Abstract
Few studies have examined longitudinal changes in human immunodeficiency virus type 1 (HIV)-specific cytotoxic T lymphocytes (CTL). To more closely define the natural history of HIV-specific CTL, we used HLA-peptide tetrameric complexes to study the longitudinal CD8+ T-cell response evolution in 16 A*0201-positive untreated individuals followed clinically for up to 14 years. As early as 1 to 2 years after seroconversion, we found a significant association between high frequencies of A*0201-restricted p17Gag/Pol tetramer-binding cells and slower disease progression (P < 0.01). We observed that responses could remain stable over many months, but any longitudinal changes that occurred were typically accompanied by reciprocal changes in RNA viral load. Phenotypic analysis with markers CD45RO, CD45RA, and CD27 identified distinct subsets of antigen-specific cells and the preferential loss of CD27+ CD45RO+ cells during periods of rapid decline in the frequency of tetramer-binding cells. In addition we were unable to confirm previous studies showing a consistent selective loss of HIV-specific cells in the context of sustained Epstein-Barr virus-specific cell frequencies. Overall, these data support a role of HIV-specific CTL in the control of disease progression and suggest that the ultimate loss of such CTL may be preferentially from the CD27+ CD45RO+ subset.
Several cross-sectional studies have suggested that there is an inverse association between the frequency of human immunodeficiency virus type 1 (HIV)-specific cytotoxic T lymphocytes (CTL) and plasma viral RNA load (5, 16, 19, 26, 28, 34), but longitudinal studies examining the role of CTL in the progression of HIV infection are rare. Two longitudinal analyses showed that Gag-specific CTL precursors were persistently elevated in nonprogressors but either absent or only transiently present in rapid progressors (22, 33). Using uncultured antigen-specific cytolysis to measure ex vivo CTL activity, two longitudinal studies showed trends toward more vigorous HIV-specific CTL responses in those individuals who progressed more slowly (4, 35). Studies addressing the levels of uncultured CTL have been difficult to clearly interpret, as the traditional lytic assay is very insensitive, requiring antigen-specific CTL to be present at above 1 in 100 peripheral blood mononuclear cells (PBMC) for the readout to be positive (14). Therefore, conclusions about the role of CTL were drawn when data were available only on those responses associated with very high levels of circulating HIV-specific CTL frequency. The development of HLA-peptide tetrameric complexes has allowed a reevaluation of these issues via a technique with much greater sensitivity (3, 28). HLA-peptide tetramers permit the direct visualization of antigen-specific T cells by flow cytometry (3). Staining is both highly sensitive (lower limit of detection of 0.02% of CD8+ T cells [28]) and highly specific such that CTL clones and lines directed to different epitope peptides bound to the same HLA molecule do not stain (9). Tetramer binding is known to correlate well with ex vivo functional activity in human studies (10–12, 25, 28, 37, 38), but a recent study using a CD4-depleted murine model has suggested that in some circumstances, tetramer-binding cells may be functionally quiescent (41).
CD45 is one of the most abundant lymphocyte cell surface glycoproteins and exists as a number of different isoforms generated by alternative splicing. It plays a role in signal transduction probably mediated by its protein tyrosine phosphatase activity, with principle substrates including Lck and Fyn (7, 27). Traditionally, CD45 high-molecular-weight isoforms (CD45RA, -RB, and -RC) have been associated with the naive T-cell phenotype, and the low-molecular-weight isoform, CD45RO, has been taken to represent an activated or memory phenotype. Activation of CD45RA+ CD45RO− T cells induces the expression of the CD45RA− CD45RO+ phenotype (1); switching back in the other direction has also been documented, but this is thought to be a rare event (23). Use of the CD45 isoforms as a marker of antigen exposure in human CD8+ T cells has recently been addressed in a study showing that costaining of CD8+ T cells with a CD27 monoclonal antibody (MAb) (member of the tumor necrosis factor receptor family [2]) and CD45RA MAb could be used to separate memory (CD45RA− CD27+) from effector (CD45RA+ CD27−) cells by flow cytometry (17). CD45RA− CD27+ cells have been shown to resemble functional memory cells that may be detected in classical CTL precursor (CTLp) limiting dilution analyses (LDA). On the contrary, CD27− CD45RA+ (RO−) effector cells, although cytolytic in direct lysis assays, may not be detected in LDA because they lack sufficient proliferative capacity and will not expand in long-term LDA cultures (17). It has been proposed that a maturation pathway exists from CD27+ CD45RA+ to CD27+ CD45RO+ to CD27− CD45RO− cells during CD8+ T-cell differentiation (18).
In this study, we used HLA-peptide tetrameric complexes to longitudinally observe the natural history of HIV- and Epstein-Barr virus (EBV)-specific CTL populations in a cohort of untreated individuals for whom clinical outcome was known. PBMC of 16 HLA A*0201-positive patients who were followed clinically for up to 14 years were investigated with four-color fluorescence-activated cell sorting analysis to determine the frequency and activation phenotype (CD45, CD27) of p17Gag, Pol, and EBV BMLF1-A*0201 tetramer-binding CD8+ T cells.
MATERIALS AND METHODS
Patients.
Sequence-specific PCR-defined HLA A*0201-positive individuals were selected from individuals within the Amsterdam cohort of HIV-infected patients on the basis of a minimum untreated follow-up of 4 years. Sixteen such A*0201-positive individuals who seroconverted for HIV between 1985 and 1987 and had been followed longitudinally for up to 14 years were identified (Table 1). Of the individuals studied, only data from patient 90 have been presented previously (22). Nine of the patients had developed AIDS (current Centers for Disease Control and Prevention criteria) by the end of the study period; from six of these nine, syncytium-inducing (SI) strains of virus were isolated during the study (Table 1). Of the seven patients who were AIDS-free at the end of the study, all had non-SI (NSI) strains of HIV throughout the study (Table 1). RNA viral load was measured by the Roche Amplicor assay (8). PBMC samples were frozen in a controlled-rate facility and cryopreserved to maximize cell viability (22). Such freezing of samples did not affect tetramer staining (29). Longitudinal samples from each individual were stained and analyzed on the same day.
TABLE 1.
Patient characteristics
| Patient | HLA-A | HLA-A | HLA-B | HLA-B | HLA-C | HLA-C | Virusa | Time (mo/yr) of:
|
|
|---|---|---|---|---|---|---|---|---|---|
| Seroconversion | Onset of AIDS | ||||||||
| 1 | *01 | *02 | *18 | *4901 | *0501 | *1203 | NSI | 11/85 | 8/94 |
| 171 | *02 | *03 | *07 | *40 | *02 | *07 | SI | 3/85 | 4/95 |
| 594 | *02 | *02 | *14 | *1501 | *0304 | *08 | SI | 9/85 | 6/93 |
| 26 | *02 | *3201 | *27 | *4001 | *02 | *0304 | SI | 4/86 | 1/94 |
| 490 | *02 | *23 | *44 | *51/52 | *02 | *04 | SI | 7/85 | 10/95 |
| 1029 | *02 | *11 | *1501 | *18 | *0303 | *07 | SI | 12/86 | 8/94 |
| 1081 | *02 | *03 | *1501 | *3701 | *0303 | *0602 | NSI | 11/85 | 1/94 |
| 1123 | *01 | *02 | *07 | *3701 | *0602 | *07 | NSI | 8/86 | 10/90 |
| 16 | *02 | *29 | *44 | *51/52 | *02 | *0501 | NSI | 4/85 | —b |
| 57 | *02 | *30 | *27 | *40 | *02 | *02 | NSI | 1/85 | — |
| 82 | *02 | *11 | *07 | *35 | *04 | *07 | NSI | 2/87 | — |
| 90 | *01 | *02 | *41 | *5701 | *0602 | *1701 | NSI | 2/85 | — |
| 434 | *02 | *6801 | *07 | *27 | *02 | *07 | NSI | 4/85 | — |
| 658 | *01 | *02 | *0801 | *40 | *02 | *07 | NSI | 9/86 | — |
| 1047 | *02 | *02 | *1501 | *1501 | *0303 | *0304 | SI | 9/86 | 3/96 |
| 1140 | *02 | *11 | *4001 | *51/52 | *0304 | *12 | NSI | 10/86 | — |
If SI strains of HIV were isolated on more than one occasion during the study, then the viral phenotype is documented as SI.
—, The individual has not yet developed an AIDS-defining diagnosis.
HLA-peptide tetrameric complexes.
Complexes were synthesized as previously described (3). Peptides used in the synthesis of the tetramers were the p17Gag peptide SLYNTVATL (Gag 77-85) (32), the Pol peptide ILKEPVHGV (Pol 476-84) (39), and the BMLF1 EBV peptide 280-8 GLCTLVAML (36). Purified HLA heavy chain and β2 microglobulin were synthesized by means of a prokaryotic expression system (pET Novagen). The heavy chain was modified by deletion of the transmembrane/cytosolic tail and C-terminal addition of a sequence containing the BirA enzymatic biotinylation site. Heavy chain, β2 microglobulin, and peptide were refolded by dilution. The 45-kDa refolded product was isolated by fast protein liquid chromatography and then biotinylated by BirA (Avidity, Denver, Colo.) in the presence of biotin (Sigma), ATP (Sigma), and Mg2+ (Sigma). Streptavidin-phycoerythrin conjugate (Sigma) was added in a 1:4 molar ratio, and the tetrameric product was concentrated to 1 mg/ml.
Flow cytometry.
Four-color flow cytometric analysis was performed by using a FACSort (Becton Dickinson) with CellQuest software (Becton Dickinson). Directly conjugated antibodies included CD45RA-fluorescein isothiocyanate (FITC) (Immunotech), CD45RO-allophycocyanin (APC) (Becton Dickinson), CD27-FITC (Becton Dickinson), and CD8-peridinin chlorophyll (PerCP) (Becton Dickinson). PBMC (106) were centrifuged at 300 × g for 5 min and resuspended in a volume of 50 μl. Tetrameric complex and directly conjugated antibodies were added, and the samples were incubated for 60 min. After two washes in cold phosphate-buffered saline, the samples were fixed in 2% formaldehyde. Controls included cryopreserved samples from HIV-negative individuals and from HIV-positive individuals with inappropriate HLA types. In line with previous reports (28, 29), the mean plus 3 standard deviations of tetramer-binding cells in the PBMC from control individuals was less than 0.02% of CD8+ T cells.
Statistics.
Statistical analyses were performed by using the chi-squared, Fisher’s exact, and related t tests and Pearson correlation coefficient.
RESULTS
High frequencies of p17Gag/Pol tetramer-binding cells correlate with slow disease progression.
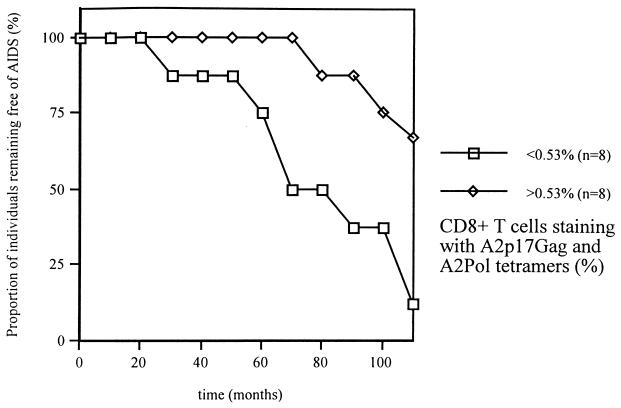
At least two-thirds of HLA A*0201-positive individuals have circulating CTL that recognize the A*0201-restricted Gag (A2Gag) epitope (77-85, SLYNTVATL). The majority (70%) of those individuals not recognizing the A2Gag epitope will recognize the A*0201-restricted Pol (A2Pol) epitope (476-84 ILKEPVHGV), so that the addition of both the A2Gag and A2Pol responses gives a representation of total A*0201-directed CTL activity (15). We investigated whether the frequency of P17Gag/Pol tetramer-binding cells was associated with subsequent clinical outcome. We defined study entry to be 1 to 2 years after seroconversion in order to allow sufficient time to establish a viral equilibrium setpoint (Table 1). We then compared disease progression between all members of the study group over the subsequent 10 years when more than 50% had developed AIDS-defining diagnoses. Confirming previous findings (28), we observed a significant inverse association between the frequency of P17Gag/Pol tetramer-binding cells and plasma RNA viral load (Pearson r = −0.61, P < 0.01) at study entry. The individuals that progressed to AIDS had significantly lower frequencies of P17Gag/Pol tetramer-binding cells at study entry than those who did not (median, 0.17% versus 1.39% of CD8+ T cells; related t test P < 0.05). Indeed, there was a significant positive association between the frequency of p17Gag/Pol tetramer-binding cells and length of AIDS-free follow-up (Pearson r = 0.76, P < 0.01). Figure 1 documents the cumulative proportion of individuals that remained AIDS free, stratified relative to the median frequency (0.53% of CD8+ T cells) of p17Gag/Pol tetramer-binding cells at study entry. Overall these data showed that high frequencies of A2Gag/Pol tetramer binding cells were associated with slower disease progression, consistent with a role for CTL in the control of viral replication.
FIG. 1.
Cumulative AIDS-free survival of members of the cohort stratified according to the frequency of p17Gag/Pol tetramer-binding cells at study entry.
Longitudinal analysis of the frequency of p17Gag/Pol/BMLF1 tetramer-binding cells.
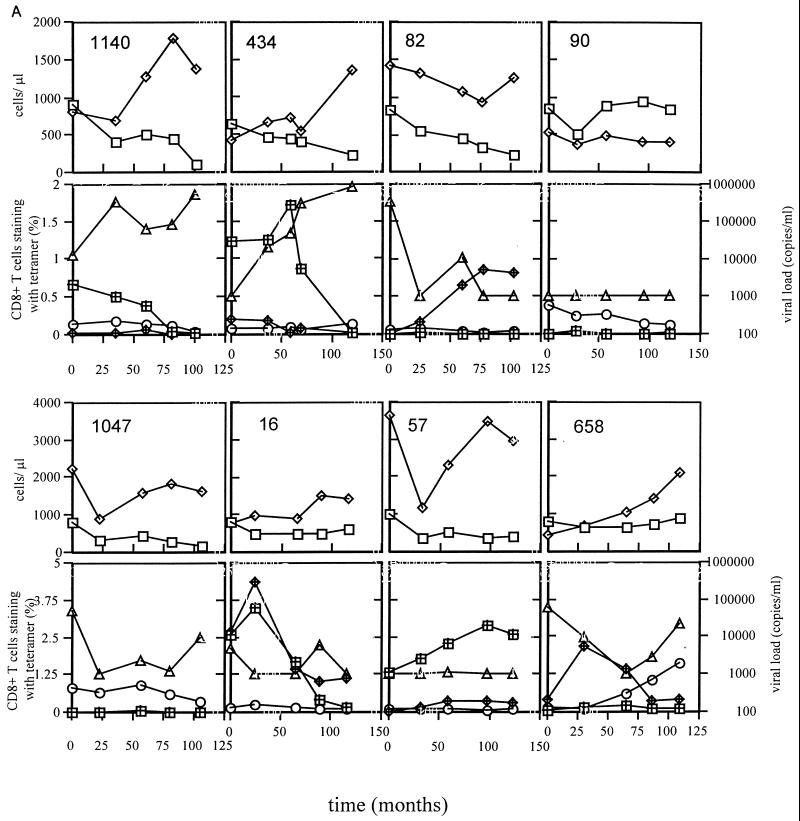
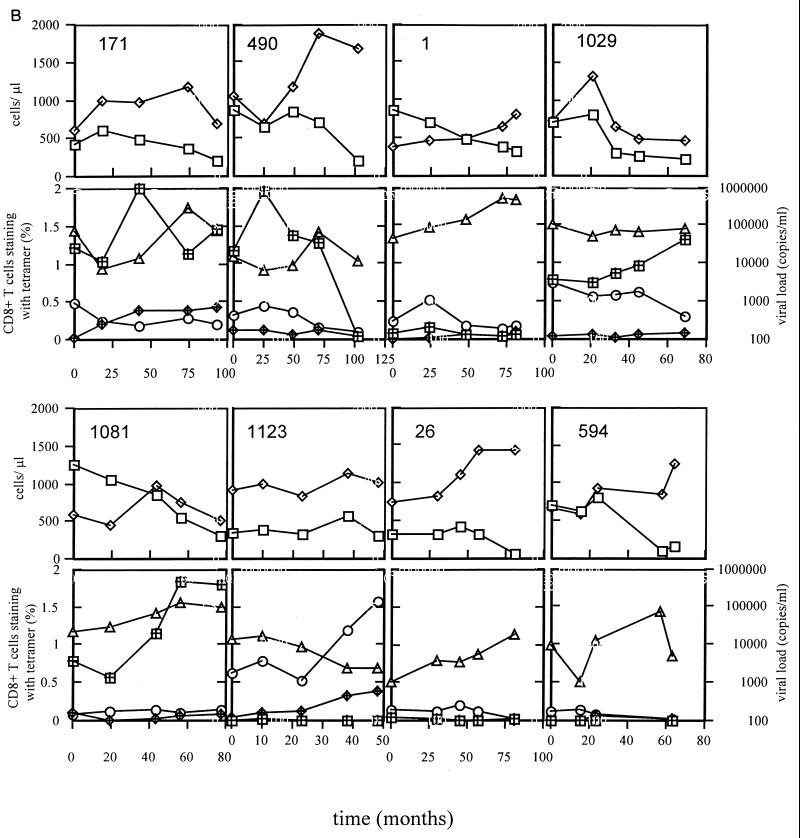
Figure 2 shows the longitudinal data from all 16 individuals studied over a median of 8.75 years (range, 4 to 10 years), including CD4 count, CD8 count, viral load, and frequencies of A*0201-restricted p17Gag/Pol/BMLF1 tetramer-binding cells. To aid comparison, the y axes all have the same scales except those for patients 1047, 16, 57, and 658, who all had higher CD4/CD8 counts and/or higher tetramer staining. The individuals who developed AIDS in the immediate months following the last sample time point were 171, 490, 1, 1029, 1081, 1123, 26, 594, and 1047; the remaining patients remained AIDS free. Twelve of the sixteen patients had declining CD4 counts during the study, and none of the four who had stable counts had progressed to AIDS. Of the nine who progressed to AIDS, six had late increases in plasma viremia preceding the onset of AIDS. The inverse association between p17Gag/Pol tetramer-binding cell frequency and plasma RNA viral load was maintained longitudinally in the majority of patients in whom tetramer-binding cells were detectable. Therefore increases in viremia were typically accompanied by decreasing p17Gag and/or Pol responses. When all time points were included from all patients in a single analysis (n = 80), the significant inverse correlation between Gag/Pol tetramer-binding cell frequency and plasma viaral RNA load was maintained (P < 0.05). A clear exception to this was observed in patient 1081, who had a late increase in viremia concomitant with an increase in the p17Gag response. It would be interesting to follow this individual further to assess whether the viremia and p17Gag responses ultimately diverge. In general, however, the responses were remarkably stable, with trends occurring over many months or years. Such relative stability of the HIV-specific CD8+ T-cell response during the chronic asymptomatic phase has been documented previously (20, 24, 40).
FIG. 2.
All individuals in panel A except patient 1047 remained asymptomatic throughout the study. Patient 1047 and all individuals in panel B developed AIDS-defining diagnoses within 2 months of the last study time point. The y axes scales are identical except those for patients 1047, 16, 57, and 658, who had higher levels of CD4/CD8 counts or tetramer staining. Tetramer staining is expressed as the percentage of CD8+ T cells staining positive with each tetramer. Symbols represent CD4+ cells (open squares), CD8+ cells (open diamonds), p17Gag tetramer staining (hashed squares), Pol tetramer staining (hashed diamonds), BMLF1 tetramer staining (open circles); and RNA viral load (open triangles). For each patient, the x axis refers to the time in months from the first sample analyzed, which in all cases was less than 2 years from seroconversion (Table 1).
CD27/CD45 expression by antigen-specific CD8+ T cells.
Before investigating the subsets of antigen-specific CD8+ T cells defined by costaining with CD27 and CD45RO MAb (17), we tested the possibility that antigen-specific CD8+ T cells could be double positive for CD45RA and CD45RO. Three of the sixteen patients were observed throughout the study using the four colour staining combination CD45RA-FITC, tetramer-phycoerythrin, CD8-PerCP, and CD45RO-APC. Whether tetramer-binding CD8+ T cells or total CD8+ T cells were analyzed, the occurrence of CD45RAbright CD45RObright double-positive cells was very rare, accounting for less than 0.5% of cells in all examples. CD45RA− CD45RO− double-negative cells were also extremely rare, although as many as 15 to 20% of cells could be CD45RAlow CD45ROlow. These cells may represent a transitional phase between one or other isoforms. In practice, however, the vast majority of CD8+ T cells and antigen-specific T cells had mutually exclusive expression of CD45RA and CD45RO.
Having excluded a significant CD45RA+ CD45RO+ double-positive population, we used CD27 MAb in combination with CD45RO MAb to distinguish specific CD8+ subpopulations and to test the possibility that Gag/Pol tetramer-binding cells become enriched during chronic HIV infection in the CD27− CD45RO− effector subset and thus not detectable by classical CTLp LDA (17). Within the antigen-specific CD8+ T cells, CD27 expression and CD45RO expression typically coexisted so that cells were usually CD27+ CD45RO+ double positive, but in many individuals antigen-specific cells could be identified with other combinations of CD27 and CD45RO expression. Table 2 shows the proportion of p17Gag tetramer-binding cells staining with CD27 and CD45RO MAbs for the five individuals with high frequencies of tetramer-positive cells (patients 1029, 1081, 171, 16, and 57) analyzed at study entry. The subsets were not exclusive to HIV tetramer-binding cells, as the BMLF1 tetramer-binding cells also split into these phenotypic subgroups (data not shown).
TABLE 2.
The majority of circulating HIV tetramer-binding CD8+ T cells were CD27+ CD45RO+ double-positive, but other phenotypic subgroups could be identified
| Patient | Absolute no. of p17Gag tetramer-binding cells/μl (SE) | % CD27− CD45RO+ | % CD27+ CD45RO+ | %CD27− CD45RO− | % CD27+ CD45RO− |
|---|---|---|---|---|---|
| 1029 | 5.5 (0.37) | 25 | 71 | 0 | 4 |
| 1081 | 2.52 (0.25) | 18 | 64 | 9 | 9 |
| 171 | 10.4 (0.275) | 12 | 55 | 11 | 22 |
| 16 | 14.8 (0.45) | 9 | 75 | 5 | 11 |
| 57 | 76.1 (1.75) | 15 | 85 | 0 | 0 |
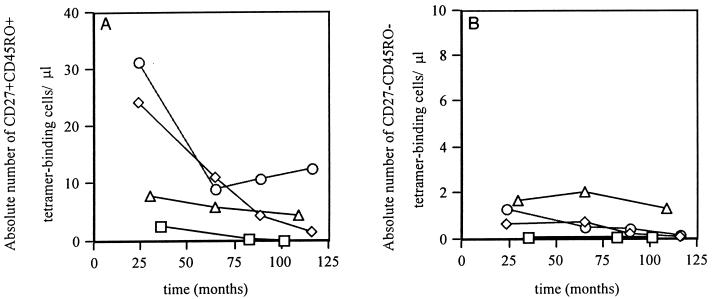
Late decreases in the frequency of Gag/Pol tetramer-binding cells typically accompanied declining CD4 counts and increases in plasma RNA viral load. In 13 of the 16 individuals, we longitudinally observed the staining of tetramer-binding CD8+ T cells with CD27 and CD45RO MAbs. Four patients had late declines in the frequency of Gag/Pol tetramer-binding cells which were typically characterized by the preferential loss of CD27+ CD45RO+ cells (Fig. 3). Loss of HIV tetramer-binding cells also occurred in the other subsets but was not as pronounced as that observed in the CD27+ CD45RO+ population (Fig. 3).
FIG. 3.
The decay of absolute numbers of circulating Gag/Pol tetramer-binding cells in the subsets CD27+ CD45RO+ (A) and CD27− CD45RO− (B). In the four patients studied with late falls in Gag/Pol tetramer-binding cells, most of the loss could be accounted for by falls in the CD27+ CD45RO+ subset. Smaller declines were also observed in the CD45RO− subset. Note that the y axes scales are different because the proportion of cells staining in the CD27− CD45RO− subset are considerably less than in the CD27+ CD45RO+ subset. Smaller declines were also observed in the CD45RO− subset.
Relative loss of HIV and EBV tetramer-binding cells.
Whether there is a selective loss of HIV-specific CTL in the face of relative preservation of other CTL responses is unclear, but several studies have suggested that this might be the case (6, 13, 30, 31). However, one study documented that loss of EBV-specific CTL in HIV-infected individuals was associated with the subsequent development non-Hodgkin’s lymphoma (21). There were three examples in the responses observed of a loss of HIV tetramer-binding cells in the face of a persistent EBV tetramer-binding response (patients 658, 434, and 26). In the remaining 11 individuals (patients 171, 490, 1, 1029, 1081, 594, 1123, 1140, 82, 16, and 57) in whom there were detectable p17Gag/Pol tetramer-binding cells and BMLF1 tetramer-binding cells, mixed patterns were observed. The p17Gag/Pol tetramer-binding cells were lost in parallel to the BMLF1 tetramer-binding cells in patients 490, 1, 594, and 1140. There was a preferential loss of BMLF1 tetramer-binding cells in patients 82 and 57. There was preservation of both p17Gag/Pol- and BMLF1-specific responses during the study in patients 171, 1029, 1081, 1123, and 16. In general, when patients’ CD4 counts fell below 100 cells/μl, all tetramer-binding responses fell below the limit of detection. Overall there was no significant difference in the probability of a preferential loss of Gag/Pol tetramer-binding cells or BMLF1 tetramer-binding cells (chi-squared and Fisher’s exact tests). These data suggest that although in some individuals there was a loss of HIV tetramer-binding cells prior to BMLF1 tetramer-binding cells, this was not a universal pattern.
DISCUSSION
We have examined the association between disease progression and the longitudinal p17Gag/Pol tetramer-binding cell frequency in 16 A*0201-positive untreated individuals followed clinically for up to 14 years. Early after seroconversion, we observed a significant association between high frequencies of such CTL and slow disease progression. This was extended to show a significant positive correlation between the frequency of p17Gag/Pol tetramer-binding cells and the length of AIDS-free follow-up. The eventual loss of HIV tetramer-binding cells that was associated with decline of CD4+ T cells and increased viremia was preferentially due to a reduction of antigen-specific cells in the CD27+ CD45RO+ subset. Finally, little evidence was obtained to support a reproducible loss of HIV-specific responses prior to EBV-specific responses.
The use of HLA peptide tetrameric complexes allowed us to overcome many of the difficulties of preexisting techniques available to determine the antigen-specific CD8+ T-cell response ex vivo. Specifically, we were able to accurately quantitate and phenotype the p17Gag/Pol-specific and EBV BMLF1-specific responses in uncultured PBMC. Consistent with our previous findings in a cross-sectional cohort (28), we observed a significant association between the frequency of p17Gag/Pol tetramer-binding cells and the length of AIDS-free follow-up. The inverse association between the frequency of p17Gag/Pol tetramer-binding cells and plasma RNA viral load was maintained longitudinally in the majority of patients, with late falls in the HIV-specific response being accompanied by increases in viremia.
Four patients had late falls in the p17Gag and/or Pol-specific responses. After first excluding the presence of large populations of CD45RA+ CD45RO+ double-positive cells, we used costaining with CD27 and CD45RO MAbs to study the dynamics of the distinct subsets of tetramer-binding CD8+ T cells. CTLp frequencies have been shown to diminish with disease progression, suggestive of a protective role for CTL (22, 33). Based on the functional properties of CD27− CD45RO− cells, virus-specific cytolytic activity may not be detected by classical LDA (17, 18). Patients that lack CTLp could still have functionally active CTL in the CD27− CD45RO− population. It has been hypothesized that during persistent viral infection, with chronic activation of CTL, virus-specific CTL could be preferentially enriched in a subpopulation of terminally differentiated CTL. Here we found that this was not the case, because in most patients the CD27+ CD45RO+ subset was the dominant population among antigen-specific cells. During the late fall in HIV-specific responses, we were able to characterize the relative loss of cells from each subset and showed that there was a preferential loss of cells from the CD27+ CD45RO+ subpopulation, consistent with previous CTLp studies (22, 33). We observed no absolute enrichment over time of CD27− CD45RO− cells, suggesting that the subset might have a short half-life. Alternatively, in persistent viral infections with ongoing stimulation, loss of CD45RO expression may be a rare event, with many cells in the CD27+ CD45RO+ subset manifesting effector function. It will be important to address the functional consequences of the staining patterns of antigen-specific CD8+ T cells by flow cytometric-guided cell sorting. In summary, the phenotype data showed that high frequencies of CD45RA+ CD45RO− antigen-specific cells can circulate in the periphery and that loss of HIV tetramer-binding cells is preferentially from the CD27+ CD45RO+ subset.
Several studies have documented selective loss of HIV-specific CTL in the context of preserved responses directed to other specificities (6, 13, 30, 31). Possible explanations have included exhaustion and/or defective clonogenic potential of HIV-specific CTL which may be secondary to loss of CD4 cell support. In only 3 of 16 patients were we able to show a similar pattern of preferential loss of HIV-specific responses prior to BMLF1-specific responses. In the remaining patients, we observed other patterns of changes in the HIV- and EBV-specific responses. It is likely that the patients will be heterogeneous with respect to the timing and degree of active EBV replication, and therefore it is perhaps not surprising that we could not document a universal pattern of decay of HIV-specific cells compared to EBV-specific cells. However, overall these data did not support a selective loss of HIV-specific cells in the presence of sustained EBV-specific responses.
This study is the first detailed longitudinal analysis of the ex vivo HIV- and EBV-specific CD8+ T-cell responses in HIV-infected individuals by using a sensitive technique that allows direct quantitation and phenotypic characterization of uncultured cells. A significant association between high frequencies of p17Gag/Pol tetramer-binding cells and slow progression was documented in the cohort of 16 untreated individuals observed for up to 14 years. These data are consistent with a role of HIV-specific CTL in the control of disease progression.
ACKNOWLEDGMENTS
This work was performed as part of the Amsterdam cohort studies on AIDS, a collaboration between the Municipal Health Service, AMC, and CLB in Amsterdam, The Netherlands.
We are grateful for support from the Medical Research Council (United Kingdom), Dutch AIDS Fund, and the Netherlands Organization for Scientific Research.
REFERENCES
- 1.Akbar A, Terry L, Timms A, Beverly P, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–2178. [PubMed] [Google Scholar]
- 2.Akiba H, Nakano H, Nishinaka S, Shindo M, Kobata T, Atsuta M, Morimoto C, Ware C F, Malinin N L, Wallach D, Yagita H, Okumura K. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J Biol Chem. 1998;273:13353–13358. doi: 10.1074/jbc.273.21.13353. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Moss P A H, Goulder P, Barouch D, McHeyzer-Williams M, Bell J I, McMichael A J, Davis M M. Direct visualization and phenotypic analysis of virus-specific T lymphocytes in HIV-infected individuals. Science. 1996;274:94–96. [Google Scholar]
- 4.Bariou C, Genetet N, Ruffault A, Michelet C, Cartier F, Genetet B. Longitudinal study of HIV-specific cytotoxic lymphocytes in HIV type 1-infected patients: relative balance between host immune response and the spread of HIV type 1 infection. AIDS Res Hum Retroviruses. 1997;13:1301–1312. doi: 10.1089/aid.1997.13.1301. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virological and immunological characterisation of long-term survivors of HIV-1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charbonneau H, Tonks N K, Walsh K A, Fischer E H. The leukocyte common antigen CD45: a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci USA. 1988;85:7182–7189. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Wolf F, Spijkerman I, Schellekens P T, Langendam M, Kuiken C, Bakker M, Roos M, Coutinho R, Miedema F, Goudsmit J. AIDS prognosis based on HIV-1 RNA, CD4+ T-cell count and function: markers with reciprocal predictive value over time after seroconversion. AIDS. 1997;11:1799–1806. doi: 10.1097/00002030-199715000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Dorrell L, Dong T, Ogg G S, McAdam S, Anzala O, Rostron T, Conlon C, McMichael A J, Rowland-Jones S. Distinct recognition of A clade human immunodeficiency virus type 1 epitopes by cytotoxic T lymphocytes generated from donors infected in Africa. J Virol. 1999;73:1708–1714. doi: 10.1128/jvi.73.2.1708-1714.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunbar P R, Chen J L, Chao D, Rust N, Teisserenc H, Ogg G S, Romero P, Weynants P, Cerundolo V. Cutting edge: rapid cloning of tumor-specific CTL suitable for adoptive immunotherapy of melanoma. J Immunol. 1999;162:6959–6962. [PubMed] [Google Scholar]
- 11.Dunbar P R, Ogg G S, Chen J, Rust N, van der Bruggen P, Cerundolo V. Direct isolation, phenotyping and cloning of low frequency antigen-specific CTL from peripheral blood. Curr Biol. 1998;8:413–416. doi: 10.1016/s0960-9822(98)70161-7. [DOI] [PubMed] [Google Scholar]
- 12.Dyer W B, Ogg G S, Demoite M-A, Jin X, Geczy A F, Rowland-Jones S L, McMichael A J, Nixon D F, Sullivan J S. Strong human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte activity in Sydney Blood Bank Cohort patients infected with nef-defective HIV type 1. J Virol. 1999;73:436–443. doi: 10.1128/jvi.73.1.436-443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geretti A M, Dings M E, van Els C A, van Baalen C A, Wijnholds F J, Borleffs J C, Osterhaus A D. Human immunodeficiency virus type 1 (HIV-1)- and Epstein-Barr virus-specific cytotoxic T lymphocyte precursors exhibit different kinetics in HIV-1-infected persons. J Infect Dis. 1996;174:34–45. doi: 10.1093/infdis/174.1.34. [DOI] [PubMed] [Google Scholar]
- 14.Gotch F M, Nixon D F, Alp N, McMichael A J, Borysiewicz L K. High frequency of memory and effector gag specific cytotoxic T lymphocytes in HIV seropositive individuals. Int Immunol. 1990;2:707. doi: 10.1093/intimm/2.8.707. [DOI] [PubMed] [Google Scholar]
- 15.Goulder P J R, Sewell A K, Lalloo D G, Price D A, Whelan J A, Evans J, Taylor G P, Luzzi G, Giangrande P, Phillips R E, McMichael A J. Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two human histocompatibility leukocyte antigens (HLA)-identical siblings with HLA-A*0201 are influenced by epitope mutation. J Exp Med. 1997;185:1423–1433. doi: 10.1084/jem.185.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenough T C, Brettler D B, Somasundaran M, Panicali D L, Sullivan J L. HIV-specific CTL, virus load and CD4+ T cell loss: evidence supporting a protective role for CTL in vivo. J Infect Dis. 1997;176:118–125. doi: 10.1086/514013. [DOI] [PubMed] [Google Scholar]
- 17.Hamann D, Baars P A, Rep M H, Hooibrink B, Kerkhof-Garde S R, Klein M R, van Lier R A W. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamann D, Roos M T L, van Lier R A W. Faces and phases of human CD8 T-cell development. Immunol Today. 1999;20:177–180. doi: 10.1016/s0167-5699(99)01444-9. [DOI] [PubMed] [Google Scholar]
- 19.Harrer T, Harrer E, Kalams S A, Elbeik T, Staprans S I, Feinberg M B, Cao Y, Ho D D, Yilma T, Caliendo A M, Johnson R P, Buchbinder S P, Walker B D. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 20.Kalams S A, Johnson R P, Trocha A K, Dynan M J, Ngo S, D’Aquila R T, Kurnick J T, Walker B D. Longitudinal analysis of TCR gene usage by HIV-1 envelope-specific CTL clones reveals a limited TCR repertoire. J Exp Med. 1994;179:1261–1271. doi: 10.1084/jem.179.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kersten M J, Klein M R, Holwerda A M, Miedema F, van Oers M H. Epstein-Barr virus-specific cytotoxic T cell responses in HIV-1 infection: different kinetics in patients progressing to opportunistic infection or non-Hodgkin’s lymphoma. J Clin Investig. 1997;99:1525–1533. doi: 10.1172/JCI119315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein M R, Van Baalen C A, Holwerda A M, Garde S R K, Bende R J, Keet I P M, Eeftinck Schattenkerk J K M, Osterhaus A D M E, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean A R, Michie C A. In vivo estimates of division and death rates of human T lymphocytes. Proc Natl Acad Sci USA. 1995;92:3707–3711. doi: 10.1073/pnas.92.9.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss P A H, Rowland-Jones S L, Frodsham P M, McAdam S, Giangrande P, McMichael A J, Bell J I. Persistent high frequency of human immunodeficiency virus-specific cytotoxic T cells in peripheral blood of infected donors. Proc Natl Acad Sci USA. 1995;92:5773–5777. doi: 10.1073/pnas.92.13.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a re-evaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 26.Musey L, Hughes J, Schaker T, Shea T, Corey L, McElrath M J. CTL responses, viral load and disease progression in early HIV infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 27.Mustelin T, Coggeshall K M, Altman A. Rapid activation of the T cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci USA. 1989;86:6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogg G, Jin X, Bonhoeffer S, Dunbar P, Nowak M, Monard S, Segal J, Cao Y, Rowland-Jones S, Cerundolo V, Hurley A, Markowitz M, Ho D, Nixon D, McMichael A. Quantitation of HIV-specific CTL and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 29.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, Markowitz M, Ho D D, McMichael A J, Nixon D F. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantaleo G, De Maria A, Koenig S, Butini L, Moss B, Baseler M, Lane H C, Fauci A S. CD8+ T lymphocytes of patients with AIDS maintain normal broad cytolytic function despite the loss of human immunodeficiency virus-specific cytotoxicity. Proc Natl Acad Sci USA. 1990;87:4818–4822. doi: 10.1073/pnas.87.12.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pantaleo G, Soudeyns H, Demarest J F, Vaccarezza M, Paolucci S, Daucher M, Cohen O J, Denis F, Biddis W E, Sekaly R P, Fauci A S. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc Natl Acad Sci USA. 1997;94:9848–9853. doi: 10.1073/pnas.94.18.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker K C, Bednarek M A, Hull L K, Utz U, Cunningham B, Zweerink H J, Biddison W E, Coligan J E. Sequence motifs important for peptide binding to the human MHC class I molecule, HLA-A2. J Immunol. 1992;149:3580–3587. [PubMed] [Google Scholar]
- 33.Pontesilli O, Klein M R, Kerkhof-Garde S R, Pakker N G, de Wolf F, Schuitemaker H, Miedema F. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J Infect Dis. 1998;178:1008–1018. doi: 10.1086/515659. [DOI] [PubMed] [Google Scholar]
- 34.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riviere Y, McChesney M B, Porrot F, Tanneau S F, Sansonetti P, Lopez O, Pialoux G, Feuillie V, Mollereau M, Chamaret S, et al. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res Hum Retroviruses. 1995;11:903–907. doi: 10.1089/aid.1995.11.903. [DOI] [PubMed] [Google Scholar]
- 36.Steven N M, Annels N E, Kumar A, Leese A M, Kurilla M G, Rickinson A B. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J Exp Med. 1997;185:1605–1617. doi: 10.1084/jem.185.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan L C, Gudgeon N, Annels N E, Hansasuta P, O’Callaghan C A, Rowland-Jones S, McMichael A J, Rickinson A B, Callan M F. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 38.Tan R, Xu X, Ogg G S, Hansasuta P, Dong T, Rostron T, Luzzi G, Conlon C P, Screaton G R, McMichael A J, Rowland-Jones S. Rapid death of adoptively transferred T cells in acquired immunodeficiency syndrome. Blood. 1999;93:1506–1510. [PubMed] [Google Scholar]
- 39.Tsomides T J, Aldovini A, Johnson R P, Walker B D, Young R A, Eisen H N. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J Exp Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson J D K, Ogg G S, Allen R L, Goulder P J, Kelleher T, Sewell A, O’Callaghan C A E, Callan M F C, Rowland-Jones S L, McMichael A J. Oligoclonal expansions of CD8+ T cells in chronic HIV infection are antigen-specific. J Exp Med. 1998;188:785–790. doi: 10.1084/jem.188.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]