Abstract
Supplemental material is available for this article.
Keywords: Deep Learning, Artificial Intelligence, Diffuse Glioma, Neuro-Oncology, Resection Cavity
Summary
The University of California San Francisco Adult Longitudinal Post-Treatment Diffuse Glioma MRI dataset is a publicly available annotated dataset featuring multimodal brain MRI scans from 298 patients with diffuse gliomas taken at two consecutive follow-ups (596 scans total), with corresponding clinical history and expert voxelwise annotations.
Key Points
■ Included segmentation masks delineate tumor subregions of enhancing tissue (ET), surrounding nonenhancing fluid-attenuated inversion recovery hyperintensity (SNFH), nonenhancing tumor core (NETC), and resection cavity (RC).
■ Longitudinal change annotations delineate change over time in SNFH and ET tumor subregions.
■ Preliminary training data provide expected baseline performance for future automated segmentation algorithms.
Introduction
Diffuse gliomas comprise a subclass of molecularly diverse brain tumors thought to arise from heterogeneous populations of neuroglial progenitor cells (1–3). Glioblastomas account for nearly three-quarters of all diffuse gliomas and carry with them dismal prognoses, with estimated 2-year survival rates of approximately 27% (1,4). Maximal safe surgical resection remains the mainstay of treatment, often in conjunction with radiation, chemotherapy, or both (2,5–7). Assessment of posttreatment gliomas is a challenging task, with MRI being the preferred imaging modality for longitudinal evaluation (6–9).
Advances in the field of artificial intelligence have been used to detect, segment, classify, and prognosticate a variety of neoplastic processes (10–13). However, implementation of clinically applicable machine learning studies has been limited by the lack of large, longitudinal, publicly accessible annotated datasets. In recent years, multiple initiatives, such as The Cancer Imaging Archive, the Multimodal Brain Tumor Segmentation Challenge, and the Radiological Society of North America AI Challenges have emerged in an attempt to support researchers seeking larger sample sizes to perform increasingly complex artificial intelligence investigations (8,14–17). Nevertheless, given the large amount of work required to collect, organize, process, and annotate datasets, as well as privacy concerns, currently available datasets remain relatively limited in clinical scope. In particular, the Multimodal Brain Tumor Segmentation Challenge datasets are limited only to pretreatment scans, despite the fact that most imaging in clinical practice is done for longitudinal follow-up during which posttreatment changes often take on highly varied appearances (8,9,18–21). To the best of our knowledge, none of the currently publicly available posttreatment glioma datasets contain expert voxelwise segmentations, though the more recently released LUMIERE (Longitudinal Glioblastoma MRI with Expert RANO Evaluation) dataset contains expert Response Assessment in Neuro-Oncology criteria (22).
To further support large-scale machine learning validations, we hereby report the public release of the University of California San Francisco Adult Longitudinal Post-Treatment Diffuse Glioma (UCSF-ALPTDG) MRI dataset. This expertly annotated, anonymized dataset consists of multimodal three-dimensional (3D) MR images of diffuse gliomas at two consecutive posttreatment time points and is readily available for training and validation of novel and existing artificial intelligence workflows.
Materials and Methods
Study Patients
This retrospective, single-center, Health Insurance Portability and Accountability Act–compliant study was undertaken with approval from the UCSF Institutional Review Board; a waiver for informed consent was granted. The sample consists of data from a prior study (8) whereby 298 patients diagnosed with diffuse glioma and with two consecutive imaging time points were retrospectively identified by software query of our institutional imaging archives (mPower; Nuance Communications), with 62 patients overlapping with a previously released dataset of preoperative diffuse glioma MRI scans (14). Of the total patients, 59% (177 of 298) were male and 41% (121 of 298) female, and the mean age of overall cohort was 51.9 years ± 14.0 (SD). As with our study sample, a slight male predominance has previously been observed with diffuse gliomas, with a 1.3:1.0 male-to-female ratio (23). According to World Health Organization 2021 criteria (24), diagnoses included 58 (19%) isocitrate dehydrogenase (IDH)–mutant astrocytomas, nine (3%) IDH–wild-type astrocytomas, 62 (21%) oligodendrogliomas, and 80 (27%) glioblastomas (Table 1). The study was limited to patients who underwent posttreatment surveillance MRI examinations within our university hospital network between January 2018 and December 2019. In patients for whom multiple consecutive follow-up time points were available, two consecutive time points with interval changes were selected for inclusion.
Table 1:
Patient Demographics and Diffuse Glioma Types
Image Acquisition and Preprocessing
All MRI scans were acquired as part of standard-of-care imaging with one of four Discovery 750 3.0-T (GE HealthCare) scanners. Typical acquisition parameters were as follows: T1 pre- and postcontrast: axial 3D inversion-recovery spoiled gradient-echo T1 (repetition time, echo time, and inversion time, 6, 2.3, and 450 msec, respectively; flip angle, 12 degrees; section thickness, 1.0 mm; matrix, 256 × 256; field of view, 25.6; number of excitations, one); T2: sagittal 3D fast spin echo (repetition time and echo time, 2200 and 100 msec; section thickness, 1.2 mm; matrix, 256 × 256; field of view, 25.6 cm; number of excitations, one); and T2 fluid-attenuated inversion recovery (FLAIR): coronal 3D fast spin echo (repetition time, echo time, and inversion time, 5700, 115, and 1650 msec, respectively; section thickness, 1.2 mm; matrix, 256 × 256; field of view, 25.6 cm; number of excitations, one). Gadoterate (Dotarem; Guerbet) at a dose of 0.2 mL/kg was used as the gadolinium-based contrast agent.
Two consecutive posttreatment scans were included for each patient, for a total of 596 scans. Preprocessing procedures included image de-identification and conversion to the Neuroimaging Informatics Technology Initiative file format, skull stripping, N4 bias correction, coregistration to the T1 postcontrast image obtained at the second posttreatment time point, and registration to the SRI24 atlas (8).
Reference Standard Voxelwise and Categorical Annotations
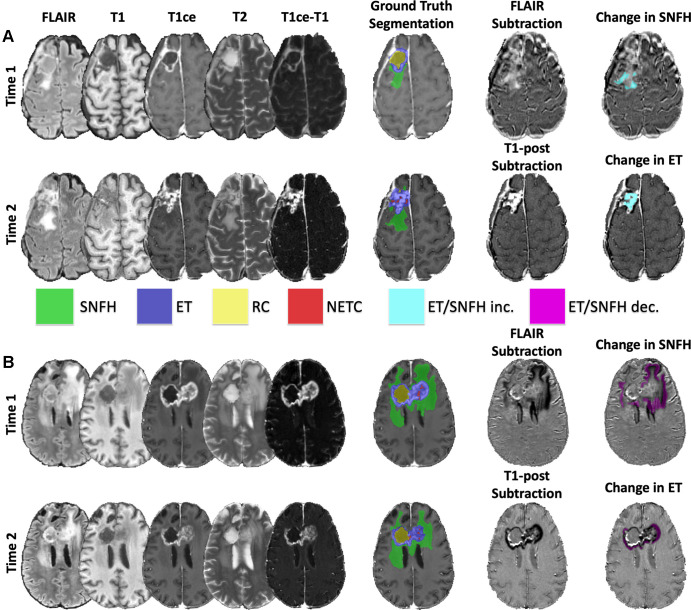
Voxelwise segmentations were performed using ITK-Snap software, version 4.0.0-alpha.3 (www.itksnap.org) (25) with a semiautomated, iterative procedure followed by manual revisions to delineate tumor subregions (8,21) (Figure). A previously developed 3D nnU-Net architecture was used to provide baseline subregion segmentations (8), which was retrained a second time after 100 cases were revised. The automated segmentations were each reviewed for accuracy and manually corrected as needed by one of three expert neuroradiologists (E.C. with 2 years, A.M.R. with 4 years, and J.D.R. with 4 years of experience in neuro-oncologic imaging, respectively); the individual who annotated each case is specified in Table S1. The four subregions consisted of enhancing tissue (ET), surrounding nonenhancing FLAIR hyperintensity (SNFH), nonenhancing tumor core (NETC), and resection cavity (RC). The RC class is new compared with the previously published study on this dataset (8), and all annotations were revised according to a new annotation protocol. Given the posttreatment nature of the scans, any T2 and FLAIR signal abnormalities, including radiation-related hyperintensity, gliosis, edema, and nonenhancing tumor, were included in the SNFH class. Any areas of thick or nodular enhancement were included in the ET class, though typical treatment-related thin linear enhancement along and within RCs and along the dura was not included in the ET class. The RC class consisted of both recent and chronic RCs. Chronic RCs, which are typically older than 3–6 months, were considered those with signal intensity isointense to cerebrospinal fluid. More recent RCs often contained air, blood, and/or proteinaceous materials and otherwise exhibited variable signal characteristics. The NETC class consisted of necrotic and nonenhancing tissue surrounded by ET and not otherwise clearly represented by a prior RC.
Representative examples of brain MRI and tumor segmentations in (A) a 55-year-old female patient with glioblastoma and (B) a 54-year-old female patient with glioblastoma. Subtraction images show representative segmentations of longitudinal (A) increases and (B) decreases in surrounding nonenhancing fluid-attenuated inversion recovery (FLAIR) hyperintensity (SNFH) and enhancing tissue (ET) in each patient, respectively. T1ce = T1 contrast-enhanced, RC = resection cavity, NETC = nonenhancing tumor core.
Separate voxelwise annotations were created to delineate regions that exhibited volumetric changes across the two time points for the SNFH and ET tissue classes. For SNFH, FLAIR sequences acquired at each posttreatment time point were subtracted from each other to guide voxelwise annotations of increasing or decreasing signal intensity. For ET, T1 postcontrast minus T1 precontrast subtraction images were first generated for each individual time point; subsequently, these newly generated enhancement subtraction images were then subtracted from each other (ie, [T1 postcontrastTime2 – T1 precontrastTime2] – [T1 postcontrastTime1 – T1 precontrastTime1]) to highlight areas of increasing or decreasing signal intensity. Compared with our previously published study (8), these longitudinal SNFH and ET change segmentations were revised to include mixed longitudinal changes (ie, areas of both increases and decreases in signal across time points in the same patient).
Volumetric changes in SNFH tissue and ET between the two time points were categorized as increased, decreased, unchanged, or a combination of increased and decreased based on volumes extracted from the final, expert neuroradiologist, manually corrected longitudinal change segmentations.
nnU-Net Convolutional Neural Network for Baseline Segmentation Performance
To provide baseline segmentation performance of this dataset, we used the nnU-Net (26), a self-configuring, 3D fully convolutional network. Networks for the four-class tissue segmentations and longitudinal changes were trained on a random set of 248 patients and tested on the remaining 50 patients. Performance was evaluated using Dice scores, volume similarities, and Hausdorff distances. In all experiments, default settings for the 3dfullres network were used with an automatically selected patch size of 128 × 128 × 128. Training was performed on a GeForce RTX 3090 graphics processing unit, version 11.2 (NVIDIA; 24 GB memory) for 1000 epochs using a combination of cross-entropy and Dice loss function (1:1). The code used for the nnU-Net implementation and pretrained networks is available at https://github.com/rachitsaluja/UCSF-ALPTDG-benchmarks.
Results
Demographics, Pathology, Treatment History, and Tumor Volumetric Information
The full scope of patient demographics, pathologic diagnoses, treatment history, tumor volumetric characteristics, and posttreatment changes over time in our cohort are reported in Tables 1 and 2, with complete information for each scan available in Table S1. Accompanying complete clinical data include genetic mutation statuses (eg, IDH, O6-methylguanine-DNA methyltransferase [MGMT], 1p19q, and ATRX); comprehensive treatment histories, including chemotherapeutic regimens; and temporal landmarks such as days from first surgery to first scan, time interval between scans, days from first scan to progression (based on expert multidisciplinary consensus in consideration of both radiologic and clinical factors), and days from first scan to death (Table S1).
Table 2:
Tumor Statistics
Briefly summarized, our dataset was composed of 27% (80 of 298) glioblastomas, 21% (62 of 298) oligodendrogliomas, and 19% (58 of 298) IDH-mutant astrocytomas. Forty-one percent (123 of 298) of patients were positive for IDH mutation status, 28% (82 of 298) were positive for O6-MGMT methylation status, and 21% (62 of 298) were positive for 1p19q codeletion status (Table 1). The median time between surgery (or MRI diagnosis if surgery was not pursued) and first scan was 766 days. The median time between scans was 65 days. A total of 21% (62 of 298) of patients had undergone gross total resections. The percentages of scans with ET, SNFH, NETC, and RC were 63% (373 of 596), 100% (596 of 596), 33% (198 of 596), and 90% (536 of 596), respectively (Table 2). There were 34% (101 of 298) with an increase in ET volume and 10% (30 of 298) with a decrease in ET volume between the time points. Likewise, 38% (112 of 298) showed an increase in SNFH, while 12% (37 of 298) demonstrated a decrease in SNFH between the evaluated time points.
Baseline Segmentation Performance with nnU-Net
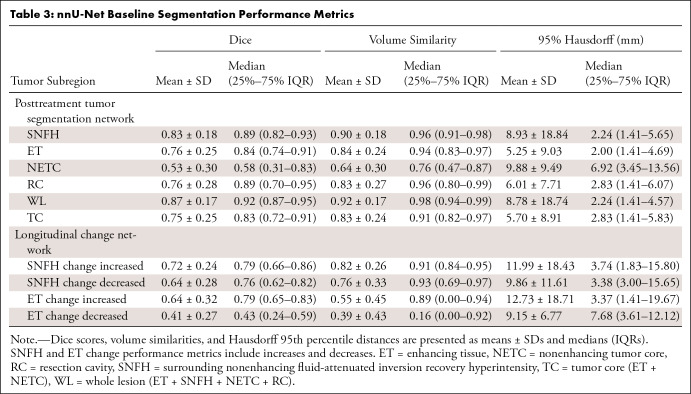
Table 3 provides segmentation performance metrics in the test set, which include mean and median Dice score, volume similarities, and Hausdorff distances for the four subregions at individual time points and for longitudinal changes in SNFH and ET across time points.
Table 3:
nnU-Net Baseline Segmentation Performance Metrics
Data Availability
The UCSF-ALPTDG dataset is hosted on the UCSF Center for Intelligent Imaging Datasets for Medical Imaging repository and is readily available for public, noncommercial use after agreeing to a data use agreement (https://imagingdatasets.ucsf.edu/dataset/2).
Discussion
Assessment of posttreatment gliomas remains a diagnostic challenge in neuro-oncologic imaging given the complex, heterogeneous appearance of treatment-related changes and variability among interpreting radiologists in classifying tissue subregions (8,20,27). Accurate longitudinal volumetric assessment of residual ET has the potential to reduce physician burden and errors in measurement while additionally generating novel, clinically relevant metrics for radiologists, neuro-oncologists, radiation oncologists, and neurosurgeons involved in the care of these patients (8,28). Given the substantial effort required to annotate subtle longitudinal changes in the posttreatment setting, the release of the UCSF-ALPTDG dataset, which includes expert voxelwise segmentations of tumor subregions and longitudinal changes, will further support future studies seeking to validate automated assessment protocols of longitudinal posttherapeutic changes in these highly morbid tumors.
This dataset has several limitations. It is derived from a single institution, protocol, and scanner model and field strength, which may limit generalizability. Additional heterogeneous, multisite data would be helpful to train a more generalizable algorithm. Although all expert annotations were curated by subspecialty trained neuroradiology attending physicians, only a single reference standard voxelwise annotation is available for each study, thus limiting assessments of interrater reliability. The different tissue classes are based on conventional structural MRI characteristics and thus combine both viable tumor and posttreatment changes. Changes in volumes of SNFH tissue and ET classes are often used as a first step in the radiologic evaluation. Of note, a recent study used longitudinal volumes of ET after laser interstitial thermal therapy to predict outcomes (29), demonstrating that volumetric changes may be helpful on their own. The second step of assessing whether these changes are related residual or recurrent tumor or treatment-related changes would be a separate determination that would require appropriate clinical information, which often remains unknown until repeat surgery. In addition, future work using more advanced imaging modalities, such as perfusion, diffusion, and/or spectroscopy, in conjunction with clinical assessments may be helpful in distinguishing treatment-related changes from progressive disease. We also recognize that due to unpredictable effects of varying acquisition parameters on signal characteristics, longitudinal changes over time were quantified solely based on volumetric change and did not account for changes in signal intensity. Furthermore, because all imaging was performed as per standard-of-care clinical workflows, the timing between follow-up scans was largely dictated by clinical factors and tumor pathology (6). This heterogeneity in follow-up intervals between scans may also limit comparisons between patients.
Publicly available annotated biomedical imaging repositories support researchers in developing effective, sophisticated machine learning algorithms (30,31). We hope that the release of the UCSF-ALPTDG dataset will bolster efforts to develop artificial intelligence techniques for longitudinal disease monitoring in this high-risk patient population for which few existing publicly available datasets feature detailed annotations of posttreatment changes (22). Disseminating this dataset should facilitate the development of robust techniques that may eventually allow for the integration of automated quantitative analyses into prospective imaging workflows. Specific use cases may include training of a more generalizable nnU-Net segmentation architecture from multisite data, prediction of survival, progression and specific areas of recurrence and infiltrative tumor, and extraction of radiomics metrics to serve as potential quantitative imaging biomarkers to predict outcomes and response to different treatments.
A.M.R. and J.D.R. are co–senior authors.
Current address: Division of Neuroradiology, Department of Radiology, Duke University Medical Center, Durham, NC.
Authors declared no funding for this work.
Disclosures of conflicts of interest: B.K.K.F. Received prior research grants from the RSNA R&E (2019-2020RMS #1909; 2018-2019 RMS #1810); consulting fees from Mendaera; honorarium payments from Neurodiem (invited author) and Elsevier (book proposal reviews); RSNA and institutional support for attending meetings (RSNA RFC stipend, institutional support stipend); vice-chair of the RSNA Resident and Fellow Committee; member of the American Board of Radiology Initial Certification Advisory Committee for Diagnostic Radiology, of the RSNA Education Council, and of the Radiology: Imaging Cancer Trainee Editorial Board; associate editor for Artificial Intelligence in Radiology with Frontiers in Radiology. E.C. Unrelated ASNR grant; unrelated Elsevier book royalties. J.M. Grant/contract from Siemens to institution; royalties from GE to author through institution; support for attending Nuance meetings; chair for RSNA’s Machine Learning Steering Committee; spouse employment at Annexon Biosciences; associate editor of Radiology: Artificial Intelligence. S.C. No relevant relationships. C.P.H. Consulting fees from GE HealthCare, Siemens Healthineers, and Kheiron Medical Technologies; participation on data safety monitoring board/advisory board for DSMB, Focused Ultrasound Foundation; DSMB, uniQure Biopharma; and DSMB, Asklepios BioPharma. L.P.S. No relevant relationships. S.M.C. AstraZeneca scientific advisory board. T.L.L. NICO Project, The Dabierre Family, Non-Invasive Characterization of Oligodrendroglioma. Coinvestigator; NIH P01CA118816 (PI Chang, Nelson, Vigneron) Noninvasive Metabolic Signatures to Improve Management of Molecular Subtypes of Glioma. Coinvestigator; Loglio Project. The Dabierre Family. Noninvasive Metabolic Signatures to Improve Management of Molecular Subtypes of Glioma. Coinvestigator; and DOD. (PI Li) Multimodal Neuroimaging for Evaluating Lower Grade Astrocytoma. Coinvestigator. All paid to institution. J.E.V.M. Grant/contract from GE HealthCare paid to UCSF. A.M.R. No relevant relationships. J.D.R. American Society of Neuroradiology Foundation Grant in Artificial Intelligence provided funding for this work, including salary/time and computing resources; consulting fees from Cortechs.ai; stock/stock options in Cortechs.ai and Subtle Medical.
Abbreviations:
- ET
- enhancing tissue
- FLAIR
- fluid-attenuated inversion recovery
- NETC
- nonenhancing tumor core
- RC
- resection cavity
- SNFH
- surrounding nonenhancing FLAIR hyperintensity
- 3D
- three-dimensional
- UCSF-ALPTDG
- University of California San Francisco Adult Longitudinal Post-Treatment Diffuse Glioma
Keywords: Deep Learning, Artificial Intelligence, Diffuse Glioma, Neuro-Oncology, Resection Cavity
References
- 1. Molinaro AM , Taylor JW , Wiencke JK , Wrensch MR . Genetic and molecular epidemiology of adult diffuse glioma . Nat Rev Neurol 2019. ; 15 ( 7 ): 405 – 417 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Claes A , Idema AJ , Wesseling P . Diffuse glioma growth: a guerilla war . Acta Neuropathol (Berl) 2007. ; 114 ( 5 ): 443 – 458 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nicholson JG , Fine HA . Diffuse Glioma Heterogeneity and Its Therapeutic Implications . Cancer Discov 2021. ; 11 ( 3 ): 575 – 590 . [DOI] [PubMed] [Google Scholar]
- 4. Gilbert MR , Wang M , Aldape KD , et al . Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial . J Clin Oncol 2013. ; 31 ( 32 ): 4085 – 4091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller JJ , Gonzalez Castro LN , McBrayer S , et al . Isocitrate dehydrogenase (IDH) mutant gliomas: A Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions . Neuro Oncol 2023. ; 25 ( 1 ): 4 – 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weller M , van den Bent M , Preusser M , et al . EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood . Nat Rev Clin Oncol 2021. ; 18 ( 3 ): 170 – 186 . [Published correction appears in Nat Rev Clin Oncol 2022;19(5):357-358.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wen PY , Weller M , Lee EQ , et al . Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions . Neuro Oncol 2020. ; 22 ( 8 ): 1073 – 1113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rudie JD , Calabrese E , Saluja R , et al . Longitudinal assessment of posttreatment diffuse glioma tissue volumes with three-dimensional convolutional neural networks . Radiol Artif Intell 2022. ; 4 ( 5 ): e210243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rudie JD , Rauschecker AM , Bryan RN , Davatzikos C , Mohan S . Emerging Applications of Artificial Intelligence in Neuro-Oncology . Radiology 2019. ; 290 ( 3 ): 607 – 618 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fields BKK , Demirjian NL , Hwang DH , et al . Whole-tumor 3D volumetric MRI-based radiomics approach for distinguishing between benign and malignant soft tissue tumors . Eur Radiol 2021. ; 31 ( 11 ): 8522 – 8535 . [DOI] [PubMed] [Google Scholar]
- 11. Demirjian NL , Varghese BA , Cen SY , et al . CT-based radiomics stratification of tumor grade and TNM stage of clear cell renal cell carcinoma . Eur Radiol 2022. ; 32 ( 4 ): 2552 – 2563 . [DOI] [PubMed] [Google Scholar]
- 12. Fields BKK , Demirjian NL , Cen SY , et al . Predicting soft tissue sarcoma response to neoadjuvant chemotherapy using an MRI-based delta-radiomics approach . Mol Imaging Biol 2023. ; 25 ( 4 ): 776 – 787 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jin J , Zhou H , Sun S , et al . Machine learning based gray-level co-occurrence matrix early warning system enables accurate detection of colorectal cancer pelvic bone metastases on MRI . Front Oncol 2023. ; 13 : 1121594 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calabrese E , Villanueva-Meyer JE , Rudie JD , et al . The University of California San Francisco Preoperative Diffuse Glioma MRI Dataset . Radiol Artif Intell 2022. ; 4 ( 6 ): e220058 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AI challenges. Radiological Society of North America website. https://www.rsna.org/education/ai-resources-and-training/ai-image-challenge. Accessed August 27, 2022.
- 16. Clark K , Vendt B , Smith K , et al . The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository . J Digit Imaging 2013. ; 26 ( 6 ): 1045 – 1057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menze BH , Jakab A , Bauer S , et al . The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS) . IEEE Trans Med Imaging 2015. ; 34 ( 10 ): 1993 – 2024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chu HH , Choi SH , Ryoo I , et al . Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging . Radiology 2013. ; 269 ( 3 ): 831 – 840 . [DOI] [PubMed] [Google Scholar]
- 19. Qian X , Tan H , Zhang J , Zhao W , Chan MD , Zhou X . Stratification of pseudoprogression and true progression of glioblastoma multiform based on longitudinal diffusion tensor imaging without segmentation . Med Phys 2016. ; 43 ( 11 ): 5889 – 5902 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kickingereder P , Isensee F , Tursunova I , et al . Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: a multicentre, retrospective study . Lancet Oncol 2019. ; 20 ( 5 ): 728 – 740 . [DOI] [PubMed] [Google Scholar]
- 21. Rudie JD , Weiss DA , Saluja R , et al . Multi-Disease Segmentation of Gliomas and White Matter Hyperintensities in the BraTS Data Using a 3D Convolutional Neural Network . Front Comput Neurosci 2019. ; 13 : 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suter Y , Knecht U , Valenzuela W , et al . The LUMIERE dataset: Longitudinal Glioblastoma MRI with expert RANO evaluation . Sci Data 2022. ; 9 ( 1 ): 768 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li K , Lu D , Guo Y , et al . Trends and patterns of incidence of diffuse glioma in adults in the United States, 1973-2014 . Cancer Med 2018. ; 7 ( 10 ): 5281 – 5290 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization . WHO Classification of Tumours: Central Nervous System Tumours . 5th ed. Lyon, France: : International Agency for Research on Cancer; , 2021. . [Google Scholar]
- 25. Yushkevich PA , Piven J , Hazlett HC , et al . User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability . Neuroimage 2006. ; 31 ( 3 ): 1116 – 1128 . [DOI] [PubMed] [Google Scholar]
- 26. Isensee F , Jaeger PF , Kohl SAA , Petersen J , Maier-Hein KH . nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation . Nat Methods 2021. ; 18 ( 2 ): 203 – 211 . [DOI] [PubMed] [Google Scholar]
- 27. Chang K , Beers AL , Bai HX , et al . Automatic assessment of glioma burden: a deep learning algorithm for fully automated volumetric and bidimensional measurement . Neuro Oncol 2019. ; 21 ( 11 ): 1412 – 1422 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vollmuth P , Foltyn M , Huang RY , et al . Artificial intelligence (AI)-based decision support improves reproducibility of tumor response assessment in neuro-oncology: An international multi-reader study . Neuro Oncol 2023. ; 25 ( 3 ): 533 – 543 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haskell-Mendoza AP , Reason EH , Gonzalez AT , et al . Automated Segmentation of Ablated Lesions Using Deep Convolutional Neural Networks: A Basis for Response Assessment Following Laser Interstitial Thermal Therapy . Neuro Oncol 2024. ; 26 ( 6 ): 1152 – 1162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gillies RJ , Kinahan PE , Hricak H . Radiomics: Images Are More than Pictures, They Are Data . Radiology 2016. ; 278 ( 2 ): 563 – 577 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bluemke DA , Moy L , Bredella MA , et al . Assessing Radiology Research on Artificial Intelligence: A Brief Guide for Authors, Reviewers, and Readers-From the Radiology Editorial Board . Radiology 2020. ; 294 ( 3 ): 487 – 489 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The UCSF-ALPTDG dataset is hosted on the UCSF Center for Intelligent Imaging Datasets for Medical Imaging repository and is readily available for public, noncommercial use after agreeing to a data use agreement (https://imagingdatasets.ucsf.edu/dataset/2).