Abstract
Objective
We aimed to describe clinical and laboratory characteristics and determine the predictors of outcome in patients with cerebral venous sinus thrombosis.
Methods
This prospective study was conducted over 2 years among hospitalized patients with cerebral venous sinus thrombosis. Patient outcome was assessed using the Modified Rankin Scale (mRS) score at 3 months. Outcome predictors were identified using logistic regression analysis.
Results
Eighty-one patients were included in this study. The median mRS outcome at 3 months was 1 (interquartile range 1–3). Poor outcomes were observed in 27.2% of patients, and the mortality rate was 9.8%. Factors associated with poor outcomes were age >60 years (relative risk [RR] 5.1), hemiparesis (RR 5.4), altered level of consciousness (RR 7.1), and transverse sinus involvement (RR 1.1). In general, mRS scores were not associated with D-dimer levels (RR 2.4). However, older patients with elevated D-dimer levels showed a significant association with poor outcomes (1.6) according to mRS scores.
Conclusion
Older age, hemiparesis, and altered consciousness levels were independent predictors of poor outcomes in patients with cerebral venous sinus thrombosis. High D-dimer level showed no association with functional disability, except in older patients.
Keywords: Cerebral venous sinus thrombosis, clinical characteristic, laboratory parameter, predictor, patient outcome, older patient
Introduction
Cerebral venous sinus thrombosis (CVT) is a common cause of stroke in young and middle-aged people, especially in women. The annual incidence of cerebral venous thrombosis (CVT) is estimated to be three to four cases per million. However, the frequency of CVT is approximately 12 cases per 100,000 deliveries among pregnant women. CVT occurs three times more frequently in women than in men. 1 The cause of CVT can be owing to congenital or acquired diseases. These may include medical conditions such as dehydration, infection, malignancy, or hematologic disorders. 2 CVT can be caused by several prothrombotic states and disorders of the clotting system. The inherited causes include protein C resistance secondary to factor V Leiden polymorphism, protein C and S resistance, and antithrombin III deficiency. Systemic lupus erythematosus and polyarteritis nodosa are common causes of secondary vasculitis, which are also relevant in young adults. Primary or idiopathic CVT is mainly caused by a hypercoagulable state, commonly owing to puerperium or dehydration. 3 Among infectious diseases, pyogenic and tuberculous meningitis are of particular concern. 4 Coagulopathy owing to SARS-CoV-2 infection has become another concern in the COVID-19 era. Several case series of CVT published in countries worldwide, including in Bangladesh, have noted this association with COVID-19.5–7
CVT has a varied presentation. Septic sinus thrombosis owing to complications of otitis media or mastoiditis is nearly always acute in nature, and patients experience severe illness and pyrexia. Most of these patients have focal neurologic symptoms and signs, as well as symptoms and signs of high intracranial pressure. Focal symptoms and signs may vary depending on the site and the cerebral venous system involved. Similarly, patients may present with seizures, cranial nerve palsies, breathing or feeding problems, urinary retention or incontinence, and altered mentation.8–11
The outcome of CVT varies from complete recovery to death, but most patients with CVT are discharged with a favorable outcome. 12 However, death and dependency are observed in 18.9% of patients, according to an international study on cerebral vein and dural sinus thrombosis. 13 Motor and sensory focal neurological deficits and multiple cranial nerve palsies are associated with poor outcomes, and the number and type of involved sinuses may influence the patient outcome. 14 Coma and cerebral hemorrhage are significantly associated with poor outcomes. 15 In addition, central nervous system infection may be related to poor outcomes at discharge. 16
The objective of the study was to describe the clinical and laboratory characteristics of CVT and to determine predictors of CVT outcome.
Methods
Study design
This was a prospective cohort study conducted at the National Institute of Neurosciences and Hospital (NINS & H) in Dhaka, Bangladesh from November 2020 to August 2022. In the present study, we assessed clinical and laboratory characteristics of CVT and determined the predictors of poor CVT outcomes.
Ethical approval was granted by the Institutional Review Board (IRB) of the study institute (NINS & H), with protocol number is IRB/NINS-127-10-20 (20 October, 2020). Written informed consent was obtained from every patient. This study was conducted according to the principles laid down in the Declaration of Helsinki. The reporting of this observational study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 17
Inclusion and exclusion criteria
We included patients (aged >18 years) presenting with clinical features of CVT for the first time and subsequently confirmed via magnetic resonance imaging (MRI) of the brain with arterial magnetic resonance angiogram (MRA) and magnetic resonance venography (MRV) with contrast.
We excluded patients who had multi-organ failure and died before recording of their information as well as those with subarachnoid hemorrhage, arteriovenous malformation, hemorrhage in the intracranial space-occupying lesion, ischemic stroke with hemorrhagic transformation, hemorrhagic encephalitis, or those who did not give their written informed consent.
Sample size and sampling technique
Because the incidence but not the prevalence of CVT is known, no power calculation was conducted to determine the sample size. For this reason, we consecutively enrolled as many patients as possible, according to our inclusion criteria. A semi-structured questionnaire was used to collect the necessary data.
Laboratory testing
For each patient, baseline investigations like complete blood count, urine testing, creatinine, alanine aminotransferase, random blood sugar, glycated hemoglobin, electrolytes, chest X-ray, and 12-lead electrocardiogram were performed. D-dimer and brain CT or MRI with brain MRV were carried out to confirm CVT. Special investigations like coagulation profile, thyroid stimulating hormone, echocardiography, reverse transcription-polymerase chain reaction for COVID-19, factor V Leiden, protein C and S, antithrombin III, homocysteine, complement (C3, C4), anti-neutrophilic antibody, anti-phospholipid antibody, and antinuclear antibody were conducted in selected cases. All patients received supportive as well as standard treatment for CVT.
Follow-up and outcome measures
Patients were followed up regularly until discharge from the hospital and at 3 months from discharge. During follow-up, vital parameters were carefully assessed and neurological examinations were performed. Patients' clinical outcome was assessed using the modified Rankin Scale (mRS) score at 3 months from discharge. Patients were divided into two groups, one with good outcome (mRS score 0–2) or the group with poor outcome (mRS 3–6, where a score of 6 indicates death owing to any cause). 18 Related factors such as demographic and clinical features were compared between the two groups.
Operational definition, signs, and symptoms
CVT includes thrombosis of the cerebral veins and dural sinuses.
Signs and symptoms of CVT included headache, vomiting, seizure, blurred vision or double vision, papilledema on fundoscopic examination, focal neurological deficit like hemiparesis, and unexplained altered sensorium.
Brain CT or MRI findings
Brain CT or MRI were used to detect evidence of hemorrhagic infarcts, particularly in the setting of multiple infarcts not confined to a single vascular territory. In CVT, both T1- and T2-weighted images show a hyperdense signal. The combination of an abnormal signal in a venous sinus combined with the absence of flow on MRV was used to confirm the diagnosis of CVT. 19
Statistical analysis
Statistical analyses were performed using IBM SPSS version 22 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean and median (interquartile range, IQR) where applicable. Categorical variables are expressed as frequency and percentage. Predictors of outcomes for CVT were analyzed using the chi-square test and Student’s t-test for qualitative and quantitative values, accordingly, followed by multivariate logistic regression analysis to determine the independent effects of those prognostic factors. To find an association of functional disability with elevated D-dimer according to different age groups and the number of sinuses involved, analysis of variance was done. A p-value <0.05, was considered statistically significant.
Results
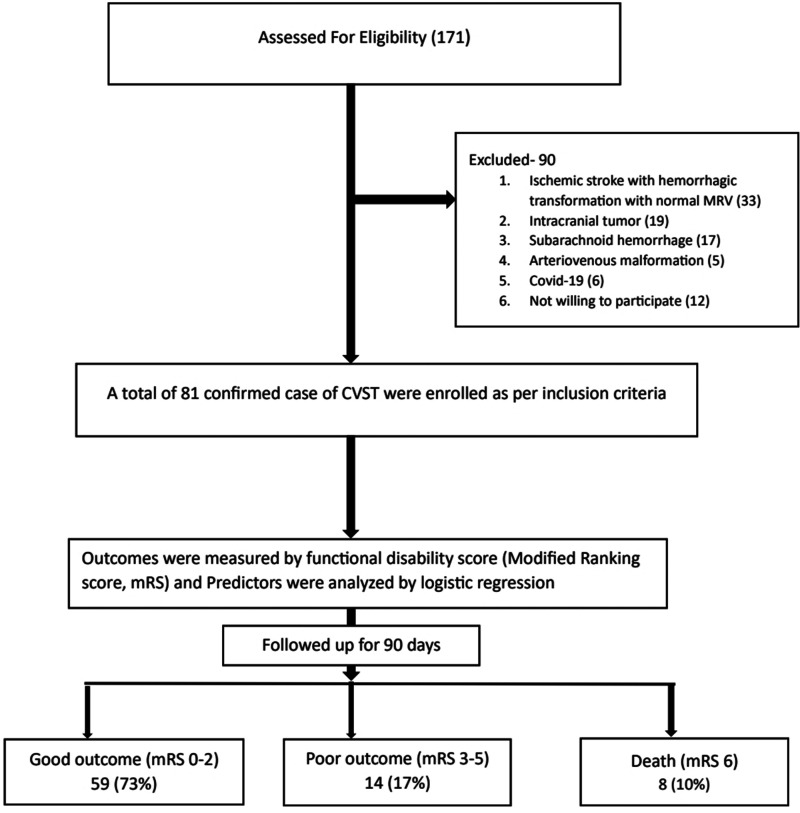
Eighty-one patients were included in the study, per the inclusion criteria, from among 171 suspected cases of CVT (Figure 1). More than 92% (n = 75) were female patients and the sex ratio was 12.5:1. Patients' ages ranged from 18 to 85 years, with a mean age of 36.86 (±13.7) years; the most prevalent age groups were 20 to 29 and 30 to 39 years (n = 24 each). The most common comorbidity was diabetes mellitus (14.8%) followed by hypertension (3.7%). Oral contraceptives (49.4%), followed by iron deficiency anemia (27.2%), puerperium (11.1%), and clinical infection (3.7%) were common risk factors for CVT. However, in 21% of cases, the risk factors were unknown. The most common symptoms were headache (97.5%), vomiting (74.1%), blurred vision (papilledema, 60.5%), altered consciousness (54.3%), hemiparesis (44.4%), convulsion (32.1%), aphasia (14.8%), and cranial nerve palsy (6.2%). The median Glasgow coma scale score was 11 (IQR 8–13) (Table 1). In laboratory investigations, the median hemoglobin level was 11.5 gm/dL (IQR 8.9–12.3), and the mean D-dimer value was 2.6 mg/L (IQR 1.4–4.5). The most commonly involved cerebral venous sinuses were the transverse sinus (84%), superior sagittal sinus (63%), sigmoid sinus (63%), and inferior jugular vein (28.4%). Hemorrhagic infarct most commonly involved the temporal lobe (41.2%) followed by the parietal lobe (25.9%) (Table 2).
Figure 1.
Flow chart showing patient enrollment and outcomes of cerebral venous sinus thrombosis (CVST). mRS, Modified Rankin Scale.
Table 1.
Baseline clinical characteristics of patients with cerebral venous sinus thrombosis.
| Characteristics | |
|---|---|
| Age, years (mean, SD) | 36.86 (13.7) |
| Age group | Frequency (percentage) |
| 10–19 | 4 (4.9) |
| 20–29 | 24 (29.6) |
| 30–39 | 24 (29.6) |
| 40–49 | 17 (21.0) |
| 50–59 | 4 (4.9) |
| 60–69 | 5 (6.2) |
| 70–79 | 2 (2.5) |
| 80–89 | 1 (1.2) |
| Sex | |
| Female | 75 (92.6) |
| Male | 6 (7.4) |
| Comorbidity | |
| Diabetes mellitus | 12 (14.8) |
| Hypertension | 3 (3.7) |
| Chronic kidney disease | 1 (1.2) |
| Hypothyroidism | 1 (1.2) |
| Malignancy | 2 (2.4) |
| Risk factors | |
| Oral contraceptives | 40 (49.4) |
| Puerperium | 9 (11.1) |
| Pregnancy | 1 (1.2) |
| Hormone replacement therapy | 2 (2.5) |
| Infection | 3 (3.7) |
| Prothrombotic state | 2 (2.5) |
| Inflammatory disease | 1 (1.2) |
| Iron deficiency anemia | 22 (27.2) |
| Clinical infection | 3 (3.7) |
| Unknown | 17 (21.0) |
| Clinical presentation | |
| Headache | 79 (97.5) |
| Vomiting | 60 (74.1) |
| Convulsion | 26 (32.1) |
| Slurred speech | 12 (14.8) |
| Hemiparesis | 36 (44.4) |
| Cranial nerve palsy | 5 (6.2) |
| Altered consciousness | 44 (54.3) |
| Blurred vision (papilledema) | 49 (60.5) |
| Glasgow Coma Scale score | 11 (IQR 8–13) |
IQR, interquartile range; SD, standard deviation.
Table 2.
Baseline laboratory characteristics of patients with cerebral venous sinus thrombosis
| Characteristics | |
|---|---|
| Hemoglobin (median, IQR) | 11.5 gm/dL (8.9–12.3) |
| D-dimer (median, IQR) | 2.6 mg/L (1.4–4.5) |
| Involved sinus | Frequency (percentage) |
| Superior sagittal sinus | 51 (63.0) |
| Transverse sinus | 68 (84.0) |
| Sigmoid sinus | 51 (63.0) |
| Straight sinus | 10 (12.3) |
| Inferior jugular vein | 23 (28.4) |
| Vein of Galen | 7 (8.6) |
| Number of involved sinuses | |
| Single sinus involvement | 19 (23.5) |
| Double sinus involvement | 19 (23.5) |
| Involvement of more than two sinuses | 44 (54.3) |
| Cortical involvement | |
| Parietal lobe | 21 (25.9) |
| Temporal lobe | 34 (41.2) |
| Frontal lobe | 11 (13.5) |
| Parieto-temporal | 49 (60.5) |
| Fronto-temporal | 32 (39.5) |
| Thalamus | 7 (8.6%) |
| Cerebellum | 5 (6.1%) |
IQR, interquartile range; mRS, Modified Rankin Scale.
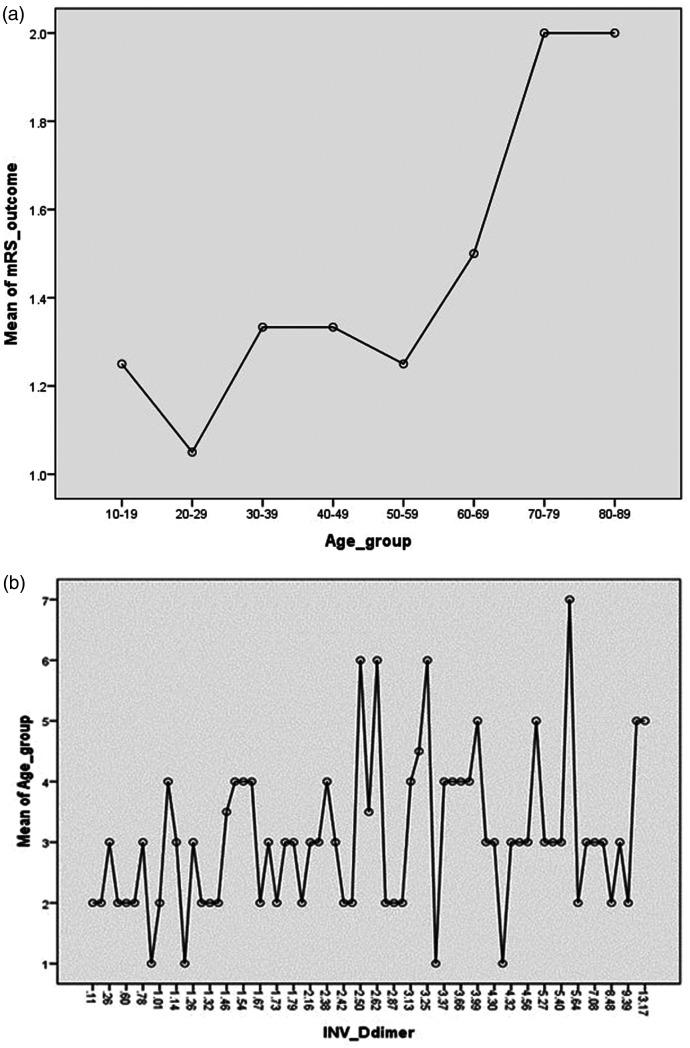
The functional disability score (mRS) at 3 months was a median of 1 (IQR 1–3). Among patients, 59 (72.8%) had a good outcome (mRS score 0–2); a poor outcome (mRS score 3–6) was observed in 22 (27.2%) patients. The mortality rate was 9.8% (Table 3). In multivariate logistic regression, factors associated with poor outcome were age >60 years (relative risk [RR], 5.1, 95% confidence interval [CI] 3.4–8.2; p-value = 0.02), hemiparesis (RR 3.5, 95% CI 1.4–8.7; p-value = 0.003), altered level of consciousness (RR 7.1, 95% CI 1.8–27.6; p-value = 0.005), and transverse sinus involvement (RR 1.1, 95% CI 1.01–1.59; p-value = 0.01). In general, the outcome was not associated with D-dimer level (RR 2.4, 95% CI 0.55–9.9; p-value = 0.23). However, older patients with elevated D-dimer levels showed a significant association with poor outcomes (RR 1.6, 95% CI 1.4–1.9; p-value 0.02) (Figure 2, Table 4).
Table 3.
Outcome of patients with cerebral venous sinus thrombosis.
| mRS at 3 months | Frequency (percentage) |
|---|---|
| Good outcome (mRS 0–2) | 59 (72.8) |
| Poor outcome (mRS 3–6) | 22 (27.2) |
| Death | 8 (9.8) |
| Median mRS (IQR) | 1 (1–3) |
mRS score 0–2 indicates good outcome, 3–6 poor outcome (6 = death).
IQR, interquartile range; mRS, Modified Rankin Scale.
Figure 2.
Association of functional disability and D-dimer with older age. (a) Mean plot showing greater functional disability (mRS score) in older age groups and (b) Mean plot showing D-dimer level is higher in the older age groups. mRS, Modified Rankin Scale.
Table 4.
Outcome predictors of cerebral venous sinus thrombosis (N = 81).
| Predictors | Univariate relative risk, 95% CI | Multivariate relative risk, 95% CI | P value |
|---|---|---|---|
| Age >60 y | 4.9 (3.6–7.8) | 5.1 (3.4–8.2) | 0.02 |
| Sex | 1.1 (0.31–7.5) | 1.4 (0.25–8.6) | 0.68 |
| Headache | 0.9 (0.7–1.9) | 1.1 (0.9–1.7) | 0.08 |
| Vomiting | 1.3 (0.5–3.9) | 1.2 (0.4–4.4) | 0.67 |
| Convulsion | 0.3 (0.2–1.7) | 0.4 (0.12–1.3) | 0.11 |
| Altered consciousness | 6.4 (1.4–24.7) | 7.1 (1.8–27.6) | 0.005 |
| Papilledema | 0.8 (0.2–2.9) | 1.03 (0.32–3.2) | 0.96 |
| Hemiparesis | 3.1 (1.5–7.2) | 3.5 (1.4–8.7) | 0.003 |
| Cranial nerve palsy | 1.5 (0.3–7.1) | 1.8 (0.43–7.7) | 0.48 |
| Iron deficiency anemia | 0.9 (0.4–3.8) | 1.2 (0.38–4.1) | 0.73 |
| D-dimer | 1.9 (0.7–8.2) | 2.4 (0.5–9.9) | 0.24 |
| Hemoglobin | 0.3 (0.2–1.5) | 0.4 (0.2–1.7) | 0.65 |
| Superior sagittal sinus | 0.8 (0.4–2.7) | 1.3 (0.5–2.9) | 0.58 |
| Transverse sinus | 1.2 (0.8–1.9) | 1.1 (1.01–1.59) | 0.01 |
| Sigmoid sinus | 0.7 (0.5–3.2) | 1.2 (0.43–3.7) | 0.68 |
| Straight sinus | 1.7 (0.8–4.7) | 2.3 (0.96–5.1) | 0.06 |
| Vein of Gallen | 1.2 (0.3–4.2) | 1.5 (0.4–4.7) | 0.53 |
| Inferior jugular vein | 1.5 (0.3–3.1) | 1.7 (0.8–3.6) | 0.2 |
| More than two sinuses involved | 0.9 (0.3–2.9) | 1.1 (0.48–2.7) | 0.90 |
CI, confidence interval.
Discussion
This prospective study showed that most patients with CVT had a good outcome, with poor outcomes observed in one-third of the study population. Older age (>60 years), hemiparesis, altered consciousness, and transverse sinus involvement could independently predict the outcome of CVT. In general, serum D-dimer levels could not predict patient outcomes. However, a significant association was found between older patients with elevated D-dimer levels and poor outcomes. The number of involved sinuses was also not associated with patient outcome. Several studies have shown that older age is an independent predictive factor of CVT and is associated with poor outcomes. Altered mental status, infarct size, likelihood of hemorrhage, delayed recanalization in the thrombotic vein, presence of malignancy, and large platelet distribution width are the most common predictors of poor outcomes in older patients12,20–24 Holay et al. 22 found that age >30 years is a predictor of poor CVT outcome whereas Chu et al. 20 reported this age to be >40 years. We found that age >60 years was a predictor of poor CVT outcome.
There is inconsistency regarding sex as a predictor of CVT outcome. Girot et al. 21 found that male sex could predict poor outcome whereas Krajíčková et al. 25 reported female sex as a predictor of poor CVT outcome. We did not find any association of CVT outcome with sex, which is consistent with the findings of Kalita et al. 26 Hemiparesis was a predictive factor of CVT outcome in our study, which is consistent with the results of numerous studies.12,21,25,27,28 Altered consciousness could predict poor CVT outcome, which is in line with previous reports.12,20,22 However, Girot et al. 21 found no association with altered consciousness levels.
The outcome of CVT may vary according to the type of cerebral venous sinus involvement as well as the number of sinuses involved. Patil et al. 3 reported a higher rate of mortality in patients with involvement of the sigmoid or transverse sinuses and/or multiple sinus involvement in male patients as well as superior sagittal sinus thrombosis in female patients. As a whole, we only found an association with transverse sinus involvement in our study. However, this observation should not be generalized because most patients had superior sagittal and transverse sinus involvement. A large-scale study is needed to confirm transverse sinus involvement as an independent risk factor for CVT. As an outcome predictor, very few studies have shown an association with D-dimer level as a predictor of a CVT diagnosis. Sun et al. 29 found that the D-dimer level on admission significantly predicts unfavorable functional outcomes in patients with CVT. We found that D-dimer was not an independent predictor of CVT outcome. However, in older patients who had malignancy, a higher D-dimer level was found to be associated with poor functional outcomes.
The pregnancy and postpartum periods are associated with complex physiological changes and adaptation mechanisms that lead to altered homeostasis and a high risk of venous thrombosis. During the postpartum period, hypercoagulability is closely related to pregnancy, cesarean delivery, infections, blood loss during delivery and dehydration, fluctuations in intracranial pressure during labor, hypertensive complications of pregnancy, and even loss of cerebrospinal fluid after lumbar puncture. 30 We documented nine cases of CVT related to the postpartum period with severe dehydration, cesarian delivery, blood loss during delivery, and septicemia being the likely causes.
Limitations
This study had several limitations. This was a single-center study with a small sample size. Power calculation was not conducted because the prevalence of CVT is not known. This study was carried out during the COVID-19 pandemic; therefore, follow-up was done via social media applications like WhatsApp and imo. D-dimer and brain MRI with MRV were not done at the follow-up visit to assess recanalization.
Conclusion
We found that CVT led to morbidity and mortality in a significant number of patients. Older age, hemiparesis, and altered consciousness level were independent predictors of poor patient outcomes. The level of D-dimer did not influence the outcome of patients with CVT, except in older patients.
Acknowledgments
We wish to thank the consultants in the radiology department for their cordial support. We also thank Professor Qazi Deen Mohammad and Professor Badrul Alam for their continuous support for our research work, even during the COVID pandemic.
Footnotes
The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
S K Jakaria Been Sayeed https://orcid.org/0000-0003-1173-4248
Reaz Mahmud https://orcid.org/0000-0002-9427-1746
References
- 1.Tadi P, Behgam B, Baruffi S. Cerebral Venous Thrombosis. In: StatPearls. Treasure Island. (FL): StatPearls Publishing; June 12, 2023. [PubMed] [Google Scholar]
- 2.Jalili M, Ghourchian S, Shahidi GA, et al. A study of factors associated with cerebral venous thrombosis. Neurol Sci 2013; 34: 321–326. DOI: 10.1007/s10072-012-0997-x. [DOI] [PubMed] [Google Scholar]
- 3.Patil VC, Choraria K, Desai N, et al. Clinical profile and outcome of cerebral venous sinus thrombosis at tertiary care center. J Neurosci Rural Pract 2014; 5: 218–224. DOI: 10.4103/0976-3147.133559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abidullah K, Mohammad H, Wazir MK, et al. Cerebral Venous Sinus Thrombosis (CVST): A Review of the Deadly Threat. JOJ Case Stud 2017; 2: 555588. [Google Scholar]
- 5.Mowla A, Shakibajahromi B, Shahjouei S, et al. Cerebral venous sinus thrombosis associated with SARS-CoV-2; a multinational case series. J Neurol Sci 2020; 419: 117183. DOI: 10.1016/j.jns.2020.117183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdalkader M, Shaikh SP, Siegler JE, et al. Cerebral Venous Sinus Thrombosis in COVID-19 Patients: A Multicenter Study and Review of Literature. J Stroke Cerebrovasc Dis 2021; 30: 105733. DOI: 10.1016/j.jstrokecerebrovasdis.2021.105733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman S, Chowdhury M, Akram A, et al. Cerebral venous sinus thrombosis (CVST), an unusual presentation of COVID-19: A case report from Bangladesh. Microbes and Infectious Diseases 2021; 2: 9–14. DOI: 10.21608/MID.2020.34404.1041. [Google Scholar]
- 8.Meyohas MC, Roullet E, Rouzioux C, et al. Cerebral venous thrombosis and dural primary infection with human immunodeficiency virus and cytomegalovirus. J Neurol Neurosurg Psychiatry 1989; 52: 1010–1011. DOI: 10.1136/jnnp.52.8.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobersen M, Kleinschmidt-DeMasters BK. Superior sagittal sinus thrombosis in a patient with acquired immunodeficiency syndrome. Arch Pathol Lab Med 1994; 118: 844–846. [PubMed] [Google Scholar]
- 10.Iranzo A, Domingo P, Cadafalch J, et al. Intracranial venous and dural sinus thrombosis due to protein S deficiency in a patient with AIDS. J Neurol Neurosurg Psychiatry 1998; 64: 688. DOI: 10.1136/jnnp.64.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lew D, Southwick FS, Montgomery WW, et al. Sphenoid sinusitis. A review of 30 cases. N Engl J Med 1983; 309: 1149–1154. DOI: 10.1056/NEJM198311103091904. [DOI] [PubMed] [Google Scholar]
- 12.Shakibajahromi B, Haghighi AB, Salehi A, et al. Clinical and radiological characteristics and predictors of outcome of cerebral venous sinus thrombosis, a hospital-based study. Acta Neurol Belg 2020; 120: 845–852. DOI: 10.1007/s13760-018-1009-6. [DOI] [PubMed] [Google Scholar]
- 13.Ferro JM, Canhao P, Stam J, ISCVT Investigators et al. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke 2004; 35: 664–670. DOI: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 14.Yadegari S, Ghorbani A, Miri SR, et al. Clinical features, risk factors, and outcome of cerebralvenous thrombosis in Tehran, Iran. J Neurosci Rural Pract 2016; 7: 554–558. DOI: 10.4103/0976-3147.185512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Bruijn SF, De Haan RJ, Stam J. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. J Neurol Neurosurg Psychiatry 2001; 70: 105–108. DOI: 10.1136/jnnp.70.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan L, Ding J, Ya J, et al. Risk factors and predictors of outcomes in 243 Chinese patients with cerebral venous sinus thrombosis: A retrospective analysis. Clin Neurol Neurosurg 2019; 183: 105384. DOI: 10.1016/j.clineuro.2019.105384. [DOI] [PubMed] [Google Scholar]
- 17.Von Elm E, Altman DG, Egger M, STROBE Initiative et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 18.Broderick JP, Adeoye O, Elm J. Evolution of the Modified Rankin Scale and Its Use in Future Stroke Trials. Stroke 2017; 48: 2007–2012. DOI: 10.1161/STROKEAHA.117.017866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulivi L, Squitieri M, Cohen H, et al. Cerebral venous thrombosis: a practical guide. Pract Neurol 2020; 20: 356–367. DOI: 10.1136/practneurol-2019-002415. [DOI] [PubMed] [Google Scholar]
- 20.Chu X, Zhang J, Zhang B, et al. Analysis of Age and Prevention Strategy on Outcome after Cerebral Venous Thrombosis. Biomed Res Int 2020; 2020: 6637692. DOI: 10.1155/2020/6637692. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Girot M, Ferro JM, Canhão P, ISCVT Investigators et al. Predictors of Outcome in Patients With Cerebral Venous Thrombosis and Intracerebral Hemorrhage. Stroke 2007; 38: 337–342. DOI: 10.1161/01.STR.0000254579.16319.35. [DOI] [PubMed] [Google Scholar]
- 22.Holay M, Khot R, Bhatti A, et al. Predictors of outcome of cerebral venous sinus thrombosis at a tertiary care centre in Central India. Panacea Journal of Medical Sciences 2018; 8: 3–9. DOI: 10.18231/2348-7682.2018.0002. [Google Scholar]
- 23.Ferro JM, Canhão P, Bousser MG, ISCVT Investigators et al. Cerebral vein and dural sinus thrombosis in elderly patients. Stroke 2005; 36: 1927–1932. DOI: 10.1161/01.STR.0000177894.05495.54. [DOI] [PubMed] [Google Scholar]
- 24.Madineni KU, Prasad SVN, Bhuma V. A study of the prognostic significance of platelet distribution width, mean platelet volume, and plateletcrit in cerebral venous sinus thrombosis. J Neurosci Rural Pract 2023; 14: 418–423. DOI: 10.25259/JNRP-2021-1-3-R2-(1431). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krajíčková D, Král J, Herzig R, et al. Factors influencing therapy choice and clinical outcome in cerebral venous sinus thrombosis. Sci Rep 2020; 10: 21633. DOI: 10.1038/s41598-020-78434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalita J, Misra UK, Singh VK, et al. Does gender difference matter in cerebral venous thrombosis? J Clin Neurosci 2022; 102: 114–119. DOI: 10.1016/j.jocn.2022.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Ortega-Gutierrez S, Holcombe A, Aksan N, et al. Association of admission clinical predictors and functional outcome in patients with Cerebral Venous and Dural Sinus Thrombosis. Clin Neurol Neurosurg 2020; 188: 105563. DOI: 10.1016/j.clineuro.2019.105563. [DOI] [PubMed] [Google Scholar]
- 28.Aarju G, Birinder Singh P, Vipin K, et al. Neurological Predictors of Functional Outcome in Cortical Venous Sinus Thrombosis. J Neurosci Rural Pract 2022; 13: 290–294. DOI: 10.1055/s-0042-1744123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun R, Yin G, Wu W, et al. Clinical value and prediction efficiency of Neutrophil-to-lymphocyte radio and D-dimer on admission for functional outcome in patients with cerebral venous sinus thrombosis. PREPRINT (Version 1). 2022. DOI: 10.21203/rs.3.rs-1446563/v1. [Google Scholar]
- 30.Bajko Z, Motataianu A, Stoian A, et al. Postpartum Cerebral Venous Thrombosis—A Single-Center Experience. Brain Sci 2021; 11: 327. DOI: 10.3390/brainsci11030327. [DOI] [PMC free article] [PubMed] [Google Scholar]