Abstract
Evidence suggests that multiple sclerosis (MS) induces a decline in motor and cognitive function and provokes a shift in gut microbiome composition in patients. Therefore, the aim of the study was to explore the effect of dance classes on the motor and cognitive functions and gut microbiota composition of MS patients. In this randomized controlled trial, 36 patients were randomly divided into two groups: the experimental group (n = 18) and the passive control group (n = 18). Supervised rock and roll and sports dance classes were performed for 12 weeks at a frequency of two times a week. Before and after the intervention, fecal samples were taken and the motor and cognitive function assessments were completed. Fecal microbiota were categorized using primers targeting the V3–V4 region of 16S rDNA. Our results revealed significant differences in mobility performance (T25‐FWT), attention and working memory (TMT B), and finger dexterity (9‐HPT) within the experimental group. Furthermore, we reported favorable shifts in gut microbial communities (an increase in Blautia stercoris and a decrease in Ruminococcus torques) within the experimental group. In conclusion, our randomized control trial on the effects of 12‐week dance classes in MS patients found significant improvements in motor and cognitive functions, with further moderate influence on gut microbiota composition.
Keywords: cognitive function, dance classes, gut microbiota, motor function, multiple sclerosis
Highlights
This study demonstrated that dance class training improved mobility, leg function performance (T25‐FW), upper extremity function (9‐HPT), and cognitive functions (TMT‐B) in MS patients.
An increase in some common and some not yet fully reviewed commensals, for example, Blautia stercoris, Clostridium algidixylanolyticum, Eubacterium xylanophilum, Megasphaera indica, and Parabacteroides faecis, was observed after dance class training in MS patients.
This study showed a significant decrease in pathogens, namely Parvimonas micra and Ruminococcus, within the experimental group; on the contrary, we reported an increased relative abundance of other pro‐inflammatory bacteria, Bilophila wadsworthia, within the control group.
1. INTRODUCTION
Multiple sclerosis (MS) is a demyelinating disease of the human central nervous system mediated by inflammation (Trapp & Nave, 2008). Progressive decreases in the physical function and mobility of the lower extremities are one of well‐studied and most significant outcomes of MS (Jeng et al., 2023). Besides, there is evidence reporting a moderate dysbiosis in the structure of the gut microbiota in MS patients compared to healthy subjects (Miyake et al., 2015; Ordoñez‐Rodriguez et al., 2023), whereas emerging research has shown that the gut microbiome plays a crucial role in maintaining overall health, including its potential influence on the immune system and neurological conditions (Menees et al., 2022; Ratsika et al., 2023).
Even though research is still in its early stages, the gut microbiome may have a significant impact on MS (Altieri et al., 2023; Bronzini et al., 2023; Dunalska et al., 2023). The shift in the bacterial composition may be related to inflammation and immune dysregulation, which are characteristic features of MS (Boussamet et al., 2022). In MS‐typical dysbiosis, holistic approaches showed a reduction of pathobionts and an increase in short‐chained fatty acid (SCFA)‐producing beneficial bacteria (Barone et al., 2021).
As reported previously, physical exercise has been shown to affect the gut microbiome in humans (Allen et al., 2018; Dziewiecka et al., 2022). Exercise can lead to changes in gut microbial diversity and composition, with some studies suggesting an increase in beneficial microbial species (Monda et al., 2017; Motiani et al., 2020; Ramos et al., 2022). However, there are still limited data on the convincing positive relationship between physical exercise and the modification of the gut microbiome in MS individuals (Mokhtarzade et al., 2021). Moreover, there are still questions regarding the intensity and type of physical exercise aimed at positively changing the gut microbiome and improving motor and cognitive functions.
Literature provides a plethora of physical exercise strategies for improving health‐related quality of life, motor function, and fatigue in MS patients (Flores et al., 2023). Despite the scarcity of specific research, there is promising potential for health‐related advantages in adopting dance classes for MS patients (Carapellotti et al., 2023; Mandelbaum et al., 2016). Importantly, apart from improved physical fitness and motor function, MS patients may also experience dancing with various other advantages, such as emotional well‐being and social interaction (Mandelbaum et al., 2016).
Therefore, the purpose of this study was to explore the effect of a 12‐week dance class training program on the gut microbiota composition, motor and cognitive functions, and physical fitness of MS patients.
2. MATERIALS & METHODS
2.1. Study subjects and recruitment
From March/2022 to May/2022, a total of 36 patients with relapsing‐remitting MS, diagnosed according to official diagnostic criteria (Thompson et al., 2018), were constitutively recruited from the Multiple Sclerosis Center at the Second Department of Neurology, Commenius University and University Hospital Bratislava, Slovakia. Due to its potential impact on fatigue, anxiety, and depression, our exclusion criteria were (i) recent relapse less than 3 months (also any worsening with the need for corticosteroids) and (ii) serious comorbidities, including psychiatric diseases.
Patients were randomized either for dance class intervention (experimental group, EXP) or passive control group (CTRL). The CTRL patients were not engaged in structured or any self‐induced physical exercise or activity (sedentary individuals). Basic demographic and clinical characteristics are shown in Table 1. Patients in both groups had been treated mostly with second‐line disease‐modifying therapy (DMT) (fingolimod, natalizumab, ocrelizumab, and cladribine); only 4 patients had been treated with first‐line DMT (interferon beta, glatiramer acetate, and dimethylfumarate).
TABLE 1.
Demographic and clinical data of experimental and control group of patients with MS.
| EXP group | CTRL group | |
|---|---|---|
| Number of MS patients | 16 | 13 |
| Age (years) | 42.8 ± 8.1 | 39.5 ± 9.0 |
| Sex (M/F) | 4/12 | 4/9 |
| BMI (kg/m2) | 26.9 ± 5.8 | 25.2 ± 4.4 |
| Disease duration (years) | 9.8 ± 6.4 | 8.7 ± 6.6 |
| EDSS | 3.31 ± 1.17 | 2.83 ± 1.39 |
Note: Values are presented as mean ± SD.
Abbreviations: BMI, Body mass index; CTRL group, control group; DMT, Disease Modifiyng Therapy; EDSS, Expanded Disability Status Scale; EXP group, Experimental group; F, Female; M, Male; MS, Multiple Sclerosis.
Data for this study were obtained in a clinical trial named Gut Microbiota Composition, Cognitive Function, and Physical Fitness in Multiple Sclerosis Patients (ClinicalTrials.gov, No: NCT06220409). This research was carried out in accordance with the Helsinki Declaration Principles for Human Experiments. Following the reading of the informed consent form, an explanation of the study steps, and talks with the investigators, all patients and controls signed the informed consent form. The research was approved by the Ethics Committee of Derer's University Hospital in Ethics Committee of Derer's University Hospital in Bratislava with No. 27/2022.
2.2. Study design
All participants underwent blood and stool sample collection, the evaluation of body composition, motor and cognitive function, and physical fitness assessment in the given order, starting at 8 a.m. For the EXP, the assessments were performed before and after 12 weeks of intervention; for the CTRL, they were performed at baseline and after 12 weeks.
2.2.1. Experimental intervention
Rock and roll classes were persuaded weekly on Tuesdays and sports dances on Thursdays under the supervision of certificated trainers. The goal of rock and roll classes was to learn and develop the basic skills and techniques of rock and roll. At the commencement of the program, we initiated the exercise routine with a total of eight repetitions, divided into two sets for each step. The basic steps were gradually combined with the dance figures. Initially, the movement was executed at a slow pace, devoid of musical accompaniment, and lacking in rhythmic buoyancy. Subsequently, the tempo increased, accompanied by a rhythmic bounce and the addition of musical elements. Once the correct technique was established, we proceeded to integrate individual steps into different combinations, which were subsequently incorporated into each practice session of the program as a designated warm‐up routine. The tempo of the music utilized during training sessions varied between 156 and 184 beats per minute (bpm), with the specific value being contingent upon the level of difficulty associated with the exercises being performed. Throughout the duration of the program, a total of five choreographies were constructed, each with a varying duration ranging from 30 to 60 s.
Sports dance classes on Thursdays aimed to teach the basics of ballroom dances (waltz, tango, Viennese waltz, quickstep) and Latin American dances (samba, chacha, rumba, jive). Both types of dance classes started with a 5‐min warm‐up, followed by 10‐min dynamic stretching, and then the 40‐min main part, which we always ended with static stretching. The patients became familiar with the various dances, as well as the traits, beats, and fundamental figures that we blended to create basic sets. Ten counts were made for each new step, followed by five counts while listening to calm music. After that, more moves were incorporated and linked to the choreography, which was performed five times: five times at a slow tempo, five times at a standard tempo, and five times while counting. The music's tempo was adjusted to fit their physical state at the time. The waltz, which has the slowest pace, opened the program, and the jive, which has one of the fastest tempos, closed it. Every training unit also included a repetition of previously taught dances. Each dance lasted 1 min and was performed five times.
To allow the examiner to track each subject's training intensity in class, each subject wore a heart rate chest belt that was synchronized with a portable Polar Team Pro (Polar Electro, Kempele, Finland). The participants should not have exercised in a prescribed heart rate range, but in the case of reaching 95% of maximum heart rate (HRmax) as assessed in the initial cardiorespiratory fitness test, they were warned for safety reasons.
2.3. Physical fitness assessment
The subjects underwent an incremental test on a bicycle ergometer (PowerCube, Ganshorn, Germany) to assess cardiorespiratory fitness (VO2max). The environmental conditions were standardized, with the temperature kept at 20°C and the relative humidity kept between 50% and 60%. Following an initial familiarization period, the warm‐up consisted of 5 min of cycling at a power output of 0.5 w.kg−1, with the load increasing by 0.25 w.kg−1 every minute until volitional exhaustion. The last step should have been held for an entire minute to record the maximum workload. We aimed to achieve maximum effort in 8–12 min (ACSM, 2013). Maximum exhaustion was confirmed when at least one of the two objective criteria were met: HRmax >220 minus age or RER peak >1.10 (Howley et al., 1995). During the testing, the procedures were overseen by one medical doctor and one exercise physiologist. The last 10 s of each workload's VO2 data were averaged.
2.4. Motor and cognitive function assessment
Neuropsychological tests were individually administered to each participant. The measures included evaluations of upper and lower motor functions, motor functions with a problem‐solving component, and measures in other categories such as cognitive as well as psychological functions. The specific tests were as follows:
The Expanded Disability Status Scale (EDSS) quantifies disability in MS patients (Kurtzke, 1983).
The Timed 25‐Foot Walk Test (T25‐FWT) and 12‐Item Multiple Sclerosis Walking Scale (MSWS‐12) evaluate mobility and leg function performance (Kieseier & Pozzilli, 2012).
Nine‐Hole Peg Test (9‐HPT) measures finger dexterity (Feys et al., 2017).
ABILHAND‐56 measures a patient's perceived difficulty using his or her hands to perform manual activities (Penta et al., 1998).
The Symbol Digit Modalities Test (SDMT) assesses psychomotor speed (Smith, 1982).
Trail Making Test A, B (TMT‐A, B) estimates cognitive functions, principally attention and working memory (Reitan, 1955).
Fatigue Scale for Motor and Cognitive Functions (FSMC) measures cognitive and motor fatigue in MS patients (Penner et al., 2009).
Patient Health Questionnaire (PHQ‐9) objectifies and assesses the degree of depression severity (Kroenke et al., 2001).
General Anxiety Disorder‐7 (GAD‐7) measures or assesses the severity of generalized anxiety disorder (Spitzer, 2006).
2.5. Body composition
The characteristics of body composition were measured before and after the intervention. We measured body weight, body height, body fat percentage, muscle mass, and visceral fat using the InBody 230 (Serial, USB, and LookingBody Basic 120). Weight in kilograms divided by height in meters squared served as the basis for calculating BMI. Waist and hip circumferences were measured with a flexible tape.
2.6. DNA extraction, NGS library preparation, and Illumina data processing
The morning fecal samples were collected before and after the intervention from the participants in a DNA/RNA Shield‐Fecal Collection Tube to maintain the stability of the nucleic acids in the fecal samples (ZymoResearch, Irvine, CA, USA). Participants were instructed on methods to prevent sample contamination during sample collection. Total DNA from fecal samples was extracted with the ZymoBIOMICS DNA/RNA kit (Zymo Research, Irvine, CA, United States) according to the manufacturer's protocol. NGS libraries were prepared with the 16S Microbiome NGS Assay (ViennaLab Diagnostics GmbH, Vienna, Austria). Details of this DNA sequencing and Illumina data processing can be found in Bielik et al. (2023).
2.7. Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics version 19 (SPSS Inc., Chicago, IL, USA). Data normality was checked by the Shapiro–Wilk test. Quantitative data were presented as mean ± standard deviation. Parametric data were analyzed using a two‐way ANOVA to account for within‐subject dependencies arising from repeated measurements over time, while concurrently assessing treatment effects between the experimental and control groups. Non‐parametric data were analyzed using the Mann–Whitney test to compare effects between groups and the Wilcoxon signed‐rank test to assess within‐group effects. The correlations between gut microbes, cognitive and motor functions, cardiorespiratory fitness, and anthropometric measurements were analyzed using the Spearman correlation coefficient. P < 0.05 was regarded as statistically significant. ClustVis was used to visualize multidimensional data by means of principal component analysis (PCA) (Metsalu & Vilo, 2015).
3. RESULTS
3.1. Body composition and physical fitness
Of the 36 patients who underwent randomization, 16 patients completed the physical training program with at least 80% of the required attendance rate in dance classes (12 females, 4 males) and 13 patients completed the study as controls (9 females, 4 males). Unfortunately, 2 patients from EXP and 5 patients from CTRL did not complete the final assessment. The changes in body composition and physical fitness characteristics are presented in Table 2. After the first familiarization week, we report that the mean peak training heart rate (THRpeak) of all rock and roll classes was 92 ± 8% of HRmax as assessed in the initial cardiorespiratory fitness test (VO2max). Similarly, the THRpeak of all sport dance classes was 92 ± 8% of HRmax. Besides that, we did not report any significant trend or shift in HR (positive or negative) during the 12 classes, either in rock and roll or in sport dance classes.
TABLE 2.
Body composition characteristics and physical fitness characteristics at baseline and after intervention in the EXP.
| EXP‐pre (n = 16) | EXP‐post (n = 16) | p‐value | |
|---|---|---|---|
| Weight (kg) | 79.09 ± 18.58 | 76.5 ± 15.96 | 0.039 |
| BMI (kg.m−2) | 26.87 ± 5.79 | 26.02 ± 4.94 | 0.050 |
| Body fat (%) | 32.91 ± 9.38 | 31.01 ± 8.76 | 0.005 |
| Body fat (kg) | 26.81 ± 12.16 | 24.24 ± 9.93 | 0.002 |
| Muscle mass (kg) | 28.98 ± 6.88 | 28.96 ± 6.54 | 0.990 |
| Waist circumference (cm) | 89.38 ± 16.24 | 86.13 ± 14.05 | 0.002 |
| WHR | 0.94 ± 0.09 | 0.92 ± 0.08 | 0.013 |
| Loadmax/kg (W.kg−1) | 1.74 ± 0.47 | 1.90 ± 0.40 | 0.017 |
| VO2max/kg (mL.kg−1.min−1) | 23.79 ± 5.80 | 26.44 ± 5.46 | 0.003 |
| HRmax (beats.min−1) | 162.75 ± 22.84 | 167.31 ± 20.02 | 0.569 |
Note: Values are presented as mean ± SD. Differences were considered significant (in bold) at p < 0.05.
Abbreviations: BMI, body mass index; EXP, experimental group; HR, heart rate; WHR, waist to hip ratio.
3.2. Motor and cognitive function
We compared the results in psychometric and motor tests at baseline and after 3 months in the EXP and CTRL of MS subjects (Table 3). The EXP did better in tasks that evaluated mobility and leg function performance (T25‐FWT; p < 0.001) (Figure 1A), upper extremity function (9‐HPT; p < 0.001), psychomotor speed (SDMT; p < 0.010) (Figure 1B), as well as attention and working memory (TMT B; p < 0.005). No statistically significant difference was detected in the rest of the observed parameters between the EXP and CTRL (Table 3).
TABLE 3.
Neuropsychological and motor function tests at baseline and after 3 months in the experimental and control group of MS patients.
| Parameter | EXP‐pre (n = 16) | EXP‐post (n = 16) | p‐value | CTRL‐pre (n = 13) | CTRL‐post (n = 13) | p‐value |
|---|---|---|---|---|---|---|
| T25‐FWT | 4.60 ± 1.08 | 2.49 ± 1.22 | <0.001 | 2.89 ± 1.11 | 2.52 ± 0.58 | 0.399 |
| 9‐HPT | 22.80 ± 10.32 | 20.08 ± 7.04 | 0.001 | 21.94 ± 3.39 | 21.63 ± 3.21 | 0.441 |
| TMT A | 29.26 ± 11.30 | 28.69 ± 12.20 | 0.712 | 29.68 ± 9.67 | 29.90 ± 6.19 | 0.875 |
| TMT B | 68.34 ± 37.54 | 55.74 ± 22.15 | 0.005 | 63.02 ± 18.00 | 65.42 ± 16.96 | 0.279 |
| PHQ‐9 | 6.06 ± 4.15 | 5.81 ± 4.18 | 0.572 | 6.62 ± 5.04 | 7.38 ± 7.22 | 0.572 |
| MSWS‐12 | 25.06 ± 11.17 | 23.19 ± 10.25 | 0.238 | 22.23 ± 11.36 | 20.85 ± 10.24 | 0.407 |
| SDMT | 61.75 ± 12.37 | 68.88 ± 14.25 | 0.010 | 57.62 ± 9.94 | 58.23 ± 9.78 | 0.664 |
| ABILHAND‐56 | 1.15 ± 0.18 | 1.13 ± 0.22 | 0.284 | 1.04 ± 0.10 | 1.05 ± 0.16 | 0.854 |
| FSMC SUM | 58.94 ± 17.18 | 57.63 ± 19.16 | 0.646 | 52.38 ± 22.61 | 51.62 ± 22.93 | 0.798 |
| FSMC cognitive | 27.56 ± 9.50 | 28.00 ± 9.62 | 0.762 | 24.69 ± 12.21 | 25.23 ± 11.23 | 0.727 |
| FSMC motor | 31.38 ± 9.47 | 29.00 ± 9.58 | 0.132 | 26.92 ± 10.61 | 26.38 ± 11.93 | 0.772 |
| GAD‐7 | 4.94 ± 3.07 | 4.13 ± 3.58 | 0.241 | 5.23 ± 3.72 | 6.69 ± 6.43 | 0.207 |
Note: Values are presented as mean ± SD. Differences were considered significant at p < 0.05.
Abbreviations: 9‐HPT, Nine‐Hole Peg Test; ABILHAND‐56, 56‐Item ABILHAND questionnaire; CTRL, control group; EXP, experimental group; FSMC, Fatigue Scale for Motor and Cognitive Functions; FSMC SUM, Fatigue Scale for Motor and Cognitive Functions total score; GAD‐7, General Anxiety Disorder‐7; MSWS‐12, 12‐Item Multiple Sclerosis Walking Scale; PHQ‐9, Patient Health Questionnaire‐9; SDMT, Symbol Digit Modalities Test; T25‐FW, Timed 25‐Foot Walk Test; TMT A, B, Trail Making Test A, B.
FIGURE 1.
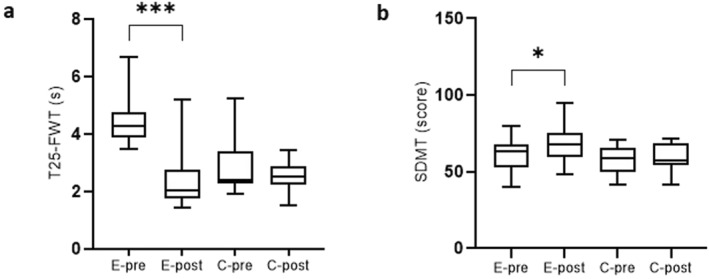
Significant change in the timed 25‐foot walk test (T25‐FWT) and symbol digit modalities test (SDMT) between the experimental and control groups at baseline and after 12 weeks. (A) Significant change in the Timed 25‐foot Walk Test. C, control group; E, experimental group; s, seconds; T25‐FW, Timed 25‐foot Walk Test; (B) Significant change in the Symbol Digit Modalities Test. C, control group; E, experimental group; SDMT, Symbol Digit Modalities Test; s, seconds.
3.3. Microbial analysis of fecal samples
In general, we reported the following significantly different results between groups: Acidaminococcales (EXP‐pre 0.4966 ± 0.6100; CTRL‐pre 1.4394 ± 1.0972; p < 0.045) and Lacticaseibacillus (EXP‐pre 0.0601 ± 0.0670; CTRL‐pre 0.0028 ± 0.0078; p < 0.005). Within the EXP, we detected significant shifts in 50 bacterial genera. Significant bacterial genera allowed discrimination between EXP at baseline and after completing the intervention when we used the Heatmap (Figure 2). Moreover, we determined significant changes in 74 bacterial species in the EXP and 45 bacterial species in CTRL. The significant differences observed within and between groups were exclusively associated with bacterial strains newly discovered and/or exhibiting very low relative abundance and lacking previously reported health effects. Significant bacterial species also allowed discrimination between EXP at baseline and after completing the intervention when we used the principal component analysis (PCA) (Figure 3).
FIGURE 2.
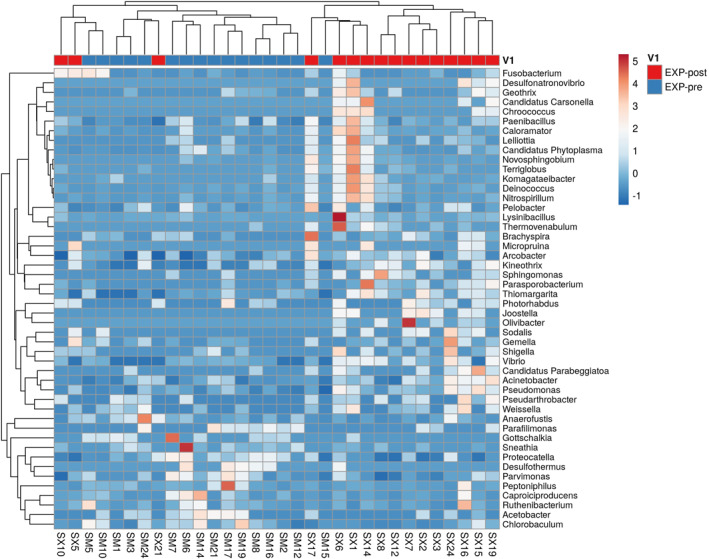
Heat map diagram of the composition of the gut microbiota for the MS patients at baseline and after an intervention. The 50 genera that acquired significant differences between baseline and after an intervention in EXP are shown. EXP, experimental group.
FIGURE 3.
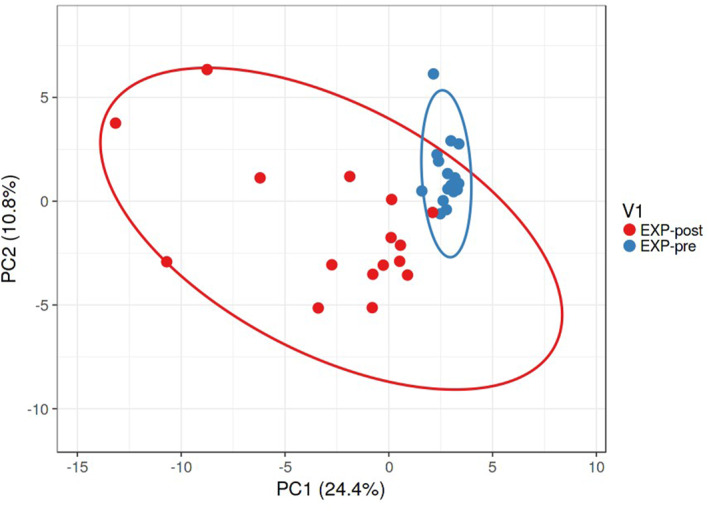
Beta‐diversity of the composition of the gut microbiota for MS patients at baseline and after an intervention, as visualized by PCA. Beta‐diversity of the composition of gut microbiota at the species level is represented by significantly altered bacterial taxa (p < 0.05) in EXP, as visualized by PCA. SVD with imputation is used to calculate the principal components. The X and Y axes show principal components 1 and 2, which explain 24.4% and 10.8% of the total variance, respectively. Prediction ellipses are such that, with a probability of 0.95, a new observation from the same group will fall inside the ellipse. N = 16 data points. EXP, experimental group.
We reported a significant altered post‐intervention taxon at species level in the EXP, including an increase in commensals, for example, Blautia stercoris, Clostridium algidixylanolyticum, Eubacterium xylanophilum, Megasphaera indica, and Parabacteroides faecis, and a decrease in pathogens, for example, Ruminococcus torques and Parvimonas micra (Table 4).
TABLE 4.
Microbiota taxa characteristics at baseline and after intervention in the EXP.
| EXP‐pre (n = 16) | EXP‐post (n = 16) | p‐value | |
|---|---|---|---|
| Commensal | |||
| Blautia stercoris | 0.0496 ± 0.0410 | 0.0980 ± 0.1556 | 0.039 |
| Clostridium algidixylanolyticum | 0.2806 ± 0.2466 | 0.5221 ± 0.2770 | 0.034 |
| Eubacterium xylanophilum | 0.0076 ± 0.0249 | 0.0553 ± 0.1253 | 0.056 |
| Megasphaera indica | 0.0005 ± 0.0011 | 0.0018 ± 0.0020 | 0.047 |
| Parabacteroides faecis | 0.0000 ± 0.0000 | 0.0012 ± 0.0022 | 0.043 |
| Pathogen | |||
| Ruminococcus torques | 0.9094 ± 1.1890 | 0.3717 ± 05078 | 0.051 |
| Parvimonas micra | 0.0151 ± 0.0108 | 0.0078 ± 0.0059 | 0.038 |
Note: Values are presented as mean ± SD. Differences were considered significant at p < 0.05.
Abbreviation: EXP experimental group.
We found significant changes in 31 bacterial genera and 45 bacterial species within the CTRL. Notably, we observed unfavorable shifts in bacterial species in the CTRL, including a decrease in commensals, for example, Anaerostipes hadrus, Blautia caecimuris, Blautia glucerasea, Blautia massiliensis, Blautia schinkii, Blautia wexlerae, Coprococcus catus, Coprococcus comes, Eubacterium limosum, and Streptococcus thermophilus, and an increase in pathogens, for example, Bilophila wadsworthia and Enterobacter cloacae (Table 5).
TABLE 5.
Microbiota taxa characteristics in the CTRL.
| CTRL‐pre (n = 13) | CTRL‐post (n = 13) | p‐value | |
|---|---|---|---|
| Commensal | |||
| Anaerostipes hadrus | 1.4107 ± 1.5869 | 0.2871 ± 0.2138 | 0.006 |
| Blautia caecimuris | 0.0343 ± 0.0423 | 0.0128 ± 0.0210 | 0.021 |
| Blautia glucerasea | 0.0482 ± 0.0290 | 0.0217 ± 0.0171 | 0.008 |
| Blautia massiliensis | 0.0275 ± 0.0289 | 0.0085 ± 0.0070 | 0.041 |
| Blautia schinkii | 3.9774 ± 2.8566 | 1.3007 ± 0.7309 | 0.001 |
| Blautia wexlerae | 2.7533 ± 2.4826 | 1.1745 ± 1.3992 | 0.040 |
| Coprococcus catus | 0.0527 ± 0.0786 | 0.0103 ± 0.0204 | 0.043 |
| Coprococcus comes | 0.4271 ± 0.3001 | 0.1762 ± 0.1437 | 0.003 |
| Eubacterium limosum | 1.9700 ± 2.3807 | 1.1471 ± 1.2697 | 0.021 |
| Streptococcus thermophilus | 0.4914 ± 0.5100 | 0.0819 ± 0.1158 | 0.013 |
| Pathogen | |||
| Bilophila wadsworthia | 0.2020 ± 0.1492 | 0.4261 ± 0.3471 | 0.025 |
| Enterobacter cloacae | 0.0072 ± 0.0260 | 0.0933 ± 0.2903 | 0.028 |
Note: Values are presented as mean ± SD. Differences were considered significant at p < 0.05.
Abbreviation: CTRL, control group.
4. DISCUSSION
Aerobic exercise has a profound impact on the physical, mental, and social well‐being of MS patients and is currently becoming established not only as a preventative measure but also as a disease‐modifying treatment for MS (Dalgas et al., 2019). In this context, a randomized control trial was used to study the effect of a 12‐week dance class training program on the gut microbiota composition, motor and cognitive functions, and physical fitness of MS patients. We hypothesized that regular physical exercise (dance classes) would improve physical fitness, motor function, and cognitive function, with an additional positive effect on the microbial composition in MS patients. We reported a positive shift in body composition, increased cardio‐respiratory fitness, improved motor test scores (T25‐FW, MSWS‐12), cognitive functions (TMT‐B, symbol coding), and extremity function (9‐HPT), as well as a positive shift in the gut microbiota composition.
The first finding of this randomized control trial was an improvement in physical fitness, motor function, and cognitive function. We reported improved body composition and aerobic capacity in MS patients who completed a 12‐week dance program. Our results are in accordance with previous studies (Cheragh Birjandi et al., 2022; Feys et al., 2019). However, research indicates that dance as an exercise alternative can be more effective than other types of structured exercise training for improving a range of physical functioning outcomes, including musculoskeletal functions such as mobility and balance (Davis et al., 2023; Fong Yan et al., 2018). Our findings demonstrated that dance class training improved mobility, leg function performance (T25‐FW), and upper extremity function (9‐HPT). We did not show statistically significant improvement in the MSWS‐12; however, data on that are equivocal (Mandelbaum et al., 2016; Ng et al., 2020; Van Geel et al., 2020).
Furthermore, the results of our intervention have shown that dance class training patients exhibited better performance in executive cognitive functions measured by TMT‐B. Additionally, we observed a nearly significant improvement in psychomotor speed measured by the SDMT, which measures information processing speed and serves as a significant predictor of cognitive impairment in MS people (Jacobsen et al., 2021; Leach et al., 2022; Marzi et al., 2023). To date, numerous publications have shown that a decline in SDMT score is correlated with patient outcomes, including employment, driving, and daily activities (Kavaliunas et al., 2019; Strober et al., 2014). Furthermore, a decrease in scores has also been associated with clinical outcomes such as changes in MRI scans (whole brain volume) and EDSS scores (Barnett et al., 2021; Matthews et al., 2023; Sedaghat et al., 2023).
Another interesting finding of this study is the favorable shift in bacterial composition in the EXP. Ordoñez‐Rodriguez et al. (2023) have conducted a systematic review of 12 studies on changes in gut microbiota in MS patients, confirming that in terms of taxonomy, alteration of the microbiota has been marked by a decrease in Firmicutes, Lachnospiraceae, Bifidobacterium, Roseburia, Coprococcus, Butyricicoccus, Lachnospira, Dorea, Faecalibacterium, and Prevotella and an increase in Bacteroidetes, Akkermansia, Blautia, and Ruminocococcus. In our study, we observed a significant decrease in pathogens, namely Parvimonas micra and Ruminococcus, within the EXP. Although Parvimonas micra may play a role in the disruption of immune regulation by promoting the release of inflammatory cytokines, the study of the relationship between this microorganism and MS is limited. However, the research on the animal model demonstrates an increase in the expression of key pro‐inflammatory cytokines, such as TNF‐α and IL‐6, after orally administering Parvimonas micra (Chang et al., 2023). Promisingly, in MS patients, pharmacological therapy has been shown to reduce the abundance of Parvimonas micra (Abdurasulova et al., 2018). Interestingly, we reported an increased relative abundance of other pro‐inflammatory bacteria, Bilophila wadsworthia, within the CTRL (Feng et al., 2017).
We also report a significant decrease in Ruminococcus torques within EXP. In the study comparing 148 Danish MS cases and 148 matched healthy control subjects, Ruminococcus torques was confirmed as the MS‐related pathogen (Thirion et al., 2023). Comparable outcomes led to an international multiple sclerosis microbiome study that revealed an enrichment of Ruminococcus torques in MS patients (Zhou et al., 2022).
An interesting finding in this study was an increase in some common and some not yet fully reviewed commensals, for example, Blautia stercoris, Clostridium algidixylanolyticum, Eubacterium xylanophilum, Megasphaera indica, and Parabacteroides faecis. These species are one of the main gut commensals and are persuasively associated with SCFA metabolism (Lopetuso et al., 2013). SCFAs, such as acetate, propionate, and butyrate, are the end products of anaerobic bacterial fermentation of indigestible carbohydrates in the gastrointestinal tract (Rojas‐Valverde et al., 2023). They have been shown to have important immunomodulatory properties that maintain gut homeostasis and suppress pro‐inflammatory cytokines (Markowiak‐Kopeć & Śliżewska, 2020; Olsson et al., 2021). In this research, we observed an increased relative abundance of Blautia stercoris within the EXP. Blautia spp. has garnered particular attention due to its association with both antimicrobial activity and the alleviation of inflammatory and metabolic diseases (Calva‐Cruz et al., 2023). Important findings of this study are the decrease in several important SCFA producers, including a number of Blautia species, namely Blautia caecimuris, Blautia glucerasea, Blautia massiliensis, Blautia schinkii, and Blautia wexlerae, within the CTRL. Accordingly, a significantly decreased proportion of Blautia spp. in MS patients was reported in the aforementioned International Multiple Sclerosis Microbiome Study (Zhou et al., 2022). Thus, together with improvements to the gut microbiome's composition, attention may need to be paid to preserve and stabilize symbiotic bacteria.
The study has several limitations. The first is the lack of reports of the patients' dietary data. We instructed all patients in both groups to follow their normal diet. Secondly, the distribution of male and female subjects and the effects of the menstrual cycle might have influenced our results. Thirdly, the limitation of this study is the frequency of exercise. We suppose that three sessions a week would bring more favorable results. At the end, the data on the physical fitness (VO2max) of MS patients in CTRL is missing.
It is important to point out that there are insufficient data to definitively link a particular bacterium or genus to MS. Although few studies have suggested a possible connection between certain gut bacteria and MS, the results are still being refined, and the underlying mechanisms remain unclear. To promote a healthier gut microbial community, exercise might positively impact the inflammatory environment and immune system dysregulation that are associated with MS. However, it is important to emphasize that research in this area is still ongoing, and the specific interactions between exercise, gut microbiome, and MS are not yet fully understood. More studies are needed to establish direct causation and fully explore the relationship between exercise, gut microbiome, and MS.
5. CONCLUSION
Our randomized control trial investigating the effects of a 12‐week dance class training program on MS patients revealed significant improvements in body composition, physical fitness, motor, and cognitive functions, along with favorable yet moderate shifts in gut microbial communities. As a result, dance class training is likely to provide a variety of physical and psychosocial benefits, making it enjoyable for MS patients.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
PATIENT CONSENT STATEMENT
Following the reading of the informed consent form, an explanation of the study steps, and talks with the investigators, all patients and controls signed informed consent.
CLINICAL TRIAL REGISTRATION
Gut Microbiota Composition, Cognitive Function, and Physical Fitness in Multiple Sclerosis Patients (ClinicalTrials.gov, No: NCT06220409).
ACKNOWLEDGMENT
The authors are grateful to all the subjects for their participation in this study. This work was supported by Grant No. APVV‐17‐0099 and APVV‐22‐0047 of the Slovak Research and Development Agency, and VEGA 1/0260/21 and VEGA 1/0618/21 by Sportdiag team o.z. This work was generously funded and supported by Hoffmann‐La Roche & Co. and the Novartis Research Foundation.
Louise Mária Adamová and Darina Slezáková contributed equally to this work.
DATA AVAILABILITY STATEMENT
The results of all analyses are included in this published article. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- Abdurasulova, I. N. , Tarasova E. A., Nikiforova I. G., Il’Ves A. G., Ivashkova E. V., Matsulevich A. V., Tatarinov A. E., et al. 2018. “[The Intestinal Microbiota Composition in Patients with Multiple Sclerosis Receiving Different Disease‐Modifying Therapies DMT].” Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 118(8. Vyp. 2): 62–69. 10.17116/JNEVRO201811808262. [DOI] [PubMed] [Google Scholar]
- Allen, J. M. , Mailing L. J., Niemiro G. M., Moore R., Cook M. D., White B. A., Holscher H. D., and Woods J. A.. 2018. “Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans.” Medicine and Science in Sports and Exercise 50(4): 747–757. 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- Altieri, C. , Speranza B., Corbo M. R., Sinigaglia M., and Bevilacqua A.. 2023. “Gut‐Microbiota, and Multiple Sclerosis: Background, Evidence, and Perspectives.” Nutrients 15(4): 942. 10.3390/NU15040942/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine (ACSM) . 2013. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins. [DOI] [PubMed] [Google Scholar]
- Barnett, M. , Bergsland N., Weinstock‐Guttman B., Butzkueven H., Kalincik T., Desmond P., Gaillard F., et al. 2021. “Brain Atrophy and Lesion Burden Are Associated with Disability Progression in a Multiple Sclerosis Real‐World Dataset Using Only T2‐FLAIR: The NeuroSTREAM MSBase Study.” NeuroImage: Clinical 32: 102802. 10.1016/J.NICL.2021.102802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone, M. , Mendozzi L., D’Amico F., Saresella M., Rampelli S., Piancone F., La Rosa F., et al. 2021. “Influence of a High‐Impact Multidimensional Rehabilitation Program on the Gut Microbiota of Patients with Multiple Sclerosis.” International Journal of Molecular Sciences 22(13): 7173. 10.3390/ijms22137173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielik, V. , Hric I., SmahovA S., Tkačiková M., Hlaváčová V., Nechalová L., Ugrayová S., and Kolenová A.. 2023. “The Effect of Physical Exercise and Dairy Probiotics (Lactobacillus Casei) on Gut Microbiome in Childhood Cancer Survivors.” Neoplasma 70(4): 588–596. 10.4149/NEO_2023_230526N287. [DOI] [PubMed] [Google Scholar]
- Boussamet, L. , Rajoka M. S. R., and Berthelot L.. 2022. “Microbiota, IgA and Multiple Sclerosis.” Microorganisms 10(3): 617. 10.3390/microorganisms10030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronzini, M. , Maglione A., Rosso R., Matta M., Masuzzo F., Rolla S., and Clerico M.. 2023. “Feeding the Gut Microbiome: Impact on Multiple Sclerosis.” Frontiers in Immunology 14: 1176016. 10.3389/FIMMU.2023.1176016/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calva‐Cruz, O. de J. , Ovando‐Vázquez C., De León‐Rodríguez A., Veana F., Espitia‐Rangel E., Treviño S., and Barba‐de la Rosa A. P.. 2023. “Dietary Supplementation with Popped Amaranth Modulates the Gut Microbiota in Low Height‐For‐Age Children: A Nonrandomized Pilot Trial.” Foods 12(14): 2760. 10.3390/FOODS12142760/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapellotti, A. M. , Meijerink H., Gravemaker‐Scott C., Thielman L., Kool R., Lewin N., and Abma T. A.. 2023. “Escape, Expand, Embrace: The Transformational Lived Experience of Rediscovering the Self and the Other while Dancing with Parkinson’s or Multiple Sclerosis.” International Journal of Qualitative Studies on Health and Well‐Being 18(1). 10.1080/17482631.2022.2143611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Y. , Huang Z., Hou F., Liu Y., Wang L., Wang Z., Sun Y., et al. 2023. “Parvimonas Micra Activates the Ras/ERK/c‐Fos Pathway by Upregulating miR‐218‐5p to Promote Colorectal Cancer Progression.” Journal of Experimental & Clinical Cancer Research 42(1): 1–17. 10.1186/S13046-022-02572-2/FIGURES/8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheragh Birjandi, K. , Sharafi J., Etemadizade A., and Ghasemi E.. 2022. “Influence of Eight Weeks of Combined Training on Adipsin and Lipoprotein Profile and Possible Relations with Depression, Anxiety and Stress in Women with Multiple Sclerosis.” Hormone Molecular Biology and Clinical Investigation 44(1): 45–51. 10.1515/HMBCI-2022-0027. [DOI] [PubMed] [Google Scholar]
- Dalgas, U. , Langeskov‐Christensen M., Stenager E., Riemenschneider M., and Hvid L. G.. 2019. “Exercise as Medicine in Multiple Sclerosis‐Time for a Paradigm Shift: Preventive, Symptomatic, and Disease‐Modifying Aspects and Perspectives.” Current Neurology and Neuroscience Reports 19(11): 88. 10.1007/S11910-019-1002-3. [DOI] [PubMed] [Google Scholar]
- Davis, E. , Webster A., Whiteside B., and Paul L.. 2023. “Dance for Multiple Sclerosis: A Systematic Review.” International Journal of MS Care 25(4): 176–185. 10.7224/1537-2073.2022-088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunalska, A. , Saramak K., and Szejko N.. 2023. “The Role of Gut Microbiome in the Pathogenesis of Multiple Sclerosis and Related Disorders.” Cells 12(13): 1760. 10.3390/CELLS12131760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziewiecka, H. , Buttar H. S., Kasperska A., Ostapiuk–Karolczuk J., Domagalska M., Cichoń J., and Skarpańska‐Stejnborn A.. 2022. “Physical Activity Induced Alterations of Gut Microbiota in Humans: A Systematic Review.” BMC Sports Science, Medicine & Rehabilitation 14(1): 122. 10.1186/S13102-022-00513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Z. , Long W., Hao B., Ding D., Ma X., Zhao L., and Pang X.. 2017. “A Human Stool‐Derived Bilophila Wadsworthia Strain Caused Systemic Inflammation in Specific‐pathogen‐free Mice.” Gut Pathogens 9(1): 59. 10.1186/S13099-017-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys, P. , Lamers I., Francis G., Benedict R., Phillips G., Larocca N., Hudson L. D., and Rudick R.. 2017. “The Nine‐Hole Peg Test as a Manual Dexterity Performance Measure for Multiple Sclerosis.” Multiple Sclerosis 23(5): 711–720. 10.1177/1352458517690824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys, P. , Moumdjian L., Van Halewyck F., Wens I., Eijnde B. O., Van Wijmeersch B., Popescu V., and Van Asch P.. 2019. “Effects of an Individual 12‐week Community‐Located „start‐To‐Run“ Program on Physical Capacity, Walking, Fatigue, Cognitive Function, Brain Volumes, and Structures in Persons with Multiple Sclerosis.” Multiple Sclerosis 25(1): 92–103. 10.1177/1352458517740211. [DOI] [PubMed] [Google Scholar]
- Flores, V. A. , Šilić P., DuBose N. G., Zheng P., Jeng B., and Motl R. W.. 2023. “Effects of Aerobic, Resistance, and Combined Exercise Training on Health‐Related Quality of Life in Multiple Sclerosis: Systematic Review and Meta‐Analysis.” Multiple Sclerosis and Related Disorders 75: 104746. 10.1016/j.msard.2023.104746. [DOI] [PubMed] [Google Scholar]
- Fong Yan, A. , Cobley S., Chan C., Pappas E., Nicholson L. L., Ward R. E., Murdoch R. E., et al. 2018. “The Effectiveness of Dance Interventions on Physical Health Outcomes Compared to Other Forms of Physical Activity: A Systematic Review and Meta‐Analysis.” Sports Medicine 48(4): 933–951. 10.1007/S40279-017-0853-5. [DOI] [PubMed] [Google Scholar]
- Howley, E. T. , Bassett D. R., and Welch H. G.. 1995. “Criteria for Maximal Oxygen Uptake: Review and Commentary.” Medicine & Science in Sports & Exercise 27(9): 1292–1301. 10.1249/00005768-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Jacobsen, C. , Zivadinov R., Myhr K. M., Dalaker T. O., Dalen I., Benedict R. H. B., Bergsland N., and Farbu E.. 2021. “Brain Atrophy and Clinical Characteristics Predicting SDMT Performance in Multiple Sclerosis: A 10‐year Follow‐Up Study.” Multiple Sclerosis Journal ‐ Experimental, Translational and Clinical 7(1): 2055217321992394. 10.1177/2055217321992394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng, B. , Šilić P., Bollaert R. E., Sandroff B. M., and Motl R. W.. 2023. “Physical Function Across the Lifespan in Adults with Multiple Sclerosis: An Application of the Short Physical Performance Battery.” Multiple Sclerosis and Related Disorders 73: 104624. 10.1016/j.msard.2023.104624. [DOI] [PubMed] [Google Scholar]
- Kavaliunas, A. , Danylaite Karrenbauer V., Gyllensten H., Manouchehrinia A., Glaser A., Olsson T., Alexanderson K., and Hillert J.. 2019. “Cognitive Function Is a Major Determinant of Income Among Multiple Sclerosis Patients in Sweden Acting Independently from Physical Disability.” Multiple Sclerosis Journal 25(1): 104–112. 10.1177/1352458517740212. [DOI] [PubMed] [Google Scholar]
- Kieseier, B. C. , and Pozzilli C.. 2012. “Assessing Walking Disability in Multiple Sclerosis.” Multiple Sclerosis 18(7): 914–924. 10.1177/1352458512444498. [DOI] [PubMed] [Google Scholar]
- Kroenke, K. , Spitzer R. L., and Williams J. B.. 2001. “The PHQ‐9: Validity of a Brief Depression Severity Measure.” Journal of General Internal Medicine 16(9): 606–613. 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke, J. F . 1983. “Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS).” Neurology 33(11): 1444–1452. 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Leach, J. M. , Cutter G., Golan D., Doniger G., Zarif M., Bumstead B., Buhse M., et al. 2022. “Measuring Cognitive Function by the SDMT Across Functional Domains: Useful but Not Sufficient.” Multiple Sclerosis and Related Disorders 60: 103704. 10.1016/J.MSARD.2022.103704. [DOI] [PubMed] [Google Scholar]
- Lopetuso, L. R. , Scaldaferri F., Petito V., and Gasbarrini A.. 2013. “Commensal Clostridia: Leading Players in the Maintenance of Gut Homeostasis.” Gut Pathogens 5(1): 23. 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbaum, R. , Triche E. W., Fasoli S. E., and Lo A. C.. 2016. “A Pilot Study: Examining the Effects and Tolerability of Structured Dance Intervention for Individuals with Multiple Sclerosis.” Disability & Rehabilitation 38(3): 218–222. 10.3109/09638288.2015.1035457. [DOI] [PubMed] [Google Scholar]
- Markowiak‐Kopeć, P. , and Śliżewska K.. 2020. “The Effect of Probiotics on the Production of Short‐Chain Fatty Acids by Human Intestinal Microbiome.” Nutrients 12(4): 1107. 10.3390/NU12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi, C. , d’Ambrosio A., Diciotti S., Bisecco A., Altieri M., Filippi M., Rocca M. A., et al. 2023. “Prediction of the Information Processing Speed Performance in Multiple Sclerosis Using a Machine Learning Approach in a Large Multicenter Magnetic Resonance Imaging Data Set.” Human Brain Mapping 44(1): 186–202. 10.1002/HBM.26106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, P. M. , Gupta D., Mittal D., Bai W., Scalfari A., Pollock K. G., Sharma V., and Hill N.. 2023. “The Association between Brain Volume Loss and Disability in Multiple Sclerosis: A Systematic Review.” Multiple Sclerosis and Related Disorders 74: 104714. 10.1016/j.msard.2023.104714. [DOI] [PubMed] [Google Scholar]
- Menees, K. B. , Otero B. A., and Tansey M. G.. 2022. Microbiome Influences on Neuro‐Immune Interactions in Neurodegenerative Disease (S. 25–57). 10.1016/bs.irn.2022.07.006. [DOI] [PubMed]
- Metsalu, T. , and Vilo J.. 2015. “ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap.” Nucleic Acids Research 43(W1): W566–W570. 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake, S. , Kim S., Suda W., Oshima K., Nakamura M., Matsuoka T., Chihara N., et al. 2015. “Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters.” PLoS One 10(9): e0137429. 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtarzade, M. , Molanouri Shamsi M., Abolhasani M., Bakhshi B., Sahraian M. A., Quinn L. S., and Negaresh R.. 2021. “Home‐Based Exercise Training Influences Gut Bacterial Levels in Multiple Sclerosis.” Complementary Therapies in Clinical Practice 45: 101463. 10.1016/j.ctcp.2021.101463. [DOI] [PubMed] [Google Scholar]
- Monda, V. , Villano I., Messina A., Valenzano A., Esposito T., Moscatelli F., Viggiano A., et al. 2017. “Exercise Modifies the Gut Microbiota with Positive Health Effects.” Oxidative Medicine and Cellular Longevity 2017: 1–8. 10.1155/2017/3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiani, K. K. , Collado M. C., Eskelinen J. J., Virtanen K. A., Löyttyniemi E., Salminen S., Nuutila P., Kalliokoski K. K., and Hannukainen J. C.. 2020. “Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia.” Medicine and Science in Sports and Exercise 52(1): 94–104. 10.1249/MSS.0000000000002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, A. , Bunyan S., Suh J., Huenink P., Gregory T., Gambon S., and Miller D.. 2020. “Ballroom Dance for Persons with Multiple Sclerosis: A Pilot Feasibility Study.” Disability & Rehabilitation 42(8): 1115–1121. 10.1080/09638288.2018.1516817. [DOI] [PubMed] [Google Scholar]
- Olsson, A. , Gustavsen S., Nguyen T. D., Nyman M., Langkilde A. R., Hansen T. H., Sellebjerg F., Oturai A. B., and Bach Søndergaard H.. 2021. “Serum Short‐Chain Fatty Acids and Associations with Inflammation in Newly Diagnosed Patients with Multiple Sclerosis and Healthy Controls.” Frontiers in Immunology 12. 10.3389/FIMMU.2021.661493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordoñez‐Rodriguez, A. , Roman P., Rueda‐Ruzafa L., Campos‐Rios A., and Cardona D.. 2023. “Changes in Gut Microbiota and Multiple Sclerosis: A Systematic Review.” International Journal of Environmental Research and Public Health 20(5): 4624. 10.3390/ijerph20054624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner, I. K. , Raselli C., Stöcklin M., Opwis K., Kappos L., and Calabrese P.. 2009. “The Fatigue Scale for Motor and Cognitive Functions (FSMC): Validation of a New Instrument to Assess Multiple Sclerosis‐Related Fatigue.” Multiple Sclerosis 15(12): 1509–1517. 10.1177/1352458509348519. [DOI] [PubMed] [Google Scholar]
- Penta, M. , Thonnard J. L., and Tesio L.. 1998. “ABILHAND: A Rasch‐Built Measure of Manual Ability.” Archives of Physical Medicine and Rehabilitation 79(9): 1038–1042. 10.1016/S0003-9993(98)90167-8. [DOI] [PubMed] [Google Scholar]
- Ramos, C. , Gibson G. R., Walton G. E., Magistro D., Kinnear W., and Hunter K.. 2022. “Systematic Review of the Effects of Exercise and Physical Activity on the Gut Microbiome of Older Adults.” Nutrients 14(3): 674. 10.3390/NU14030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratsika, A. , Cruz Pereira J. S., Lynch C. M. K., Clarke G., and Cryan J. F.. 2023. “Microbiota‐Immune‐Brain Interactions: A Lifespan Perspective.” Current Opinion in Neurobiology 78: 102652. 10.1016/j.conb.2022.102652. [DOI] [PubMed] [Google Scholar]
- Reitan, R. M . 1955. “The Relation of the Trail Making Test to Organic Brain Damage.” Journal of Consulting Psychology 19(5): 393–394. 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- Rojas‐Valverde, D. , Bonilla D. A., Gómez‐Miranda L. M., Calleja‐Núñez J. J., Arias N., and Martínez‐Guardado I.. 2023. “Examining the Interaction between Exercise, Gut Microbiota, and Neurodegeneration: Future Research Directions.” Biomedicines 11(8): 2267. 10.3390/BIOMEDICINES11082267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat, S. , Jang H., Athertya J. S., Groezinger M., Corey‐Bloom J., and Du J.. 2023. “The Signal Intensity Variation of Multiple Sclerosis (MS) Lesions on Magnetic Resonance Imaging (MRI) as a Potential Biomarker for Patients’ Disability: A Feasibility Study.” Frontiers in Neuroscience 17: 1145251. 10.3389/FNINS.2023.1145251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A . 1982. Symbol Digit Modalities Test—Revised. Los Angeles: Western Psychological Services. [Google Scholar]
- Spitzer, R. , Kroenke K., Williams J. B., and Löwe B.. 2006. “A Brief Measure for Assessing Generalized Anxiety Disorder ‐ the GAD‐7.” Archives of Internal Medicine 166(10): 1092–1097. 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Strober, L. , Chiaravalloti N., Moore N., and Deluca J.. 2014. “Unemployment in Multiple Sclerosis (MS): Utility of the MS Functional Composite and Cognitive Testing.” Multiple Sclerosis 20(1): 112–115. 10.1177/1352458513488235. [DOI] [PubMed] [Google Scholar]
- Thirion, F. , Sellebjerg F., Fan Y., Lyu L., Hansen T. H., Pons N., Levenez F., et al. 2023. “The Gut Microbiota in Multiple Sclerosis Varies with Disease Activity.” Genome Medicine 15(1): 1–17. 10.1186/S13073-022-01148-1/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, A. J. , Banwell B. L., Barkhof F., Carroll W. M., Coetzee T., Comi G., Correale J., et al. 2018. “Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria.” The Lancet Neurology 17(2): 162–173. 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- Trapp, B. D. , and Nave K.‐A.. 2008. “Multiple Sclerosis: An Immune or Neurodegenerative Disorder?” Annual Review of Neuroscience 31(1): 247–269. 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Van Geel, F. , Van Asch P., Veldkamp R., and Feys P.. 2020. “Effects of a 10‐week Multimodal Dance and Art Intervention Program Leading to a Public Performance in Persons with Multiple Sclerosis ‐ A Controlled Pilot‐Trial.” Multiple sclerosis and related disorders 44: 102256. 10.1016/J.MSARD.2020.102256. [DOI] [PubMed] [Google Scholar]
- Zhou, X. , Baumann R., Gao X., Mendoza M., Singh S., Katz Sand I., Xia Z., et al. 2022. “Gut Microbiome of Multiple Sclerosis Patients and Paired Household Healthy Controls Reveal Associations with Disease Risk and Course.” Cell 185(19): 3467–3486.e16. 10.1016/J.CELL.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The results of all analyses are included in this published article. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.


