This cohort study examines 20 years of data to determine how rates of endophthalmitis after intraocular procedures have changed over time and how the treatment of those patients has changed.
Key Points
Question
How have the rates of endophthalmitis after intraocular procedures changed over time, and how has the treatment of those patients changed?
Findings
This cohort study showed the rate of postprocedure endophthalmitis has decreased 75% over the past 20 years. A similar reduction was seen in the rate of prompt vitrectomy as a primary treatment for endophthalmitis.
Meaning
Although fewer patients are experiencing endophthalmitis, these results suggest recent rates are associated with a reduced rate of prompt vitrectomy.
Abstract
Importance
Long-term trend analyses of overall endophthalmitis rates and treatment patterns are scarce. It is also unknown if the deviation from the recommendations of the Endophthalmitis Vitrectomy Study toward decreased utilization of vitrectomy is associated with different vision outcomes.
Objective
To determine whether the rate of endophthalmitis after intraocular procedures or the primary treatment (prompt vitrectomy vs tap and inject) for endophthalmitis has changed over the past 20 years.
Design, Setting, and Participants
This cohort study examined data for cohorts created by querying for different intraocular procedures, including intravitreal injections and surgeries for cataract removal, glaucoma, retinal conditions, and corneal transplants from 2000 to 2022. The data source was a US administrative medical claims database comprising commercial and Medicare Advantage insurance plans. Any intraocular procedure with at least 6 months of data available before and 6 weeks after the procedure was eligible. Exclusion criteria consisted of any previous diagnosis of endophthalmitis or another intraocular procedure during the follow-up period.
Main Outcome Measure
The main outcomes were rate of postprocedure endophthalmitis and relative rate of prompt vitrectomy (vs tap and inject) as the primary method of treatment.
Results
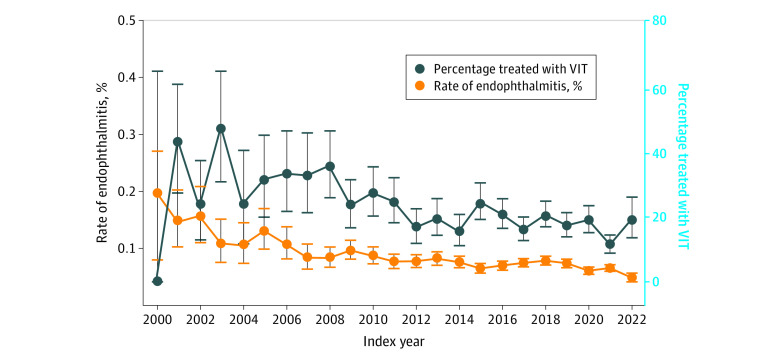
Among 2 124 964 patients, the mean (SD) age was 71.4 (10.2) years; 1 230 320 were female and 894 414 male. Over 22 years, 5 827 809 intraocular procedures were analyzed with 4305 cases of endophthalmitis found for an overall endophthalmitis rate of 0.07%. The yearly rate of endophthalmitis varied but generally declined from a high of 7 cases per 3502 procedures (0.20%) in 2000 to a low of 163 cases per 332 159 procedures (0.05%) in 2022. The percentage of cases treated with prompt vitrectomy also varied but generally declined over time with a high of 17 of 35 (48.6%) in 2003 and a low of 60 of 515 (11.6%) in 2021. Multivariable analysis of the endophthalmitis incidence rate ratio (IRR) showed a per-year decrease of 2.7% (IRR, 0.97; 95% CI, 0.97-0.98; P < .001) over the study period. A similar analysis also showed that the incidence rate of prompt surgical treatment decreased by 3.8% per year throughout the study period (IRR, 0.96; 95% CI, 0.95-0.97; P < .001).
Conclusions and Relevance
This study found that the incidence of endophthalmitis following intraocular procedures appears to have decreased substantially over the past 20 years while prompt vitrectomy is being used less frequently as primary treatment than in the past.
Introduction
Since the dawn of intraocular surgery, endophthalmitis has been a feared, sight-threatening postoperative complication. Tremendous effort has been expended in the hopes of reducing its incidence. This effort has had considerable success over the past century of ophthalmic surgery. With the advent of modern sterilization, the rate of postsurgical endophthalmitis in the mid-1900s was near 1%.1 Over the next 50 years, this rate continued to decrease through the turn of this century.2 Since then, the analysis of endophthalmitis rates has largely fragmented into specialty- and procedure-focused studies, complicating the comparison of rates across differing populations, time periods, and even definitions of endophthalmitis.
Tracking the treatment patterns for endophthalmitis over time has had similar difficulties. Prompt vitrectomy for endophthalmitis after cataract surgery for light perception (LP) vision or worse has been the standard of care since the publication of the Endophthalmitis Vitrectomy Study (EVS) in 1995.3 Despite prompt surgery being the better evidence-based option for this specific cohort of patients with endophthalmitis, studies describing treatment patterns suggest this guideline is regularly not followed.4,5,6 Also, without additional studies to assess the generalizability of the EVS results to other types of procedures, there are no clear mandates for how to treat non-cataract surgery–related endophthalmitis with or without LP vision. Further clouding this picture is the suggested possibility that prompt surgery may benefit patients with presenting visual acuity better than LP.7,8
This analysis used a US medical claims database with more than 20 years of data to assess the rate of endophthalmitis after intraocular procedures and gauge how that rate has changed over time. Similarly, we also evaluated the frequency of prompt vitrectomy (vs tap and inject procedures) used as primary treatment of endophthalmitis and how that rate has changed over time.
Methods
Dataset
A retrospective cohort study was performed using Optum’s deidentified Clinformatics Data Mart database. The database contains all outpatient medical claims (office visits, procedures, and medications given) as well as demographic data and some laboratory values for each beneficiary enrolled in commercial and Medicare Advantage insurance plans. The subset of data available for this study included all patients in the database from January 1, 2000, to June 30, 2022. This study was deemed exempt from review by the University of Pennsylvania’s institutional review board because of the deidentified nature of the database. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies was followed.
Cohort Creation
All intraocular procedures (eg, cataract removals, retina surgery, intravitreal injections, etc) as defined by Current Procedural Terminology (CPT) codes were analyzed (eTable 1 in Supplement 1). Each procedure was considered a unique observation, and the date of the procedure was used as the index date. Final inclusion into the study occurred if the patient had at least 180 days in the insurance plan before and 42 days after the index date. Exclusion occurred for any procedure in which a patient had a second procedure within 42 days following the index date and for any history of previous endophthalmitis before the index date. At any point that an individual was diagnosed with endophthalmitis, all procedures that occurred after the initial endophthalmitis diagnosis were removed from the analysis. Specific to intravitreal injections, any injection that was coded with a bilateral code (CPT code modifier 50) or billed for both a right and left injection on the same day was considered 2 separate injections. Also, because previous work has shown that steroids and various anti–vascular endothelial growth factor agents confer different risks for postinjection endophthalmitis, each injection was categorized based on the medication injected.9,10
Outcome Measures and Statistical Analysis
The primary outcome for this study was the incident rate of developing endophthalmitis after an intraocular procedure. Secondary outcomes included the rate of prompt vitrectomy (VIT) as the primary treatment for endophthalmitis. Cases of endophthalmitis were defined by having a new endophthalmitis diagnosis code in conjunction with a procedure code to treat endophthalmitis. A secondary outcome was the percentage of patients who were treated with VIT compared with an injection of antibiotics alone or a tap-and-inject (TAP) procedure. Prompt vitrectomy was only considered the primary mode of treatment if the vitrectomy occurred the same day or the day following the date of the endophthalmitis diagnosis. CPT codes were used to define both the injection and surgical procedures. Endophthalmitis cases were required to occur 1 to 42 days after the index date. Those cases that occurred on the same day as the index date were excluded because of an inability to distinguish if the intraocular procedure preceded the endophthalmitis-defining procedure.
Overall and yearly rates of endophthalmitis were generated as were rates of those treated by VIT or TAP. Multivariable Poisson regression was performed for incident rate ratio (IRR) calculations and to assess potential risk factors for endophthalmitis, including index year, age, sex, race and ethnicity, geographic location, procedure type (cataract, glaucoma, corneal transplant, retina, intravitreal injections, and combined), and medication injected for intravitreal injections. This type of modeling was chosen as it is the preferred method for dealing with outcomes that involve counts and cannot go below zero, as is the case with endophthalmitis. Procedure type was coded within each type of surgery as a binary (yes/no) variable (ie, a combined cataract-glaucoma procedure would have been included in the following categories: cataract surgery, glaucoma surgery, and combined). Given the size of the cataract surgery (without including combined procedures) and intravitreal injection cohorts, the yearly rates of postprocedure endophthalmitis also were calculated separately for each cohort as they would likely be the primary drivers of any overall rate trend. Other categories of procedures did not have enough observations to make useful inferences on a yearly basis. Optum’s Clinformatics Data Mart database uses a proprietary algorithm derived from geographic location and last name to assign race and ethnicity.
Statistical analysis was performed using SAS version 9.4 (SAS Institute). Statistical significance was assessed at the 95% significance level and all tests were 2-tailed, but no correction for multiple testing was applied.
Results
Postprocedure Endophthalmitis Rates
After inclusion and exclusion criteria were applied, 5 827 809 intraocular procedures from 2 124 964 patients were analyzed (Table 1). Of these, 4305 patients developed endophthalmitis, resulting in an overall endophthalmitis rate of 0.07% (Table 2). The mean (SD) age of patients with endophthalmitis was older than those without (73.8 [11.0] vs 71.4 [10.2] years; P < .001). No differences were seen in the rate among males vs females (risk difference [RD], 0.00; 95% CI, −0.01 to 0.02; P = .17) or among the races and ethnicities (RR from −0.03 to −0.5; 95% CI, −0.08 to 0.01; P = .08) of individuals who got endophthalmitis. The yearly rate generally decreased over time from a high of 7 cases per 3502 procedures (0.20%) in 2000 to a low of 163 cases per 332 159 procedures (0.05%) in 2022 (P < .001) (Figure 1 and eTable 2 in Supplement 1). Procedure/specialty-specific rates of endophthalmitis included a rate of 0.08% for cataract surgery, 0.21% for corneal transplants (RD vs cataracts, 0.13; 95% CI, 0.08 to 0.19; P = .004), 0.17% for combined surgeries (RD, 0.09; 95% CI, −0.06 to 0.13; P = .26), 0.16% for retina surgery (RD, 0.08; 95% CI, 0.06 to 0.10; P = .006), 0.16% for glaucoma surgeries (RD, 0.08; 95% CI, 0.05 to 0.11; P = .005), and 0.06% for intravitreal injections (RD, −0.02; 95% CI, −0.02 to −0.01; P = .048). The rate of endophthalmitis was also different among various intravitreal injection agents with steroids being the highest rate (0.25%), followed in order by aflibercept (0.07%), ranibizumab (0.06%), bevacizumab (0.05%), and brolicizumab (0.04%) (P < .001).
Table 1. Characteristics of Patients Who Had Procedures Between 2000 and 2022.
| Characteristic | No. of patients (N = 2 124 964) | No. with endophthalmitis (n = 4304) | Endophthalmitis rate, %a | Rate difference (95% CI) | P value |
|---|---|---|---|---|---|
| Age, mean (SD), y | 71.4 (10.2) | 73.8 (11.0) | NA | NA | <.001 |
| Gender | |||||
| Female | 1 230 320 | 2477 | 0.20 | Reference | .17 |
| Male | 894 414 | 1827 | 0.20 | 0.00 (−0.01 to 0.02) | |
| Race and ethnicityb | |||||
| Asian | 64 991 | 108 | 0.17 | −0.05 (−0.08 to −0.01) | .08 |
| Black | 203 290 | 373 | 0.18 | −0.03 (−0.05 to −0.01) | |
| Hispanic | 196 616 | 357 | 0.18 | −0.03 (−0.05 to −0.01) | |
| White | 1 524 825 | 3223 | 0.21 | Reference | |
| Unknown | 135 242 | 244 | 0.18 | −0.03 (−0.05 to −0.01) | |
| Geographic location | |||||
| Northeast | 240 028 | 521 | 0.22 | Reference | .007 |
| Mountain | 220 960 | 475 | 0.21 | −0.00 (−0.03 to 0.02) | |
| Pacific | 267 758 | 555 | 0.21 | −0.01 (−0.04 to 0.02) | |
| South Atlantic | 498 684 | 1093 | 0.22 | 0.00 (−0.02 to 0.02) | |
| Southern Midwest | 373 667 | 688 | 0.18 | −0.03 (−0.06 to −0.01) | |
| Upper Midwest | 521 401 | 968 | 0.19 | −0.03 (−0.05 to −0.01) | |
| Unknown | 2466 | 5 | 0.20 | −0.01 (−0.19 to 0.16) |
Abbreviation: NA, not applicable.
Rates are higher than elsewhere in the article because this was a per-person instead of a per-procedure analysis as 1 person can have multiple procedures assessed.
Optum’s Clinformatics Data Mart database uses a proprietary algorithm derived from geographic location and last name to assign race and ethnicity.
Table 2. Rate of Endophthalmitis and Type of Primary Treatment by Procedure Type.
| Procedure typea | No. with endophthalmitis (n = 4305) | No. of procedures (n = 5 827 809) | Endophthalmitis rate, % | Rate difference (95% CI) | P valueb | VIT, No. (%) | Rate difference (95% CI) | P valuec |
|---|---|---|---|---|---|---|---|---|
| Cataract | 1659 | 2 051 926 | 0.08 | Reference | .35 | 392 (23.6) | Reference | .93 |
| Corneal transplants | 61 | 28 646 | 0.21 | 0.13 (0.08 to 0.19) | .004 | 17 (27.9) | 4.24 (−7.20 to 15.68) | .85 |
| Glaucoma | 148 | 91 718 | 0.16 | 0.08 (0.05 to 0.11) | .005 | 36 (24.3) | 0.70 (−6.51 to 7.90) | .96 |
| Retina surgery | 250 | 155 863 | 0.16 | 0.08 (0.06 to 0.10) | .006 | 49 (19.6) | −4.03 (−9.36 to 1.30) | .59 |
| Combined surgeriesd | 103 | 59 079 | 0.17 | 0.09 (−0.06 to 0.13) | .26 | 24 (23.3) | −0.33 (−8.74 to 8.09) | .83 |
| Any intravitreal injection | 2290 | 3 554 119 | 0.06 | −0.02 (−0.02 to −0.01) | .048 | 407 (17.8) | −5.86 (−8.43 to −3.28) | .46 |
| Individual intravitreal medications | ||||||||
| Bevacizumab | 926 | 1 733 916 | 0.05 | NA | NA | NA | NA | NA |
| Ranibizumab | 297 | 507 487 | 0.06 | NA | NA | NA | NA | NA |
| Aflibercept | 706 | 983 725 | 0.07 | NA | NA | NA | NA | NA |
| Brolucizumab | 3 | 7909 | 0.04 | NA | NA | NA | NA | NA |
| Steroids | 173 | 69 886 | 0.25 | NA | NA | NA | NA | NA |
| Other/unknown | 185 | 251 196 | 0.07 | NA | NA | NA | NA | NA |
| Total | 4305 | 5 827 809 | 0.07 | NA | NA | NA | NA | NA |
Abbreviations: NA, not applicable; VIT, prompt vitrectomy.
Surgeries that included more than 1 type of surgery (eg, cataract and glaucoma) are counted in more than 1 category and also in the combined category. As such, the sum of each procedure group will be higher than the total number of procedures performed.
Comparing endophthalmitis rate between the surgery cohort and the no-surgery cohort. Generalized estimating equations were applied to account for repeated measurement correlation.
Comparing VIT rate between the surgery cohort and the no-surgery cohort. Generalized estimating equations were applied to account for repeated measurement correlation.
Combined surgeries happened when any 2 categories of procedure types occurred on the same day.
Figure 1. Rate of Endophthalmitis and Percentage Treated With Prompt Vitrectomy (VIT) by Year.
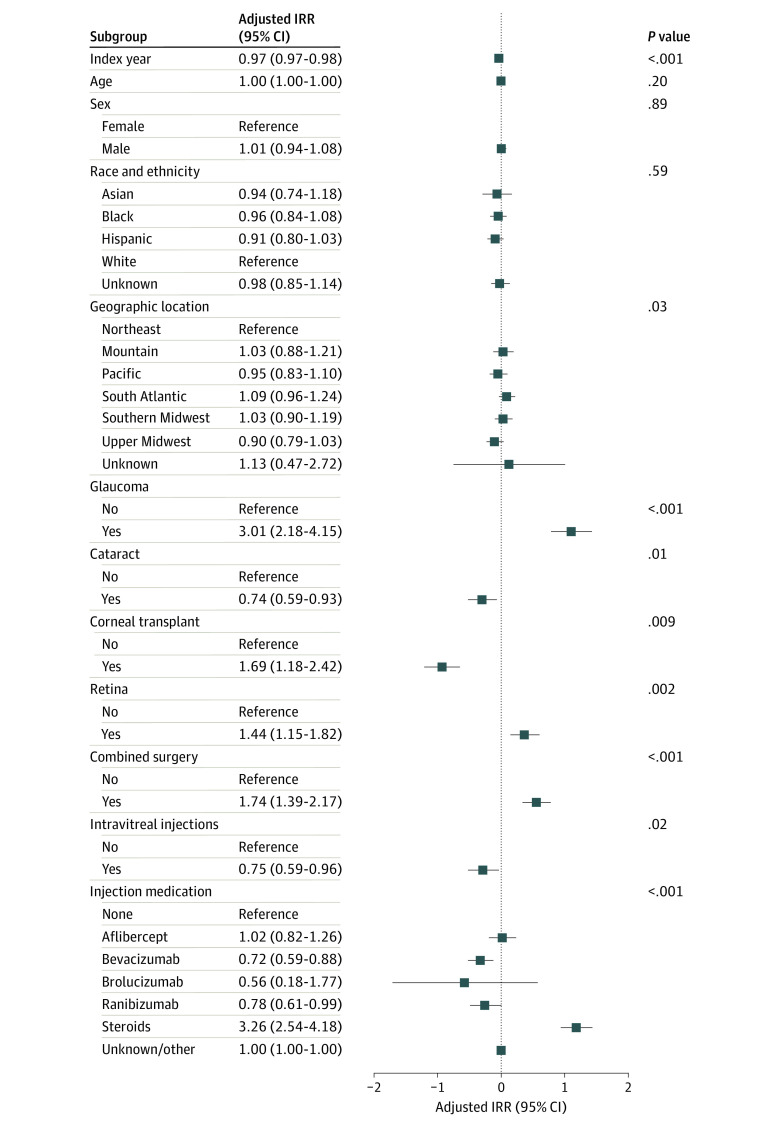
Univariate analysis showed that over the period of observation, the IRR for endophthalmitis decreased by 3.2% each year (IRR, 0.97; 95% CI, 0.96-0.98; P < .001). Multivariable analysis of the endophthalmitis IRR showed that after controlling for all other variables, a per-year decrease of 2.7% (IRR, 0.97; 95% CI, 0.97-0.98; P < .001) was found (Figure 2 and eTable 3 in Supplement 1). Compared with patients who did not have the specified type of procedure, patients who had a glaucoma surgery (IRR, 3.01; 95% CI, 2.18-4.15; P < .001), corneal transplant (IRR, 1.69; 95% CI, 1.18-2.41; P = .009), retina (IRR, 1.44; 95% CI, 1.15-1.82; P = .002), or a combined surgery (IRR, 1.74; 95% CI, 1.39-2.17; P < .001) had higher IRRs for endophthalmitis. Patients who had cataract (IRR, 0.74; 95% CI, 0.59-0.93; P = .01) or injection (IRR, 0.75; 95% CI, 0.59-0.96; P = .02) procedures had a reduced IRR for endophthalmitis compared with other procedure categories. The type of medication injected in intravitreal injections was also associated with endophthalmitis. The adjusted IRRs for bevacizumab (IRR, 0.72; 95% CI, 0.59-0.88) and ranibizumab (IRR, 0.78; 95% CI, 0.61-0.99) were lower and steroids were higher (IRR, 3.26; 95% CI, 2.54-4.18) compared with procedures not involving injections (P < .001 for all comparisons).
Figure 2. Forest Plot of the Multivariable Results for Incident Rate Ratios (IRRs) of Endophthalmitis.
The overall rate of postcataract endophthalmitis was 0.08% with 1568 cases occurring after 1 997 431 cataract procedures. The rate of endophthalmitis generally declined from 2000 when it was 0.22% to a low of 0.05% in 2022 (RD, −0.17; 95% CI, −0.33 to −0.00; P = .047). The overall rate of post–intravitreal injection endophthalmitis was 0.06% with 2290 cases of endophthalmitis cases occurring after 3 556 207 injections. Before 2005, intravitreal injections were relatively uncommon with varying rates of endophthalmitis. However, starting in 2005, again, a general decline in endophthalmitis rates was seen with the highest rate of 0.28% occurring in 2005 and the lowest of 0.04% in 2022 (RD, −1.41; 95% CI, −4.21 to 1.40; P = .33) (Table 3). After controlling for other factors, including the type of drug injected for intravitreal injections, a 2% and 4% per-year decrease was seen in endophthalmitis rates after cataract and intravitreal injections, respectively (cataract IRR, 0.98, 95% CI, 0.97 to 0.99; P < .001; injection IRR, 0.96, 95% CI, 0.95 to 0.98; P < .001).
Table 3. Rates of Endophthalmitis After Cataract Surgeries and Intravitreal Injections by Year.
| Calendar year | No. of cataract procedures | No. with endophthalmitis | Endophthalmitis rate, % | Rate difference (95% CI) | P valuea | No. of intravitreal injections | No. with endophthalmitis | Endophthalmitis rate, % | Rate difference (95% CI) | P valuea |
|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 3170 | 7 | 0.22 | Reference | NA | 3 | 0 | 0.00 | NA | NA |
| 2001 | 22 051 | 32 | 0.15 | −0.08 (−0.25 to 0.10) | .39 | 42 | 0 | 0.00 | NA | NA |
| 2002 | 26 148 | 37 | 0.14 | −0.08 (−0.25 to 0.09) | .36 | 276 | 4 | 1.45 | Reference | NA |
| 2003 | 27 482 | 30 | 0.11 | −0.11 (−0.28 to 0.06) | .19 | 886 | 2 | 0.23 | −1.22 (−4.05 to 1.60) | .40 |
| 2004 | 29 414 | 28 | 0.10 | −0.13 (−0.29 to 0.04) | .14 | 1756 | 1 | 0.06 | −1.39 (−4.20 to 1.42) | .33 |
| 2005 | 33 412 | 38 | 0.11 | −0.11 (−0.27 to 0.06) | .21 | 4653 | 13 | 0.28 | −1.17 (−3.98 to 1.64) | .41 |
| 2006 | 38 282 | 28 | 0.07 | −0.15 (−0.31 to 0.02) | .08 | 10 940 | 23 | 0.21 | −1.24 (−4.05 to 1.57) | .39 |
| 2007 | 46 716 | 32 | 0.07 | −0.15 (−0.32 to 0.01) | .07 | 18 450 | 20 | 0.11 | −1.34 (−4.15 to 1.47) | .35 |
| 2008 | 62 711 | 48 | 0.08 | −0.14 (−0.31 to 0.02) | .09 | 33 110 | 25 | 0.08 | −1.37 (−4.18 to 1.43) | .34 |
| 2009 | 76 560 | 65 | 0.09 | −0.14 (−0.30 to 0.03) | .11 | 50 938 | 48 | 0.09 | −1.35 (−4.16 to 1.45) | .34 |
| 2010 | 78 627 | 52 | 0.07 | −0.15 (−0.32 to 0.01) | .07 | 70 971 | 75 | 0.11 | −1.34 (−4.15 to 1.46) | .35 |
| 2011 | 89 486 | 65 | 0.07 | −0.15 (−0.31 to 0.02) | .08 | 104 167 | 65 | 0.06 | −1.39 (−4.20 to 1.42) | .33 |
| 2012 | 102 763 | 82 | 0.08 | −0.14 (−0.31 to 0.02) | .09 | 140 232 | 98 | 0.07 | −1.38 (−4.19 to 1.43) | .34 |
| 2013 | 95 260 | 77 | 0.08 | −0.14 (−0.30 to 0.02) | .10 | 135 138 | 93 | 0.07 | −1.38 (−4.19 to 1.42) | .34 |
| 2014 | 107 679 | 94 | 0.09 | −0.13 (−0.30 to 0.03) | .11 | 176 105 | 109 | 0.06 | −1.39 (−4.20 to 1.42) | .33 |
| 2015 | 120 773 | 88 | 0.07 | −0.15 (−0.31 to 0.02) | .08 | 224 341 | 129 | 0.06 | −1.39 (−4.20 to 1.42) | .33 |
| 2016 | 144 080 | 118 | 0.08 | −0.14 (−0.30 to 0.03) | .10 | 287 008 | 164 | 0.06 | −1.39 (−4.20 to 1.42) | .33 |
| 2017 | 168 413 | 146 | 0.09 | −0.13 (−0.30 to 0.03) | .11 | 354 028 | 228 | 0.06 | −1.39 (−4.19 to 1.42) | .33 |
| 2018 | 147 246 | 135 | 0.09 | −0.13 (−0.29 to 0.03) | .12 | 346 235 | 227 | 0.066 | −1.38 (−4.19 to 1.42) | .33 |
| 2019 | 151 217 | 101 | 0.07 | −0.15 (−0.32 to 0.01) | .07 | 382 640 | 284 | 0.074 | −1.38 (−4.18 to 1.43) | .34 |
| 2020 | 146 502 | 104 | 0.07 | −0.15 (−0.31 to 0.01) | .07 | 420 487 | 226 | 0.054 | −1.40 (−4.20 to 1.41) | .33 |
| 2021 | 196 276 | 116 | 0.06 | −0.16 (−0.33 to 0.00) | .053 | 556 764 | 353 | 0.063 | −1.39 (−4.19 to 1.42) | .33 |
| 2022b | 83 163 | 45 | 0.05 | −0.17 (−0.33 to −0.00) | .047 | 237 037 | 103 | 0.043 | −1.41 (−4.21 to 1.40) | .33 |
| Total | 1 997 431c | 1568c | 0.08 | NA | NA | 355 6207 | 2290 | 0.06 | NA | NA |
Abbreviation: NA, not applicable.
Generalized estimating equations were applied to account for correlation because of multiple observations coming from a single patient.
Only 5 months of data were available for analysis.
Totals are different from Table 2 because this table does not include cataracts that were part of a combined procedure.
Prompt Vitrectomy Rates
The proportion of cases treated by VIT (vs TAP) also generally decreased over time, ranging from a high of 17 of 35 (48.6%) in 2003 to a low of 60 of 515 (11.6%) in 2021. The patients who received VIT varied across procedure types, ranging from 407 of 2290 (17.8%) for postinjection endophthalmitis to 17 of 61 (27.9%) for corneal transplant–related endophthalmitis (P = .22) (Table 2). Univariate analysis showed that the IRR for VIT (vs TAP) decreased over the observation period by 4.1% per year (IRR, 0.96; 95% CI, 0.95-0.97; P < .001).
Multivariable analysis also showed that the incidence rate of prompt surgical treatment decreased 3.8% per year throughout the study (IRR, 0.96; 95% CI, 0.95-0.97; P < .001) (eTable 2 in Supplement 1). Males were slightly less likely to receive prompt surgery (IRR, 0.87; 95% CI, 0.75-1.00; P = .046). Geographic location was also associated with prompt surgery because patients with endophthalmitis in both the upper and southern Midwest states were more likely to get prompt surgery than patients in the Northeast (upper Midwest IRR, 1.38; 95% CI, 1.04-1.81; southern Midwest IRR, 1.55; 95% CI, 1.17-2.06; P = .004) (eTable 3 in Supplement 1).
Discussion
In a study covering 22 years of observation from across the US and more than 5.8 million intraocular procedures, the rate of endophthalmitis varied dramatically, starting at 0.2% initially in 2000 and decreasing by 75% to a low of 0.05% in 2022. Over the entire analysis period, the rate of postprocedure endophthalmitis decreased by an adjusted average of 2.7% per year, even after accounting for the percentages of procedures that were performed that typically have lower rates of endophthalmitis. Also of note is the fact that while the number of procedures was increasing every year, the rate of endophthalmitis continued to decrease. The rate of VIT as primary treatment for endophthalmitis also varied considerably but generally declined during the study period from a high of 48.5% of cases in 2003 to 11.7% in 2021. After controlling for other factors, the rate of VIT as a treatment for endophthalmitis also decreased by 3.8% per year over the whole period.
The reduction in the rate of postprocedure endophthalmitis over time has been similarly reported in a recent nationwide study from France.11 The report showed the rate of endophthalmitis after intraocular procedures was 0.05% from 2009 to 2018. This is lower than the rate found in our study over the same time frame (our 2009-2018 data: 0.08%; 2428 endophthalmitis cases/3 172 355 procedures). This difference seems to be driven mostly by the rate of endophthalmitis after intravitreal injections, which was considerably higher in our dataset (0.06% vs 0.02%). Why this difference exists between our studies is not clear but may be related to the relative higher use of intraocular steroids in the US, which are known to carry a much higher risk of endophthalmitis.9
Surgeries involving glaucoma procedure conferred the highest risk for endophthalmitis in our analysis. Sabharwal et al12 recently reported on Medicare data from 2016 to 2019 showing an elevated risk ratio of 1.5 and an endophthalmitis rate of 0.12% for all glaucoma surgeries, with trabeculectomies and tube shunts having a higher risk than microinvasive glaucoma surgery (MIGS) procedures. In our analysis, both the IRR and the overall rate were higher at 3.0 and 0.16%, respectively. Some of these contrasts may be explained by the different time periods evaluated and dissimilar surgical case mix. Certainly, early in our study period, nearly all glaucoma surgeries in our population were trabeculectomies and tube shunts, with only recent years having MIGS procedures constituting a larger percentage of the glaucoma procedures performed.
Consistent with previous studies, we found a relatively low rate of endophthalmitis after cataract surgery. Although the study by Sabharwal et al12 focused on rates after glaucoma surgery, they also found a postcataract endophthalmitis rate of 0.08%, which is identical to the rate we found. Interestingly, a different Medicare study using data from 2011 to 2019 found a higher rate of endophthalmitis (0.13%) after cataract surgery but used a longer window (90 days vs 42 days) to define endophthalmitis.13 These rates are all higher than one reported from an Intelligent Research in Sight Registry study (0.05%).14 This difference may reflect a difference in how registry data are acquired compared with medical claims data because endophthalmitis cases are often cared for at tertiary care centers, which may not report their data to the Intelligent Research in Sight Registry.
The lowest rate of endophthalmitis across all procedures in our analysis was found after intravitreal injections. Previous studies using US administrative claims data have assessed the rate of endophthalmitis after intravitreal injections, all of which found lower rates than that presented in this analysis.9,10,15 This is likely due to the previous studies using only a 15-day window to observe for endophthalmitis and the current analysis using a 42-day window, which was thought necessary for comparison purposes across procedure types. These results corroborate and continue an earlier finding using the same database that the rate of postinjection endophthalmitis had decreased through 201610 and now has continued through 2022. This improvement is likely secondary to multiple additive improvements over time in procedure safety, including discontinuing preinjection and postinjection antibiotics, stressing the importance of limiting aerosolized oral bacterial contamination (no-talk technique) and the increasing use of prefilled syringes.
Our study also found that the use of VIT as the primary treatment option has steadily declined over the past 20 years. We also found the type of procedure leading to the endophthalmitis was not associated with having prompt surgery, suggesting patients who had noncataract procedures were just as likely to have prompt surgery as those undergoing other types of procedures. Although presenting visual acuity is unavailable in the database used in this study, other studies support the conclusion that VIT for endophthalmitis is being performed less often. One survey of practice trends found only 75% of retina surgeons would perform VIT on LP eyes, despite the findings of the EVS.3,16 Furthermore, several case series have shown that even fewer LP eyes are getting VIT in practice, one as low as only 10% of LP eyes.4,5,6 This trend is concerning because the EVS offers clear evidence of the benefit of VIT in LP eyes, and more recent studies have suggested eyes with better than LP vision may benefit as well.7,8
Limitations
Several limitations of this study need to be considered when evaluating its results. First, because of the deidentified nature of medical claims data, we are unable to verify clinical data with a medical record review, nor do we have access to laboratory results to determine causative organisms. This also impacts our ability to determine whether patients “should” have had VIT or not based on their presenting visual acuity or other factors that may limit the ability to perform surgery (eg, corneal clarity) with regard to the EVS guidelines. Next, the data used in this study come from a single medical claims database and may not generalize to other populations (ie, individuals who are uninsured or insured through other means, such as the Veterans Health Administration). Third, we did not include the rates of endophthalmitis after trauma. One of the primary questions of interest was the rate of endophthalmitis in controlled settings. While intracameral antibiotics have been considered as a possible way of decreasing the rate of endophthalmitis, given the design of this study, evaluating the role of intracameral antibiotics was outside the scope of this investigation. Because the risk of introduction of outside infectious contaminates is much higher in traumatic cases, we felt this represented a risk outside of the control of the proceduralist and did not include them in this analysis.
Conclusions
The rate of endophthalmitis in our study has steadily decreased over the past 20 years. However, the rate of VIT as treatment for postoperative endophthalmitis has also been steadily decreasing over this time. Additional work will need to be done to evaluate the reason behind this change in management and the clinical impact this change in management has on outcomes.
eTable 1. Codes used within this study
eTable 2. Number and rates of endophthalmitis cases and types of treatment by year over time
eTable 3. Multivariable analysis of incident rate ratios for endophthalmitis rates and immediate surgery
Data sharing statement
References
- 1.Allen HF, Mangiaracine AB. Bacterial endophthalmitis after cataract extraction: a study of 22 infections in 20,000 operations. Arch Ophthalmol. 1964;72:454-462. doi: 10.1001/archopht.1964.00970020454003 [DOI] [PubMed] [Google Scholar]
- 2.Eifrig CWG, Flynn HW Jr, Scott IU, Newton J. Acute-onset postoperative endophthalmitis: review of incidence and visual outcomes (1995-2001). Ophthalmic Surg Lasers. 2002;33(5):373-378. doi: 10.3928/1542-8877-20020901-06 [DOI] [PubMed] [Google Scholar]
- 3.Endophthalmitis Vitrectomy Study Group . Results of the Endophthalmitis Vitrectomy Study: a randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113(12):1479-1496. [PubMed] [Google Scholar]
- 4.Yannuzzi NA, Si N, Relhan N, et al. Endophthalmitis after clear corneal cataract surgery: outcomes over two decades. Am J Ophthalmol. 2017;174:155-159. doi: 10.1016/j.ajo.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 5.Gower EW, Keay LJ, Stare DE, et al. Characteristics of endophthalmitis after cataract surgery in the United States Medicare population. Ophthalmology. 2015;122(8):1625-1632. doi: 10.1016/j.ophtha.2015.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pijl BJ, Theelen T, Tilanus MAD, Rentenaar R, Crama N. Acute endophthalmitis after cataract surgery: 250 consecutive cases treated at a tertiary referral center in the Netherlands. Am J Ophthalmol. 2010;149(3):482-7.e1, 2. doi: 10.1016/j.ajo.2009.09.021 [DOI] [PubMed] [Google Scholar]
- 7.Mason LB, Mason JO III, Friedman DA, Mason JO IV; European Society of Medicine . Postoperative bacterial endophthalmitis: tap/inject versus sutureless vitrectomy. Published 2017. Accessed February 15, 2024. https://esmed.org/MRA/mra/article/view/999
- 8.Dib B, Morris RE, Oltmanns MH, Sapp MR, Glover JP, Kuhn F. Complete and early vitrectomy for endophthalmitis after cataract surgery: an alternative treatment paradigm. Clin Ophthalmol. 2020;14:1945-1954. doi: 10.2147/OPTH.S253228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanderBeek BL, Bonaffini SG, Ma L. The association between intravitreal steroids and post-injection endophthalmitis rates. Ophthalmology. 2015;122(11):2311-2315.e1. doi: 10.1016/j.ophtha.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bavinger JC, Yu Y, VanderBeek BL. Comparative risk of endophthalmitis after intravitreal injection with bevacizumab, aflibercept, and ranibizumab. Retina. 2019;39(10):2004-2011. doi: 10.1097/IAE.0000000000002351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baudin F, Benzenine E, Mariet AS, et al. Epidemiology of acute endophthalmitis after intraocular procedures: a national database study. Ophthalmol Retina. 2022;6(6):442-449. doi: 10.1016/j.oret.2022.01.022 [DOI] [PubMed] [Google Scholar]
- 12.Sabharwal J, Dai X, Dun C, et al. Early endophthalmitis incidence and risk factors after glaucoma surgery in the Medicare population from 2016 to 2019. Ophthalmology. 2024;131(2):179-187. [DOI] [PubMed] [Google Scholar]
- 13.Zafar S, Dun C, Srikumaran D, et al. Endophthalmitis rates among Medicare beneficiaries undergoing cataract surgery between 2011 and 2019. Ophthalmology. 2022;129(3):250-257. doi: 10.1016/j.ophtha.2021.09.004 [DOI] [PubMed] [Google Scholar]
- 14.Pershing S, Lum F, Hsu S, et al. Endophthalmitis after cataract surgery in the United States: a report from the Intelligent Research in Sight Registry, 2013-2017. Ophthalmology. 2020;127(2):151-158. doi: 10.1016/j.ophtha.2019.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vo LV, Lapakko ZJ, Leder HA, et al. Certification and credentials of intravitreal injection proceduralists in the United States. Ophthalmol Retina. 2021;5(5):487-489. doi: 10.1016/j.oret.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 16.Fliney GD, Pecen PE, Cathcart JN, Palestine AG. Trends in treatment strategies for suspected bacterial endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):833-838. doi: 10.1007/s00417-018-3910-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Codes used within this study
eTable 2. Number and rates of endophthalmitis cases and types of treatment by year over time
eTable 3. Multivariable analysis of incident rate ratios for endophthalmitis rates and immediate surgery
Data sharing statement