Abstract
Objectives
The clinical activity score (CAS) and European severity scale (ESS) are established clinical tools to assess thyroid eye disease (TED) but are limited in terms of subjectivity and their reliability in non-Caucasian individuals, and can underestimate significant disease in the posterior orbit. Preliminary data from pilot studies have shown that diffusion-weighted imaging (DWI) using extraocular muscle (EOM) apparent diffusion coefficient (ADC) measurements may provide complementary information in TED. This study expands on previous research to assess for correlations between clinical scores and EOM-ADCs in stratifying disease activity and severity in a large patient cohort from an ethnically diverse population.
Methods
A retrospective review of TED clinics between 2011 and 2021 identified 96 patients with a documented CAS and ESS and an orbital MRI that included DWI. From regions of interest manually placed on EOM bellies, the highest ADC was computed for each patient and analysed for correlations and associations with CAS and ESS using Spearman Rank correlation and Mann–Whitney U tests, and any potential discriminatory cut-offs using Receiver Operator Curve analyses. A p-value < 0.05 indicated statistical significance.
Results
EOM-ADCs showed a positive association with CAS (p ≤ 0.001). EOM-ADCs were higher in sight-threatening compared to mild disease (p ≤ 0.01). A cut-off of 995 mm2/s achieved AUC = 0.7744, equating to 77% sensitivity and 67% specificity for discrimination between mild-moderate and sight-threatening disease.
Conclusion
EOM-ADCs correlate with higher scores of disease severity and activity in TED. Besides providing quantitative data to support clinical tools, EOM-ADC cut-offs may identify patients at risk of developing sight-threatening diseases.
Critical relevance statement
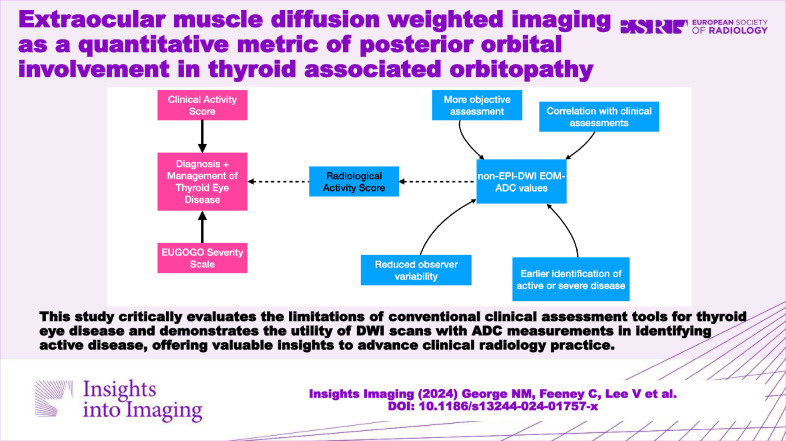
This study critically evaluates the limitations of conventional clinical assessment tools for TED and demonstrates the utility of DWI scans with ADC measurements in identifying active disease, offering valuable insights to advance clinical radiology practice.
Key Points
Conventional tools for TED assessment have subjective limitations.
ADCs from non-echoplanar diffusion-weighted imaging correlate with clinical activity.
Non-echoplanar diffusion-weighted imaging offers quantitative assessment to aid clinical practice reliability.
Graphical Abstract
Keywords: Diffusion magnetic resonance imaging, Echo-planar imaging, Thyroid eye disease, Disease severity
Introduction
Thyroid eye disease (TED) is an autoimmune condition usually, but not invariably, associated with Graves’ hyperthyroidism. The underlying pathophysiology is complex but typically manifests with the expansion of orbital and periorbital tissues, especially intra and extraconal fat and extraocular muscles (EOMs) [1]. This disease follows a sequential time course lasting a few years in most patients, with a clinically active phase initially followed by an inactive chronic phase. Some degree of cosmetic disfigurement is almost universal in patients with moderate/severe disease, and sight-threatening disease can lead to diplopia and visual loss through corneal ulceration and optic nerve compression [1]. About one-third of patients benefit from timely immunosuppression during to active phase to modify the disease course and lessen residual disease burden [1]. Therefore, determining TED activity and severity accurately is important to guide optimal management.
The clinical activity of TED is frequently assessed by the Clinical activity score (CAS), a composite score of binary measures (yes or no) for different clinical symptoms and signs of inflammation in the anterior orbit (e.g., pain, conjunctival redness, swelling), with a minimum score of 0 and maximum score of 10 [2, 3]. TED severity is also assessed using the European severity scale (ESS) developed by the European Group on Graves Orbitopathy (EUGOGO) which classifies patients into mild, moderate/severe, and sight-threatening groups based on soft tissue signs, eyelid measurement, ocular motility, corneal integrity, and the degree of proptosis [2]. Despite being useful clinical tools, both assessments are observer-dependent and are limited in their assessment of the posterior orbit [4]. The criteria of the scores are binary, which may be too simplistic and not reflect the spectrum of changes that can occur [5].
Magnetic resonance imaging (MRI) is superior to computerised tomography (CT) when it is available for the evaluation of TED due to its superior soft tissue contrast resolution [6, 7]. Besides providing anatomic information concerning muscle and fat measurements and apical crowding, MRI can provide information analogous to active disease by demonstrating changes in signal intensity of involved tissues including EOM [5].
In the evaluation of active TED, the STIR intensity ratio (SIR) plays a crucial role in MRI [8]. STIR sequences are particularly sensitive to fluid content and inflammation, making them well-suited for detecting active disease manifestations such as orbital oedema and inflammation. The SIR, calculated by comparing signal intensities in affected and unaffected orbits, provides a quantitative measure of the inflammatory activity in the thyroid orbit.
Whilst STIR is a valuable tool, diffusion-weighted imaging (DWI) MRI has also gained attention in the assessment of TED. Preliminary research has found significant changes in MRI DWI in patients with TED compared to healthy controls [9]. It has been shown to provide measures of the diffusion of water in soft tissues and non-echoplanar diffusion-weighted imaging (non-EPI DWI) is more resistant to susceptibility artefacts including at bone-air interfaces than echoplanar DWI, which makes it useful for orbital evaluation. Higher apparent diffusion coefficient (ADC) indicates greater facilitated diffusion which can have several mechanisms including tissue oedema or inflammation in benign tissues [9, 10]. We have previously shown the utility of non-echoplanar DWI (non-EPI-DWI) in guiding clinical decisions, especially when the CAS and ESS were unreliable [11].
Previous studies have provided insights into the use of clinical scores and DWI scans in the management of TED [11]; however, these are limited by their sample size. With a larger cohort of patients from a diverse multiethnic population, this study is likely to reflect the situation in clinical practice in terms of encompassing varied presentations of TED and challenges in clinical assessment. This study specifically examines the associations between EOM-ADCs with CAS and ESS as well identify any potential discriminatory cut-offs for clinically useful groups.
Materials and methods
Patients were identified from three multidisciplinary (MDT) TED clinics at Imperial College Healthcare NHS Trust Hospitals (Western Eye (WEH) and Charing Cross Hospitals (CXH)) and at London North-West University Hospital Trust (Central Middlesex Hospital (CMH)). Anonymised patient databases were evaluated from the inception of the clinics (CMH (Nov 2011), WEH (Jan 2016) and CXH (Jan 2017)) until March 3rd 2021. Patients were selected for analysis based on the inclusion and exclusion criteria as outlined below. The study was approved by the local Audit Committee and adhered to the tenets of the Declaration of Helsinki.
Inclusion criteria
Adult (> 18 years of age).
A diagnosis of TED using standard criteria.
At least one orbital MRI examination including a non-EPI DWI with corresponding ADC map.
CAS within six weeks of MRI scan (or two measurements before and after the scan within three months of the scan).
Imaging evaluation
MR imaging was performed on a 1.5-T MRI scanner (Magnetom Avanto; Siemens) using a standard Head Matrix coil. In all patients, a 3-mm-thick Half-Fourier Acquisition Single-Shot Turbo Spin-Echo (HASTE) non-EPI DWI sequence was acquired in the coronal plane (Repetition Time (TR) = 900 ms; Echo Time (TE) = 118 ms; matrix = 192 × 86; Field of View (FOV) = 145 mm; 18 averages; EPI spacing = 6.28 ms; bandwidth = 465 Hz/pixel; b factors, 0 and 1000 s/mm2). ADC maps were automatically generated by the MRI scanner software. Conventional axial T1 weighted and coronal short tau inversion recovery (STIR) sequences had also been performed and were used for anatomic reference.
ADC evaluation
A specialist head and neck radiologist (K.B., R.L., G.M.) examined the MR images to select the coronal non-EPI-DWI section that best illustrated EOMs with the highest ADC on visual analysis. This was typically identified in the slice showing the largest diameter of the muscle belly and/or adjacent sections. Using the B0 image initially for optimal muscle delineation (anatomic reference), circular or oval freehand regions of interest (ROIs) were contoured within the inner border of the active EOMs, copied to the ADC map, and the ADC for the ROI was extracted (Fig. 1). The highest EOM-ADC from all the ADCs acquired for the patient was used for subsequent analysis.
Fig. 1.
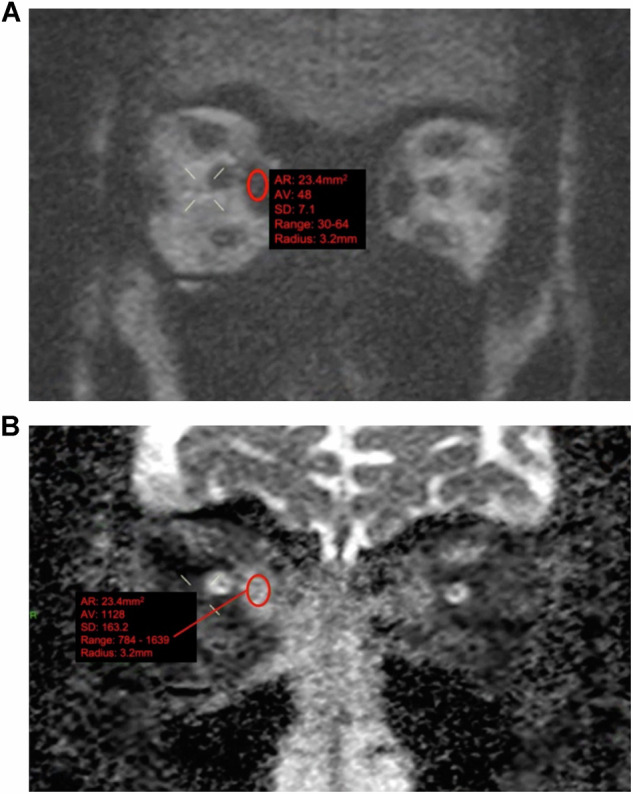
Representative examples of non-echoplanar diffusion-weighted imaging (non-EPI-DWI) of orbital EOMs in a patient with TED. Coronal orbital/EOM non-EPI-DWI MRI scan at a b value of 1000 (A) and apparent diffusion coefficient (ADC) image (B) show moderate-to-marked enlargement of the extraocular muscles and increased ADC values, notably at the right medial rectus (ADC value = 1128). Contour representing the freehand region-of-interest outline within the inner border of the active EOM
Subgroup analyses
The following subgroups were selected for group-wise analyses since they reflect the clinical classification of patients applied in routine clinical practice.
Receiver operator characteristics (ROC)
To evaluate the potential use of ADC as a diagnostic test for dystrophic optic neuropathy or to exclude mild/possible TED, ROC curves for comparing clinically active vs. inactive TED (i.e., CAS < 3 and CAS ≥ 3).
Statistical analysis
Statistical analyses were performed using GraphPad Prism Software, Version 9.1.0. Demographic data were summarised descriptively including using median and interquartile range (IQR) as data was non-normally distributed data. To assess for a correlation between EOM-ADC and CAS, which is an ordinal scale with up to ten levels, the Spearman’s Rank correlation test was used (rs, with 95% confidence intervals (CI) and p-values). For testing for associations between EOM-ADC and groups classified by CAS and ESS, the Mann–Whitney U test was used. Receiver operator curve (ROC) analyses were performed to assess diagnostic performances of EOM-ADCs for differentiating clinical subgroups as described, and diagnostic performance data were recorded for the cut-off achieving the highest overall accuracy. p-values < 0.05 were considered statistically significant.
Results
Patient characteristics
There were 352 patients (median 46 (range: 36.0–55.0) years; 278 [79.0%] female; 83 [23.7%] current smokers) who attended the three MDTED clinics over the study period. After applying the inclusion criteria, 256 (72.7%) patients were excluded, most of which were due to the absence of a suitable DWI-MRI scan with a concurrent CAS recorded. Ninety-six (27.3%) patients met the inclusion criteria, who had a median age of 50 (range: 39.8–58.3) years, 78.1% were female and 24% were current smokers. Most patients were either hyperthyroid (46.3%) or euthyroid (41.1%) at presentation. The study cohort was ethnically diverse with 34.3% Caucasian, 30.2% Afro-Caribbean and 11.5% Asian. Demographic data are shown in Table 1.
Table 1.
Patient demographics for radiology cohort analysis
| Radiology Cohort n = 96 | |
|---|---|
| Age at first TED clinic (years) | |
| Median (IQR) | 50 (39.8–58.3) |
| No. (%) Female | 75 (78.1) |
| Ethnicitya | |
| No. (%) Caucasian | 33 (34.3) |
| No. (%) Afro-Caucasian | 29 (30.2) |
| No. (%) Asian | 11 (11.5) |
| Smoking Statusb | |
| No. (%) current smokers | 23 (24.0) |
| No. (%) never smoked | 13 (13.5) |
| No. (%) ex-smokers | 57 (59.4) |
| No. (%) positive family historyc | 20 (22.2) |
| Thyroid status at first clinicd | |
| No. (%) Euthyroid | 39 (41.1) |
| No. (%) Hyperthyroid | 44 (46.3) |
| No. (%) Hypothyroid | 9 (9.5) |
| Antithyroid Medication | |
| No. (%) Carbimazole | 34 (37.4) |
| No. (%) Thyroxine | 13 (14.3) |
| No. (%) IV Methylprednisolone | 57 (59.4) |
| No. (%) Second-line immunosuppression | 21 (22.1) |
| Baseline Clinical Scores at first presentation | |
| Median CAS (IQR) | 2 (1–3) |
| EUGOGO Severity Scale | |
| No. (%) Mild | 35 (36.5) |
| No. (%) Moderate-Severe | 51 (53.1) |
| No. (%) Sight-Threatening | 10 (10.4) |
| TSHR Antibody Status | 3.95 (155–8.975) |
a Data unrecorded n = 23,
b Data unrecorded n = 3,
c Data unrecorded n = 6,
d Data unrecorded n = 5,
e Data unrecorded n = 5. Data was recorded from the inception of the clinics until March 2021
Association and correlation between ADC and CAS (disease activity)
The results for EOM-ADCs according to CAS score are shown in Fig. 2 including median values. There was a positive correlation between the EOM-ADC and CAS (rs = 0.362, 95% CI (0.1773–0.5218), p ≤ 0.001) (Fig. 2A). Comparing the clinically relevant groups, CAS ≥ 3 (n = 29) had a higher ADC (1332 (range: 934–1428) mm2/s) than the CAS < 3 (n = 76) (997 (range: 830–1131) mm2/s) (p ≤ 0.001) (Fig. 2B).
Fig. 2.
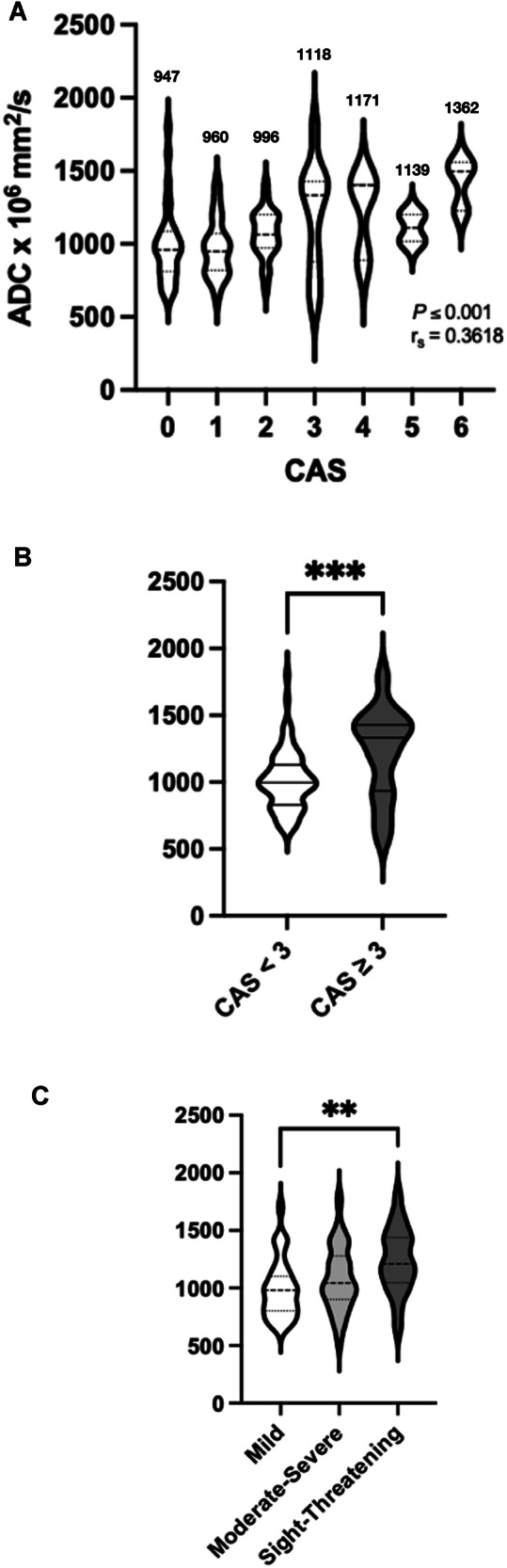
Correlation between CAS and EUGOGO Severity Scale, and ADC with a clinically meaningful group-wise comparison of ADC and CAS in the overall cohort. Violin plots demonstrate a positive Spearman rank correlation coefficient between CAS and ADC (n = 105) as well as the median value of each CAS score (in mm2/s) (A) and significantly greater ADC values in CAS ≥ 3 (n = 29) compared with CAS < 3 (n = 76) groups (B) (***p ≤ 0.001, 2-tailed). Greater ADC values in the sight-threatening severity cohort compared with those in the mild severity cohort (**p ≤ 0.01, 2-tailed) with no significant difference between the other groups (C)
Association between ADC and EUGOGO severity scale (disease severity)
EOM-ADCs along the severity scale of the cohort were as follows: 980 (range: 800–1100) mm2/s, 1040 (range: 890–1255) mm2/s and 1210 (range: 1044–1438) mm2/s for mild (n = 35), moderate-severe (n = 51) and sight-threatening groups (n = 10) respectively. There was a positive trend for a higher ADC according to ESS which was statistically significant between mild and sight-threatening groups (p < 0.01) (Fig. 2C).
ROC analysis
To differentiate clinically active disease from inactive TED (i.e., CAS < 3 and CAS ≥ 3), an ADC cut-off of 1062 mm2/s achieved 60% sensitivity and 72% specificity in diagnosing active disease (area under the curve (AUC) = 0.718, 95% CI 0.63–0.80, p ≤ 0.0001) (Fig. 3A). The estimated ADC cut-off to differentiate between mild-moderate disease and sight-threatening disease was 995 mm2/s to give 77% sensitivity and 67% specificity (AUC = 0.7744, 95% CI, (0.63–0.92), p ≤ 0.01) (Fig. 3B).
Fig. 3.
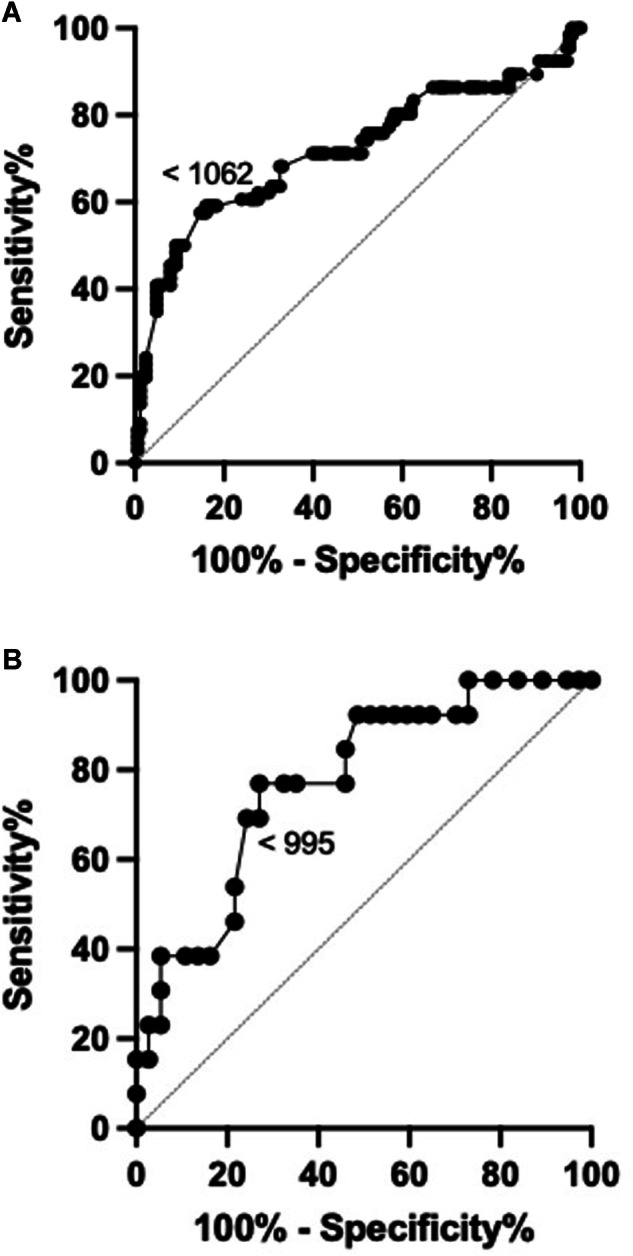
ROCs for the ADC values obtained in subjects with CAS < 3 and CAS ≥ 3 (area under the curve = 0.7178; 95% CI, (0.63–0.80); p ≤ 0.0001) (A). ROCs for the ADC values were obtained in subjects with mild-moderate disease and sight-threatening disease (area under the curve = 0.7744, 95% CI, 0.63–0.92; p ≤ 0.01) (B). A diagonal line represents a line of no discrimination between disease states. The arrowed number represents the cut-off ADC values
Discussion
CAS and ESS are common clinical scores used to assess the activity and severity of TED [2, 3]. CAS is a useful clinical tool in decision-making for TED patients, however, this clinical measure can over or underestimate true disease activity, even by clinicians with experience [12]. The CAS considers each TED symptom and sign as having equal weighting in scoring and does not include important signs of TED, such as diplopia and proptosis at the initial assessment. The ESS is used to assess the severity of TED which is the functional deficit caused by the disease [2, 3]. This includes the assessment of ocular motility and the clinical proptosis measurement. However, there can be poor intra-observer agreement in clinical proptosis measurements [13]. This raises concerns about the sensitivity of this clinical score as an indicator of TED severity [13]. Furthermore, some patients with functionally severe eye disease may have minimal mild proptosis only, which can be explained in terms of a variation between individuals in how much the orbital septal tissues can be distended axially, and those with less distensible orbital septae may be more likely to develop complications as a result of higher retrobulbar orbital pressures developing similar to a chronic compartment syndrome. Levels of proptosis are also dependent on the ethnicity of the patient which influences the anatomy of the bony orbit [14].
Orbital MRI is an important diagnostic tool in patients with clinically evident TED as it can provide both anatomic and functional imaging information and includes retrobulbar structures (posterior orbit) that are inaccessible to clinical evaluation.
The findings of this study are consistent with previously published data that document a positive association between increased extraocular muscle EOM-ADC values of involved EOM in TED and higher clinical measures of disease activity based on CAS and higher disease severity based on the ESS [6, 7, 10]. Therefore, EOM-ADCs potentially have clinical utility by providing objective quantitative data regarding muscle inflammatory changes in patients with TED, which in turn correlate with disease activity and severity. This is important for several reasons. Firstly, clinical assessment tools currently used to assess disease activity in TED, such as CAS, evaluate signs and symptoms of periorbital inflammation which are binary and inherently subjective. Furthermore, specific features, such as erythema, are difficult to assess in darker-skinned individuals, which in turn limits the reliability of CAS in such cases. In the present study over 40% of patients were non-Caucasian [12, 14, 15]. In addition, CAS does not evaluate the posterior orbit directly, including the EOMs and retroorbital fat, so it can over or underestimate the true disease activity, even if performed by clinicians with experience [12]. For example, some individuals with minimal signs of periorbital inflammation in TED may have severe active inflammation in the EOMs. Consequently, an accurate overview of the activity of TED requires both clinical assessments, e.g. using CAS, and orbital imaging. The present study suggests that EOM-ADCs could be utilised for this purpose.
The underlying pathological mechanism for the elevated ADC measurements in patients with TED is not closely established and could represent increased oedema alone or additional pathophysiological processes [16]. Furthermore, different acute and chronic effects may contribute at different time points. The temporal changes of EOM-ADCs in patients with TED were not addressed in this study, although there is some preliminary data to suggest that ADCs may not normalise in patients in the late phase inactive of TED [11].
In this study, higher ADC values were associated with higher CASs and were higher in the sight-threatening cohort compared to the mild severity group. Although this study showed no statistically significant difference in EOM-ADCs in TED with moderate severity compared to the other groups, it is possible that there are different processes predominating in this group including chronic inflammation and fibrosis, which may have different influences on diffusion in EOMs, which in turn may influence ADCs.
The ROC analyses suggest that EOM-ADC values have the potential to differentiate between clinically active and inactive TED, as well as mild to moderate severity of disease and sight-threatening severity of TED (Fig. 3). The EOM-ADC cut-offs may be used to identify those patients who may require earlier intervention, e.g. immunosuppression therapy for high-risk patients. Furthermore, in patients with apparently low CAS, which reflects minimal anterior orbital symptoms and signs of inflammation, the finding of a very high EOM-ADC, which reflects posterior orbital inflammation, may be used to justify closer clinical monitoring with earlier interval imaging.
Study limitations
There were several study limitations. First, the DWI-MRI protocol was introduced at different hospital sites at different time points due to the real-life healthcare evolution of the practice. Therefore, many TED patients who had MRI imaging before the protocol introduction had to be excluded from the study. Given the retrospective nature of the study, we had to exclude patients where clinical parameters such as concurrent CAS were not recorded. Second, ADC was calculated by manually drawing ROIs on enlarged EOMs by three experienced radiologists, but at different sites and scanners. Steps were also taken to ensure inter-scan variability was minimised by standardising the imaging parameters, but small differences are likely to remain. This could be improved by scanning the same individual at both sites to evaluate the inter-observer agreement, but this was out of the scope of this study. Previous studies in TED assessment have shown good inter-observer agreement with ADC measurements [8]. Third, there has been some preliminary research into the potential utility of other MRI-derived indices as a surrogate of disease activity including EOM T2 signal intensity, T2 relaxation, and fat fraction estimates [5]. These indices are still experimental and were not evaluated in this study partly due to its retrospective design although it is possible that a combination of MRI indices may have the highest predictive value for disease activity or severity in TED patients.
Conclusion
Our results show a trend between clinical activity and severity with EOM-ADC across the spectrum of TED. This has clinical utility as EOM-ADC can provide quantitative data pertaining to disease activity in the posterior orbit in TED, which differs from clinically derived measures of disease activity and severity that are subjective and focused on signs of anterior orbital inflammation.
Acknowledgements
We would like to acknowledge all staff at Eye, Endocrinology and Radiology Departments at Central Middlesex Hospital, Western Eye Hospital and Charing Cross Hospital, especially our colleagues at the London Group of the European Group on Graves Orbitopathy (EUGOGO) including Drs Rashmi Akshikar, Kleo Alexiadou, Ahmad Aziz, Vassiliki Bravis, Rajini Jain, Kate Lazarus, and Karim Meeran.
Abbreviations
- ADC
Apparent diffusion coefficient
- CAS
Clinical activity score
- EOM
Extra ocular muscles
- ESS
European Severity Scale
- MRI
Magnetic resonance imaging
- non-EPI-DWI
Non-echoplanar diffusion-weighted imaging
- TED
Thyroid eye disease
Authors contributions
N.M.G. collected and analysed the data, and was the first author of the manuscript. C.F. assisted in analysing the data, and was a major contributor in writing the manuscript. V.L. was a major contributor in developing the project, as well as writing the manuscript. P.A., A.A. assisted in collecting the data. G.M. and R.L. assisted in collecting the radiological data. K.B. was a major contributor in developing and overseeing the project, as well as writing the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable
Data availability
Patient data was collected from three Thyroid Eye Clinics at different NHS Healthcare Trusts, and was kept anonymous in line with patient confidentiality.
Declarations
Ethics approval and consent to participate
The study was approved by the local Audit Committee and adhered to the tenets of the Declaration of Helsinki.
Competing interests
V.L.: Principal Investigator for the Horizon Viridian and Sling Trials. Thyroid Eye Disease Amsterdam Declaration UK Implementation (TEAMeD-5) Ophthalmology Lead. British Oculoplastic Surgery Society (BOPSS) National Lead for Thyroid Eye Disease. Advisory Board for Amgen Limited. The remaining authors declare that they have no competing interests.
Consent for publication
Not Applicable
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McAlinden C (2014) An overview of thyroid eye disease. Eye Vis (Lond) 1. 10.1186/s40662-014-0009-8 [DOI] [PMC free article] [PubMed]
- 2.Barrio-Barrio J, Sabater AL, Bonet-Farriol E, Velázquez-Villoria Á, Galofré JC (2015) Graves’ ophthalmopathy: VISA versus EUGOGO classification, assessment, and management. J Ophthalmol 2015:249125–16. 10.1155/2015/249125 [DOI] [PMC free article] [PubMed]
- 3.Manjandavida F, Chahar S (2020) An update on thyroid eye disease: current knowledge, preferred practice patterns, and future therapies. Kerala J Ophthalmol 32:10. 10.4103/kjo.kjo_87_19 10.4103/kjo.kjo_87_19 [DOI] [Google Scholar]
- 4.Mourits MP, Prummel MF, Wiersinga WM et al (1997) Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol 47:9–14. 10.1046/j.1365-2265.1997.2331047.x 10.1046/j.1365-2265.1997.2331047.x [DOI] [PubMed] [Google Scholar]
- 5.Das T, Roos JCP, Patterson AJ et al (2019) T2-relaxation mapping and fat fraction assessment to objectively quantify clinical activity in thyroid eye disease: an initial feasibility study. Eye (Lond) 33. 10.1038/s41433-018-0304-z [DOI] [PMC free article] [PubMed]
- 6.Gonçalves ACP, Gebrim EMMS, Monteiro MLR (2012) Imaging studies for diagnosing Graves’ orbitopathy and dysthyroid optic neuropathy. Clinics 67:1327–1334 10.6061/clinics/2012(11)18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gontarz-Nowak K, Szychlińska M, Matuszewski W et al (2020) Current knowledge on graves’ orbitopathy. J Clin Med 10:16. 10.3390/jcm10010016 10.3390/jcm10010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lingam RK, Mundada P, Lee V (2018) Novel use of non-echo-planar diffusion weighted MRI in monitoring disease activity and treatment response in active Grave’s orbitopathy: an initial observational cohort study. Orbit 37:325–330. 10.1080/01676830.2017.1423343 10.1080/01676830.2017.1423343 [DOI] [PubMed] [Google Scholar]
- 9.Kilicarslan R, Alkan A, Ilhan MM, Yetis H, Aralasmak A, Tasan E (2015) Graves’ ophthalmopathy: The role of diffusion-weighted imaging in detecting involvement of extraocular muscles in early period of disease. Br J Radiol 88. 10.1259/bjr.20140677 [DOI] [PMC free article] [PubMed]
- 10.Baliyan V, Das CJ, Sharma R et al (2016) Diffusion weighted imaging: technique and applications. World J Radiol 8:785. 10.4329/wjr.v8.i9.785 10.4329/wjr.v8.i9.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feeney C, Lingam RK, Lee V et al (2020) Non-EPI-DWI for detection, disease monitoring, and clinical decision-making in thyroid eye disease. AJNR Am J Neuroradiol 41:1466–1472. 10.3174/ajnr.A6664 10.3174/ajnr.A6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolman PJ (2018) Grading severity and activity in thyroid eye disease. Ophthalmic Plast Reconstr Surg 34:S34–S40. 10.1097/IOP.0000000000001150 10.1097/IOP.0000000000001150 [DOI] [PubMed] [Google Scholar]
- 13.Bingham CM, Sivak-Callcott JA, Gurka MJ et al (2016) Axial globe position measurement: a prospective multicenter study by the International Thyroid Eye Disease Society. Ophthalmic Plast Reconstr Surg 32. 10.1097/IOP.0000000000000437 [DOI] [PMC free article] [PubMed]
- 14.Ross DS, Burch HB, Cooper DS et al (2016) 2016 American Thyroid Association Guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 26. 10.1089/thy.2016.0229 [DOI] [PubMed]
- 15.Mawn LA, Dolman PJ, Kazim M et al (2018) Soft tissue metrics in thyroid eye disease: an international thyroid eye disease society reliability study. Ophthalmic Plast Reconstr Surg 34:544–546. 10.1097/IOP.0000000000001080 10.1097/IOP.0000000000001080 [DOI] [PubMed] [Google Scholar]
- 16.Ferreira TA, Saraiva P, Genders SW et al (2018) CT and MR imaging of orbital inflammation. Neuroradiology 60:1253. 10.1007/S00234-018-2103-4 10.1007/S00234-018-2103-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Patient data was collected from three Thyroid Eye Clinics at different NHS Healthcare Trusts, and was kept anonymous in line with patient confidentiality.