Abstract
Tranexamic acid (TXA) is a synthetic antifibrinolytic agent that has been used for several decades to reduce blood loss during surgery and after trauma. TXA was traditionally used to reduce bleeding in various clinical settings such as menorrhagia, hemophilia, or other bleeding disorder. Numerous studies have demonstrated the efficacy of TXA in reducing blood loss and the need for transfusions. Interest in the potential applications of TXA beyond its traditional use has been growing recently, with studies investigating the use of TXA in postpartum hemorrhage, cardiac surgery, trauma, neurosurgery, and orthopedic surgery. Despite its widespread use and expanding indications, data regarding the safe and appropriate use of TXA is lacking. Recent clinical trials have found various potential risks and limitations in the long-term benefits of TXA. This narrative review summarizes the clinical applications and limitations of TXA.
Keywords: Blood transfusion, Cardiac surgical procedures, Hemorrhage, Neurosurgery, Orthopedic procedures, Postpartum hemorrhage, Tranexamic acid, Trauma
Introduction
Tranexamic acid (TXA) is a synthetic antifibrinolytic agent developed in the 1960s that has been used to manage bleeding disorders for decades. TXA reduces bleeding by preventing the breakdown of fibrin clots and reducing blood loss through inhibiting the activation of plasminogen to plasmin, which is 8–10 times more potent than ε-aminocaproic acid [1–5].
TXA was first used in women with heavy menstrual bleeding and patients with hereditary bleeding disorders [1]. However, subsequent studies showed that TXA could significantly reduce the risk of mortality from bleeding after trauma or surgery by decreasing the risk of hemorrhage and the need for transfusions [2,3]. Owing to its blood-saving effect, TXA has been used to treat bleeding disorders, including trauma, obstetrics and gynecological diseases, and gastrointestinal bleeding, even in the surgical field. Currently, TXA is included in the World Health Organization (WHO) Model List of Essential Medicines, and interest in the use of TXA in various clinical settings, such as postpartum hemorrhage (PPH), surgical hemorrhage, and trauma, is growing [1–5]. A recent large clinical trial (Perioperative Ischemic Evaluation–3; POISE-3) revealed that TXA significantly reduces the incidence of life-threatening or major bleeding in patients with a risk of bleeding and cardiovascular events undergoing noncardiac surgery [6].
However, data on the clinical use and safety of TXA are limited except in areas with large-scale clinical trials. Moreover, recent clinical trials have reported disappointing results that suggest that TXA is not associated with favorable long-term outcomes or functional improvement. The clinical use of TXA also has potential risks, such as seizures and thrombosis, and limitations (e.g., the optimal dosage or timing of administration is unknown). This narrative review summarizes the various clinical uses of TXA and its limitations based on previous clinical trials that have been conducted to date.
Coagulation cascade, fibrinolysis system, and mechanism of action of TXA
The coagulation cascade and fibrinolytic system are complementary systems to maintain hemostasis and balance between coagulation and bleeding in the body [1,5]. When the endothelial wall of a blood vessel is damaged, platelet activation leads to vasoconstriction and platelet aggregation, which promotes the formation of platelet plugs and thrombin through the activation of the coagulation cascade. Thrombin-activated fibrinogen forms fibrin crosslinks to strengthen blood clots composed of red blood cells, white blood cells, platelets, fibrinogen, fibrin, and plasminogen. Simultaneously, local fibrinolysis prevents systemic spread resulting from uncontrolled thrombus generation. The local activation of plasminogen activator (PA) in the vascular endothelium converts plasminogen to plasmin, which results in fibrinolysis at the site of thrombus formation. Likewise, the coagulation process is tightly regulated by a counteracting fibrinolytic system to prevent excessive coagulation, which can lead to thrombosis or the formation of blood clots in blood vessels. The fibrinolytic system is tightly regulated to prevent bleeding due to excessive activation. However, coagulopathy can result from systemic fibrinolytic activation in cases such as delivery, trauma, surgery, or exposure to cardiopulmonary bypass (CPB) or extracorporeal circulation, which can promote excessive activation of fibrinolysis beyond a person’s ability to regulate it physiologically.
TXA is an antifibrinolytic agent that prevents fibrin degradation by binding to the lysine-binding site where plasminogen and plasmin bind to fibrin, thereby inhibiting its fibrinolytic activity. TXA does not directly affect the coagulation cascade but indirectly supports the coagulation process by inhibiting the fibrinolytic system and stabilizing clots, which helps prevent excessive hemorrhage [5].
Pharmacology of TXA
The peak blood concentration of TXA is reached within 3 h of oral administration and within 1 h of intravenous administration [1,7–9]. The volume of TXA distribution is approximately 9–12 liters, and its protein binding rate is low, at approximately 3% [7]. TXA is deposited in tissues and diffuses rapidly into the joint fluid or synovial membrane, resulting in the maintenance of similar blood and joint cavity concentrations [1,7]. TXA crosses the blood-brain barrier, and its concentration in the cerebrospinal fluid is 1/10 of its blood concentration after intravenous administration of 10 mg/kg TXA [1,7]. In pregnant women, the placental concentration of TXA is 4–31 μg/ml when the plasma concentration is 10–53 μg/ml [1,7]. In addition, TXA is safe to administer in women who are breastfeeding [1,7].
The half-life of TXA is approximately 2–3 h, but its biological half-life is approximately 80 min [1,5,7–9]. After intravenous administration of 10 mg/kg TXA, 30% is excreted within an hour, and 90% is excreted within 24 h in an unchanged form by the kidneys [1,5,7–9]. The renal clearance rate equals the plasma clearance rate from 110 to 116 ml/min; therefore, for patients with impaired renal function, the dose should be reduced because of increased plasma concentrations [1,5,7–9]. According to pharmacokinetic studies of patients with chronic kidney disease (CKD) undergoing cardiac surgery, while CKD itself is not contraindicated, an optimized dosing regimen based on the CKD stage and glomerular filtration rate should be implemented because the plasma concentration of TXA increases over a long period in patients with CKD stage 3 or higher [1,10,11].
Studies comparing the effect of fibrinolysis according to the plasma concentration of TXA found that for 100% inhibition of fibrinolysis, the plasma concentration was 100 µg/ml, while for 80% inhibition of fibrinolysis, the plasma concentration was 10–20 µg/ml [8,11]. Generally, 80% inhibition of fibrinolysis is considered sufficient for clinical use [1]. However, a higher plasma concentration may be required to maintain the effectiveness of TXA in patients in a hyperfibrinolytic state, such as those undergoing CPB surgery [2]. Therefore, efforts should be made to maximize the antifibrinolytic effects and minimize the adverse effects by adjusting the dose and timing of TXA administration according to the patient’s coagulation status, clinical situation, and surgical procedure.
Applications of TXA
Obstetric fields
Although TXA was developed to reduce PPH, which is an important cause of maternal mortality and morbidity, the extent to which hyperfibrinolysis affects bleeding in severe PPH remains unknown [2]. Likewise, clinical trials using TXA in the field of obstetrics have shown ambiguous results in terms of overall outcomes (Table 1).
Table 1.
Clinical Trials on the Use of Tranexamic Acid in the Obstetric Field
| Clinical trial | Subjects | Definition and treatment | Results |
|---|---|---|---|
| WOMAN trial [12] | Women with PPH from low- and middle-income countries (n = 20,060) | -TXA 1–2 g or placebo | -Mortality rate: 1.5% vs. 1.9% for the placebo (RR: 0.81, 95% CI [0.65, 1.0], P = 0.045) |
| -If given within 3 h: 1.2% vs. 1.7% for the placebo (RR: 0.69, 95% CI [0.52, 0.91], P = 0.008) | |||
| TRAAP1 [15] | Vaginal delivery (n = 4,079) | -PPH: blood loss ≥ 500 ml in a collector bag | -No significant differences in PPH (8.1% vs. 9.8% for the placebo; RR: 0.83, 95% CI [0.68, 1.01], P = 0.07) |
| -TXA 1 g vs. placebo | |||
| TRAAP2 [16] | Cesarean delivery (n = 4,551) | -PPH: blood loss > 1,000 ml or an allogeneic RBC transfusion within 2 d of delivery | -A lower likelihood of PPH (26.7% vs. 31.6% for the placebo; adjusted RR: 0.84, 95% CI [0.75, 0.94], P = 0.003) |
| -TXA 1 g vs. placebo | -Average difference in blood loss: 100 ml | ||
| -No clinical differences |
WOMAN trial: World Maternal Antifibrinolytic trial, TRAAP1: Tranexamic Acid for Preventing Postpartum Hemorrhage Following a Vaginal Delivery study, TRAAP2: Tranexamic Acid for Preventing Postpartum Hemorrhage Following a Cesarean Delivery study, PPH: postpartum hemorrhage, TXA: tranexamic acid, RBC: red blood cell, RR: relative risk.
The WOMAN (World Maternal Antifibrinolytic) trial was a large-scale randomized clinical trial (RCT) involving 20,060 pregnant women with PPH in low- and middle-income countries [12]. The relative risk of death due to hemorrhage was decreased by 19% in the group that received 1–2 g TXA compared to the control group (mortality rate due to bleeding 1.5% vs. 1.9% for the placebo). The best effects were seen when TXA was administered within 3 h of delivery, reducing the relative risk of mortality due to hemorrhage by 31% (mortality rate due to bleeding 1.2% vs. 1.7% for the placebo). Despite criticism that these results would not be achievable in developed countries with better healthcare environments and infrastructure, the WHO recommends using TXA as a standard treatment for PPH in all cases [13,14]. However, the TRAAP1 (Tranexamic Acid for Preventing Postpartum Hemorrhage Following a Vaginal Delivery) study, a RCT of 4,079 pregnant women, revealed that the administration of 1 g TXA did not decrease the incidence of PPH, defined as a blood loss of ≥ 500 ml (8.1% vs. 9.8% for the placebo) [15]. The TRAAP2 (Tranexamic Acid for Preventing Postpartum Hemorrhage Following a Cesarean Delivery) study assessed the incidence of PPH in 4,551 patients who underwent a cesarean section. Although this RCT showed a decreased incidence of PPH in the group treated with 1 g TXA (26.7% vs. 31.6% for the placebo), the average difference in blood loss between the two groups was 100 ml; thus, the clinical advantage of TXA could not be determined [16]. Moreover, recent systematic reviews and meta-analyses have reported that TXA does not appear to reduce the amount of bleeding after cesarean sections, blood transfusions, or hysterectomies [17,18]. In addition, a recent retrospective study that included a propensity-matched analysis showed no difference in maternal morbidity and mortality between the women with PPH who received TXA early and those who received TXA late or not at all (odds ratio [OR]: 0.92, 95% CI [0.66, 1.27]) [19].
Therefore, goal-directed treatment protocols using functional coagulation assays with viscoelastography are required to accurately and effectively treat PPH during pregnancy. For example, a TXA dose of 600–650 mg within 1 h of childbirth was predicted to be sufficient to prevent PPH in a recent population pharmacodynamic analysis study [20,21]. A recent WHO recommendation for PPH treatment emphasized that 1 g TXA (intravenously over 10 min; 100 mg/min) should be administered in all cases of PPH as soon as possible within 3 h after onset [13,14].
Cardiac surgery
Owing to numerous results showing that TXA helps reduce bleeding and transfusions in cardiac surgery regardless of the presence or absence of extracorporeal circulation, guidelines have consistently recommended the use of TXA in cardiac surgery [22,23]. Recent guidelines strongly recommend the use of TXA to reduce the amount of bleeding and blood transfusions (Class IA, Level A), and these advantages can be found even in off-pump coronary artery bypass graft (CABG) surgery (Class, IIA, Level B–R) [24]. TXA has also been reported to be helpful in patients undergoing deep hypothermic circulatory arrest, which can promote fibrinolysis [25].
The use of TXA has rapidly increased since the withdrawal of aprotinin by the Food and Drug Administration in 2008. However, reports of serious complications, especially adverse neurological events such as seizures or thrombosis, have also increased [1,26]. The International Society for Minimally Invasive Cardiothoracic Surgery recommends that the total amount of TXA not exceed 100 mg/kg in patients aged ≥ 50 years who undergo on-pump cardiac surgery to avoid potential neurotoxic complications [27]. Several clinical trials have been conducted to evaluate the effectiveness of TXA and the risk of complications (Table 2). The Aspirin and Tranexamic Acid for Coronary Artery Surgery (ATACAS) trial addressed the potential risk for thrombosis associated with TXA [28,29]. This trial confirmed that TXA could reduce the incidence of blood transfusions or reoperation due to major hemorrhage or cardiac tamponade in CABG surgery (1.4% vs. 2.8% for the placebo; P = 0.001) without an increase in the 30-day mortality rate or complications due to thrombosis (myocardial infarction, cerebral infarction, pulmonary embolism, renal failure, intestinal embolism, etc.) [28]. Additionally, no differences in major adverse cardiovascular events were found at the 1-year follow-up [29]. Interestingly, the dose of TXA was reduced by half, from the initial 100 mg/kg to 50 mg/kg owing to the risk of seizures during the ATACAS trial. The incidence of seizures was significantly higher in all patients, regardless of whether 50 mg/kg or 100 mg/kg TXA was used (0.7% vs. 0.1% for the placebo; P = 0.002) [28]. However, the risk of seizures seemed to be dose-dependent, with a higher risk associated with higher doses. A meta-analysis conducted in 2019 showed that both low-dose TXA (< 50 mg/kg bolus only or ≤ 10 mg/kg bolus + 1 mg/kg/h infusion) and high-dose TXA (≥ 50 mg/kg bolus only or > 10 mg/kg bolus + 1 mg/kg/h infusion) resulted in a reduction in the amount of blood transfused [30]. However, high-dose TXA was associated with a 4.83-fold greater risk of seizures than low-dose TXA [30].
Table 2.
Clinical Trials on the Use of Tranexamic Acid in Cardiac Surgery
| Clinical trial | Subjects | Design | Notes |
|---|---|---|---|
| ATACAS [28] | CABG (n = 4,631) | -2-by-2 factorial design (aspirin or placebo and tranexamic acid or placebo) | -No significant differences in death or thrombotic complications (16.7% vs. placebo 18.1%; RR: 0.92, 95% CI [0.81, 1.05], P = 0.22) |
| -Death and thrombotic complications (nonfatal myocardial infarction, stroke, pulmonary embolism, renal failure, or bowel infarction) within 30 d of surgery | -Major hemorrhage or cardiac tamponade leading to reoperation (1.4% vs. 2.8% for the placebo, P = 0.001) | ||
| -Higher risk of seizures (0.7% and 0.1% for the placebo, P = 0.002) | |||
| OPTIMAL [34] | Cardiac surgery with cardiopulmonary bypass (n = 3,079) | -High-dose (n = 1,525): 30 mg/kg bolus + 16 mg/kg/h maintenance dose + 2 mg/kg prime | -Significant reduction in transfusion (high-dose 21.8% vs. low-dose 26.0%; RR: 0.84, 1-sided 97.55% CI [−∞, 0.96], P = 0.004) |
| Aged ≤ 70 yr; mean age 53 yr | -Low-dose (n = 1,506): 10 mg/kg bolus + 2 mg/kg/h maintenance dose + 1 mg/kg prime | -Met criteria for noninferiority: complications included seizures, thrombotic events, kidney dysfunction, 30-d mortality (high-dose 17.6% vs. low-dose 16.8%; 1-sided 97.55% CI [−∞,3.9%], P = 0.03 for noninferiority) |
ATACAS: Aspirin and Tranexamic Acid for Coronary Artery Surgery trial, OPTIMAL: Outcome Impact of Different Tranexamic Acid Regimens in Cardiac Surgery with Cardiopulmonary Bypass trial, CABG: coronary artery bypass graft, RR: relative risk.
Therefore, the doses of TXA currently recommended for cardiac surgery are decreasing. Moreover, this is supported by a population-based pharmacokinetic analysis in patients with CKD, which showed that with a lower dose of TXA as 25–30 mg/kg bolus, the plasma concentration reaches high enough levels for 100% inhibition of fibrinolysis [31]. Because of the possible risk of complications due to elevated plasma concentrations of TXA in patients with CKD, the dose must be optimized in this patient population [32,33]. Recent studies have suggested that high-dose TXA (30 mg/kg bolus + 16 mg/kg/h infusion + 2 mg/kg priming) be used in patients with high-risk bleeding, and low-dose TXA (10 mg/kg bolus + 1–2 mg/kg/h infusion + 1 mg/kg priming) be used in patients with low-risk bleeding [33,34]. However, dosing strategies linked to the objective clinical effect of TXA at therapeutic concentrations and viscoelastography are needed [2].
Trauma
An imbalance in the physiological response to fibrinolysis, which can lead to hyper- or hypofibrinolysis, can occur in trauma patients [35–37]. Major blood loss due to fibrinolysis-related coagulation dysfunction is a cause of increased mortality [38]. This is a significant controllable cause of death after trauma that can be assessed quickly and accurately using recent developments in viscoelastic coagulation assays [38]. When evaluating trauma patients using rotational thromboelastometry, 60% of patients showed hyperfibrinolysis, and these patients had a significantly higher 28-day mortality rate than normal patients (12% vs. 1% of controls; P < 0.001) [39]. Laboratory study has shown that TXA inhibits this type of hyperfibrinolysis [40].
Several large-scale clinical trials have been conducted to evaluate the effects of TXA in patients with trauma (Table 3). The Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage (CRASH-2) trial showed that TXA reduced mortality in trauma patients, particularly mortality due to hemorrhage (4.9% vs. 5.7% for the placebo) [41]. The best results were seen when TXA was administered within 3 h and if possible, within 1 h of trauma [41]. However, most subjects in the CRASH-2 trial did not receive treatment based on the latest management guidelines for trauma patients, and less than 2% of the subjects received rapid blood transfusions or specialized intensive care or underwent damage-control surgery or angiography. Therefore, further research is needed to determine the effects of TXA on trauma patients in specialized trauma management. The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) study showed that TXA does not affect the long-term survival rate of severely injured trauma patients [42]. Although the use of TXA during hospitalization in patients with hyperfibrinolysis confirmed by thromboelastography improved the 6-h survival rate, long-term follow-up in terms of the survival rate and reduction in 24-h transfusion requirements showed no benefit. Moreover, complications, such as acute renal failure or multi-organ failure, have increased despite the absence of coagulation-related complications. Recent studies have also cast doubt on the effectiveness of TXA in trauma patients given the lack of support for long-term survival benefits after early administration (before arrival at the hospital) [43,44].
Table 3.
Clinical Trials on the Use of Tranexamic Acid in Trauma Patients
| Clinical trial | Subjects | Design | Notes |
|---|---|---|---|
| CRASH-2 [41] | -274 hospitals in 40 countries | TXA (loading dose 1 g over 10 min, then infusion of 1 g over 8 h) vs. placebo | -Reduced mortality (14.5% vs. 16.0% for the placebo; RR: 0.91, 95% CI [0.85, 0.97], P = 0.004) |
| -Adult trauma patients with or at risk of significant bleeding (n = 20,211) | -Death from hemorrhage (4.9% vs. 5.7% for the placebo; RR: 0.85, 95% CI [0.76, 0.96], P = 0.008) | ||
| -The best results were found when administered within 3 h of trauma and, if possible, within 1 h | |||
| PROPPR [42] | Hyperfibrinolysis (LY30 >3%) (n = 93) | TXA (n = 31) vs. no-TXA (n = 62); propensity score matching (1:2) for demographics, admission vitals, and injury severity | -Lower 6-h mortality rate (34% vs. 13% for the placebo, P = 0.04) and greater volume of 24-h transfusions (15 vs. 10 units for the placebo, P = 0.03) |
| -No difference in the 12-h (P = 0.24), 24-h (P = 0.25), or 30-d mortality (P = 0.82). | |||
| -No difference in the 24-h transfusion of RBC (P = 0.11) or platelets (P = 0.13), time to achieve hemostasis (P = 0.65), rebleeding requiring intervention (P = 0.13), or thrombotic complications (P = 0.98). |
CRASH-3: trial of the effects of tranexamic acid on death and disability in patients with TBI (traumatic brain injury), PROPPR: Pragmatic, Randomized Optimal Platelet and Plasma Ratios, TXA: tranexamic acid, RR: relative risk, RBC: red blood cell.
Therefore, adjusting the dose according to fibrinolysis status using repeated viscoelastic coagulation assays is necessary for the appropriate use of TXA in trauma patients [2]. Since there are currently no pharmacokinetic data on TXA in trauma patients, it is recommended that 1 g TXA be administered as a loading dose, followed by a continuous infusion over 4–8 h according to the presence or absence of hyperfibrinolysis by viscoelastic testing [2]. A study on the effects of TXA in the context of an advanced trauma patient management system (prehospital antifibrinolytics for traumatic coagulopathy and hemorrhage; PATCH) is currently being conducted [45].
Neurosurgery
TXA can be administered to reduce bleeding in patients undergoing neurosurgery, as these patients are at a high risk of mortality even with a small amount of bleeding. TXA has been reported to reduce blood loss and transfusion requirements without side effects related to thrombosis in brain tumor and complex skull base surgeries [46,47]. Many studies have also reported that TXA reduces the incidence of fatal bleeding and rebleeding after surgery for cerebral aneurysms or subarachnoid hemorrhages by up to 40% [48,49].
However, given the increased risk of cerebral infarction, long-term benefits may be minimal [49]. Clinical trials on TXA in neurosurgery have shown disappointing results (Table 4). According to the Tranexamic Acid for Hyperacute Primary Intracerebral Hemorrhage (TICH-2) study, the use of TXA had no significant effect on the 90-day long-term survival rate or functional improvement in patients with cerebral hemorrhage [50]. Therefore, despite the benefit of reducing bleeding in patients undergoing brain surgery, the use of TXA in neurosurgery is associated with the potential risks of thrombosis and cerebral infarction; thus, the routine use of TXA is not recommended in patients with subarachnoid hemorrhage [51]. The use of TXA in patients with traumatic brain injury has also shown no long-term benefits due to the continuous changes in fibrinolysis after trauma. The CRASH-3 study, which investigated the effects of TXA on death and disability in patients with traumatic brain injury, showed an increase in the short-term survival rate in patients with mild traumatic brain injury but showed no benefits in terms of long-term functional recovery [52]. A recent study also reported that early TXA administration (before arrival at the hospital) in patients with brain damage had no effect on the long-term survival rate or functional recovery [53].
Table 4.
Clinical Trials on the Use of Tranexamic Acid in Neurosurgery
| Clinical trial | Subject | Design | Notes |
|---|---|---|---|
| TICH-2 [50] | -Intracerebral hemorrhage from acute stroke (n = 2,325) | -International, randomized, placebo-controlled trial | -No difference in functional status (aOR: 0.88, 95% CI [0.76, 1.03], P = 0.11) or fatality (22% vs. 21% for the placebo; adjusted hazard ratio: 0.92, 95% CI [0.77, 1.10], P = 0.37) at 90 d |
| -TXA (n = 1,161, loading dose 1 g over 10 min then infusion of 1 g over 8 h) vs. placebo (n = 1,164) within 8 h of symptom onset | -Fewer deaths by day 7 (9% vs. 11% for the placebo; aOR: 0.73, 95% CI [0.53, 0.99], P = 0.041) and serious adverse events | ||
| CRASH-3 [52] | -175 hospitals in 29 countries | -International, multi-center, randomized, placebo-controlled trial | -Patients with a mild-to-moderate head injury |
| -Adults with TBI (within 3 h of injury, GCS score of 12 or lower, or any ICH on CT scan) and no major extracranial bleeding (n = 12,737) | -TXA (n = 6,406, loading dose 1 g over 10 min) vs. placebo (n = 6,331) within 3 h of injury | : Reduced death (RR: 0.78, 95% CI [0.64, 0.95]) | |
| : Early treatment was more effective (P = 0.005) | |||
| -Patients with a severe head injury | |||
| : No difference in death (RR: 0.99, 95% CI [0.91, 1.07]; P = 0.030) without obvious effect on treatment time | |||
| -No difference in the risk of vascular occlusive events (RR: 0.98, 95% CI [0.74, 1.28]) | |||
| -Similar risk of seizures (RR: 1.09, 95% CI [0.90, 1.33]) |
TICH-2: Tranexamic acid for hyperacute primary IntraCerebral Hemorrhage study, CRASH-3: trial of the effects of tranexamic acid on death and disability in patients with TBI (traumatic brain injury), GCS: Glasgow Coma Scale, ICH: intracranial bleeding, CT: computed tomography, TXA: tranexamic acid, aOR: adjusted odd ratio, RR: relative risk.
Although no standardized method of TXA application in neurosurgery currently exists, the immediate bolus administration of 1 g TXA after diagnosis of a cerebral aneurysm followed by the intermittent administration of 1 g every 6 h until the completion of surgery or coiling procedure or until 72 h, is recommended [54].
Orthopedic surgery
TXA has been found to effectively reduce bleeding and transfusion volume in orthopedic surgeries [55–57]. According to the retrospective cohort study of five Canadian hospitals, the most common surgical use of TXA was orthopedic surgery (41%), particularly for pelvic and hip surgeries, except for cardiac surgery [56]. According to the clinical practice guidelines for total joint arthroplasty [58,59], TXA is actively recommended for hip or knee joint replacement surgery because it can effectively reduce bleeding and transfusion requirements, regardless of the administration route (i.e., intravenous, oral, topical application, etc.), without the risk of side effects due to thrombosis (Table 5). In particular, 1 g TXA administered intravenously is the usual dose for arthroplasty (median dose), although all of the doses evaluated in clinical trials showed promising results [58,59]. Administering TXA before skin incision is most effective at reducing bleeding, but additional administrations during the surgery showed no benefit [58,59]. The use of TXA in hip fractures also reduces the amount of blood loss (mean difference: –273 ml, 95% CI [–353, –193], P < 0.001) and transfusion volume (RR: 0.66, 95% CI [0.56, 0.78], P < 0.003), without increased risk of thrombosis (risk ratio: 1.38, 95% CI [0.74, 2.55], P = 0.31) [57].
Table 5.
Summary of the Clinical Practice Guidelines for TXA Use for Orthopedic Surgery to Reduce the Risk of Transfusion and/or Blood Loss [59]
| Evidence | Purpose | Recommendation |
|---|---|---|
| Strongly recommended | Route | Intravenous, topical, oral, or combinations; all methods are effective |
| Dose | The dose of tranexamic acid does not significantly affect the effectiveness | |
| Multiple doses | No difference | |
| Moderately recommended | Timing | Before incision |
| Risk | Does not increase the risk of arterial or VTE in patients with a history of VTE, myocardial infarction, cerebrovascular accident, transient ischemic attack, and/or vascular stent placement |
TXA: tranexamic acid, VTE: venous thromboembolism.
The optimal plasma concentration of TXA for orthopedic surgery is > 10 μg/ml [56,60,61]. TXA can be administered via various routes, but intravenous injection of a 10–20 mg/kg bolus or multiple divided doses is most commonly used [59,62]. One meta-analysis showed that combined intra-articular and intravenous injections were more effective than intra-articular injections alone [30].
Complications from TXA in orthopedics are rare. The risk of complications, such as venous thrombosis or myocardial infarction, has not been found to increase even when TXA is used during arthroplasty in patients with coronary artery disease or those who have had coronary stents > 8 years [63]. In addition, the TXA dose used in orthopedic surgery (10–20 mg/kg) has not been found to increase the risk of seizures [64]. However, caution is advised in patients with a high risk of complications due to thrombosis, such as those with a history of recent cerebral infarction, coronary stent insertion, and hypercoagulation, as no large-scale prospective study has been conducted to date.
Clinical application of tranexamic acid and its limitations
The indications and dosing strategies suggested by previous studies for TXA are summarized in Table 6 and Fig. 1. However, as noted in this review, the clinical application of TXA has limitations and potential risks that must be considered. First, TXA may not be effective in all populations. Effectiveness may depend on the timing of TXA administration to prevent the progression of bleeding or the development of coagulopathy, the dose of TXA required to achieve the optimal balance between antifibrinolytic and prothrombotic effects, the mechanism of bleeding, and the patients’ comorbidities. Second, as TXA is associated with an increased risk of seizures, caution is advised, particularly at high doses. Third, caution is advised when administering TXA to patients at risk of thrombotic complications. Fourth, as TXA is excreted by the kidneys, dose adjustments and caution are required in patients with renal dysfunction. Finally, data on the use of TXA in specific patient populations, such as those with renal or hepatic impairment, are lacking. Additionally, studies on potential drug interactions with, for instance, anticoagulants and antiplatelet agents are also lacking.
Table 6.
Clinical Application of Tranexamic Acid
| Applications | Indications | Dose | |
|---|---|---|---|
| Postpartum hemorrhage [12–16] | Vaginal birth: EBL > 500 ml | -1 g (100 mg/ml) intravenously over 10 min (1 ml/min) | |
| Cesarean section: EBL > 1,000 ml | -A second dose of 1 g can be administered if bleeding continues after 30 min or restarts within 24 h of the first dose | ||
| -All cases of postpartum hemorrhage | -As soon as possible after postpartum hemorrhage onset (but no later than 3 h from birth) | ||
| Cardiac surgery [32–34] | High risk of bleeding | Without renal insufficiency | -30 mg/kg bolus + 16 mg/kg/h infusion + 2 mg/kg priming |
| With renal insufficiency | Minor: 25–30 mg/kg bolus + 11–16 mg/kg/h infusion | ||
| Mild: 25–30 mg/kg bolus + 5–10 mg/kg/h infusion | |||
| Severe: 25–30 mg/kg bolus + 3–5 mg/kg/h infusion | |||
| Low risk of bleeding | Without renal insufficiency | 10 mg/kg bolus + 1 mg/kg/h infusion + 1 mg/kg priming | |
| With renal insufficiency | Minor: 10–15 mg/kg bolus + 3.75 mg/kg/h infusion | ||
| Mild: 10–15 mg/kg bolus + 2.50 mg/kg/h infusion | |||
| Severe: 10–15 mg/kg bolus + 1.25 mg/kg/h infusion | |||
| Trauma patients [41] | Trauma patients with or without a risk of hyperfibrinolysis | 1 g loading dose over 10 min | |
| Perform a viscoelastic test for 4–8 h | |||
| If hypofibrinolysis: discontinue | |||
| If hyperfibrinolysis: 1 g infusion over 4–8 h | |||
| Neurosurgery [51,54] | Intracranial hemorrhage [50] | Loading dose 1 g with or without an infusion of 1 g over 8 h | |
| Traumatic brain injury [52] | |||
| Tumor resection surgery [46] | 10–20 mg/kg over 20 min followed by 1–10 mg/kg/h infusion | ||
| Spine surgery [47] | |||
| Cerebral aneurysm | Caution for potential complication of thrombosis is necessary (ischemia) | ||
| Subarachnoid hemorrhage | |||
| Orthopedic surgery [58,59] | Hip fracture | Intravenous: 10 mg/kg for loading over 30 min before skin incision followed by 1 mg/kg/h for maintenance infusion | |
| Total knee arthroplasty | Optionally repeated 10–15 mg/kg (after 3 h or via infusion) | ||
| Spine surgeries | Topical with closure: 1–3 g diluted in 30–100 ml NS | ||
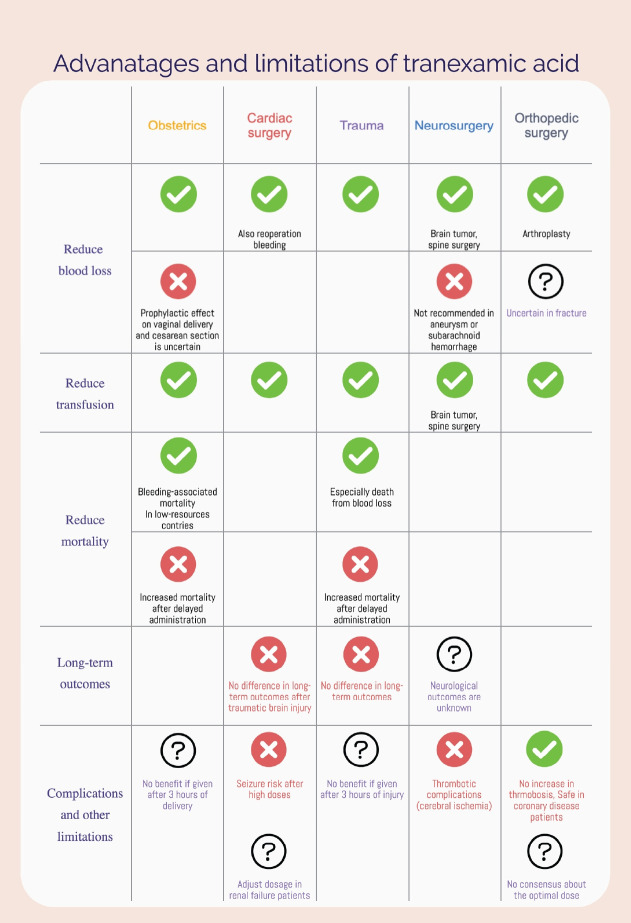
Fig. 1.
Advantages and limitations of tranexamic acid (TXA). Although TXA decreases the risk of bleeding and transfusion, the long-term outcomes of TXA administration remain uncertain. Due to its limitations and potentially hazardous risks, TXA should be used with caution and under medical supervision, and the benefits and risks should be weighed carefully for each patient.
Conclusion
TXA is an antifibrinolytic agent that has gradually been applied in various settings beyond its traditional use in menstrual bleeding or hemophilia. TXA has been actively used in obstetrics, trauma, and various surgeries. However, the long-term outcomes associated with TXA administration are uncertain, and limitations and potentially hazardous risks remain concerning. The beneficial effects and complications of TXA appear to be dose-dependent, with variable pharmacokinetics and pharmacodynamics depending on the route of administration, dose, timing, and patient characteristics. Therefore, TXA should be administered with caution under medical supervision. The benefits and risks of TXA should be carefully weighed for each patient according to the indications. The optimal dose and timing of TXA administration remain under investigation and may vary depending on the type and severity of bleeding. Further research is needed to select patients for whom the use of TXA is appropriate and to determine the optimal dosage in various clinical settings.
Footnotes
Funding
None.
Conflicts of Interest
Ki Tae Jung has been an editor for the Korean Journal of Anesthesiology since 2020. However, he was not involved in any process of review for this article, including peer reviewer selection, evaluation, or decision-making. There were no other potential conflicts of interest relevant to this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author Contributions
Dong Joon Kim (Resources; Writing – original draft; Writing – review & editing)
Su Yeon Cho (Resources; Writing – original draft; Writing – review & editing)
Ki Tae Jung (Conceptualization; Data curation; Supervision; Visualization; Writing – original draft; Writing – review & editing)
References
- 1.Tengborn L, Blomback M, Berntorp E. Tranexamic acid--an old drug still going strong and making a revival. Thromb Res. 2015;135:231–42. doi: 10.1016/j.thromres.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Patel PA, Wyrobek JA, Butwick AJ, Pivalizza EG, Hare GM, Mazer CD, et al. Update on applications and limitations of perioperative tranexamic acid. Anesth Analg. 2022;135:460–73. doi: 10.1213/ANE.0000000000006039. [DOI] [PubMed] [Google Scholar]
- 3.Ockerman A, Vanassche T, Garip M, Vandenbriele C, Engelen MM, Martens J, et al. Tranexamic acid for the prevention and treatment of bleeding in surgery, trauma and bleeding disorders: a narrative review. Thromb J. 2021;19:54. doi: 10.1186/s12959-021-00303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franchini M, Mannucci PM. The never ending success story of tranexamic acid in acquired bleeding. Haematologica. 2020;105:1201–5. doi: 10.3324/haematol.2020.250720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayeux J, Alwon K, Collins S, Hewer I. Tranexamic acid in anesthetic management of surgical procedures. AANA J. 2016;84:201–9. [PubMed] [Google Scholar]
- 6.Devereaux PJ, Marcucci M, Painter TW, Conen D, Lomivorotov V, Sessler DI, et al. Tranexamic acid in patients undergoing noncardiac surgery. N Engl J Med. 2022;386:1986–97. doi: 10.1056/NEJMoa2201171. [DOI] [PubMed] [Google Scholar]
- 7.Earnshaw C, Poole M. Tranexamic acid. Anaesth Tut Week. 2019;406:1–7. [Google Scholar]
- 8.Andersson L, Nilsoon IM, Colleen S, Granstrand B, Melander B. Role of urokinase and tissue activator in sustaining bleeding and the management thereof with EACA and AMCA. Ann N Y Acad Sci. 1968;146:642–58. doi: 10.1111/j.1749-6632.1968.tb20322.x. [DOI] [PubMed] [Google Scholar]
- 9.Andersson L, Nilsson IM, Nilehn JE, Hedner U, Granstrand B, Melander B. Experimental and clinical studies on AMCA, the antifibrinolytically active isomer of p-aminomethyl cyclohexane carboxylic acid. Scand J Haematol. 1965;2:230–47. doi: 10.1111/j.1600-0609.1965.tb01300.x. [DOI] [PubMed] [Google Scholar]
- 10.Nuttall GA, Gutierrez MC, Dewey JD, Johnson ME, Oyen LJ, Hanson AC, et al. A preliminary study of a new tranexamic acid dosing schedule for cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:230–5. doi: 10.1053/j.jvca.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Koster A, Faraoni D, Levy JH. Antifibrinolytic therapy for cardiac surgery: an update. Anesthesiology. 2015;123:214–21. doi: 10.1097/ALN.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 12.WOMAN Trial Collaborators Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–16. doi: 10.1016/S0140-6736(17)30638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. Updated WHO recommendation on tranexamic acid for the treatment of postpartum haemorrhage [Internet]. Geneva: WHO; 2017 Oct [cited 2023 Jul 7]. Available from https://apps.who.int/iris/bitstream/handle/10665/259379/WHO-RHR-17.21-eng.pdf. [PubMed]
- 14.Vogel JP, Oladapo OT, Dowswell T, Gulmezoglu AM. Updated WHO recommendation on intravenous tranexamic acid for the treatment of post-partum haemorrhage. Lancet Glob Health. 2018;6:e18–9. doi: 10.1016/S2214-109X(17)30428-X. [DOI] [PubMed] [Google Scholar]
- 15.Sentilhes L, Winer N, Azria E, Senat MV, Le Ray C, Vardon D, et al. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med. 2018;379:731–42. doi: 10.1056/NEJMoa1800942. [DOI] [PubMed] [Google Scholar]
- 16.Sentilhes L, Senat MV, Le Lous M, Winer N, Rozenberg P, Kayem G, et al. Tranexamic acid for the prevention of blood loss after cesarean delivery. N Engl J Med. 2021;384:1623–34. doi: 10.1056/NEJMoa2028788. [DOI] [PubMed] [Google Scholar]
- 17.Simonazzi G, Bisulli M, Saccone G, Moro E, Marshall A, Berghella V. Tranexamic acid for preventing postpartum blood loss after cesarean delivery: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2016;95:28–37. doi: 10.1111/aogs.12798. [DOI] [PubMed] [Google Scholar]
- 18.Bellos I, Pergialiotis V. Tranexamic acid for the prevention of postpartum hemorrhage in women undergoing cesarean delivery: an updated meta-analysis. Am J Obstet Gynecol. 2022;226:510–23. doi: 10.1016/j.ajog.2021.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Gillissen A, Henriquez DD, van den Akker T, Caram-Deelder C, Wind M, Zwart JJ, et al. The effect of tranexamic acid on blood loss and maternal outcome in the treatment of persistent postpartum hemorrhage: a nationwide retrospective cohort study. PLoS One. 2017;12:e0187555. doi: 10.1371/journal.pone.0187555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmadzia HK, Luban NL, Li S, Guo D, Miszta A, Gobburu JV, et al. Optimal use of intravenous tranexamic acid for hemorrhage prevention in pregnant women. Am J Obstet Gynecol. 2021;225:85. doi: 10.1016/j.ajog.2020.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Ahmadzia HK, Guo D, Dahmane E, Miszta A, Luban NL, et al. Population pharmacokinetics and pharmacodynamics of Tranexamic acid in women undergoing caesarean delivery. Br J Clin Pharmacol. 2021;87:3531–41. doi: 10.1111/bcp.14767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 23.Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Ferraris VA, Ferraris SP, Saha SP, Hessel EA, 2nd, Haan CK, Royston BD, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83(5 Suppl):S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 24.Tibi P, McClure RS, Huang J, Baker RA, Fitzgerald D, Mazer CD, et al. STS/SCA/AmSECT/SABM update to the clinical practice guidelines on patient blood management. J Extra Corpor Technol. 2021;53:97–124. doi: 10.1182/ject-2100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn KT, Yamanaka K, Iwakura A, Hirose K, Nakatsuka D, Kusuhara T, et al. Usefulness of intraoperative continuous infusion of tranexamic acid during emergency surgery for type A acute aortic dissection. Ann Thorac Cardiovasc Surg. 2015;21:66–71. doi: 10.5761/atcs.oa.13-00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koster A, Schirmer U. Re-evaluation of the role of antifibrinolytic therapy with lysine analogs during cardiac surgery in the post aprotinin era. Curr Opin Anaesthesiol. 2011;24:92–7. doi: 10.1097/ACO.0b013e32833ff3eb. [DOI] [PubMed] [Google Scholar]
- 27.Menkis AH, Martin J, Cheng DC, Fitzgerald DC, Freedman JJ, Gao C, et al. Drug, devices, technologies, and techniques for blood management in minimally invasive and conventional cardiothoracic surgery: a consensus statement from the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS) 2011. Innovations (Phila) 2012;7:229–41. doi: 10.1097/IMI.0b013e3182747699. [DOI] [PubMed] [Google Scholar]
- 28.Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376:136–48. doi: 10.1056/NEJMoa1606424. [DOI] [PubMed] [Google Scholar]
- 29.Myles PS, Smith JA, Kasza J, Silbert B, Jayarajah M, Painter T, et al. Tranexamic acid in coronary artery surgery: one-year results of the aspirin and tranexamic acid for coronary artery surgery (ATACAS) trial. J Thorac Cardiovasc Surg. 2019;157:644–52. doi: 10.1016/j.jtcvs.2018.09.113. [DOI] [PubMed] [Google Scholar]
- 30.Guo J, Gao X, Ma Y, Lv H, Hu W, Zhang S, et al. Different dose regimes and administration methods of tranexamic acid in cardiac surgery: a meta-analysis of randomized trials. BMC Anesthesiol. 2019;19:129. doi: 10.1186/s12871-019-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grassin-Delyle S, Tremey B, Abe E, Fischler M, Alvarez JC, Devillier P, et al. Population pharmacokinetics of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Br J Anaesth. 2013;111:916–24. doi: 10.1093/bja/aet255. [DOI] [PubMed] [Google Scholar]
- 32.Jerath A, Yang QJ, Pang KS, Looby N, Reyes-Garces N, Vasiljevic T, et al. Tranexamic acid dosing for cardiac surgical patients with chronic renal dysfunction: a new dosing regimen. Anesth Analg. 2018;127:1323–32. doi: 10.1213/ANE.0000000000002724. [DOI] [PubMed] [Google Scholar]
- 33.Hodgson S, Larvin JT, Dearman C. What dose of tranexamic acid is most effective and safe for adult patients undergoing cardiac surgery? Interact Cardiovasc Thorac Surg. 2015;21:384–8. doi: 10.1093/icvts/ivv134. [DOI] [PubMed] [Google Scholar]
- 34.Shi J, Zhou C, Pan W, Sun H, Liu S, Feng W, et al. Effect of high- vs low-dose tranexamic acid infusion on need for red blood cell transfusion and adverse events in patients undergoing cardiac surgery: the OPTIMAL randomized clinical trial. JAMA. 2022;328:336–47. doi: 10.1001/jama.2022.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossetto A, Vulliamy P, Lee KM, Brohi K, Davenport R. Temporal transitions in fibrinolysis after trauma: adverse outcome is principally related to late hypofibrinolysis. Anesthesiology. 2022;136:148–61. doi: 10.1097/ALN.0000000000004036. [DOI] [PubMed] [Google Scholar]
- 36.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg. 2016;222:347–55. doi: 10.1016/j.jamcollsurg.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coats TJ, Morsy M. Biological mechanisms and individual variation in fibrinolysis after major trauma. Emerg Med J. 2020;37:135–40. doi: 10.1136/emermed-2019-209181. [DOI] [PubMed] [Google Scholar]
- 38.Brill JB, Brenner M, Duchesne J, Roberts D, Ferrada P, Horer T, et al. The role of TEG and ROTEM in damage control resuscitation. Shock. 2021;56:52–61. doi: 10.1097/SHK.0000000000001686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11:307–14. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 40.Dirkmann D, Gorlinger K, Gisbertz C, Dusse F, Peters J. Factor XIII and tranexamic acid but not recombinant factor VIIa attenuate tissue plasminogen activator-induced hyperfibrinolysis in human whole blood. Anesth Analg. 2012;114:1182–8. doi: 10.1213/ANE.0b013e31823b6683. [DOI] [PubMed] [Google Scholar]
- 41.CRASH-2 trial collaborators. Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 42.Khan M, Jehan F, Bulger EM, OʼKeeffe T, Holcomb JB, Wade CE, et al. Severely injured trauma patients with admission hyperfibrinolysis: is there a role of tranexamic acid? Findings from the PROPPR trial. J Trauma Acute Care Surg. 2018;85:851–7. doi: 10.1097/TA.0000000000002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almuwallad A, Cole E, Ross J, Perkins Z, Davenport R. The impact of prehospital TXA on mortality among bleeding trauma patients: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2021;90:901–7. doi: 10.1097/TA.0000000000003120. [DOI] [PubMed] [Google Scholar]
- 44.Guyette FX, Brown JB, Zenati MS, Early-Young BJ, Adams PW, Eastridge BJ, et al. Tranexamic acid during prehospital transport in patients at risk for hemorrhage after injury: a double-blind, placebo-controlled, randomized clinical trial. JAMA Surg. 2020;156:11–20. doi: 10.1001/jamasurg.2020.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitra B, Bernard S, Gantner D, Burns B, Reade MC, Murray L, et al. Protocol for a multicentre prehospital randomised controlled trial investigating tranexamic acid in severe trauma: the PATCH-Trauma trial. BMJ Open. 2021;11:e046522. doi: 10.1136/bmjopen-2020-046522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hooda B, Chouhan RS, Rath GP, Bithal PK, Suri A, Lamsal R. Effect of tranexamic acid on intraoperative blood loss and transfusion requirements in patients undergoing excision of intracranial meningioma. J Clin Neurosci. 2017;41:132–8. doi: 10.1016/j.jocn.2017.02.053. [DOI] [PubMed] [Google Scholar]
- 47.Mebel D, Akagami R, Flexman AM. Use of tranexamic acid is associated with reduced blood product transfusion in complex skull base neurosurgical procedures: a retrospective cohort study. Anesth Analg. 2016;122:503–8. doi: 10.1213/ANE.0000000000001065. [DOI] [PubMed] [Google Scholar]
- 48.de Faria JL, da Silva Brito J, Costa E Silva LT, Kilesse CT, de Souza NB, Pereira CU, et al. Tranexamic acid in neurosurgery: a controversy indication-review. Neurosurg Rev. 2021;44:1287–98. doi: 10.1007/s10143-020-01324-0. [DOI] [PubMed] [Google Scholar]
- 49.Baharoglu MI, Germans MR, Rinkel GJ, Algra A, Vermeulen M, van Gijn J, et al. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2013;2013:CD001245. doi: 10.1002/14651858.CD001245.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391:2107–15. doi: 10.1016/S0140-6736(18)31033-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Relke N, Chornenki NL, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract Thromb Haemost. 2021;5:e12546. doi: 10.1002/rth2.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.CRASH-3 trial collaborators Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394:1713–23. doi: 10.1016/S0140-6736(19)32233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowell SE, Meier EN, McKnight B, Kannas D, May S, Sheehan K, et al. Effect of out-of-hospital tranexamic acid vs placebo on 6-month functional neurologic outcomes in patients with moderate or severe traumatic brain injury. JAMA. 2020;324:961–74. doi: 10.1001/jama.2020.8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hillman J, Fridriksson S, Nilsson O, Yu Z, Saveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg. 2002;97:771–8. doi: 10.3171/jns.2002.97.4.0771. [DOI] [PubMed] [Google Scholar]
- 55.Gandhi R, Evans HM, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184. doi: 10.1186/1756-0500-6-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Houston BL, Fergusson DA, Falk J, Krupka E, Perelman I, Breau RH, et al. Prophylactic tranexamic acid use in non-cardiac surgeries at high risk for transfusion. Transfus Med. 2021;31:236–42. doi: 10.1111/tme.12780. [DOI] [PubMed] [Google Scholar]
- 57.Qi YM, Wang HP, Li YJ, Ma BB, Xie T, Wang C, et al. The efficacy and safety of intravenous tranexamic acid in hip fracture surgery: a systematic review and meta-analysis. J Orthop Translat. 2019;19:1–11. doi: 10.1016/j.jot.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Y, Jiang C, Li Q. A systematic review and meta-analysis comparing combined intravenous and topical tranexamic acid with intravenous administration alone in THA. PLoS One. 2017;12:e0186174. doi: 10.1371/journal.pone.0186174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Bini SA, Clarke HD, et al. Tranexamic acid in total joint arthroplasty: the endorsed clinical practice guides of the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. Reg Anesth Pain Med. 2019;44:7–11. doi: 10.1136/rapm-2018-000024. [DOI] [PubMed] [Google Scholar]
- 60.Pilbrant A, Schannong M, Vessman J. Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol. 1981;20:65–72. doi: 10.1007/BF00554669. [DOI] [PubMed] [Google Scholar]
- 61.Picetti R, Shakur-Still H, Medcalf RL, Standing JF, Roberts I. What concentration of tranexamic acid is needed to inhibit fibrinolysis? A systematic review of pharmacodynamics studies. Blood Coagul Fibrinolysis. 2019;30:1–10. doi: 10.1097/MBC.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavenski K, Ward SE, Hare GM, Freedman J, Pulendrarajah R, Pirani RA, et al. A rationale for universal tranexamic acid in major joint arthroplasty: overall efficacy and impact of risk factors for transfusion. Transfusion. 2019;59:207–16. doi: 10.1111/trf.14995. [DOI] [PubMed] [Google Scholar]
- 63.Zak SG, Tang A, Sharan M, Waren D, Rozell JC, Schwarzkopf R. Tranexamic acid is safe in patients with a history of coronary artery disease undergoing total joint arthroplasty. J Bone Joint Surg Am. 2021;103:900–4. doi: 10.2106/JBJS.20.01226. [DOI] [PubMed] [Google Scholar]
- 64.Kirksey MA, Wilson LA, Fiasconaro M, Poeran J, Liu J, Memtsoudis SG. Tranexamic acid administration during total joint arthroplasty surgery is not associated with an increased risk of perioperative seizures: a national database analysis. Reg Anesth Pain Med. 2020;45:505–8. doi: 10.1136/rapm-2020-101301. [DOI] [PubMed] [Google Scholar]