Abstract
Modification of adenovirus to achieve tissue specific targeting for the delivery of therapeutic genes requires both the ablation of its native tropism and the introduction of specific, novel interactions. Inactivation of the native receptor interactions, however, would cripple the virus for growth in production cells. We have developed an alternative receptor, or pseudoreceptor, for the virus which might allow propagation of viruses with modified fiber proteins that no longer bind to the native adenovirus receptor (coxsackievirus/adenovirus receptor [CAR]). We have constructed a membrane-anchored single-chain antibody [m-scFv(HA)] which recognizes a linear peptide epitope (hemagglutinin [HA]). Incorporation of HA within the HI loop of the fiber protein enabled the modified virus to transduce pseudoreceptor expressing cells under conditions where fiber-CAR interaction was blocked or absent. The pseudoreceptor mediated virus transduction with an efficiency similar to that of CAR. In addition, the HA epitope mediated virus transduction through interaction with the m-scFv(HA) when it was introduced into penton base. These findings indicate that cells expressing the pseudoreceptor should support production of HA-tagged adenoviruses independent of retaining the fiber-CAR interaction. Moreover, they demonstrate that high-affinity targeting ligands may function following insertion into either penton base or fiber.
The utility of adenovirus as a vector to deliver therapeutic genes would be greatly enhanced if the virus could be specifically targeted to the tissue of interest. Incorporation of tissue-specific ligands into viral coat proteins which lack their native cell recognition activity could allow selective delivery of transgenes to tissues that bind those ligands, while heterologous tissues normally susceptible to infection would not be modified. This approach requires the identification of appropriate ligands and a strategy for introducing them into the targeted virus. Redirecting virus to the target tissue will also require knocking out those interactions that contribute to the native tropism of the virus, and so an understanding of the molecular basis of the latter is critical.
Infection by adenovirus is facilitated by an initial attachment of the virus to its target cell through an interaction of the fiber protein with a cellular receptor. A fiber receptor for adenoviruses of subgroups A, C, D, E, and F has been identified as the CAR (coxsackievirus/adenovirus receptor) protein (2, 23, 26). The importance of the fiber protein for infection is demonstrated by the ability of soluble fiber protein to block virus entry (3, 20) and by the enhanced infectivity of the virus on cells expressing CAR (11, 16). In addition, viruses with chimeric fiber proteins have been shown to acquire the tropism of the virus from which the fiber knob is derived (25). The fiber knob binds to the N-terminal immunoglobulin-like domain of CAR (8).
Following attachment via CAR, internalization of the virus is promoted by the interaction of penton base protein with αv integrins (31). This interaction may elicit critical cell signaling events in addition to linking the virus to an endocytic pathway (17, 18). Although the penton base-αv integrin interaction does not mediate direct binding of subgroup C adenoviruses (serotypes 2 and 5 [Ad2 and Ad5]), the infectivity of these viruses is reduced on cells that express little or no αv integrins or when the interaction is blocked by competitors (13, 31). Mutation of the RGD motif within penton base results in a delay of virus replication (1). The lack of contribution of the penton base-αv integrin interaction to initial binding of virus might be a consequence of the 30-fold-lower affinity of this interaction relative to that of fiber for CAR (31) or result from a steric hindrance imposed by the long fiber of subgroup C viruses. The importance of both the fiber- and penton base-mediated interactions in vivo is highlighted by the finding that the failure of adenovirus to efficiently transduce mature airway epithelial cells correlates with absence of both αv integrins and the CAR receptor (21, 36).
While the two-step model of adenovirus entry involving fiber-mediated attachment and penton base-mediated internalization is supported by a number of studies and is a useful guide for attempting to alter the native tropism of the virus, variations of this model should be noted. The subgroup D virus Ad9, which has a much shorter fiber (16 nm, versus 37 nm for Ad2), attaches directly to cells via either its fiber or penton base (22). The fiber protein of Ad9 binds to CAR, but infection of many cell types is not inhibited by competing soluble fiber, whereas antibodies to αv integrin or penton base do block binding. In addition, Ad2 has been shown to bind cells in the absence of CAR via an interaction of penton base with β2 integrins (14). An interaction between penton base and αv integrins was still required for Ad2 to enter these cells. These findings demonstrate that penton base can mediate direct binding if it binds to β2 in the context of Ad2 or if it binds to αv in the context of Ad9.
While there may be additional components of the native tropism of adenovirus, abolishing the high-affinity binding of fiber to CAR is essential for development of a targeted vector. Since growth of Ad5-based vectors is dependent on fiber binding to the CAR receptor on production cells, a modified virus that no longer bound CAR would require an alternative means of attaching to these cells. We developed an alternative receptor (pseudoreceptor) for adenovirus to mediate CAR-independent transduction. The pseudoreceptor consists of a membrane-anchored single-chain antibody [m-scFv(HA)] which recognizes a linear peptide epitope from the hemagglutinin (HA) protein of influenza virus. This approach might allow a variety of modified viruses to be grown in a single production cell line so long as they express the HA epitope in a context which allows binding to the pseudoreceptor. In addition the HA epitope could serve as a model for a tissue-specific ligand to examine its function following insertion into various sites in different adenovirus coat proteins. In this report we show that the m-scFv(HA) can mediate transduction of adenoviruses carrying the HA epitope within the HI loop of fiber. Moreover, insertion of the HA epitope within penton base allowed the m-scFv(HA) to mediate entry of viruses in the absence of attachment via the fiber receptor. These results suggest that packaging cell lines expressing this type of pseudoreceptor will be useful in producing tissue-specific targeted adenoviral (Ad) vectors in which CAR binding has been ablated.
MATERIALS AND METHODS
Cells.
293 cells and CHO cells were obtained from the American Type Culture Collection and maintained in Dulbecco’s modified Eagle’s medium (Life Technologies, Gaithersburg, Md.) supplemented with 10% calf serum (HyClone, Logan, Utah). 12CA5 hybridoma cells (7, 35) were grown in RPMI (Life Technologies) with 10% fetal calf serum.
Peptides and recombinant proteins.
HA and FLAG peptides (Sigma, St. Louis, Mo.) were reconstituted in phosphate-buffered saline (PBS) at 1 mM. N-terminally fluoresceinated peptides HA* and scrHA*, having the sequences AYPYDVPDYA and AYDPYDPYVA, respectively, were obtained from Genosys (The Woodlands, Tex.) and also reconstituted at 1 mM in PBS. Soluble Ad5 fiber was purified as described previously (22).
Ad vectors.
Ad vectors used in this study are deleted for E1A and partially deleted for E1B and E3. AdG.PB(HA) and AdG.PB(FLAG), which have HA and FLAG epitopes, respectively, inserted into penton base have been described previously, together with the baculoviruses expressing the HA or FLAG-tagged penton base proteins (33). AdZ.F2K(HA) and AdZ.F2K(FLAG) have the HA and FLAG peptide epitopes, respectively, inserted in the HI loop of fiber. The fiber background into which these epitopes were inserted is designated F2K and is a chimera of the Ad5 protein in which the C-terminal domain, beginning at residue 371, has been replaced by the corresponding sequence from Ad2. The SpeI site within the HI loop of F2K was used to insert the epitopes in the form of oligonucleotide duplexes, using the oligonucleotides CTAGAGACTACAAGGACGACGATGATAAGA and CTAGTCTTATCATCGTCGTCCTTGTAGTCT for FLAG and CTAGTTATCCATATGATGTTCCAGATTATGCTT and CTAGAAGCATAATCTGAAACATCATATGGATAA for HA. The FLAG epitope was inserted into the plasmid pNSF2K, which differs from pNS (34) by the F2K fiber modification, to generate pNSF2K(FLAG), while the HA epitope was inserted into the plasmid pASF2K to generate pASF2K(HA). Plasmids pNSF2K and pASF2K carry Ad sequences that extend to the right end of the genome but differ in that the Ad sequence in pNSF2K starts with the NdeI site at map unit 86.5, while pASF2K starts with the AgeI site at 73.5 but lacks the E3 region removed as an XbaI fragment. The virus AdZ.F2K(FLAG) was generated by transfection of SalI-linearized pNSF2K(FLAG) into 293 cells infected with AdZ.11A as previously described (34). The virus AdZ.F2K(HA) was generated by transfection of 293 cells with a plasmid carrying the complete genome of the virus (10). This plasmid was isolated following cotransfection of Escherichia coli with SalI-DrdI-digested pASF2K(HA) and a second linearized plasmid carrying the left end of AdZ (extending to the SpeI site at map unit 75.4) and 371 nucleotides representing the right end of the Ad genome.
The virus AdSc(HA) contains the gene for the membrane-anchored anti-HA scFv under control of the cytomegalovirus (CMV) promoter in the E1 region. The promoter, splice sequence, and poly(A)/stop signal are the same as in pSc(HA) (Fig. 1). The virus AdF expresses green fluorescent protein from E1; AdCAR, which expresses CAR in E1, has been described previously (12).
FIG. 1.
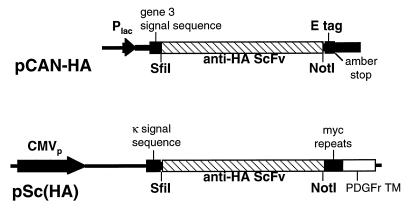
Schematic diagram of scFv expression cassettes in pCAN-HA and pSc(HA). pCAN-HA carries the scFv for expression from the Plac promoter. The scFv is flanked by an N-terminal signal sequence from gene 3 and a C-terminal E-tag epitope. The scFv was moved as an SfiI/NotI fragment to pSc(HA) where expression is driven by the CMV promoter. The N-terminal signal sequence in this construct is from Igκ light chain, and the scFv is anchored in the membrane via the C-terminal PDGF receptor transmembrane (PDGFr TM) sequence from which it is separated by a Myc epitope spacer.
Construction of anti-HA scFv.
mRNA was prepared from 12CA5 hybridoma cells by using the Ultraspec RNA system (Biotecx Laboratories, Houston, Tex.) followed by an Oligotex spin column (Qiagen, Chatsworth, Calif.). Reverse transcription-PCR amplification and assembly of the scFv were done as described in detail by Gilliland et al. (9). Briefly, the Moloney murine leukemia virus enzyme (Life Technologies) was used to catalyze separate reverse transcription reactions with the immunoglobulin kappa (Igκ) chain-specific (CTTCCACTTGACATTGATGTCTTTG) and IgG2b-specific (CAAGTCACAGTCACTGGCTCAGG) primers (9), and poly(G) tails were added to the cDNA products by using terminal deoxynucleotide transferase (Stratagene, La Jolla, Calif.). The cDNA was amplified by using Pfu polymerase (Stratagene) and Igκ chain- or IgG2b-specific antisense primers (CGTCATGTCGACGGATCCAAGCTTCAAGAAGCACACGACTGAGGCAC and CGTCATGTCGACGGATCCAAGCTTGTCACCATGGAGTTAGTTTGGGC, respectively) in combination with a poly(C)-containing primer (CGTCGATGAGCTCTAGAATTCGCATGTGCAAGTCCGATGGTCCCCCCCCCCCCCC) for the sense strand. PCR products were digested with HindIII and XbaI (sites underlined in primer sequences), cloned into pBluescript (Stratagene) and sequenced. The light-chain variable region (VL) was amplified with a primer pair which introduced an SfiI site upstream of the mature N terminus and (Gly4Ser)2 at the C terminus. The heavy-chain variable region (VH) was amplified with a primer pair which added (Gly4Ser)2 at the N terminus and a NotI site at the C terminus. Products from these amplifications were assembled into a single chain in a second PCR in the presence of the SfiI/N-terminal VL primer and the C-terminal VH/NotI primer. Assembly was mediated by the overlapping sequences encoding the Gly4Ser motifs at the C and N termini of the initial VL and VH products, respectively. This product, which has the structure VL-(Gly4Ser)3-VH, was cloned into pCANTAB-5E (Pharmacia Biotech, Piscataway, N.J.) as an SfiI/NotI fragment to generate pCAN-HA (Fig. 1).
Expression and functional assay for soluble anti-HA scFv.
Bacterial clones transformed with pCAN-HA were cultured overnight at 30°C in Luria-Bertani (LB) medium supplemented with 2% (wt/vol) glucose and 1 μg ampicillin per ml (LBGA). Three ml of the overnight culture was added to 30 ml of LBGA and grown for 1 h at 30°C. The bacteria were collected as a pellet by centrifugation (1,500 × g for 20 min) and resuspended in 40 ml of LB containing 1 mM isopropyl-β-d-thiogalactopyranoside and ampicillin. Following a 4-h incubation at 30°C, the bacteria were pelleted as before, resuspended in 1 ml of ice-cold 1× TES (0.2 M Tris-HCl [pH 8.0], 0.5 mM EDTA, 0.5 M sucrose), and then diluted with 1.5 ml of 0.2× TES, vortexed, and incubated on ice for 30 min. The debris was pelleted at 17,500 × g for 15 min at 4°C, and the supernatant, containing soluble scFv, was collected.
Lysates were prepared by three freeze-thaw cycles of Tn5 cells that had been infected with baculoviruses expressing Ad penton base protein containing HA or FLAG epitopes inserted adjacent to the RGD motif (33). The lysates were diluted fivefold in TBST (100 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% Tween 20) and supplemented with bovine serum albumin and sodium dodecyl sulfate (SDS) at final concentrations of 4 and 0.2% (wt/vol), respectively. Aliquots of lysate (250 μl) were combined with 200 μl of the bacterial extracts or buffer alone and incubated at 4°C for 90 min.
Antibody-coated agarose beads were prepared by diluting 20-μl aliquots of a 50:50 mixture of protein A-agarose and protein G-agarose (both from Boehringer Mannheim, Indianapolis, Ind.) with 400 μl of TBST. Separate aliquots received 25 μl of tissue culture supernatant from the anti-HA-expressing hybridoma cells, 10 μl of an anti-E peptide antiserum (Pharmacia Biotech), or 2.5 μl (2.5 μg) of the M2 anti-FLAG antibody (Sigma) and were incubated at 4°C for 90 min. The beads were washed three times in buffer A (50 mM Tris [pH 7.5], 150 mM NaCl, 1% Triton X-100 [Sigma], 0.1% SDS) and resuspended in penton base-containing lysates or mixes of the lysates with bacterial scFv extracts. After a 4-h incubation at 4°C, the beads were pelleted, washed three times with buffer A, and then washed once with 25 mM Tris (pH 6.8). Samples were eluted with 2× SDS sample buffer by heating at 100°C for 5 min and analyzed by electrophoresis on a 10% polyacrylamide gel (PAGE). After transfer to nitrocellulose, samples were incubated with an antipenton antiserum at a dilution of 1:5,000 in PBS containing 5% (wt/vol) nonfat dry milk and 0.1% (vol/vol) Tween 20. Bound antibody was detected using a peroxidase-conjugated anti-rabbit antiserum (Boehringer Mannheim) and the Amersham ECL (enhanced chemiluminescence) reagent.
Expression and analysis of m-scFv(HA).
The plasmid used to express the anti-HA scFv as a membrane-anchored protein in tissue culture cells is based on pRC/CMVp puro (GenVec Inc., Rockville, Md.). pRC/CMVps puro has a murine immunoglobulin splice acceptor site inserted downstream of the splice donor sequence of the CMV promoter. The SfiI site from the simian virus 40 sequence has also been removed. A HindIII/XbaI fragment containing the membrane-anchored scFv coding sequence from pHook-3 (Invitrogen, Carlsbad, Calif.) was isolated following PCR using primers with the sequences AAGCTTGGGTACGATATCCACCATGGAGACA and TCTAGACTACACCGGTTTCTTCTGCCAAAGCATGAT. This fragment was inserted at the corresponding sites between the CMV promoter and the bovine growth hormone poly(A) site in pRC/CMVps puro. The sequence encoding the HA epitope present at the N terminus of the scFv expressed from pHook-3 was removed by PCR amplification with primers AAGCTTGGGTACGATATCCACCATGGAGACA and GGCCGGCTGGGCCCCGTCACCAGTGGAACCTGGAA and subsequent substitution of this product as a HindIII-SfiI fragment into the parental plasmid. The anti-HA scFv was then substituted as an SfiI-NotI fragment upstream of the platelet-derived growth factor (PDGF) receptor transmembrane sequence to give plasmid pSc(HA) (Fig. 1).
293 cells were transfected with FspI-linearized pSc(HA) by a calcium phosphate method, and cells were subsequently passaged in the presence of 1 μg of puromycin (Sigma) per ml to select for clones. CHO cells were transfected with FspI-linearized pSc(HA) by using Pfx-4 (Invitrogen) according to the manufacturer’s protocol, and clones were selected in the presence of 10 μg of puromycin per ml. Binding of the fluoresceinated peptides HA* and scrHA* to cells was assayed by incubating cells in complete medium containing 10 mM HEPES and 100 nM peptide for 45 min at 4°C. After aspiration of the peptide solution, cells were rinsed once with PBS and then incubated in PBS for observation by fluorescence microscopy. For fluorescence-activated cell sorting (FACS) analysis, cells were dislodged from tissue culture plates in the presence of PBS containing 5 mM EDTA, centrifuged at 500 × g, and resuspended at 1.5 × 106 cells/ml in complete medium containing 10% serum and 10 mM HEPES. Cells were incubated with HA* or scrHA* as described above and then washed once in PBS before resuspension in the original volume of PBS containing 1 μg of propidium iodide per ml. Competition with unlabeled peptides was performed by preincubating cells for 45 min at 4°C in the presence of 100 μM HA peptide or FLAG peptide prior to addition of HA*.
Virus transduction assays.
Cells were seeded on 24-well plates for transduction assays. All incubations were at 37°C. Prior to infection, wells were incubated for 1 h in 200 μl of complete medium with 10 mM HEPES alone or containing Ad5 fiber (5 μg/ml), HA peptide (100 μM), FLAG peptide (100 μM), or combinations thereof. These additions remained present during virus infection when virus was added in a volume of 20 μl of complete medium-HEPES and incubated with cells for 1 h. The medium was then aspirated from cells, which were washed twice with PBS before addition of complete medium. Cell lysates were prepared 16 to 20 h later by addition of 200 μl of 1× reporter lysis buffer (Promega, Madison, Wis.).
In experiments utilizing AdCAR and AdSc(HA), CHO cells were seeded in 24-well plates and incubated for 1 h with 104 particles of either virus per cell. Cells were then incubated at 37°C for 24 h and exposed to AdZ.F2K(HA) at 100 or 1,000 particles per cell for 1 h. Cells were harvested 20 h later as described above to assay for β-galactosidase.
β-Galactosidase and β-glucuronidase assays.
Lysates assayed for β-galactosidase were first incubated at 50°C for 60 min. β-Galactosidase or β-glucuronidase activity was measured by adding 4 μl of diluted lysate to 60 μl of the appropriate substrate (Tropix, Bedford, Mass.). At a specific interval of 10 to 20 min later, light emission accelerator was added and luminescence was measured by integration for 10 s.
RESULTS
Expression of functional anti-HA scFv in E. coli.
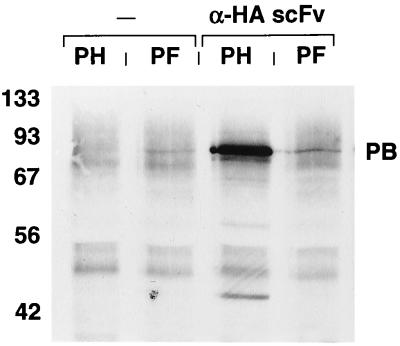
m-scFv(HA) was constructed as outlined by Gilliland et al. (9). An SfiI site was introduced at the 5′ terminus and a NotI site was introduced at the 3′ terminus to allow cloning into the bacterial expression vector pCANTAB-5E, to generate pCAN-HA (Fig. 1). Periplasmic extracts from bacterial clones transformed with this vector were examined for anti-HA binding activity. Extracts were added to lysates containing baculovirus expressed Ad penton base proteins that were modified by insertion of either the HA epitope or a FLAG epitope. These mixtures were then added to agarose beads coated with an anti-E peptide antibody which recognizes an epitope incorporated at the C terminus of the soluble scFv molecules. Proteins eluted from the beads were analyzed by Western blotting using an antipenton antiserum. The anti-HA scFv sample mediated binding of HA-tagged but not FLAG-tagged penton base (Fig. 2). The additional bands present in the HA-tagged penton sample which migrate just above the 42- and 56-kDa markers are degradation products of penton base. The HA-tagged penton base did not bind to the beads in the absence of scFv. The presence of penton base in the FLAG-tagged lysate was confirmed in assays using beads coated with an anti-FLAG antibody (not shown). This result indicated that a functional anti-HA scFv had been recovered.
FIG. 2.
Western blot showing activity of soluble anti-HA scFv. Lysates from baculovirus-infected cells were incubated directly with anti-E antibody-coated agarose beads (−) or following preincubation with periplasmic extracts from E. coli transformed with pCAN-HA (α-HA scFv). These lysates were derived from cells infected with baculoviruses encoding HA-tagged (PH) or FLAG-tagged (PF) Ad penton base protein. Eluted proteins were subjected to SDS-PAGE, transferred to nitrocellulose, and probed with an antipenton antiserum. At the left are shown the positions of molecular weight standards in kilodaltons. The prominent band corresponding to epitope-tagged penton base is identified (PB).
Expression of m-scFv(HA) on the surface of 293 cells.
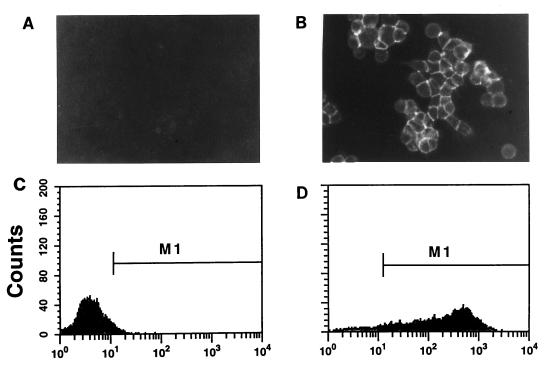
The anti-HA scFv gene was introduced into pSc(HA) as an SfiI/NotI fragment (Fig. 1). In this vector, the scFv is transcribed from the CMV promoter as a spliced mRNA encoding a cleaved, N-terminal signal sequence upstream of the SfiI site and a spacer region (Myc epitopes) and transmembrane sequence (PDGF receptor) downstream of the NotI site. Cells expanded from single, puromycin-resistant 293 (293-HA) cell colonies were assayed for the ability to bind HA*. The discrete fluorescent outlines at the periphery of 293-HA cells exposed to HA* confirmed the presence of functional anti-HA binding activity at the cell surface (Fig. 3B). No fluorescent cells were observed when 293-HA cells were incubated with the control peptide scrHA* (Fig. 3A) or when 293 cells were exposed to HA* (not shown). FACS analysis of 293-HA cells following incubation with the HA* peptide revealed a marked increase in cell fluorescence. The median fluorescence for cells within M1 was 246, versus 13 for cells exposed to scrHA* (Fig. 3C and D). The FACS profiles of unstained 293 cells, 293 cells reacted with HA*, and 293-HA cells exposed to scrHA* were indistinguishable (data not shown).
FIG. 3.
Binding of fluoresceinated HA peptide to m-scFv(HA)-expressing cells. 293-HA cells were observed by fluorescence microscopy following incubation with 100 nM HA* or the control peptide scrHA* (A and B). Cells were seeded to allow examination of discrete clusters of cells, and virtually all of the cells observed by light microscopy were found to fluoresce when exposed to HA*. For FACS analysis, cells detached in the presence of 5 mM EDTA were resuspended at 1.5 × 106 cells/ml and incubated with 100 nM HA* or scrHA* (C and D).
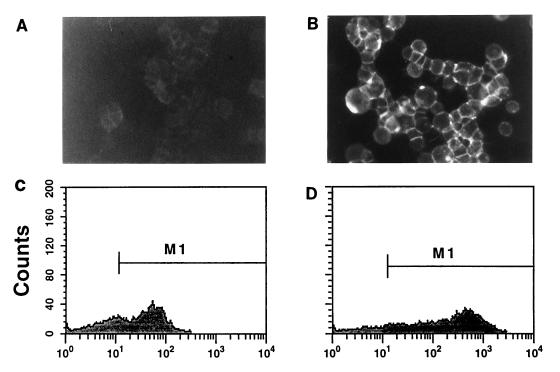
Competition with unlabeled peptides was used to further examine the binding specificity of the receptor on 293-HA cells (Fig. 4). Preincubation of cells with HA peptide followed by presence of HA during exposure to HA* dramatically reduced binding; a faint but discrete signal could still be detected at the cell surface (Fig. 4). FACS analysis showed that the presence of HA caused the median fluorescence to drop from 246 to 44 (Fig. 3 and 4). In contrast, FLAG peptide had no effect on binding of HA* (Fig. 4).
FIG. 4.
Specific inhibition of HA* binding to m-scFv(HA)-expressing cells by HA peptide. HA* peptide binding was performed as described for Fig. 3 except that cells were preincubated with unlabeled HA (A and C) or FLAG (B and D) peptide at 100 μM for 1 h and these remained present during incubation with HA*.
Transduction of 293-HA cells by virus expressing the HA epitope in fiber.
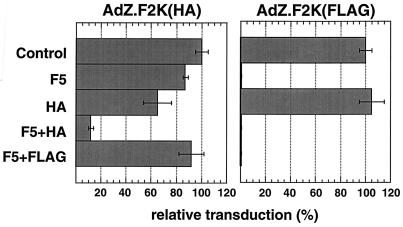
To test the functionality of the membrane-anchored anti-HA scFv as a receptor for adenovirus, 293-HA cells were incubated with either AdZ.F2K(HA) or AdZ.F2K(FLAG), which have, respectively, an HA or FLAG epitope inserted in the HI loop of fiber, at 1 focus-forming unit (FFU) per cell. Transduction was assayed the following day by measuring β-galactosidase activity, and typical results are depicted in Fig. 5. AdZ.F2K(FLAG) transduction of 293-HA cells was nearly completely blocked by fiber alone. In contrast, transduction by AdZ.F2K(HA) was inhibited only 13% by fiber. This also contrasts with transduction of 293 cells by AdZ.F2K(HA), which was reduced by more than 95% in the presence of fiber (not shown), indicating that the scFv receptor mediated the transduction of 293-HA cells which was resistant to fiber. HA peptide alone inhibited transduction of 293-HA cells by AdZ.F2K(HA) by 35% but had no effect on transduction of these cells with AdZ.F2K(FLAG) (Fig. 5). When 293-HA cells were incubated with a combination of fiber and HA peptide, their transduction with AdZ.F2K(HA) was inhibited by 88% (Fig. 5). Combining FLAG peptide with fiber, on the other hand, gave no additional inhibition of AdZ.F2K(HA) entry versus fiber alone. These results suggest that the m-scFv(HA) receptor expressed on 293-HA cells can bind to the HA epitope within fiber and substitute for CAR in mediating virus entry.
FIG. 5.
Transduction of 293-HA cells by AdZ.F2K(HA) or AdZ.F2K(FLAG). Virus was added to cells at 1 FFU/cell and incubated for 1 h at 37°C before being replaced with fresh medium (Control). Prior to addition of virus, cells were incubated for 1 h with soluble Ad5 fiber at 5 μg/ml (F5), HA peptide at 100 μM (HA), a combination of the two (F5+HA), or a combination of 5 μg of Ad5 fiber per ml and 100 μM FLAG peptide (F5+FLAG). Infections were done as for the control but in the continued presence of competitor. At 20 h postinfection, cells were lysed and tested for β-galactosidase activity by using an ECL assay. After subtraction of background, the activities in the control samples for each virus were taken as 100% and those of the other samples were expressed relative to this. Shown are the means and error bars for the duplicate infections performed in this experiment.
Transduction mediated by interaction of HA-tagged fiber with the m-scFv(HA) can occur in the absence of CAR.
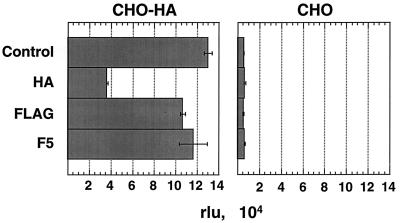
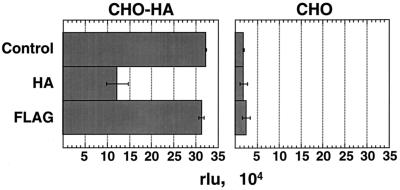
Since 293-HA cells express CAR, the question remained whether the function of the m-scFv(HA) in mediating transduction was still in some way dependent on coexpression with CAR. The pseudoreceptor was introduced into CHO cells, which do not express CAR, and its expression was confirmed by FACS analysis of the resulting CHO-HA cells. Presence of the m-scFv(HA) resulted in a 22-fold increase in transduction of CHO-HA cells versus CHO cells by AdZ.F2K(HA) (Fig. 6). As expected, soluble fiber inhibited neither the transduction of CHO-HA cells nor the low level of transduction seen in CHO cells. HA peptide, however, resulted in a 70% decrease in β-galactosidase activity for CHO-HA cells but had no effect on CHO cell infection. The control virus, AdZ.F2K(FLAG), showed no increase in transduction of CHO-HA cells above the low level seen on CHO cells (not shown). Thus, expression of the m-scFv(HA) specifically increased transduction by virus bearing the HA epitope, and this enhancement could be competed specifically with free HA peptide. This result indicates that the ability of AdZ.F2K(HA) to transduce cells via interaction with the anti-HA scFv is not dependent on presence of the CAR protein.
FIG. 6.
Transduction of CHO-HA cells by AdZ.F2K(HA). Infections of CHO-HA and CHO cells (at 5 FFU/cell) and β-galactosidase assays were performed as described for Fig. 5. To highlight the relative transduction of CHO-HA versus CHO cells, the results are shown as relative luminescence units (rlu), determined as the average from duplicate infections.
Comparison of efficiency of virus transduction mediated by CAR or m-scFv(HA).
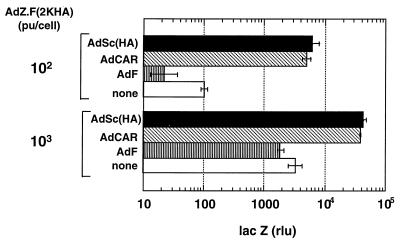
The enhanced transduction of CHO-HA cells relative to CHO cells by AdZ.F2K(HA) indicates the functionality of m-scFv(HA) as a viral receptor but not how efficient this interaction is relative to that between CAR and fiber. To address the latter, we transduced CHO cells separately with AdCAR and AdSc(HA), vectors expressing CAR and m-scFv(HA), respectively, and then examined the subsequent transduction of these cells by AdZ.F2K(HA). Since AdCAR and AdSc(HA) have similar expression cassettes for the two transgenes, transduction of cells with equal particles of these viruses should give comparable expression for the two receptors. Cells were incubated with 104 particles of AdCAR, AdSc(HA), or the control virus AdF per cell. After 24 h, the cells were incubated with 100 or 103 particles of AdZ.F2K(HA). Exposure of CHO cells to AdF did not enhance their transduction by AdZ.F2K(HA). At a dose of 100 particles of AdZ.F2K(HA) per cell, CHO cells expressed only background levels of lacZ; lacZ expression was significantly elevated at 103 particles, consistent with an inefficient transduction of these cells. CHO cells exposed to AdCAR at 104 particles/cell showed an enhanced transduction by AdZ.F2K(HA) of 50-fold or more at 100 particles/cell and 10-fold or more at 1,000 particles/cell (Fig. 7). The important finding is that AdSc(HA) was as effective as AdCAR in rendering CHO cells susceptible to AdZ.F2K(HA). Thus, it appears that the interaction between m-scFv(HA) and the HA epitope is as effective as that between CAR and its binding site in mediating transduction of CHO cells by AdZ.F2K(HA).
FIG. 7.
Transduction of CHO cells with AdZ.F2K(HA) following exposure to AdCAR or AdSc(HA). CHO cells were incubated with AdCAR or AdSc(HA) at 104 particles (pu)/cell for 1 h. Twenty-four hours later, the cells were exposed to AdZ.F2K(HA) at 100 or 1,000 particles/cell for 1 h. Cell lysates were prepared 20 h later and assayed for β-galactosidase activity.
Transduction mediated by attachment via an HA epitope in penton base.
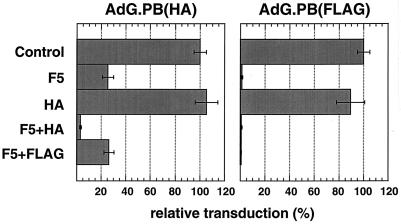
Viruses with HA or FLAG epitopes inserted into the penton base have been described previously (33). We wanted to examine whether the HA epitope introduced into the penton base of AdG.PB(HA) could contribute to transduction by interacting with the scFv. In the presence of blocking fiber, the transduction of 293-HA cells with AdG.PB(HA) was inhibited by 75% (Fig. 8). Fiber inhibited transduction by the control virus AdG.PB(FLAG), on the other hand, by 99%. Although HA peptide had no significant effect on the transduction of 293-HA cells by either virus, when it was combined with fiber the inhibition of AdG.PB(HA) transduction increased to 97%. FLAG peptide, by contrast, did not augment the partial inhibition seen with fiber alone (Fig. 8). This result indicates that the HA peptide inserted adjacent to the RGD motif in penton base can promote the transduction of cells expressing the m-scFv(HA).
FIG. 8.
Transduction of 293-HA cells by AdG.PB(HA) or AdG.PB(FLAG). Infections were done at 1 FFU/cell as described for Fig. 5. Cells were lysed at 20 h postinfection to assay for β-glucuronidase, and activities are expressed as percentages of those found for the unblocked control for each virus. Average values and error bars for duplicate infections are shown.
Transduction of AdG.PB(HA) was also assayed on CHO and CHO-HA cells which do not express the fiber receptor. A 17-fold increase in β-glucuronidase activity was observed for CHO-HA cells versus that seen on CHO cells (Fig. 9). Competition with HA peptide reduced this transduction by 62%, while FLAG peptide had no effect. AdG.PB(FLAG) induced levels of β-glucuronidase activity in both CHO and CHO-HA cells comparable to that seen for AdG.PB(HA) on CHO cells (not shown). The enhanced transduction of CHO-HA cells by virus bearing the HA epitope within penton base demonstrates that incorporation of a ligand in this coat protein can mediate virus entry through a novel receptor.
FIG. 9.
Transduction of CHO-HA cells by AdG.PB(HA). CHO-HA and CHO cells were infected at 5 FFU/cell as described for Fig. 5. β-Glucuronidase activities are reported in relative light units (rlu) and shown as the average value for duplicate infections.
DISCUSSION
We have shown that a synthetic molecule composed of a membrane-anchored scFv can function as a receptor for attachment of adenovirus. The fiber protein appears to mediate docking of virus at the cell surface, a function which enhances subsequent interactions required for internalization of the virus and plays an important role in virus tropism. Thus, introduction of a heparin-binding domain at the C terminus of fiber created an adenovirus with enhanced infectivity for multiple cell types which are only poorly infected by the unmodified virus (32). Likewise, addition of an RGD motif with high affinity for αv integrins to the C terminus of fiber enhanced infection of cells that expressed the integrins but lacked the fiber receptor (34). Adenovirus has also been retargeted to cells that lack the fiber receptor by coating the virus with neutralizing antifiber Fabs, either as part of a complex with a cellular ligand or as part of a bispecific antibody. These approaches have led to adenovirus transduction via binding to the folate receptor (5), fibroblast growth factor 2 receptor (24), or epidermal growth factor receptor (19). The variety of cellular proteins successfully targeted as alternative fiber receptors argues against any specific receptor function beyond providing a docking site. In fact, the one limiting variable appears to be the affinity of an alternative receptor for the ligand (34).
The critical role that fiber-receptor interactions have been shown to play in determining adenovirus tropism supports the approach of introducing novel ligands into the fiber protein. Retargeting of adenovirus, however, will require abolishing the native receptor binding activity of fiber. Exogenously added complexes, described above, which simultaneously block fiber-receptor interactions and introduce a new targeting specificity, provide one approach to overcoming the native tropism of the virus. Another is to produce viruses which lack the fiber protein (6, 15, 27). Genetically modifying the fiber protein so that it no longer binds CAR would eliminate the need for a postproduction modification step and could preserve the activity of the virus versus fiberless configurations where the ratios of particles to infectious units are elevated. The pseudoreceptor and corresponding cell line which we have produced should enable growth of an adenovirus which retains a modified fiber that does not bind the native receptor but can still be used as a scaffold for introducing foreign ligands. Incorporation of the HA epitope along with the targeting ligand would also allow a variety of differentially targeted viruses to be grown in a single production cell line. The virus used in the present study had the HA epitope inserted into the HI loop. We had previously found that the FLAG epitope functioned in this location and tolerance for an RGD motif in the HI loop has also been reported recently (4). The m-scFv(HA) cell line should allow testing the feasibility of inserting a targeting ligand at other sites within fiber.
The AdZ.F2K(HA) virus was able to transduce 293-HA cells by using either CAR or the m-scFv(HA). Only the combination of soluble fiber plus HA peptide was effective in blocking infection by this virus to the extent that fiber alone blocked AdZ.F2K(FLAG) or adenovirus with a wild-type fiber. We also found that the pseudoreceptor functioned in CHO-HA cells, ruling out any essential contribution of CAR to entry of virus mediated by the m-scFv(HA). The activity of the m-scFv(HA) as a fiber receptor despite the absence of a cytoplasmic domain is consistent with evidence that the cytoplasmic domain of CAR is dispensable for its function as a native fiber receptor (16). Receptors for fiber can also function with a glycophosphatidylinositol membrane anchor, as seen from targeting the folate receptor (5) and modification of CAR (28).
For Ad2 and Ad5, the interaction of penton base with αv integrins does not promote direct binding of virus to cells but rather appears to be dependent on an interaction between fiber and its receptor. The inability of penton base to mediate direct binding of virus to cells might be due to (i) steric interference by the fiber protein, (ii) the 30-fold-lower affinity of penton base for αv integrin relative to that of fiber for CAR, or (iii) the accessibility of the target bound by penton base. Ad9, which has a short-shafted fiber, can bind directly to αv integrins via penton base (22). Ad2, on the other hand, does bind directly to β2 integrins via its penton base (14). It is not clear to what extent the steric constraints and the affinity of this interaction differ from that of penton base and αv integrin. Bispecific antibodies attached to a ligand inserted into penton base of an Ad5 variant have been used to transduce cells through binding to αv integrins, E-selectin, or CD3 in the absence of a fiber-receptor interaction (29, 30, 33).
Given that a fiber-independent interaction between penton base and a cell surface protein can promote virus attachment under some conditions, we examined whether insertion of an HA epitope within penton base could mediate transduction of cells expressing the m-scFv(HA). Transduction of CHO-HA cells by AdG.PB(HA) was 17-fold greater than in CHO cells, and HA peptide blocked this transduction by 62%. By comparison, transduction of CHO-HA cells by AdZ.F2K(HA) was 22-fold greater than in CHO cells, and HA peptide blocked this transduction by 70%. These results suggest that the Ad5-based virus carrying the HA epitope in penton can bind and transduce m-scFv(HA)-expressing cells in the absence of a fiber-CAR interaction. In addition, the effectiveness of a high-affinity ligand inserted in penton base is similar to that of one inserted in fiber. Thus, the long fiber of this virus does not completely block a direct interaction of penton base with a cellular receptor. The potential contribution of the m-scFv(HA) in sterically enabling this interaction cannot be ignored. However, these results strongly suggest that the incorporation of a ligand into the penton base can mediate transduction of cells expressing a novel receptor.
ACKNOWLEDGMENTS
We thank Alena Lizonova for advice on isolation of cell clones, Barb Aughtman for FACS analysis, Susan Delphin for cell culture assistance, and Lou Cantolupo and Yuan Li for help in making AdZ.F2K(HA).
REFERENCES
- 1.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 3.Defer C, Belin M T, Caillet-Boudin M L, Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dmitriev I, Krasnykh V, Miller C R, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel D T. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas J T, Rogers B E, Rosenfeld M E, Michael S I, Feng M, Curiel D T. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 6.Falgout B, Ketner G. Characterization of adenovirus particles made by deletion mutants lacking the fiber gene. J Virol. 1988;62:622–625. doi: 10.1128/jvi.62.2.622-625.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, Wilson I A, Lerner R A, Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freimuth P, Springer K, Berard C, Hainfeld J, Bewley M, Flanagan J. Coxsackievirus and adenovirus receptor amino-terminal immunoglobulin V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J Virol. 1999;73:1392–1398. doi: 10.1128/jvi.73.2.1392-1398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilliland L K, Norris N A, Marquardt H, Tsu T T, Hayden M S, Neubauer M G, Yelton D E, Mittler R S, Ledbetter J A. Rapid and reliable cloning of antibody variable regions and generation of recombinant single chain antibody fragments. Tissue Antigens. 1996;47:1–20. doi: 10.1111/j.1399-0039.1996.tb02509.x. [DOI] [PubMed] [Google Scholar]
- 10.Graham F L. Covalently closed circles of human adenovirus DNA are infectious. EMBO J. 1984;3:2917–2922. doi: 10.1002/j.1460-2075.1984.tb02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmi S, Geertsen R, Mezzacasa A, Peter I, Dummer R. The presence of human coxsackievirus and adenovirus receptor is associated with efficient adenovirus-mediated transgene expression in human melanoma cell cultures. Hum Gene Ther. 1998;9:2363–2373. doi: 10.1089/hum.1998.9.16-2363. [DOI] [PubMed] [Google Scholar]
- 12.Hidaka C, Milano E, Leopold P L, Bergelson J M, Hackett N R, Finberg R W, Wickham T J, Kovesdi I, Roelvink P, Crystal R G. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J Clin Investig. 1999;103:579–587. doi: 10.1172/JCI5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S, Endo R I, Nemerow G R. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S, Kamata T, Takada Y, Ruggeri Z M, Nemerow G R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legrand V, Spehner D, Schlesinger Y, Settelen N, Pavirani A, Mehtali M. Fiberless recombinant adenoviruses: virus maturation and infectivity in the absence of fiber. J Virol. 1999;73:907–919. doi: 10.1128/jvi.73.2.907-919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leon R P, Hedlund T, Meech S J, Li S, Schaack J, Hunger S P, Duke R C, DeGregori J. Adenoviral-mediated gene transfer in lymphocytes. Proc Natl Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li E, Stupack D, Bokoch G M, Nemerow G R. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J Virol. 1998;72:8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li E, Stupack D, Klemke R, Cheresh D A, Nemerow G R. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller C R, Buchsbaum D J, Reynolds P N, Douglas J T, Gillespie G Y, Mayo M S, Raben D, Curiel D T. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 1998;58:5738–5748. [PubMed] [Google Scholar]
- 20.Philipson L, Lonberg-Holm K, Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968;2:1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roelvink P W, Kovesdi I, Wickham T J. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roelvink P W, Lizonova A, Lee J G, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers B E, Douglas J T, Ahlem C, Buchsbaum D J, Frincke J, Curiel D T. Use of a novel cross-linking method to modify adenovirus tropism. Gene Ther. 1997;4:1387–1392. doi: 10.1038/sj.gt.3300541. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson S C, Rollence M, Marshall-Neff J, McClelland A. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J Virol. 1997;71:4782–4790. doi: 10.1128/jvi.71.6.4782-4790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von Seggern D J, Chiu C Y, Fleck S K, Stewart P L, Nemerow G R. A helper-independent adenovirus vector with E1, E3, and fiber deleted: structure and infectivity of fiberless particles. J Virol. 1999;73:1601–1608. doi: 10.1128/jvi.73.2.1601-1608.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Bergelson J M. Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J Virol. 1999;73:2559–2562. doi: 10.1128/jvi.73.3.2559-2562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wickham T J, Haskard D, Segal D, Kovesdi I. Targeting endothelium for gene therapy via receptors up-regulated during angiogenesis and inflammation. Cancer Immunol Immunother. 1997;45:149–151. doi: 10.1007/s002620050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wickham T J, Lee G M, Titus J A, Sconocchia G, Bakacs T, Kovesdi I, Segal D M. Targeted adenovirus-mediated gene delivery to T cells via CD3. J Virol. 1997;71:7663–7669. doi: 10.1128/jvi.71.10.7663-7669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 32.Wickham T J, Roelvink P W, Brough D E, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 33.Wickham T J, Segal D M, Roelvink P W, Carrion M E, Lizonova A, Lee G M, Kovesdi I. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J Virol. 1996;70:6831–6838. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickham T J, Tzeng E, Shears L L, 2nd, Roelvink P W, Li Y, Lee G M, Brough D E, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson I A, Niman H L, Houghten R A, Cherenson A R, Connolly M L, Lerner R A. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- 36.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Investig. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]