Abstract
We previously showed that B16 melanoma cells produce ecotropic melanoma-associated retrovirus (MelARV) which encodes a melanoma-associated antigen recognized by MM2-9B6 monoclonal antibody. The biological significance of MelARV in melanoma formation remains unknown. We found that infection of normal melanocytes with MelARV resulted in malignant transformation. It is likely that MelARV emerged from the defective Emv-2 provirus, a single copy of ecotropic provirus existing in the genome of C57BL/6 mice. In the present study, we cloned and sequenced the full-length MelARV genome and its insertion sites and we completed sequencing of the Emv-2 provirus. Our data show that MelARV has a typical full-length retroviral genome with high homology (98.54%) to Emv-2, indicating a close relationship between both viruses. MelARV probably emerged as a result of recombination between Emv-2 and an endogenous nonecotropic provirus. Some observed differences in the gag and pol regions of MelARV might account for the restoration of productivity and infectivity of a novel retrovirus that somatically emerged during melanoma formation. MelARV does not contain any oncogene and therefore might induce transformation by insertional mutagenesis. We sequenced two insertion sites of MelARV. The first insertion site represents the 3′ coding region of the c-maf proto-oncogene at 67.0 centimorgans (cM) on chromosome 8. The c-maf proto-oncogene encodes a basic leucine zipper protein homologous to c-fos and c-jun. Insertion of MelARV in BL6 melanoma cells resulted in the up-regulation of c-maf. It is noteworthy that the Emv-2 provirus is also inserted into a noncoding region at 61.0 cM on the same chromosome 8. The second insertion site is the 3′ noncoding region of the DNA polymerase gamma (PolG) gene on chromosome 7. The expression of PolG was not affected by the MelARV insertion. Further investigation of the biological significance of MelARV in melanoma formation is being undertaken.
All strains of laboratory mice have proved to contain endogenous retroviral sequences in their genomes (13, 16, 19, and 20). These sequences encode retroviruses that are morphologically classified as A, B, and C type (19). Type A retroviruses are known as intracisternal A particles (IAPs), which assemble on intracellular membranes and bud into cisternae of the endoplasmic reticulum. A mouse contains about 1,000 copies of IAP proviral elements per haploid genome (21). IAPs are generally absent in normal tissues but are abundant in some malignant cells such as plasmacytomas, neuroblastomas, and teratocarcinomas (21). The B-type retrovirus group includes as infectious agents only the mouse mammary tumor viruses (MMTVs) which induce mammary adenocarcinoma formation in some strains of mice. Several genetic loci that are associated with production of MMTV, and mammary tumors have been identified (19). C-type murine retroviruses were initially identified in murine lymphomas/leukemias, which were thereafter termed murine leukemia viruses (MuLVs). According to their ability to invade cells of different species, MuLVs are divided into several classes: ecotropic, xenotropic, or polytropic. Generally, a murine genome contains numerous copies of xenotropic or polytropic proviruses. Most inbred strains of mice, however, contain one to two ecotropic proviruses, while the AKR strain has three ecotropic proviruses and this strain of mice is characterized by high production of ecotropic MuLV early in life and high incidence of development of spontaneous lymphomas. Some strains of mice such as DBA/2, C3H, or C57BL/6 produce virus spontaneously only late in life or not at all. Usually, a very low incidence of spontaneous lymphomas is observed in these mice (19). We have recently found that a B16 melanoma that spontaneously originated in C57BL/6 mice produces A- and C-type retroviral particles (25, 26, 30). We have cloned and sequenced an IAP from BL6 melanoma cells (25). A 9-base motif in the R region showed that the melanoma-derived IAP differs from other previously sequenced IAPs and thus it was termed melanoma-associated IAP (25). B16 melanoma and all its high- and low-metastatic sublines produce numerous C-type retroviral particles that encode an antigen that is recognizable by MM2-9B6 monoclonal antibody (MAb) (24). This antigen is also expressed by other melanomas (JB/RH and JB/MS) that originated in C57BL/6 mice but not by normal or malignant cells of different histological types. Therefore, this antigen has been termed melanoma-associated antigen (MAA). It was found that MM2-9B6 MAb directed against MAA could lead to eradication of pulmonary and liver metastases (7, 11). We found that MAA is encoded by the env region of a C-type retrovirus (26). This retrovirus was identified as B-tropic ecotropic retrovirus (24) and has been designated melanoma-associated retrovirus (MelARV) (26). It is of note that the Cloudman S91 melanoma that was spontaneously developed in DBA/2 mice and the UV-induced K1735 melanoma of C3H mice also produce ecotropic retroviruses. However, the retrovirus-encoded antigen in these melanomas does not react with MM2-9B6 MAb (24). It is possible that the env region of the MelARV from melanomas of DBA/2 and C3H mice does not contain an epitope that is present in the MelARV from C57BL/6 mice and thus the MM2-9B6 MAb fails to react with these melanomas. MuLVs were initially found in murine lymphoma/leukemia. The finding of MuLVs in murine melanomas raises the question of what their biological significance is and whether they play a role in melanoma formation. The ecotropic MelARV in B16 melanoma cells probably originated from the endogenous ecotropic provirus Emv-2 that exists in all cells of C57BL/6 mice. However, this provirus is defective and is unable to generate replication-competent retrovirus. The defect in Emv-2 was mapped to nucleotide 3576 by the substitution of alanine for proline in the pol gene (17). It is possible that the MelARV in melanomas of C57BL/6 mice emerged as a result of a recombination between ecotropic Emv-2 and nonecotropic sequences during malignant transformation or tumor progression. MelARV in melanomas of other strains of mice might result from recombination between different partners. For example, in Cloudman S91 melanoma of DBA/2 mice, MelARV might be a descendant of Emv-3 that resides in the genome of DBA/2 mice. In K1735 melanoma, MelARV might contain env sequences from Emv-4 or Emv-5 of C3H mice. This might explain the differences between the reactions of melanomas of C57BL/6 and those of other strains of mice to MM2-9B6 MAb.
Therefore, it is of interest to clone and sequence MelARV and determine how unique or how common the MelARV is to the already well-characterized and sequenced ecotropic MuLVs. In the present study we have cloned and sequenced the complete genome of MelARV from B16 melanoma. In order to understand the origin of MelARV, it is important to compare the MelARV nucleotide and amino acid sequences with those of the endogenous ecotropic Emv-2 provirus that might be a precursor of the MelARV. However, Emv-2 was only partially sequenced (17). Therefore, we also sequenced the entire genome of Emv-2 and performed a full comparative analysis of MelARV and Emv-2 as well as the previously sequenced Emv-11 of AKR mice (12).
The role of MuLVs in malignant transformation has been mostly investigated in malignancies of hematopoetic origins. The existence of ecotropic MuLV in melanoma cells raises the question of whether MelARV production is incidental to or rather, whether it plays a pathogenic role in melanoma formation and progression. To test this possibility, we recently infected two melanocyte culture lines derived from C57BL/6 mice with MelARV from cell-free supernatant of BL6 melanoma cells. Normal melanocyte cells are able to grow in vitro only in the presence of 12-O-tetradecanolphorbol-13-acetate (TPA). In most cases, infection of melanocytes with MelARV resulted in the appearance of MAAs recognized by MM2-9B6 MAb without changes in their morphology and their dependence on TPA. In two cases, melanocytes showed morphological changes and were able to grow in vitro in the absence of TPA and after inoculation into mice, formation of the progressively growing highly pigmented tumors was observed. These MelARV-transformed melanomas were termed Meli-A and Meli-BL, respectively (27).
It is believed that there are two major mechanisms used by murine retroviruses to cause malignancies: (i) some MuLVs, like Abelson virus, contain an oncogene and can induce malignant transformation at a high frequency, practically in each infected cell, and (ii) the vast majority of MuLVs do not carry an oncogene, but instead they integrate into cellular genomes, which results in the insertional mutagenesis of certain cellular proto-oncogenes or tumor suppressor genes (for a review, see reference 37). Based on the relatively low frequencies of malignant transformation of normal melanocytes, it seems unlikely that the MelARV derived from B16 melanoma is a transforming retrovirus. It is more likely that the MelARV is a typical MuLV and its ability to transform melanocytes depends on its ability to insert into a cellular proto-oncogene(s) or tumor suppressor gene(s) and to change its expression or functions. Analysis of retrovirus insertion sites in MuLV-induced lymphoma/leukemia has been found to be a very fruitful approach in identification of numerous genes that are involved in leukemogenesis (1).
It is possible that cloning and sequencing of the MelARV insertion sites in murine melanomas might shed a new light on understanding the genetic mechanisms of melanoma formation. In the present study, we have attempted to clone and sequence the insertion sites of MelARV in B16 melanoma cells.
MATERIALS AND METHODS
Cell lines and plasmids.
The B16BL6 melanoma line (hereafter referred as BL6 line) was selected from B16F10 melanoma cells for high invasiveness and was kindly provided by Isaiah Fidler (M.D. Anderson Cancer Center, Houston, Tex.) (10). The BL6-8 clone was isolated from the parental B16BL6 line by limiting dilution (8). These melanoma cells were tested and proved to be free of the following murine viruses: respiratory enteric orphan virus type 3, pneumonia virus, K virus, Theiler’s murine encephalomyelitis virus, Sendai virus, minute virus of mice, mouse adenovirus, mouse hepatitis virus, lymphocytic choriomeningitis virus, ectromelia virus, and lactate dehydrogenase-elevating virus (Microbiological Associates, Walkersville, Md.). They were maintained in vitro in RPMI 1640 supplemented with 10% fetal bovine serum, glutamine, streptomycin, and penicillin (all from Life Technologies, Inc., Gaithersburg, Md.). The melanocyte line Melan-A was generated from the skin of 18-day-old embryos of C57BL/6 (2) and was a gift from D. Bennett (St. George’s Hospital Medical School, London, United Kingdom). Melan-A melanocytes were maintained in Bennett’s medium, which is minimum essential medium supplemented with 5% fetal calf serum, sodium pyruvate, sodium, bicarbonate, 2-mercaptoethanol (100 μM), antibiotics, and TPA (200 μM), adjusted to pH 6.9. Another melanocyte line, Melan-BL, was established from the skin of newborn C57/BL6 mice and kindly provided by R. Halaban (Yale University, New Haven, Conn.). Melan-BL was maintained in Ham’s F-10 medium supplemented with 5% FCS, 10% horse serum, and 100 nM TPA. Melan-A melanocytes that were transformed by v-rasHa transfection (40) were termed Mel-ras. Meli-A1 and Meli-BL melanomas were obtained by infection of the Melan-A line and Melan-BL lines with MelARV (27). All melanomas were maintained in complete RPMI 1640 medium. Plasmid pB6eco-fl was kindly provided by Steven King (Wayne State University, Detroit, Mich.). The plasmid contains on a pBR322 backbone a 20-kb insert, of which 8.8 kb is the full-length genome of the Emv-2 ecotropic retrovirus. The rest of the insert represents partial cellular flanking sequence (17).
Sequencing of pB6eco-fl.
The nucleotide sequence of the Emv-2 provirus and its cellular flanking regions was determined by sequencing the pB6eco-fl plasmid. DNA sequencing was performed with the chain terminator method (39) on an ABI 377 sequencer by a “crawling-along” strategy (38). A pair of primers that bind to ecotropic env gene were used to start the “walking” sequencing strategy in a “back-to-back” manner. Specifically, one primer (TATACGTCTCTGGACATG) lies at nucleotides 6507 to 6524 and directs the sequencing reaction toward the upstream portion of the viral genome and its 5′ flanking region. Another primer (GGTCATGTCCAGAGACG) binds the same stretch of nucleotides but extends toward the opposite downstream direction. The newly obtained sequences with these primers were then utilized to design primers for the next round of sequencing. By repeating this procedure, each clone that was subject to sequencing was eventually sequenced to completion on both strands.
Isolation of viral RNA.
Supernatant (100 ml) was freshly collected from BL6-8 cell culture and centrifuged at 2,000 × g for 10 min and then filtered through a 0.2-um-pore-size microfilter. The filtrated supernatant was then subject to ultracentrifugation at 25,000 rpm (Beckman SW28 rotor) for 3 h at 4°C. The pellet was recovered and resuspended in 1 ml of RNase-free, 10 mM Tris-HCl (pH 8.8), 1 mM EDTA, and 1% Nonidet P-40 and then extracted three times with 1 ml of phenol-chloroform-isoamyl alcohol (25:24:1). The resultant RNA was precipitated with ethanol and resolved in 100 μl of DEPC treated H2O.
Long template RT-PCR amplification, cloning, and sequencing.
Long template reverse transcriptase-PCR (RT-PCR) was performed with the One-step RT-PCR kit and the Elongase thermostable DNA polymerase (both from Life Technologies, Inc., Md.) according to the manufacturer’s manual. Briefly, 5 μg of viral RNA was applied to a reaction mixture containing 1× buffer, 5 U of Elongase, 1 μl of RT-Taq enzyme mixture and 0.2 μM of each primer. The RT-PCR primers were designed based on the consensus sequence of ecotropic retrovirus that was originally determined on AKV MuLV (12). The upstream primer (AACAAGGAAGTACACAGAGGCTGG) was derived from the U3 region and the downstream primer (GCAGTCAATCACTCTGAGGAGACC) was from the U5 region. The cycling parameters were as follows: 50°C for 30 min for reverse transcription, followed by 35 cycles of 94°C for 30 s, 57°C for 30 s, and 70°C for 5 min.
The recovery and cloning of the long-template RT-PCR product were performed with the TOPO XL PCR Cloning Kit (Invitrogen, Calif.). Briefly, the amplified DNA was recovered from 0.7% agarose gel after electrophoresis, purified with a spin column provided in the kit, and ligated to a precut vector pCR-XL-TOPO (Invitrogen) with two overhung T’s. The ligation reaction was then electroperforated into competent Escherichia coli cells and plated on LB-agar petri dishes. The resultant colonies were screened by hybridizing with an ecotropic retrovirus-specific env probe (pEc-B4) to obtain ecotropic retrovirus containing clones (3, 4). The RT-PCR and cloning were repeated to assure the accuracy of the amplification.
A crawling-along strategy similar to that used in pB6eco-fl sequencing was applied to sequence the positive clones obtained from the above cloning procedures. Plasmid DNA was purified with the Plasmid Miniprep Kit from Qiagen (California). The first round of sequencing was started from both ends of the viral inserts, primed by the following sequencing primers, respectively: the M13 reverse sequencing primer and the T7 sequencing primer (both from Life Technologies). The newly obtained sequences with these primers were then utilized to design primers for the next round of sequencing. By repeating this procedure, the insert of each clone that was subject to sequencing was eventually sequenced to completion on both strands. Sequence analysis was executed with the PC/Gene software (IntelliGenetics) and the BLAST program provided by National Center for Biotechnology Information, available on the Internet.
Inverse PCR.
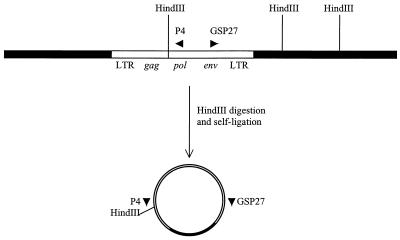
High-molecular-weight DNA was isolated from BL6-8 cells by proteinase K digestion and phenol-chloraform extraction and resuspended in H2O (38). DNA (1 μg) was subject to HindIII restriction enzyme digestion, followed by phenol-chloroform extraction. The HindIII-digested DNA was then added to a self-ligation reaction with T4 DNA ligase to generate a circular molecule that sequentially contains the 3′ cellular flanking sequence, partial pol region, the gag region, and the env region of MelARV. Two inverse primers P4 (GAAAGTCGGAGAGCCAGGTGGACC) and GSP27 (TGGGTCCTACTATTTGGCCGC) (Fig. 1) were designed in accordance with the nucleotide sequences of the pol and env regions of MelARV that were determined in this study. The PCR was performed with Taq DNA polymerase and the following cycling parameters: 94°C for 30 s, 57°C for 30 s, 72°C for 3 min for 30 cycles.
FIG. 1.
Inverse PCR used to clone MelARV flanking sequences. (A) Design of inverse PCR. HindIII restriction sites are marked. P4 and GSP27, two primers used in the inverse PCR. (B) Result of inverse PCR.
To directly sequence the products of inverse PCRs, the amplified bands were recovered from an agarose gel with a GenElute Agarose Spin Column (Suppleco), according to the manufacturer’s instruction. The purified DNA samples were then subject to a sequencing reaction containing a sequencing primer that is nested to the inverse PCR primer.
Northern blot analysis.
Northern analysis was performed as described previously (26). Cellular total RNA was isolated by the phenol-chloroform extraction method (38). The extracted RNAs were separated on 1% agarose gel containing formaldehyde and then transferred to a nylon membrane. The c-maf probe used in the Northern blot hybridization that represents the 5′ 300-bp portion of the murine c-maf gene was kindly given by L. Glimcher (Harvard Medical School, Boston, Mass.). After the blot membrane hybridized with the c-maf probe was exposed to an X-ray film, it was stripped with 1% sodium dodecyl sulfate and a 0.01× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) solution at 100°C twice for 5 min, followed by a rehybridization with a murine DNA polymerase gamma full-length cDNA probe that was a gift from H. P. Zassenhaus (St. Louis University, St. Louis, Mo.).
Nucleotide sequence accession number.
The sequence of the MelARV genome was deposited in GenBank under accession no. U63133.
RESULTS
Nucleotide sequence of MelARV.
Retroviral particles were obtained from the cell-free supernatant of cultured BL6-8 melanoma cells, and the retroviral RNA was isolated and used as a template for RT-PCR amplification. With a long-template RT-PCR method, we cloned and sequenced the full-length cDNA copy of the MelARV genome. The complete sequence of the MelARV genome consists of 8,274 nucleotides (nt). It contains 5′ and 3′ long terminal repeats (LTRs) and gag-pol and env coding regions that are 5,205 and 2,010 nt in length, respectively. The primary structure of these regions appears to have full coding capacity as a retrovirus. The coding sequences for all typical retroviral proteins, such as a gag gene coding for matrix protein (p15), protein p12, and capsid protein (p30), a pol gene coding for Pol protein, and an env gene coding for SU protein (gp70) and TM protein (p15E), are found to be organized in the typical order along the genome. Sequence analysis revealed that MelARV is a typical ecotropic retrovirus that does not contain an intraviral EcoRI restriction site. A PstI site was found only in the LTRs, and HindIII site was found in the pol region. Furthermore, the env region contains sequences that are typical for ecotropic retroviruses. However, the U3 region of MelARV compared to that of fully sequenced Emv-11 contains a 99-bp deletion.
Comparative nucleotide sequence analysis of MelARV and Emv-2 provirus.
It is believed that the Emv-2 locus exists in all cells of C57B/6 mice and is the only ecotropic endogenous provirus in normal cells of this origin (13). The sequence information of this provirus, obviously, is of importance since it could serve as one of the best references in comparative studies of other ecotropic viruses that originate in B16 melanomas. The sequence of the Emv-2 virus, however, was only partially determined by King et al. (17, 18), with a total length of 2,422 bp, covering areas that distribute discontinuously along the Emv-2 genome (bp 1 to 452, 2670 to 3719, 4168 to 4360, 4568 to 4632, 6004 to 6078, 6543 to 6641, 7111 to 7363, 7824 to 7922, and 8191 to 8326). Therefore, we attempted to fully sequence the Emv-2 genome cloned in the pB6eco plasmid. Our sequence analysis indicates that Emv-2 has a total length of 8,274 nt, with a perfect match to the partial sequences of Emv-2 virus previously reported by King et al. (17, 18). To characterize the Emv-2 sequence, it was compared with the Emv-11 virus (also designated AKV-623) (12), which is the only completely sequenced ecotropic retrovirus according to GenBank and has been widely used as a standard reference in structural and functional studies on endogenous retroviruses. The alignment of these two sequences between Emv-2 and Emv-11 reveals an overall homology as high as 98.38% (8,140 identical nt out of 8,274 nt in total) (Table 1). The most striking nucleotide difference is a 99-bp deletion in the Emv-2 U3 region, corresponding to nt 8035 to 8133 in the Emv-11, which is virtually one of two copies of the enhancer sequence in this region. In the coding regions, it is independently confirmed here that the pol region of Emv-2 carries a G to C mutation at the nt 3576, which has been considered responsible for the deficiency of the Emv-2 virus due to the resultant Ala to Pro substitution (17). The other variations, as shown in Table 1, basically scatter around the entire genome, many of which lead to amino acid substitutions.
TABLE 1.
Sequence variations among MelARV, Emv-2, and Emv-11 viruses in all regions
| Genome region and nucleotide positiona | Emv-11 (AKR) | MelARV (B16) | Emv-2 (C57B/6) | ||
|---|---|---|---|---|---|
| 5′UTRb | |||||
| 129 | C | C | T | ||
| 263-4 | TG | Deleted | Deleted | ||
| 333 | C | T | T | ||
| 433 | G | A | G | ||
| 468 | T (inserted) | ||||
| 598 | C | T | T | ||
| Gag-p15 | |||||
| 646 | A (Gln) | A (Gln) | A (Gln) | ||
| 809 | T | T | C | ||
| 929 | C | A | C | ||
| 1009 | C (Pro) | T (Leu) | C (Pro) | ||
| Gag-p12 | |||||
| 1144 | T (Leu) | C (Pro) | C (Pro) | ||
| Gag-p30 | |||||
| 1285 | T (Leu) | C (Pro) | T (Leu) | ||
| 1376 | G | A | G | ||
| 1389 | C | T | C | ||
| 1394 | T | C | T | ||
| 1397 | A | C | A | ||
| 1406 | A | G | A | ||
| 1409 | C | T | T | ||
| 1434 | T (Trp) | C (Arg) | T (Trp) | ||
| 1439 | T | C | T | ||
| 1442 | T | C | T | ||
| 1445 | C | T | C | ||
| 1454 | A | G | A | ||
| 1457 | A | G | A | ||
| 1469 | T | G | T | ||
| 1472 | C | A | C | ||
| 1475 | G | A | G | ||
| 1478 | G | A | G | ||
| 1481 | G | A | G | ||
| 1499 | G | A | G | ||
| 1502 | A | G | A | ||
| 1506 | C | A | C | ||
| 1511 | G | G | A | ||
| 1514 | T | A | T | ||
| 1532 | G | A | G | ||
| 1544-5 | AC | GT | AC | ||
| 1550 | C | T | C | ||
| 1553 | C | T | C | ||
| 1556 | G | A | G | ||
| 1559-60 | TG (Asp) | CA (Asn) | TG (Asp) | ||
| 1562-63 | CG (Ala) | TT (Ser) | CG (Ala) | ||
| 1565 | T | C | T | ||
| 1568 | T | C | T | ||
| 1571 | T | C | T | ||
| 1598 | C | T | C | ||
| 1601 | C | A | C | ||
| 1606-9 | AAAG (GlnArg) | CTGA (ProGlu) | AAAG (GlnArg) | ||
| 1646 | T | C | T | ||
| 1652 | A | A | G | ||
| 1719 | A (Asn) | A (Asn) | G (Asp) | ||
| 1727 | C | T | C | ||
| 1884 | A (Ser) | G (Gly) | A (Ser) | ||
| 1970 | A | G | G | ||
| 2024 | G | A | G | ||
| Gag-p10 | |||||
| 2109 | G (Gly) | A (Arg) | G (Gly) | ||
| 2119 | G (Arg) | T (Met) | G (Arg) | ||
| Pol | |||||
| 2375 | G | C | C | ||
| Genome region and nucleotide positiona | Emv-11 (AKR) | MelARV (B16) | Emv-2 (C57B/6) | ||
| 2504 | C | T | C | ||
| 2538 | C | T | C | ||
| 2643 | T (Tyr) | C (His) | C (His) | ||
| 2668 | T (Val) | C (Ala) | C (Ala) | ||
| 2690 | C | T | T | ||
| 2824 | A (Lys) | G (Arg) | G (Arg) | ||
| 2978 | G | G | A | ||
| 3129 | A (Arg) | G (Gly) | A (Arg) | ||
| 3161 | A | G | A | ||
| 3219 | C | T | C | ||
| 3221 | A | G | A | ||
| 3288 | A (Ile) | T (Leu) | A (Ile) | ||
| 3298 | A | G | A | ||
| 3302 | C | T | C | ||
| 3308 | T | C | T | ||
| 3311 | G | A | G | ||
| 3314 | C | A | C | ||
| 3341-2 | GT | TC | GT | ||
| 3346 | T (Leu) | A (Gln) | T (Leu) | ||
| 3356 | A | G | A | ||
| 3365 | G | A | G | ||
| 3368 | T | C | T | ||
| 3393 | C (Leu) | A (Ile) | C (Leu) | ||
| 3395 | T | C | T | ||
| 3410 | C | T | C | ||
| 3413 | G | A | G | ||
| 3416 | T | C | C | ||
| 3425 | T | C | T | ||
| 3428 | C | T | C | ||
| 3431 | A | G | A | ||
| 3433-4 | AA (Lys) | GG (Arg) | AA (Lys) | ||
| 3461 | C | T | C | ||
| 3485 | G | A | G | ||
| 3488-90 | TAC (Thr) | CGT (Val) | TAC (Thr) | ||
| 3494 | G | A | G | ||
| 3500 | C | T | C | ||
| 3542 | T | C | T | ||
| 3575 | A | G | A | ||
| 3576 | G (Ala) | G (Ala) | C (Pro) | ||
| 3614 | C | T | C | ||
| 3665 | C | T | C | ||
| 3785 | C | T | C | ||
| 3812 | T | C | C | ||
| 3824 | T | C | T | ||
| 3839 | T | C | T | ||
| 3966 | C (Leu) | A (Ile) | C (Leu) | ||
| 4005 | C (Leu) | A (Ile) | C (Leu) | ||
| 4070 | C | A | C | ||
| 4077 | G (Glu) | A (Lys) | G (Glu) | ||
| 4172 | C | T | C | ||
| 4223 | G | T | G | ||
| 4549 | C (Ser) | A (Thr) | C (Ser) | ||
| 4583 | G | A | G | ||
| 4983 | T (Ser) | A (Thr) | T (Ser) | ||
| 5185 | C (Pro) | C (Pro) | T (Leu) | ||
| 5336 | T | G | T | ||
| 5375 | T | C | T | ||
| 5409 | C | T | T | ||
| 5453 | T | C | C | ||
| 5575 | A (Gln) | G (Arg) | G (Arg) | ||
| 5597 | A | G | A | ||
| 5765 | G | A | G | ||
| Env-gp70 | |||||
| 5879 | A (Thr) | G (Ala) | G (Ala) | ||
| 5915 | A (Thr) | T (Ser) | T (Ser) | ||
| 6116 | C (Pro) | T (Ser) | C (Pro) | ||
| 6184 | C | C | C | ||
| 6214 | A | C | C | ||
| 6222 | G (Gly) | A (Glu) | G (Gly) | ||
| 6224 | G (Gly) | G (Gly) | A (Arg) | ||
| 6271 | A | G | G | ||
| 6349 | G | G | C | ||
| 6419 | G (Glu) | A (Lys) | G (Glu) | ||
| 7057 | T | C | C | ||
| Env-p15E | |||||
| 7475 | T (Phe) | C (Leu) | T (Phe) | ||
| 7769-70 | GA (Glu) | GG (Gly) | GA (Glu) | ||
| 3′UTR | |||||
| 7875 | T | C | C | ||
| 8006 | C | C | C | ||
| 8012 | C | C | C | ||
| 8014 | A (inserted) | ||||
| 8035-134 | Deleted | Deleted | |||
| 8298 | G | A | A |
The nucleotide positions are numbered in accordance with the system used by Herr in the nucleotide sequence of the AKV-623 provirus (12). Changes in deduced amino acid sequences are noted in brackets following the corresponding nucleotides.
UTR, untranslated region.
The highly productive MelARV probably emerged in B16 melanoma cells from the endogenous ecotropic Emv-2 provirus. Also of note is that the deduced Gag proteins of Emv-2 and MelARV reveal a greater difference than those between Emv-11 and Emv-2 (2.05% versus 0.37%). Most of their amino acid differences are due to alterations present in the coding region of the major core protein, p30 (nt 1280 to 2068) (Table 1). The p30 protein contains N/B tropism determinants (35). Because the Emv-2 is an N-tropic virus (18), these alterations must occur to restore efficient viral growth in the Fv-1b/b strain of C57B/6 mice. Hence, our data suggest that the changes in p30 are a part of the molecular basis for the restoration of the productivity of the MelARV during its emergence in melanoma or premalignant cells.
The variation rate between the env genes of Emv-2 and MelARV is the lowest (0.4%) compared with the gag and pol other two coding regions (3.4 and 1.56%, respectively) (Table 1). However, seven of eight nucleotide changes in the env region of MelARV give rise to an amino acid alteration. As a result, the deduced amino acid sequences of MelARV and Emv-2 Env proteins reveal a moderate heterology of 1.05%, which is significantly higher than the difference between Emv-11 and Emv-2. In addition, five of the seven changed amino acids are present in gp70 (Table 1), which is the very molecule that is most likely engaged in extracellular molecular interactions. We previously found that the env gene of MelARV encodes the cell surface MAA recognizable by MM2-9B6 MAb, which has long been used as a powerful tool in experimental studies of B16 melanoma (9, 24, and 25). According to our data, it is likely that structural modifications in MelARV gp70 caused by the observed sequence changes resulted in the emergence of the specific MAA epitope.
Since the highly productive MelARV that emerged in B16BL6 melanoma cells is an ecotropic retrovirus, it could be that the MelARV is a variant virus derived from the Emv-2 locus during the development of melanoma. This hypothesis is supported by the fact that MelARV, like Emv-2, also carries the 99-bp deletion in its U3 region, but Emv-11 which represents an ecotropic virus of AKR mice has a duplication of this sequence. As a concomitant result, the difference in the LTRs between Emv-2 and MelARV (0.36%) appears much lower than that between MelARV and Emv-11 (9.27%) (Table 2).
TABLE 2.
Nucleotide and amino acid sequence variation rates between MelARV, Emv-2, and Emv-11
| Comparison groups | % Differences (no. of variations/total no. of residues)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nucleotides
|
Deduced amino acids
|
||||||||
| UTRc | gag | pol | env | Overall | Gag | Pol | Env | Overall | |
| MelARV vs Emv-2 | 0.36 (4/1100) | 3.22 (52/1614) | 1.59 (57/3591) | 0.40 (8/2010) | 1.46 (121/8274) | 2.05 (11/537) | 1.09 (13/1196) | 1.05 (7/669) | 1.29 (31/2402) |
| MelARV vs Emv-11 | 9.73b (107/1100) | 3.16 (51/1614) | 1.78 (64/3591) | 0.55 (11/2010) | 2.33 (193/8274) | 2.05 (11/537) | 1.25 (15/1196) | 1.20 (8/669) | 1.42 (34/2402) |
| Emv-2 vs Emv-11 | 9.73 (107/1100) | 0.42 (7/1614) | 0.36 (6/3591) | 0.30 (6/2010) | 1.61 (133/8274) | 0.37 (2/537) | 0.50 (6/1196) | 0.45 (3/669) | 0.46 (11/2402) |
All residue changes, deletions, and insertions are taken into consideration in calculation of the variation rates.
Variation rate includes deletions. If excluding the deletions, the rate would be 0.72% (8/1100).
UTR, untranslated region.
As shown in Table 2, the overall difference between Emv-2 and MelARV is 1.49% at the nucleotide level and 1.29% at the deduced amino acid level. Most noteworthy among all the differences is probably the C to G reversion at position 3576 in the pol region of MelARV (Table 1), consistent with the previous conclusion that the mutation at this position contributes to the deficiency of the Emv-2 provirus (17).
Identification of MelARV insertion sites.
Sequence analysis indicates that MelARV does not contain an oncogene. Its possible involvement in malignant transformation could be due to its insertion in cellular oncogenes or suppressor genes and the resultant changes in the expression or functions of these genes or their products. Therefore, we attempted to clone and sequence the MelARV insertion sites in BL6 melanoma cells. Based on our Southern blot analysis of the HindIII-digested genomic DNA, we estimated that the BL6 melanoma cell line contains at least four ecotropic proviruses in its genome (26, 30). One of them is Emv-2, which was shown as a 7.0-kb band on the Southern blot and was found in DNAs from all normal cells (30), consistent with all earlier studies (13, 28). In addition, several HindIII-digested fragments shorter than 7.0 kb (about 6.5 and 6.0 kb) and another of about 11.0 kb were found when DNA from BL6 melanoma cells was analyzed. By sequencing the pB6eco-fl plasmid, we obtained the 5′ and 3′ flanking sequences of Emv-2 proviruses, with a length of 7 and 8 kb, respectively. Within the 15-kb flanking region sequenced so far, no significant homology has been found to sequences in any of the GenBank collections and no open reading frame longer than 40 amino acids has been identified, suggesting that Emv-2 is inserted into a region that lacks coding capacity.
Since Emv-2 is a single copy of an ecotropic provirus in C57B/6 mice that is defective and unable to produce replication-competent retroviral particles, we reasoned that MelARV might have been produced from some or all of the other three ecotropic proviral loci in the BL6 melanoma genome. To characterize these integration sites, we applied inverse PCR on the artificially circulated genomic DNA derived from the BL6-8 melanoma clone. The isolated DNA was digested with the HindIII restriction enzyme, circulated, and used as a template in the inverse PCR. Two amplification products were obtained from the inverse PCR, with sizes of 1.6 and 2.1 kb, respectively. The respective sizes of these two bands are in accordance with flanking sequences of the 6.5- and 6.0-kb bands of the MelARVs on the HindIII-digested Southern blot pattern previously described (26).
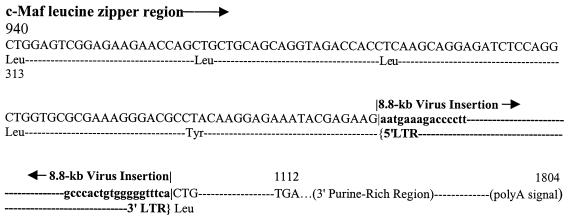
Nucleotide sequences of the inverse PCR products were determined. The 1.6-kb band contains a 230-bp nonviral sequence. A search of GenBank showed that it represents the 3′ end of the c-maf proto-oncogene (GenBank accession no. S74567). As shown in Fig. 2, MelARV is inserted between the nt 1973 and 1974. The c-maf gene codes for a basic region/leucine zipper protein that belongs to the AP-1 family of transcription factors (15, 22, 31). The virus is integrated at the end of the fifth zipper, in the same transcription direction as the c-maf gene. This gene is also characterized by its long 3′ nontranslational sequence (NTS). As a result of the insertion, the last leucine codon of the leucine zippers, the downstream 22 codons of the c-maf gene, and the 700-bp 3′ NTS are separated from their upstream sequences. It is known that the c-maf proto-oncogene gene is at 61.0 centimorgans (cM) on mouse chromosome 8, to which the Emv-2 provirus has also been localized at 67.0 cM (6). Thus, one of the MelARV proviruses and Emv-2 are inserted in the same chromosome.
FIG. 2.
MelARV (lowercase) insertion in the c-maf proto-oncogene (uppercase). The insertion borders are marked with vertical lines. All the leucines that form the zipper structure are shown. Omitted sequences are represented by dotted lines. Nucleotides of the c-maf gene are numbered from the A of the start codon.
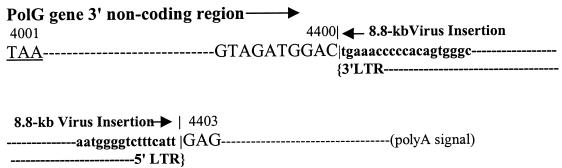
The 2.1-kb inverse PCR product represents a virus-cell junction at the 3′ end of the DNA polymerase gamma gene (PolG) (GenBank accession no. U53584). PolG catalyzes replication of mitochondrial DNA. This copy of MelARV is inserted into chromosome 7 where it is known the PolG gene is located (37). The insertion occurs in the opposite transcription direction to PolG and in the 3′ nontranslational region of the gene, 400-bp downstream from the stop codon TAA (Fig. 3).
FIG. 3.
MelARV insertion in the PolG gene. Sequence of the PolG gene (uppercase) downstream from the translation stop codon (underlined) and the insertion junctions are shown. The viral sequence is shown in lowercase. The insertion borders are marked with vertical lines. Nucleotides are numbered in accordance with that of the PolG gene sequence in GenBank (accession no. U53584).
Analysis of c-maf and DNA polymerase gamma gene expression in melanoma cells.
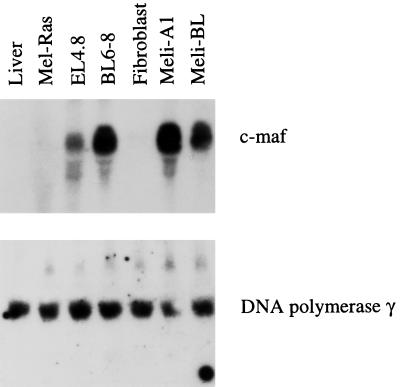
To examine the potential impact of retroviral insertion on the expression of the involved host genes, Northern blot analysis was performed (Fig. 4). RNA from EL4.8 T-lymphoma cells provided by L. Glimcher (Harvard Medical School, Boston, Mass.) served as a positive control. It was found that expression of c-maf gene was up-regulated in BL6-8, Meli-A1, and Meli-BL melanoma cell lines. No c-maf expression was found in normal liver cells and fibroblasts. It is of note that the Mel-ras melanoma cells, which were derived from the Melan-A melanocyte line transformed by the v-rasHa gene did not express the c-maf proto-oncogene (Fig. 4). Normal melanocytes also show no expression of c-maf (data not shown). When the same Northern blot membrane was stripped and rehybridized with a DNA polymerase gamma probe, all tested lines showed an equal amount of messages for the gene, indicating that expression of the DNA polymerase gamma gene was not affected by the retroviral integration (Fig. 4).
FIG. 4.
Expression of the c-maf and DNA polymerase gamma genes in melanoma cells. RNA was extracted from melanoma cells (BL6-8), melanomas transformed by MelARV (Meli-A1 and Meli-BL), Mel-Ras melanoma cells transformed by v-rasHa, and normal C57BL/6 fibroblasts or liver cells. RNA from EL4-8 T lymphocytes was used as a positive control. An equal amount of loaded RNAs was shown by hybridization with a beta-actin probe (data not shown).
DISCUSSION
We have cloned and sequenced the full-length genome of the ecotropic B-tropic MelARV, which is produced by B16 melanoma cells. B16 melanoma cells were originally derived from C57B/6 mice that contain only one single copy of the ecotropic Emv-2 provirus that has features of N tropism. It is very likely that the Emv-2 provirus took part in the molecular process that resulted in the emergence of MelARV during malignant transformation or melanoma progression. To compare the primary structures of these two related viruses, we also completely sequenced the Emv-2 provirus.
Our comparative study exhibits very high homologies between MelARV and Emv-2 as well as Emv-11. However, in comparison with Emv-11, the U3 region of MelARV contains only one direct repeat. It is known that the tandem repeat elements of MuLVs function as an enhancer and they direct a high level of transcription in a tissue-specific manner (23, 36). It is possible that the lack of 99 bp in the LTR of MelARV might regulate the tissue specificity of this retrovirus.
Emv-2 is a defective ecotropic provirus. In a previous study, a mutation that inactivated the ecotropic MuLV reverse transcriptase was mapped to nucleotide 3576, as a result of an alanine to proline substitution in the presumed tether region between the polymerase and the RNase H domains (14, 17, 18). Accordingly, a correction of the mutation at this position must occur in the proviruses to restore its capability of producing viral particles. Comparative analysis on the sequence data obtained in our study proved that this has been the case in MelARV, which has an alanine rather than a proline at the position. In addition, the Emv-2 locus is known to code for an N-tropic ecotropic virus (35). For this virus to efficiently grow in its Fv-1b/b host such as C57BL/6 cells, its major core protein (p30) must be changed. Indeed, we found that clustered alterations did occur in the deduced p30 sequence of MelARV, reflected as a significantly higher variation rate in the region between Emv-2 and MelARV than between Emv-2 and Emv-11.
Considering the requirement for multiple changes in both p30 and pol genes to resume the correct functions of their products, we propose that the MelARV originated as a result of recombination between Emv-2 and an N-tropic nonecotropic endogenous virus in C57BL/6 mice. This hypothesis is further supported by the feature that the differences between the Emv-2 and MelARV sequences are clustered in certain areas rather than randomly spanning around the entire genome. Consistent with our data, the emergence of productive ecotropic viruses by recombination was also found in MuLV recovered from radiation-induced thymomas of C57B/6 or from aged DBA/2 mice (33, 34).
The envelope protein of retrovirus is a critical molecule during viral infection and other molecular interactions. The env gene of MelARV has been found to code for MAA, recognizable by MM2-9B6 MAb (26). The recognition specificity is not found on normal or other malignant cells of C57B/6 origin, indicating that either the Env protein of Emv-2 is not expressed or correctly presented on the cell surface or the expressed protein structure does not contain the recognizable epitopes. There are seven amino acid differences between the SU proteins of the Emv-2 and the MelARV, as demonstrated in this study. These amino acid differences might be responsible for the appearance of the epitope that is specific for MelARV. It is of note that the env regions of Emv-2 and Emv-11 are more homologous and manifest differences in only three amino acids.
The biological significance of MelARV remains to be further clarified. We previously demonstrated that in vitro infection of a normal melanocyte line with MelARV could induce malignant transformation (27). However, this transformation was a relatively rare event, and in most cases infection of melanocytes resulted in expression of MAA but without malignant transformation. Our current data show that MelARV does not contain any oncogene and the MelARV-induced transformation could be a result of viral insertion. We have previously demonstrated that MelARV has been inserted in at least three locations in the genome of B16 melanoma cells (26, 30). In this study, cloning and sequencing of the insertion sites revealed that one copy of MelARV is inserted in the c-maf proto-oncogene and that the insertion results in overexpression of the gene. This result suggests that the viral insertion in the melanoma cell lines might indeed play an active role in melanoma formation by affecting host genes such as c-maf. c-maf is the cellular counterpart of the v-maf oncogene, which was identified in the avian retrovirus AS42 that was isolated from a spontaneous musculoaponeurotic fibrosarcoma of a chickensso (15, 31). Products of both genes are basic leucine zipper proteins. They belong to the AP-1 family of transcription factors and are able to form homodimers or heterodimers with Fos and Jun oncoproteins. Overexpression of c-maf gene can cause malignant transformation of transfected chicken fibroblasts (15). Moreover, frequent dysregulation of the c-maf proto-oncogene at 16q23 by translocation to an immunoglobulin locus was recently found in human multiple myeloma cells (5). Insertion of MelARV in the 3′ end of the c-maf coding sequence truncates the last leucine zipper repeat as well as the extraordinarily long 3′ noncoding sequence. Although the mechanism of the c-maf overexpression resulting from this integration remains unclear, several possibilities should be taken into consideration. While it was suggested that the last leucine zipper is not critical to its transcription-activating activity (22), truncation of a long 3′ noncoding region could stabilize the mRNA and, in turn, could be important for oncogenic activation, as was found with the c-fos gene (29). Furthermore, the viral LTR region contains numerous transcription regulatory elements such as enhancers, which might also have an impact on expression of the inserted genes. Indeed, it was demonstrated that retrovirus insertion downstream of the c-myc gene elevated its transcription when the provirus was inserted in the same transcription orientation as c-myc (32). In melanoma cells, MelARV is inserted downstream of c-maf in the same orientation and thus might affect c-maf expression.
A MelARV provirus is also inserted in the 3′ noncoding sequence of the PolG gene, in the opposite transcription direction. The PolG gene encodes DNA polymerase gamma, which catalyzes the replication of mitochondria DNA. Our Northern blot analysis showed that the expression of this gene was not affected by viral integration.
In summary, the complete genomes of MelARV and Emv-2 have been sequenced. Our data reported here provide clues to further understanding the molecular events that occurred during the emerging of the ecotropic retrovirus as an MelARV. This study, by uncovering the MelARV insertion sites, has also opened an avenue to further exploring the potential molecules involved in melanoma formation and progression.
ACKNOWLEDGMENTS
This work was in part supported by Public Health Service grant CA59903.
We thank Steven R. King for providing the pB6eco-fl plasmid, Laurie Glimcher for providing the c-maf probe, and H. Peter Zassenhaus for providing the DNA polymerase gamma probe. We also thank Ronald C. Montelaro for comments and fruitful discussions.
REFERENCES
- 1.Askew D S, Bartholomew C, Ihle J N. Insertional mutagenesis and the transformation of hematopoietic stem cells. Hematol Pathol. 1993;7:1–22. [PubMed] [Google Scholar]
- 2.Bennett D C, Cooper P J, Hart I R. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumor promoter for growth. Int J Cancer. 1987;39:414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- 3.Chan H, Bryan T, Moore J, Staal S, Rowe W, Martin M. Identification of ecotropic proviral sequences in inbred mouse strains with a cloned subgenomic DNA fragment. Proc Natl Acad Sci USA. 1980;77:5779–5783. doi: 10.1073/pnas.77.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chattopadhyay S, Lander M, Rands E, Lowy D. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci USA. 1980;77:5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chesi M, Bergsagel P L, Shonukan O O, Martelli M L, Brents L A, Chen T, Schrock E, Ried T, Kuehl W M. Frequent dysregulation of the c-maf proto-oncogene at 16q23 by translocation to an Ig locus in multiple myeloma. Blood. 1998;91:4457–4463. [PubMed] [Google Scholar]
- 6.Copeland N G, Jenkins N A, Gilbert D J, Eppig J T, Maltais L J, Miller J C, Dietrich W F, Weaver A, Lincoln S E, Steen R G, Nadeau J H, Lander E S. A genetic linkage map of the mouse: current applications and future prospects. Science. 1993;262:57–66. doi: 10.1126/science.8211130. [DOI] [PubMed] [Google Scholar]
- 7.Eisenthal A, Lafreniere R, Lefor A, Rosenberg S A. Effect of anti-B16 melanoma monoclonal antibody on established murine B16 melanoma liver metastases. Cancer Res. 1987;47:7140–7145. [PubMed] [Google Scholar]
- 8.Gorelik E, Peppoloni S, Overton T, Herberman R. Increase in H-2 antigen expression and immunogenicity of BL6 melanoma cells treated with N-methyl-N′-nitro-nitrosoguanidine. Cancer Res. 1985;45:5341–5347. [PubMed] [Google Scholar]
- 9.Gorelik E, Jay G, Kim M, Hearing V J, DeLeo A, McCoy P J. Effects of H-2Kb gene on expression of melanoma-associated antigen and lectin-binding sites on BL6 melanoma cells. Cancer Res. 1991;51:5212–5218. [PubMed] [Google Scholar]
- 10.Hart I. The selection and characterization of an invasive variant of the BL6 melanoma. Am J Pathol. 1979;97:587–591. [PMC free article] [PubMed] [Google Scholar]
- 11.Hearing V J, Leong S P, Vieira W D, Law L W. Suppression of established pulmonary metastases by murine melanoma-specific monoclonal antibodies. Int J Cancer. 1991;47:148–153. doi: 10.1002/ijc.2910470126. [DOI] [PubMed] [Google Scholar]
- 12.Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984;49:471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins N, Copeland N, Taylor B. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus J. Virol. 1982;43:26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson M S, McClure M A, Feng D-F, Gray J, Doolittle R F. Computer analysis of retroviral pol genes: assignment of enzymatic functions to specific sequences and homologies with nonviral enzymes. Proc Natl Acad Sci USA. 1986;83:7648–7652. doi: 10.1073/pnas.83.20.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataoka K, Nishizawa M, Kawai S. Structure-function analysis of the maf oncogene product, a member of the b-Zip protein family. J Virol. 1993;67:2133–2141. doi: 10.1128/jvi.67.4.2133-2141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keshet E, Schiff R, Itin A. Mouse retrotransposons: a cellular reservoir of long terminal repeat (LTR) elements with diverse transcriptional specificities. Adv Cancer Res. 1991;56:215–251. doi: 10.1016/s0065-230x(08)60482-0. [DOI] [PubMed] [Google Scholar]
- 17.King S, Berson B, Risser R. Mechanism of interaction between endogenous ecotropic murine leukemia viruses in (BALB/c × C57BL/6) hybrid cells. Virology. 1988;163:1–11. doi: 10.1016/0042-6822(88)90388-1. [DOI] [PubMed] [Google Scholar]
- 18.King S R, Horowitz J M, Risser R. Nucleotide conservation of endogenous ecotropic murine leukemia proviruses in inbred mice: implications for viral origin and dispersal. Virology. 1987;157:543–547. doi: 10.1016/0042-6822(87)90298-4. [DOI] [PubMed] [Google Scholar]
- 19.Kozak C A. Retroviruses as chromosomal genes in the mouse, Adv. Cancer Res. 1985;44:295–336. doi: 10.1016/s0065-230x(08)60030-5. [DOI] [PubMed] [Google Scholar]
- 20.Kozak C A, Ruscetti S. Retroviruses in rodents. In: Levy J A, editor. The Retroviridae. New York, N.Y: Plenum Press; 1992. pp. 405–481. [Google Scholar]
- 21.Kuff E, Leuders K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- 22.Kurschner C, Morgan J. The maf proto-oncogene stimulates transcription from multiple sites in a promoter that directs Purkinje neuron-specific gene expression. Mol Cell Biol. 1995;15:246–254. doi: 10.1128/mcb.15.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrenz-Smith S C, Massey A C, Innes D J, Thomas C Y. Pathogenic determinants in the U3 region of recombinant murine leukemia viruses isolated from CWD and HRS/J mice. J Virol. 1994;68:5174–5183. doi: 10.1128/jvi.68.8.5174-5183.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leong S, Muller J, Yetter R, Gorelik E, Takami T, Hearing V J. Expression and modulation of a retrovirus-associated antigen by murine melanoma cells. Cancer Res. 1988;48:4954–4958. [PubMed] [Google Scholar]
- 25.Li M, Muller J, Rao V, Hearing V J, Leuders K, Gorelik E. Loss of intracisternal A-type retroviral particles (IAPs) in BL6 melanoma cells transfected with MHC class I genes. J Gen Virol. 1996;77:2757–2765. doi: 10.1099/0022-1317-77-11-2757. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Muller J, Xu F, Hearing V J, Gorelik E. Inhibition of melanoma-associated antigen expression and ecotropic retrovirus production in B16BL6 melanoma cells transfected with major histocompatibility complex class I genes. Cancer Res. 1996;56:4464–4474. [PubMed] [Google Scholar]
- 27.Li M, Xu F, Muller J, Hearing V J, Gorelik E. Ecotropic C-type retrovirus of B16 melanoma and malignant transformation of normal melanocytes. Int J Cancer. 1998;76:430–436. doi: 10.1002/(sici)1097-0215(19980504)76:3<430::aid-ijc23>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 28.Lowy D R, Rands E, Chattopadhyay S K, Garon C F, Hager G L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci USA. 1980;77:614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller A D, Curran T, Verma I M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984;36:51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- 30.Muller J, Khan A S, Li M, Rao V, Hearing V J, Gorelik E. Phenotypic changes and loss of melanoma-specific endogenous C-type retroviruses in BL6 melanoma cells transfected with the H-2Kb gene. Melanoma Res. 1996;6:101–111. doi: 10.1097/00008390-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Nishizawa M, Kataoka K, Goto N, Fujiwara T, Kawai S. v-maf, a viral oncogene that encodes a “leucine zipper” motif. Proc Natl Acad Sci USA. 1989;86:7711–7715. doi: 10.1073/pnas.86.20.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne G S, Bishop J M, Varmus H E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982;295:209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- 33.Rassart E, Sander-Minstry P, Lemay G, DesGroseillers L, Jolicoeur P. New class of leukemogenic ecotropic recombinant murine leukemia virus isolated from radiation-induced thymomas of C57BL/6 mice. J Virol. 1983;45:565–575. doi: 10.1128/jvi.45.2.565-575.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rassart E, Shang M, Boie Y, Jolicoeur P. Studies on emerging radiation leukemia virus variants in C57BL/Ka mice. J Virol. 1986;58:96–106. doi: 10.1128/jvi.58.1.96-106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risser T, Horowitz J M, McCubrey J. Endogenous mouse leukemia viruses. Annu Rev Genet. 1983;17:85–121. doi: 10.1146/annurev.ge.17.120183.000505. [DOI] [PubMed] [Google Scholar]
- 36.Rosen C A, Haseltine W A, Lenz J, Ruprecht T, Cloyd M W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985;55:862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg N, Jolicoeur P. Retroviral pathogenesis. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1997. pp. 475–585. [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 39.Sanger F S, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsukamoto K, Ueda M, Hearing V J. Melanogenesis in murine melanocytes is suppressed by infection with the v-rasHa oncogene. Pigment Cell Res Suppl. 1992;2:181–184. doi: 10.1111/j.1600-0749.1990.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 41.Zullo S J, Butler L, Zahorchak R J, Macville M, Wilkes C, Merril C R. Localization by fluorescence in situ hybridization (FISH) of human mitochondrial polymerase gamma (PolG) to human chromosome band 15q24→q26, and of mouse mitochondrial polymerase gamma (Polg) to mouse chromosome band 7E, with confirmation by direct sequence analysis of bacterial artificial chromosomes (BACs) Cytogenet Cell Genet. 1997;78:281–284. doi: 10.1159/000134672. [DOI] [PubMed] [Google Scholar]