Abstract
Pyraclostrobin, a strobilurin-derived fungicide, causes oxidative stress and DNA damage in the organism. Taurine plays an important role in metabolic processes such as osmoregulatory, cytoprotective, and antioxidant effects. The study aimed to investigate the protective effect of taurine in Sprague Dawley male rats exposed to pyraclostrobin. The rats were separated into 6 groups and were found 8 animals in each group. Rats were given 30 mg/kg pyraclostrobin and pyraclostrobin together with three different taurine concentrations (50, 100, and 200 mg/kg) via oral gavage for 28 days. While pyraclostrobin increased biochemical parameters, lipid peroxidation, and DNA damage, it decreased glutathione levels and enzyme activities of catalase and superoxide dismutase. Pyraclostrobin increased apoptotic, proinflammatory, and CYP2E1 mRNA expression levels, whereas antiapoptotic gene Bcl-2 mRNA expression levels decreased in liver tissue. Additionally, pyraclostrobin caused histopathological alterations in tissues. Taurine in a dose-dependent manner reversed the changes caused by pyraclostrobin. As a result, taurine exhibited a cytoprotective effect by showing antioxidant, anti-inflammatory, and antiapoptotic activities against oxidative damage caused by pyraclostrobin.
Keywords: pyraclostrobin, taurine, rat, antioxidant, anti-inflammatory, antiapoptotic
Graphical Abstract
Graphical Abstract.

Introduction
Pyraclostrobin, a fungicide belonging to the strobilurin group, is a carbamate ester of methyl [2-({[1-(4-chlorophenyl)-1H-pyrazol-3-yl]oxy}methyl)phenyl]methoxycarbamate.1 Pyraclostrobin blocks electron transfer between cytochrome b and c1, inhibiting mitochondrial respiration, which leads to the death of the target fungus.2 It has been reported that substances derived from strobilurin generally cause genotoxicity, immunotoxicity, cardiotoxicity, neurotoxicity, and endocrine disruption.3 Additionally, studies have shown that pyraclostrobin induces DNA damage and oxidative stress, harming mitochondrial structure in zebrafish,4,5 exhibits in vitro cytotoxic and genotoxic effects in human blood lymphocytes, significantly reduces lifespan in aged bees, and causes DNA damage in D. melanogaster.6–8
Taurine synthesis, derived from the amino acid cysteine, occurs in mammals through the cysteine sulfinic acid pathway in the pancreas.9 The physicochemical properties of taurine potentially make it an good modulator for several fundamental processes, including protein phosphorylation modulation, lipid metabolism, osmoregulation, bile acid conjugation, antioxidant response, membrane stabilization, regulation of calcium ions and glucose.10 Additionally, taurine plays an important role in reducing lipid peroxidation (LPO), thus protecting cells against tissue damage.11 Particularly, taurine is considered fundamental in sustaining homeostasis, generally due to its function as an intracellular osmolyte in the renal and brain medulla.12 Besides, its potential in regulating cell volume, its osmolytic activity may also contribute to protein folding in the endoplasmic reticulum.13,14 Furthermore, various studies have indicated taurine’s suppressive effects on oxidative stress, infection, inflammation, along with its antimicrobial, antidiabetic, and antitumor activities.15
Fungicides are frequently used by growers for the improvement of agriculture and economic benefits. However, the uncontrolled and unconscious application of fungicides can lead to serious health problems when ingested by humans and animals. Antioxidant substances used against these chemicals help to mitigate their adverse effects. This study aimed to determine, for the first time in vivo, the potential protective effect of taurine against oxidative stress induced in rats administered with pyraclostrobin. To this end, various biochemical parameters, LPO, antioxidant status, DNA damage, and histopathological changes were assessed. Additionally, the mRNA expression levels of apoptotic markers inflammatory cytokines, and CYP2E1 in the liver tissue were measured.
Material and methods
Animals
In the study, 48 male Sprague Dawley rats weighing 200–300 g were used, obtained from the Experimental Animals Unit of Afyon Kocatepe University. The animals were divided into groups and housed for 7 days at a suitable humidity (50%–55%) and temperature (25 °C) for acclimatization before the commencement of the experimental phase. During the experimental stage, the rats were given ad libitum access to rat feed and drinking water. Approval for the conduct of the study was obtained from the Local Ethics Committee with the number 49533702/195.
Experimental design
Pyraclostrobin used in the experimental phase was obtained from BASF (Seltima®, BASF, Istanbul, Turkey), and taurine was sourced from Sigma-Aldrich (MO, USA). The dosages of pyraclostrobin16,17 and taurine18 were determined by reviewing previously conducted studies. The doses of taurine used are the dose ranges specified in the studies and where the best antioxidant effect is observed. Additionally, the use of taurine in these dosage ranges will show at what levels the protective effect can occur depending on increasing doses. The pyraclostrobin concentration in the commercial formulation used in the study was examined using high-performance liquid chromatography according to CIPAC 657 method, revealing a content of 110.04 g/L pyraclostrobin. Animals were randomly divided into 6 groups of 8 each, and the following treatments were administered daily for 28 days:
Group I - Control group: standard feed and drinking water.
Group II - Corn oil group: corn oil (0.5 mL) administered via gastric lavage.
Group III - Pyraclostrobin group: Pyraclostrobin at 30 mg/kg (dissolved in 0.5 mL corn oil) and administered via gastric lavage.
Group IV - Pyraclostrobin + Taurine 50 group: Pyraclostrobin at 30 mg/kg and 50 mg/kg taurine (dissolved in 0.5 mL water), both administered via gastric lavage.
Group V – Pyraclostrobin + Taurine 100 group: Pyraclostrobin at 30 mg/kg and 100 mg/kg taurine (dissolved in 0.5 mL water), both administered via gastric lavage.
Group VI – Pyraclostrobin + Taurine 200 group: Pyraclostrobin at 30 mg/kg and 200 mg/kg taurine (dissolved in 0.5 mL water), both administered via gastric lavage.
Following the completion of the experimental phase, the rats were anesthetized using ketamine (13 mg/kg i.m.) and xylazine (87 mg/kg i.m.). Subsequently, 3–5 cc of blood was collected intracardially using a syringe after opening the thoracic cage. After blood collection, cervical dislocation was performed on the animals, and necessary tissue samples were obtained. The blood samples were centrifuged at 3,000 rpm (10 min) to separate the plasma, which was stored (−80 °C) until analysis. After the sacrifice of the rats, surgical methods were used to collect heart, liver, kidney, testis, and brain tissues for examinations. For the determination of superoxide dismutase (SOD) and catalase (CAT) enzyme activities, an equal volume of KH2PO4 buffer solution was added to the blood samples from which plasma was obtained, and centrifuged at 3,000 rpm for 5 min (3 times). The supernatant was throwed, and 0.5 mL of KH2PO4 buffer was put to 0.5 mL of erythrocytes and transferred to eppendorf tubes.19 Additionally, for biochemical analyses, 5 mL of cold KH2PO4 (50 mM, pH = 7) was added to the tissues (0.5 g) collected. They were then homogenized using mechanical and ultrasonic homogenizers (20 kHz). After this process, the tissues in the solution were centrifuged at 5,000 rpm for 15 min, and the obtained supernatants were used for analyses.
Biochemical analyses
The levels of enzymes (aspartate aminotransferase -AST, alanine aminotransferase-ALT, alkaline phosphatase-ALP), blood urea nitrogen (BUN), and creatinine in the plasma samples were measured spectrophotometrically (Shimadzu 1,601 UV–Vis, Tokyo, Japan) using commercial kits supplied by Human, Max-Planck-Ring, Wiesbaden, Germany. The determination of DNA damage was carried out using a commercially available iQuant™ ssDNA ELISA kit (ABP Biosciences, Rockville, MD, USA).
Determination of oxidative stress parameters
MDA levels in blood were determined according to the method of Ohkawa et al.,20 and in tissues according to the method of Draper and Hadley.21 GSH levels were measured following the method of Beutler et al.22 Activities of SOD and CAT in erythrocytes and tissues were determined according to the methods of Sun et al.23 and Sinha,24 respectively. Additionally, protein content in tissues was determined using the method of Lowry et al.25 and hemoglobin amount was determined using the colorimetric method of Drabkin and Austin.26
Histopathological evaluation
Brain, heart, liver, kidney, and testis tissues were collected from different regions in sizes of approximately 0.5–1 cm2, with 3–5 pieces each, and placed in tissue processing cassettes. These prepared samples were then immersed in 10% neutral formalin and left for 5 days before undergoing classical tissue processing. To remove excess formaldehyde, the tissues were rinsed in running water for 30 min and then dehydrated through increasing concentrations of alcohol series (each for 1 h). Following this, they were cleared in xylene. The tissues were held in xylene for 1.5 h and then in soft paraffin for 3 h, changing every hour, and later in hard paraffin for 2 h. After this process, the tissues were embedded in paraffin blocks. Sections of 5 μm thickness were obtained from the blocked samples using a microtome, transferred to standard slides for evaluation, and grouped for staining. For histopathological assessment, tissues were stained with Hematoxylin–Eosin (H&E). The stained samples were evaluated under a microscope to assess differences observed in tissues among groups.
Molecular analyses
Liver samples obtained from the rats were placed in RNA later solution (ThermoFisher Scientific) and stored at −80 °C until analysis. RNA isolation from the tissues was performed using the GeneJet RNA Purification Kit (ThermoFisher Scientific). The quality and quantity of the RNAs were determined using a Multiskan™ FC Microplate Photometer (Thermo) at A260/A280 UV wavelengths. Isolated RNAs were calculated to make up a total of 1 μg for cDNA synthesis. To remove DNA, DNase I (Thermo Scientific) was added, and the total volume was brought up to 10 μL with DEPC (diethyl pyrocarbonate). Subsequently, the samples were incubated for 30 min at 37 °C and then for 10 min at 65 °C. cDNA was obtained from the DNase I-treated RNA using the cDNA Synthesis Kit (RevertAid H Minus Single Strand, Thermo Scientific).
Primers used in the experiment were specific for Rattus norvegicus (NCBI) and were designed for β-actin, p53, Bcl-2, Bax, Caspase-3, Caspase-8, Caspase-9, NFκB, TNF-α, and CYP2E1 mRNA sequences, using FastPCR 6.0 software.27 The primer sequences, their total base lengths, and GenBank accession numbers are provided in Table 1.
Table 1.
Genes, oligonucleotide sequences, sizes and gene bank numbers used for molecular analysis.
| Gens | Oligonucleotide sequences | Sizes (bp) | Gene bank numbers | |
|---|---|---|---|---|
| β-actin | F | GAGGGAAATCGTGCGTGACAT | 452 | NC_005111.4 |
| R | ACATCTGCTGGAAGGTGGACA | |||
| p53 | F | TGCAGAGTTGTTAGAAGGCCCA | 397 | NM_030989.3 |
| R | GTCACCATCAGAGCAACGCTC | |||
| Caspase-3 | F | ACCCTGAAATGGGCTTGTGTA | 427 | NM_012922.2 |
| R | GCCATATCATCGTCAGTTCCAC | |||
| Bcl-2 | F | GGGTATGATAACCGGGAGATCG | 508 | NM_016993.1 |
| R | ACTCAGTCATCCACAGAGCGA | |||
| NFκB | F | TCCCCAAGCCAGCACCCCAGC | 334 | NM_199267.2 |
| R | GGCCCCCAAGTCTTCATCAGC | |||
| Caspase-8 | F | TTGCTGAACGTCTGGGCAACG | 502 | NM_022277.1 |
| R | TCGTCGATCCTTCCCAGCAAGC | |||
| Caspase-9 | F | AGAAACACCCAGGCCGGTGGA | 327 | NM_031632.1 |
| R | ACCACGAAGCAGTCCAGGGCAC | |||
| TNF-α | F | CGAGTGACAAGCCCGTAGCC | 368 | NM_012675.3 |
| R | GGATGAACACGCCAGTCGCC | |||
| Bax | F | AGGACGCATCCACCAAGAAGC | 363 | NM_017059.2 |
| R | CAGTGAGGACTCCAGCCACAA | |||
| CYP2E1 | F | TGAGATATGGGCTCCTGATCC | 293 | AF061442.1 |
| R | ATCTGGAAACTCATGGCTGTC | |||
In the liver samples taken from the rats, mRNA expressions of the housekeeping gene (β-actin) and the targeted genes were determined using a real-time PCR device. Following the amplification process, the relative changes in mRNA expression levels of the target genes were calculated using the 2−ΔΔCt method, based on the cycle threshold (Ct) values obtained from the amplification curves.28 The calculated value for each gene was substituted into the 2−ΔΔCt formula to determine the fold change in mRNA expression levels, either as an increase or decrease. The β-actin gene was used as an endogenous control, and correction (normalization) of the expression levels of other genes was applied based on the β-actin gene levels of each sample.
Statistical analyses
For statistical analysis, the SPSS software (Statistical Package for the Social Sciences ver. 22.0, SPSS Inc, Chicago, Illinois, USA) was used. Results were presented as mean ± standard deviation. Prior to statistical analysis, a test for normal distribution was conducted to ascertain the suitability of the data for normal distribution, and it was determined that the data were normally distributed. For statistical analysis, one-way analysis of variance (One way ANOVA) was employed for comparisons between groups, and the post-hoc Duncan test was utilized to determine significances. A P-value <0.05 was taken into account statistically significant.
Results
The effect of pyraclostrobin and taurine on biochemical parameters
In the rat plasma, it was found that the levels of AST (Fig. 1A), ALT (Fig. 1B), ALP (Fig. 1C), BUN (Fig. 1D), and creatinine (Fig. 1E) were the highest in the pyraclostrobin group when compared with the control group (P < 0.001). However, in the groups given taurine at 50, 100, and 200 mg/kg along with pyraclostrobin, these elevated levels were observed to decrease in a dose-dependent manner (P < 0.001). Additionally, it was noted that these levels did not undergo significant changes in the oil group (P > 0.05).
Fig. 1.
The effect of pyraclostrobin (PYR) and taurine (T) administered at 50 (T50), 100 (T100), and 200 (T200) mg/kg in combination with PYR on plasma levels of aspartate aminotransferase (AST; A), alanine aminotransferase (ALT; B), alkaline phosphatase (ALP; C), blood urea nitrogen (BUN; D), and creatinine (E) in rats. a,b,c,d,eDifferent letters indicate statistical significance (P < 0.001).
Effect of pyraclostrobin and taurine on oxidative stress parameters
It was determined that MDA levels (Table 2) measured in the whole blood, liver, kidney, brain, heart, and testis tissues of rats were significantly higher in the pyraclostrobin group compared to the control group (P < 0.001). In groups administered with taurine along with pyraclostrobin, a dose-dependent decrease in these elevated MDA levels was observed (especially at 100 and 200 mg/kg doses) (P < 0.001). On the other hand, a decrease in GSH levels (Table 3) was observed following pyraclostrobin administration (P < 0.001), while applications of taurine along with pyraclostrobin increased the reduced GSH levels, especially at higher doses (100 and 200 mg/kg). The SOD (Table 4) and CAT (Table 5) enzyme activity levels measured in erythrocytes, liver, kidney, brain, heart, and testis tissues were found to be at the lowest in groups treated with pyraclostrobin when compared to the control (P < 0.001). In groups given taurine along with pyraclostrobin, an increase in these reduced SOD and CAT levels was observed, especially at higher doses (100 and 200 mg/kg) (P < 0.001). Additionally, in the oil group, these parameter values did not create a significant difference when compared to the control (P > 0.05).
Table 2.
The effect of pyraclostrobin (PYR) and pyraclostrobin plus taurine (T) administered at 50 (T50), 100 (T100) and 200 (T200) mg/kg on malondialdehyde (MDA) levels in whole blood, liver, kidney, brain, heart and testis tissues of male rats.
| Groups | Blood (nmol/mL) | Liver (nmol/g tissue) | Kidney (nmol/g tissue) | Brain (nmol/g tissue) | Heart (nmol/g tissue) | Testis (nmol/g tissue) |
|---|---|---|---|---|---|---|
| Control | 6.93 ± 0.51a | 10.67 ± 0.91a | 8.54 ± 1.09a | 4.38 ± 0.84a | 4.78 ± 1.25a | 5.33 ± 1.35a |
| Oil | 7.42 ± 0.30a | 10.92 ± 0.58a | 9.09 ± 1.00a | 4.43 ± 0.53a | 6.41 ± 1.04a | 6.62 ± 0.90a |
| PYR | 19.59 ± 4.17a | 35.08 ± 5.67a | 31.37 ± 6.63a | 28.46 ± 4.45a | 19.70 ± 3.69a | 21.40 ± 3.42a |
| PYR + T50 | 19.44 ± 3.01a | 29.80 ± 4.10a | 24.23 ± 6.17a | 26.38 ± 3.27a | 16.57 ± 3.18a | 19.66 ± 3.82a |
| PYR + T100 | 12.77 ± 1.92a | 25.23 ± 3.20a | 18.27 ± 2.60a | 22.84 ± 2.81a | 13.00 ± 2.18a | 17.27 ± 3.35a |
| PYR + T200 | 9.23 ± 1.21a | 23.04 ± 2.38a | 16.50 ± 2.75a | 18.76 ± 3.08a | 9.19 ± 1.24a | 14.21 ± 3.01a |
Mean ± standard deviation (n = 8).
aValues with different letters in the same column are statistically significant (P < 0.001).
Table 3.
The effect of pyraclostrobin (PYR) and pyraclostrobin plus taurine (T) administered at 50 (T50), 100 (T100) and 200 (T200) mg/kg on glutathione (GSH) levels in whole blood, liver, kidney, brain, heart and testis tissues of male rats.
| Groups | Blood (nmol/mL) | Liver (nmol/g tissue) | Kidney (nmol/g tissue) | Brain (nmol/g tissue) | Heart (nmol/g tissue) | Testis (nmol/g tissue) |
|---|---|---|---|---|---|---|
| Control | 81.40 ± 7.45a | 46.90 ± 5.11a | 25.63 ± 2.22a | 16.34 ± 2.06a | 27.79 ± 2.08a | 19.66 ± 2.95a |
| Oil | 78.71 ± 9.32a | 46.71 ± 6.19a | 24.96 ± 2.39a | 15.98 ± 2.26a | 26.69 ± 2.60a | 19.64 ± 2.42a |
| PYR | 24.96 ± 2.69a | 17.24 ± 0.99a | 11.59 ± 1.36a | 6.13 ± 0.52a | 11.28 ± 1.33a | 6.86 ± 0.71a |
| PYR + T50 | 33.96 ± 3.45a | 25.22 ± 2.09a | 12.64 ± 1.50a | 6.74 ± 0.87a | 15.43 ± 1.35a | 8.14 ± 0.72a |
| PYR + T100 | 53.07 ± 5.97a | 30.98 ± 2.47a | 16.01 ± 1.65a | 10.09 ± 1.21a | 17.51 ± 1.98a | 12.54 ± 2.69a |
| PYR + T200 | 67.65 ± 7.68a | 37.40 ± 3.70a | 19.76 ± 1.08a | 13.35 ± 1.78a | 21.19 ± 2.79a | 15.87 ± 2.36a |
Mean ± standard deviation (n = 8).
aValues with different letters in the same column are statistically significant (P < 0.001).
Table 4.
The effect of pyraclostrobin (PYR) and pyraclostrobin plus taurine (T) administered to male rats at 50 (T50), 100 (T100) and 200 (T200) mg/kg on superoxide dismutase (SOD) activities in erythrocyte, liver, kidney, brain, heart and testis tissues.
| Groups | Erythrocyte (U/gHb) | Liver (U/μg protein) | Kidney (U/μg protein) | Brain (U/μg protein) | Heart (U/μg protein) | Testis (U/μg protein) |
|---|---|---|---|---|---|---|
| Control | 17.96 ± 3.20a | 4.10 ± 0.56a | 3.37 ± 0.73a | 1.96 ± 0.64a | 2.66 ± 0.49a | 2.54 ± 0.20a |
| Oil | 16.60 ± 3.73a | 3.85 ± 0.57a | 3.14 ± 0.57a | 1.90 ± 0.55a | 2.66 ± 0.43a | 2.56 ± 0.12a |
| PYR | 6.97 ± 0.83a | 0.44 ± 0.06a | 1.52 ± 0.24a | 0.40 ± 0.11a | 0.50 ± 0.08a | 0.35 ± 0.05a |
| PYR + T50 | 10.30 ± 1.36a | 1.39 ± 0.58a | 1.61 ± 0.23a | 0.93 ± 0.27a | 0.84 ± 0.08a | 0.80 ± 0.09a |
| PYR + T100 | 13.19 ± 2.03a | 1.97 ± 0.63a | 1.94 ± 0.53a | 1.14 ± 0.42a | 1.46 ± 0.29a | 1.29 ± 0.13a |
| PYR + T200 | 15.48 ± 2.61a | 2.83 ± 0.38a | 2.67 ± 0.75a | 1.63 ± 0.47a | 2.10 ± 0.46a | 1.55 ± 0.29a |
Mean ± standard deviation (n = 8).
aValues with different letters in the same column are statistically significant (P < 0.001).
Table 5.
The effect of pyraclostrobin (PYR) and pyraclostrobin plus taurine (T) administered to male rats at 50 (T50), 100 (T100) and 200 (T200) mg/kg on catalase (CAT) activities in erythrocyte, liver, kidney, brain, heart and testis tissues.
| Groups | Erythrocyte (U/gHb) | Liver (U/μg protein) | Kidney (U/μg protein) | Brain (U/μg protein) | Heart (U/μg protein) | Testis (U/μg protein) |
|---|---|---|---|---|---|---|
| Control | 20.32 ± 3.82a | 6.14 ± 0.71a | 5.23 ± 0.84a | 1.41 ± 0.40a | 2.29 ± 1.07a | 1.38 ± 0.30a |
| Oil | 19.95 ± 3.97a | 5.91 ± 0.43a | 5.20 ± 0.81a | 1.38 ± 0.25a | 2.29 ± 1.14a | 1.28 ± 0.14a |
| PYR | 4.87 ± 0.94a | 0.76 ± 0.11a | 0.77 ± 0.11a | 0.30 ± 0.05a | 0.41 ± 0.02a | 0.32 ± 0.07a |
| PYR + T50 | 7.73 ± 1.64a | 1.09 ± 0.14a | 1.06 ± 0.39a | 0.65 ± 0.06a | 1.07 ± 0.19a | 0.50 ± 0.05a |
| PYR + T100 | 12.7 ± 2.62a | 1.66 ± 0.37a | 1.51 ± 0.41a | 0.81 ± 0.04a | 1.27 ± 0.19a | 0.82 ± 0.08a |
| PYR + T200 | 14.81 ± 3.69a | 3.14 ± 0.50a | 2.51 ± 0.33a | 1.00 ± 0.17a | 1.36 ± 0.20a | 1.05 ± 0.09a |
Mean ± standard deviation (n = 8).
aValues with different letters in the same column are statistically significant (P < 0.001).
Effect of pyraclostrobin and taurine on anti-inflammatory and apoptotic gene expressions and DNA fragmentation
Following the treatment of pyraclostrobin, an increase in the mRNA expression levels of apoptotic genes Bax (Fig. 2A), Caspase 3 (Fig. 2B), Caspase 8 (Fig. 2C), Caspase 9 (Fig. 2D), and p53 (Fig. 2E) was observed in the rat liver tissues, while the mRNA expression of the antiapoptotic gene Bcl-2 (Fig. 2F) decreased (P < 0.001). The mRNA expression levels of genes included in the inflammatory process, TNF-α (Fig. 2G) and NFκB (Fig. 2H), as well as CYP2E1 (Fig. 2I), which plays a significant role in biotransformation, were found to increase with pyraclostrobin administration (P < 0.001). However, taurine administrations in a dose dependent manner along with pyraclostrobin regulated these gene expressions stimulated by pyraclostrobin. Furthermore, the mRNA expression levels of these genes in the oil group were not found to change compared to the control. At the end of the study, ssDNA levels were measured in ng/μL from the blood samples of the animals and evaluated as a percentage (Fig. 2J). The highest DNA fragmentation damage among the groups was observed in the group treated with pyraclostrobin, while taurine administration (especially at 100 and 200 mg/kg) was seen to reduce the DNA fragmentation observed with pyraclostrobin (P < 0.05).
Fig. 2.
The effect of pyraclostrobin (PYR) and taurine (T) administered at 50, 100, and 200 mg/kg in combination with PYR on Bax (A), Caspase 3 (B), Caspase 8 (C), Caspase 9 (D), p53 (E), Bcl-2 (F), TNF-α (G), NFkB (H), CYP2E1(I) mRNA expression, and ssDNA levels (J) in rat liver tissue. a,b,c,dDifferent letters indicate statistical significance (P < 0.001).
Effect of pyraclostrobin and taurine on histopathological changes in tissues
Pyraclostrobin caused histopathological changes in rat tissues. Hyperemia in the vessels, vacuolization in neurons, and focal areas of glial cell infiltration were observed in the brain tissue (Fig. 3A3). In the heart tissue (Fig. 4A3), areas of hyaline degeneration in myocardial cells were found, and hyperemia in the central veins, degenerative changes in hepatocytes in the pericentral area, and sinusoidal dilation were observed in the liver tissue (Fig. 5A3). Additionally, vacuolization in glomeruli, degenerative changes in tubules, and expansion in Bowman’s space were found in the kidney tissue (Fig. 6A3) and vacuolization in the lumen of seminiferous tubules, decreased density of spermatozoa in the interstitial area, and irregular appearances in the basal membrane were shown in the testis tissue (Fig. 7A3). In groups administered with 50 (Figs 3–7A4), 100 (Figs 3–7A5), and 200 (Figs 3–7A6) mg/kg taurine along with pyraclostrobin, these changes were found to decrease with increasing doses of taurine. No histopathological changes were detected in the tissues of animals in the group given oil (Figs 3–7A2) compared to control (Figs 3–7A1). Additionally, the statistical evaluation of the histopathological examination of the tissues was shown in Table 6.
Fig. 3.

Histopathological assessment of the effect of pyraclostrobin (PYR) and taurine (T) administered at 50, 100, and 200 mg/kg in combination with PYR on brain tissues in rats. A1-control, A2-oil, A3-PYR, A4-PYR + T50, A5-PYR + T100, A6-PYR + T200. The thick arrow indicated hyperemia in vessels, the thin arrow indicated vacuolization in neurons and the arrowhead indicated focal areas of glia cell infiltration. All figures are stained with H&E. 20×, and 100 μm were used as the original magnifications.
Fig. 4.

Histopathological assessment of the effect of pyraclostrobin (PYR) and taurine (T) administered at 50, 100, and 200 mg/kg in combination with PYR on heart tissues in rats. A1-control, A2-oil, A3-PYR, A4-PYR + T50, A5-PYR + T100, A6-PYR + T200. The arrow indicated areas of hyaline degeneration in myocardial cells. All figures are stained with H&E. 20×, and 100 μm were used as the original magnifications.
Fig. 5.

Histopathological assessment of the effect of pyraclostrobin (PYR) and taurine (T) administered at 50, 100, and 200 mg/kg in combination with PYR on liver tissues in rats. A1-control, A2-oil, A3-PYR, A4-PYR + T50, A5-PYR + T100, A6-PYR + T200. The thick arrow indicated hyperemia in central veins, the thin arrow indicated degenerative changes in hepatocytes in the pericentral area and the arrowhead indicated sinusoidal dilation. All figures are stained with H&E. 20×, and 100 μm were used as the original magnifications.
Fig. 6.

Histopathological assessment of the effect of pyraclostrobin (PYR) and taurine (T) administered at 50, 100, and 200 mg/kg in combination with PYR on kidney tissues in rats. A1-control, A2-oil, A3-PYR, A4-PYR + T50, A5-PYR + T100, A6-PYR + T200. The thick arrow indicated vacuolization in glomeruli, the thin arrow indicated expansion in Bowman’s space in glomeruli and the arrowhead indicated degenerative changes in tubules. All figures are stained with H&E. 20×, and 100 μm were used as the original magnifications.
Fig. 7.
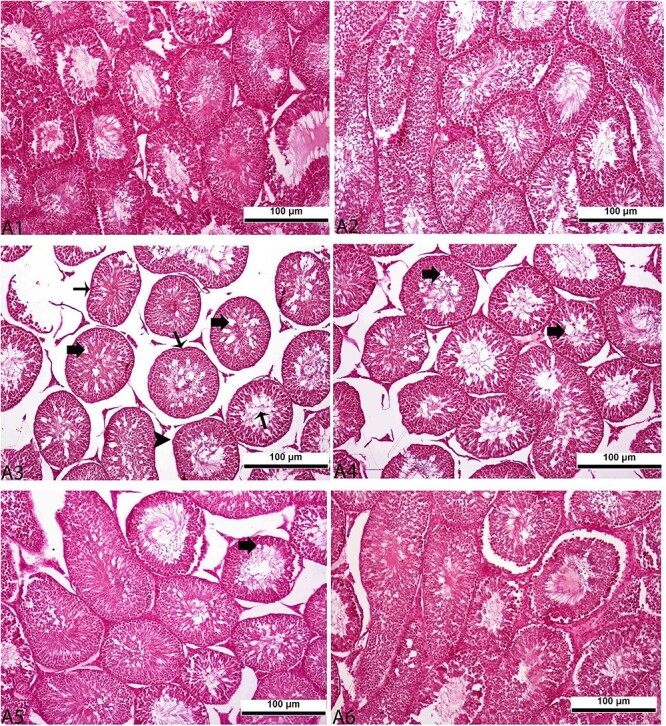
Histopathological assessment of the effect of pyraclostrobin (PYR) and taurine (T) administered at 50, 100, and 200 mg/kg in combination with PYR on testis tissues in rats. A1-control, A2-oil, A3-PYR, A4-PYR + T50, A5-PYR + T100, A6-PYR + T200. The thick arrow indicated vacuolization in the lumen of seminiferous tubules, the thin arrow indicated a decrease in spermatozoa density in the interstitial area and the arrowhead indicated irregular appearances in the basal membrane. All figures are stained with H&E. 20×, and 100 μm were used as the original magnifications.
Table 6.
Statistical evaluation of histopathological findings in brain, heart, liver, kidney and testis tissues of male rats administered pyraclostrobin (PYR) and PYR plus taurine (T) at 50 (T50), 100 (T100) and 200 (T200) mg/kg.
| Tissue | Histopathological Finding | Control | Oil | PYR | PYR + T 50 | PYR + T 100 | PYR + T 200 |
|---|---|---|---|---|---|---|---|
| Brain | Hyperemia in the veins | 0.00 ± 0.00a | 0.00 ± 0.00a | 3.40 ± 0.89a | 2.00 ± 0.97a | 1.13 ± 0.76a | 0.93 ± 0.49a |
| Focal glia cell infiltrations | 0.00 ± 0.00a | 0.00 ± 0.00a | 2.90 ± 0.83a | 2.66 ± 0.73a | 1.53 ± 0.83a | 0.76 ± 0.32a | |
| Vacuolization formations in neurons | 0.00 ± 0.00a | 0.00 ± 0.00a | 2.56 ± 0.40a | 2.00 ± 0.27a | 1.13 ± 0.41a | 0.80 ± 0.36a | |
| Heart | Hyaline degeneration | 0.00 ± 0.00a | 0.00 ± 0.00a | 3.90 ± 0.54a | 2.60 ± 0.86a | 1.86 ± 0.77a | 0.70 ± 0.36a |
| Liver | Sinusoidal dilatation | 0.00 ± 0.00a | 0.00 ± 0.00a | 4.06 ± 0.51a | 2.66 ± 0.92a | 1.86 ± 0.83a | 0.46 ± 0.12a |
| Hyperemia in the vena centralis | 0.00 ± 0.00a | 0.00 ± 0.00a | 3.56 ± 0.98a | 2.00 ± 0.27a | 1.86 ± 0.97a | 0.46 ± 0.12a | |
| Degeneration in hepatocytes | 0.00 ± 0.00a | 0.00 ± 0.00a | 3.56 ± 0.75a | 2.16 ± 0.83a | 1.53 ± 0.53a | 0.49 ± 0.13a | |
| Kidney | Enlargement of the bowman’s space in the glomeruli | 0.00 ± 0.00a | 0.00 ± 0.00a | 2.90 ± 0.54a | 2.00 ± 0.47a | 1.30 ± 0.31a | 0.36 ± 0.12a |
| Vacuolization in the glomeruli | 0.00 ± 0.00a | 0.00 ± 0.00a | 3.40 ± 0.69a | 1.60 ± 0.43a | 1.03 ± 0.28a | 0.56 ± 0.18a | |
| Degenerative changes in the tubules | 0.00 ± 0.00a | 0.00 ± 0.00a | 3.06 ± 1.03a | 2.06 ± 0.53a | 0.96 ± 0.43a | 0.56 ± 0.18a | |
| Testis | Vacuolization formations in the seminiferous tubules lumen | 0.00 ± 0.00a | 0.00 ± 0.00a | 2.73 ± 0.51a | 2.11 ± 0.95a | 1.20 ± 0.61a | 0.63 ± 0.31a |
| Irregular appearance of seminiferous tubules basement membrane | 0.00 ± 0.00a | 0.00 ± 0.00a | 2.73 ± 0.81a | 2.00 ± 0.82a | 1.15 ± 0.51a | 0.46 ± 0.22a | |
| Decreased spermatocyte density in the seminiferous tubules lumen | 0.00 ± 0.00a | 0.00 ± 0.00a | 3.23 ± 0.98a | 2.00 ± 0.97a | 1.46 ± 0.27a | 0.70 ± 0.36a |
Mean ± standard deviation (n = 8).
aValues with different letters in the same column are statistically significant (P < 0.05).
Discussion
Strobilurin fungicides demonstrate toxic effects on the liver and kidney, causing increased levels in function parameters such as aminotransferases, phosphatase, urea, and creatinine.17,29 In a study, administration of 117.25 mg/kg azoxystrobin to rats for 14 days resulted in elevated serum ALT, AST, ALP, BUN, and creatinine levels, whereas concurrent administration of sesame oil (5 mL/kg) reduced these values.29 Zemheri et al.17 reported that 30 mg/kg pyraclostrobin administration to rats for 30 days enhanced serum AST, ALT, and ALP values, while concurrent administration of resveratrol at doses of 10 and 20 mg/kg for protective purposes decreased these values. Similarly, in this study, pyraclostrobin administration increased AST, ALT, ALP, BUN, and creatinine values, whereas taurine administration at increasing doses decreased these values. This suggests that pyraclostrobin disrupts normal metabolic processes in the liver and kidney functions, leading to an increase in these parameters, and taurine, due to its antioxidant and cell-protective effects,11,18 exerts a protective effect on the liver and kidneys, thereby mitigating the adverse effects of pyraclostrobin.
Oxidation of polyunsaturated fatty acids in cells by •H2O2 or •OH radicals leads to the formation of LPO, and their enzymatic or chemical degradation results in the production of malondialdehyde (MDA).30 GSH and related enzymatic systems (such as SOD and CAT) play a important role in the detoxification of LPO and free radicals. It has been reported that treatment of pyraclostrobin at a dose of 30 mg/kg for 30 days to rats increased MDA levels, decreased GSH levels, and additionally reduced SOD and CAT enzyme activities in blood and tissues. Concurrent administration of resveratrol (10 and 20 mg/kg) with pyraclostrobin reversed these values, indicating the antioxidant effect of resveratrol in inhibiting LPO and playing an active role in the antioxidant system.17 Another study highlighted that administration of azoxystrobin, a strobilurin fungicide (117.25 mg/kg), along with 5 mL/kg sesame oil for 14 days, increased plasma MDA levels and decreased plasma GSH levels, whereas sesame oil reduced these values. The study suggested that fungicides weaken the activity of xanthine oxidase and cause the production of main free radicals in LPO, superoxide anion, and hydroxyl radical, while sesame oil exhibited a protective effect due to its antioxidant properties. Additionally, researches have shown that trifloxystrobin, another strobilurin fungicide, increased MDA levels and decreased GSH levels, as well as SOD and CAT activities in earthworms,31 and that azoxystrobin administration in silver-cheeked toadfish increased LPO and decreased SOD activity.32 Consistent with these studies, the current study determined that pyraclostrobin increased MDA levels and decreased GSH levels and antioxidant enzyme activities in rat tissues, while taurine administration (especially at 100 and 200 mg/kg doses) normalized these levels. This indicates that taurine, due to its antioxidant effect,11,18 reduces LPO induced by pyraclostrobin and improves antioxidant enzyme activities.
Strobilurins, among the most commonly used fungicides, are reported to be very toxic, particularly to certain aquatic organisms.33 In a study assessing the toxicity of strobilurins on zebrafish embryos, it was reported that various amounts of three strobilurins (picoxystrobin, pyraclostrobin, and trifloxystrobin,) exposed to the embryos 96 h post-fertilization caused differential changes in mRNA levels of genes related to the antioxidant system (Nrf2, Ucp2, SOD, and CAT) and the immune system (TNF-α, IL-1b, IL-8, and C1C).34 Similarly, another study investigating the toxic effects of strobilurins on zebrafish larvae reported a significant increase in the relative expression of Bax, Caspase-9, and p53 following pyraclostrobin exposure (0.1, 10, and 100 μg/L).35 However, in vivo studies comparing the toxicity and potential mechanisms of strobilurins remain limited. An in vivo study reported that treatment of 30 mg/kg pyraclostrobin to rats for 30 days upregulated liver Bax, CYP2E1, Caspase-8, Caspase-9, Caspase-3, p53, NFκB, and TNF-α mRNA expressions, and downregulated Bcl-2 mRNA expression. Nevertheless, concurrent administration of resveratrol (10 and 20 mg/kg) reversed the expression levels of these genes.17 Similarly, in the present in vivo study, it was determined that taurine administration at increasing doses downregulated liver Bax, Caspase-3, Caspase-8, Caspase-9, p53, CYP2E1, NFκB, and TNF-α mRNA expressions induced by pyraclostrobin and upregulated Bcl-2 mRNA expression. This suggests that taurine prevents excessive hepatocyte apoptosis induced through the mitochondrial signaling pathway (antiapoptotic effect), reduces the induced inflammatory process (anti-inflammatory), and inhibits oxidative stress by decreasing the increased CYP2E1 activity.
In an in vitro study, it was reported that the administration of pyraclostrobin (10, 20, 40, and 80 μmol/L for 6 h) in RAW264.7 macrophages under alkaline conditions caused dose-dependent extensive DNA strand breaks emanating from the heads of comet-like structures, shaping DNA damage in macrophage cells.36 Similarly, an in vivo study indicated that administration of pyraclostrobin (30 mg/kg for 30 days) resulted in the formation of ssDNA breaks and the shaping of DNA damage.17 In a study investigating the effect of high dietary intake of taurine on apoptosis and atherosclerosis in the main left coronary artery of rabbits, it was reported that ssDNA breaks formed as a result of an atherogenic diet in rabbits decreased with taurine administration, demonstrating a protective effect.37 In the current study, pyraclostrobin was found to cause an increase in ssDNA levels by inducing DNA strand breaks, whereas taurine administration reduced these DNA breaks. This indicates that taurine has an effect in reducing DNA damage induced by pyraclostrobin.
In a study investigating the tissue distribution of pyraoxystrobin, a strobilurin fungicide, in rats, it was reported that following a single oral dose of 500 and 1,000 mg/kg, peak concentrations in most tissues, except for liver, spleen, and lungs, occurred at 28 h. The distribution of pyraoxystrobin concentration was noted to be primarily in blood-rich tissues such as the heart, liver, and kidneys, with the distribution pattern being heart > liver > kidney > brain > spleen > lung > muscle > testis. It was also found that pyraoxystrobin was detectable in the brain, suggesting it could cross the blood–brain barrier. Additionally, its presence in the testis even after a single oral administration indicated that the compound could cross the testicular barrier due to its lipophilic properties and small molecular weight.38 There are limited studies on the histopathological changes caused by pyraclostrobin in tissues. Zemheri et al.17 reported in their study that administration of pyraclostrobin in rats led to increased degenerative changes and binuclear hepatocyte formations in pericentral hepatocytes of the liver, vacuolization in glomerulus, and degeneration in tubular epithelial cells of the kidneys. These changes were reduced in groups administered with 10 and 20 mg/kg resveratrol as a protective measure, in a dose-dependent manner. Moreover, studies have reported organ toxicities caused by pyraclostrobin in fishes39 and bees.7 In the current study, pyraclostrobin was found to cause histopathological changes that could affect organ functions in the brain, heart, liver, kidney, and testis tissues of rats. However, increasing doses of taurine, with its antioxidant and cell-protective effects,11,18 were determined to reduce these changes.
Conclusion
In the study conducted, it was determined that administration of pyraclostrobin in rats led to increased levels of certain biochemical parameters (AST, ALT, ALP, BUN, and creatinine), enhanced LPO, DNA fragmentation, and elevated expression levels of proinflammatory, apoptotic, and CYP2E1 genes. Additionally, a reduce in GSH and antioxidant enzyme activities was observed, along with histopathological changes indicative of damage in the brain, heart, liver, kidney, and testis tissues. Conversely, administration of taurine at doses of 50, 100, and 200 mg/kg was found to mitigate these adverse changes caused by pyraclostrobin. As a result, taurine exhibited cytoprotective effects, including antioxidant, anti-inflammatory, and antiapoptotic activities against oxidative damage induced by pyraclostrobin. From this perspective, it appears that taurine supplementation could potentially offer a protective effect against oxidative damage caused by fungicide exposure, particularly strobilurins, in terms of human and animal health. Additionally, when the results obtained in the study are evaluated, the fact that it is not clear whether it can be applied to other animal models and whether it can show its effect with these doses shows the limitations of the study.
Acknowledgments
This study was Master thesis of the first author and was orally represented in the 3th International Congress on Biological and Health Sciences (April 14-16, 2023).
Contributor Information
Ibrahim Serim, Faculty of Veterinary Medicine, Department of Pharmacology and Toxicology, Afyon Kocatepe University, 03200, Afyonkarahisar, Turkey.
Hasan Huseyin Demirel, Afyon Kocatepe University, Bayat Vocational School, 03200, Afyonkarahisar, Turkey.
Fahriye Zemheri-Navruz, Faculty of Science, Department of Molecular Biology and Genetics, Bartın University, 74100, Bartın, Turkey.
Sinan Ince, Faculty of Veterinary Medicine, Department of Pharmacology and Toxicology, Afyon Kocatepe University, 03200, Afyonkarahisar, Turkey.
Author contributions
Ibrahim Serim: Data curation, Investigation, Methodology, Writing—review & editing. Hasan Huseyin Demirel: Data curation, Investigation, Methodology; Fahriye Zemheri-Navruz: Investigation, Methodology; Sinan Ince: Supervision, Visualization, Writing—original draft, Writing—review & editing, Methodology, Funding acquisition, Project administration, Conceptualization.
Conflict of interest statement. The authors declare no competing interests.
Funding
This study was financially supported by a grant from the Afyon Kocatepe University Scientific Research Council of Turkey (Project No: 20.SAG.BIL.29).
Data availability
All data generated or analyzed during this study are included in the manuscript.
References
- 1. Mercader JV, Suárez-Pantaleón C, Agulló C, Abad-Somovilla A, Abad-Fuentes A. Production and characterization of monoclonal antibodies specific to the strobilurin pesticide pyraclostrobin. J Agric Food Chem. 2008:56(17):7682–7690. [DOI] [PubMed] [Google Scholar]
- 2. Fulcher JM, Wayment DG, White PM Jr, Webber CL III. Pyraclostrobin wash-off from sugarcane leaves and aerobic dissipation in agricultural soil. J Agric Food Chem. 2014:62(10):2141–2146. [DOI] [PubMed] [Google Scholar]
- 3. Wang X, Li X, Wang Y, Qin Y, Yan B, Martyniuk CJ. A comprehensive review of strobilurin fungicide toxicity in aquatic species: emphasis on mode of action from the zebrafish model. Environ Pollut. 2021:275:116671. [DOI] [PubMed] [Google Scholar]
- 4. Zhang C, Wang J, Zhang S, Zhu L, du Z, Wang J. Acute and subchronic toxicity of pyraclostrobin in zebrafish (Danio rerio). Chemosphere. 2017:188:510–516. [DOI] [PubMed] [Google Scholar]
- 5. Li H, Zhao F, Cao F, Teng M, Yang Y, Qiu L. Mitochondrial dysfunction-based cardiotoxicity and neurotoxicity induced by pyraclostrobin in zebrafish larvae. Environ Pollut. 2019:251:203–211. [DOI] [PubMed] [Google Scholar]
- 6. Luz AL, Kassotis CD, Stapleton HM, Meyer JN. The high-production volume fungicide pyraclostrobin induces triglyceride accumulation associated with mitochondrial dysfunction, and promotes adipocyte differentiation independent of PPARγ activation, in 3T3-L1 cells. Toxicology. 2018:393:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eduardo da Costa Domingues C, Bello Inoue LV, Mathias da Silva-Zacarin EC, Malaspina O. Foragers of Africanized honeybee are more sensitive to fungicide pyraclostrobin than newly emerged bees. Environ Pollut. 2020:266:115267. [DOI] [PubMed] [Google Scholar]
- 8. Navruz FZ, Köstekçi ÖÇ, Ince S. Effect of pyraclostrobin-based herbicide on DNA damage and reproductive performance in drosophila melanogasters. J Appl Biol Sci. 2023:17(2):266–275. [Google Scholar]
- 9. Jong CJ, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids. 2012:42(6):2223–2232. [DOI] [PubMed] [Google Scholar]
- 10. Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009:11(12):2985–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kp AD, Martin A. Recent insights into the molecular regulators and mechanisms of taurine to modulate lipid metabolism: a review. Crit Rev Food Sci Nutr. 2023:63(23):6005–6017. [DOI] [PubMed] [Google Scholar]
- 12. Navruz FZ, Köstekçi ÖÇ, Ince S. Taurine reduced reproductive performance and DNA damage induced by lead in Drosophila melanogaster. Int J Vet Anim Res. 2023:6(2):48–51. [Google Scholar]
- 13. Obukohwo OM. Physio-pharmacological potentials of taurine: a review in animal and human studies. Asian J Biol Sci. 2023:16(4):452–463. [Google Scholar]
- 14. Rais N, Ved A, Shadab M, Ahmad R, Shahid M. Taurine, a non-proteinous essential amino acid for human body systems: an overview. Arab Gulf J Sci Res. 2023:41(1):48–66. [Google Scholar]
- 15. Marcinkiewicz J, Kontny E. Taurine and inflammatory diseases. Amino Acids. 2014:46(1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tuttle AH, Salazar G, Cooper EM, Stapleton HM, Zylka MJ. Choice of vehicle affects pyraclostrobin toxicity in mice. Chemosphere. 2019:218:501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zemheri-Navruz F, Ince S, Arslan-Acaroz D, Acaroz U, Demirel HH, Demirkapi EN. Resveratrol alleviates pyraclostrobin-induced lipid peroxidation, oxidative stress, and DNA damage in rats. Environ Sci Pollut Res. 2023:30(3):6414–6423. [DOI] [PubMed] [Google Scholar]
- 18. Ince S, Arslan-Acaroz D, Demirel HH, Varol N, Ozyurek HA, Zemheri F, Kucukkurt I. Taurine alleviates malathion induced lipid peroxidation, oxidative stress, and proinflammatory cytokine gene expressions in rats. Biomed Pharmacother. 2017:96:263–268. [DOI] [PubMed] [Google Scholar]
- 19. Winterbourn CC, Hawkins RE, Brain M, Carrell RW. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975:55:337–341. [PubMed] [Google Scholar]
- 20. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by Thiobarbituric acid reaction. Anal Biochem. 1979:95(2):351–358. [DOI] [PubMed] [Google Scholar]
- 21. Draper HH, Hardley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990:186:421–431. [DOI] [PubMed] [Google Scholar]
- 22. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963:61:882–888. [PubMed] [Google Scholar]
- 23. Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988:34(3):497–500. [PubMed] [Google Scholar]
- 24. Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972:47(2):389–394. [DOI] [PubMed] [Google Scholar]
- 25. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951:193(1):265–275. [PubMed] [Google Scholar]
- 26. Drabkin DL, Austin JH. Spectrophotometric studies. II. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem. 1935:112(1):51–65. [Google Scholar]
- 27. Kalendar R, Lee D, Schulman AH. FastPCR software for PCR, in silico PCR, and oligonucleotide assembly and analysis. Methods Mol Biol. 2014:1116:271–302. [DOI] [PubMed] [Google Scholar]
- 28. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001:29(9):e45–e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziada RM, Abdulrhman SM, Nahas AA. Hepato-nephro-toxicity induced by premium fungicide and protective effect of sesame oil. Egypt J Hosp Med. 2020:81(7):2445–2450. [Google Scholar]
- 30. Cheng J, Wang F, Yu DF, Wu PF, Chen JG. The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur J Pharmacol. 2011:650(1):184–194. [DOI] [PubMed] [Google Scholar]
- 31. Wu R, Zhou T, Wang J, Wang J, du Z, Li B, Juhasz A, Zhu L. Oxidative stress and DNA damage induced by trifloxystrobin on earthworms (Eisenia fetida) in two soils. Sci Total Environ. 2021:797:149004. [DOI] [PubMed] [Google Scholar]
- 32. Crupkin AC, Fulvi AB, Iturburu FG, Medici S, Mendieta J, Panzeri AM, Menone ML. Evaluation of hematological parameters, oxidative stress and DNA damage in the cichlid Australoheros facetus exposed to the fungicide azoxystrobin. Ecotoxicol Environ Saf. 2021:207:111286. [DOI] [PubMed] [Google Scholar]
- 33. Liu L, Jiang C, Wu ZQ, Gong YX, Wang GX. Toxic effects of three strobilurins (trifloxystrobin, azoxystrobin and kresoxim-methyl) on mRNA expression and antioxidant enzymes in grass carp (Ctenopharyngodon idella) juveniles. Ecotoxicol Environ Saf. 2013:98:297–302. [DOI] [PubMed] [Google Scholar]
- 34. Li H, Cao F, Zhao F, Yang Y, Teng M, Wang C, Qiu L. Developmental toxicity, oxidative stress and immunotoxicity induced by three strobilurins (pyraclostrobin, trifloxystrobin and picoxystrobin) in zebrafish embryos. Chemosphere. 2018:207:781–790. [DOI] [PubMed] [Google Scholar]
- 35. Kumar N, Willis A, Satbhai K, Ramalingam L, Schmitt C, Moustaid-Moussa N, Crago J. Developmental toxicity in embryo-larval zebrafish (Danio rerio) exposed to strobilurin fungicides (azoxystrobin and pyraclostrobin). Chemosphere. 2020:241:124980. [DOI] [PubMed] [Google Scholar]
- 36. Han S, Lu J, Gao J, Cheng J, Xu W, Tao L, Zhang Y. Pyraclostrobin induced AMPK/mTOR pathways mediated autophagy in RAW264. 7 macrophages. J Environ Sci Health B. 2021:56(9):793–800. [DOI] [PubMed] [Google Scholar]
- 37. Zulli A, Lau E, Wijaya BP, Jin X, Sutarga K, Schwartz GD, Learmont J, Wookey PJ, Zinellu A, Carru C, et al. High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery: association with reduced CCAAT/enhancer binding protein homologous protein and total plasma homocysteine but not lipidemia. Hypertension. 2009:53(6):1017–1022. [DOI] [PubMed] [Google Scholar]
- 38. Lin LH, Duan MY, Chen G, You XH, Liu CL, Guo XJ. An LC-MS/MS method for determination of novel fungicide pyraoxystrobin in rat plasma and tissues: Toxicokinetics and tissue distribution study. Talanta. 2015:136:183–189. [DOI] [PubMed] [Google Scholar]
- 39. Huang X, Yang S, Li B, Wang A, Li H, Li X, Luo J, Liu F, Mu W. Comparative toxicity of multiple exposure routes of pyraclostrobin in adult zebrafish (Danio rerio). Sci Total Environ. 2021:777:145957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the manuscript.




