Abstract
Background/Aims
Gastric subepithelial tumors (SETs) are often encountered during the upper gastrointestinal endoscopic screening. We assessed the prevalence of gastric SETs and the risk factors for their progression.
Methods
We reviewed the electronic medical records of 30,754 patients who underwent upper gastrointestinal endoscopic screening at our medical center between January 2013 and December 2016.
Results
Among the 30,754 patients examined, 599 (1.9%) had gastric SETs. The prevalence increased with age and was 9.56% in patients aged ≥70 years. In total, 262 patients underwent serial endoscopy for more than 6 months. The median age was 68 years (interquartile range [IQR], 61–74), and the number of females was 167 (63.7%). During a median follow-up of 58 months (IQR, 38–75), 22 patients (8.4%) showed significant changes in tumor size. An irregular border (odds ratio, 4.623; 95% confidence interval, 1.093–19.558; p=0.037) was a significant risk factor for progression. Seven patients underwent surgical or endoscopic resections. The pathologies of gastric SETs included leiomyomas (n=3), gastrointestinal stromal tumors (n=2), and lipomas (n=2).
Conclusions
The prevalence of gastric SETs increases with age. Most gastric SETs do not progress during long-term endoscopic examinations, and the risk of an increase in size is low in asymptomatic small SETs without irregular borders.
Keywords: Gastrointestinal endoscopy, Irregular border, Risk factors, Stomach, Subepithelial tumor
Graphical abstract
INTRODUCTION
Subepithelial tumors (SETs) are protruding lesions in the cavity of the gastrointestinal tract that are covered by an intact mucosal surface. SETs can be classified as benign lesions, including leiomyomas, granular cell tumors, lipomas, and malignant or premalignant lesions, such as gastrointestinal stromal tumors (GISTs), neuroendocrine tumors, and glomus tumors.1
SETs are often detected during upper gastrointestinal endoscopic screenings. Lim et al.2 reported that SETs were detected in 0.76% of patients who underwent upper gastrointestinal endoscopy at the Health Promotion Center, and the stomach was the most common site for SETs, followed by the esophagus and duodenum. Lee et al.3 also found that the prevalence of gastric SETs among healthy individuals was 1.7% and increased with age.
Differential diagnosis of gastric SETs by conventional endoscopy with standard biopsy is difficult because special techniques are required to break open the lesions for tissue sampling.4 Some methods for histologic diagnosis of gastric SETs include bite-on-bite biopsy, mucosal cutting biopsy, endoscopic ultrasound (EUS)-guided fine-needle biopsy, endoscopic mucosal resection, and endoscopic submucosal dissection.1,4 However, in the case of small SETs, it is not easy to conduct an active examination considering the complications associated with the procedure. In addition, because little is known about the natural history of gastric SETs, proper management has not been clearly established.5 The present study aimed to determine the natural course of gastric SETs and assess the risk factors for progression.
METHODS
Patients and data collection
A total of 30,754 patients underwent upper gastrointestinal endoscopic screening at our medical center between January 2013 and December 2016. We reviewed the electronic medical records of patients with gastric SETs. Demographic data and clinical information, including age, sex, location, initial size, size increment, mucosal changes, irregular borders, and follow-up duration, were collected. SETs size was determined based on the maximum diameter obtained using EUS or open biopsy forceps (6 mm). Based on a previous study, a significant increase in size was defined as >25% and a >5 mm increase in the longest diameter.2 We compared the characteristics of the stable SETs group with those of the progressive SETs group and then analyzed the risk factors associated with progression.
Statistical analysis
Chi-squared or Fisher exact tests were used to compare categorical variables. The Mann-Whitney U test was used to analyze continuous variables. Multivariate logistic regression analysis was performed using risk factors significantly (p<0.1) associated with the progression of SETs in the univariate analysis. Statistical analyses were performed using IBM SPSS for Windows ver. 21.0 (IBM Corp.).
Ethics statement
The Institutional Review Board (IRB) of Presbyterian Medical Center approved the protocol and waived the requirement for informed consent due to the retrospective nature of the study (IRB No: 2022-09-062). All the clinical investigations were conducted in accordance with the principles of the Declaration of Helsinki.
RESULTS
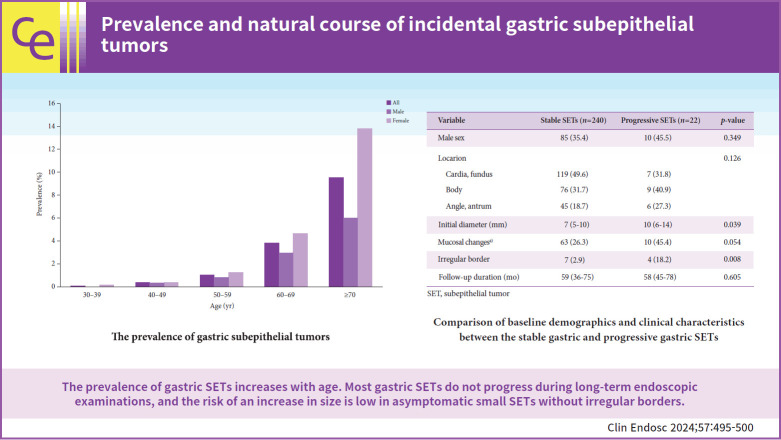
Among the 30,754 healthy examinees, 599 (1.9%) were diagnosed with gastric SETs. The prevalence of SETs was higher in females (2.5%) than in males (1.4%). The prevalence of gastric SETs increased with age. The prevalence by age group was as follows: 0.06%, 0.37%, 1.05%, 3.81%, and 9.56% of patients aged 30–39, 40–49, 50–59, 60–69, and ≥70 years, respectively (Fig. 1). Fifteen patients underwent surgical or endoscopic resections. The median age at diagnosis was 62 years (interquartile range [IQR], 61–70 years), with 11 female patients. The number of tumor locations was one in the cardia and fundus, 11 in the body, and three in the angle and antrum. The median tumor diameter at initial diagnosis was 13 mm (IQR, 10–15 mm). The pathologies of the gastric SETs were leiomyomas (n=6), lipomas (n=3), GISTs (n=2), inflammatory fibroid polyps (n=1), ectopic pancreas (n=1), and chronic gastritis (n=2).
Fig. 1.
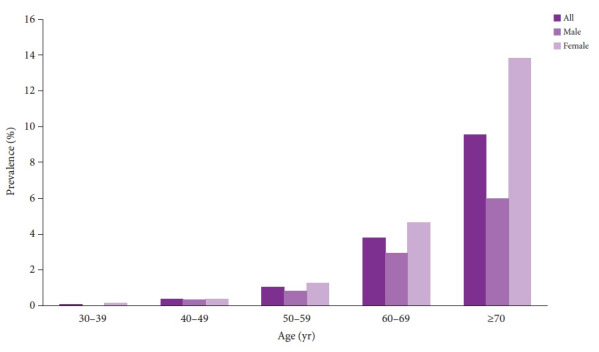
The prevalence of gastric subepithelial tumors.
A total of 262 patients who underwent serial endoscopy for more than 6 months were included in the final analysis to assess the risk factors for progression (Table 1). The median age at diagnosis was 68 years (IQR, 61–74 years), and 167 (63.7%) of the patients were female. The median follow-up period was 58 months (IQR, 38–75 months). The proportions of tumor locations were 48.1%, 32.4%, and 19.5% in the cardia and fundus, body, and angle and antrum, respectively. The median tumor diameter at initial diagnosis was 7 mm (IQR, 5–10 mm). Of the 262 patients, 22 (8.4%) showed a significant change in tumor size. Seven patients underwent surgical (n=2) or endoscopic resection (n=5): three were diagnosed with leiomyoma, two with GISTs, and two with lipoma. Among the two GISTs, one was classified as low-risk and the other as intermediate-risk. Multivariate logistic regression analysis showed that the independent risk factor associated with progression was irregular borders (odds ratio, 4.623; 95% confidence interval, 1.093–19.558; p=0.037) (Table 2).
Table 1.
Comparison of baseline demographics and clinical characteristics between the stable gastric and progressive gastric SETs
| Variable | Stable SETs (n=240) | Progressive SETs (n=22) | p-value |
|---|---|---|---|
| Age (yr) | 68 (60–74) | 66 (63–73) | 0.930 |
| 30–39 | 1 (0.4) | 0 (0) | |
| 40–49 | 14 (5.8) | 0 (0) | |
| 50–59 | 40 (16.7) | 2 (9.1) | |
| 60–69 | 76 (31.7) | 12 (54.5) | |
| ≥70 | 109 (45.4) | 8 (36.4) | |
| Male sex | 85 (35.4) | 10 (45.5) | 0.349 |
| Location | 0.126 | ||
| Cardia, fundus | 119 (49.6) | 7 (31.8) | |
| Body | 76 (31.7) | 9 (40.9) | |
| Angle, antrum | 45 (18.7) | 6 (27.3) | |
| Initial diameter (mm) | 7 (5–10) | 10 (6–14) | 0.039 |
| Mucosal changesa) | 63 (26.3) | 10 (45.4) | 0.054 |
| Irregular border | 7 (2.9) | 4 (18.2) | 0.008 |
| Follow-up duration (mo) | 59 (36–75) | 58 (45–78) | 0.605 |
Values are presented as median (interquartile range) or number (%).
SET, subepithelial tumor.
Mucosal changes: erythema, erosion, and ulcer.
Table 2.
Results of univariate and multivariate logistic regression analyses of risk factors for the progression of gastric subepithelial tumors
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 1.005 (0.963–1.050) | 0.807 | ||
| Male sex | 0.658 (0.273–1.586) | 0.351 | ||
| Location | ||||
| Cardia, fundus | Reference | |||
| Body | 2.013 (0.720–5.632) | 0.183 | ||
| Angle, antrum | 2.267 (0.723–7.109) | 0.161 | ||
| Initial diameter | 1.090 (0.998–1.190) | 0.054 | 1.061 (0.967–1.163) | 0.210 |
| Mucosal changesa) | 2.341 (0.964–5.685) | 0.060 | 1.686 (0.644–4.417) | 0.288 |
| Irregular border | 7.397 (1.978–27.654) | 0.003 | 4.623 (1.093–19.558) | 0.037 |
OR, odds ratio; CI, confidence interval.
Mucosal changes: erythema, erosion, and ulcer.
DISCUSSION
In this study, we found that the prevalence of gastric SETs among healthy individuals was 1.94% and increased with age. In addition, there was no significant change in 91.6% of the gastric SETs during a median follow-up of 58 months. Our findings are similar to those of previous studies. Kim et al.6 found that the prevalence of gastric SETs in healthy participants was 1.51%, and no increase in size was detected in 93% of gastric SETs. A single-center study of 948 patients demonstrated that 8.5% of gastric SETs showed significant change at a median follow-up of 24 months.7 Lee et al.3 reported that the prevalence of gastric SETs was 2.4% of patients aged ≥70 years and increased with age. Our results showed that the prevalence was 9.56% of patients aged ≥70 years. Although the prevalence of gastric SETs increases with age, there is no significant association between age and lesion progression.
Most gastric SETs are identified incidentally during routine endoscopy. Most gastric SETs are considered benign lesions; however, some lesions, such as GISTs or neuroendocrine tumors, are malignant.8 Gastric SETs remain difficult to manage because determining the possibility of malignancy before resection is challenging.9 Although surgical excision has been considered the gold standard treatment for gastric SETs, the European Society of Gastrointestinal Endoscopy (ESGE) recommends endoscopic resection as an alternative treatment for gastric SETs that protrude into the gastric lumen.10 Recently, gastric SETs diagnosed as growing outward have been safely treated by endoscopic subserosal dissection.11
The natural course of gastric SETs is still unclear; thus, the appropriate management strategy is controversial.12 Kim et al.6 proposed biennial endoscopic follow-up for small SETs <1 cm, annual follow-up for SETs 1 to 2 cm, and close follow-up every 6 months or less for large SETs or SETs with malignant potential. ESGE recommends surveillance of gastric SETs without definite diagnosis, with endoscopy at 3 to 6 months, then 2 to 3 years intervals for lesions <1 cm and 1 to 2 years intervals for lesions 1 to 2 cm in size.10
Several studies have investigated risk factors associated with changes in gastric SETs. Min et al.13 reported that old age, tumor site outside the cardia, large size, and ulceration or dimpling on the surface were independent predictive factors for GISTs. Nishida et al.14 proposed surface ulceration, irregular borders, and increased size during follow-up as high-risk features. In this study, irregular borders were also a significant risk factor for tumor progression.
Our study had some limitations. As the study data were collected retrospectively, other critical clinical variables affecting the risk factors for the progression of gastric SETs may have been missed. In addition, the gastric SET size was estimated using open biopsy forceps; therefore, this method may differ between endoscopists and the actual size of the lesion.
In conclusion, we found that the progression of incidental gastric SETs was only approximately 8% during a long-term follow-up period of approximately 5 years. Regular follow-up endoscopies can be performed for asymptomatic small SETs without irregular borders. The prevalence of gastric SETs on screening endoscopy was 1.94% and increased with age. As the prevalence of gastric SETs is approximately 10% in patients aged ≥70 years, gastric SETs are expected to increase significantly in the future as life expectancy increases. Nationwide collaborative research is needed to assess the burden of gastric SETs and the risk factors for disease progression.
Footnotes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
This research was supported by Saehim Cancer Patient Support Association, Presbyterian Medical Center.
Acknowledgments
We express our gratitude to In Su Park in Presbyterian Medical Center for his assistance in data collection.
Author Contributions
Conceptualization: DHH, MAY, JWC; Data curation: DHH, JSS; Formal analysis: DHH, WDL; Investigation: DHH, JSS, WDL; Methodology: MAY, JWC; Project administration: MAY, JSS; Resources: DHH, MAY, JSS; Software: JSS, WDL; Supervision: JWC; Validation: MAY, WDL; Visualization: DHH, JSS, WDL; Writing–original draft: DHH, JWC; Writing–review & editing: all authors.
REFERENCES
- 1.Cho JW, Korean ESD Study Group Current guidelines in the management of upper gastrointestinal subepithelial tumors. Clin Endosc. 2016;49:235–240. doi: 10.5946/ce.2015.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim YJ, Son HJ, Lee JS, et al. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol. 2010;16:439–444. doi: 10.3748/wjg.v16.i4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Lee HL, Ahn YW, et al. Prevalence of gastric subepithelial tumors in Korea: a single center experience. Korean J Gastroenterol. 2015;66:274–276. doi: 10.4166/kjg.2015.66.5.274. [DOI] [PubMed] [Google Scholar]
- 4.Goto O, Kaise M, Iwakiri K. Advancements in the diagnosis of gastric subepithelial tumors. Gut Liver. 2022;16:321–330. doi: 10.5009/gnl210242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho J, Han J, Choi M, et al. Correlation between endoscopic resection outcomes and endosonographic findings in gastric tumors with muscularis propria origin. Medicine (Baltimore) 2022;101:e29947. doi: 10.1097/MD.0000000000029947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim B, Kang S, Lee E, et al. Gastric subepithelial tumor: long-term natural history and risk factors for progression. Surg Endosc. 2022;36:5232–5242. doi: 10.1007/s00464-021-08901-4. [DOI] [PubMed] [Google Scholar]
- 7.Kim MY, Jung HY, Choi KD, et al. Natural history of asymptomatic small gastric subepithelial tumors. J Clin Gastroenterol. 2011;45:330–336. doi: 10.1097/MCG.0b013e318206474e. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Li B, Li L, et al. Current status of endoscopic resection of gastric subepithelial tumors. Am J Gastroenterol. 2019;114:718–725. doi: 10.14309/ajg.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 9.Kim SY, Kim KO. Management of gastric subepithelial tumors: the role of endoscopy. World J Gastrointest Endosc. 2016;8:418–424. doi: 10.4253/wjge.v8.i11.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deprez PH, Moons LMG, OʼToole D, et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412–429. doi: 10.1055/a-1751-5742. [DOI] [PubMed] [Google Scholar]
- 11.Han J, Cho J, Song J, et al. Endoscopic subserosal dissection for gastric tumors: 18 cases in a single center. Surg Endosc. 2022;36:8039–8046. doi: 10.1007/s00464-022-09229-3. [DOI] [PubMed] [Google Scholar]
- 12.Song JH, Kim SG, Chung SJ, et al. Risk of progression for incidental small subepithelial tumors in the upper gastrointestinal tract. Endoscopy. 2015;47:675–679. doi: 10.1055/s-0034-1391967. [DOI] [PubMed] [Google Scholar]
- 13.Min YW, Park HN, Min BH, et al. Preoperative predictive factors for gastrointestinal stromal tumors: analysis of 375 surgically resected gastric subepithelial tumors. J Gastrointest Surg. 2015;19:631–638. doi: 10.1007/s11605-014-2708-9. [DOI] [PubMed] [Google Scholar]
- 14.Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3–14. doi: 10.1007/s10120-015-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]