Abstract
Background/Objective
Follicular thyroid cancer without an intrathyroidal primary cancer is rare. We present a patient with multifocal pulmonary metastatic follicular thyroid cancer without apparent cancer within her thyroid.
Case Report
A 44-year-old woman was referred to the thyroid cancer clinic via telemedicine for evaluation of intrapulmonary thyroid tissue. Her past medical history included Roux-en-Y gastric bypass and hysterectomy with bilateral oophorectomy. Six months prior, abdominal computed tomography (CT) showed incidental bilateral lung nodules. Chest CT demonstrated 4 solid left and 1 solid right lung nodules. Lung nodule core biopsy revealed benign thyroid tissue. Thyroid ultrasound showed bilateral subcentimeter anechoic nodules. Chest CT 6 months after initial CT demonstrated stable lung nodules. The levels of thyroid-stimulating hormone, serum thyroglobulin, and thyroglobulin antibody were 1.63 mIU/L (reference range, 0.3-5.5 mIU/L), 40.9 ng/mL (reference range, 0-35 ng/mL), and <1 IU/mL (reference range, <4), respectively. Positron emission tomography/CT showed fluorodeoxyglucose-avid lung lesions measuring 1.5, 1.1, and 2.2 cm and other subcentimeter pulmonary nodules. Repeat lung core biopsy showed thyroid tissue with microfollicular architecture, favoring metastatic follicular carcinoma with neuroblastoma-RAS gene (NRAS) mutation. Total thyroidectomy performed showed multinodular hyperplasia without thyroid cancer. Her postoperative radioiodine scan demonstrated bilateral iodine-avid pulmonary nodules, a serum thyroglobulin level of 179.8 ng/mL, a thyroid-stimulating hormone level of 151.3 mIU/L, and undetectable serum thyroglobulin antibody. She received 261 mCi of radioactive iodine. Fourteen months later, chest CT revealed decreased lung nodules and a serum thyroglobulin level of 0.7 ng/mL.
Discussion
Approximately 2 cases of multifocal pulmonary follicular thyroid cancer without a primary source and no other site of metastasis have been reported.
Conclusion
Pulmonary follicular thyroid cancer without a primary source and no other site of metastasis is extremely rare.
Key words: follicular thyroid cancer, pulmonary metastasis, ectopic thyroid tissue
Highlights
-
•
Thyroid cancer should be considered in the diagnosis of intrapulmonary thyroid tissue.
-
•
Malignant struma ovarii should be considered in females with ectopic thyroid tissue.
-
•
PET/CT or biopsies augmented by molecular testing may assist in diagnosis.
Clinical Relevance
Pulmonary thyroid tissue is uncommon. This constellation should raise suspicion for differentiated thyroid cancer.
Introduction
Pulmonary thyroid tissue is a rare finding. Both benign and malignant thyroid tissues can be found in the lungs. Ectopic thyroid tissue develops during aberrant thyroid migration during embryologic development. The prevalence of ectopic thyroid tissue is estimated to be 1 in 100 000 to 300 000 people.1 Ectopic thyroid tissue in the mediastinum accounts for less than 1% of all ectopic thyroid cases.2
Several patients with ectopic thyroid tissue are asymptomatic and are diagnosed after incidental pulmonary nodules are found on imaging, whereas others may present with hoarseness, shortness of breath, cough, hemoptysis, difficulty swallowing, foreign body sensation, and superior vena cava syndrome.3
Metastatic disease without an intrathyroidal primary cancer is rare. These patients may have had a microcarcinoma that was undetected on thyroidectomy pathology or may have had a follicular carcinoma that was misdiagnosed as a follicular adenoma due to unrecognized capsular or vascular invasion. Here, we report a rare case of a patient with multifocal pulmonary metastatic follicular thyroid cancer without apparent cancer within her thyroid.
Case Report
A 44-year-old woman was referred to the multidisciplinary thyroid cancer clinic via telemedicine appointment for evaluation of intrapulmonary thyroid tissue. Her past medical history was notable for endometriosis, polycystic ovary syndrome, and Roux-en-Y gastric bypass in 2005, hysterectomy with bilateral oophorectomy in 2014, and stomach pouch tightening in 2021. Six months prior, she had presented to a local emergency room with abdominal pain. Abdominal computed tomography (CT) showed incidental bilateral lung base nodules and no intra-abdominal pathology. Chest CT demonstrated 4 solid left lower lung nodules measuring 15.2, 13.5, 10.4, and 5.9 mm and a solid right lower lung nodule measuring 6.3 mm. Two weeks after her chest CT, biopsy of the largest left lung nodule was performed locally where pathology was read as benign thyroid tissue. The thyroid-stimulating hormone (TSH) level was 2.31 mIU/L (reference range, 0.3-5.0 mIU/L). Thyroid ultrasound performed locally 1 month later showed 2 simple-appearing anechoic nodules measuring >1 cm in the right and left thyroid with a sonographic appearance of colloid cysts. Follow-up chest CT 6 months after her initial CT and lung biopsy demonstrated stable lung nodules. At this time, the levels of serum TSH, thyroglobulin, and thyroglobulin antibody were 1.63 mIU/L, 40.9 ng/mL, and <1 IU/mL, respectively. Her lung nodule biopsy pathology slides were reviewed at our center and showed well-differentiated metastatic follicular thyroid carcinoma. Immunohistochemical stains were positive for TTF-1 and thyroglobulin and negative for BRAF V600E. Thyroid ultrasound was performed at our center, which revealed a 7-mm left cyst and 10-mm right cyst. Three weeks later, a positron emission tomography (PET)/CT scan was completed, showing fluorodeoxyglucose-avid lung lesions measuring 1.5 cm in the left lower lobe (standardized uptake value [SUV], 4), 1.1 cm in the left lower lobe (SUV, 3.1), and 2.2 cm in the left lower lobe (SUV, 2.6) with additional subcentimeter pulmonary nodules too small to accurately characterize by PET (Fig. 1). The patient requested a second lung biopsy at our center because of the discrepancy in pathology reports. Left lung nodule core biopsy performed at our center 1 week after her PET/CT showed thyroid tissue with microfollicular architecture, favoring metastatic follicular carcinoma (Fig. 2). Next-generation sequencing of this tissue revealed neuroblastoma-RAS gene (NRAS) c.181C>A, p.Q61K (NM_002524.4) mutation. Two months later, she was evaluated in-person by endocrine surgery at which time it was noted on physical examination that her thyroid was normal in size without a palpable nodule. Ten days later, she underwent total thyroidectomy with surgical pathology showing multinodular hyperplasia without thyroid cancer. Surgical pathology slides from her complete hysterectomy were reviewed retrospectively and showed no evidence of malignant struma ovarii. After thyroidectomy, she was started on Cytomel 25 mcg daily. Two weeks after discontinuation of Cytomel, she underwent thyroid hormone withdrawal radioiodine scanning, which showed intense radioiodine uptake in multiple pulmonary nodules in both lungs (Fig. 3). The serum thyroglobulin level at this time was 179.8 ng/mL (TSH level, 151.3 mIU/L) with no detectable serum thyroglobulin antibody. She received 261 mCi of radioactive iodine, and Synthroid 200 mcg daily was initiated. Her posttherapy whole-body scan redemonstrated multifocal radiotracer uptake as seen on the pretherapy radioiodine scan with no new foci of activities. Follow-up chest CT 2 months later demonstrated a decrease in the lung nodules size with a left lower lobe nodule decreasing from 10 mm to 5 mm, left lower lobe nodule decreasing from 16 mm to 11 mm, left lower lobe nodule decreasing from 12 mm to 8 mm, and right lower lobe nodule decreasing from 6 mm to 3 mm. At this time, her serum thyroglobulin level decreased to 2 ng/mL (TSH level, 0.01 mIU/L) without serum thyroglobulin antibodies. Eight months after radioactive iodine treatment, her serum thyroglobulin level decreased further to 0.9 ng/mL (TSH level, <0.01 mIU/L), and serum thyroglobulin antibody remained <1 IU/mL. Fourteen months after radioactive iodine treatment, chest CT revealed a further decrease in the size of the largest lung nodule to 5.5 mm, and the other nodules were ≤4 mm (Fig. 4). Her serum thyroglobulin level decreased to 0.7 ng/mL with no thyroglobulin antibody and a TSH level of 0.08 mIU/L (reference range, 0.45-5.33 mIU/L).
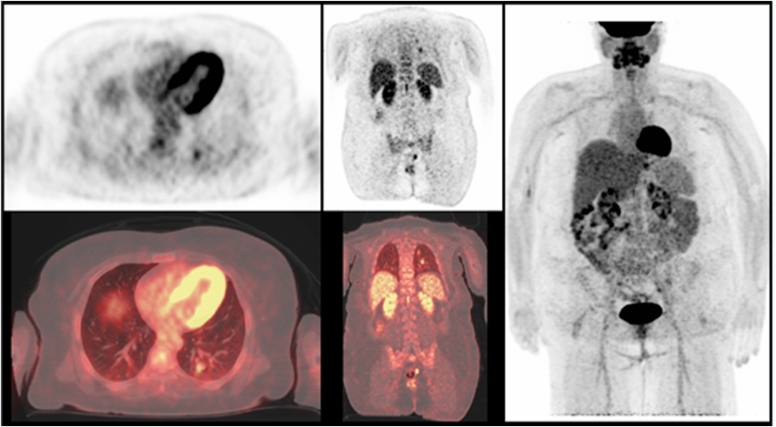
Fig. 1.
Positron emission tomography/computed tomography scan before radioactive iodine therapy demonstrating fluorodeoxyglucose-avid lung nodules.
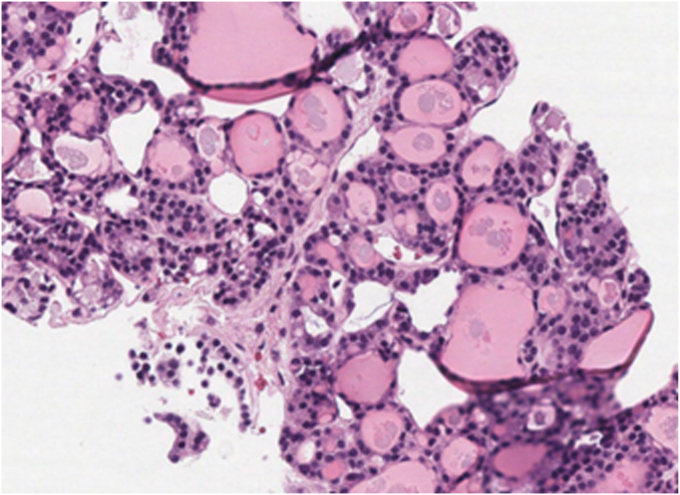
Fig. 2.
Hematoxylin-eosin stain (magnification, ×200). Pulmonary nodule core biopsy demonstrating thyroid tissue with microfollicular architecture.
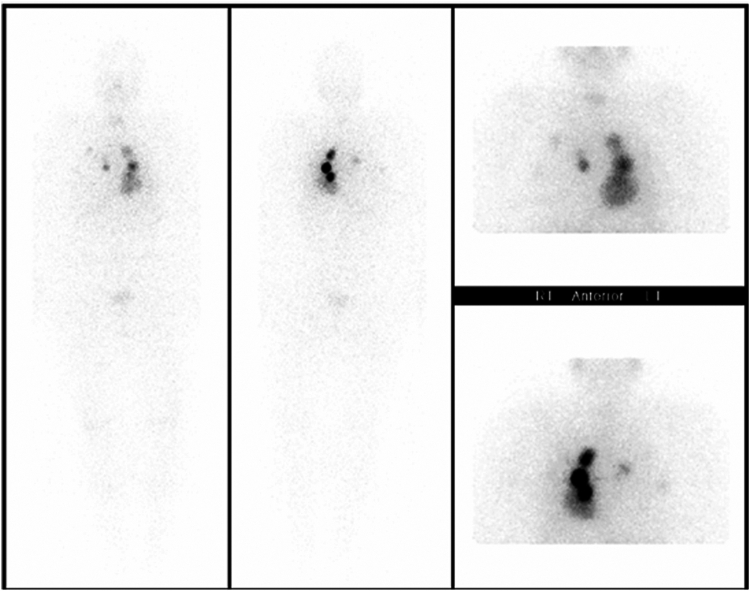
Fig. 3.
Preradioactive iodine therapy whole-body scan demonstrating multifocal bilateral iodine-avid pulmonary metastases.
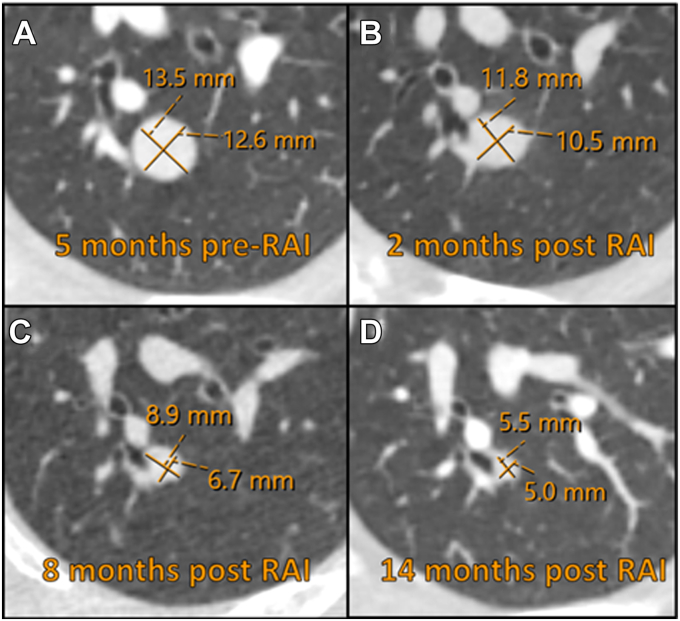
Fig. 4.
Computed tomography scans 5 months before radioactive iodine therapy (A) and 2 (B), 8 (C), and 14 (D) months after the therapy demonstrating a decrease in the pulmonary nodule size.
Discussion
Herein, we present a 44-year-old euthyroid woman who was incidentally found to have bilateral lung nodules with core biopsy initially read locally as benign thyroid tissue. Further evaluation at our center showed this to be follicular thyroid cancer. Next-generation sequencing of this tissue revealed NRAS c.181C>A, p.Q61K (NM_002524.4) mutation. Positron emission tomography/CT demonstrated fluorodeoxyglucose-avid lung lesions. Total thyroidectomy was performed with no thyroid cancer noted. Her thyroid hormone withdrawal radioiodine scan showed bilateral iodine-avid lung metastases and an elevated thyroglobulin level of 179.8 ng/mL. Fourteen months after receiving radioactive iodine therapy, her pulmonary nodules decreased in size, and the thyroglobulin level decreased to 0.7 ng/mL.
It is very rare to have multifocal pulmonary metastatic follicular thyroid cancer presenting without a primary intrathyroidal cancer or other sites of metastases. In our literature search, this is the third report of such a case.4,5 The differential diagnosis of intrapulmonary thyroid tissue includes benign thyroid tissue, thyroid cancer metastasis, thymoma, neuroma, and germ cell tumors.3 Malignant struma ovarii is part of the differential diagnoses of intrapulmonary thyroid tissue with case reports of patients presenting with pulmonary metastases and no intrathyroidal thyroid cancer.6,7 Because of this, our patient’s hysterectomy with bilateral oophorectomy pathology slides were obtained and reviewed, showing no evidence of malignant struma ovarii.
In lung nodules measuring >1 cm, a PET SUV cutoff of ≥2.5 has been used to discriminate malignant pulmonary nodules from benign pulmonary nodules.8 Our patient had an SUV max of >2.5. In case reports of patients with ectopic benign intrapulmonary thyroid tissue who underwent PET, their SUV was ≤1.8.9,10
The location of lung nodules can also provide a clue in differentiating benign from malignant pulmonary nodules. As in our patient, previous studies have shown that metastatic lung nodules are more commonly located in the lower and lateral lungs.8,11
Molecular testing can provide additional risk assessment. NRAS mutations have been found in both benign and malignant thyroid follicular neoplasms. These mutations are thought to occur early in the development of follicular thyroid cancer.12 NRAS mutations play a key role in causing thyroid dedifferentiation, thwarting apoptosis resulting in propagation of thyroid cancer cells in the presence of TSH and promoting cell growth even in low or absent TSH levels.13,14 In the study by Bae et al,15 prediction of malignancy in NRAS mutation–positive follicular neoplasm specimens had a sensitivity of 37%, specificity of 83%, positive predictive value of 67%, and negative predictive value of 58%. Jang et al16 and Fukahori et al17 showed that NRAS codon 61 mutation that was identified in our patient is significantly associated with distant metastases in patients with follicular thyroid cancer.
Differentiating between benign ectopic thyroid tissue and follicular thyroid cancer metastasis can be challenging, as demonstrated in this case. Microcarcinomas within the thyroid can be present despite entire histopathologic examination of the thyroid gland. Differentiating between a follicular carcinoma and follicular adenoma in the thyroid can also be difficult because of unrecognized capsular or vascular invasion or designation of this invasion as being too minimal. This limitation was circumvented in this case because the histopathologic studies of the core lung nodule biopsy and thyroidectomy specimen were reviewed by our tertiary center’s expert thyroid cancer pathologist and augmented by molecular testing and imaging to establish the diagnosis of follicular thyroid cancer.
Conclusion
Thyroid cancer should always be considered in the differential diagnosis of intrapulmonary thyroid tissue. Differentiating between ectopic thyroid tissue and metastasis can be difficult. PET/CT imaging examinations or core or excision biopsies augmented by molecular testing may assist in differentiating between these 2 processes. Pulmonary thyroid tissue is uncommon. This constellation should raise suspicion for differentiated thyroid cancer.
Disclosure
The authors have no conflicts of interest to disclose.
Acknowledgment
This case was presented as a poster at the American Thyroid Association meeting in September 2023.
References
- 1.Raji Y., Gupta S., Pucar D., Keshavamurthy J.H. Ectopic thyroid: the great mimicker. Lung India. 2018;35(3):248–250. doi: 10.4103/lungindia.lungindia_141_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S.Y. A case of right paratracheal ectopic thyroid, mimicking metastasis on CT and 18F-FDG PET CT. Open J Med Imaging. 2013;3:82. [Google Scholar]
- 3.Ko H.H., Cho S.W., Lee H.S., Kim H.S., Nam E.S., Cho S.J. Ectopic intrapulmonary thyroid: a case report. Korean J Thorac Cardiovasc Surg. 2013;46(3):237–239. doi: 10.5090/kjtcs.2013.46.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng H., Yang L., Xiong J., Peng J., Ruan Q. Multiple thyroid nodules in the lung: metastasis or ectopia? Diagn Pathol. 2015;10:61. doi: 10.1186/s13000-015-0299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito Y., Yabuta T., Hirokawa M., et al. Distant and lymph node metastases of thyroid nodules with no pathological evidence of malignancy: a limitation of pathological examination. Endocr J. 2008;55(5):889–894. doi: 10.1507/endocrj.k08e-116. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi M., Derebew L., Boucai L., Kishore P. Retrospective diagnosis of malignant struma ovarii after discovery of pulmonary metastasis. AACE Clin Case Rep. 2021;7(5):320–322. doi: 10.1016/j.aace.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruel I.F., Fierrard H., Vercellino L., et al. Pulmonary metastasis of struma ovarii: a case report. Clin Nucl Med. 2010;35(9):692–694. doi: 10.1097/RLU.0b013e3181e9fb1b. [DOI] [PubMed] [Google Scholar]
- 8.Khalaf M., Abdel-Nabi H., Baker J., Shao Y., Lamonica D., Gona J. Relation between nodule size and 18F-FDG-PET SUV for malignant and benign pulmonary nodules. J Hematol Oncol. 2008;1:13. doi: 10.1186/1756-8722-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng L., Jiang X., Jiang Y. Ectopic thyroid as multiple nodules in bilateral lung lobes: a case report. Gland Surg. 2020;9(3):806–811. doi: 10.21037/gs-19-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan J., Kuang J., Li Y., et al. Rare ectopic thyroid tissue as multiple bilateral pulmonary nodules: a case report and literature review. J Cardiothorac Surg. 2022;17(1):205. doi: 10.1186/s13019-022-01962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa T. [CT of metastatic pulmonary tumor: morphology, HRCT and histological correlation] Nihon Igaku Hoshasen Gakkai Zasshi. 1996;56(14):1032–1038. [PubMed] [Google Scholar]
- 12.Luvhengo T.E., Bombil I., Mokhtari A., et al. Multi-omics and management of follicular carcinoma of the thyroid. Biomedicines. 2023;11(4):1217. doi: 10.3390/biomedicines11041217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh A., Ham J., Po J.W., Niles N., Roberts T., Lee C.S. The genomic landscape of thyroid cancer tumourigenesis and implications for immunotherapy. Cells. 2021;10(5):1082. doi: 10.3390/cells10051082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Vita G., Bauer L., da Costa V.M., et al. Dose-dependent inhibition of thyroid differentiation by RAS oncogenes. Mol Endocrinol. 2005;19(1):76–89. doi: 10.1210/me.2004-0172. [DOI] [PubMed] [Google Scholar]
- 15.Bae J.S., Choi S.K., Jeon S., et al. Impact of NRAS mutations on the diagnosis of follicular neoplasm of the thyroid. Int J Endocrinol. 2014;2014 doi: 10.1155/2014/289834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang E.K., Song D.E., Sim S.Y., et al. NRAS codon 61 mutation is associated with distant metastasis in patients with follicular thyroid carcinoma. Thyroid. 2014;24(8):1275–1281. doi: 10.1089/thy.2014.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukahori M., Yoshida A., Hayashi H., et al. The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: new insights from a single center and a large patient cohort. Thyroid. 2012;22(7):683–689. doi: 10.1089/thy.2011.0261. [DOI] [PubMed] [Google Scholar]