Abstract
An implantable loop recorder (ILR) is now widely used for differential diagnosis of unexplained syncope or recurrent syncope with unknown causes. In the inherited arrhythmia syndromes, ILR may be useful for management of the therapeutic strategies; however, there is no obvious evidence to uncover arrhythmic syncope by ILR in long-QT syndrome (LQTS) patients. Here we experienced a 19-year-old female patient with LQTS type 1 who had recurrent syncope even after beta-blocker therapy but no arrhythmias were documented, and some episodes might be due to non-cardiogenic causes. Implantable cardioverter defibrillator (ICD) therapy was also recommended; however, she could not accept ICD but was implanted with ILR for further continuous monitoring. Two years later, she suffered syncope during a brief run, and ILR recorded an electrocardiogram at that moment. Thus a marked QT interval prolongation as well as T-wave alternance resulting in development of torsades de pointes could be detected. Although ILR is just a diagnostic tool but does not prevent sudden cardiac death, most arrhythmic events in LQTS are transient and sometimes hard to be diagnosed as arrhythmic syncope. ILR may provide direct supportive evidence to select the optimal therapeutic strategy in cases where syncope is difficult to diagnose.
Learning objective
Long-QT syndrome (LQTS) patients often suffer recurrent syncope even after beta-blocker therapy, but torsades de pointes (TdP) is not always detected by standard 12‑lead electrocardiogram or Holter monitoring, and some syncope might be non-cardiogenic. In this case, implantable loop recorder (ILR) documented the evidence of QT interval prolongation and beat-by-beat T-wave alternance subsequent TdP. Thus, ILR may provide useful evidence for the optimal treatment strategy in LQTS cases where syncope is difficult to diagnose.
Keywords: Implantable loop recorder, Torsades de pointes, Long-QT syndrome, Syncope, Diagnosis, T-wave alternance
Introduction
Long QT syndrome (LQTS) is characterized by QT interval prolongation of electrocardiogram (ECG) and polymorphic ventricular tachycardia named as torsades de pointes (Tdp) leading to syncope and sudden cardiac death (SCD) [1]. The typical TdP usually terminates spontaneously and patients suffer syncope or convulsive seizures, which are associated with increased risk of subsequent cardiac arrest. Almost half of symptomatic LQTS patients experience their first cardiac event by age 12 years and 90 % by age 40 years [2]. On the other hand, syncopal attacks observed in children or adolescents are not only caused by TdP but also by many other reasons: vasovagal (neurocardiogenic) syndrome, structural heart diseases, orthostatic hypotension, cerebral diseases, epilepsy, and seizure. Thus, differential diagnosis of syncope is important for risk stratification and optimal medical therapies in children and adolescents with LQTS.
An implantable loop recorder (ILR) is now widely used for differential diagnosis of unexplained syncope or recurrent syncope with unknown causes. In the inherited arrhythmia syndromes, ILR may be useful for guiding management [3], but TdP may be captured in only 5 % of LQTS patients with ILR [4]. However, there are no reports examining the efficacy of ILR for LQTS, and its prognostic effect is unknown. Here we report a case of congenital LQTS type 1 (LQT1) who had been implanted with an ILR due to recurrent syncope even after beta-blocker therapy. ILR detected a typical TdP and contributed to the decision for a further intensive therapeutic strategy.
Case report
A 19-year-old female had her first syncopal episode while swimming as a 7-year-old, and her ECG showed longer QTc interval (480 ms). Genetic testing identified a pathogenic variant in KCNQ1 (c.1032 G > A, p.A344A splicing error), thus LQT1 was diagnosed. She started medication (propranolol 60 mg/day) as well as restriction of competitive sports.
Although she continued with a beta-blocker therapy, syncope recurred during mild exercise such as riding a bicycle. However, her consciousness soon recovered and ECG at emergency hospital could not detect any arrhythmias or abnormal QT prolongation. Furthermore, she sometimes had dizziness or faintness without doing exercise.
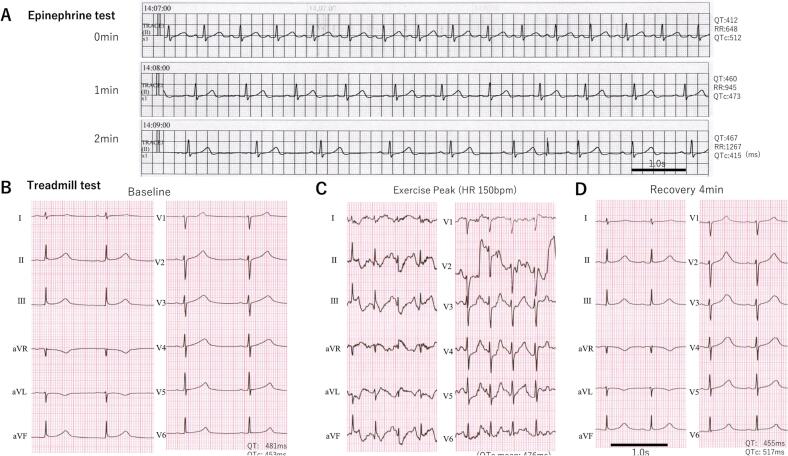
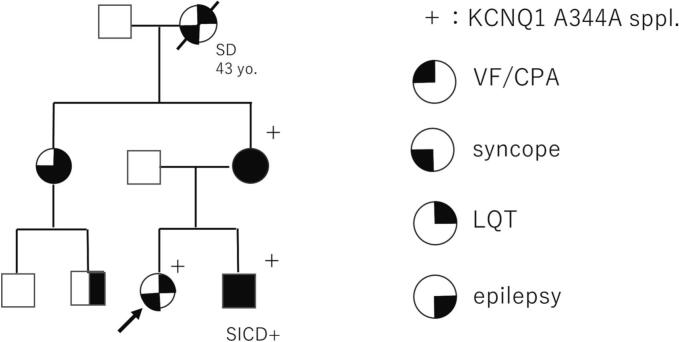
She was hospitalized for more detailed clinical examination when she was 18 years old. An epinephrine stress test under propranolol (75 mg/day) was performed to confirm the effect of a beta-blocker but failed to evaluate the efficacy because a significant bradycardia and dysphoria occurred immediately after epinephrine bolus injection (Fig. 1A). Treadmill exercise testing showed a QT prolongation at baseline (Fig. 1B) and its further increase after exercise (Fig. 1C,D). Although no ventricular arrhythmias were induced after the examination, she had dizziness, hyperventilation, and transient disturbance of consciousness. Magnetic resonance imaging of the head and electroencephalogram showed no abnormal findings for suspicion of a dissociative disorder or non-convulsive status epilepticus. Head-up tilt testing induced symptoms and reflex syncope was diagnosed. She had a family history of LQTS (Fig. 2) and her brother with the same LQT1 suffered out-of-hospital cardiac arrest and was soon resuscitated; he was implanted with an implantable cardioverter defibrillator (ICD). From these findings, it was concluded that not all her symptoms might be due to arrhythmias, and she was diagnosed as a higher risk LQTS patient. ICD implantation was also considered but she could not accept implantation of ICD. Then, we recommended her to be implanted with an ILR for continuous monitoring.
Fig. 1.
(A) Monitoring electrocardiogram (ECG) (lead II) at epinephrine provocation test, soon after epinephrine 0.1 μg/kg bolus injection (0 min) followed by 0.1 μg/kg/min continuous injection (1– 2 min). (B–D) A standard 12-lead ECG at baseline before the treadmill exercise testing (B), during peak exercise by the standard Bruce protocol (C) and recovery 4 min after exercise testing (D) when the patient was 19 years old and taking the beta-blocker, propranolol 75 mg/day.
Fig. 2.
Family pedigree of this patient.
+: genotype positive; SICD+: subcutaneous implantable cardioverter defibrillator was implanted; SD: sudden death; VF/CPA: ventricular fibrillation or cardiopulmonary arrest; LQT: long-QT syndrome.
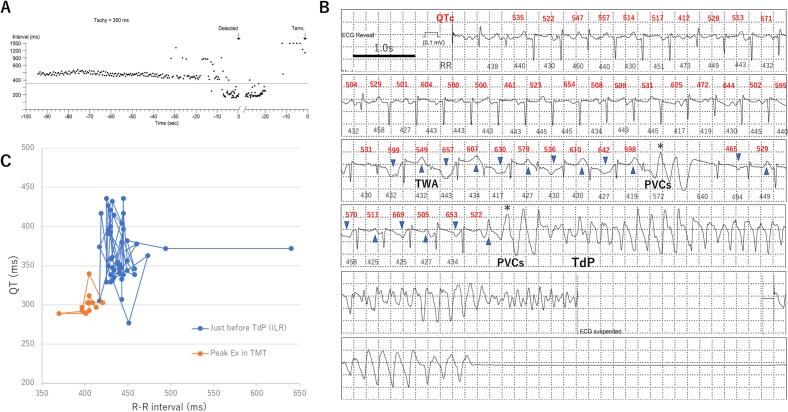
During follow up, the patient had a total 13 episodes of symptoms such as dizziness, faintness, and palpitations, but no serious arrhythmias were recorded by ILR. Two years after the ILR implantation, even though she continued medication, she suffered syncope for about 3 min while briefly running at a train station. She was sent to our hospital, where no abnormal vital signs on physical examination were observed at admission, and cardiac function was preserved on echocardiography, but an ECG showed marked QT prolongation (QTc: 514 ms). ILR had stored the data from 90 s before the event (Fig. 3A), in which the R-R interval was continuously under 500 msec (heart rate over 120 bpm) followed by premature ventricular contractions and tachycardia. Detailed ECG just before the event is shown in Fig. 3B, in which the QT (QTc) interval was significantly prolonged over 500 ms and the beat-by-beat T wave morphology alternance could be seen followed by spontaneous premature ventricular contractions, resulting in occurrence of TdP for about 2.5 min and then it spontaneously terminated. The QT (QTc) interval just before the event was significantly prolonged and the beat-by-beat alternance was more manifested compared to in the treadmill exercise testing (Fig. 3C). Since symptomatic TdP occurred under a beta-blocker, she was implanted with a subcutaneous-ICD as a secondary prevention of SCD.
Fig. 3.
(A) Continuous R-R interval 90s before the event recorded by implantable loop recorder (ILR). (B) ILR recorded occurrence of torsades de pointes (TdP), manifestation of T-wave alternance (TWA) as well as beat-by-beat dynamic change of the QT (QTc) interval followed by premature ventricular contractions (PVCs) leading to polymorphic ventricular tachycardia. (C) Superimposed QT/RR relationship at the peak exercise during treadmill test (orange) and at just before TdP event (blue).
Discussion
ILR for LQTS
Recently, ILR implantation has been recommended for patients with clinical signs of suspected cardiogenic syncope, but in whom comprehensive evaluation cannot identify the cause of the syncope or specific treatment cannot be determined [5]. However, there is no obvious evidence of ILR for congenital LQTS except for the Andersen-Tawil syndrome [6].
In a recent review paper [4], only 1 (5 %) of 18 LQTS patients who had been implanted with an ILR had TdP detected, in contrast, the others were asymptomatic or only had non-lethal arrhythmias that did not require intensive treatment during follow up. On the other hand, the frequency of arrhythmic events in LQTS is not high and no one has died after ILR implantation. Use of ILR for LQTS patients with unknown syncope may have a benefit to detect serious ventricular arrhythmias, which helps us to implement more advanced pharmacological and/or non-pharmacological therapies.
This patient, to the best of our knowledge, is the first LQT1 case to have TdP detected by ILR. The treadmill exercise testing under treatment with a beta-blocker had been evaluated before, but even in the peak exercise (heart rate > 150 bpm), it could not induce any arrhythmias or T-wave alternance (Fig. 1B). On the other hand, ILR could detect the dynamic beat-by-beat change of the T-wave morphology and prolongation of QT (QTc) interval followed by TdP (Fig. 3B,C). These findings suggest that the treadmill exercise testing by the Bruce protocol could not completely reproduce the arrhythmogenic situations in this case, thus ILR may be useful to detect arrhythmic events in the case of sudden increased sympathetic nerve activity.
Therapeutic strategies for LQTS
Beta-blocker is the most effective pharmacological therapy to prevent arrhythmic events and SCD in LQTS, particularly for LQT1 and LQT2. This patient had taken propranolol (maximum 75 mg/day) after diagnosis of LQT1 when she was 7 years old, however, she had recurrence of syncope although she sometimes forgot to take the medicine. On the other hand, not all beta-blockers are the same for LQTS [7], and nadolol is considered more effective compared to other beta-blockers not only in LQT2 but also in LQT1. Thus, the patient's beta-blocker was consequently changed to nadolol (60 mg/day) from propranolol, but it is still uncertain whether she could be free from syncope even if she had taken nadolol.
The JCS/JHRS guidelines [8] recommend ICD for LQTS patients with at least two of the following three findings: 1) history of TdP or syncope, 2) family history of SCD, and 3) refractory to beta-blocker therapy. On the other hand, ESC guidelines more strongly indicate ICD for those who had symptoms while receiving beta-blocker therapy [6].
This patient had already been diagnosed with LQT1 and had several syncope or near syncope events even after taking a beta-blocker, some of them were considered as typical arrhythmic events in LQTS. Furthermore, her younger brother also had a lethal cardiac event resulting in implantation of ICD. Consequently, the patient might be better indicated for ICD before using ILR because she had recurrent syncope even after beta-blocker therapy.
However, this patient was a younger female, and could not easily accept ICD implantation despite repetitive syncope. Moreover, she might have some non-cardiogenic syncope or symptoms due to hyperventilation, dissociative disorders, and reflex syncope. Device infection, lead problems due to long-term implantation, and cosmetic concerns also need to be considered. Taken together, after careful consideration of the benefits and disadvantages of ICD implantation in the young patient, we used ILR to uncover whether her symptoms are really arrhythmogenic or not.
Genotype and variant specific risk n LQTS
About half of patients with KCNQ1 mutations are asymptomatic, and 10–40 % of patients do not have predominant QT prolongation on resting ECG. This patient was identified with c.1032G > A, a splicing variant [9] and most common variant in Japanese LQT1 [10], in which there is an intermediate arrhythmic risk in patients with LQT1 in Japan.
In this patient's family, as shown in Fig. 2, most of the phenotype-positive (LQTS) family members had a history of syncope, and her grandmother died suddenly (etiology unknown) due to discontinuation of beta-blocker therapy. Furthermore, her younger brother suffered cardiac arrest during exercise without discontinuation of beta-blocker. These findings suggest that the patient's arrhythmic risk is relatively high, and that careful observation and advanced therapies need to be considered.
Conclusions
ILR is just a diagnostic tool and not able to prevent SCD. However, most arrhythmic events in LQTS are transient, and ILR may provide supportive evidence to select the optimal therapeutic strategy in cases whose syncope is difficult to diagnose.
Consent statement
Written informed consent was obtained from the patient.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors are grateful to Kazuko Hattori for her excellent clinical support. This work has been partially supported by a grant from Health Science Research Grant from the Ministry of Health, Labor and Welfare of Japan (21FC1004, 23FC1003 to T.A.).
References
- 1.Wilde A.A.M., Schwartz P.J. Long QT syndrome, a diagnosis that warrants expert opinion and expert centers. J Am Coll Cardiol. 2023;81:487–489. doi: 10.1016/j.jacc.2022.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz P.J., Crotti L., Insolia R. Long-QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. 2012;5:868–877. doi: 10.1161/CIRCEP.111.962019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avari Silva J.N., Bromberg B.I., Emge F.K., Bowman T.M., Van Hare G.F. Implantable loop recorder monitoring for refining management of children with inherited arrhythmia syndromes. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balfe C., Durand R., Crinion D., Ward D., Sheahan R. The evidence for the implantable loop recorder in patients with inherited arrhythmia syndromes: a review of the literature. Europace. 2022;24:706–712. doi: 10.1093/europace/euab256. [DOI] [PubMed] [Google Scholar]
- 5.Takase B, Ikeda T, Shimizu W, Abe H, Aiba T, Chinushi M, Koba S, Kusano K, Niwano S, Takahashi N, Takatsuki S, Tanno K, Watanabe E, Yoshioka K, Amino M et al. JCS/JHRS 2022 Guideline on diagnosis and risk assessment of arrhythmia. Circ J 2023. Sep 11. doi:10.1253/circj.CJ-22-0827. Online ahead of print. [DOI] [PubMed]
- 6.Zeppenfeld K., Tfelt-Hansen J., de Riva M., Winkel B.G., Behr E.R., Blom N.A., et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997–4126. doi: 10.1093/eurheartj/ehac262. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman M.J., Priori S.G., Dubin A.M., Kowey P., Linker N.J., Slotwiner D., et al. Beta-blocker therapy for long QT syndrome and catecholaminergic polymorphic ventricular tachycardia: are all beta-blockers equivalent? Heart Rhythm. 2017;14:e41–e44. doi: 10.1016/j.hrthm.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Nogami A., Kurita T., Abe H., Ando K., Ishikawa T., Imai K., et al. JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. J Arrhythm. 2021;37:709–870. doi: 10.1002/joa3.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray A., Donger C., Fenske C., Spillman I., Richard P., Dong Y.B., et al. Splicing mutations in KCNQ1: a mutation hot spot at codon 344 that produces in frame transcripts. Circulation. 1999;100:1077–1084. doi: 10.1161/01.cir.100.10.1077. [DOI] [PubMed] [Google Scholar]
- 10.Itoh H., Dochi K., Shimizu W., Denjoy I., Ohno S., Aiba T., et al. A common mutation of long QT syndrome type 1 in Japan. Circ J. 2015;79:2026–2030. doi: 10.1253/circj.CJ-15-0342. [DOI] [PubMed] [Google Scholar]