1. INTRODUCTION
Recent research has highlighted the increasing use of surrogate endpoints in interventional trials. 1 The United States Food and Drug Administration (US FDA) defines a surrogate endpoint, or an “intermediate clinical endpoint,” as: “…a marker, such as a laboratory measurement, radiographic image, physical sign, or another measure, that is not itself a direct measurement of clinical benefit, and—(A) is known to predict clinical benefit and could be used to support traditional approval of a drug or biological product; or (B) is reasonably likely to predict clinical benefit and could be used to support the accelerated approval of a drug or biological product under section 506(c).” 2 Among the “Table of Surrogate Endpoints” provided by FDA, preterm birth (PTB) is listed as a surrogate endpoint to accelerate the approval of investigational therapies for spontaneous PTB (sPTB). 3 Time to sPTB from the onset of spontaneous preterm labor (sPTL) is a surrogate endpoint that may also be reasonably likely to predict neonatal morbidity/mortality. Currently, there are no FDA‐approved therapies for reducing the risk of neonatal morbidity/mortality resulting from sPTB. To understand the potential for predicting neonatal outcomes in future studies, a rapid review was conducted to synthesize the quantitative evidence on the strength of surrogacy for PTB and time to delivery from sPTL diagnosis to sPTB.
2. METHODS
The Cochrane rapid review guidance 4 was used to identify systematic literature reviews (SLRs), randomized controlled trials (RCTs) and observational cohort studies in Ovid MEDLINE, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews. Searches were executed on February 16, 2023, and used predefined Population, Intervention, Comparators, Outcomes, Study design criteria (Appendix Table A1). Searches were restricted to English language and to articles published between 2002 and 2023. Searches for RCTs were restricted to those conducted in North America, while searches for SLRs were expanded to include any geographic region. Additional searches for observational studies conducted in North America or Europe and published between 2018 and 2023 were conducted. These latter search restrictions were applied to reflect studies following up on the retosiban program, now terminated. 5 , 6 This was the only late phase development program in the US known to have conducted observational studies published in the last 5 years, which were relevant to the research question; as such, observational studies sponsored by GlaxoSmithKline were eligible for consideration. Supplemental hand searches of reference lists, publicly available FDA documents, and the American College of Obstetricians and Gynecologists websites were conducted.
The population of interest included individuals with a singleton and uncomplicated pregnancy, with/without a history of singleton sPTB, and reported delivery at <37 weeks' gestational age, or, reported time to delivery from sPTL diagnosis to sPTB. Interventions included preventative agent(s) for the prolongation of pregnancy; or treatment of sPTL. Outcomes included short‐term neonatal outcomes (defined as the time from PTB to 28 days beyond the expected due date at 40 weeks' gestational age) including morbidity and mortality (as defined by the literature). Studies were included if either one or both surrogate endpoints of interest and, simultaneously, any one or more clinically meaningful neonatal outcomes (i.e., neonatal morbidity/mortality) were reported. For this rapid review, strength of surrogacy was defined as any empirical measures of association between the surrogate endpoints of interest and clinically meaningful outcomes of interest (morbidity, mortality); empirical measures of interest included coefficients derived from correlational analyses or using regression techniques.
One reviewer screened and extracted data; a second reviewer independently verified ≥ 10% of all screenings and extractions. Strength of surrogacy was determined based on a quantitative assessment of the correlation or predictive capability between each surrogate endpoint of interest and neonatal morbidity/mortality. Risk of bias was assessed using the second version of the Cochrane risk‐of‐bias tool for RCTs, the adapted Newcastle‐Ottawa scale for observational studies, and the second version of the Assessing the Methodological Quality of Systematic Reviews tool for SLRs.
3. RESULTS
Thirty‐one articles (one observational study, 7 one pooled analysis of trial data, 8 four RCTs, 9 , 10 , 11 , 12 and 25 SLRs 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 ) were included (Appendix Figure A1). Study periods within the identified RCTs ranged from 1999 through 2021; within the identified SLRs, they ranged from 1957 through 2017; from 2003 to 2011 for the pooled analysis of trial data 8 ; and from 2000 to 2011 for the observational study. 7
Interventions in the four RCTs 9 , 10 , 11 , 12 included two trials 9 , 10 for 17‐hydroxyprogesterone caproate (17‐OHPC), one for progesterone gel, 11 and one for vaginal progesterone compared to intramuscular 17‐OHPC. 12 Across the included studies, the most commonly reported neonatal morbidity outcomes were respiratory distress syndrome, necrotizing enterocolitis, bronchopulmonary dysplasia, and intraventricular hemorrhage. Neonatal mortality outcomes included perinatal loss, early infant death (defined as death after birth until 28 days of life occurring in live‐born neonates delivered < 240/7 weeks' gestation), and neonatal death (defined as death < 28 days).
Among the RCTs included (Figure 1) 9 , 10 , 11 , 12 results were inconsistent and no strength of surrogacy assessment was conducted; three RCTs 9 , 10 , 11 had low risk of bias and one RCT 12 had a high risk of bias. The pooled analysis study 8 compared vaginal progesterone to other procedural interventions such as cervical pessary and cerclage in three separate trials, each evaluating different treatment protocols. This study 8 reported no significant differences in sPTB at <37 weeks' gestational age, neonatal morbidity, or perinatal loss; magnitudes of association were inconsistent, and considerable variability was evident for the neonatal outcomes reported. One observational study, 7 rated “good quality,” illustrated lower frequency of neonatal morbidity/mortality events as weeks' gestational age at birth increased; a strength of surrogacy assessment was not conducted.
Figure 1.
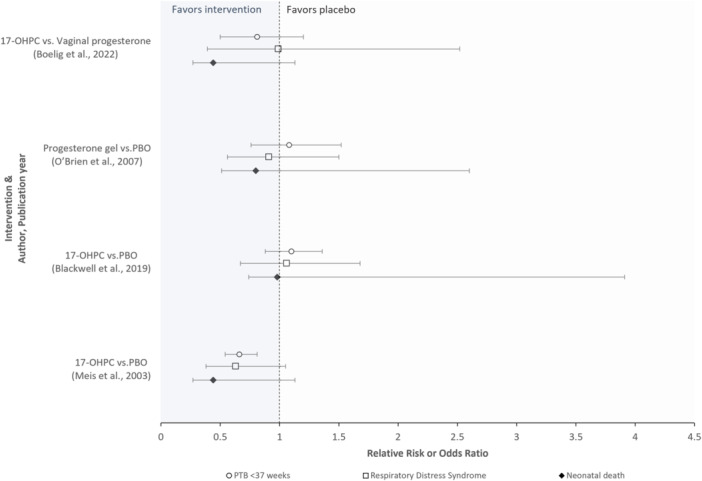
Randomized controlled trials evaluating treatment effects on the occurrence of preterm birth and, separately, neonatal morbidity/mortality outcomes (n = 4 studies). The O'Brien et al. study reported odds ratios; all other studies reported relative risks. Abbreviations: 17‐OHPC, 17‐hydroxyprogesterone caproate, PBO, Placebo, PTB, preterm birth. Interpretation: Area < 1 on the x‐axis favors the intervention, while the area > 1 favors placebo. Most trials reported no statistically significant differences between study groups across surrogate and neonatal clinical endpoints; although Meis et al. reported a statistically significant reduction attributed to the intervention for the surrogate endpoint (i.e., preterm birth), significant reductions were not reported for the neonatal clinical endpoints evaluated. The strength of surrogacy was not evaluated in any study.
Among the 25 SLRs 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 included (Figure 2), 21 were systematic reviews of RCTs, two 28 , 34 included RCTs and observational studies, and two 23 , 27 included only observational cohort studies 23 or case‐control studies. 27 Most SLRs assessed progesterone‐based interventions, but some summarized the evidence on the use of other treatments or procedures such as tocolytic drugs, 20 , 34 , 35 , 36 cervical pessary, 27 , 38 cervical cerclage, 28 , 37 corticosteroids, 23 hormones, 13 and others (e.g., omega 3 fatty acids, 14 probiotics, 24 and ethanol 25 ). Among the 25 SLRs 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 included, most SLRs assessed progesterone‐based interventions, 16 , 17 , 18 , 19 , 26 , 29 , 30 , 31 , 32 , 33 , 37 followed by treatments or procedures such as tocolytic drugs, cervical pessary, cervical cerclage, corticosteroids, hormones, and other agents (e.g., omega 3 fatty acids, probiotics etc.). Like the results from the RCTs in this review, the conclusions of the SLRs were inconsistent and absent any strength of surrogacy assessment; 80% of the SLRs included had low or critically low quality (Figure 2).
Figure 2.
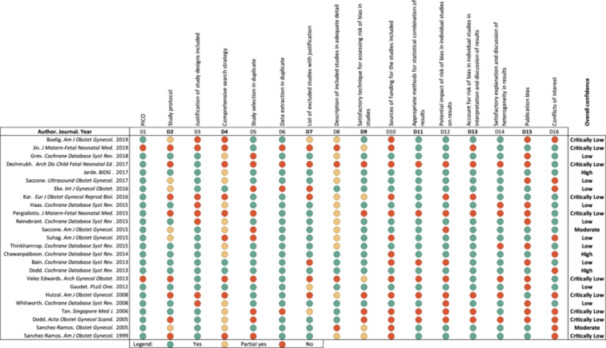
AMSTAR‐2 quality assessment of systematic literature reviews (n = 25 studies). Interpretation: Overall confidence rating: High, no or one noncritical weakness; Moderate, more than one noncritical weakness; Low, one critical flaw with or without noncritical weaknesses; Critically Low, more than one critical flaw with or without noncritical weaknesses. Abbreviations: AMSTAR, Assessing the Methodological Quality of Systematic Reviews; PICO, Population Intervention Comparator Outcomes.
4. DISCUSSION
The absence of a direct quantitative assessment, defined as any empirical measure of association between the surrogate endpoints of interest and neonatal morbidity/mortality, among the studies in this rapid literature review, which spanned the last 20 years, precludes the ability to draw conclusions on the strength of surrogacy. Such information, if available, would facilitate a deeper understanding by which these surrogate endpoints can reasonably predict neonatal morbidity/mortality. Reporting guidelines for surrogate endpoints are under development, which could improve transparency in the reporting of such endpoints, especially for clinical trials, thus facilitating the interpretation of future trial results. 39 , 40 Although a rapid review approach was undertaken which may have resulted in some studies or other relevant data being missed by design, this review highlights the need for empirical evidence to better support the use of these surrogate measures particularly given their role as key efficacy endpoints in investigational studies assessing therapeutic intervention for the prevention of sPTB.
AUTHOR CONTRIBUTIONS
Shiraz El Adam: Writing—original draft; methodology; validation; visualization; writing—review and editing; project administration; supervision. Karissa Johnston: Methodology; writing—review and editing; supervision. Maanasa Venkataraman: Methodology; validation; visualization; writing—review and editing. Vanessa Perez Patel: Conceptualization; investigation; methodology; writing—review & editing; supervision.
CONFLICTS OF INTEREST STATEMENT
Shiraz El Adam, Karissa Johnston, and Maanasa Venkataraman are employees of Broadstreet HEOR and received consultancy fees from Organon. Vanessa Perez Patel is an employee of Organon.
TRANSPARENCY DECLARATION
The authors had full access to all studies included in this rapid review. VPP affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study have been explained. As only previously published data was included in this study, ethics approval was not required.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Damien J. Croft, MD, (Organon, Jersey City, NJ, USA) for providing medical input during the conduct of the rapid review. This study was funded by Organon. The authors were solely responsible for the final content of the manuscript and decision to submit for publication.
APPENDIX A.
Table A1.
Population, intervention, comparators, outcomes, and study design (PICOS) criteria.
| Population | Individuals with a singleton and uncomplicated pregnancy, with/without a history of singleton sPTB, and: |
Or
| |
| Note: individuals with complicated pregnancies and neonates with congenital or chromosomal conditions were excluded. | |
| Intervention/comparator |
|
| No exclusions were planned based on intervention or comparator. | |
| Outcomes | Short‐term neonatal outcome (short‐term defined as the time from PTB to 28 days beyond the expected due date at 40 weeks' gestational age): |
| |
| Study design |
|
Note: The retosiban program, now terminated, was the only late phase development program in the US which was known to have conducted observational studies published in the last 5 years (from the time of search execution) which were relevant to the research question; as such, observational studies sponsored by GlaxoSmithKline were eligible for consideration.
Abbreviations: CDSR, Cochrane Database of Systematic Reviews; CENTRAL, Cochrane Central Register of Controlled Trials; MEDLINE, Medical Literature Analysis and Retrieval System Online; PICOS, Population, Intervention, Comparator, Outcomes, Study design; PTB, preterm birth; PTL, preterm labor; sPTB, spontaneous preterm birth; sPTL, spontaneous preterm labor; US, United States.
Figure A1.
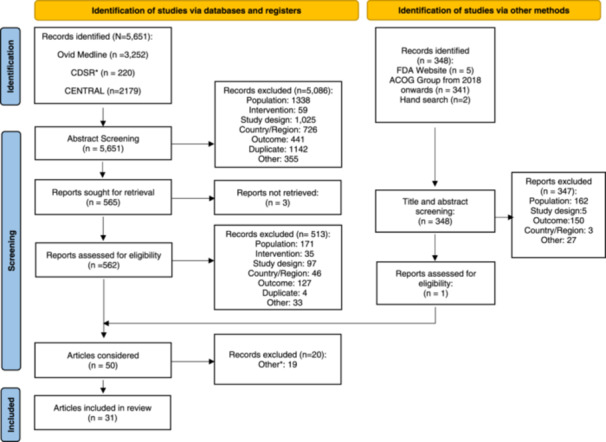
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Flow Diagram. From: Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. Notes: *Articles did not report treatment effect simultaneously in surrogate endpoint(s) and morbidity/mortality outcomes.
Adam SE, Johnston K, Venkataraman M, Patel VP. Surrogate endpoints for neonatal outcome: a rapid review. Health Sci Rep. 2024;7:e2279. 10.1002/hsr2.2279
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
REFERENCES
- 1. Ciani O, Manyara AM, Davies P, et al. A framework for the definition and interpretation of the use of surrogate endpoints in interventional trials. EClinicalMedicine. 2023;65:102283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kraus VB, Simon LS, Katz JN, et al. Proposed study designs for approval based on a surrogate endpoint and a post‐marketing confirmatory study under FDA's accelerated approval regulations for disease modifying osteoarthritis drugs. Osteoarth Cartil. 2019;27(4):571‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. U.S. Food and Drug Administration . Table of surrogate endpoints that were the basis of drug approval or licensure. 2022. https://www.fda.gov/drugs/development-resources/table-surrogate-endpoints-were-basis-drug-approval-or-licensure
- 4. Garritty C, Gartlehner G, Nussbaumer‐Streit B, et al. Cochrane rapid reviews methods group offers evidence‐informed guidance to conduct rapid reviews. J Clin Epidemiol. 2021;130:13‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saade GR, Shennan A, Beach KJ, et al. Randomized trials of retosiban versus placebo or atosiban in spontaneous preterm labor. Am J Perinatol. 2021;38(S 01):e309‐e317. [DOI] [PubMed] [Google Scholar]
- 6. GlaxoSmithKline . A Phase III Efficacy and Safety Study of Intravenous Retosiban Versus Placebo for Women in Spontaneous Preterm Labor (NEWBORN‐1); ClinicalTrials.gov Identifier: NCT02377466. 2015. https://classic.clinicaltrials.gov/ct2/show/NCT02377466
- 7. Pimenta J, Ebeling M, Montague T, et al. A retrospective database analysis of neonatal morbidities to evaluate a composite endpoint for use in preterm labor clinical trials. Am J Perinatol Rep. 2018;08(1):e25‐e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alfirevic Z, Owen J, Carreras Moratonas E, Sharp AN, Szychowski JM, Goya M. Vaginal progesterone, cerclage or cervical pessary for preventing preterm birth in asymptomatic singleton pregnant women with a history of preterm birth and a sonographic short cervix. Ultrasound Obstet Gynecol. 2013;41(2):146‐151. [DOI] [PubMed] [Google Scholar]
- 9. Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 Alpha‐Hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379‐2385. [DOI] [PubMed] [Google Scholar]
- 10. Blackwell SC, Gyamfi‐Bannerman C, Biggio JR Jr., et al. 17‐OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG Study): A multicenter, international, randomized Double‐Blind trial. Am J Perinatol. 2020;37(2):127‐136. [DOI] [PubMed] [Google Scholar]
- 11. O'Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double‐blind, placebo‐controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):687‐696. [DOI] [PubMed] [Google Scholar]
- 12. Boelig RC, Schoen CN, Frey H, et al. Vaginal progesterone vs intramuscular 17‐hydroxyprogesterone caproate for prevention of recurrent preterm birth: a randomized controlled trial. Am J Obstet Gynecol. 2022;226(5):722.e1‐722.e12. [DOI] [PubMed] [Google Scholar]
- 13. Bain E, Heatley E, Hsu K, Crowther CA. Relaxin for preventing preterm birth. Cochrane Database Syst Rev. 2013;(8):CD010073. [DOI] [PubMed] [Google Scholar]
- 14. Kar S, Wong M, Rogozinska E, Thangaratinam S. Effects of omega‐3 fatty acids in prevention of early preterm delivery: a systematic review and meta‐analysis of randomized studies. Eur J Obstet Gynecol Reprod Biol. 2016;198:40‐46. [DOI] [PubMed] [Google Scholar]
- 15. Whitworth M, Quenby S. Prophylactic oral betamimetics for preventing preterm labour in singleton pregnancies. Cochrane Database Syst Rev. 2008;2008(1):CD006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dodd JM, Crowther CA, Cincotta R, Flenady V, Robinson JS. Progesterone supplementation for preventing preterm birth: a systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2005;84(6):526‐533. [DOI] [PubMed] [Google Scholar]
- 17. Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst Rev. 2013;(7):CD004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reinebrant HE, Pileggi‐Castro C, Romero CL, et al. Cyclo‐oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst Rev. 2015;2015(6):CD001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. American J Obste Gynecol MFM. 2019;1(1):50‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chawanpaiboon S, Laopaiboon M, Lumbiganon P, Sangkomkamhang US, Dowswell T. Terbutaline pump maintenance therapy after threatened preterm labour for reducing adverse neonatal outcomes. Cochrane Database Syst Rev. 2014;(3):CD010800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hutzal CE, Boyle EM, Kenyon SL, et al. Use of antibiotics for the treatment of preterm parturition and prevention of neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 2008;199(6):620.e1‐620.e8. [DOI] [PubMed] [Google Scholar]
- 22. Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P, Ota E. Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity. Cochrane Database Syst Rev. 2015;2015(6):CD002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deshmukh M, Patole S. Antenatal corticosteroids in impending preterm deliveries before 25 weeks' gestation. Arch Dis Childh Fetal Neon Edit. 2018;103(2):F173‐F176. [DOI] [PubMed] [Google Scholar]
- 24. Grev J, Berg M, Soll R. Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2018;12(12):CD012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haas DM, Morgan AM, Deans SJ, Schubert FP. Ethanol for preventing preterm birth in threatened preterm labor. Cochrane Database Syst Rev. 2015;2015(11):CD011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eke AC, Chalaan T, Shukr G, Eleje GU, Okafor CI. A systematic review and meta‐analysis of progestogen use for maintenance tocolysis after preterm labor in women with intact membranes. Int J Gynecol Obst. 2016;132(1):11‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin Z, Chen L, Qiao D, et al. Cervical pessary for preventing preterm birth: a meta‐analysis. J Mate Fetal Neo Med. 2019;32(7):1148‐1154. [DOI] [PubMed] [Google Scholar]
- 28. Pergialiotis V, Vlachos DG, Prodromidou A, Perrea D, Gkioka E, Vlachos GD. Double versus single cervical cerclage for the prevention of preterm births. J Mater Fetal Neon Med. 2015;28(4):379‐385. [DOI] [PubMed] [Google Scholar]
- 29. Saccone G, Khalifeh A, Elimian A, et al. Vaginal progesteronevsintramuscular 17α‐hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth in singleton gestations: systematic review and meta‐analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;49(3):315‐321. [DOI] [PubMed] [Google Scholar]
- 30. Saccone G, Suhag A, Berghella V. 17‐alpha‐hydroxyprogesterone caproate for maintenance tocolysis: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213(1):16‐22. [DOI] [PubMed] [Google Scholar]
- 31. Sanchez‐Ramos L, Kaunitz AM, Delke I. Progestational agents to prevent preterm birth: a meta‐analysis of randomized controlled trials. Obstet Gynecol. 2005;105(2):273‐279. [DOI] [PubMed] [Google Scholar]
- 32. Suhag A, Saccone G, Berghella V. Vaginal progesterone for maintenance tocolysis: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213(4):479‐487. [DOI] [PubMed] [Google Scholar]
- 33. Velez Edwards DR, Likis FE, Andrews JC, et al. Progestogens for preterm birth prevention: a systematic review and meta‐analysis by drug route. Arch Gynecol Obstet. 2013;287(6):1059‐1066. [DOI] [PubMed] [Google Scholar]
- 34. Gaudet LM, Singh K, Weeks L, Skidmore B, Tsertsvadze A, Ansari MT. Effectiveness of terbutaline pump for the prevention of preterm birth. A systematic review and meta‐analysis. PLoS One. 2012;7(2):e31679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanchez‐Ramos L, Kaunitz AM, Gaudier FL, Delke I. Efficacy of maintenance therapy after acute tocolysis: A meta‐analysis. Am J Obstet Gynecol. 1999;181(2):484‐490. [DOI] [PubMed] [Google Scholar]
- 36. Tan TC, Devendra K, Tan LK, Tan HK. Tocolytic treatment for the management of preterm labour: a systematic review. Singapore Med J. 2006;47(5):361‐366. [PubMed] [Google Scholar]
- 37. Jarde A, Lutsiv O, Park C, et al. Effectiveness of progesterone, cerclage and pessary for preventing preterm birth in singleton pregnancies: a systematic review and network meta‐analysis. BJOG. 2017;124(8):1176‐1189. [DOI] [PubMed] [Google Scholar]
- 38. Yost NP, Bloom SL, McIntire DD, Leveno KJ. Hospitalization for women with arrested preterm labor: a randomized trial. Obstet Gynecol. 2005;106(1):14‐18. [DOI] [PubMed] [Google Scholar]
- 39. Manyara AM, Davies P, Stewart D, et al. Protocol for the development of SPIRIT and CONSORT extensions for randomised controlled trials with surrogate primary endpoints: SPIRIT‐SURROGATE and CONSORT‐SURROGATE. BMJ Open. 2022;12(10):e064304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manyara AM, Davies P, Stewart D, et al. Definitions, acceptability, limitations, and guidance in the use and reporting of surrogate end points in trials: a scoping review. J Clin Epidemiol. 2023;160:83‐99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.


