Abstract
Studies have highlighted a potential link between malignancies and immunoglobulin A nephropathy (IgAN). In such studies, the treatment of malignancy improved the symptoms of IgAN. Here, we report a patient case involving a history of hypertension, tobacco use disorder, and chronic kidney disease (CKD) presenting with hematuria with acute renal failure secondary to IgAN per renal biopsy. Prompted by this association, a malignancy workup was performed including computed tomography (CT) body imaging and biopsies of mediastinal and cervical lymph nodes which revealed a metastatic adenocarcinoma. Current knowledge includes a general mechanism behind the development of IgAN that points toward glomerular deposition of tumor-specific immunoglobulin A (IgA) immunoglobulins. However, the association of IgAN and malignancy has no definitive management guidelines. This clinical case serves as an important contribution in the hopes of future development of guidelines regarding the surveillance and management of IgAN in the setting of malignancy.
Keywords: secondary IgA nephropathy, metastatic lung adenocarcinoma, renal failure secondary to IgA nephropathy, retropharyngeal mass
Background and Research
Immunoglobulin A nephropathy (IgAN) is a form of glomerulonephritis, where immunoglobulin A (IgA) deposits in the glomeruli of the kidneys. This induces an inflammatory process that ultimately results in mesangial deposits visible on electron microscopy. Primary IgAN is the most common form of this disease, accounting for about 90% of cases. 1
Secondary IgAN is associated with numerous underlying conditions, ranging from infectious, hepatic, autoimmune, and malignancies. The association with malignancy is particularly of note. Studies have shown improved symptoms of IgAN after treatment of coexisting malignancies. Youn et al 2 describe a case in which successful treatment of early gastric cancer led to significant reduction of hematuria and proteinuria. Similarly, Wang et al 3 illustrate a case in which a patient with lung adenocarcinoma had improved symptoms of his IgAN with additional cycles of chemotherapy. At one of his later follow-ups, they found that the patient had no evidence of lung cancer recurrence and found that all the symptoms of his IgAN had resolved. It was proposed that IgAN could even perhaps be a paraneoplastic manifestation of lung adenocarcinoma. Jeong et al 4 show after a patient was given 3 cycles of chemotherapy for non-Hodgkin lymphoma, she showed no evidence of hematuria or proteinuria. Masutani et al 5 highlighted a case of a 52-year-old woman who presented with biopsy-proven IgAN 2 years after diagnosis and partial response to treatment of advanced lung adenocarcinoma with gefitinib. It was elected to continue treatment with gefitinib, and 16 months later, she continued to have proteinuria and hematuria.
Immunoglobulin A nephropathy as a risk factor for development of cancer is debated in the literature. A study by Ryu et al looked at 1155 patients with biopsy-proven glomerulonephritis. It was found that patients greater than 50 with biopsy-proven glomerulonephritis were 3 times more likely to develop cancer 1 month out from their diagnosis compared with the general population. 6 In a Swedish population-based cohort study, cancer risk was compared among 3882 biopsy-verified IgAN patients diagnosed during 1974 to 2011 with 19 341 reference individuals. They were followed until 2015. This study found no support for IgAN being a paramalignant condition. However, it did note an increased risk of cancer in IgAN patients with end-stage renal disease (ESRD), specifically non-melanoma skin cancer (NMSC) and kidney cancer. 7 The results show 6 extra cancer cases per 100 IgAN patients with ESRD per 10 years. This goes up to >17 more cases if including NMSC as well.
The literature to date presents a possibility that IgAN is associated with malignancy, whether as an independent risk factor for its development or as a paramalignant condition. Yet, there is no consensus on routine screening for malignancy in patients with IgAN. Based on these studies, further research is needed to determine the optimal cancer screening strategies for these patients. We present an unfortunate case where lung adenocarcinoma presented as renal failure secondary to IgAN.
Case Presentation
Patient is a 58-year-old Caucasian male with a history of 35 pack-year tobacco use disorder hypertension, pre-diabetes, chronic kidney disease (CKD) of unknown stage, left lower extremity deep vein thrombosis recently started on apixaban who presented to a local charity clinic with frank hematuria. He was found to have a creatinine of 5.9, previously 2.5 two weeks prior. He was diagnosed and admitted to the hospital with acute on chronic kidney injury. Eliquis was stopped, and the patient received an inferior vena cava filter. During this procedure, he was found to have an occluded right internal jugular vein with patent collateral vessels confirmed with ultrasound. Hematology was consulted for hypercoagulability, and the workup was unremarkable (negative for factor V Leiden, antithrombin III [ATIII] deficiency, and anticardiolipin antibodies). Renal biopsy was performed. Overall, 22 glomeruli were visualized on light microscopy. The 18 intact glomeruli demonstrated mesangial matrix expansion with diffuse mesangial hypercellularity. In addition, 8 of the intact glomeruli demonstrated cellular/fibrocellular crescent formation (Figure 3). Occasional mesangial electron-dense deposits are identified on electron microscopy, accompanied by the increase in mesangial matrix. The tubular basement membranes were without deposits. Patient developed metabolic acidosis and was initiated on hemodialysis. Owing to patient’s risk factors, recent thrombosis, and renal failure, malignancy was investigated. Computed tomography (CT) chest revealed a large retropharyngeal mass at the level of C1-C6, right upper lobe pulmonary nodules, and right hilar lymphadenopathy concerning for malignancy (Figure 1). Fine needle aspiration of cervical and subcarinal lymph nodes revealed poorly differentiated metastatic carcinoma (Figure 2). Interventional pulmonology was consulted for endobronchial ultrasound and biopsy of lung nodules and mediastinal lymph nodes. Biopsy of pulmonary nodules and mediastinal lymph nodes re-demonstrated poorly differentiated metastatic carcinoma. Positive biopsies were sent to Next-Gen Sequencing for genetic analysis which showed that 92% of collected samples with similar characteristics were found to be lung adenocarcinoma. He was then planned for outpatient chemotherapy induction. His clinical course was further complicated by Superior Vena Cava Syndrome which required thrombectomy and stent placement, which delayed initiation of chemotherapy. Patient ultimately refused all medical treatment, including hemodialysis, and died from cardiopulmonary arrest with pulseless electrical activity.
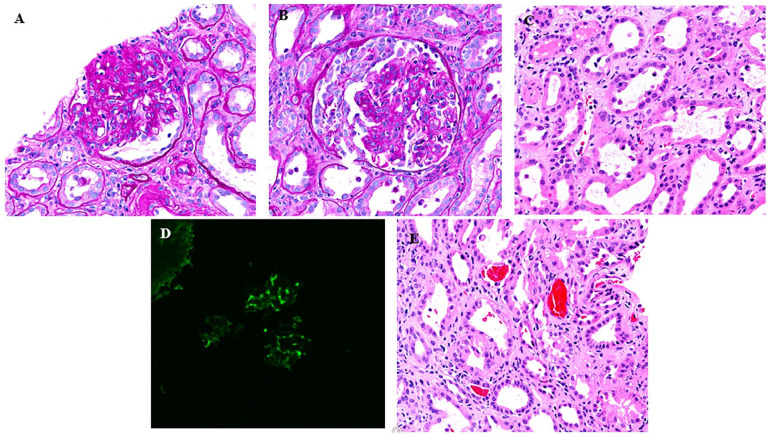
Figure 3.
(A) Biopsy of kidney demonstrating segmental sclerosis, (B) biopsy image demonstrating crescent, (C) tubular injury, (D) immunofluorescence pattern demonstrating IgA nephropathy, and (E) red blood cell casts.
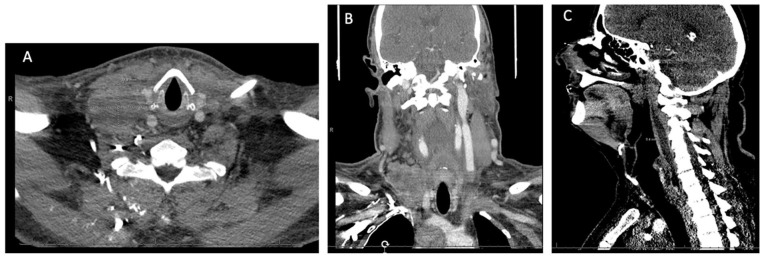
Figure 1.
Computed tomography head and neck showing a large retropharyngeal mass at the level of C1-C6: (A) axial view, (B) coronal view, and (C) sagittal view.
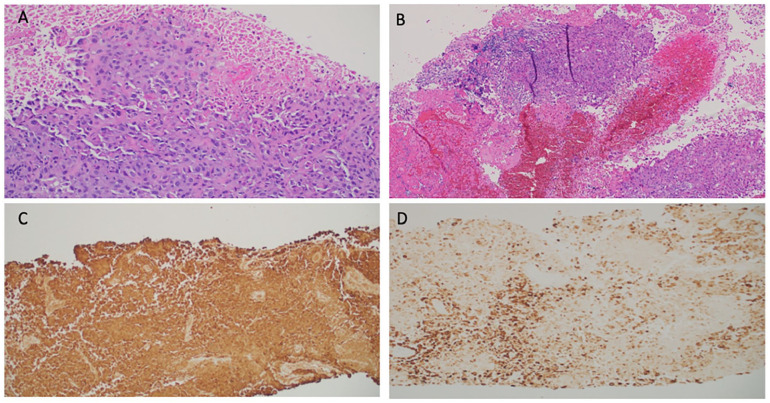
Figure 2.
(A) Biopsy of right cervical lymph node showing metastatic poorly differentiated carcinoma (20× original magnification), (B) fine needle aspiration of subcarinal lymph node showing metastatic carcinoma with squamous differentiation (10× original magnification), (C) pancytokeratin stain of right cervical lymph node, and (D) CK7 stain of right cervical lymph node (Images courtesy of Pramila Moideen, MD, Medical College of Georgia Department of Pathology).
Discussion and Conclusion
Secondary Immunoglobulin A Nephropathy
Immunoglobulin A nephropathy is the most common glomerulonephritis worldwide. It is presented in 2 main variants: primary IgAN and secondary IgAN, with the latter variant being associated with a variety of underlying conditions, including infections, hepatic issues, gastrointestinal (GI) diseases, and malignancies. Accurately estimating the true incidence of secondary IgAN is difficult, but it is most associated with cirrhosis. 8 Importantly, there is no consistent definition for secondary IgAN across the literature, and there are no distinctive histological features that can distinguish between the primary and secondary forms of the disease. In certain case reports of secondary IgAN, some of the identified causes may be more reflective of chance association rather than true causation.
Immunoglobulin A Nephropathy and Malignancy
Current literature suggests a potential connection between IgAN and malignancy. Whereas paraneoplastic glomerulonephritis is uncommon, respiratory, and GI tract carcinomas are more commonly observed. 9 Since both tracts produce IgA1 and IgA2, invasion of the tracts by malignant cells allows for increased circulation of these isotype IgA in the systemic circulation, resulting in subsequent mesangial deposits. This finding is further strengthened by the fact that mesangial IgA deposits are typically of the secretory and polymeric type, which is the form of IgA secreted by mucosal surfaces.
Additional malignancies that are commonly associated with IgAN include cutaneous T-cell lymphoma, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, and renal cell carcinoma. Previous cases have highlighted an association between IgAN and renal cell carcinoma and have demonstrated that plasma cells deposited mesangial IgA within the renal cell carcinoma. 10 Published papers by Wang et al, 3 Youn et al, 2 and Jeong et al 4 have all shown improvement of IgAN with treatment of concurrent malignancies. In addition, a Swedish population-based cohort study demonstrated an increased risk of cancer in IgAN patients with ESRD. 7 Another published report described a case of clinical IgAN in which further workup revealed recurrent gastric adenocarcinoma. 11
Workup
Patients who present with new onset hematuria should warrant further workup. The diagnostic approach should begin with urinalysis, which provides an array of useful information to distinguish various differentials. 12 If the patient’s medical history, physical exam, and renal findings decrease the likelihood of an acute kidney injury, imaging modalities such as ultrasound, abdominopelvic CT scan, or cystoscopy can be used to identify urinary tract abnormalities. When considering the causes of glomerular hematuria, it is crucial to be cognizant of the presence of abnormal serologies that are suggestive of a systemic disease contributing to the patient’s presentation. Elevated levels of antinuclear antibodies (ANAs), antineutrophil cytoplasmic antibodies (ANCAs), antiglomerular basement membrane antibodies, antidouble-stranded DNA, and cryoglobulins are some markers that can provide insight into the underlying cause and aid in subsequent diagnostic and treatment plans. In addition, urinary red blood cell (RBC) morphology can be used to differentiate between a glomerular and non-glomerular bleed. The presence of greater than 25% dysmorphic RBCs has significant specificity for glomerulonephritis, with glomerular hematuria being an important indication for renal biopsy. 13 Nonetheless, due to the invasive nature of the test, biopsy is not recommended in isolated glomerular hematuria, as the clinical course for these patients is reversible and prognosis is typically excellent. In contrast, renal biopsy is warranted when glomerular hematuria presents with risk factors for progressive disease (eg, rise in serum creatinine, proteinuria, and new onset hypertension).
Considering this patient’s history of tobacco use, further investigation should include screening for lung cancer. The United States Preventive Services Task Force (USPSTF) currently recommends annual screening for lung cancer via low-dose CT in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years. 14 In addition, this patient presented with concerning findings of cervical lymphadenopathy, renal failure, the occurrence of an unprovoked thrombosis while on apixaban, and an unremarkable hypercoagulability workup. The combination of these findings prompted workup for malignancy, which revealed metastatic lung adenocarcinoma.
Screening for Malignancy
This case, as well as other cases in the literature, has demonstrated occurrences of IgAN in conjunction with malignancies, particularly involving mucosal and renal regions. These presentations of IgAN alongside malignancies could suggest that the association may not be coincidental; rather, in certain malignancies, IgAN might be a paraneoplastic feature. This connection, however, has not been significant enough to warrant cancer screenings in all patients with IgAN. Moreover, we recognize the infeasibility of conducting widespread screening for all types of malignancies in patients with IgAN. Nonetheless, we strongly advise the consideration of malignancy in patients who have IgAN and risk factors for neoplasia (eg, smoking, tobacco users, and prior GI and lung malignancies). Along with diligently following known and accepted cancer screening guidelines, we recommend using clinical judgment and expertise to guide focused screening efforts.
Acknowledgments
The authors acknowledge the Department of Internal Medicine, Department of Oncology, Department of Pathology, and Department of Nephrology at the Medical College of Georgia for their support and guidance.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Samer Yassin  https://orcid.org/0009-0009-1028-5963
https://orcid.org/0009-0009-1028-5963
References
- 1. Lai KN, Tang SC, Schena FP, et al. IgA nephropathy. Nat Rev Dis Primers. 2016;2:16001. [DOI] [PubMed] [Google Scholar]
- 2. Youn HJ, Kim JS, Lee JM, et al. A case of IgA nephropathy associated with early gastric cancer. Korean J Nephrol. 2001;20:728-731. [Google Scholar]
- 3. Wang J, Liu Y, Liu N, Gao M, Yuan H. Paraneoplastic immunoglobulin a nephropathy in a patient with lung adenocarcinoma: a case report and literature review. J Int Med Res. 2021;49(4):996868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeong YS, Lee HJ, Kim JS, et al. A case of IgA nephropathy associated with non-Hodgkin’s lymphoma. Korean J Nephrol. 1998;17:813-817. [Google Scholar]
- 5. Masutani K, Fujisaki K, Maeda H, et al. Tubulointerstitial nephritis and IgA nephropathy in a patient with advanced lung cancer treated with long-term gefitinib. Clin Exp Nephrol. 2008;12(5):398-402. [DOI] [PubMed] [Google Scholar]
- 6. Ryu J, Ryu HJ, Kim S, et al. Comparison of cancer prevalence between patients with glomerulonephritis and the general population at the time of kidney biopsy. PLoS ONE. 2019;14(10):e0224024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rehnberg J, Ludvigsson JF, Carrero JJ, et al. Cancer risk in patients with immunoglobulin A nephropathy: a Swedish population-based cohort study. Nephrol Dial Transplant 2022;37:749-759. [DOI] [PubMed] [Google Scholar]
- 8. Boitan B, Stancu S, Stefan G, et al. Secondary versus primary IgA nephropathy: are there any differences? Nephrol Dial Transplant. 2018;33:402-415.28482048 [Google Scholar]
- 9. Bacchetta J, Juillard L, Cochat P, et al. Paraneoplastic glomerular diseases and malignancies. Crit Rev Oncol Hematol. 2009;70(1):39-58. [DOI] [PubMed] [Google Scholar]
- 10. Mimura I, Tojo A, Kinugasa S, et al. Renal cell carcinoma in association with IgA nephropathy in the elderly. Am J Med Sci. 2009;338(5):431-432. [DOI] [PubMed] [Google Scholar]
- 11. Kocyigit I, Dortdudak S, Eroglu E, et al. Immunoglobulin A nephropathy could be a clue for the recurrence of gastric adenocarcinoma. Nefrologia. 2013;33:853-855. [DOI] [PubMed] [Google Scholar]
- 12. Patel J, Chambers CV, Gomella LG. Hematuria: etiology and evaluation for the primary care physician. Can J Urol. 2008;15(suppl 1):54-62. [PubMed] [Google Scholar]
- 13. Hamadah AM, Gharaibeh K, Mara KC, et al. Urinalysis for the diagnosis of glomerulonephritis: role of dysmorphic red blood cells, nephrology dialysis transplantation. 2018;33:1397-1403. [DOI] [PubMed] [Google Scholar]
- 14. US Preventive Services Task Force. Screening for lung cancer: US preventive services task force recommendation statement. JAMA. 2021;325:962-970. [DOI] [PubMed] [Google Scholar]