Abstract
A 20-year-old man was presented with ulcerative gastritis and duodenitis complicated by pyloric stenosis. Helicobacter pylori infection was excluded, and the lesions did not respond to treatment with proton pump inhibitors. No other parts of the intestinal tract showed signs of inflammation. Histopathological review showed signs of chronic inflammation with granuloma formation. A tentative diagnosis of isolated upper gastrointestinal (UGI) Crohn’s disease was performed. However, additional work-up revealed significantly positive IgG4 staining as well as elevated IgG4 serum levels. Since granulomatous disease is unlikely in IgG4-related disease, an eventual diagnosis of overlapping IgG4-related disease and Crohn’s disease (CD) was performed. Treatment with systemic steroids and anti-TNF in combination with azathioprine led to rapid symptomatic improvement. In this article, we review the available literature on IgG4-related gastroduodenitis, granulomatous gastritis, and upper GI CD. We suggest the possibility that IgG4-infiltration may be a marker of severely active inflammatory bowel disease rather than a separate disease entity.
Keywords: granulomatous disease, IgG4-related disease, Crohn’s disease, gastritis, anti-TNF, upper GI tract
Introduction
Gastroduodenal inflammation is a common finding when performing esophagogastroduodenoscopy (EGD). In many patients, an obvious cause, such as Helicobacter pylori (HP) or non-steroidal anti-inflammatory drug (NSAID)-associated peptic ulcer disease, can be identified (1). First-line treatment consists of proton pump inhibitors (PPIs) or HP eradication (2). However, some patients do not respond, and a broad range of more rare disease entities need to be explored. We report a case of treatment-refractory ulcerative gastritis and duodenitis complicated by pyloric stenosis, in which we evaluated a broad differential diagnosis based on the clinical course and pathological findings.
Case report
A 20-year-old man with a previously unremarkable medical history presented to the outpatient clinic with epigastric pain and 10 kg weight loss over the past 6 months. He also reported early satiety during meals, nausea without vomiting, and looser stools for a couple of months. The patient was an active smoker and had regular alcohol consumption, but denied illegal substance use. He worked as a logistic assistant in the shipping industry. He did not recently use NSAIDs or any maintenance medical treatment. Familial history included colorectal cancer (paternal grandfather, at the age of 60) and a perianal fistula (father). Vital parameters were normal, and physical examination showed no abdominal abnormalities. No pathological lymph nodes were palpable.
Initial laboratory testing showed no anemia. White blood cell count was within normal range and there was mild thrombocytosis (394,000 10E3/μL). The serum electrolytes were within normal limits, except for mild hypomagnesemia (0.61 mmol/L, 0.70–1.05 mmol/L). There was mild elevation of aspartate aminotransferase (AST) (84 U/L; reference <37 U/L) and alanine aminotransferase (ALT) (92 U/L; reference <40 U/L) with no alterations in other liver function tests. IgE titer was remarkably elevated (758 kU/L; reference 0–100 kU/L). Fecal calprotectin was slightly elevated (130.9 mg/kg, reference <50 mg/kg).
A few months earlier, the patient had already undergone an EGD for his complaints at a different center. This revealed a bumpy and erosive appearance of the gastric mucosa and bulboduodenal ulcerations with stenosing effect, causing gastric outlet subobstruction. The biopsies showed chronic active, HP-negative gastritis and bulbitis, in the absence of PPI intake. There were no signs of malignancy, and no granulomas were observed. Pantoprazole 40 mg BID was started.
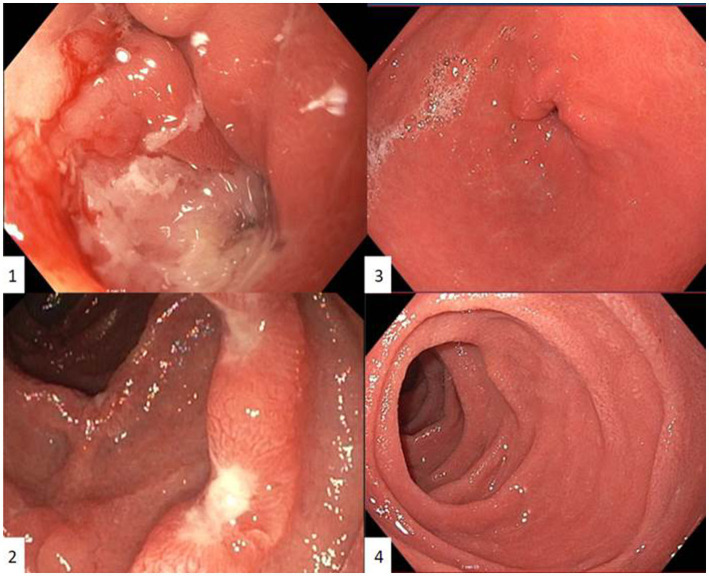
Control EGD after presentation at our center showed similar macroscopic findings despite PPI treatment: diffuse gastritis with gastric outlet stenosis due to large ulcers at the transition between pylorus and bulbus, which could only be passed with a nasogastric endoscope (diameter 5.4 millimeter) (Figure 1). Repeated extensive biopsy sampling confirmed persistent acute bulbitis and HP-negative, chronic active gastritis. Periodic acid-Schiff staining gave no arguments for Whipple’s disease.
Figure 1.
First esophagogastroduodenoscopy in our centre showing an ulcerative gastritis with gastric outlet stenosis due to large ulcers at the transition between pylorus and bulbus, which could only be passed with a nasogastric endoscope (diameter 5.4 millimeter) (1) and duodenal ulcerations with intermediate normal appearing mucosa (2). Control esophagogastroduodenoscopy six months after induction with Infliximab and azathioprine showing no residual inflammation in the antrum (3) or duodenum (4). Pyloric opening and transition between pylorus and bulbus could be easily passed with a gastric endoscope (diameter 9.9 millimeter).
An abdominal computed tomography (CT), followed by additional investigations including ileocolonoscopy with ileal and colonic biopsies and a magnetic resonance (MR) enterography, did not show other locations of intestinal inflammation. An additional video capsule endoscopy to screen for more distal small bowel inflammation could not be performed because the capsule could not be advanced beyond the pyloric stenosis despite endoscopic maneuvers.
Screening for Zollinger–Ellison (ZE) syndrome showed a mildly elevated serum gastrin level (339 ng/L) on PPI and 68Ga-DOTA-1-NaI3-octreotide (DOTANOC) positron emission tomography (PET)-CT showed no elevated somatostatin receptor expression. Endoscopic ultrasound (EUS) of the pancreas and MR enterography showed no arguments for a primary neuro-endocrine tumor. There were no histopathological arguments for autoimmune gastritis, and anti-intrinsic factor antibodies and anti-parietal cell antibodies were negative. Intestinal tuberculosis was excluded by a negative interferon-gamma release assay (i.e., QuantiFERON-TB) and normal X-ray of the thorax. Serum calcium and serum angiotensin-converting enzyme (ACE) levels were within normal limits, which made sarcoidosis less likely.
After this profound work-up, the patient was referred to the IBD unit of our hospital for further investigation. In order to establish a diagnosis, a repeated EGD with biopsies showed erosions in the stomach with the known stenosis of the pylorus, a large ulcer at the transition from the pylorus to the bulbus and multiple punched-out ulcerations in the duodenum. The gastric biopsies now showed a few small non-caseating granulomas, suggestive of Crohn’s disease (CD), since other causes of granulomatous gastritis (GG), i.e., sarcoidosis, malignancy, and infectious diseases such as tuberculosis or Whipple’s disease, had already been thoroughly ruled out (Figure 1). In addition, because of persistent ulcerative gastritis with stenosis, despite high doses of PPI, immunoglobulin G4 (IgG4) staining was performed to rule out IgG4-related disease, and the number of IgG4-positive plasmocytes was found to be significantly elevated on both gastric and duodenal biopsies. In the corpus of the stomach, the number of IgG4-positive plasma cells was highest, with more than 50 IgG4-positive plasma cells per high power field (HPF) and an IgG4/IgG ratio above 40%. An elevated IgG4 was seen in serum (194 mg/dL, reference values 8–140 mg/dL). Based on these clinical, serological, radiological, and histopathological findings, the presumptive diagnosis of an overlap of gastroduodenal CD and IgG4 disease was performed, as granulomas are unlikely in IgG4-related disease. Initially, systemic steroids were refused by the patient. Budesonide (9 milligram/day) was started without any effect on the complaints. Consequentially, a methylprednisolone 32 mg/day tapering course was started, and because of the suspected upper gastrointestinal (UGI) CD, anti-tumor-necrosis factor alpha (anti-TNFα) treatment with adalimumab was initiated early in the disease course. This approach led to rapid symptomatic improvement.
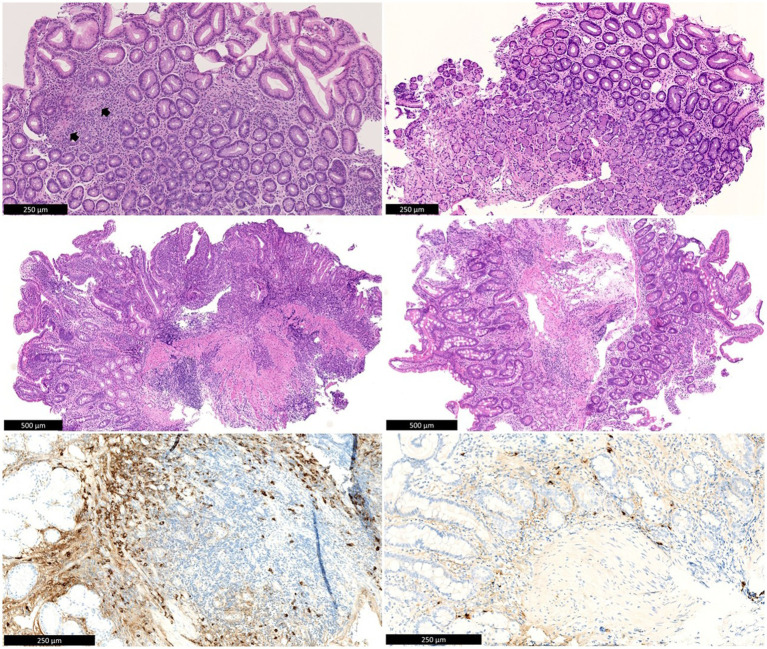
Figure 1.
Top left: Antral mucosa with chronic inactive gastritis and two non-necrotic granulomas (arrows). Middle left: Duodenal bulb mucosa with active chronic inflammation with erosion. Below left: IgG4 immunohistochemistry shows numerous IgG4-positive plasma cells in the duodenal mucosa (>100/HPF). There is background staining in the stroma, as is often seen with high serum IgG4 levels. Top right: Antral mucosa after treatment, without significant inflammation. Middle right: Duodenal mucosa after treatment, without significant inflammation. Below left: IgG4 immunohistochemistry shows rare residual IgG4-positive plasma cells in the duodenal mucosa. There is also much weaker background staining in the stroma compared to before treatment.
A control EGD (performed when the patient was still under CS) confirmed a clear endoscopic response with resolution of large ulcerations but still persistent diffuse gastroduodenitis with erosions. Because of the positive IgG4 staining, after a review of the literature, it was decided to switch adalimumab to infliximab in combination with azathioprine. After induction with Infliximab, EGD now showed an increased response, with only some residual erythema in the antrum and now a normal pyloric opening that could be passed with a gastric endoscope (diameter 9.9 millimeter). No abnormalities were visualized in the duodenal bulbus. However, active chronic inflammation with increased IgG4-positive plasma cells (>100 IgG4-positive plasma cells/HPF) remained present in the duodenal biopsies. Gastric biopsies showed inactive chronic gastritis with up to 14 IgG4-positive plasma cells/HPF. Control EGD 6 months after induction showed no macroscopic abnormalities, with only mild chronic and inactive antritis in the biopsies without granulomas (Picture 1). IgG4-plasmocyte count was also normalized (only 7/HPF in the duodenum and 2/HPF in the gastric biopsies).
Review of literature
In this case report, we suggest an overlap between gastric CD and IgG4-related disease (IgG4-RD). A review of literature was performed below to summarize the diagnosis and treatment of both entities.
Granulomatous gastritis
GG is rare, with a prevalence between 0.08 and 0.35%. Histopathological evaluation reveals non-necrotizing epithelioid cell granulomas. GG is commonly classified as an uncommon inflammation pattern and not as a distinct diagnosis. Therefore, it is unclear whether GG should be called idiopathic or if extensive work-up is needed to rule out underlying causes (3). For example, GG has been linked to CD, sarcoidosis, foreign bodies, neoplasms, and vasculitis. Furthermore, multiple infectious diseases are demonstrated in GG, i.e., aspergillosis, tuberculosis, parasites, histoplasmosis, and Whipple disease. CD, sarcoidosis, and tuberculosis are most prevalent (4).
Liang et al. investigated more than 142,000 gastric biopsies. GG was confirmed in 0.19%. There was no correlation between HP and GG, with a significantly higher amount of HP in the non-GG biopsies (p < 0.001) (4). On the contrary, other studies suggest HP infection as a possible causal factor for GG pathogenesis, in which eradication can lead to granuloma resolution (5, 6). Liang et al. (4) identified an isolated GG in 32% of the cases, of which 59.3% eventually were diagnosed with CD. GG-associated CD was more prevalent in male and young patients. Furthermore, single, small, or antral granulomas are more likely in CD (4). Shapiro et al. (3) suggest also taking into account the background inflammatory pattern of the gastric mucosa to help categorize GG.
Upper gastrointestinal (UGI) Crohn’s disease
CD is a chronic inflammatory bowel disease characterized by a segmental and transmural involvement of the bowel wall that can affect any part of the intestinal tract. Crohn’s gastritis is commonly associated with duodenitis, is referred to as “gastroduodenal CD,” and is defined according to Montreal’s Classification as L4 (7). Gastroduodenal CD is reported in 0.5 to 4.0% of all CD cases (8–11). Isolated gastric involvement is rare, affecting less than 0.07% of all CD cases (12). Almost 60% of patients with gastroduodenal CD have had previous inflammation elsewhere in the GI tract, and one-third of patients with isolated upper GI CD (UGI-CD) at diagnosis will develop distal disease later in life (8, 10, 13). UGI-CD is most frequently diagnosed in the fourth decade of life, although some reports show that the disease is more common in children than in adults (8, 9, 13). A cross-sectional study has demonstrated that proximal CD affects younger and non-smoking patients. In these patients, concomitant ileal disease and stenosing behavior are more present and are associated with a higher probability of undergoing abdominal surgery. Upper GI CD can also be associated with colonic inflammation, although less frequently (14).
Diagnosis
UGI-CD is diagnosed based on a combination of clinical presentation, biochemical signs of inflammation, and endoscopic evaluation with biopsy and histopathological evaluation (12, 15). Nugent and Roy grouped these investigations and formulated diagnostic criteria for gastroduodenal CD: (1) demonstration of non-caseating granulomatous inflammation in the stomach or duodenum in the absence of other systemic granulomatous disorders, with or without more distal intestinal inflammation; (2) endoscopic or radiographic findings of diffuse inflammation in the stomach or duodenum consistent with CD in a patient with confirmed CD of the GI tract (11).
Endoscopic evaluation is indicated in every CD patient with upper GI symptoms according to international guidelines by the European Crohn’s and Colitis Organization (ECCO) and the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) (16). However, due to the potential asymptomatic presentation, upper GI disease might be missed without routine endoscopic evaluation. For example, Annunziata et al. reported upper CD involvement in 16% of the patients, of whom 63% were asymptomatic (17). Routine EGD identifies mild macroscopic inflammation in 30 to 64% of CD patients and microscopic inflammation in up to 70% of patients (9).
UGI-CD is most frequently localized at the antrum and proximal duodenum. The proximal stomach is usually not affected (10, 18). Endoscopic lesions are not specific and are similar to CD lesions in the more distal parts of the GI tract. These include mucosal edema, longitudinal or irregular erosions, and ulcers that may be deep and punched out. Also, nodularity of the mucosa, cobblestone or bamboo-joint-like appearance, stenoses and fistulae are described (10, 16, 19).
Histologic features of CD include patchy or focal chronic inflammation in combination with focal crypt architectural abnormalities, preservation of mucin at active sites and the presence of non-caseating granulomas (20). None of these findings are pathognomonic, and biopsy findings are often non-specific in upper GI CD (8). The main histological findings described in the CD of the stomach and duodenum are acute and chronic inflammation, focal inflammatory changes, lymphoid aggregates, mucosal-muscular fibrosis, chronic HP-negative gastritis, focal gastritis, epithelioid granulomas, and duodenitis with or without granulomas. Because of the focal/patchy distribution of the disease, biopsies should be taken from macroscopically normal areas as well as affected areas in the stomach. Prevalence of non-caseous granuloma varies according to different publications, and percentages between 0 and 83% of the cases are reported. The finding of granuloma is not a definitive criterion of CD (8, 19, 21). Exclusion of other causes of granulomatous lesions is therefore necessary (8, 16).
The main differential diagnoses of UGI-CD are peptic ulcer disease, gastrinoma, ZE syndrome, Ménétrier disease, lymphoma, tuberculosis, sarcoidosis, gastric syphilis, plastic lymphoma, amyloidosis, and collagen diseases (8, 22). Intestinal tuberculosis should be ruled out, particularly in endemic regions. Gastric and duodenal tuberculosis are rare (0.4–2% and 2–2.5%, respectively) and are usually associated with pulmonary tuberculosis or immunodeficiencies (10). In Ménétrier’s disease, the entire stomach can be involved and ulcerations do occur. However, Ménétrier disease does not cause transmural damage. Last but not least, malignant and infiltrative processes need to be ruled out by the histological findings (8).
Treatment
The first line of treatment consists of PPIs with or without steroids (8). PPIs are only a complementary treatment as they do not affect chronic inflammation (10). Further, the medical treatment for UGI-CD does not differ substantially from the rest of the CD locations. Thiopurines can be used as a maintenance treatment (8).
Additionally, in the era of biological treatment, treatment with anti-TNF has shown good results. In the ACCENT I trial, 56% of patients with gastroduodenal CD responded to infliximab within 2 weeks of therapy, similar to the response observed in other locations of intestinal CD inflammation (23). Adalimumab is a valid treatment option as well, with a satisfactory response, according to several case reports (17, 24).
Short, secondary, gastroduodenal strictures can be treated with endoscopic balloon dilation, intestinal resection, or strictureplasty on a case-to-case basis (25). Gastric outlet obstruction, fistula, upper GI hemorrhage, and abscess formation are indications for surgical treatment (8). The most common indication for surgery is small bowel obstruction, requiring surgery in up to 91% of patients. Options for surgical management of complicated duodenal CD include bypass, stricturoplasty, or resection. However, resectional surgery has become exceptional due to excessive morbidity (26, 27).
IgG4-related upper GI disease
IgG4-RD was described for the first time in the early 2000s and is characterized by fibrotic lesions in multiple organs such as salivary glands, lacrimal glands, pancreas, or retroperitoneum (28, 29). It can mimic many inflammatory, infectious, and malignant diseases (30). Upper GI IgG4-RD is rare, but exact numbers of prevalence are lacking. It is unclear whether the risk of gastric malignancy is increased in gastric IgG4-RD. In a Japanese observational study, two out of eight patients with gastric IgG4-RD had a concurrent malignancy, but the study size was too small to conclude (31).
Diagnosis
Diagnosing upper GI IgG4-RD is difficult, as it can mimic peptic ulcers, gastrointestinal stromal tumors (GISTs), submucosal tumors, and malignancy. Recently, even a case report of gastric IgG4-RD presenting as a collagenous gastritis was published (32). This diagnostic difficulty may delay adequate treatment and pose a risk of unnecessary treatment and even resection. This is illustrated by a systematic review by Sawada et al., showing that the initial diagnosis was mistaken as gastric cancer, submucosal tumor, GIST, or peptic ulcer disease in >50% of the patients, and resection occurred in 47.6% of the gastric IgG4-RD. CT abdomen showed a submucosal tumor and diffuse or focal wall thickening in most of the patients. Serum IgG4 levels were only available in half of the patients, with a median of 430 mg/dL (33).
For the diagnosis of IgG4-RD, a cutoff of serum IgG4 > 135 mg/dL is in generally widely accepted. A retrospective study of Masaki et al. in 132 patients showed a sensitivity and specificity of 97.0 and 79.6%, respectively, for this cutoff. Serum IgG4/IgG ratios above 8% have a sensitivity of 95.5% and a specificity of 87.5% for IgG4-RD (34).
The histopathological diagnosis of upper GI IgG4-RD is still a matter of debate. Uchino et al. used >10 IgG4-positive plasma cells per HPF and an IgG4/IgG-positive ratio > 40% as a cutoff for IgG4-high cases, based on 2020 revised comprehensive diagnostic criteria for IgG4-RD (29, 35). Importantly, there are organ-specific criteria for IgG4-RD, but these are not established for upper GI IgG4-RD. The ratio of IgG4/IgG-positive cells is more important for diagnosis than the absolute numbers because, in rheumatoid arthritis, atopic dermatitis and ANCA-associated vasculitis infiltration by IgG4-positive cells can be observed (29, 36).
A consensus statement on the pathology of IgG4-related disease by Deshpande et al. has put forward three main pathological features for the diagnosis of IgG4-related disease: storiform fibrosis, lymphoplasmacytic infiltration, and obliterative phlebitis. Plasma cells and lymphocytes are polyclonal (37). Eosinophils are common whereas neutrophilic infiltration, necrosis, and granuloma are atypical. Although only seen in 29–42% of the patients, bottom-heavy lymphoplasmacytic mucosal infiltration or bottom-heavy plasmacytosis (BPH) is characteristic for IgG4-gastritis (31, 35). In patients with known IgG4-RD, gastric biopsies of 31 patients were analyzed by Uchino et al. and were compatible with IgG4-high cases, i.e., both IgG4/IgG4 ratio > 40% and > 10 IgG4-positive plasma cells/HPF, in 10 cases (out of 25 eligible biopsies). In six cases, BHP was identified in the IgG4-high cases. Only one patient, under treatment with corticosteroids, out of the IgG4-low group showed BPH. It is noted that the evaluation of BHP can be difficult due to the disoriented sectioning (35). Uchino et al. (35) also found that permeation of plasma cells between non-atrophic fundic glands and plasmocytic aggregation in the muscularis mucosae is useful for the diagnosis of GI IgG4-related disease, as it was only rarely observed in the control cases. Moreover, striated inflammation in the muscularis propria is proposed as a characteristic feature of IgG4-related gastritis, as shown in surgically resected specimens (31).
Treatment of IgG4-related disease in general
No randomized clinical trials are available on the treatment of IgG4-RD, and certainly not in the field of upper GI Ig4-RD.
In general, corticosteroids are used as first-line treatment (38). A Japanese consensus statement paper for the treatment of autoimmune IgG4-related pancreatitis suggested a dose of 0.6 milligrams per kilogram (mg/kg) prednisolone per day for 2 to 4 weeks for the treatment of pancreatitis, followed by tapering over 3 to 6 months to a maintenance dose of 2.5–5 mg/day up to 3 years (39). Approximately 2 weeks after the start of corticosteroids, a follow-up serological assessment should be performed to objectify the decline in serum IgG4 (30).
Disease flare-ups are common despite treatment with glucocorticoids. Several publications have reported on the use of methotrexate, mycophenolate mofetil, and azathioprine as potential maintenance treatment options (38). In patients with refractory or recurrent disease, rituximab can be considered (38). Anti-TNF treatment has been described in two non-IBD patients with IgG4-RD with a good response (i.e., infliximab in IgG4-related orbital disease and adalimumab in IgG4-related colitis) (40, 41).
Link between IgG4-RD and IBD
The role of IgG4 levels in patients with underlying IBD remains unclear. Published results are heterogenous when it comes to described associations between IgG4 and IBD, as shown in Table 1 (42–51). No research on upper GI IgG4-RD is available. In some patients with UC, IgG4 infiltration was associated with a worse outcome (44, 46). Whether IgG4 infiltration is a marker for aggressive IBD rather than a separate disease entity in these patients is still unknown. To date, a possible correlation is mainly suggested in patients with UC. Although little data are available to conclude.
Table 1.
Studies reporting on associations between serum/mucosal IgG4 and IBD.
| Author, year | Study design | Serum or mucosal IgG4 levels | IBD pts (UC/CD) | Control group | Findings |
|---|---|---|---|---|---|
| Koutroumpakis et al. (42) | Observational, monocentric study | Serum IgG4 (Ref 9–89 mg/dL) | N: 1193 (788 CD, 405 UC) | / |
|
| Faria et al. (43) | Cross-sectional study | Serum IgG4 > 140 mg/dL; Colon tissue ≥10 IgG4+ plasma cells per field | N: 56 (26 CD, 30 UC) | / |
|
| Wang et al. (44) | Case–control study | Serum IgG4 > 1.50 g/L; Mucosal >10 IgG4+ plasma cells/HPF | N: 232 (28 CD, 104 UC) | 45 healthy controls |
|
| Raina et al. (45) | Retrospective | Mucosal >10 IgG4+ plasma cells/HPF in rectal biopsies | N: 54 (13 CD, 18 UC, 23 UC + PSC) | 11 controls (aspecific diarrhea or microscopic colitis) |
|
| Şimşek et al. (46) | Retrospective | Mucosal >10 IgG4+ plasma cells/HPF | N: 72 (17 CD, 55 UC) | / |
|
| Keyashian et al. (47) | Retrospective | Mucosal IgG4+ plasma cells/HPF in rectal biopsies | N: 134 UC | / |
|
| Navaneethan et al. (48) | Retrospective | Serum IgG4 > 112 mg/dL | N: 50 PSC pts. of which 42 had UC | / |
|
| Tavaghi et al. (49) | Retrospective | Serum IgG4-levels | N: 73 PSC pts. of which 51 had IBD | / |
|
| Navaneethan et al. (50) | Prospective | Serum IgG4 ≥ 112 mg/dL | N: 124 UC pts. with symptomatic pouchitis | / |
|
| Virk et al. (51) | Retrospective | Mucosal >10 IgG4+ plasma cells/HPF in pretreatment colonic biopsies | N: 78 (50 UC, 38 CD) | / |
|
IBD, inflammatory bowel disease; CD, Crohn’s disease, UC, ulcerative colitis, pts, patients; PSC, primary sclerosing cholangitis; HPF, high power field.
Discussion
A thorough work-up remains necessary in patients presenting with refractory, ulcerative gastroduodenal inflammation, and differentiating between GG, upper GI IgG4-RD, and upper GI CD remains challenging. We report a case in which IgG4-RD gastroduodenitis and upper GI CD were overlapping. Granulomas are typical but not necessary in diagnosing CD after eliminating other causes such as sarcoidosis and tuberculosis. As was the case in our patient, GG-associated CD is more prevalent in male and young patients. On the other hand, granulomas are uncommon in IgG4-RD. However, due to persisting ulcerative gastroduodenitis with stenosis despite high doses of pantoprazole, an IgG4 stain and serum IgG4 were requested, which was positive. As shown in the literature, IgG4-RD should be ruled out not only in ulcerative disease but also in patients with a submucosal tumor or suspicion of GIST to avoid unnecessary surgery. First-line treatment for both CD and IgG4-RD consists of corticosteroids with tapering. Our patient received anti-TNF and azathioprine as a maintenance treatment based on the existing literature for CD, which is in favor of anti-TNF and/or azathioprine, and IgG4-RD, which favors an immunomodulator such as azathioprine. This approach led to an excellent response clinically, endoscopically, and histologically.
Physicians should be aware of the potential existence of IgG4-RD in the upper GI tract in refractory disease. Nonetheless, literature on diagnosis, prognosis, and treatment of upper GI IgG4-RD is scarce and the meaning of increased IgG4 in serum or gastric/duodenal biopsies remains the subject of further research.
Especially in IBD patients, mucosal IgG4-infiltration may be a marker and risk factor for aggressive disease rather than a separate disease entity, which is to date mostly suggested in UC. More research in this field is necessary to unravel this question and further explore the actual role of IgG4-elevation of IBD patients.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
VD: Writing – original draft. JG: Writing – review & editing. AH: Writing – review & editing. TL: Writing – review & editing.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1388940/full#supplementary-material
References
- 1.Sepulveda AR, Patil M. Practical approach to the pathologic diagnosis of gastritis. Arch Pathol Lab Med. (2008) 132:1586–93. doi: 10.5858/2008-132-1586-PATTPD, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Scarpignato C, Gatta L, Zullo A, Blandizzi C, SIF-AIGO-FIMMG Group. Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners . Effective and safe proton pump inhibitor therapy in acid-related diseases - a position paper addressing benefits and potential harms of acid suppression. BMC Med. (2016) 14:179. doi: 10.1186/s12916-016-0718-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro JL, Goldblum JR, Petras RE. A clinicopathologic study of 42 patients with granulomatous gastritis. Is there really an "idiopathic" granulomatous gastritis? Am J Surg Pathol. (1996) 20:462–70. doi: 10.1097/00000478-199604000-00009, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Liang Y, Cui S, Polydorides AD. Clinicopathological characteristics and aetiological factors of granulomatous gastritis. Histopathology. (2021) 79:1040–50. doi: 10.1111/his.14462, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto M, Haruma K, Yoshihara M, Sumioka M, Kamada T, Ito M, et al. Isolated granulomatous gastritis successfully treated by Helicobacter pylori eradication: a possible association between granulomatous gastritis and Helicobacter pylori. J Gastroenterol. (2003) 38:371–5. doi: 10.1007/s005350300066, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Ectors NL, Dixon MF, Geboes KJ, Rutgeerts PJ, Desmet VJ, Vantrappen GR. Granulomatous gastritis: a morphological and diagnostic approach. Histopathology. (1993) 23:55–61. doi: 10.1111/j.1365-2559.1993.tb01183.x, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. (2006) 55:749–53. doi: 10.1136/gut.2005.082909, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingle SB, Adgaonkar BD, Jamadar NP, Siddiqui S, Hinge CR. Crohn's disease with gastroduodenal involvement: diagnostic approach. World J Clin Cases. (2015) 3:479–83. doi: 10.12998/wjcc.v3.i6.479, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner D, Griffiths AM. Esophageal, gastric, and duodenal manifestations of IBD and the role of upper endoscopy in IBD diagnosis. Curr Gastroenterol Rep. (2009) 11:234–7. doi: 10.1007/s11894-009-0036-0, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Pimentel AM, Rocha R, Santana GO. Crohn's disease of esophagus, stomach and duodenum. World J Gastrointest Pharmacol Ther. (2019) 10:35–49. doi: 10.4292/wjgpt.v10.i2.35, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kefalas CH. Gastroduodenal Crohn's disease. Proc Bayl Univ Med Center. (2003) 16:147–51. doi: 10.1080/08998280.2003.11927896, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingle SB, Pujari GP, Patle YG, Nagoba BS. An unusual case of Crohn's disease with isolated gastric involvement. J Crohns Colitis. (2011) 5:69–70. doi: 10.1016/j.crohns.2010.10.001, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Reynolds HL, Stellato TA. Crohn's disease of the foregut. Surg Clin North Am. (2001) 81:117–35, viii. doi: 10.1016/S0039-6109(05)70276-0, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Lazarev M, Huang CR, Bitton A, Cho JH, Duerr RH, McGovern DP, et al. Relationship between proximal Crohn's disease location and disease behavior and surgery: a cross-sectional study of the IBD genetics consortium. Am J Gastroenterol. (2013) 108:106–12. doi: 10.1038/ajg.2012.389, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomollon F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al. 3rd European evidence-based consensus on the diagnosis and Management of Crohn's disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. (2017) 11:3–25. doi: 10.1093/ecco-jcc/jjw168, PMID: [DOI] [PubMed] [Google Scholar]
- 16.van Hogezand RA, Witte AM, Veenendaal RA, Wagtmans MJ, Lamers CB. Proximal Crohn's disease: review of the clinicopathologic features and therapy. Inflamm Bowel Dis. (2001) 7:328–37. doi: 10.1097/00054725-200111000-00010, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Annunziata ML, Caviglia R, Papparella LG, Cicala M. Upper gastrointestinal involvement of Crohn's disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci. (2012) 57:1618–23. doi: 10.1007/s10620-012-2072-0, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Chateau T, Damico F, Zallot C, Mathieu N, Peyrin-Biroulet L. Crohn's disease only visible on small bowel capsule endoscopy: a new entity. Dig Dis Sci. (2021) 66:2712–6. doi: 10.1007/s10620-020-06553-3, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Sakuraba A, Iwao Y, Matsuoka K, Naganuma M, Ogata H, Kanai T, et al. Endoscopic and pathologic changes of the upper gastrointestinal tract in Crohn's disease. Biomed Res Int. (2014) 2014:610767: 1–6. doi: 10.1155/2014/610767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. (2019) 13:144–164K. doi: 10.1093/ecco-jcc/jjy113, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Davis KG. Crohn's disease of the foregut. Surg Clin North Am. (2015) 95:1183–93, vi. doi: 10.1016/j.suc.2015.07.004, PMID: [DOI] [PubMed] [Google Scholar]
- 22.Schwartzberg DM, Brandstetter S, Grucela AL. Crohn's disease of the esophagus, duodenum, and stomach. Clin Colon Rectal Surg. (2019) 32:231–42. doi: 10.1055/s-0039-1683850, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. (2002) 359:1541–9. doi: 10.1016/S0140-6736(02)08512-4 [DOI] [PubMed] [Google Scholar]
- 24.Gaggar S, Scott J, Thompson N. Pyloric stenosis associated Crohn's disease responding to adalimumab therapy. World J Gastrointest Pharmacol Ther. (2012) 3:97–9. doi: 10.4292/wjgpt.v3.i6.97, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rieder F, Latella G, Magro F, Yuksel ES, Higgins PD, Di Sabatino A, et al. European Crohn's and colitis organisation topical review on prediction, diagnosis and Management of Fibrostenosing Crohn's disease. J Crohns Colitis. (2016) 10:873–85. doi: 10.1093/ecco-jcc/jjw055, PMID: [DOI] [PubMed] [Google Scholar]
- 26.Karateke F, Menekse E, Das K, Ozyazici S, Demirturk P. Isolated duodenal Crohn's disease: a case report and a review of the surgical management. Case Rep Surg. (2013) 2013:421961: 1–3. doi: 10.1155/2013/421961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah J, Etienne D, Reddy M, Kothadia JP, Shahidullah A, Baqui AAMA. Crohn's disease manifesting as a duodenal obstruction: an unusual case. Gastroenterol Res. (2018) 11:436–40. doi: 10.14740/gr1105, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. (2001) 344:732–8. doi: 10.1056/NEJM200103083441005 [DOI] [PubMed] [Google Scholar]
- 29.Umehara H, Okazaki K, Kawa S, Takahashi H, Goto H, Matsui S, et al. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol. (2021) 31:529–33. doi: 10.1080/14397595.2020.1859710, PMID: [DOI] [PubMed] [Google Scholar]
- 30.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. (2015) 385:1460–71. doi: 10.1016/S0140-6736(14)60720-0 [DOI] [PubMed] [Google Scholar]
- 31.Notohara K, Kamisawa T, Uchida K, Zen Y, Kawano M, Kasashima S, et al. Gastrointestinal manifestation of immunoglobulin G4-related disease: clarification through a multicenter survey. J Gastroenterol. (2018) 53:845–53. doi: 10.1007/s00535-017-1420-4, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Tiftikci A, Koc ES, Cicek B. IgG4-related disease: an unusual presentation as collagenous gastritis. Gastrointest Endosc. (2023) 98:450–1. doi: 10.1016/j.gie.2023.04.2097, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Sawada H, Czech T, Silangcruz K, Kozai L, Obeidat A, Wien EA, et al. Clinicopathological characteristics of gastric IgG4-related disease: systematic scoping review. J Gastroenterol Hepatol. (2022) 37:1865–72. doi: 10.1111/jgh.15980, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Masaki Y, Kurose N, Yamamoto M, Takahashi H, Saeki T, Azumi A, et al. Cutoff values of serum IgG4 and histopathological IgG4+ plasma cells for diagnosis of patients with IgG4-related disease. Int J Rheumatol. (2012) 2012:580814: 1–5. doi: 10.1155/2012/580814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchino K, Notohara K, Uehara T, Kuraishi Y, Itakura J, Matsukawa A. Utility of gastric biopsy in diagnosing IgG4-related gastrointestinal disease. Pathol Int. (2021) 71:124–34. doi: 10.1111/pin.13059, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Strehl JD, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol. (2011) 64:237–43. doi: 10.1136/jcp.2010.085613, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. (2012) 25:1181–92. doi: 10.1038/modpathol.2012.72, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. (2012) 366:539–51. doi: 10.1056/NEJMra1104650 [DOI] [PubMed] [Google Scholar]
- 39.Kamisawa T, Okazaki K, Kawa S, Shimosegawa T, Tanaka M. Research Committee for Intractable Pancreatic D, Japan pancreas S. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol. (2010) 45:471–7. doi: 10.1007/s00535-010-0221-9, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Karim F, Paridaens D, Westenberg LEH, Guenoun J, Verdijk RM, van Hagen PM, et al. Infliximab for IgG4-related orbital disease. Ophthalmic Plast Reconstr Surg. (2017) 33:S162–5. doi: 10.1097/IOP.0000000000000625 [DOI] [PubMed] [Google Scholar]
- 41.Naghibi M, Ahmed A, Al Badri AM, Bateman AC, Shepherd HA, Gordon JN. The successful treatment of IgG4-positive colitis with adalimumab in a patient with IgG4-related sclerosing disease – a new subtype of aggressive colitis? J Crohns Colitis. (2013) 7:e81–4. doi: 10.1016/j.crohns.2012.05.003, PMID: [DOI] [PubMed] [Google Scholar]
- 42.Koutroumpakis F, Phillips AE, Yadav D, Machicado JD, Ahsan M, Ramos Rivers C, et al. Serum IgG4 subclass deficiency defines a distinct, commonly encountered, severe inflammatory bowel disease subtype. Inflamm Bowel Dis. (2021) 27:855–63. doi: 10.1093/ibd/izaa230, PMID: [DOI] [PubMed] [Google Scholar]
- 43.Faria RJ, Clemente CM, Carneiro FP, Santos-Neto L. Can IgG4 levels identify the ulcerative colitis subtype of inflammatory bowel disease? Gastroenterol Res. (2015) 8:178–85. doi: 10.14740/gr648w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang ZJ, Zhu M, Luo CX, Zhen Y, Mu JX, Zhang WY, et al. High level of IgG4 as a biomarker for a new subset of inflammatory bowel disease. Sci Rep. (2018) 8:8. doi: 10.1038/s41598-018-28397-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raina A, Yadav D, Regueiro M, Krasinskas AM, Saul MI, Sapienza DAM, et al. Mucosal IgG4 cell infiltration in ulcerative colitis is linked to disease activity and primary Sclerosing cholangitis. Inflamm Bowel Dis. (2013) 19:1232–7. doi: 10.1097/MIB.0b013e318281344d, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Şimşek HD, Basyigit S, Aktas B, Vargol E, Şimşek GG, Küçükazman M, et al. Comparing the type and severity of inflammatory bowel disease in relation to IgG4 immunohistochemical staining. Acta Gastro Belg. (2016) 79:216–21. [PubMed] [Google Scholar]
- 47.Keyashian K, Duregon E, Brinkerhoff BT, Bradley L, Larson B, Lim J, et al. Uses and limitations of IgG4 positive plasma cells in evaluating ulcerative colitis. J Gastrointestin Liver Dis. (2017) 26:428–9. doi: 10.15403/jgld.2014.1121.264.igg, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Navaneethan U, Venkatesh PG, Choudhary M, Shen B, Kiran RP. Elevated immunoglobulin G4 level is associated with reduced colectomy-free survival in patients with primary sclerosing cholangitis and ulcerative colitis. J Crohns Colitis. (2013) 7:e35–41. doi: 10.1016/j.crohns.2012.04.006, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Taghavi SA, Majd SK, Sianati M, Sepehrimanesh M. Prevalence of IgG-4-associated cholangiopathy based on serum IgG-4 levels in patients with primary sclerosing cholangitis and its relationship with inflammatory bowel disease. Turk J Gastroenterol. (2016) 27:547–52. doi: 10.5152/tjg.2016.16344, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Navaneethan U, Venkatesh PGK, Kapoor S, Kiran RP, Remzi FH, Shen B. Elevated serum IgG4 is associated with chronic antibiotic-refractory Pouchitis. J Gastrointest Surg. (2011) 15:1556–61. doi: 10.1007/s11605-011-1587-6, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Virk R, Shinagare S, Lauwers GY, Yajnik V, Stone JH, Deshpande V. Tissue IgG4-positive plasma cells in inflammatory bowel disease: a study of 88 treatment-naive biopsies of inflammatory bowel disease. Mod Pathol. (2014) 27:454–9. doi: 10.1038/modpathol.2013.121, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.