clinical profiles [108]. Although piracetam was marketed 50 years ago, its mechanism of action is still poorly understood. Originally marketed by UCB Pharma in 1971, piracetam was the first nootropic drug to modulate cognitive function without causing sedation or psychostimulation. The mechanism presumably comprises the restoration of cell membrane fluidity in elderly animals and humans, modulation of cholinergic and glutamatergic transmission, improvement of neuroplasticity, and facilitation of microcirculation [109]. Levetiracetam and other S-enantiomers, but not piracetam, show a high affinity to a brain-specific stereoselective binding site, later described as SV2A protein. Preclinical studies revealed that piracetam is more efficient in improving learning and memory, while levetiracetam shows a much higher efficacy than piracetam in preventing seizures. Piracetam has a moderate or no inhibitory effect on generalized tonic or clonic seizures. Still, it reduces the incidence and duration of spike-wave discharges in the model of absence seizures and the number of spikes/min in the cobalt-induced focal epilepsy model. Despite its negligible effects in most screening tests, it can significantly improve the efficiency of other ASMs via non-pharmacokinetic interactions [110]. Clinically, piracetam is mainly used in the treatment of post-stroke aphasia and age-related cognitive disorders, vertigo, dyslexia, and sickle cell anemia, and in very high doses it is also effective against cortical myoclonus [111]. On the other hand, levetiracetam is a widely used ASM in focal-onset, myoclonic and generalized tonic-clonic seizures. Further studies have supported the notion that piracetam-derived drugs can be roughly divided into two categories– cognitive enhancers and ASMs. To this end, a systematic review of the pharmacology of piracetam and piracetam-related drugs revealed that piracetam, oxiracetam, aniracetam, pramiracetam, and phenylpiracetam were used in the treatment of various cognitive impairments; this group of medications, phenylpiracetam was used for a wider range of indications showing more potency than piracetam [112]. Other piracetam-related drugs such as levetiracetam, seletracetam, and brivaracetam demonstrated antiepileptic activity, whereas their procognitive effects remained unclear. Although SV2A is regarded as the primary molecular target for antiseizure effects of levetiracetam and brivaracetam, new ligands of SV2A have been designed that show procognitive but not antiseizure properties [113]. Very recently, a highly selective SV2A ligand SDI-118 devoid of anticonvulsant activity was reported to display significant cognitive-enhancing effects in various animal tests and the first-in-human randomized controlled trial [114]. Overall, elucidation of the intriguing contribution of SV2A in anticonvulsive and/or procognitive effects of racetams warrants further studies.
Neurochemical mechanisms of gabapentin and pregabalin
Gabapentin has been synthesized as an analog of GABA, which is an inhibitory neurotransmitter in the central nervous system. Gabapentin, however, does not show any action similar to GABA or does not evoke any effect on the GABA receptors [115]. The mechanism of action of gabapentin consists of suppressing the subunit α2δ1 of the voltage-gated calcium channel, which results in the inhibition of calcium influx into the neuron and decreases its excitability. Pregabalin was synthesized in 2004 as a drug similar to gabapentin in its pharmacodynamics, but more potent in the suppression of the subunit α2δ1 of the voltage-gated calcium channel. The abundantly expressed in the nervous system membrane-anchored extracellular α2δ glycoproteins form auxiliary subunits of the voltage-gated calcium channels, which are essential components of excitable cells and, among others, regulate presynaptic neurotransmitter release. Mutations and polymorphisms of human (CACNA2D1-4) genes encoding for the four known α2δ proteins (isoforms α2δ1 to α2δ4) are linked with several central nervous system disorders including epilepsy [116, 117]. Using the cellular α2δ subunit triple-knockout/knockdown model, it has been demonstrated that the α2δ subunits play a crucial role in the formation and organization of glutamatergic synapses, especially in the presynaptic differentiation. There is also convincing research evidence that the α2δ subunits may be engaged in critical steps during synapse maturation [118]. Both α2δ1 and α2δ2 are the targets for gabapentin and pregabalin. Interestingly, when administered acutely, these drugs produce only a slight inhibition of the calcium channel currents. In contrast, their chronic administration inhibits the currents and impairs the trafficking of the α2δ subunits resulting in the inhibition of synaptic transmission [119]. Recent reports indicate that α2δ1 protein can interact not only with the voltage-dependent calcium channels but also with other presynaptic proteins, including the NMDAR, neurexin-1α and adhesion molecules (thrombospondins) (Table 2) It has been suggested that these interactions may contribute to the mechanisms of gabapentinoids therapeutic effects in neuropathic pain [120]. Other investigators speculated that the thrombospondin/α2 δ axis is important for the correct functioning of the cortico-thalamo-cortical circuits and that disturbances in this axis may contribute to the pathomechanisms of absence epilepsy [Celli et al., 2017]. AMPARs are complexes composed of glutamate A1 (GluA1), GluA2, GluA3, and GluA4 subunits which form functionally different heterotetramers [7]. Li et al. (2021) demonstrated that α2δ1 is a key AMPA receptor-interacting protein that controls the subunit composition and Ca2+ permeability of the postsynaptic AMPA receptors. They reported that α2δ1 inhibits the glutamate GluA1/GluA2 heteromeric assembly and increases the GluA2 retention in the endoplasmic reticulum, and these effects can be reversed by gabapentin. These results point to an important role of α2δ1 in modulating synaptic functions because the AMPA receptors which lack the GluA2 subunits are Ca2+-permeable and their presence is linked with some neurologic disorders [121]. Zhou et al. (2021) showed the interdependence of α2δ-1 and PKC phosphorylation in regulating synaptic trafficking and activity of NMDAR. Via the inhibition of α2δ1, pregabalin interferes with the phosphorylation of NMDAR, in this way affecting neuroplasticity, which, among others, is the key element of epileptogenesis [122]. Gabapentin dose-dependently reduced neuronal injury induced by cerebral ischemia-reperfusion preventing the oxidative stress-related autophagy via the activation of the phosphoinositide 3 kinase (PI3K)/Akt/mammalian (or mechanistic) target of rapamycin (mTOR) (PI3K/Akt/mTOR) pathway [123]. Of note, both the oxidative stress and PI3K/Akt/mTOR pathway are also linked with molecular mechanisms of epileptogenesis [124]. (Table 2).
Table 2.
The α2δ1-mediated effects of gabapentinoids
| Molecular target | Mechanism | Effect | References |
|---|---|---|---|
| VGCCs | inhibition of calcium influx into the neuron and decreases its excitability | anti-seizures | [115] |
| presynaptic proteins engaged in the organization of glutamatergic synapses | interference with the formation and maturation of glutamatergic synapses | anti-epileptogenic? | [118] |
| neurexin-1α, and adhesion molecules (thrombospondins) | inhibition of aberrant excitatory synaptogenesis |
analgesia, detrimental effect in the absence epilepsy |
[8, 120, 122] |
| AMPAR | controlling the subunit composition and Ca2+ permeability of postsynaptic AMPARs | analgesia | [121] |
| NMDAR | interference with the phosphorylation of NMDAR, in this way, affecting neuroplasticity | anti-epileptogenic? | [122] |
| PI3K/Akt/mTOR pathway | preventing the oxidative stress-related autophagy via the activation of the PI3K/Akt/mTOR pathway |
neuroprotective, anti-epileptogenic? |
[123] |
VGCCs - voltage-gated Ca2 + channels, AMPAR - α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, NMDAR– N-methyl-D-aspartate receptor, PI3K/Akt/mTOR - the phosphoinositide 3 kinase (PI3K)/Akt/mammalian (or mechanistic) target of the rapamycin (mTOR) pathway
The above-mentioned molecular mechanisms suggest that gabapentinoids may have modifying effects on the course of epilepsy development. Beyond the α2δ1 binding sites, gabapentin and pregabalin may show different affinities to other neuronal molecular targets. Thus, it was reported that gabapentin is a potent activator of the heteromeric KCNQ2/3 voltage-gated potassium channel and the homomeric KCNQ3 and KCNQ5 channels, whereas pregabalin at higher concentrations displays opposite effects [125].
Pharmacological profiles of gabapentin and pregabalin in rodent models of seizures and epilepsy
Gabapentin and pregabalin show similar, but not identical pharmacological profiles in animal seizure models. Both drugs display anti-seizure activity in primary generalized tonic-clonic seizures (maximal electroshock seizure threshold test [MEST]– gabapentin [126] and MES– pregabalin–focal seizures (6-Hz test; 32 or 44 mA), and focal seizures in kindling. They are inactive in the genetic models of absence seizures (GAERS or WAG/Rij rat strains). Gabapentin has been reported to decrease the duration of hyperthermia-induced seizures in Scn1a mutant rats [127] but had no significant effect on seizure frequency in the lethargic (lh/lh) mouse model of absence seizures [128].
Interaction profile of gabapentin when combined with other ASMs– a preclinical perspective
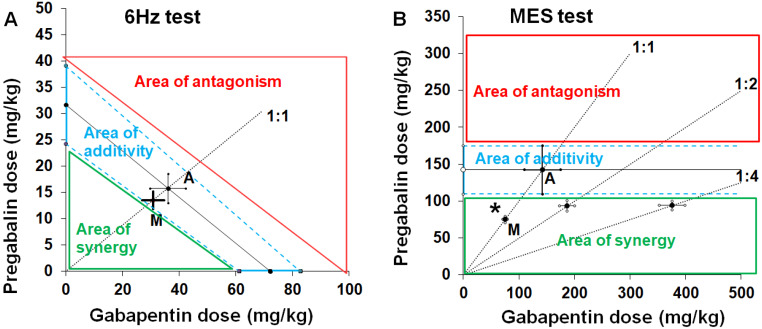
Gabapentin is considered to be “virtually ineffective” in the mouse MES model, but its interaction profile with other ASMs in this seizure model was determined with type II isobolographic analysis (Table 1). Preclinical studies provided evidence that gabapentin interacted both synergistically and additively with commonly used ASMs (including carbamazepine, phenytoin, phenobarbital, lamotrigine, valproate, oxcarbazepine, topiramate, tiagabine, and talampanel) in the mouse MES model [126, 129]. Because both pregabalin and gabapentin are characterized by similar molecular mechanisms of action, it was surprising to observe that the combination of pregabalin with gabapentin triggered a synergistic interaction in the mouse MES model with type II isobolographic analysis (Fig. 2B) [130], and simultaneously, an additive interaction in the mouse 6 Hz corneal stimulation-induced (32 mA) seizure model with type I isobolographic analysis (Fig. 2A) [77]. In the mouse PTZ-induced seizure model, gabapentin produced synergistic interactions with vigabatrin [131] and oxcarbazepine [132], and an additive interaction with tiagabine [133], felbamate, and loreclezole [134]. In the mouse 6 Hz corneal stimulation-induced (32 mA) seizure model, gabapentin combined with lacosamide triggered either a supra-additive interaction or an additive interaction [77]. Gabapentin combined with levetiracetam or retigabine triggered supra-additive interactions in the mouse 6 Hz corneal stimulation-induced (32 mA) seizure model [77]. (Fig. 2A-1B).
Fig. 2.
Isobolograms illustrating interactions between gabapentin and pregabalin in the 6 Hz corneal stimulation-induced seizure (A) and maximal electroshock-induced (MES) seizure (B) tests in mice. A. The type I isobolographic analysis was used to assess the interactions between two fully effective drugs in the mouse 6 Hz test (A). The isobologram for the combination of two fully active drugs, pregabalin and gabapentin in the 6 Hz test was presented earlier [77], and partly modified to display isobolographically areas of synergy, additivity and antagonism, where the experimentally derived ED50 mix value can be placed. The ED50 values for pregabalin and gabapentin when used alone in the 6 Hz test are placed on the ordinate (Y) and abscissa (X) of the Cartesian plot system of coordinates, respectively. The line connecting these ED50 values on the X and Y axes is the line of additivity. The diagonal line starting from the beginning of the Cartesian plot system and crossing the line of additivity indicates the proportion of the two-drug mixture (at the fixed-ratio of 1:1). For the interaction of pregabalin with gabapentin in the 6 Hz test, the experimentally derived ED50 mix (as the point M on the graph) is placed in the area of additivity, close to the point A, which reflects the theoretically calculated ED50 add value, predicted to exert additive interaction between drugs. B. The type II isobolographic analysis was conducted to evaluate the interaction between drugs of which gabapentin was ineffective in the mouse MES test (B). The isobologram for the combination of pregabalin (a fully active drug) and gabapentin (a virtually ineffective drug) in the MES test was presented earlier [130], and partly modified to display isobolographically areas of synergy, additivity and antagonism, where the experimentally derived ED50 mix value can be placed. The ED50 value for pregabalin when used alone in the mouse MES test is placed on the ordinate of the Cartesian plot system of coordinates. The line parallel to X axis starting from the ED50 for pregabalin is the line of indifference (additivity). The diagonal lines starting from the beginning of the Cartesian plot system and crossing the line of additivity indicate the proportions of the drugs in the mixture (at the fixed-ratios of 1:1, 1:2 and 1:4). For the interaction of pregabalin with gabapentin in the MES test, the experimentally derived ED50 mix (as the point M on the graph for the fixed-ratio of 1:1) is placed in the area of synergy, significantly below the area of additivity (*p < 0.05), which reflects the theoretically calculated ED50 add value, predicted to exert additive interaction between drugs
Interaction profile of pregabalin when combined with other ASMs– a preclinical perspective
Pregabalin evoked additive interactions with phenobarbital, oxcarbazepine, lamotrigine, topiramate, carbamazepine, valproate, and levetiracetam in the mouse MES model [135–138]. Pregabalin combined with lacosamide, levetiracetam, or retigabine triggered synergistic interactions in the mouse 6 Hz corneal stimulation-induced (32 mA) seizure model [77]. Pregabalin combined with gabapentin produced an additive interaction in the mouse 6 Hz corneal stimulation-induced (32 mA) seizure model [77], but the same two-drug combination triggered a synergistic interaction in the mouse MES model [130].
Interaction profile of pregabalin when combined in three-drug mixtures– a preclinical perspective
In contrast to several two-drug combinations, only a few three-drug combinations were evaluated experimentally in the mouse MES model. Experimental evidence indicates that the combination of pregabalin with phenobarbital and phenytoin (with a fixed ratio of 1:1:1) produced synergy in the mouse MES model [139]. Similarly, in the case of the combinations of pregabalin with phenobarbital and lamotrigine, pregabalin with phenobarbital and oxcarbazepine, and pregabalin with phenobarbital and topiramate [140], pregabalin with lacosamide and topiramate, pregabalin with lacosamide and oxcarbazepine, pregabalin with oxcarbazepine and topiramate [141], all the six combinations evoked synergistic interactions in the mouse MES model. Only the combination of pregabalin with lacosamide and lamotrigine produced an additive interaction in the mouse MES model [141]. To date, no other three-drug combinations of ASMs, containing levetiracetam and/or gabapentin have been tested experimentally in the animal seizure models.
Clinical use of gabapentinoids in the treatment of epilepsy
Gabapentin is a narrow-spectrum ASM approved for the treatment of focal epilepsy as monotherapy or adjunctive therapy in adults and children ≥ 12 years and in the treatment of neuropathic pain. The recommended dose in adults is 900–3600 mg/day. Gabapentin is also used off-label for generalized anxiety disorder and panic disorder, obsessive-compulsive disorder, addictions, bipolar disorder, essential tremor, and migraine prophylaxis [142, 143]. The medication is approved in Europe for initial monotherapy [97]. However, in a large comparative study, gabapentin was found to be less effective than lamotrigine [144]. The distribution of the drug and its non-linear absorption pharmacokinetics requires its dosing three times daily [145].
Pregabalin is indicated as the adjunctive therapy in epilepsy with focal seizures in adults. The recommended dose is 300–600 mg/day [146]. Other indications include neuropathic pain and generalized anxiety disorders. Off-label indications are similar to those of gabapentin [142]. Pregabalin is also used in the treatment of symptoms of fibromyalgia [147]. In focal seizures, pregabalin has been found to be less beneficial than lamotrigine as the first-choice drug [148] and probably should not be used as the first-line ASM [97]. However, the results of the study in which the conversion of previous treatment to pregabalin monotherapy was assessed were favorable for the drug [149]. Pregabalin shows more beneficial pharmacokinetics than gabapentin. The oral availability of pregabalin (90%) is higher than that of gabapentin (60%) and its pharmacokinetics is linear which allows for its use in a twice-daily regimen. The conversion of treatment from gabapentin to pregabalin may increase adherence to therapy, as it is usually more convenient for the patients to take their medications twice daily instead of three times per day. The scheme of drug replacement is 6:1, where a gabapentin daily dose of 1800 mg is the equivalent of a 300 mg dose of pregabalin [150].
Gabapentinoids (gabapentin and pregabalin) do not provoke any major drug-drug interactions. They are usually well tolerated; however, the side effects that may occur during treatment are somnolence, dizziness, weight gain, and peripheral edema [146]. Treatment with gabapentinoids also poses a risk of abuse and dependence, especially in patients abusing other drugs and substances in the past [142, 146]. Gabapentinoids can also pose a serious risk for respiratory depression, especially in patients using opioids subjects with pulmonary diseases, or in the elderly [150].
Drug combinations including levetiracetam, pregabalin, or gabapentin in therapy of epilepsy– a clinical perspective
If the treatment of epilepsy with the first ASM fails due to its ineffectiveness, the substitution monotherapy or adjunctive therapy options are equivalent. Adjunctive therapy for epilepsy is preferred when the first antiepileptic drug is well tolerated and only partially effective, or the drug to be added to the therapy has not been tested in monotherapy [151]. The adjuvant drug should not have any adverse pharmacokinetic interactions with the first antiepileptic medication or other concomitant drugs [152]. Rational polytherapy should maximize the efficacy and minimize the side effects [153]. The presented scientific data indicate that the combination of two ASMs with different mechanisms of action is better in balancing tolerance and effectiveness, and the combination of drugs with similar mechanisms is associated with increased side effects [154]. In human clinical trials, the synergistic effect of only one combination of lamotrigine and valproate has been confirmed [155, 156]. Regardless of the rather limited use of gabapentin and the widespread use of racetams in the treatment of epilepsy, medications from both these therapeutic groups are an important element of drug combinations in drug-resistant epilepsy. This is facilitated by the fact that representatives of both these groups are characterized by the lowest interaction potential among all ASMs [157].
Clinical studies indicated that the two-drug combination of gabapentin with carbamazepine produced seizure freedom in 37 patients with focal and generalized seizures [158, 159]. Additionally, the combinations of levetiracetam with carbamazepine, levetiracetam with lamotrigine, and levetiracetam with valproate successfully protected 23, 19, and 16 patients with focal and generalized seizures, respectively [159]. The most successful triple therapy regimens were those containing the combinations of levetiracetam with lamotrigine and valproate, lamotrigine with carbamazepine or lamotrigine with topiramate [159] or gabapentin with carbamazepine and topiramate [158].
Clinical possibilities for evaluating drug combinations of ASMs
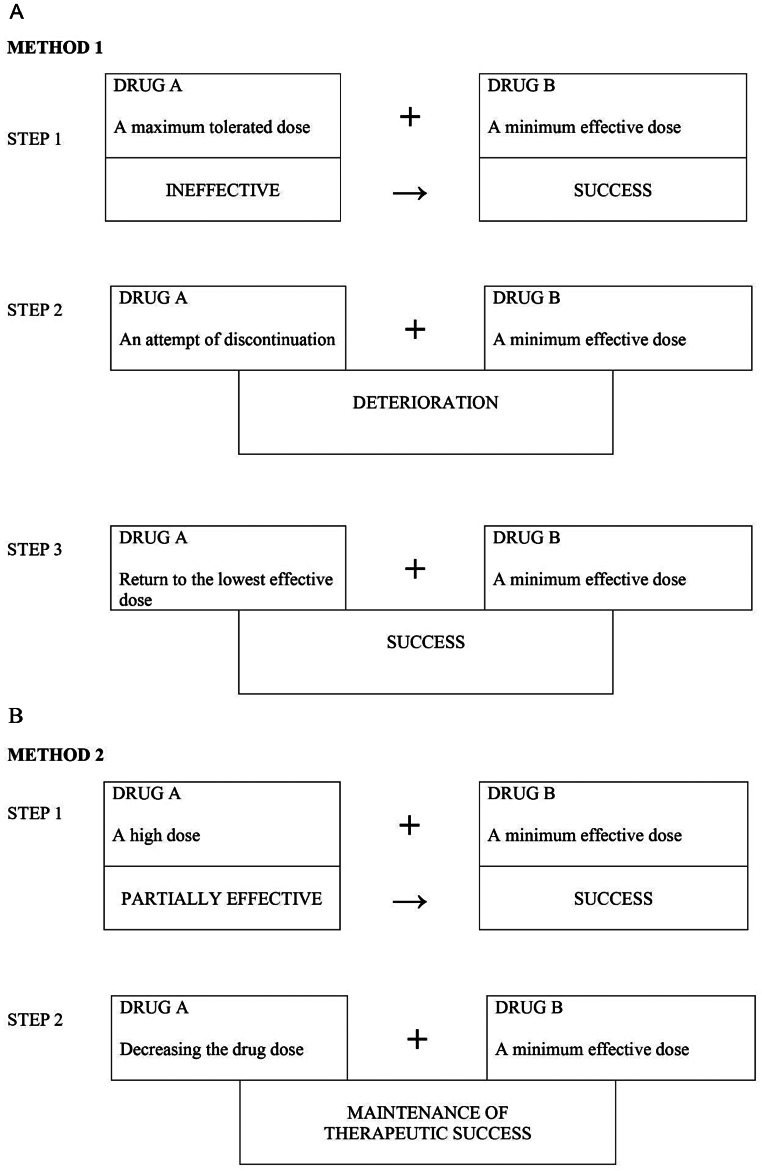
A clinical method of assessing the value of a drug combination was proposed by Stephen et al. [160]. The method evaluated the effectiveness of a combination of drug A and drug B in a situation where previously neither drug A nor drug B were effective in monotherapy when administered in maximum tolerated doses. Czapinski et al. (2014) proposed two other methods of clinical evaluation of the effectiveness of drug combinations (Fig. 3AB).
Fig. 3.
Two proposed methods of clinical evaluation of drug combinations.A. Method 1 assumes the ineffectiveness of drug A at the maximum tolerated dose and a therapeutic success after adding the minimum effective dose of drug B (first step), an unsuccessful attempt at discontinuing drug A (second step) and a repeated therapeutic success after reinstating drug A but at the minimum effective dose (third step). B. Method 2 assumes a partial effectiveness of drug A used at a high dose and a therapeutic success after adding a minimal effective dose of drug B (first step) and the maintenance of the said effect after lowering the dose of the first drug without attempting to discontinue its administration (step two)
An antagonistic combination in terms of adverse effects was considered when the result obtained before using the second medication was higher than after adding the second drug. Based on the proposed research method Czapinski et al. observed that the combination of levetiracetam + lamotrigine was synergistic in effectiveness and antagonistic in adverse symptoms and that the combination of topiramate + levetiracetam was synergistic in effectiveness and antagonistic in adverse symptoms in patients with focal impaired awareness automatism seizures progressing to bilateral tonic-clonic seizures. Furthermore, the combination of oxcarbazepine + levetiracetam was synergistic in effectiveness and antagonistic in adverse symptoms. Employing the above method, Czapinski et al. have found clinical evidence of the efficacy synergism and adverse symptoms antagonism in several combinations including gabapentin or levetiracetam: levetiracetam + lamotrigine, topiramate + levetiracetam, levetiracetam + oxcarbazepine, gabapentin + lamotrigine [147].
Discussion
It appears that combining ASMs with various mechanisms of action is more efficacious than using combinations of ASMs sharing the same or comparable mechanisms [161]. The results from combinations of gabapentin (or pregabalin) with other ASMs seem to support this assumption. For instance, synergistic combined treatments of gabapentin with carbamazepine, valproate, phenytoin, oxcarbazepine, topiramate, lamotrigine, or tiagabine involve ASMs affecting various brain targets [161]. Also, levetiracetam, in certain dose ratios, proved to be synergistic when combined with carbamazepine, oxcarbazepine, topiramate, felbamate, and retigabine. The same conclusion may be drawn when analyzing 3-ASM combinations. Considering the 6-Hz seizure model, synergistic interactions of levetiracetam with numerous ASMs, including gabapentinoids, need to be accentuated. Furthermore, the lack of synergy shown by the combined treatment of gabapentin + pregabalin in this test may be explained based on their identical mechanisms of action. However, at one fixed dose ratio, both the ASMs combined evoked a synergistic anticonvulsant interaction which is, however, not further seen at different dose ratios in the MES test. At present, there is no good explanation for this surprisingly beneficial interaction among these ASMs in this seizure model.
There is a good correlation between the experimental and clinical data on the nature of interactions among ASMs. Practically, only one contradictory result exists regarding the combined treatment of carbamazepine + lamotrigine shown as antagonistic by isobolography and claimed as positive from the clinical point of view [158]. This discrepancy probably arises from a different understanding of antagonism in experimental studies which may be more demanding. This problem has been discussed in detail in a review by Błaszczyk et al. (2018) [162]. Anyway, all the positive combinations of ASMs derived from experimental studies are highly likely to be clinically efficient although several limitations must be taken into consideration. In fact, among the undesired effects, only neurotoxicity was considered, and some other peripheral untoward actions might have been missed. Moreover, the experimental data were obtained after acute injections of ASMs, so a possibility thus arises that in clinical conditions more pharmacokinetic events may be encountered in case of a chronic drug administration. After all, the experiments were carried out on non-epileptic animals, so the results were dependent on the normal brain. Nevertheless, experimental convulsions induced in non-epileptic rodents may be ascribed to different seizure models– for instance, the results from MES may have the best predictive value for generalized tonic-clonic seizures [163], from the PTZ test– for myoclonic and to a certain degree for absences [164], and from 6-Hz– for drug-resistant focal seizures [165]. In light of the 6-Hz test, all synergistic 3-drug combinations need to be especially underlined.
Conclusions
Summing up, preclinical data indicate that gabapentinoids and levetiracetam combined with many other ASMs exhibit a synergistic anticonvulsant activity with generally low neurotoxic potential. Clinical data, although obtained from a limited number of patients, seem to support preclinical findings. Knowledge of the ASMs mechanism of action may guide add-on or substitution decisions but is of limited predictive value for the efficacy and tolerability of these drugs. Therefore, developing a better understanding of ASM synergy could lead to considerably better outcomes for patients, and could enable them to avoid some of the ‘trial and error’ effects of different drug combinations that many patients currently undergo. The beneficial pharmacological profile of racetams and gabapentinoids suggests that the presynaptic release machinery appears to be a particularly promising target for further ASMs.
Future directions
Recent decades have witnessed spectacular progress in better understanding the pathophysiology of epilepsy and potential molecular targets for pharmacotherapy of the heterogenous group disorders. However, the relationship between the mechanism of actions of ASMs and their clinical efficacies has been unraveled in some cases only. Likewise, the knowledge of the basic mechanisms of ASMs modestly, if at all, affects a clinician’s decision on polytherapy. The majority of screening tests identify chemical compounds that after single administration suppress acute seizures in rodents. Such compounds may limit the number of seizures but do not necessarily have disease-modifying properties. It is commonly accepted that pathological transformation of neuronal circuits underlies epilepsy, thus the future development of ASMs should be able to target neuroplastic processes and restore normal neurotransmission. As discussed in this article, the presynaptic mechanisms of some ASMs have been partly disclosed, but further studies are warranted for elucidating the interrelationships between the ASM’s molecular targets, e.g., the SV2A and α2δ1 proteins, via various presynaptic signaling pathways. Overall, presynaptic machinery remains still an unexplored and promising area for identifying new potential targets for new ASMs.
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- ASMs
Antiseizure Medications
- DNM1
Dynamin 1
- EAA
Excitatory Amino Acid
- EPSCs
Excitatory Postsynaptic Currents
- FDA
US Food and Drug Administration
- GABA -γ
Aminobutyric Acid
- GABA AR
GABA A Receptor
- GABA-T
GABA Transaminase
- GAERS
Genetic Absence Epilepsy Rats from the Strasbourg
- GAT-1
GABA transporter 1
- GPR55
G Protein-coupled Receptor 55
- GTP
Guanosine Triphosphate
- HCN1
Potassium/Sodium Hyperpolarization-activated Nyclic Nucleotide-gated channel 1
- IK(DR)
Delayed-Rectifier K + current
- IK(M)
Depolarization-Induced M-type K + current
- INa
Voltage-gated Na + current
- IP3R
1,4,5-Trisphosphate Receptor
- IPSCs
Inhibitory Postsynaptic Currents
- KO
Knockout phenotype
- LTP
Long-Term Potentiation
- MES
Maximal Electroshock
- mTORC1
mammalian Target Of Rapamycin Complex 1
- NMDAR
N-Methyl-D-Aspartate Receptor
- PI3K/Akt/Mtor
the phosphoinositide 3 kinase (PI3K)/Akt/mammalian (or mechanistic) target of the rapamycin (mTOR) pathway
- PPAR-γ
Peroxisome Proliferator-Activated Receptor gamma
- Prrt2
Presynaptic proline-rich transmembrane protein 2
- PTZ
Pentylenetetrazol
- SC
Sub Cutaneous
- SVs
Synaptic Vesicles
- SNAP25
Synaptosomal Associated Protein 25
- SNARE
“SNAP REceptors”
- v-SNARE
vesicle-associated SNARE
- SV2A
Synaptic Vesicle protein type 2 A
- STX7
Syntaxin 7
- SWDs
Spontaneous spike-Wave Discharges
- TRPV-1
Transient Receptor Potential Vanilloid 1 channel
- VGCCs
Voltage-Gated Ca2 + Channels
- VGSCs
Voltage-Gated Na + Channels
Funding
Not applicable.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
All authors have contributed equally to the work.
Declarations
Conflict of interest
S.J.C. received financial support from Bayer, GlaxoSmithKline, Janssen, Novartis, and Sanofi-Aventis for lecturing. S.J.C. and J.Ł are also recipients of an unrestricted grant from GlaxoSmithKline. P.C. has participated in 72 industry-sponsored clinical trials as the principal investigator and in seven clinical trials as the country coordinator. He has received financial support from pharmaceutical companies for organizing 29 editions of the conference “Psychosocial Aspects of Epilepsy” and financial support from Allergan, Teva, and UCB Pharma for lecturing. E.K.C.C. received financial support from Allergan, Novartis, Teva, Angelini Pharma, and Pfizer for lecturing and has participated in 22 industry-sponsored clinical trials as a sub-investigator.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Falco-Walter J, Epilepsy-Definition. Classification, pathophysiology, and Epidemiology. Semin Neurol. 2020;40:617–23. 10.1055/s-0040-1718719. 10.1055/s-0040-1718719 [DOI] [PubMed] [Google Scholar]
- 2.Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689–701. 10.1016/S0140-6736(18)32596-0. 10.1016/S0140-6736(18)32596-0 [DOI] [PubMed] [Google Scholar]
- 3.Löscher W, Potschka H, Sisodiya SM, Vezzani A. Drug Resistance in Epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev. 2020;72:606–38. 10.1124/pr.120.019539. 10.1124/pr.120.019539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia. 2003;44(Suppl 7):2–8. 10.1046/j.1528-1157.44.s7.10.x. 10.1046/j.1528-1157.44.s7.10.x [DOI] [PubMed] [Google Scholar]
- 5.Löscher W. Animal models of seizures and Epilepsy: past, Present, and future role for the Discovery of Antiseizure drugs. Neurochem Res. 2017;42:1873–88. 10.1007/s11064-017-2222-z. 10.1007/s11064-017-2222-z [DOI] [PubMed] [Google Scholar]
- 6.Löscher W, White HS. Animal models of drug-resistant Epilepsy as Tools for deciphering the Cellular and Molecular mechanisms of Pharmacoresistance and discovering more effective treatments. Cells. 2023;12:1233. 10.3390/cells12091233. 10.3390/cells12091233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanada T. Ionotropic glutamate receptors in Epilepsy: a review focusing on AMPA and NMDA receptors. Biomolecules. 2020;10:464. 10.3390/biom10030464. 10.3390/biom10030464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celli R, Fornai F. Targeting Ionotropic Glutamate receptors in the treatment of Epilepsy. Curr Neuropharmacol. 2021;19:747–65. 10.2174/1570159X18666200831154658. 10.2174/1570159X18666200831154658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyrer J, Maljevic S, Scheffer IE, Berkovic SF, Petrou S, Reid CA. Ion Channels in Genetic Epilepsy: from genes and mechanisms to Disease-targeted therapies. Pharmacol Rev. 2018;70:142–73. 10.1124/pr.117.014456. 10.1124/pr.117.014456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dossi E, Huberfeld G. GABAergic circuits drive focal seizures. Neurobiol Dis. 2023;180:106102. 10.1016/j.nbd.2023.106102. 10.1016/j.nbd.2023.106102 [DOI] [PubMed] [Google Scholar]
- 11.Löscher W, Klein P. The Pharmacology and Clinical Efficacy of Antiseizure medications: from bromide salts to Cenobamate and Beyond. CNS Drugs. 2021;35:935–63. 10.1007/s40263-021-00827-8. 10.1007/s40263-021-00827-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie X, Lancaster B, Peakman T, Garthwaite J. Interaction of the antiepileptic drug lamotrigine with recombinant rat brain type IIA na + channels and with native na + channels in rat hippocampal neurones. Pflugers Arch. 1995;430:437–46. 10.1007/BF00373920. 10.1007/BF00373920 [DOI] [PubMed] [Google Scholar]
- 13.Curia G, Biagini G, Perucca E, Avoli M. Lacosamide: a new approach to target voltage-gated sodium currents in epileptic disorders. CNS Drugs. 2009;23:555–68. 10.2165/00023210-200923070-00002. 10.2165/00023210-200923070-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol. 1989;25:582–93. 10.1002/ana.410250610. 10.1002/ana.410250610 [DOI] [PubMed] [Google Scholar]
- 15.Sills GJ, Rogawski MA. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology. 2020;168:107966. 10.1016/j.neuropharm.2020.107966. 10.1016/j.neuropharm.2020.107966 [DOI] [PubMed] [Google Scholar]
- 16.Czapiński P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Curr Top Med Chem. 2005;5:3–14. 10.2174/1568026053386962. 10.2174/1568026053386962 [DOI] [PubMed] [Google Scholar]
- 17.Magheru C, Magheru S, Coltau M, Hoza A, Moldovan C, Sachelarie L, et al. Antiepileptic drugs and their dual mechanism of action on carbonic anhydrase. J Clin Med. 2022;11:2614. 10.3390/jcm11092614. 10.3390/jcm11092614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozsoy HZ. Anticonvulsant effects of Carbonic anhydrase inhibitors: the enigmatic link between Carbonic anhydrases and Electrical Activity of the brain. Neurochem Res. 2021;46:2783–99. 10.1007/s11064-021-03390-2. 10.1007/s11064-021-03390-2 [DOI] [PubMed] [Google Scholar]
- 19.Ibeas Bih C, Chen T, Nunn AVW, Bazelot M, Dallas M, Whalley BJ. Molecular targets of Cannabidiol in Neurological disorders. Neurotherapeutics. 2015;12:699–730. 10.1007/s13311-015-0377-3. 10.1007/s13311-015-0377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gáll Z, Kelemen K, Tolokán A, Zolcseak I, Sável I, Bod R, et al. Anticonvulsant Action and Long-Term effects of Chronic Cannabidiol Treatment in the Rat Pentylenetetrazole-Kindling Model of Epilepsy. Biomedicines. 2022;10:1811. 10.3390/biomedicines10081811. 10.3390/biomedicines10081811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitkänen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–86. 10.1016/S1474-4422(10)70310-0. 10.1016/S1474-4422(10)70310-0 [DOI] [PubMed] [Google Scholar]
- 22.Curia G, Lucchi C, Vinet J, Gualtieri F, Marinelli C, Torsello A, et al. Pathophysiogenesis of mesial temporal lobe epilepsy: is prevention of damage antiepileptogenic? Curr Med Chem. 2014;21:663–88. 10.2174/0929867320666131119152201. 10.2174/0929867320666131119152201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fon EA, Edwards RH. Molecular mechanisms of neurotransmitter release. Muscle Nerve. 2001;24:581–601. 10.1002/mus.1044. 10.1002/mus.1044 [DOI] [PubMed] [Google Scholar]
- 24.Neher E. Vesicle pools and Ca2 + microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–99. 10.1016/s0896-6273(00)80983-6. 10.1016/s0896-6273(00)80983-6 [DOI] [PubMed] [Google Scholar]
- 25.Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–53. 10.1038/375645a0. 10.1038/375645a0 [DOI] [PubMed] [Google Scholar]
- 26.Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–18. 10.1016/0092-8674(93)90376-2. 10.1016/0092-8674(93)90376-2 [DOI] [PubMed] [Google Scholar]
- 27.Stout KA, Dunn AR, Hoffman C, Miller GW. The synaptic vesicle glycoprotein 2: structure, function, and Disease Relevance. ACS Chem Neurosci. 2019;10:3927–38. 10.1021/acschemneuro.9b00351. 10.1021/acschemneuro.9b00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendoza-Torreblanca JG, Vanoye-Carlo A, Phillips-Farfán BV, Carmona-Aparicio L, Gómez-Lira G. Synaptic vesicle protein 2A: basic facts and role in synaptic function. Eur J Neurosci. 2013;38:3529–39. 10.1111/ejn.12360. 10.1111/ejn.12360 [DOI] [PubMed] [Google Scholar]
- 29.Bradberry MM, Chapman ER. All-optical monitoring of excitation-secretion coupling demonstrates that SV2A functions downstream of evoked Ca2 + entry. J Physiol. 2022;600:645–54. 10.1113/JP282601. 10.1113/JP282601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamberger H, Weckhuysen S, De Jonghe P. STXBP1 as a therapeutic target for epileptic encephalopathy. Expert Opin Ther Targets. 2017;21:1027–36. 10.1080/14728222.2017.1386175. 10.1080/14728222.2017.1386175 [DOI] [PubMed] [Google Scholar]
- 31.Spoto G, Valentini G, Saia MC, Butera A, Amore G, Salpietro V, et al. Synaptopathies in Developmental and Epileptic encephalopathies: a focus on pre-synaptic dysfunction. Front Neurol. 2022;13:826211. 10.3389/fneur.2022.826211. 10.3389/fneur.2022.826211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsen KE, Walker MC, Cock HR. Characterization of the tetanus toxin model of refractory focal neocortical epilepsy in the rat. Epilepsia. 2005;46:179–87. 10.1111/j.0013-9580.2005.26004.x. 10.1111/j.0013-9580.2005.26004.x [DOI] [PubMed] [Google Scholar]
- 33.Mainardi M, Pietrasanta M, Vannini E, Rossetto O, Caleo M. Tetanus neurotoxin-induced epilepsy in mouse visual cortex. Epilepsia. 2012;53. 10.1111/j.1528-1167.2012.03510.x. e132-136. [DOI] [PubMed]
- 34.Vannini E, Restani L, Dilillo M, McDonnell LA, Caleo M, Marra V. Synaptic vesicles Dynamics in Neocortical Epilepsy. Front Cell Neurosci. 2020;14:606142. 10.3389/fncel.2020.606142. 10.3389/fncel.2020.606142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prichard KL, O’Brien NS, Murcia SR, Baker JR, McCluskey A. Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic vesicle recycling and implications in neurological diseases. Front Cell Neurosci. 2021;15:754110. 10.3389/fncel.2021.754110. 10.3389/fncel.2021.754110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Consortium EEPINOMICS-RES, Epilepsy Phenome/Genome Project, Epi4K Consortium. De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. Am J Hum Genet. 2014;95:360–70. 10.1016/j.ajhg.2014.08.013. 10.1016/j.ajhg.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y-Y, Chen X-N, Fan X-X, Zhang Y-J, Gu J, Fu X-W, et al. Upregulated dynamin 1 in an acute seizure model and in epileptic patients. Synapse. 2015;69:67–77. 10.1002/syn.21788. 10.1002/syn.21788 [DOI] [PubMed] [Google Scholar]
- 38.Odell LR, Jones NC, Chau N, Robertson MJ, Ambrus JI, Deane FM, et al. The sulfonadyns: a class of aryl sulfonamides inhibiting dynamin I GTPase and clathrin mediated endocytosis are anti-seizure in animal models. RSC Med Chem. 2023;14:1492–511. 10.1039/d2md00371f. 10.1039/d2md00371f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Zhang H, Yang L, Chen Y, Li J, Yang M, et al. Syntaxin 7 modulates seizure activity in epilepsy. Neurobiol Dis. 2023;181:106118. 10.1016/j.nbd.2023.106118. 10.1016/j.nbd.2023.106118 [DOI] [PubMed] [Google Scholar]
- 40.Ohno Y, Tokudome K. Therapeutic role of synaptic vesicle glycoprotein 2A (SV2A) in modulating Epileptogenesis. CNS Neurol Disord Drug Targets. 2017;16:463–71. 10.2174/1871527316666170404115027. 10.2174/1871527316666170404115027 [DOI] [PubMed] [Google Scholar]
- 41.Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, et al. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc Natl Acad Sci U S A. 1999;96:15268–73. 10.1073/pnas.96.26.15268. 10.1073/pnas.96.26.15268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janz R, Goda Y, Geppert M, Missler M, Südhof TC. SV2A and SV2B function as redundant Ca2 + regulators in neurotransmitter release. Neuron. 1999;24:1003–16. 10.1016/s0896-6273(00)81046-6. 10.1016/s0896-6273(00)81046-6 [DOI] [PubMed] [Google Scholar]
- 43.Gorter JA, van Vliet EA, Aronica E, Breit T, Rauwerda H, Lopes da Silva FH, et al. Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J Neurosci. 2006;26:11083–110. 10.1523/JNEUROSCI.2766-06.2006. 10.1523/JNEUROSCI.2766-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Vliet EA, Aronica E, Redeker S, Boer K, Gorter JA. Decreased expression of synaptic vesicle protein 2A, the binding site for levetiracetam, during epileptogenesis and chronic epilepsy. Epilepsia. 2009;50:422–33. 10.1111/j.1528-1167.2008.01727.x. 10.1111/j.1528-1167.2008.01727.x [DOI] [PubMed] [Google Scholar]
- 45.Kaminski RM, Gillard M, Leclercq K, Hanon E, Lorent G, Dassesse D, et al. Proepileptic phenotype of SV2A-deficient mice is associated with reduced anticonvulsant efficacy of levetiracetam. Epilepsia. 2009;50:1729–40. 10.1111/j.1528-1167.2009.02089.x. 10.1111/j.1528-1167.2009.02089.x [DOI] [PubMed] [Google Scholar]
- 46.Ciruelas K, Marcotulli D, Bajjalieh SM. Synaptic vesicle protein 2: a multi-faceted regulator of secretion. Semin Cell Dev Biol. 2019;95:130–41. 10.1016/j.semcdb.2019.02.003. 10.1016/j.semcdb.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sills GJ. SV2A in epilepsy: the plot thickens. Epilepsy Curr. 2010;10:47–9. 10.1111/j.1535-7511.2009.01351.x. 10.1111/j.1535-7511.2009.01351.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaminski RM, Matagne A, Leclercq K, Gillard M, Michel P, Kenda B, et al. SV2A protein is a broad-spectrum anticonvulsant target: functional correlation between protein binding and seizure protection in models of both partial and generalized epilepsy. Neuropharmacology. 2008;54:715–20. 10.1016/j.neuropharm.2007.11.021. 10.1016/j.neuropharm.2007.11.021 [DOI] [PubMed] [Google Scholar]
- 49.Nowack A, Malarkey EB, Yao J, Bleckert A, Hill J, Bajjalieh SM. Levetiracetam reverses synaptic deficits produced by overexpression of SV2A. PLoS ONE. 2011;6:e29560. 10.1371/journal.pone.0029560. 10.1371/journal.pone.0029560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossi R, Arjmand S, Bærentzen SL, Gjedde A, Landau AM. Synaptic vesicle glycoprotein 2A: features and functions. Front Neurosci. 2022;16:864514. 10.3389/fnins.2022.864514. 10.3389/fnins.2022.864514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge Y-X, Lin Y-Y, Bi Q-Q, Chen Y-J. Brivaracetam prevents the over-expression of synaptic vesicle protein 2A and rescues the deficits of hippocampal long-term potentiation in vivo in chronic temporal lobe Epilepsy rats. Curr Neurovasc Res. 2020;17:354–60. 10.2174/1567202617666200514114917. 10.2174/1567202617666200514114917 [DOI] [PubMed] [Google Scholar]
- 52.Xing H, Han X, Xu S, Sun Z, Yang S. Brivaracetam modulates short-term synaptic activity and low-frequency spontaneous brain activity by delaying synaptic vesicle recycling in two distinct rodent models of epileptic seizures. J Mol Neurosci. 2022;72:1058–74. 10.1007/s12031-022-01983-2. 10.1007/s12031-022-01983-2 [DOI] [PubMed] [Google Scholar]
- 53.Wu P-P, Cao B-R, Tian F-Y, Gao Z-B. Development of SV2A ligands for Epilepsy Treatment: a review of Levetiracetam, Brivaracetam, and Padsevonil. Neurosci Bull 2023. 10.1007/s12264-023-01138-2. [DOI] [PMC free article] [PubMed]
- 54.Meehan AL, Yang X, Yuan L-L, Rothman SM. Levetiracetam has an activity-dependent effect on inhibitory transmission. Epilepsia. 2012;53:469–76. 10.1111/j.1528-1167.2011.03392.x. 10.1111/j.1528-1167.2011.03392.x [DOI] [PubMed] [Google Scholar]
- 55.Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A. 2004;101:9861–6. 10.1073/pnas.0308208101. 10.1073/pnas.0308208101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gillard M, Fuks B, Leclercq K, Matagne A. Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anti-convulsant properties. Eur J Pharmacol. 2011;664:36–44. 10.1016/j.ejphar.2011.04.064. 10.1016/j.ejphar.2011.04.064 [DOI] [PubMed] [Google Scholar]
- 57.Contreras-García IJ, Cárdenas-Rodríguez N, Romo-Mancillas A, Bandala C, Zamudio SR, Gómez-Manzo S, et al. Levetiracetam mechanisms of Action: from molecules to systems. Pharmaceuticals (Basel). 2022;15:475. 10.3390/ph15040475. 10.3390/ph15040475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wood MD, Gillard M. Evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia. 2017;58:255–62. 10.1111/epi.13638. 10.1111/epi.13638 [DOI] [PubMed] [Google Scholar]
- 59.Hung T-Y, Wu S-N, Huang C-W. The Integrated effects of Brivaracetam, a selective Analog of Levetiracetam, on ionic currents and neuronal excitability. Biomedicines. 2021;9:369. 10.3390/biomedicines9040369. 10.3390/biomedicines9040369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niespodziany I, André VM, Leclère N, Hanon E, Ghisdal P, Wolff C. Brivaracetam differentially affects voltage-gated sodium currents without impairing sustained repetitive firing in neurons. CNS Neurosci Ther. 2015;21:241–51. 10.1111/cns.12347. 10.1111/cns.12347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Heylen L, Partoens M, Mills JD, Kaminski RM, Godard P, et al. Connectivity mapping using a Novel sv2a loss-of-function zebrafish Epilepsy Model as a powerful strategy for anti-epileptic drug Discovery. Front Mol Neurosci. 2022;15:881933. 10.3389/fnmol.2022.881933. 10.3389/fnmol.2022.881933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okada M, Fukuyama K, Shiroyama T, Ueda Y. Brivaracetam prevents astroglial l-glutamate release associated with hemichannel through modulation of synaptic vesicle protein. Biomed Pharmacother. 2021;138:111462. 10.1016/j.biopha.2021.111462. 10.1016/j.biopha.2021.111462 [DOI] [PubMed] [Google Scholar]
- 63.Fukuyama K, Okada M. Brivaracetam and Levetiracetam suppress Astroglial L-Glutamate Release through Hemichannel via inhibition of synaptic vesicle protein. Int J Mol Sci. 2022;23:4473. 10.3390/ijms23094473. 10.3390/ijms23094473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wood M, Daniels V, Provins L, Wolff C, Kaminski RM, Gillard M. Pharmacological Profile of the Novel antiepileptic drug candidate padsevonil: interactions with synaptic vesicle 2 proteins and the GABAA receptor. J Pharmacol Exp Ther. 2020;372:1–10. 10.1124/jpet.119.261149. 10.1124/jpet.119.261149 [DOI] [PubMed] [Google Scholar]
- 65.Klitgaard H, Matagne A, Gobert J, Wülfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. 10.1016/s0014-2999(98)00410-5. 10.1016/s0014-2999(98)00410-5 [DOI] [PubMed] [Google Scholar]
- 66.Matagne A, Margineanu D-G, Kenda B, Michel P, Klitgaard H. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol. 2008;154:1662–71. 10.1038/bjp.2008.198. 10.1038/bjp.2008.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klitgaard H, Matagne A, Nicolas J-M, Gillard M, Lamberty Y, De Ryck M, et al. Brivaracetam: Rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment. Epilepsia. 2016;57:538–48. 10.1111/epi.13340. 10.1111/epi.13340 [DOI] [PubMed] [Google Scholar]
- 68.Leclercq K, Matagne A, Provins L, Klitgaard H, Kaminski RM. Pharmacological Profile of the Novel antiepileptic drug candidate padsevonil: characterization in Rodent Seizure and Epilepsy models. J Pharmacol Exp Ther. 2020;372:11–20. 10.1124/jpet.119.261222. 10.1124/jpet.119.261222 [DOI] [PubMed] [Google Scholar]
- 69.Bleakley LE, McKenzie CE, Reid CA. Efficacy of antiseizure medication in a mouse model of HCN1 developmental and epileptic encephalopathy. Epilepsia. 2023;64:e1–8. 10.1111/epi.17447. 10.1111/epi.17447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luszczki JJ, Andres MM, Czuczwar P, Cioczek-Czuczwar A, Ratnaraj N, Patsalos PN, et al. Pharmacodynamic and pharmacokinetic characterization of interactions between levetiracetam and numerous antiepileptic drugs in the mouse maximal electroshock seizure model: an isobolographic analysis. Epilepsia. 2006;47:10–20. 10.1111/j.1528-1167.2006.00364.x. 10.1111/j.1528-1167.2006.00364.x [DOI] [PubMed] [Google Scholar]
- 71.Luszczki JJ, Andres-Mach MM, Ratnaraj N, Patsalos PN, Czuczwar SJ. Levetiracetam and felbamate interact both pharmacodynamically and pharmacokinetically: an isobolographic analysis in the mouse maximal electroshock model. Epilepsia. 2007;48:806–15. 10.1111/j.1528-1167.2006.00964.x. 10.1111/j.1528-1167.2006.00964.x [DOI] [PubMed] [Google Scholar]
- 72.Luszczki JJ, Zagaja M, Miziak B, Florek-Luszczki M, Czuczwar SJ. Synergistic Interaction of Retigabine with Levetiracetam in the Mouse Maximal Electroshock-Induced Seizure Model: A Type II Isobolographic Analysis. Pharmacology. 2015;96:11–5. 10.1159/000430822. [DOI] [PubMed]
- 73.Dudra-Jastrzebska M, Andres-Mach MM, Ratnaraj N, Patsalos PN, Czuczwar SJ, Luszczki JJ. Isobolographic characterization of the anticonvulsant interaction profiles of levetiracetam in combination with clonazepam, ethosuximide, phenobarbital and valproate in the mouse pentylenetetrazole-induced seizure model. Seizure. 2009;18:607–14. 10.1016/j.seizure.2009.06.009. 10.1016/j.seizure.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 74.Dudra-Jastrzebska M, Andres-Mach MM, Sielski M, Ratnaraj N, Patsalos PN, Czuczwar SJ, et al. Pharmacodynamic and pharmacokinetic interaction profiles of levetiracetam in combination with gabapentin, tiagabine and vigabatrin in the mouse pentylenetetrazole-induced seizure model: an isobolographic analysis. Eur J Pharmacol. 2009;605:87–94. 10.1016/j.ejphar.2008.12.046. 10.1016/j.ejphar.2008.12.046 [DOI] [PubMed] [Google Scholar]
- 75.Shandra A, Shandra P, Kaschenko O, Matagne A, Stöhr T. Synergism of lacosamide with established antiepileptic drugs in the 6-Hz seizure model in mice. Epilepsia. 2013;54:1167–75. 10.1111/epi.12237. 10.1111/epi.12237 [DOI] [PubMed] [Google Scholar]
- 76.Wojda E, Wlaz A, Patsalos PN, Luszczki JJ. Isobolographic characterization of interactions of levetiracetam with the various antiepileptic drugs in the mouse 6 hz psychomotor seizure model. Epilepsy Res. 2009;86:163–74. 10.1016/j.eplepsyres.2009.06.003. 10.1016/j.eplepsyres.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 77.Luszczki JJ, Panasiuk A, Zagaja M, Karwan S, Bojar H, Plewa Z, et al. Polygonogram and isobolographic analysis of interactions between various novel antiepileptic drugs in the 6-Hz corneal stimulation-induced seizure model in mice. PLoS ONE. 2020;15:e0234070. 10.1371/journal.pone.0234070. 10.1371/journal.pone.0234070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Metcalf CS, Gagangras S, Bulaj G, White HS. Synergistic effects of the galanin analog 810-2 with the antiseizure medication levetiracetam in rodent seizure models. Epilepsia. 2022;63:3090–9. 10.1111/epi.17420. 10.1111/epi.17420 [DOI] [PubMed] [Google Scholar]
- 79.Metcalf CS, Klein BD, Smith MD, Pruess T, Ceusters M, Lavreysen H, et al. Efficacy of mGlu2 -positive allosteric modulators alone and in combination with levetiracetam in the mouse 6 hz model of psychomotor seizures. Epilepsia. 2017;58:484–93. 10.1111/epi.13659. 10.1111/epi.13659 [DOI] [PubMed] [Google Scholar]
- 80.Metcalf CS, Klein BD, Smith MD, Ceusters M, Lavreysen H, Pype S, et al. Potent and selective pharmacodynamic synergy between the metabotropic glutamate receptor subtype 2-positive allosteric modulator JNJ-46356479 and levetiracetam in the mouse 6-Hz (44-mA) model. Epilepsia. 2018;59:724–35. 10.1111/epi.14005. 10.1111/epi.14005 [DOI] [PubMed] [Google Scholar]
- 81.Matsumura N, Nakaki T. Isobolographic analysis of the mechanisms of action of anticonvulsants from a combination effect. Eur J Pharmacol. 2014;741:237–46. 10.1016/j.ejphar.2014.08.001. 10.1016/j.ejphar.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 82.Czuczwar SJ, Kaplanski J, Swiderska-Dziewit G, Gergont A, Kroczka S, Kacinski M. Pharmacodynamic interactions between antiepileptic drugs: preclinical data based on isobolography. Expert Opin Drug Metab Toxicol. 2009;5:131–6. 10.1517/17425250802677826. 10.1517/17425250802677826 [DOI] [PubMed] [Google Scholar]
- 83.Berenbaum MC. What is synergy? Pharmacol Rev. 1989;41:93–141. [PubMed] [Google Scholar]
- 84.Löscher W, Richter A. Piracetam and levetiracetam, two pyrrolidone derivatives, exert antidystonic activity in a hamster model of paroxysmal dystonia. Eur J Pharmacol. 2000;391:251–4. 10.1016/s0014-2999(00)00105-9. 10.1016/s0014-2999(00)00105-9 [DOI] [PubMed] [Google Scholar]
- 85.Lyseng-Williamson KA. Levetiracetam: a review of its use in epilepsy. Drugs. 2011;71:489–514. 10.2165/11204490-000000000-00000. 10.2165/11204490-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 86.Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530–8. 10.1016/S1474-4422(18)30107-8. 10.1016/S1474-4422(18)30107-8 [DOI] [PubMed] [Google Scholar]
- 87.Tomson T, Battino D, Bromley R, Kochen S, Meador KJ, Pennell PB, et al. Global survey of guidelines for the management of Epilepsy in pregnancy: a report from the International League against Epilepsy Task Force on women and pregnancy. Epilepsia Open. 2020;5:366–70. 10.1002/epi4.12420. 10.1002/epi4.12420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nucera B, Brigo F, Trinka E, Kalss G. Treatment and care of women with epilepsy before, during, and after pregnancy: a practical guide. Ther Adv Neurol Disord. 2022;15:17562864221101687. 10.1177/17562864221101687. 10.1177/17562864221101687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vossler DG, Bainbridge JL, Boggs JG, Novotny EJ, Loddenkemper T, Faught E, et al. Treatment of Refractory Convulsive Status Epilepticus: a Comprehensive Review by the American Epilepsy Society Treatments Committee. Epilepsy Curr. 2020;20:245–64. 10.1177/1535759720928269. 10.1177/1535759720928269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chamberlain JM, Kapur J, Shinnar S, Elm J, Holsti M, Babcock L, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020;395:1217–24. 10.1016/S0140-6736(20)30611-5. 10.1016/S0140-6736(20)30611-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trinka E, Leitinger M. Management of Status Epilepticus, Refractory Status Epilepticus, and super-refractory Status Epilepticus. Continuum (Minneap Minn). 2022;28:559–602. 10.1212/CON.0000000000001103. 10.1212/CON.0000000000001103 [DOI] [PubMed] [Google Scholar]
- 92.Steinhoff BJ, Klein P, Klitgaard H, Laloyaux C, Moseley BD, Ricchetti-Masterson K, et al. Behavioral adverse events with brivaracetam, levetiracetam, perampanel, and topiramate: a systematic review. Epilepsy Behav. 2021;118:107939. 10.1016/j.yebeh.2021.107939. 10.1016/j.yebeh.2021.107939 [DOI] [PubMed] [Google Scholar]
- 93.Hansen CC, Ljung H, Brodtkorb E, Reimers A. Mechanisms underlying aggressive Behavior Induced by antiepileptic drugs: focus on Topiramate, Levetiracetam, and Perampanel. Behav Neurol 2018;2018:2064027. 10.1155/2018/2064027. [DOI] [PMC free article] [PubMed]
- 94.Marson A, Burnside G, Appleton R, Smith D, Leach JP, Sills G, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet. 2021;397:1375–86. 10.1016/S0140-6736(21)00246-4. 10.1016/S0140-6736(21)00246-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marson A, Burnside G, Appleton R, Smith D, Leach JP, Sills G, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet. 2021;397:1363–74. 10.1016/S0140-6736(21)00247-6. 10.1016/S0140-6736(21)00247-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bermeo-Ovalle A. SANAD II: dear Levetiracetam, the honeymoon is over. Epilepsy Curr. 2022;22:18–21. 10.1177/15357597211052129. 10.1177/15357597211052129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abou-Khalil BW, Update on Antiseizure Medications. 2022. Continuum (Minneap Minn) 2022;28:500–35. 10.1212/CON.0000000000001104. [DOI] [PubMed]
- 98.Feyissa AM. Brivaracetam in the treatment of epilepsy: a review of clinical trial data. Neuropsychiatr Dis Treat. 2019;15:2587–600. 10.2147/NDT.S143548. 10.2147/NDT.S143548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kwan P, Trinka E, Van Paesschen W, Rektor I, Johnson ME, Lu S. Adjunctive brivaracetam for uncontrolled focal and generalized epilepsies: results of a phase III, double-blind, randomized, placebo-controlled, flexible-dose trial. Epilepsia. 2014;55:38–46. 10.1111/epi.12391. 10.1111/epi.12391 [DOI] [PubMed] [Google Scholar]
- 100.Brigo F, Lattanzi S, Nardone R, Trinka E. Intravenous brivaracetam in the treatment of Status Epilepticus: a systematic review. CNS Drugs. 2019;33:771–81. 10.1007/s40263-019-00652-0. 10.1007/s40263-019-00652-0 [DOI] [PubMed] [Google Scholar]
- 101.Asadi-Pooya AA, Sperling MR, Chung S, Klein P, Diaz A, Elmoufti S, et al. Efficacy and tolerability of adjunctive brivaracetam in patients with prior antiepileptic drug exposure: a post-hoc study. Epilepsy Res. 2017;131:70–5. 10.1016/j.eplepsyres.2017.02.007. 10.1016/j.eplepsyres.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 102.Steinhoff BJ, Bacher M, Bucurenciu I, Hillenbrand B, Intravooth T, Kornmeier R, et al. Real-life experience with brivaracetam in 101 patients with difficult-to-treat epilepsy-A monocenter survey. Seizure. 2017;48:11–4. 10.1016/j.seizure.2017.03.010. 10.1016/j.seizure.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 103.Abraira L, Salas-Puig J, Quintana M, Seijo-Raposo IM, Santamarina E, Fonseca E, et al. Overnight switch from levetiracetam to brivaracetam. Safety and tolerability. Epilepsy Behav Rep. 2021;16:100504. 10.1016/j.ebr.2021.100504. 10.1016/j.ebr.2021.100504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nicolas J-M, Hannestad J, Holden D, Kervyn S, Nabulsi N, Tytgat D, et al. Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia. 2016;57:201–9. 10.1111/epi.13267. 10.1111/epi.13267 [DOI] [PubMed] [Google Scholar]
- 105.Theochari E, Cock H, Lozsadi D, Galtrey C, Arevalo J, Mula M. Brivaracetam in adults with drug-resistant epilepsy and psychiatric comorbidities. Epilepsy Behav. 2019;90:129–31. 10.1016/j.yebeh.2018.11.032. 10.1016/j.yebeh.2018.11.032 [DOI] [PubMed] [Google Scholar]
- 106.Hirsch M, Hintz M, Specht A, Schulze-Bonhage A. Tolerability, efficacy and retention rate of Brivaracetam in patients previously treated with Levetiracetam: a monocenter retrospective outcome analysis. Seizure. 2018;61:98–103. 10.1016/j.seizure.2018.07.017. 10.1016/j.seizure.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 107.Yates SL, Fakhoury T, Liang W, Eckhardt K, Borghs S, D’Souza J. An open-label, prospective, exploratory study of patients with epilepsy switching from levetiracetam to brivaracetam. Epilepsy Behav. 2015;52:165–8. 10.1016/j.yebeh.2015.09.005. 10.1016/j.yebeh.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 108.Genton P, Van Vleymen B. Piracetam and levetiracetam: close structural similarities but different pharmacological and clinical profiles. Epileptic Disord. 2000;2:99–105. 10.1684/j.1950-6945.2000.tb00363.x [DOI] [PubMed] [Google Scholar]
- 109.Winblad B. Piracetam: a review of pharmacological properties and clinical uses. CNS Drug Rev. 2005;11:169–82. 10.1111/j.1527-3458.2005.tb00268.x. 10.1111/j.1527-3458.2005.tb00268.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fischer W, Kittner H, Regenthal R, Russo E, De Sarro G. Effects of piracetam alone and in combination with antiepileptic drugs in rodent seizure models. J Neural Transm (Vienna). 2004;111:1121–39. 10.1007/s00702-004-0155-6. 10.1007/s00702-004-0155-6 [DOI] [PubMed] [Google Scholar]
- 111.Genton P, Guerrini R, Remy C. Piracetam in the treatment of cortical myoclonus. Pharmacopsychiatry. 1999;32(Suppl 1):49–53. 10.1055/s-2007-979237. 10.1055/s-2007-979237 [DOI] [PubMed] [Google Scholar]
- 112.Malykh AG, Sadaie MR. Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders. Drugs. 2010;70:287–312. 10.2165/11319230-000000000-00000. 10.2165/11319230-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 113.Löscher W, Gillard M, Sands ZA, Kaminski RM, Klitgaard H. Synaptic vesicle glycoprotein 2A ligands in the treatment of Epilepsy and Beyond. CNS Drugs. 2016;30:1055–77. 10.1007/s40263-016-0384-x. 10.1007/s40263-016-0384-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Botermans W, Koole M, Van Laere K, Savidge JR, Kemp JA, Sunaert S, et al. SDI-118, a novel procognitive SV2A modulator: first-in-human randomized controlled trial including PET/fMRI assessment of target engagement. Front Pharmacol. 2022;13:1066447. 10.3389/fphar.2022.1066447. 10.3389/fphar.2022.1066447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rogawski MA, Bazil CW. New molecular targets for antiepileptic drugs: alpha(2)delta, SV2A, and K(v)7/KCNQ/M potassium channels. Curr Neurol Neurosci Rep. 2008;8:345–52. 10.1007/s11910-008-0053-7. 10.1007/s11910-008-0053-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ablinger C, Geisler SM, Stanika RI, Klein CT, Obermair GJ. Neuronal α2δ proteins and brain disorders. Pflugers Arch. 2020;472:845–63. 10.1007/s00424-020-02420-2. 10.1007/s00424-020-02420-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hessenberger M, Haddad S, Obermair GJ. Pathophysiological Roles of Auxiliary Calcium Channel α2δ subunits. Handb Exp Pharmacol. 2023. 10.1007/164_2022_630. 10.1007/164_2022_630 [DOI] [PubMed] [Google Scholar]
- 118.Schöpf CL, Ablinger C, Geisler SM, Stanika RI, Campiglio M, Kaufmann WA, et al. Presynaptic α2δ subunits are key organizers of glutamatergic synapses. Proc Natl Acad Sci U S A. 2021;118:e1920827118. 10.1073/pnas.1920827118. 10.1073/pnas.1920827118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dolphin AC. Calcium channel α2δ subunits in epilepsy and as targets for antiepileptic drugs. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic mechanisms of the epilepsies. 4th ed. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- 120.Taylor CP, Harris EW. Analgesia with Gabapentin and Pregabalin May involve N-Methyl-d-Aspartate receptors, neurexins, and Thrombospondins. J Pharmacol Exp Ther. 2020;374:161–74. 10.1124/jpet.120.266056. 10.1124/jpet.120.266056 [DOI] [PubMed] [Google Scholar]
- 121.Li L, Chen S-R, Zhou M-H, Wang L, Li D-P, Chen H, et al. α2δ-1 switches the phenotype of synaptic AMPA receptors by physically disrupting heteromeric subunit assembly. Cell Rep. 2021;36:109396. 10.1016/j.celrep.2021.109396. 10.1016/j.celrep.2021.109396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou M-H, Chen S-R, Wang L, Huang Y, Deng M, Zhang J, et al. Protein kinase C-Mediated phosphorylation and α2δ-1 interdependently regulate NMDA receptor trafficking and activity. J Neurosci. 2021;41:6415–29. 10.1523/JNEUROSCI.0757-21.2021. 10.1523/JNEUROSCI.0757-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yan BC, Wang J, Rui Y, Cao J, Xu P, Jiang D, et al. Neuroprotective Effects of Gabapentin Against Cerebral Ischemia Reperfusion-Induced Neuronal Autophagic Injury via Regulation of the PI3K/Akt/mTOR signaling pathways. J Neuropathol Exp Neurol. 2019;78:157–71. 10.1093/jnen/nly119. 10.1093/jnen/nly119 [DOI] [PubMed] [Google Scholar]
- 124.Berdichevsky Y, Dryer AM, Saponjian Y, Mahoney MM, Pimentel CA, Lucini CA, et al. PI3K-Akt signaling activates mTOR-mediated epileptogenesis in organotypic hippocampal culture model of post-traumatic epilepsy. J Neurosci. 2013;33:9056–67. 10.1523/JNEUROSCI.3870-12.2013. 10.1523/JNEUROSCI.3870-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Manville RW, Abbott GW. Gabapentin is a potent activator of KCNQ3 and KCNQ5 potassium channels. Mol Pharmacol. 2018;94:1155–63. 10.1124/mol.118.112953. 10.1124/mol.118.112953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luszczki JJ, Swiader M, Parada-Turska J, Czuczwar SJ. Tiagabine synergistically interacts with gabapentin in the electroconvulsive threshold test in mice. Neuropsychopharmacology. 2003;28:1817–30. 10.1038/sj.npp.1300243. 10.1038/sj.npp.1300243 [DOI] [PubMed] [Google Scholar]
- 127.Hayashi K, Ueshima S, Ouchida M, Mashimo T, Nishiki T, Sendo T, et al. Therapy for hyperthermia-induced seizures in Scn1a mutant rats. Epilepsia. 2011;52:1010–7. 10.1111/j.1528-1167.2011.03046.x. 10.1111/j.1528-1167.2011.03046.x [DOI] [PubMed] [Google Scholar]
- 128.Hosford DA, Wang Y. Utility of the lethargic (lh/lh) mouse model of absence seizures in predicting the effects of lamotrigine, vigabatrin, tiagabine, gabapentin, and topiramate against human absence seizures. Epilepsia. 1997;38:408–14. 10.1111/j.1528-1157.1997.tb01729.x. 10.1111/j.1528-1157.1997.tb01729.x [DOI] [PubMed] [Google Scholar]
- 129.Borowicz KK, Swiader M, Luszczki J, Czuczwar SJ. Effect of gabapentin on the anticonvulsant activity of antiepileptic drugs against electroconvulsions in mice: an isobolographic analysis. Epilepsia. 2002;43:956–63. 10.1046/j.1528-1157.2002.34301.x. 10.1046/j.1528-1157.2002.34301.x [DOI] [PubMed] [Google Scholar]
- 130.Luszczki JJ, Filip D, Florek-Luszczki M. Interactions of Pregabalin with gabapentin, levetiracetam, tiagabine and vigabatrin in the mouse maximal electroshock-induced seizure model: a type II isobolographic analysis. Epilepsy Res. 2012;98:148–56. 10.1016/j.eplepsyres.2011.09.002. 10.1016/j.eplepsyres.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 131.Luszczki JJ, Ratnaraj N, Patsalos PN, Czuczwar SJ. Isobolographic and behavioral characterizations of interactions between vigabatrin and gabapentin in two experimental models of epilepsy. Eur J Pharmacol. 2008;595:13–21. 10.1016/j.ejphar.2008.07.051. 10.1016/j.ejphar.2008.07.051 [DOI] [PubMed] [Google Scholar]
- 132.Luszczki JJ, Andres MM, Czuczwar SJ. Synergistic interaction of gabapentin and oxcarbazepine in the mouse maximal electroshock seizure model–an isobolographic analysis. Eur J Pharmacol. 2005;515:54–61. 10.1016/j.ejphar.2005.03.046. 10.1016/j.ejphar.2005.03.046 [DOI] [PubMed] [Google Scholar]
- 133.Luszczki JJ, Czuczwar SJ. Isobolographic profile of interactions between tiagabine and gabapentin: a preclinical study. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:434–46. 10.1007/s00210-004-0867-z. 10.1007/s00210-004-0867-z [DOI] [PubMed] [Google Scholar]
- 134.Luszczki JJ, Czuczwar SJ. Isobolographic characterisation of interactions among selected newer antiepileptic drugs in the mouse pentylenetetrazole-induced seizure model. Naunyn Schmiedebergs Arch Pharmacol. 2005;372:41–54. 10.1007/s00210-005-1088-9. 10.1007/s00210-005-1088-9 [DOI] [PubMed] [Google Scholar]
- 135.Luszczki J. Additive interaction of pregabalin with phenytoin in the mouse maximal electroshock-induced seizure model: an isobolographic analysis. Annales UMCS 2009;Sectio DDD: Pharmacia. 2009:31–8.
- 136.Luszczki J. Interactions between Pregabalin and phenobarbital in the mouse maximal electroshock-induced seizure model: an isobolographic analysis. J Pre-Clin Clin Res 2009:103–9.
- 137.Luszczki JJ. Interaction of Pregabalin with carbamazepine in the mouse maximal electroshock-induced seizure model: a type I isobolographic analysis for non-parallel dose-response relationship curves. Adv Med Sci. 2010;55:43–52. 10.2478/v10039-010-0005-8. 10.2478/v10039-010-0005-8 [DOI] [PubMed] [Google Scholar]
- 138.Luszczki JJ, Filip D, Czuczwar SJ. Additive interactions of pregabalin with lamotrigine, oxcarbazepine and topiramate in the mouse maximal electroshock-induced seizure model: a type I isobolographic analysis for non-parallel dose-response relationship curves. Epilepsy Res. 2010;91:166–75. 10.1016/j.eplepsyres.2010.07.009. 10.1016/j.eplepsyres.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 139.Luszczki JJ, Mazurkiewicz LP, Wroblewska-Luczka P, Wlaz A, Ossowska G, Szpringer M, et al. Combination of phenobarbital with phenytoin and Pregabalin produces synergy in the mouse tonic-clonic seizure model: an isobolographic analysis. Epilepsy Res. 2018;145:116–22. 10.1016/j.eplepsyres.2018.06.003. 10.1016/j.eplepsyres.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 140.Luszczki JJ, Podgórska D, Kozińska J, Jankiewicz M, Plewa Z, Kominek M, et al. Polygonogram with isobolographic synergy for three-drug combinations of phenobarbital with second-generation antiepileptic drugs in the tonic-clonic seizure model in mice. Pharmacol Rep. 2021;73:111–21. 10.1007/s43440-020-00164-5. 10.1007/s43440-020-00164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Załuska-Ogryzek K, Marzęda P, Wróblewska-Łuczka P, Florek-Łuszczki M, Plewa Z, Bojar H, et al. Interactions among Lacosamide and Second-Generation antiepileptic drugs in the Tonic-Clonic Seizure Model in mice. Int J Mol Sci. 2021;22:5537. 10.3390/ijms22115537. 10.3390/ijms22115537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Greenblatt HK, Greenblatt DJ. Gabapentin and Pregabalin for the treatment of anxiety disorders. Clin Pharmacol Drug Dev. 2018;7:228–32. 10.1002/cpdd.446. 10.1002/cpdd.446 [DOI] [PubMed] [Google Scholar]
- 143.Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, et al. EFNS guideline on the drug treatment of migraine–revised report of an EFNS task force. Eur J Neurol. 2009;16:968–81. 10.1111/j.1468-1331.2009.02748.x. 10.1111/j.1468-1331.2009.02748.x [DOI] [PubMed] [Google Scholar]
- 144.Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–15. 10.1016/S0140-6736(07)60460-7. 10.1016/S0140-6736(07)60460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010;49:661–9. 10.2165/11536200-000000000-00000. 10.2165/11536200-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 146.Calandre EP, Rico-Villademoros F, Slim M. Alpha2delta ligands, gabapentin, Pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use. Expert Rev Neurother. 2016;16:1263–77. 10.1080/14737175.2016.1202764. 10.1080/14737175.2016.1202764 [DOI] [PubMed] [Google Scholar]
- 147.Tzellos TG, Toulis KA, Goulis DG, Papazisis G, Zampeli VA, Vakfari A, et al. Gabapentin and Pregabalin in the treatment of fibromyalgia: a systematic review and a meta-analysis. J Clin Pharm Ther. 2010;35:639–56. 10.1111/j.1365-2710.2009.01144.x. 10.1111/j.1365-2710.2009.01144.x [DOI] [PubMed] [Google Scholar]
- 148.Kwan P, Brodie MJ, Kälviäinen R, Yurkewicz L, Weaver J, Knapp LE. Efficacy and safety of pregabalin versus lamotrigine in patients with newly diagnosed partial seizures: a phase 3, double-blind, randomised, parallel-group trial. Lancet Neurol. 2011;10:881–90. 10.1016/S1474-4422(11)70154-5. 10.1016/S1474-4422(11)70154-5 [DOI] [PubMed] [Google Scholar]
- 149.French J, Kwan P, Fakhoury T, Pitman V, DuBrava S, Knapp L, et al. Pregabalin monotherapy in patients with partial-onset seizures: a historical-controlled trial. Neurology. 2014;82:590–7. 10.1212/WNL.0000000000000119. 10.1212/WNL.0000000000000119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chincholkar M. Gabapentinoids: pharmacokinetics, pharmacodynamics and considerations for clinical practice. Br J Pain. 2020;14:104–14. 10.1177/2049463720912496. 10.1177/2049463720912496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Semah F, Thomas P, Coulbaut S, Derambure P. Early add-on treatment vs alternative monotherapy in patients with partial epilepsy. Epileptic Disord. 2014;16:165–74. 10.1684/epd.2014.0650. 10.1684/epd.2014.0650 [DOI] [PubMed] [Google Scholar]
- 152.Zaccara G, Perucca E. Interactions between antiepileptic drugs, and between antiepileptic drugs and other drugs. Epileptic Disord. 2014;16:409–31. 10.1684/epd.2014.0714. 10.1684/epd.2014.0714 [DOI] [PubMed] [Google Scholar]
- 153.Verrotti A, Lattanzi S, Brigo F, Zaccara G. Pharmacodynamic interactions of antiepileptic drugs: from bench to clinical practice. Epilepsy Behav. 2020;104:106939. 10.1016/j.yebeh.2020.106939. 10.1016/j.yebeh.2020.106939 [DOI] [PubMed] [Google Scholar]
- 154.Margolis JM, Chu B-C, Wang ZJ, Copher R, Cavazos JE. Effectiveness of antiepileptic drug combination therapy for partial-onset seizures based on mechanisms of action. JAMA Neurol. 2014;71:985–93. 10.1001/jamaneurol.2014.808. 10.1001/jamaneurol.2014.808 [DOI] [PubMed] [Google Scholar]
- 155.Poolos NP, Warner LN, Humphreys SZ, Williams S. Comparative efficacy of combination drug therapy in refractory epilepsy. Neurology. 2012;78:62–8. 10.1212/WNL.0b013e31823ed0dd. 10.1212/WNL.0b013e31823ed0dd [DOI] [PubMed] [Google Scholar]
- 156.Brodie MJ, Yuen AW. Lamotrigine substitution study: evidence for synergism with sodium valproate? 105 Study Group. Epilepsy Res. 1997;26:423–32. 10.1016/s0920-1211(96)01007-8. 10.1016/s0920-1211(96)01007-8 [DOI] [PubMed] [Google Scholar]
- 157.Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: general features and interactions between antiepileptic drugs. Lancet Neurol. 2003;2:347–56. 10.1016/s1474-4422(03)00409-5. 10.1016/s1474-4422(03)00409-5 [DOI] [PubMed] [Google Scholar]
- 158.Stephen LJ, Brodie MJ. Seizure freedom with more than one antiepileptic drug. Seizure. 2002;11:349–51. 10.1053/seiz.2002.0711. 10.1053/seiz.2002.0711 [DOI] [PubMed] [Google Scholar]
- 159.Stephen LJ, Forsyth M, Kelly K, Brodie MJ. Antiepileptic drug combinations–have newer agents altered clinical outcomes? Epilepsy Res. 2012;98:194–8. 10.1016/j.eplepsyres.2011.09.008. 10.1016/j.eplepsyres.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 160.Stephen LJ, Sills GJ, Brodie MJ. Lamotrigine and topiramate may be a useful combination. Lancet. 1998;351:958–9. 10.1016/S0140-6736(05)60613-7. 10.1016/S0140-6736(05)60613-7 [DOI] [PubMed] [Google Scholar]
- 161.Deckers CL, Czuczwar SJ, Hekster YA, Keyser A, Kubova H, Meinardi H, et al. Selection of antiepileptic drug polytherapy based on mechanisms of action: the evidence reviewed. Epilepsia. 2000;41:1364–74. 10.1111/j.1528-1157.2000.tb00111.x. 10.1111/j.1528-1157.2000.tb00111.x [DOI] [PubMed] [Google Scholar]
- 162.Błaszczyk B, Miziak B, Czuczwar P, Wierzchowska-Cioch E, Pluta R, Czuczwar SJ. A viewpoint on rational and irrational fixed-drug combinations. Expert Rev Clin Pharmacol. 2018;11:761–71. 10.1080/17512433.2018.1500895. 10.1080/17512433.2018.1500895 [DOI] [PubMed] [Google Scholar]
- 163.Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–68. 10.1016/j.seizure.2011.01.003. 10.1016/j.seizure.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 164.Löscher W, Hönack D, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy Res. 1991;8:171–89. 10.1016/0920-1211(91)90062-k. 10.1016/0920-1211(91)90062-k [DOI] [PubMed] [Google Scholar]
- 165.Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–27. 10.1016/s0920-1211(01)00302-3. 10.1016/s0920-1211(01)00302-3 [DOI] [PubMed] [Google Scholar]
- 166.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9:68–82. 10.1038/nrd2997. 10.1038/nrd2997 [DOI] [PubMed] [Google Scholar]
- 167.Metcalf CS, West PJ, Thomson KE, Edwards SF, Smith MD, White HS, et al. Development and pharmacologic characterization of the rat 6 hz model of partial seizures. Epilepsia. 2017;58:1073–84. 10.1111/epi.13764. 10.1111/epi.13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Detrait ER, Leclercq K, Matagne A, Klitgaard H. Protective activity of brivaracetam in the 6 hz model of partial epilepsy: comparison with levetiracetam older antiepileptic drugs. Epilepsia. 2008;49:177–319. 10.1111/j.1528-1167.2008.01871_7.x. 10.1111/j.1528-1167.2008.01871_7.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
All authors have contributed equally to the work.