Abstract
Purpose
To evaluate the diagnostic performance of image-based artificial intelligence (AI) studies in predicting muscle-invasive bladder cancer (MIBC). (2) To assess the reporting quality and methodological quality of these studies by Checklist for Artificial Intelligence in Medical Imaging (CLAIM), Radiomics Quality Score (RQS), and Prediction model Risk of Bias Assessment Tool (PROBAST).
Materials and methods
We searched Medline, Embase, Web of Science, and The Cochrane Library databases up to October 30, 2023. The eligible studies were evaluated using CLAIM, RQS, and PROBAST. Pooled sensitivity, specificity, and the diagnostic performances of these models for MIBC were also calculated.
Results
Twenty-one studies containing 4256 patients were included, of which 17 studies were employed for the quantitative statistical analysis. The CLAIM study adherence rate ranged from 52.5% to 75%, with a median of 64.1%. The RQS points of each study ranged from 2.78% to 50% points, with a median of 30.56% points. All models were rated as high overall ROB. The pooled area under the curve was 0.85 (95% confidence interval (CI) 0.81–0.88) for computed tomography, 0.92 (95% CI 0.89–0.94) for MRI, 0.89 (95% CI 0.86–0.92) for radiomics and 0.91 (95% CI 0.88–0.93) for deep learning, respectively.
Conclusion
Although AI-powered muscle-invasive bladder cancer-predictive models showed promising performance in the meta-analysis, the reporting quality and the methodological quality were generally low, with a high risk of bias.
Critical relevance statement
Artificial intelligence might improve the management of patients with bladder cancer. Multiple models for muscle-invasive bladder cancer prediction were developed. Quality assessment is needed to promote clinical application.
Key Points
Image-based artificial intelligence models could aid in the identification of muscle-invasive bladder cancer.
Current studies had low reporting quality, low methodological quality, and a high risk of bias.
Future studies could focus on larger sample sizes and more transparent reporting of pathological evaluation, model explanation, and failure and sensitivity analyses.
Graphical Abstract
Keywords: Magnetic resonance imaging, Urinary bladder neoplasms, Neoplasm staging, Muscle-invasive bladder neoplasms, Artificial intelligence
Introduction
Bladder cancer (BCa) constitutes a significant global health challenge, with an estimated 550,000 new cases and 200,000 deaths worldwide annually [1]. Muscle-invasive bladder cancer (MIBC) is a particularly aggressive form of BCa, defined by the invasion of the tumor into or beyond the superficial muscularis propria of the bladder wall [2]. This subtype is characterized by higher mortality rates, earlier metastasis, and a worse prognosis compared to non-muscle-invasive bladder cancer (NMIBC) [3, 4]. Identifying MIBC promptly is crucial, as it necessitates more aggressive treatments, including radical cystectomy (RC) and adjuvant therapy, which are critical for improving patient outcomes [4, 5].
Clinically, cystoscopy with transurethral resection of bladder tumor (TURBT) is usually the diagnostic approach for identifying MIBC in patients suspected of BCa. While effective, this invasive procedure can occasionally under-sample muscular tissue, resulting in false negative rates of approximately 10% to 15% [6]. The Vesical Imaging-Reporting and Data System (VI-RADS), based on multiparametric magnetic resonance imaging (MRI), has emerged as a valuable non-invasive alternative, offering high sensitivity and specificity in differentiating MIBC from NMIBC [7, 8]. However, the utility of VI-RADS is limited by the long acquisition times, high costs of MRI examinations, and dependence on the subjective experience of the radiologist interpreting the images.
Recent advancements in artificial intelligence (AI), particularly in radiomics and deep learning (DL), provide a promising avenue for the pre-operative identification of MIBC. AI techniques can analyze medical images by extracting hand-crafted radiomic features or using self-learned DL features to predict disease status through sophisticated classification algorithms [9–11]. These technologies have shown potential in enhancing the accuracy and efficiency of MIBC diagnosis [12–32]. Despite the promise, there is a wide variation in the reported results across studies [33, 34]. Furthermore, the overall quality of these studies has not been thoroughly assessed, especially concerning critical methodological aspects such as patient selection, model development, and performance evaluation [33, 34], hindering the clinical application of AI techniques in identifying MIBC.
In this study, we aimed to (1) systematically review and evaluate the diagnostic performances of current AI studies on the prediction of MIBC and (2) use the Checklist for Artificial Intelligence in Medical Imaging (CLAIM), Radiomics Quality Score (RQS), and Prediction model Risk of Bias Assessment Tool (PROBAST) to comprehensively assess the reporting quality and methodological quality of these models [35–37].
Materials and methods
Literature search strategy and study selection
The study protocol is registered in the International Prospective Register of Systematic Reviews (CRD42023446035). This systematic review was conducted according to the recommendations published in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for Diagnostic Test Accuracy statement [38]. PRISMA checklist is provided in the Supplementary material 1.
Medline via PubMed, Embase via Ovid, Web of Science, and The Cochrane Library were searched for eligible studies from inception to October 30, 2023 using a combination of the following terms: “bladder cancer”, “muscle invasion or staging”, “radiomics or deep learning”, and “computed tomography or magnetic resonance imaging or ultrasound”. The language was restricted to English. The detailed search queries were displayed in Supplementary Material 2. In addition, we screened the bibliographies of initially searched articles for additional relevant studies.
After the removal of duplicated studies, two researchers with 2 and 7 years of experience in genitourinary imaging screened the titles and abstracts of the identified studies. Studies were excluded if the type of the article was one of the following: review, editorial, abstract, and case report. The remaining studies were full-text assessed. To be included, the articles must have fulfilled the following: (1) population: patients with primary BCa; (2) index test: development or validation of radiomics or DL models using computed tomography (CT), MR, or ultrasound images; (3) outcomes: the muscle invasion status confirmed by at least one pathological evaluation method; (4) original articles. Studies were excluded from meta-analysis if they lacked adequate data sufficient to reconstruct the 2 × 2 contingency table.
Data extraction
Relevant data were extracted from each eligible publication using a standardized form recording the following information: study year, data collection strategy, number of centers, target population, prediction level, sample size, MIBC ratio, gold standard, internal validation method, external validation method, modality, annotation method, number of readers per case, reader agreement, feature extraction method, number of extracted features, number of selected features, and final classifier algorithm. The feature number of DL models was the number of neurons in the first fully-connected layer since the convolutional layers were considered as feature extractors. The following diagnostic accuracy measures were also recorded for meta-analysis: true positive, true negative, false positive, and false negative. When a study involved training and test cohorts, the diagnostic performance in the test cohort was selected for the model’s prediction power; When a study involved external and internal cohorts, the diagnostic performance in the external cohort was selected for the model’s prediction power. If several prediction models were developed in one study, the model with the best performance was chosen.
CLAIM, RQS, and PROBAST evaluation
The same two researchers independently assessed all eligible publications with CLAIM, RQS, and PROBAST. When a discrepancy occurred, an agreement was reached after discussions with two senior researchers. The consensus data were used in the following analyses. For CLAIM, the study reporting was evaluated by a total of 42 items. The item adherence rate was the percentage of adhering studies over all applicable studies among the item, while the study adherence rate was the percentage of adhering items over all applicable items among the study. The RQS, which consists of 16 criteria, is a recently accepted tool to measure the methodological rigor of radiomics workflow. The total RQS points are the sum of points from checkpoint 1, checkpoint 2, and checkpoint 3, with the ideal RQS points being 100% (36/36.00). For PROBAST, the risk of bias (ROB) was assessed across four domains: participants, predictors, outcome, and analysis. Signaling questions within each domain were answered with one out of five options: “yes, “ “probably yes, “ “probably no, “ “no, “ “no information”. If there was any “no/probably no” in signaling questions, the domain was labeled as having high ROB. If all signaling question is “yes/probably yes”, the domain was labeled as having low ROB. If there was no “no/probably no” but any “no information” in signaling questions, the domain was labeled as having unclear ROB. The overall ROB of the four domains was then determined using the same criteria.
Statistical analysis
Heterogeneity was evaluated using the following methods: (1) the Cochran Q test, with a p-value of < 0.05 indicating significant heterogeneity, and (2) the Higgins I2 test. I2 values of 0–25%, 25–50%, 50–75%, and > 75% represent very low, low, medium, and high heterogeneity, respectively. The weight of each study was calculated with the inverse variance method, in which the weight given to each study is chosen to be the inverse of the variance of the effect estimate, minimizing the uncertainty of the pooled effect estimate. In the case of medium and high heterogeneity, the random-effect model was favored over the fixed-effect model. Diagnostic accuracy was assessed using the hierarchical summary receiver operating characteristic (HSROC) curves and areas under the HSROC (AUC). Sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio were also calculated. Meta-regression was performed to explore the potential sources of heterogeneity.
All calculations were performed with a 95% confidence interval (95% CI). A difference was considered statistically significant when the p-value was smaller than 0.05. We used the “metandi” and “midas” modules in Stata 17 for statistical analyses [39, 40].
Results
Characteristics of included studies
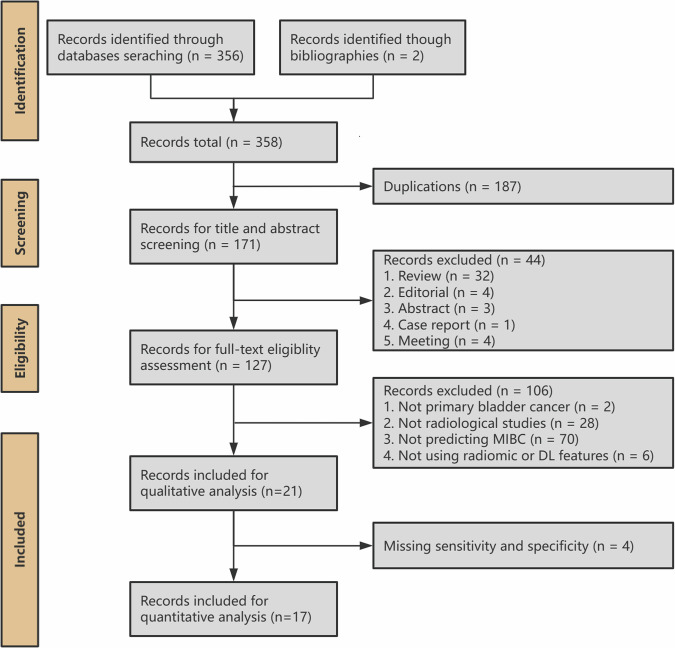
A flowchart depicting the study selection process is shown in Fig. 1. The search strategy identified 171 studies after removing duplicates. Among these, 21 studies met the inclusion criteria. The included studies are summarized in Table 1 and Fig. 2.
Fig. 1.
Systematic review flow diagram designed according to PRISMA
Table 1.
Characteristics of included studies
| Citation | Study year | Data collection | Number of centers | Population | Prediction level | Sample size | MIBC ratio | Source of gold standard | Internal validation | External validation |
|---|---|---|---|---|---|---|---|---|---|---|
| Xu et al [12] | 2017 | Retrospective | 1 | BCa | Lesion | 118 | 0.7119 | TURBT | No validation | No validation |
| Garapati et al [13] | 2017 | Retrospective | 1 | BCa | Lesion | 84 | 0.4881 | Lack information | 2-fold cross-validation | No validation |
| Xu and Zhang et al [14] | 2018 | Retrospective | 1 | BCa | Patient | 54 | 0.5556 | Surgery | 100-run 10-fold cross-validation | No validation |
| Zheng et al [15] | 2019 | Retrospective | 1 | UC | Patient | 199 | 0.4372 | RC or TURBT | Bootstrapping | Public dataset |
| Xu and Yao et al [16] | 2019 | Retrospective | 1 | high-grade UC | Patient | 218 | 0.6468 | RC | Random split | No validation |
| Wang et al [17] | 2020 | Retrospective | 2 | BCa | Patient | 106 | 0.566 | RC, PC, or TURBT | No validation | Geographical validation |
| Zhou et al [18] | 2021 | Retrospective | 1 | BCa | Patient | 100 | 0.5 | Surgery | Random split | No validation |
| Zhang et al [19] | 2021 | Retrospective | 2 | UC | Patient | 441 | 0.2766 | RC or TURBT | Random split | Geographical validation |
| Zheng and Xu et al [20] | 2021 | Retrospective | 1 | UC | Patient | 185 | 0.3351 | Surgery | Random split | No validation |
| Yang et al [21] | 2021 | Retrospective | 1 | UC | Patient | 369 | 0.3252 | RC | Random split | No validation |
| Gao et al [22] | 2021 | Retrospective | 1 | BCa | Patient | 104 | 0.4135 | Surgery | Random split | No validation |
| Chen et al [23] | 2022 | Retrospective | 1 | UC | Patient | 173 | 0.2486 | RC, PC, or TURBT | Random split | No validation |
| Zou et al [24] | 2022 | Retrospective + prospective | 3 | BCa | Patient | 468 | 0.2863 | TURBT | Random split | Prospective validation; geographical validation |
| Zhang and Li et al [25] | 2022 | Retrospective | 1 | BCa | Patient | 342 | 0.231 | RC or TURBT | Random split | No validation |
| Cui et al [26] | 2022 | Retrospective | 1 | BCa | Patient | 188 | 0.5 | RC, PC, or TURBT | Random split | No validation |
| Zhang and Wu et al [27] | 2022 | Retrospective | 2 | BCa | Patient | 441 | 0.2766 | RC or TURBT | 10-run 10-fold cross-validation | Geographical validation |
| Liu et al [28] | 2022 | Retrospective | 1 | UC | Patient | 206 | 0.2718 | Surgery | 10-run 5-fold cross-validation | No validation |
| Sarkar et al [29] | 2023 | Retrospective | 1 | UC | Patient | 65 | 0.6307 | RC | 10-fold cross-validation | No validation |
| Li et al [30] | 2023 | Retrospective | 2 | UC | Lesion | 121 | 0.3305 | RC, PC, or TURBT | 5-fold cross-validation | Geographical validation |
| Wang and Li et al [31] | 2023 | Retrospective | 1 | UC | Patient | 191 | 0.3717 | RC or TURBT | Random split | No validation |
| Li and Cao et al [32] | 2023 | Retrospective | 2 | UC | Lesion | 215 | 0.2884 | RC, PC, or TURBT | Random split | Geographical validation |
BCa bladder cancer, MIBC muscle-invasive bladder cancer, UC urothelial carcinoma, TURBT transurethral resection of bladder tumor, RC radical cystectomy, PC partial cystectomy
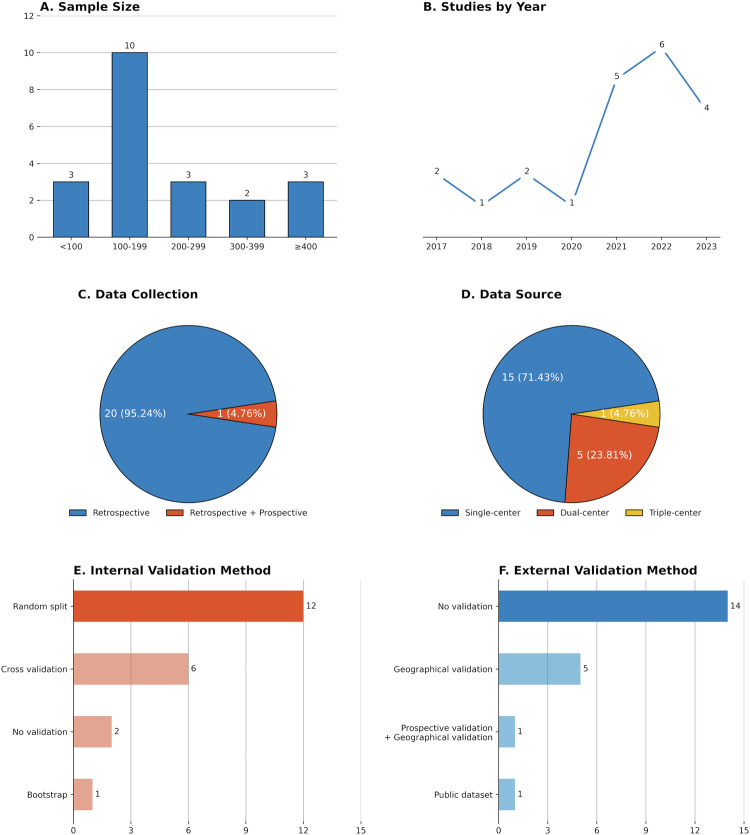
Fig. 2.
Overview of study characteristics. A Aggregate number of patients included in the study; B Year of publication; C Data collection strategy; D Data source; E Internal validation method; F External validation method
The included 21 studies were published between September 2017 and May 2023, of which approximately two-thirds (15/21, 71.4%) were published within the past three years. Most of the studies (20/21, 95.2%) were retrospectively designed except for one [24]. The population of these studies varied. Ten studies included patients with BCa [12–14, 17, 18, 22, 24–27], one study analyzed patients with high-grade urothelial carcinoma [16], and other studies analyzed patients with urothelial carcinoma [15, 16, 19–21, 23, 28–32]. Four studies performed lesion-level prediction while others performed patient-level prediction [12, 13, 30, 32]. The sample size of the studies ranged from 54 to 468 patients, with a total of 4256 patients and 4388 lesions analyzed in 21 studies. The prevalence of MIBC ranged from 23.1% to 71.2%, with a median of 37.2%. The reference standard for the diagnosis of MIBC also varied. Ten studies diagnosed MIBC by specimens from different surgical techniques of TURBT, RC, or partial cystectomy (PC) [15, 17, 19, 23, 25–27, 30–32]. Three studies only included RC patients [16, 21, 29]. Two studies only included TURBT patients [12, 24]. However, there were still five studies that did not specify the source of the specimen [13, 14, 20, 22, 28]. The most common internal validation method was random-split validation [16, 18–26, 31, 32], while most studies (15/21, 71.4%) did not perform external validation except for six studies [17, 19, 24, 27, 30, 32].
AI technique details of the included studies
The AI technique details of the included studies are summarized in Table 2. All the studies analyzed a single modality. The most common modality was MRI (n = 12), followed by CT (n = 8) and ultrasound (n = 1). Manual annotation (n = 15) was the major method for delineating the region of interest (ROI), while other studies used semi-automatic (n = 4) or fully-automatic (n = 2) segmentation algorithms. Fourteen studies extracted hand-crafted radiomic features, five studies extracted self-learned DL features [13, 19, 21, 24, 29, 32], and two studies extracted both types of features [23, 30]. Extracted feature counts ranged from 63 to 23,688 in the included studies, and the selected feature counts ranged from 6 to 2048.
Table 2.
Methodological characteristics of the included studies
| Citation | Modality | Annotation | Number of readers per case | Reader agreement after annotation | Feature extraction | Number of extracted features | Number of selected features | Final classifier | AUC |
|---|---|---|---|---|---|---|---|---|---|
| Xu et al [12] | MRI | Manual; 3D | 2 | Yes | Radiomics | 63 | 30 | Support vector machine | / |
| Garapati et al [13] | CT | Automatic; 3D | 1 | No | Radiomics | 91 | Lack information | Random forest | / |
| Xu and Zhang et al [14] | MRI | Manual; 2D | 1 | No | Radiomics | 1104 | 19 | Support vector machine | 0.9857 |
| Zheng et al [15] | MRI | Semi-automatic; 3D | 1 | Yes | Radiomics | 2062 | 23 | LASSO | / |
| Xu and Yao et al [16] | MRI | Manual; 3D | 1 | No | Radiomics | 156 | 21 | Random forest | 0.907 |
| Wang et al [17] | MRI | Manual; 2D | 2 | Yes | Radiomics | 1404 | 36 | Logistic regression | 0.672 |
| Zhou et al [18] | CT | Semi-automatic; 3D | 2 | No | Radiomics | 1223 | 6 | Support vector machine; logistic regression | 0.782 |
| Zhang et al [19] | CT | Semi-automatic; 3D | 2 | No | Deep learning | Lack information | Lack information | FGP-Net | 0.791 |
| Zheng and Xu et al [20] | MRI | Manual; 3D | 1 | No | Radiomics | 2436 | 21 | LASSO | 0.906 |
| Yang et al [21] | CT | Manual; 2D | 2 | Yes | Deep learning | 49 | 49 | Neural network | 0.998 |
| Gao et al [22] | US | Manual; 2D | 2 | Yes | Radiomics | 5936 | 30 | Naïve Bayes | 0.84 |
| Chen et al [23] | CT | Manual; 3D | Lack information | No | Both | 1735; 2048 | 30 | Logistic regression | 0.884 |
| Zou et al [24] | MRI | Automatic; 2D | 2 | Yes | Deep learning | 2048 | 2048 | Inception V3 | 0.856 |
| Zhang and Li et al [25] | MRI | Manual; 3D | 2 | Yes | Radiomics | 23,688 | 9 | Logistic regression | 0.931 |
| Cui et al [26] | CT | Manual; 3D | 1 | No | Radiomics | 102 | 8 | AdaBoost | 0.894 |
| Zhang and Wu et al [27] | CT | Semi-automatic; 3D | 2 | No | Radiomics | 1218 | 8 | Logistic regression | 0.784 |
| Liu et al [28] | MRI | Manual; 2D | 1 | No | Radiomics | 4128 | 28 | LASSO | / |
| Sarkar et al [29] | CT | Manual; 3D | 1 | Lack information | Deep learning | 2048–9216 | Lack information | Linear discriminant analysis | / |
| Li et al [30] | MRI | Manual; 3D | 1 | No | Both | 851; Lack information | Lack information | Neural network | 0.932 |
| Wang and Li et al [31] | MRI | Manual; 3D | 2 | No | Radiomics | 1070 | 6 | Logistic regression | 0.711 |
| Li and Cao et al [32] | MRI | Manual; 3D | 1 | No | Deep learning | 2048 | 2048 | ResNet | 0.861 |
US ultrasound, LASSO least absolute shrinkage and selection operator
Quality evaluation
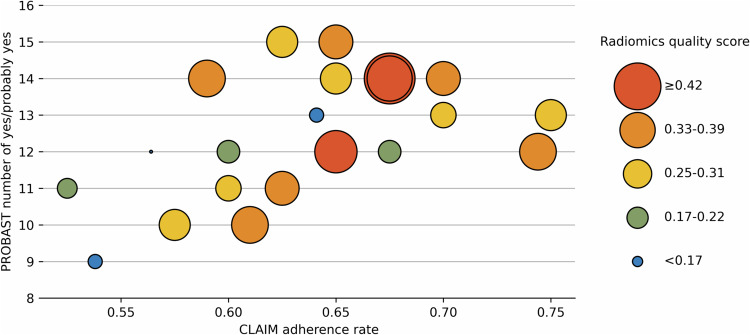
The CLAIM study adherence rates, RQS points, and number of yes/probably yes in PROBAST of each study were shown in Fig. 3.
Fig. 3.
Diagram showing reporting quality, methodological quality, and risk of bias of each study. The x-axis is the CLAIM adherence rate. The y-axis is the number of yes or probably yes in the PROBAST evaluation. The size of each point is the RQS points
CLAIM
The study adherence rates on CLAIM of each study are shown in Fig. 4. The study adherence rate on CLAIM ranged from 52.5% to 75%, with a median of 64.1%. Five studies had a CLAIM study adherence rate lower than 60.0% [12, 13, 25, 28, 29]. The detailed CLAIM evaluation can be found in Supplementary Material 3.
Fig. 4.
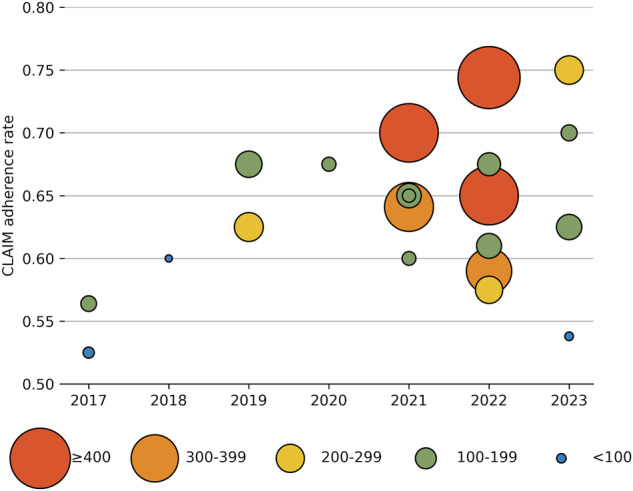
Diagram showing reporting quality by year and sample size. The x-axis is the year of publication. The y-axis is the CLAIM adherence rate. The size of each point is the sample size
The item adherence rates for CLAIM are displayed in Table 3. The item adherence rate for CLAIM ranged from 0.0% to 100.0%, with a median of 85.7%. A total of 14 items had poor adherence rates (CLAIM item adherence rate < 60.0%), including data preprocessing (item 9, 42.9%), de-identification (item 12, 0.0%), missing data handling (item 13, 9.5%), alternative reference standard choosing (item 15, 6.3%), pathological evaluation standard (item 16, 4.7%), pathological evaluation method (item 17, 0.0%), pathological evaluation variability measurement (item 18, 0.0%), sample size estimation (item 19, 0.0%), model parameters initialization method (item 24, 14.3%), sensitivity analysis (item 30, 0.0%), explainability or interpretability method (item 31, 23.8%), external data validation (item 32, 33.3%), failure analysis (item 37, 14.3%), and full study protocol (item 41, 0.0%).
Table 3.
Reporting quality assessment using the CLAIM
| Section | Item | Adherence studies | Applicable studies | Adherence rate | Section | Item | Adherence studies | Applicable studies | Adherence rate |
|---|---|---|---|---|---|---|---|---|---|
| Title or Abstract | item_1 | 21 | 21 | 100% | Methods—Model | item_22 | 20 | 21 | 95.24% |
| item_2 | 20 | 21 | 95.24% | item_23 | 18 | 21 | 85.71% | ||
| Introduction | item_3 | 21 | 21 | 100% | item_24 | 3 | 21 | 14.29% | |
| item_4 | 20 | 21 | 95.24% | Methods—Training | item_25 | 21 | 21 | 100% | |
| Methods—Study Design | item_5 | 21 | 21 | 100% | item_26 | 21 | 21 | 100% | |
| item_6 | 21 | 21 | 100% | item_27 | 0 | 0 | / | ||
| Methods—Data | item_7 | 21 | 21 | 100% | Methods—Evaluation | item_28 | 21 | 21 | 100% |
| item_8 | 19 | 21 | 90.48% | item_29 | 18 | 21 | 85.71% | ||
| item_9 | 9 | 21 | 42.86% | item_30 | 0 | 21 | 0% | ||
| item_10 | 1 | 1 | 100% | item_31 | 5 | 21 | 23.81% | ||
| item_11 | 21 | 21 | 100% | item_32 | 7 | 21 | 33.33% | ||
| item_12 | 0 | 21 | 0% | Results—Data | item_33 | 13 | 21 | 61.9% | |
| item_13 | 2 | 21 | 9.52% | item_34 | 18 | 21 | 85.71% | ||
| Methods—Ground Truth | item_14 | 16 | 21 | 76.19% | Results—Model performance | item_35 | 21 | 21 | 100% |
| item_15 | 1 | 16 | 6.25% | item_36 | 18 | 21 | 85.71% | ||
| item_16 | 1 | 21 | 4.76% | item_37 | 3 | 21 | 14.29% | ||
| item_17 | 0 | 21 | 0% | Discussion | item_38 | 21 | 21 | 100% | |
| item_18 | 0 | 21 | 0% | item_39 | 15 | 21 | 71.43% | ||
| Methods—Data Partitions | item_19 | 0 | 21 | 0% | Other information | item_40 | 19 | 21 | 90.48% |
| item_20 | 21 | 21 | 100% | item_41 | 0 | 21 | 0% | ||
| item_21 | 21 | 21 | 100% | item_42 | 13 | 21 | 61.9% |
RQS
The RQS points of each study are shown in Fig. 5, and the median RQS points for each criterion are displayed in Table 4. The RQS points of each study ranged from 2.78% to 50% points, with a median of 30.56% points. The detailed RQS evaluation and checklist can be found in Supplementary Material 3.
Fig. 5.
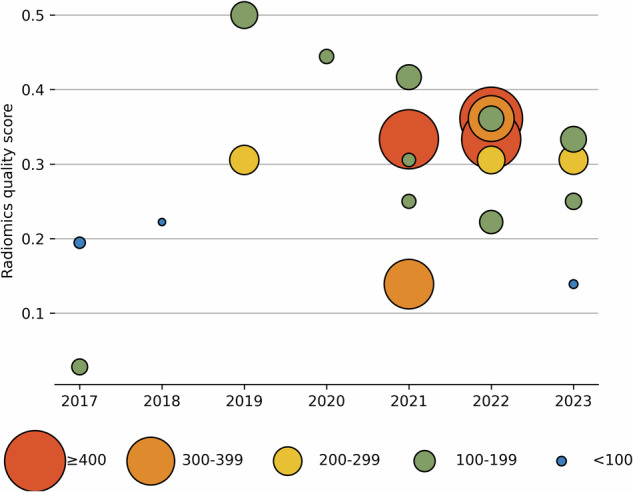
Diagram showing methodological quality by year and sample size. The x-axis is the year of publication. The y-axis is the RQS points. The size of each point is the sample size
Table 4.
Methodological quality assessment using the RQS
| Criterion | Points range | Median points (percentage) |
|---|---|---|
| Image protocol quality | 0–2 | 1 (50%) |
| Multiple segmentations | 0–1 | 1 (50%) |
| Phantom study on all scanners | 0–1 | 0 (0%) |
| Imaging at multiple time points | 0–1 | 0 (0%) |
| Feature reduction or adjustment for multiple testing | −3 to 3 | 3 (100%) |
| Multivariable analysis with non-radiomics features | 0–1 | 0 (0%) |
| Detect and discuss biological correlates | 0–1 | 0 (0%) |
| Cut-off analyses | 0–1 | 0 (0%) |
| Discrimination statistics | 0–2 | 1 (50%) |
| Calibration statistics | 0–2 | 0 (0%) |
| Prospective study registered in a trial database | 0–7 | 0 (0%) |
| Validation | −5 to 5 | 2 (40%) |
| Comparison to ‘gold standard' | 0–2 | 0 (0%) |
| Potential clinical utility | 0–2 | 0 (0%) |
| Cost-effectiveness analysis | 0–1 | 0 (0%) |
| Open science and data | 0–4 | 0 (0%) |
| Total | −8 to 36 | 11 (30.6%) |
Median points percentage was calculated by dividing the scored points by the ideal points
Most of the studies (18/21, 85.7%) had presented their image protocols except for three [13, 18, 29], while no study reported the use of a public protocol. Less than half of the studies (6/21, 28.6%) did not perform multiple segmentations to control the inter- or intra-rater variability of feature extraction [13, 14, 24, 29, 30, 32], no study analyzed inter-scanner differences and temporal variabilities of the features. All studies that used radiomic features and one study that used both radiomic and DL features reduced the dimension of features, while most DL-only studies (4/21, 19.0%) did not perform feature selection on DL features. Seven studies (33.3%) combined clinical information with radiomic models [15–18, 20, 25, 31], and nine (42.9%) compared the radiomic models with radiologist’s diagnosis or VI-RADS category [15–17, 19, 20, 23, 28, 31, 32]. The models performed better than radiologists in internal validation, but their generalizability to external validation data was not as good as experienced radiologists. Only one study discussed the relevance between radiomic features and clinical/histological phenotypes [22]. Most studies (20/21, 95.2%) reported the discrimination statistics except for one [29], but only less than half of the studies (8/21, 38.1%) reported calibration statistics [15, 17–20, 25, 27, 30] as well as (9/21, 42.9%) the cut-off analysis [15, 17, 19, 20, 25–27, 31, 32]. Only one study (4.8%) used prospectively collected data [24]. Only one did not (1/21, 4.8%) perform validation of radiomic signatures [12], and seven (33.3%) externally validated their models on data from other institutes [15, 17, 19, 24, 27, 30, 32]. Only eight studies (38.1%) used decision curve analysis to determine the clinical utility of models [15, 17–20, 25, 26, 30]. Finally, no study conducted cost-effectiveness analyses or shared code or representative data for model development and inference.
PROBAST
The results of the PROBAST evaluation are shown in Fig. 6 and Table 5. In total, all studies were rated as having a high overall risk of bias. Models in two studies were rated as unclear ROB in participants domain due to poor-documented eligibility criteria [13, 29]. In the predictors domain, models in four studies were rated as high ROB [24, 26, 28, 29], and models in ten studies were rated as unclear ROB [14–16, 18, 20, 21, 23, 30–32]. The annotation was done by more than one rater but the inter-rater variability was not analyzed, resulting in probably different predictor definitions for all participants. Blind annotation was the major source of high or unclear ROB. Raters in two studies annotated the image with knowledge of pathological evaluation results [24, 26], and twelve studies did not specify whether the annotation was done blindly [14–16, 18, 20, 21, 23, 28–32]. In the outcome domain, only one study was rated as low ROB [16] while the others were rated as unclear ROB. Most of the studies (20/21) did not report how MIBC was determined in pathological evaluation, how many pathologists were enrolled, and whether the annotation was done blindly. In the analysis domain, all models were rated as having a high overall ROB. Inadequate sample size and poor model performance examination both contributed to the potential high ROB of these AI models in this domain. The detailed PROBAST evaluation can be found in Supplementary Material 3.
Fig. 6.
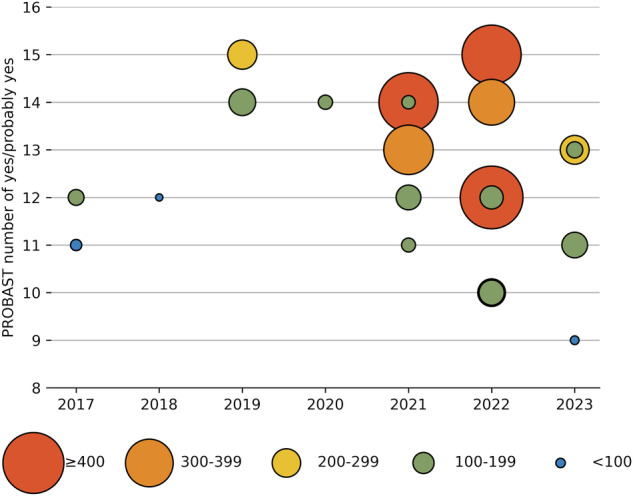
Diagram showing the risk of bias by year and sample size. The x-axis is the year of publication. The y-axis is the number of yes or probably yes in the PROBAST evaluation. The size of each point is the sample size
Table 5.
Risk of bias assessment using the PROBAST
| Signaling question | Yes or probably yes | No information | No or probably no |
|---|---|---|---|
| Domain 1: participants | |||
| Were appropriate data sources used? | 21 (100%) | 0 (0%) | 0 (0%) |
| Were all inclusions and exclusions of participants appropriate? | 19 (90.48%) | 2 (9.52%) | 0 (0%) |
| Domain 2: predictors | |||
| Were predictors defined and assessed in a similar way for all participants? | 18 (85.71%) | 1 (4.76%) | 2 (9.52%) |
| Were predictor assessments made without knowledge of outcome data? | 7 (33.33%) | 12 (57.14%) | 2 (9.52%) |
| Are all predictors available at the time the model is intended to be used? | 21 (100%) | 0 (0%) | 0 (0%) |
| Domain 3: outcome | |||
| Was the outcome determined appropriately? | 17 (80.95%) | 4 (19.05%) | 0 (0%) |
| Was a prespecified or standard outcome definition used? | 1 (4.76%) | 20 (95.24%) | 0 (0%) |
| Were predictors excluded from the outcome definition? | 21 (100%) | 0 (0%) | 0 (0%) |
| Was the outcome defined and determined in a similar way for all participants? | 1 (4.76%) | 20 (95.24%) | 0 (0%) |
| Was the outcome determined without knowledge of predictor information? | 1 (4.76%) | 20 (95.24%) | 0 (0%) |
| Was the time interval between predictor assessment and outcome determination appropriate? | 17 (80.95%) | 4 (19.05%) | 0 (0%) |
| Domain 4: analysis | |||
| Were there a reasonable number of participants with the outcome? | 1 (4.76%) | 1 (4.76%) | 19 (90.48%) |
| Were continuous and categorical predictors handled appropriately? | 21 (100%) | 0 (0%) | 0 (0%) |
| Were all enrolled participants included in the analysis? | 20 (95.24%) | 0 (0%) | 1 (4.76%) |
| Were participants with missing data handled appropriately? | 2 (9.52%) | 19 (90.48%) | 0 (0%) |
| Was selection of predictors based on univariable analysis avoided? | 16 (76.19%) | 0 (0%) | 5 (23.81%) |
| Were complexities in the data accounted for appropriately? | 21 (100%) | 0 (0%) | 0 (0%) |
| Were relevant model performance measures evaluated appropriately? | 8 (38.1%) | 0 (0%) | 13 (61.9%) |
| Were model overfitting, underfitting, and optimism in model performance accounted for? | 7 (33.33%) | 0 (0%) | 14 (66.67%) |
| Do predictors and their assigned weights in the final model correspond to the results from the reported multivariable analysis? | 21 (100%) | 0 (0%) | 0 (0%) |
Clinical value of AI in predicting MIBC
Four studies were excluded for not providing detailed information about sensitivity and specificity in the meta-analysis [12, 13, 15, 28]. A total of seventeen studies were included in the meta-analysis. Seven studies evaluated the diagnostic performance using CT [18, 19, 21, 23, 26, 27, 29], nine using MRI [14, 16, 17, 20, 24, 25, 30–32] and one using ultrasound [22]. The pooled sensitivity, specificity, and AUC for CT were 0.82 (95% CI 0.72–0.89), 0.79 (95% CI 0.72–0.84), and 0.85 (95% CI 0.81–0.88). For MRI, the pooled sensitivity, specificity and AUC were 0.84 (95% CI 0.75–0.91), 0.79 (95% CI 0.77–0.92), and 0.92 (95% CI 0.89–0.94). Ten studies assessed MIBC prediction using radiomics [14, 16–18, 22, 25–27, 31], six using deep learning [19, 21, 24, 29, 30, 32] and one using both [23]. The pooled sensitivity, specificity, and AUC for radiomics were 0.84 (95% CI 0.76–0.90), 0.82 (95% CI 0.74–0.87), and 0.89 (95% CI 0.86–0.92). In terms of deep learning, the pooled sensitivity, specificity and AUC were 0.81 (95% CI 0.68–0.89), 0.87 (95% CI 0.74–0.94), and 0.91 (95% CI 0.88–0.93). The forest plots and AUC curves are illustrated in Supplementary Material 2.
Discussion
AI techniques have been widely studied in MIBC identification. Our systematic review comprehensively evaluated the reporting quality, methodological quality, and ROB of current AI studies for MIBC prediction. The results showed that the overall quality of these studies was poor, with a median CLAIM study adherence rate of 64.1%, a median RQS points percentage of 30.6%, and a high ROB among all studies. The meta-analysis showed current MIBC-predictive AI models had good performance with an AUC of 0.85 (95% CI 0.81–0.88) for CT, 0.92 (95% CI 0.89–0.94) for MRI, 0.89 (95% CI 0.86–0.92) for radiomics and 0.91 (95% CI 0.88–0.93) for deep learning. The current results indicate that AI models have a high potential for predicting MIBC but are far from useful tools in clinical practice.
Two systematic reviews have previously evaluated radiomic studies for MIBC prediction using RQS [33, 34]. Most of the RQS results in our study were similar to theirs. However, a difference in the “Comparison to Gold standard” part was observed. In the previous reviews, most studies were assigned two points for comparing the models with the current gold standard. In our review, less than half of the studies were assigned two points. To show the added value of radiomics, we believe that the “gold standard” refers to the commonly-used non-invasive methods in current clinical practice for detecting MIBC (i.e., manual image interpretation with or without VI-RADS category) [4], thus we only assigned two points to nine studies that had compared the models with manual interpretations. The results of the nine studies showed the AI models usually performed better than radiologists in internal validation, but their generalizability to external validation data was not as good as experienced radiologists [15–17, 19, 20, 26, 28, 31, 32].
Using CLAIM and PROBAST, our systematic review identified some unique quality-reducing items in MIBC-predictive AI studies. Firstly, for the pathology gold standard, only one study reported how MIBC was confirmed through histopathological investigation [16]. Most studies only reported the source of the specimen, and none of the studies reported the details of the histopathological investigation, including the number of pathologists, the inter-reader agreement, and the blindness of assessment. The results of the meta-analysis showed different pathological gold standards significantly contributed to the heterogeneity of sensitivity and specificity. About half of the studies used multiple surgical techniques to obtain the specimen, however, none of them reported the criteria for choosing the reference standard for individual patients or compared the model performance in patients who underwent TURBT with that in patients who underwent RC or PC. Secondly, statistical concerns were poorly considered in current studies. No study calculated the minimal sample size. When internally validating the model, only a few studies avoided over-pessimistic or over-optimistic evaluation of model performance by using cross-validation or bootstrapping. Many studies used cross-validation to select the best hyperparameter or the best model in the training set. However, nested cross-validation is needed to evaluate the model performance while selecting optimal hyperparameters [41]. In addition, only a few studies evaluated the calibration of AI models. Thirdly, current studies lacked analyses beyond performance evaluation. The explainability was poorly discussed, especially in radiomic studies, and few studies performed failure analysis or sensitivity analysis. Finally, no study reported the de-identification method for the clinical, pathological, and image data. Blind assessment during the pathological and radiological evaluation was also poorly reported.
There are some limitations in this study. First, only a subset of eligible studies met the selection criteria for meta-analyses, and significant heterogeneity existed among studies, thus it is important to interpret meta-analysis results with caution. Second, we only used RQS, PROBAST, and CLAIM in the evaluations. The quality of radiomics has always been a hot topic, which affects the repeatability and reproducibility of radiomics and limits its widespread clinical application. Therefore, various checklists and tools have been proposed for quality evaluation. Recently, the CheckList for EvaluAtion of Radiomics research (CLEAR) and METhodological RadiomICs Score (METRICS) were proposed and were regarded as better alternatives to CLAIM and RQS for radiomic studies [42, 43]. However, our studies did not include these newly developed tools. Thirdly, CLAIM, RQS, and PROBAST contain some elements of subjective judgment, and borderline results may impact overall interpretation.
In conclusion, although AI techniques show high diagnostic performance in predicting MIBC, the insufficient quality of studies suggests that these AI models are not currently available for clinical use. Future studies could focus on more transparent reporting of pathological evaluation, larger sample size, and additional analyses, such as prediction explanation, failure analysis, and sensitivity analysis.
Supplementary Information
Abbreviations
- AI
Artificial intelligence
- AUC
Area under hierarchical summary receiver operating characteristic curve
- BCa
Bladder cancer
- CI
Confidence interval
- CLAIM
Checklist for Artificial Intelligence in Medical Imaging
- CT
Computed tomography
- DL
Deep learning
- HSROC
Hierarchical summary receiver operating characteristic
- MIBC
Muscle-invasive bladder cancer
- MRI
Magnetic resonance imaging
- NMIBC
Non-muscle-invasive bladder cancer
- PC
Partial cystectomy
- PROBAST
Prediction model Risk of Bias Assessment Tool
- RC
Radical cystectomy
- ROB
Risk of bias
- RQS
Radiomics Quality Score
- TURBT
Transurethral resection of bladder tumor
- VI-RADS
Vesical Imaging-Reporting and Data System
Authors contributions
C.H.: screen studies, information extraction, checklist assessments, writing—original draft. H.X.: information extraction, methodology, checklist assessments, writing—original draft. Y.C.: conceptualization, methodology, visualization, checklists assessments, writing—review & editing. L.Y.: conceptualization, investigation, checklists assessments, writing—review & editing. E.Y.: checklists assessments, conceptualization, investigation, resources, writing—review & editing. J.Y.: funding acquisition, resources, writing—review & editing. B.S.: funding acquisition, resources, writing—review & editing. All authors read and approved the final manuscript.
Funding
This study has received funding from the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant number ZYGD22004), and the National Key R&D Program of China (Grant number 2021YFF0501504).
Data availability
All data were provided in the supplementary material.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
B.S. is a deputy editor for Insights into Imaging, they have not participated in the selection nor review processes for this article. The remaining authors declare that they have no competing interests.
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunlei He and Hui Xu contributed equally to this work.
Supplementary information
The online version contains supplementary material available at 10.1186/s13244-024-01780-y.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Hansel DE, Amin MB, Comperat E et al (2013) A contemporary update on pathology standards for bladder cancer: transurethral resection and radical cystectomy specimens. Eur Urol 63:321–332 10.1016/j.eururo.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Capoun O et al (2022) European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol 81:75–94 10.1016/j.eururo.2021.08.010 [DOI] [PubMed] [Google Scholar]
- 4.Witjes JA, Bruins HM, Cathomas R et al (2021) European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol 79:82–104 10.1016/j.eururo.2020.03.055 [DOI] [PubMed] [Google Scholar]
- 5.Flaig TW, Spiess PE, Abern M et al (2022) NCCN Guidelines(R) Insights: Bladder Cancer, Version 2.2022. J Natl Compr Canc Netw 20:866–878 10.6004/jnccn.2022.0041 [DOI] [PubMed] [Google Scholar]
- 6.Cumberbatch MGK, Foerster B, Catto JWF et al (2018) Repeat transurethral resection in non-muscle-invasive bladder cancer: a systematic review. Eur Urol 73:925–933 10.1016/j.eururo.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 7.Woo S, Panebianco V, Narumi Y et al (2020) Diagnostic performance of vesical imaging reporting and data system for the prediction of muscle-invasive bladder cancer: a systematic review and meta-analysis. Eur Urol Oncol 3:306–315 10.1016/j.euo.2020.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arita Y, Yoshida S, Shigeta K et al (2023) Diagnostic value of the Vesical Imaging-reporting and Data System in bladder urothelial carcinoma with variant histology. Eur Urol Oncol 6:99–102 10.1016/j.euo.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 9.Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soffer S, Ben-Cohen A, Shimon O, Amitai MM, Greenspan H, Klang E (2019) Convolutional neural networks for radiologic images: a radiologist’s guide. Radiology 290:590–606 10.1148/radiol.2018180547 [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Liu Y, Zhang X et al (2017) Preoperative prediction of muscular invasiveness of bladder cancer with radiomic features on conventional MRI and its high-order derivative maps. Abdom Radiol (NY) 42:1896–1905 10.1007/s00261-017-1079-6 [DOI] [PubMed] [Google Scholar]
- 13.Garapati SS, Hadjiiski L, Cha KH et al (2017) Urinary bladder cancer staging in CT urography using machine learning. Med Phys 44:5814–5823 10.1002/mp.12510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Zhang X, Tian Q et al (2019) Quantitative identification of nonmuscle-invasive and muscle-invasive bladder carcinomas: a multiparametric MRI radiomics analysis. J Magn Reson Imaging 49:1489–1498 10.1002/jmri.26327 [DOI] [PubMed] [Google Scholar]
- 15.Zheng J, Kong J, Wu S et al (2019) Development of a noninvasive tool to preoperatively evaluate the muscular invasiveness of bladder cancer using a radiomics approach. Cancer 125:4388–4398 10.1002/cncr.32490 [DOI] [PubMed] [Google Scholar]
- 16.Xu SS, Yao QY, Liu Q et al (2020) Combining DWI radiomics features with transurethral resection promotes the differentiation between muscle-invasive bladder cancer and non-muscle-invasive bladder cancer. Eur Radiol 30:1804–1812 10.1007/s00330-019-06484-2 [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Xu X, Zhang X et al (2020) Elaboration of a multisequence MRI-based radiomics signature for the preoperative prediction of the muscle-invasive status of bladder cancer: a double-center study. Eur Radiol 30:4816–4827 10.1007/s00330-020-06796-8 [DOI] [PubMed] [Google Scholar]
- 18.Zhou Q, Zhang Z, Ang X, Zhang H, Ouyang J (2021) A nomogram combined with radiomics features, albuminuria, and metabolic syndrome to predict the risk of myometrial invasion of bladder cancer. Transl Cancer Res 10:3177–3191 10.21037/tcr-21-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang GMY, Wu Z, Xu LL et al (2021) Deep learning on enhanced CT images can predict the muscular invasiveness of bladder cancer. Front Oncol 11:654685 10.3389/fonc.2021.654685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Z, Xu F, Gu Z et al (2021) Combining multiparametric MRI radiomics signature with the Vesical Imaging-Reporting and Data System (VI-RADS) score to preoperatively differentiate muscle invasion of bladder cancer. Front Oncol 11:619893 10.3389/fonc.2021.619893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang YH, Zou XH, Wang YX, Ma XL (2021) Application of deep learning as a noninvasive tool to differentiate muscle-invasive bladder cancer and non-muscle-invasive bladder cancer with CT. Eur J Radiol 139:109666 10.1016/j.ejrad.2021.109666 [DOI] [PubMed] [Google Scholar]
- 22.Gao RZ, Wen R, Wen DY et al (2021) Radiomics analysis based on ultrasound images to distinguish the tumor stage and pathological grade of bladder cancer. J Ultrasound Med 40:2685–2697 10.1002/jum.15659 [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Gong M, Zhou D et al (2022) CT-based deep learning radiomics signature for the preoperative prediction of the muscle-invasive status of bladder cancer. Front Oncol 12:1019749 10.3389/fonc.2022.1019749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou Y, Cai L, Chen C et al (2022) Multi-task deep learning based on T2-weighted images for predicting muscular-invasive bladder cancer. Comput Biol Med 151:106219 10.1016/j.compbiomed.2022.106219 [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Li X, Yang L et al (2023) Multi-sequence and multi-regional MRI-based radiomics nomogram for the preoperative assessment of muscle invasion in bladder cancer. J Magn Reson Imaging 58:258–269 10.1002/jmri.28498 [DOI] [PubMed] [Google Scholar]
- 26.Cui Y, Sun Z, Liu X, Zhang X, Wang X (2022) CT-based radiomics for the preoperative prediction of the muscle-invasive status of bladder cancer and comparison to radiologists’ assessment. Clin Radiol 77:e473–e482 10.1016/j.crad.2022.02.019 [DOI] [PubMed] [Google Scholar]
- 27.Zhang G, Wu Z, Zhang X et al (2022) CT-based radiomics to predict muscle invasion in bladder cancer. Eur Radiol 32:3260–3268 10.1007/s00330-021-08426-3 [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Xu X, Wang H et al (2023) The additional value of tri-parametric mri in identifying muscle-invasive status in bladder cancer. Acad Radiol 30:64–76 10.1016/j.acra.2022.04.014 [DOI] [PubMed] [Google Scholar]
- 29.Sarkar S, Min K, Ikram W et al (2023) Performing automatic identification and staging of urothelial carcinoma in bladder cancer patients using a hybrid deep-machine learning approach. Cancers 15:1673 10.3390/cancers15061673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Qiu Z, Cao K et al (2023) Predicting muscle invasion in bladder cancer based on MRI: a comparison of radiomics, and single-task and multi-task deep learning. Comput Methods Programs Biomed 233:107466 10.1016/j.cmpb.2023.107466 [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Li W, Wang K et al (2023) Integrating radiomics with the Vesical Imaging-reporting and Data System to predict muscle invasion of bladder cancer. Urol Oncol 41:294.e291–294.e298 10.1016/j.urolonc.2022.10.024 [DOI] [PubMed] [Google Scholar]
- 32.Li J, Cao K, Lin H et al (2023) Predicting muscle invasion in bladder cancer by deep learning analysis of MRI: comparison with Vesical Imaging-reporting and Data System. Eur Radiol 33:2699–2709 10.1007/s00330-022-09272-7 [DOI] [PubMed] [Google Scholar]
- 33.Boca B, Caraiani C, Telecan T et al (2023) MRI-based radiomics in bladder cancer: a systematic review and radiomics quality score assessment. Diagnostics 13:2300 10.3390/diagnostics13132300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozikowski M, Suarez-Ibarrola R, Osiecki R et al (2022) Role of radiomics in the prediction of muscle-invasive bladder cancer: a systematic review and meta-analysis. Eur Urol Focus 8:728–738 10.1016/j.euf.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 35.Mongan J, Moy L, Kahn Jr CE (2020) Checklist for Artificial Intelligence in Medical Imaging (CLAIM): a guide for authors and reviewers. Radiol Artif Intell 2:e200029 10.1148/ryai.2020200029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762 10.1038/nrclinonc.2017.141 [DOI] [PubMed] [Google Scholar]
- 37.Wolff RF, Moons KGM, Riley RD et al (2019) PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 170:51–58 10.7326/M18-1376 [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben Dwamena (2007) MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies. Statistical Software Components S456880. Revised 05 Feb 2009.
- 40.Deeks JJBP, Leeflang MM, Takwoingi Y (eds) Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy.Version 2.0 (updated July 2023). Cochrane, 2023. Available from https://training.cochrane.org/handbook-diagnostic-test-accuracy/current [DOI] [PMC free article] [PubMed]
- 41.Bradshaw TJ, Huemann Z, Hu J, Rahmim A (2023) A guide to cross-validation for artificial intelligence in medical imaging. Radiol Artif Intell 5:e220232 10.1148/ryai.220232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kocak B, Baessler B, Bakas S et al (2023) CheckList for EvaluAtion of Radiomics research (CLEAR): a step-by-step reporting guideline for authors and reviewers endorsed by ESR and EuSoMII. Insights Imaging 14:75 10.1186/s13244-023-01415-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kocak B, Akinci D’Antonoli T, Mercaldo N et al (2024) METhodological RadiomICs Score (METRICS): a quality scoring tool for radiomics research endorsed by EuSoMII. Insights Imaging 15:8 10.1186/s13244-023-01572-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data were provided in the supplementary material.