Abstract

Chemiresistive gas sensors based on semiconducting metal oxides typically rely on noble metal catalysts to enhance their sensitivity and selectivity. However, noble metal catalysts have several drawbacks for practical utilization, including their high cost, their propensity for spontaneous agglomeration, and poisoning effects with certain types of gases. As such, in the interest of commercializing the chemiresistive gas sensor technology, we propose an alternative design for a noble-metal-free sensing material through the case study of Co-doped ceria (Co–CeO2) catalysts embedded in a SnO2 matrix. In this investigation, we utilized electrospinning and subsequent calcination to prepare Co–CeO2 catalyst nanoparticles integrated with SnO2 nanofibers (NFs) with uniform particle distribution and particle size regulation down to the sub-2 nm regime. The resulting Co–CeO2@SnO2 NFs exhibited superior gas sensing characteristics toward isoprene (C5H8) gas, a significant biomarker for monitoring the onset of various diseases through breath diagnostics. In particular, we identified that the Co–CeO2 catalysts, owing to the transition metal doping, facilitated the spillover of chemisorbed oxygen species to the SnO2 sensing body. This resulting in the sensor having a 27.4-fold higher response toward 5 ppm of C5H8 (compared to pristine SnO2), exceptionally high selectivity, and a low detection limit of 100 ppb. The sensor also exhibited high stability for prolonged response–recovery cycles, attesting to the strong anchoring of Co–CeO2 catalysts in the SnO2 matrix. Based on our findings, the transition metal-doped metal oxide catalysts, such as Co–CeO2, demonstrate strong potential to completely replace noble metal catalysts, thereby advancing the development of the commercially viable chemiresistive gas sensors free from noble metals, capable of detecting target gases at sub-ppm levels.
Keywords: cerium oxide, doping, metal oxide, electrospinning, gas sensor
The high valuation of the gas sensor market at USD 2.90 billion in 2023, coupled with optimistic predictions to reach USD 5.49 billion by 2030, reflects the burgeoning demand for cutting-edge chemiresistive sensors based on metal oxides.1 Importantly, to simultaneously achieve high sensitivity and selectivity standards to satisfy this demand,2 the metal oxides serving as the sensing layer are often decorated with sensitizers to enhance surface reactivity between the metal oxide surface and target analyte. This enhancement stems from the operational principle of metal oxide-based sensors, which involves measuring the resistance change of the sensing layer as a result of the interaction between target gases and adsorbed oxygen species (O2–, O–, O2–) on the oxide surface.3 In this regard, maximizing the number of adsorbed oxygen species at a given operating temperature is crucial for bolstering the surface reactivity of metal oxides toward the target gas. One reliable strategy is to functionalize noble metal nanoparticles (NPs) onto the oxide surface, as these catalytic NPs promote the oxidation of atmospheric oxygen molecules through a spillover process.4−6 However, noble metal NPs still face critical issues, such as deactivation of the catalytic activity due to the poisoning effect, a propensity for aggregation at elevated temperatures, and the scarcity of noble metals in the Earth’s crust.7−10 These challenges emphasize the necessity for alternative non-noble metal-based sensitizers to provide practical solutions, aiming to enhance sensing performance and achieve cost-effectiveness for commercialization.
To this end, lanthanides and their derivatives have gained considerable attention in various applications due to their distinct potential and advantages as alternatives to noble metals, which are more abundant in nature and exhibit superior resistance to gas poisoning.11−13 In particular, ceria (CeO2) is promising due to its vastly different redox properties, high oxygen conductivity, and thermochemical stability.14,15 Based on these, CeO2 has been reputed to be an ideal material for chemiresistive sensors, as both the main sensing layer and the sensitizer.16,17 In addition, doping with transition metal atoms provides additional avenues to modify the surface chemistry of CeO2 and improve their catalytic activity as heterogeneous catalysts.18,19 As a sensitizer in chemiresistive sensor applications, transition metal-doped CeO2 as a sensitizer offers three major advantages. First, dopants modify the electronic structure of CeO2 to reduce the oxygen vacancy formation energy, facilitating the dissociation of chemisorbed oxygens and generating active oxygen species.20 Second, transition metals and CeO2 introduce additional active sites, enhancing oxidation activity and selectivity when interacting with target analytes. Third, strong metal–oxygen interactions between transition metal atoms and the oxygen atoms of the CeO2 matrix contribute to increased stability and cyclability. Nevertheless, these CeO2 catalysts have rarely been utilized as reaction promoters in chemiresistive sensors due to the lack of reliable functionalization strategies. It is imperative that these sensitizers be uniformly functionalized on the metal oxide-based sensing layer with a high degree of control if the objective is to elucidate the direct influence of transition metal doping on maximizing the reactivity of surface oxygens.
Functionalizing catalytic sensitizers on one-dimensional (1D) metal oxide nanofibers (NFs) by electrospinning is an emerging strategy to enhance the catalytic performance and obtain a nanostructure with a large surface area and high porosity.21−23 Previous studies have demonstrated the functionalization of host oxide NFs with as-prepared perovskite oxides and ex-solution catalysts, leading to improved sensing properties.24−26 However, achieving high dispersity and small sizes in electrospun sensing layers remained difficult, primarily due to the high temperatures necessary for the bulk synthesis of CeO2 catalysts. These include methods such as coprecipitation, hydrothermal, and combustion,27−31 which often lead to excessive grain growth and resultant particle sizes of 100 nm or larger. Aside from the issues with particle size, incorporating powder-form CeO2 catalysts into the electrospinning solutions introduces the risk of poor redispersion, particle aggregation, and nozzle blockages, all of which lead to a poorly regulated final product.
In this work, we demonstrate the precipitation synthesized Co-doped CeO2 NPs decorated onto SnO2 NFs (Co–CeO2@SnO2 NFs), with the former serving as the sensitizer and the latter as the chemiresistive 1D sensing layer. A liquid-phase reaction with organic solvents (ethanol, ethylene glycol) as surface capping agents produced sub-2 nm Co–CeO2 colloids as the capping agents effectively weakened interparticle interactions and suppressed grain growth. The colloidal solution could conveniently be added to the electrospinning solution by simply mixing Co–CeO2, Sn precursors, and polymer, followed by electrospinning and calcination for the surface functionalization of Co–CeO2 NPs on SnO2 NFs. To evaluate the effectiveness of the Co–CeO2 sensitizers in SnO2 sensing layers, we comprehensively evaluated the sensing performance of the Co–CeO2@SnO2 NF-based sensor. The improved sensing performance for the target gas isoprene (C5H8) indicates that Co doping in the CeO2 contributes to enhanced catalytic activity in comparison to undoped CeO2. In addition, density functional theory (DFT) calculations, O2 temperature-programmed desorption (O2-TPD) analyses, and X-ray photoelectron spectroscopy (XPS) analyses were conducted to investigate the role of the Co–CeO2 sensitizers in promoting the chemisorption behavior of oxygen species, their catalytic enhancement, and their effect on the enhanced sensing performance. These complementary techniques provided valuable insights and an understanding of the interactions between the Co–CeO2 sensitizers and the target gases, contributing to the optimization and refinement of the sensor design. Moreover, our sensor exhibits sensing capabilities suitable for monitoring exhaled C5H8 gas, which serves as a biomarker in breath diagnostics, such as metabolic byproducts or blood cholesterol levels, with potential for real-world implementation. Altogether, our findings empower the development of robust chemiresistive gas sensors through effective sensitization by non-noble and cost-effective catalyst loading.
Results and Discussion
Figure 1 illustrates the synthesis procedures for obtaining 2 nm Co-doped CeO2 NPs (referred to as Co–CeO2 NPs) as the catalysts for sensitizing the SnO2-based chemiresistive sensors and their functionalization on the metal oxide NFs through electrospinning and subsequent calcination. Figure 1a describes how the Co–CeO2 NPs could be obtained by the precipitation process (refer to Notes S1–S6). Here, ethanol and ethylene glycol were used for capping the surfaces of particles, effectively weakening the interparticle interactions and thus suppressing particle aggregation. In a typical synthesis, the Ce and Co precursors were dissolved in the mixed solvent, the pH of which was modulated using ammonium hydroxide, and a specific amount of hydrogen peroxide was added to trigger the reaction. After 24 h of reaction, the Co–CeO2 NPs were obtained as a stable colloid. Then, the as-prepared colloidal solution was added to an electrospinning solution consisting of Sn precursors and the polymer matrix. The solution was electrospun into a polymer NF membrane containing Co–CeO2 NPs and Sn species, which was calcined in an ambient atmosphere at 600 °C for 1 h to finally obtain the Co–CeO2-decorated SnO2 NFs (Figure 1b).
Figure 1.
Schematic illustration of the overall synthesis process. (a) Synthesis of Co–CeO2 NPs by precipitation process. (b) Functionalization of the Co–CeO2 NPs on SnO2 NFs through electrospinning.
We first characterized the prepared colloidal Co–CeO2 NPs to evaluate their applicability as a gas-sensitizing catalyst. Electron microscopy (EM) analyses were conducted on the as-obtained Co–CeO2 NPs to assess their morphologies. For comparison, CeO2 NPs have also been prepared using a similar protocol, just without Co, as the reference sample. The STEM images (Figures 2a,b and S1–S3) show that the average particle size of Co–CeO2 NPs was determined to be 1.94 nm, with a standard deviation of 0.23, indicating that the organic solvents (ethanol and ethylene glycol) had regulated the formation of Co–CeO2 colloids. The size distribution was similar to that of pristine CeO2 NPs, implying that the morphology of NPs was not significantly affected by Co doping. In a high-resolution TEM image, Co–CeO2 NPs show a diffraction pattern with a lattice spacing of 0.31 nm, which corresponds to the (111) plane of CeO2 (Figures 2c and S4), confirming that the crystal structure of the matrix was maintained after doping.32,33 Moreover, X-ray diffraction (XRD) patterns of the Co–CeO2 NPs showed prominent peaks corresponding to the cubic structure of CeO2 (JCPDF # 34-0394), not any different from that of pristine CeO2, with a relatively high degree of crystallinity (Figure 2d). Notably, no peaks related to Co were observed, which suggests that there is almost no phase-separated Co, or at least the amount is below the detection limit of the XRD equipment. Scherrer analysis of peak broadening reveals that the average crystallite sizes of 1.98 nm for Co–CeO2 NPs, which is similar to 2.04 nm for pristine CeO2 NPs, estimated from the peaks corresponding to (111), (220), and (311) planes (Figure S5 and Table S1). Taken together, we could confirm that the Co atoms were incorporated into the CeO2 lattice without significant alterations to their crystallographic properties.
Figure 2.
Characterization of Co–CeO2 NPs. (a) Low magnification STEM image of Co–CeO2 NPs. (b) High magnification STEM image of Co–CeO2 NPs. (c) HRTEM image and inset showing the fast Fourier transform (FFT) patterns of Co–CeO2 NPs. (d) XRD patterns showing the reflections of the CeO2 structure. (e) EDS mapping images of Ce, O, and Co in the Co–CeO2 NPs (scale bar 5 nm). (f) Ex situ XPS spectra of pristine CeO2 and Co–CeO2 NPs in the vicinity of Co 2p. (g) EPR spectrum of CeO2 and Co–CeO2 NPs. (h) UV–vis absorbance spectra and optical photograph of suspension of CeO2 and Co–CeO2 NPs (inset). (i) Tauc plots of CeO2 and Co–CeO2 NPs.
In conjunction with the EM-based physical morphology characterizations, the chemical properties of Co–CeO2 NPs were studied using spectroscopic and diffraction analysis methods. Low-magnification energy-dispersive X-ray spectroscopy (EDS) mapping revealed the homogeneous distribution of cerium, oxygen, and cobalt throughout the entire particles without apparent separation (Figure 2e). XPS measurements were also conducted on the Co–CeO2 NPs, revealing distinct peaks corresponding to Co 2p and indicating the concomitant presence of Co3+ and Co2+ valence states at 780.4 and 782.5 eV, respectively (Figure 2f).34,35 With increasing Co doping levels from 0 (pristine CeO2) to 7 at% Co, the intensity of the Co 2p peak showed a correlative increase (Figure S6a). In the Ce 3d region, the Ce 3d spectrum consisted of five spin–orbit split doublets (u: 3d3/2, v: 3d5/2) split by 18.6 eV. Ce cations in Co–CeO2 NPs were in a mixed valence state of Ce3+ and Ce4+, even though an ideally stoichiometric CeO2 should consist solely of Ce4+ (Figure S6b). This is because the oxygen vacancies in CeO2 NPs promote the formation of Ce3+ in order to maintain charge neutrality.36 Moreover, it was observed that the intensity of the O 1s peak corresponding to adsorbed oxygen species on Co–CeO2 NPs increased with higher Co doping levels (Figure S6c). This increase in adsorbed oxygen species provides enhanced catalytic activity in gas interaction reactions to improve the overall sensing performance. In addition, the presence of oxygen vacancies in CeO2 promotes greater adsorption of oxygen species, facilitating the redox interaction between adsorbed oxygens produced on oxygen vacancies and reducing gas.37 Therefore, it is evident that the Co dopants promote the formation of additional active oxygen species and provide the CeO2-based sensitizer with favorable catalytic properties.
Since the Co dopants indubitably influenced the surface electronic structure of CeO2, electron paramagnetic resonance (EPR) characterization was performed to obtain a detailed analysis of the chemical states of Ce species and oxygen vacancies CeO2 and in Co–CeO2 (Figure 2g). The reference EPR spectrum obtained from pristine CeO2 NPs exhibited a low and broad signal, whereas intense and narrow resonance signals appeared at g = 2.20 in the case of Co–CeO2 NPs, indicating the presence of Co ions.38 Additionally, other resonance signals observed at g = 1.96 and g = 2.04 suggest increased amounts of Ce3+ and oxygen vacancies in Co–CeO2, respectively.36,39,40 These findings indicate that Co–CeO2 exhibits multiple valences of Ce3+ and Ce4+ as well as abundant oxygen vacancies, resulting in enhanced activity for the oxidation of reducing gases. Furthermore, ultraviolet–visible (UV–vis) spectroscopy was performed to quantitatively evaluate the overall influence of the Co dopants on the electronic properties of CeO2, with significant differences in absorbance spectra revealed between CeO2 and Co–CeO2 NP samples. Visually, the dispersed CeO2 and Co–CeO2 NP samples exhibited distinct pale yellow and orange colors, respectively (inset of Figure 2h), while maintaining a colloidal state without aggregation, with both samples exhibiting high transmittances (>95%) in the visible spectrum (Figure S7). The Tauc plots (inset) indicated an estimated decrease in the band gap energy from 2.38 eV for pristine CeO2 to 2.10 eV for Co-doped CeO2, owing to the energy levels provided by the Co dopant (Figure 2i). Specifically, the introduction of Co leads to an increased concentration of Ce3+ states, resulting in localized energy levels that are closer to the conduction band, reducing the band gap.41,42 Altogether, the electronically modulated Co–CeO2 NPs present a more favorable opportunity for facilitating surficial interactions with gas molecules.
The main advantage of this synthetic route, in comparison to high-temperature protocols, is that a high-quality colloidal solution of evenly doped catalyst NPs can be prepared at a relatively high concentration of approximately 1.4 × 1015 particles/mL. Importantly, these Co–CeO2 NPs do not aggregate in the electrospinning solution owing to the organic-capped NP surfaces. This allows the highly concentrated Co–CeO2 NPs to be uniformly dispersed, facilitating their incorporation into the electrospinning solution. As a result, a relatively high concentration of NPs can be used for the synthesis of chemiresistive sensing materials with high catalyst loading to facilitate a more efficient sensing behavior. As a result, through facile electrospinning and calcination processes, Co–CeO2 NPs could be uniformly functionalized onto SnO2 NFs. The scanning electron microscope (SEM) images in Figures 3a, S8, and S9 show the resultant Co–CeO2 NPs-decorated SnO2 NFs (Co–CeO2@SnO2 NFs) with a 1D fiber structure. Simultaneously, this procedure introduced porous sites and voids between the polycrystalline SnO2 nanograin structures that facilitate gas diffusion, which was also confirmed by the TEM images in Figures 3b and S10. This morphological characteristic can be attributed to the hindrance of grain growth inhibition caused by well-dispersed Co–CeO2 NPs, which effectively prevents the Ostwald ripening of SnO2 during the calcination and results in a less dense structure.24 Consequently, the Co–CeO2@SnO2 NFs showed a slight increase in the Brunauer–Emmett–Teller (BET) surface area and pore volume (20.2–28.9 m2 g–1 and 0.053–0.113 m3 g–1) in comparison to pristine SnO2 NFs (14.4 m2 g–1 and 0.038 m3 g–1), as presented in Figure S11 and Table S2. The external regions of the 1D nanostructure were denser than the core regions, prompting the concentration of Co–CeO2 NPs closer to the fiber surface. Ultimately, these aspects collectively make the Co–CeO2@SnO2 NFs one of the most rationally designed nanostructures for facilitating gas sensing applications.
Figure 3.
Characterization of the Co–CeO2@SnO2 NF. (a) SEM image of Co–CeO2@SnO2 NF after calcination. (b) TEM image of Co–CeO2@SnO2 NF. (c) HRTEM image of the Co–CeO2@SnO2 NF in (b) and magnified images (inset). (d) STEM image and EDS mapping images of overlay, Sn, O, Ce, and Co in the Co–CeO2@SnO2 NF (scale bar 5 nm). Ex situ XPS spectra of (e) Sn 3d, (f) O 1s, (g) Ce 3d, and (h) Co 2p in Co–CeO2@SnO2 NFs. (i) XRD patterns of pristine SnO2 NFs and Co–CeO2@SnO2 NFs.
Notably, the integrity of the Co–CeO2 NP catalysts was not compromised during the fabrication process despite the involvement of a heat treatment step. For instance, the high-resolution TEM (HRTEM) image of the Co–CeO2@SnO2 NFs revealed two populations of grains having an interplanar spacing of either 0.34 or 0.27 nm, corresponding to the SnO2 (110) and Co–CeO2 (200) lattice planes, respectively (Figure 3c).43 STEM imaging analyses accompanied by EDS mapping were also performed, as shown in Figures 3d and S12. The STEM image of Co–CeO2@SnO2 NFs clearly shows distinct contrast variations within the SnO2 layers, indicating the homogeneous loading of the Co–CeO2 phases on the surface of SnO2 NFs by electrospinning and subsequent calcination process. The contrast differences among the grains indicate the presence of dissimilar elements with higher atomic numbers, such as cerium (ZCe = 58), leading to brighter appearances compared to lower atomic numbers, such as tin (ZSn = 50), from which we could discern the presence and distribution of Co–CeO2 phases on the SnO2 NFs. The mapping results further show the uniform distributions of Sn and O elements, corresponding to the SnO2 NFs. The presence of Ce and Co elements corresponding to Co–CeO2 NPs appears to be localized on the SnO2 surface. In addition, XPS spectra of the Co–CeO2@SnO2 NFs showed characteristics of doublet peaks of Sn4+ at 486.8 and 495.2 eV, assigned to Sn 3d5/2 and Sn 3d3/2 peaks, respectively (Figure 3e),44 while the deconvoluted O 1s spectra revealed two main peaks at 530.6 and 531.4 eV, each attributed to the lattice O atoms (Olatt) in SnO2 and adsorbed oxygen (O–) species, respectively (Figure 3f).45 The XPS spectra of Ce 3d and Co 2p in Co–CeO2@SnO2 NFs are shown in Figure 3g,h, with no noticeable anomalies in comparison to the previously discussed XPS data for Co–CeO2 NPs.
Moreover, we systematically investigated the crystallographic properties of the different SnO2 NFs with varying concentrations of Co–CeO2 additives. The relative weight percentages (wt %) of reference CeO2 and Co–CeO2 NPs in the prepared samples relative to the SnO2 support layers were as follows: 0.1_CeO2@SnO2 (0.1 wt % of CeO2 with respect to SnO2 matrix), 0.01_Co–CeO2@SnO2, 0.1_Co–CeO2@SnO2, and 0.5_Co–CeO2@SnO2 (0.01, 0.1, and 0.5 wt % of Co–CeO2 concentration relative to SnO2 NFs). Note that, at these low levels of loading, the XRD diffraction pattern of the Co–CeO2 NPs could not be discerned from the SnO2 data (Figures 3i and S13). Therefore, we focused on the average crystallite sizes of SnO2 grains in the pristine SnO2 NF, 0.1_CeO2@SnO2 NF, 0.01_Co–CeO2@SnO2 NF, 0.1_Co–CeO2@SnO2 NF, and 0.5_Co–CeO2@SnO2 NF samples, calculated by the Scherrer equation using (110), (101), (200), and (211) planes. As a result, the grain sizes were shown to remain in similar ranges of 12.7–15.7 nm, indicating that the structural properties were not significantly altered by the incorporation of Co–CeO2 NPs (Figure S14). Taken together, it is evident that the proposed electrospinning-based strategy for loading precipitation-prepared Co–CeO2 NPs on SnO2 NFs is effective for preparing oxide sensing materials decorated with catalytic NPs in a general sense.
The sensitizing role of the Co–CeO2 catalyst NPs in the SnO2 sensing layers was experimentally demonstrated by performing a series of chemiresistive sensing measurements in the presence of the target gas C5H8, essential for health monitoring and physiological assessment. For this experiment, the sensing materials, e.g., Co–CeO2@SnO2 NFs, were first drop-coated on prefabricated alumina sensor substrates with interdigitated gold electrodes. Afterward, their sensing characteristics were analyzed using our homemade sensor setup with regulated gas injection through mass flow controllers (Figure S15). We hypothesized that the introduction of redox-active Co-doped CeO2 in the oxide-based sensing layer would greatly boost the sensing performance to levels that are not anticipated in unadulterated CeO2 or CeO2 decorated with a single-phase catalyst. To explore this, we evaluated the sensing capabilities of pristine SnO2, CeO2@SnO2, and Co–CeO2@SnO2 NFs. For Co–CeO2@SnO2 NFs, comparative experiments were conducted with various loading amounts of Co–CeO2 NPs for optimization.
After analyzing the temperature-dependent sensing properties toward the target analyte in the temperature range from 200 to 450 °C (Figure S16), we determined the optimal temperature for sensing to be 350 °C based on the response values. At this temperature, we performed dynamic gas sensing experiments using five different sensors (i.e., pristine SnO2 NFs, 0.1_CeO2@SnO2 NFs, 0.01_CeO2@SnO2 NFs, 0.1_CeO2@SnO2 NFs, and 0.5_Co–CeO2@SnO2 NFs) monitoring the resistance changes in the respective sensors upon exposure to 5 ppm of C5H8 (Figure 4a). Initially, the 0.1_CeO2@SnO2 NFs showed a baseline resistance of 5.5 kΩ, which is 3.2-fold higher than that of pristine SnO2 NFs (1.7 kΩ) due to the greater number of chemisorbed oxygen species on the surfaces of SnO2 NFs contributed by the CeO2 NPs. Furthermore, the baseline resistances of the 0.01, 0.1, and 0.5_Co–CeO2@SnO2 NFs gradually increased from 16.1 to 90.1 kΩ, then to 2.9 MΩ, indicating a higher density of chemisorbed oxygen molecules on the Co–CeO2@SnO2 NFs as a result of spillover effects induced by catalytic Co–CeO2 NPs. The spillover effect of the Co–CeO2 NPs was formally examined by comparative ultraviolet photoelectron spectroscopy (UPS) measurements performed on pristine SnO2 NFs, 0.1_CeO2@SnO2 NFs, and 0.1_Co–CeO2@SnO2 NFs. The work function of pristine SnO2 NFs was measured as 5.48 eV (Figure S17a–d), which decreased to 5.33 and 5.11 eV with the incorporation of CeO2 and Co–CeO2, respectively (Figure S17e–l). This decrease suggests the transfer of electrons from the Co–CeO2 NPs to the SnO2 sensing layers, supporting the formation of a heterojunction between SnO2 and Co–CeO2. Furthermore, as indicated by the prior EPR and UV–vis absorbance results (Figure 2f,g), it can be inferred that Co–CeO2 NPs exhibit multivalence, such as Ce3+ states, and an increased formation of oxygen vacancies. This results in a decrease in the bandgap while forming localized defect energy levels that are in closer proximity to the conduction band.41,42 This can result in activated electron layers that facilitate the adsorption of oxygen species, ultimately improving the sensing performance of Co–CeO2@SnO2 NFs and further reinforcing their effectiveness as a gas sensor. Moreover, the additional generation of oxygen adsorption sites by Co–CeO2 NPs promotes the uptake of oxygen species, facilitating the occurrence of the oxygen spillover phenomenon. Consequently, this raises the concentration of chemisorbed oxygen on the surface of SnO2 decorated with Co–CeO2 NPs.
Figure 4.
Chemiresistive sensing performance of Co–CeO2 NPs functionalized SnO2 NFs. (a) Electrical resistance profiles of the pristine SnO2 NFs, 0.1_CeO2@SnO2 NFs, and 0.01, 0.1, 0.5_Co–CeO2@SnO2 NFs sensors at 5 ppm of C5H8 gas. (b) Dynamic C5H8 gas response in a concentration ranging from 0.1 to 5 ppm at 350 °C and (c) their corresponding plot of response versus gas concentration. (d) Linear regression results of responses extracted from the response values of SnO2, 0.1_CeO2@SnO2, and 0.1_Co–CeO2@SnO2 NFs toward sub-ppm level (0.1, 0.2, 0.4, 0.6, and 0.8, 1 ppm) C5H8 gases. (e) Response and recovery times of SnO2, 0.1_CeO2@SnO2, and 0.1_Co–CeO2@SnO2 NFs toward 5 ppm of C5H8 gas. (f) Selectivity chart of SnO2, 0.1_CeO2@SnO2, and 0.1_Co–CeO2@SnO2 NFs toward 7 different analytes at 5 ppm. (g) Long-term reliability tests of SnO2, 0.1_CeO2@SnO2, and 0.1_Co–CeO2@SnO2 NFs toward 27 cycles of exposure to 5 ppm of C5H8 under dry air. (h) Comparison of response values of the state-of-the-art C5H8 sensors compared with that of the Co–CeO2@SnO2 NF-based sensor.
Upon exposure to 5 ppm of C5H8, the 0.1_Co–CeO2@SnO2 NFs showed an excellent response (Rair/Rgas) of 120.38, which was 27.4-fold higher than that of pristine SnO2 NFs (Rair/Rgas = 4.39). In contrast, the response of 0.1_CeO2@SnO2 NFs was enhanced only 3.4-fold (Rair/Rgas = 15.12) compared to pristine SnO2 NFs. This difference is attributed to the heterojunctions between the Co–CeO2 and SnO2, in which the Co dopant played an important role in amplifying the spillover effect. Furthermore, upon dynamic exposure of C5H8 in the range from 0.1 to 5 ppm (Figures 4b and S18), we found that the 0.1_Co–CeO2@SnO2 NFs exhibited superior C5H8 response over those based on 0.01_Co–CeO2@SnO2, 0.5_Co–CeO2@SnO2 NFs, across the entire concentration range, demonstrating that the optimal amount of the catalytic sensitizers results in a significant improvement of their sensing characteristics. With fewer Co–CeO2 NPs as sensitizers, the surface reactivity of the SnO2 sensing layer is not sufficiently improved. Conversely, excessive loading of the catalysts could block the active sites within the SnO2 layer, resulting in a notable degradation of sensing properties. Furthermore, we prepared different sensors with varying amounts of Co doping in CeO2 while maintaining the same weight percent of the Co–CeO2 NPs in SnO2 NFs to determine the appropriate Co doping levels for optimized gas sensing performance (Figure S19). After conducting comparative evaluations, we determined that the optimum condition was found to be 3 mol % Co-doped CeO2 functionalized SnO2 NFs (0.1_Co–CeO2@SnO2 NFs) for detecting 5 ppm of C5H8 at 350 °C.
Considering the potential development of the Co–CeO2@SnO2 NF-based sensors into a commercially viable product, we comprehensively evaluated their sensing performance using multiple different performance metrics, as follows. During the dynamic changes in electrical resistance, an apparent linear relationship was observed between the response and concentration range of 0.1–5 ppm for the sensors (Figure 4c). This relationship indicates that the Co–CeO2@SnO2 NFs could be utilized to reliably detect C5H8 gas, especially in the low concentration range from 0.1 to 1 ppm. The detection limit (LOD) was estimated based on the response value exceeding 1.2 for sub-ppm-level C5H8 gases (Figure 4d). The Co–CeO2@SnO2 NFs showed a detection limit value of 100 ppb C5H8, surpassing the LOD exhibited by pristine SnO2 NFs (130 ppb) and CeO2@SnO2 NFs (120 ppb), owing to the effective modulation of electrical resistance through the accelerated spillover of chemisorbed oxygen induced by the Co dopant. The response and recovery times of the sensors were calculated for 5 ppm of C5H8 exposure (Figure 4e). The response times of the sensors were improved from 12 s for pristine SnO2 NFs to 7 and 5 s for CeO2- and Co–CeO2-decorated SnO2 NFs, respectively. Likewise, the recovery rates were also improved in 0.1_Co–CeO2@SnO2 (514 s) compared to 0.1_CeO2@SnO2 (>610 s) and pristine SnO2 (>610 s). This can be attributed to the contribution of increased porosity in the NF structure, which provides more channels for gas diffusion as well as active surface sites.46 Furthermore, the gas selectivity of the 0.1_Co–CeO2@SnO2 NFs was investigated against other types of gases (Figure 4f). In particular, the 0.1_Co–CeO2@SnO2 NFs showed excellent C5H8 selectivity against potential interfering gases such as toluene (C7H8), acetone (C3H6O), ethanol (C2H6O), carbon monoxide (CO), formaldehyde (HCHO), and ammonia (NH3). On the contrary, pristine SnO2 NFs could not dependably isolate the C5H8 response from those of the interfering gases. Moreover, 0.1_Co–CeO2@SnO2 NFs also showed excellent stability against 27 cyclic tests toward 5 ppm of C5H8 (Figure 4g). Interestingly, even 5 weeks after the initial sample preparation, the 0.1_Co–CeO2@SnO2 NF-based sensor showed stable sensitivity performance during 193 cycles of exposure to 5 ppm of C5H8, as shown in Figure S20. In addition, as C5H8 is an important biomarker for breath diagnostics, the accurate detection of this species under highly humid conditions, such as exhaled breath, is crucial.47,48 The effects of different humidity levels on our sensors can be observed in Figure S21. The dynamic resistance traces of our Co–CeO2@SnO2 sensors toward 5 ppm of C5H8 at 0, 25, 50, and 75% relative humidity show a slight decrease in response in humid air, attributed to the adsorption of water molecules that impede the reactions between C5H8 with the chemisorbed oxygen species of Co–CeO2@SnO2 NFs.2,10,49 Nonetheless, this demonstrates that the C5H8 sensing performances of Co–CeO2@SnO2 NFs remain viable under various humid environments. Altogether, as shown in the summary table in Figure 4h and Table S3 comparing the sensing performances of Co–CeO2@SnO2 NFs to other reported C5H8 sensors,50−57 the Co–CeO2 NPs have effectively sensitized the SnO2 sensing layers, enabling the selective detection of C5H8 gas with exceptional sensing performance. Most notably, this sensor efficiently responds to C5H8 using non-noble metals in lieu of noble metal catalysts, making significant progress in the realm of highly sensitive chemiresistive gas sensors. Despite comparing the gas detection performance of SnO2 NFs with added noble metal Pt catalysts, the 0.1_Co–CeO2@SnO2 NF-based sensors still demonstrate superior C5H8 sensitivity (Figure S22).
Having established the effectiveness of the Co–CeO2 catalysts, we further investigated the detailed sensitizing mechanisms through additional XPS analyses and O2-TPD profiles, helping reveal the underlying chemistry of the chemisorption of oxygen species and selective gas adsorption facilitated by the Co–CeO2 sensitizers. Indeed, the adsorption and activation of oxygen species on the Co–CeO2@SnO2 NFs were critical steps for facilitating efficient chemiresistive sensing. The properties of oxygen species were inevitably affected by oxygen vacancies and the presence of Co–CeO2 NPs on the surfaces of SnO2 sensing layers. The O 1s XPS spectra (Figures 5a and S23) revealed asymmetrical peaks corresponding to oxygen species chemically adsorbed by surface oxygen vacancies (Oads at 531.4 eV) and lattice oxygen (Olatt at 530.6 eV).45 The Oads/Olatt ratio, calculated based on the respective integrated peak areas, showed significant increases in 0.1_Co–CeO2@SnO2 (0.85) and 0.1_CeO2@SnO2 (0.68) compared to pristine SnO2 (0.52). This indicates that the 0.1_Co–CeO2@SnO2-based sensor, with a larger number of surface oxygen species, would exhibit the most sensitive response based on the aforementioned sensing performances. It is sufficient to demonstrate that a high density of oxygen species is chemisorbed on the surface of SnO2 NFs, and based on the aforementioned O 1s XPS results of Co-doped CeO2 from Figure S6c, the relative increase in the proportion of reactive oxygen species can be attributed to the enhanced spillover effects of decorated Co-doped CeO2.18,58 Meanwhile, comparing the desorbed oxygen profiles for each sample through O2-TPD analyses revealed that the oxygen species contribute significantly toward the gas response (Figure 5b). In the case of 0.1_Co–CeO2@SnO2, noticeable oxygen desorption was observed as a broad shoulder in the temperature range of 150–350 °C. On the other hand, the pristine SnO2 only showed a peak related to the desorption of chemisorbed oxygen (∼400 °C). It should be noted that below 150 °C, the dominant species are in their molecular form (O2–), while the atomic species (O– and O2–) are prevalent in the higher temperature region.58−60 Consequently, these increased reactive oxygen atomic species formulated upon introducing the Co–CeO2 NPs would serve as primary active sites for the adsorbed analytes to enhance the C5H8 response.
Figure 5.
Investigation of catalytic role of Co–CeO2 sensitizers. (a) Ex situ XPS spectra of pristine SnO2, 0.1_CeO2@SnO2, and 0.1_Co–CeO2@SnO2 in the vicinity of O 1s peaks. (b) O2-TPD profiles of pristine SnO2, 0.1_CeO2@SnO2, and 0.1_Co–CeO2@SnO2. (c,d) The optimized binding configurations of CeO2 (left) and Co–CeO2 (right) toward the C5H8 molecule were calculated by DFT calculation (O: red, Ce: brown, Co: blue, C: dark brown, and H: white). (e,f) Side-view image of the charge density difference plot for CeO2 (left) and Co–CeO2 (right) interacting with C5H8 molecule (regions of charge accumulation in yellow and charge depletion in cyan). (g) Comparison of the calculated adsorption energy of C5H8 at CeO2, Co–CeO2, and their charge transfer values for the cases of C5H8.
To support the experimental findings and rationalize the underlying mechanism behind the enhanced sensing properties in Co–CeO2@SnO2, we conducted DFT calculations to investigate the adsorption energies and conducted Bader charge analyses (Figures 5c–g and S24). First, adsorption energies of C5H8 on both CeO2 and Co–CeO2 surfaces were calculated for different possible configurations (Table S4). The results revealed that the adsorption energy of C5H8 on Co–CeO2 (−83.5 kJ mol–1) was higher compared to that of CeO2 (−67.6 kJ mol–1), indicating more favorable adsorption of the target analyte on the Co–CeO2 surface (Figure 5g). Additionally, the charge density difference plot in Figure 5e,f, along with the Bader charge analysis in Figure 5g, indicates an increase in the absolute value of charge transfer from 0.0263 e for CeO2 to 0.0401 e for Co–CeO2. In chemiresistive sensors, adsorption and dissociation processes involve charge transfer between the target analyte and the catalytic layers, which affects the resistance of the sensing layers. Thus, a larger magnitude of charge transfer in Co-doped CeO2 would indicate a significant reinforcement in the C5H8 adsorption and activation behavior compared to pristine CeO2 NPs. Based on these findings, the improved sensing performance of Co–CeO2@SnO2 NFs toward C5H8 can be attributed to the improved catalytic properties of Co–CeO2. Upon incorporation of Co–CeO2 NPs SnO2, the sensitizer starts to facilitate the dissociation of chemisorbed oxygen species on the sensing body and promotes favorable surficial interactions with C5H8. Therefore, it was anticipated that the Co–CeO2@SnO2 NF-based chemiresistive sensor would demonstrate a significant improvement in overall sensing performance compared to pristine SnO2 NFs as well as any other pre-existing sensing materials. Additionally, Fe–CeO2 and Ni–CeO2 catalysts were functionalized onto SnO2 as sensitizers and sensor measurements were conducted (Figure S25). We confirmed that using Fe–CeO2 and Ni–CeO2 as sensitizers also positively affects sensitivity enhancement compared to pristine SnO2 NFs and CeO2@SnO2 NFs. However, the performance improvement was most pronounced when using Co–CeO2 catalysts. Therefore, we chose to utilize the combination of Co–CeO2, which offers the most superior sensing performance, albeit at a higher cost compared to other transition metals such as Fe and Ni. Nevertheless, the use of Co-doped CeO2 remains still advantageous in terms of cost compared to noble metal elements (Pt, Pd, Ag, Au) (Table S5). Based on the aforementioned sensitizing mechanisms of the Co–CeO2 NPs on SnO2 NFs, it is important to emphasize that our transition metal-doped CeO2 NPs offer an efficient approach for designing high-performance chemiresistive sensors without noble catalysts.
Conclusion
In the development of metal oxide-based chemiresistive gas sensors, incorporating catalytic sensitizers has traditionally relied on noble metal catalysts to enhance reactivity between the target gas and the sensing layer. While noble metals offer high catalytic activity, oxidation resistance, and corrosion resistance, their scarcity drives up sensor costs, and their susceptibility to agglomeration and poisoning effects can degrade sensing performance. To address these challenges, we propose an alternative sensitizer design using transition metal Co-doped ceria (Co–CeO2) catalysts. Doping CeO2 with Co enhances the spillover effect and increases oxygen adsorption, thereby accelerating the diffusion of oxygen species into the sensing layer. These catalysts were effectively integrated onto nanostructured SnO2 NFs using electrospinning (Co–CeO2@SnO2 NFs). The Co–CeO2@SnO2 NF-based sensor demonstrates low detection limits (100 ppb), rapid sensing speed (5 s), and long-term stability over repeated sensing cycles for up to 550 min, indicating comprehensive improvement across all sensor performance metrics. Thus, this study presents a robust and rational design for chemiresistive sensors that effectively detect C5H8 through cost-effective sensitization, offering viable alternatives to noble metal catalysts. This achievement is expected to establish a foundational paradigm for future sensitizer designs, significantly advancing the field of high-performing gas sensors.
Experimental Section
Materials
Tin chloride dihydrate (SnCl2··2H2O, 98%), N,N-dimethylformamide (DMF, 99.8%), acetic acid (99.7%), and polyvinylpyrrolidone (PVP, Mw = 1,300,000), and chloroplatinic acid hexahydrate (H2PtCl6·6H2O) were purchased from Sigma-Aldrich. Ethanol (99.5%), ethylene glycol (99.5%), ammonium hydroxide (30%), and hydrogen peroxide (34.5%) were obtained from Samchun. Cerium nitrate hexahydrate (Ce(NO3)3·6H2O, 99.95%) was purchased from GFS Chemicals. Cobalt nitrate hexahydrate (Co(NO3)2·6H2O, 99.9%) was received from Junsei. All chemicals were utilized as received without undergoing additional purification processes.
Preparation of CeO2 NPs
26.04 g of cerium nitrate hexahydrate was dissolved in a mixture of 60 mL of ethylene glycol and 240 mL of ethanol. The solution was stirred with a magnetic bar at 300 rpm for 30 min. Then, 1 mL of hydrogen peroxide was added to the mixed cerium precursor solution and stirred at 150 rpm for 20 min. Ammonium hydroxide was added to the solution to adjust the pH to 8.0–9.0. Then, the reaction temperature increased to 50 °C, and air was bubbled into the solution. The reaction was carried out for 24 h. As a result of this process, a yellow precipitate was formed. The solutions were washed with ethanol and centrifuged at 5000 rpm three times, each time for 10 min. Then, 8 mL of distilled water was added to the solution and mixed using a shaker for 5 min. After the mixing, a yellowish transparent solution was obtained.
Preparation of Co–CeO2 NPs
Co–CeO2 NPs were synthesized using the same method as the fabrication of CeO2 NPs. Initially, cerium nitrate hexahydrate was dissolved in the mixture of solvents. In addition, the Co ion precursor was prepared in an amount that corresponds to the desired doping mol % concentrations relative to cerium (1, 3, 5, and 7%). Subsequently, a controlled amount of the Co ion precursor was injected into the reaction mixture using a micropipette. Afterward, the resulting solution was subjected to the same procedure described earlier for CeO2 synthesis. Inductively coupled plasma optical emission spectroscopy (ICP-OES) was used to measure the Co content on Co–CeO2 quantitatively.
Preparation of SnO2 NFs, CeO2@SnO2 NFs, Co–CeO2@SnO2 NFs, and Pt@SnO2 NFs
SnO2 NFs were produced using electrospinning, with an initial solution formulated by dissolving 1.0 g of tin chloride dihydrate in 8 mL of DMF solvent. Next, 1.4 g of PVP polymer was added to the composite solution and stirred for 24 h. The resultant precursor solution was subsequently loaded into 20 mL plastic syringes and electrospun using a pump operating at a feeding rate of 5 μL min–1, with a voltage of 16 kV, while maintaining a distance of 10 cm between the syringe nozzle tip and the drum collector. Following electrospinning, the as-spun composite fiber mats consisting of Sn precursor and PVP were calcined at 600 °C for 1 h, with a heating rate of 5 °C min–1, in an ambient atmosphere to obtain crystalline SnO2 NFs. For CeO2-decorated SnO2 NFs (CeO2@SnO2 NFs) and Co–CeO2-decorated SnO2 NFs (Co–CeO2@SnO2 NFs), different amounts of CeO2 NPs and Co–CeO2 NPs, respectively, were incorporated into the electrospinning solution containing the Sn precursor and PVP matrix polymer. For Pt catalyst-loaded SnO2 NFs, a 0.2 M aqueous solution containing the Pt ion precursor was prepared, and a controlled amount of this solution was mixed with the electrospinning solution. The fabrication of CeO2-decorated SnO2 NFs and Co–CeO2-decorated SnO2 NFs, and Pt catalyst-loaded SnO2 NFs followed the same experimental procedures for electrospinning and calcination steps without further modifications.
Fabrication of Gas Sensor and Evaluation of Sensing Performance
Gas sensors were fabricated on an alumina (Al2O3) substrate to measure the resistance variation of the prepared materials upon exposure to air and target analytes. Interdigitated Au electrodes with a width of 25 μm and a distance of 70 μm, along with a Pt heating wire on the other side, were printed on the alumina support, which had dimensions of 2.5 × 2.5 mm and a thickness of 0.2 μm. Next, 10 mg of the prepared materials were dispersed in 500 μL of ethanol, and the dispersed solutions were drop-coated onto the front side of the substrate. The resistance of the prepared gas sensors was evaluated using a custom-made gas sensor testing system. The system included a 16-channel multiplexer (34972A, Agilent), solenoid valves, and mass flow controllers (MFC). Before the gas was introduced, all the sensors were equilibrated under ambient conditions for 5 h. The exposure to gases followed a 10 min on/off interval. The humidity of the reference gas was controlled by redirecting a fraction of the gas stream into a water bubbler maintained at 37 °C. Subsequently, this humidified gas was mixed with the balance gas using the MFC. The operation temperature was controlled by adjusting the direct current (DC) voltage applied to the Pt heater using a DC power supply (E3654A, Agilent). The response and recovery times are characterized as the duration required for the resistance to attain 90% of the ultimate response ((R – Rmin)/Rmax ≥ 0.9) and the duration for the resistance to return to 10% of the final response ((R – Rmin)/Rmax ≤ 0.1), respectively. The collected data were processed by calculating the resistance ratio of Rair/Rgas, where Rair represents the baseline resistance in ambient air and Rgas corresponds to the resistance during gas injection. This ratio serves as the gas response of the sensors, indicating their sensitivity to the target gas.
Characterization
Ultraviolet–visible (UV–vis) spectra were obtained using a Lambda 1050 (PerkinElmer). The crystal structures of the SnO2, CeO2-decorated SnO2, and Co–CeO2-decorated SnO2 were confirmed using a high-resolution powder X-ray diffractometer (XRD) (RIGAKU, SmartLab) equipped with Cu Kα radiation (λ = 1.5418 Å). The average crystallite size of CeO2, Co–CeO2, SnO2, CeO2-decorated SnO2, and Co–CeO2-decorated SnO2 was calculated using the Scherrer equation, which is defined as follows: d = 0.9λ/βcos θ, where d is the average crystallite size, λ is the wavelength of the incident beam (0.154 nm), θ is the Bragg angle, and β is the line broadening factor at half of the maximum intensity (fwhm) in radians. The morphologies of the samples were characterized using a scanning electron microscope (SEM, SU5000, Hitachi), while the microstructures were investigated using a 300 keV field emission transmission electron microscope (TEM) (F30 supertwin, Tecnai). Scanning transmission electron microscopy (STEM) and energy dispersive spectroscopy (EDS) elemental mapping analysis were conducted with aberration-corrected TEM (JEMARM200F, JEOL). X-ray photoelectron spectroscopy (XPS) (Axis-Supra, Kratos) with Al K radiation was employed to investigate the chemical bonding states. Ultraviolet photoelectron spectroscopy (UPS) measurements were performed using an AXIS-Supra with a He I discharge lamp emitting photons with an energy of 21.2 eV. The doping amount of Co compared to Ce was measured using an Agilent ICP-OES 720 instrument. The Brunauer–Emmett–Teller (BET) surface area with pore size distribution was measured using N2 adsorption/desorption isotherms used by a Tristar 3020 instrument from Micromeritics. O2 temperature-programmed desorption (O2-TPD) experiments were performed using AutoChem II to quantitatively evaluate the amount of desorbed oxygen. In each experiment, 100 mg of samples was positioned on a quartz bed and subjected to pretreatment with air at room temperature. The temperature was then gradually increased up to 500 °C at a ramping rate of 10 °C min–1. Electron paramagnetic resonance (EPR) measurements were conducted at room temperature utilizing a JES-FA100 instrument, maintaining consistent conditions such as a microwave frequency of 9.21 GHz, microwave power of 1 mW, and modulation frequency of 100 kHz. The g-factor was derived by computation using the equation hν = gβH, wherein h denotes Planck’s constant, H represents the applied magnetic field, and β stands for Bohr’s magneton.
DFT Calculations
DFT calculations were performed using Vienna Ab initio Simulation Program (VASP) software version 5.4.1.61,62 The Perdew–Burke–Ernzerhof (PBE) exchange–correlation functional and projector-augmented wave (PAW) pseudopotentials were utilized in the calculations.63 To account for van der Waals interactions, the PBE-D3 method of Grimme was used, and dipole corrections were implemented to alleviate artificial potential gradients near periodic boundaries.64 The bulk structure of CeO2 was obtained from the Materials Project database.65 The CeO2 surface was cleaved to expose the (111) facet,66 and a 9 atomic layers model with a 20 Å vacuum gap perpendicular to the surface was prepared, consisting of 27 Ce atoms and 54 O atoms. The Co–CeO2 surface was obtained by substituting one Co atom in the CeO2 surface, resulting in a structure comprising 1 Co atom, 26 Ce atoms, and 54 O atoms. During the optimization process, the bottom 6 atomic layers were kept fixed. The convergence criteria for electronic relaxation and ionic relaxation were established at 1 × 10–5 eV and 0.01 eV/Å, respectively. The cutoff energy of the plane wave basis was set to 480 eV, and a 3 × 3 × 1 Monkhorst–Pack k-point sampling was applied for the Brillouin zone. The binding energy (Ebinding) can be calculated using the following equation:
where  , Esurface,
and
, Esurface,
and 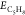 represent the
energies of the surface with
C5H8, the surface without C5H8, and a molecule of C5H8, respectively.
The charge density difference plots were obtained using the following
equation:
represent the
energies of the surface with
C5H8, the surface without C5H8, and a molecule of C5H8, respectively.
The charge density difference plots were obtained using the following
equation:
Acknowledgments
This work was supported by the Korea Technology and Information Promotion Agency for SMEs (Grant No. 00141845). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government, Ministry of Science and ICT (Development of Nanofiber Yarn Based Compound Sensor as a Comprehensive Wearable Healthcare Solution) (Grant No. RS-2024-00357296). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2023- 00236572).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.4c03168.
Additional characterization data (SEM, XRD, XPS, TEM, STEM, EDS mapping, N2 sorption isotherms, BJH pore size measurement, UPS) for the materials developed, particle size distribution of Co–CeO2 NPs, transmittance spectra of CeO2, Co–CeO2 NPs, the average crystallite size of the materials developed calculated by the Scherrer equation, schematic illustrations of the gas sensor measurement system, data for control experiments (Fe–CeO2@SnO2 NFs, Ni–CeO2@SnO2 NFs, Pt@SnO2 NFs), DFT-optimized binding configurations of CeO2 and Co–CeO2 toward the C5H8 molecule, detailed theoretical values from the DFT calculation, comparison of the performances of sensing properties of the state-of-the-art C5H8 sensors compared with that of the Co–CeO2@SnO2 NF-based sensor reported in the literature, the price comparison table: price of metal per kilogram in USD as of April 16, 2024, a supplementary note detailing the preparation of Co–CeO2 NPs (PDF)
Author Contributions
J.W.B. designed the project, including the experiments with characterization, and wrote the manuscript. S.H. and J.K. conducted the computational analyses. J.A. and C.P. contributed to writing the manuscript and conducting formal analysis. S.E.L. and H.J.P. contributed to material synthesis. J.S.N., Y.H.K., and J.-S.J. contributed to the formal analysis. E.S. and M.K. contributed to formal analysis and gas sensing tests. I.-D.K. supervised this work and revised the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Gas Sensor Market Size, Share and Growth Report, 2030 (Grand View Research), https://www.grandviewresearch.com/industry-analysis/gas-sensors-market. accessed 2023 August 28.
- Das S.; Jayaraman V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. 10.1016/j.pmatsci.2014.06.003. [DOI] [Google Scholar]
- Barsan N.; Weimar U. Conduction model of metal oxide gas sensors. J. Electroceramics 2001, 7, 143–167. 10.1023/A:1014405811371. [DOI] [Google Scholar]
- Kim S.-J.; Choi S.-J.; Jang J.-S.; Kim N.-H.; Hakim M.; Tuller H. L.; Kim I.-D. Mesoporous WO3 nanofibers with protein-templated nanoscale catalysts for detection of trace biomarkers in exhaled breath. ACS Nano 2016, 10, 5891–5899. 10.1021/acsnano.6b01196. [DOI] [PubMed] [Google Scholar]
- Kim D.-H.; Cha J.-H.; Shim G.; Kim Y. H.; Jang J.-S.; Shin H.; Ahn J.; Choi S.-Y.; Kim I.-D. Flash-thermochemical engineering of phase and surface activity on metal oxides. Chem 2022, 8, 1014–1033. 10.1016/j.chempr.2021.12.003. [DOI] [Google Scholar]
- Zhu L.-Y.; Ou L.-X.; Mao L.-W.; Wu X.-Y.; Liu Y.-P.; Lu H.-L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano-Micro Lett. 2023, 15, 89. 10.1007/s40820-023-01047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal., B 2010, 100, 403–412. 10.1016/j.apcatb.2010.08.023. [DOI] [Google Scholar]
- Hansen T. W.; DeLariva A. T.; Challa S. R.; Datye A. K. Sintering of catalytic nanoparticles: particle migration or Ostwald ripening?. Acc. Chem. Res. 2013, 46, 1720–1730. 10.1021/ar3002427. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Zhu Y.; Lin Q.; Zhang L.; Zhang X.; Wang H. Noble-metal single-atoms in thermocatalysis, electrocatalysis, and photocatalysis. Energy Environ. Sci. 2021, 14, 2954–3009. 10.1039/D1EE00247C. [DOI] [Google Scholar]
- Korotcenkov G.; Cho B. Instability of metal oxide-based conductometric gas sensors and approaches to stability improvement (short survey). Sens. Actuators, B 2011, 156, 527–538. 10.1016/j.snb.2011.02.024. [DOI] [Google Scholar]
- Azimi G.; Dhiman R.; Kwon H.-M.; Paxson A. T.; Varanasi K. K. Hydrophobicity of rare-earth oxide ceramics. Nat. Mater. 2013, 12, 315–320. 10.1038/nmat3545. [DOI] [PubMed] [Google Scholar]
- Branco J. B.; Brito P. E.; Ferreira A. C. Methanation of CO2 over nickel-lanthanide bimetallic oxides supported on silica. Chem. Eng. J. 2020, 380, 122465. 10.1016/j.cej.2019.122465. [DOI] [Google Scholar]
- Shi X.; Cao B.; Liu J.; Zhang J.; Du Y. Rare-Earth-Based Metal–Organic Frameworks as Multifunctional Platforms for Catalytic Conversion. Small 2021, 17 (22), 2005371. 10.1002/smll.202005371. [DOI] [PubMed] [Google Scholar]
- Montini T.; Melchionna M.; Monai M.; Fornasiero P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. 10.1021/acs.chemrev.5b00603. [DOI] [PubMed] [Google Scholar]
- Su Z.; Yang W.; Wang C.; Xiong S.; Cao X.; Peng Y.; Si W.; Weng Y.; Xue M.; Li J. Roles of oxygen vacancies in the bulk and surface of CeO2 for toluene catalytic combustion. Environ. Sci. Technol. 2020, 54, 12684–12692. 10.1021/acs.est.0c03981. [DOI] [PubMed] [Google Scholar]
- Jasinski P.; Suzuki T.; Anderson H. U. Nanocrystalline undoped ceria oxygen sensor. Sens. Actuators, B 2003, 95, 73–77. 10.1016/S0925-4005(03)00407-6. [DOI] [Google Scholar]
- Yuan W.; Zhang H.; Liu Q.; Li G.; Gao X. Surface modification of Li(Li0.17Ni0.2Co0. 05Mn0.58)O2 with CeO2 as cathode material for Li-ion batteries. Electrochim. Acta 2014, 135, 199–207. 10.1016/j.electacta.2014.04.181. [DOI] [Google Scholar]
- Elias J. S.; Risch M.; Giordano L.; Mansour A. N.; Shao-Horn Y. Structure, bonding, and catalytic activity of monodisperse, transition-metal-substituted CeO2 nanoparticles. J. Am. Chem. Soc. 2014, 136, 17193–17200. 10.1021/ja509214d. [DOI] [PubMed] [Google Scholar]
- Parastaev A.; Muravev V.; van Hoof A. J.; Kimpel T. F.; Kosinov N.; Hensen E. J. Boosting CO2 hydrogenation via size-dependent metal–support interactions in cobalt/ceria-based catalysts. Nat. Catal. 2020, 3, 526–533. 10.1038/s41929-020-0459-4. [DOI] [Google Scholar]
- Kong J.; Xiang Z.; Li G.; An T. Introduce oxygen vacancies into CeO2 catalyst for enhanced coke resistance during photothermocatalytic oxidation of typical VOCs. Appl. Catal., B 2020, 269, 118755. 10.1016/j.apcatb.2020.118755. [DOI] [Google Scholar]
- Koo W.-T.; Choi S.-J.; Kim S.-J.; Jang J.-S.; Tuller H. L.; Kim I.-D. Heterogeneous sensitization of metal–organic framework driven metal@ metal oxide complex catalysts on an oxide nanofiber scaffold toward superior gas sensors. J. Am. Chem. Soc. 2016, 138, 13431–13437. 10.1021/jacs.6b09167. [DOI] [PubMed] [Google Scholar]
- Baek J. W.; Kim Y. H.; Ahn J.; Kim D.-H.; Shin H.; Ko J.; Park S.; Park C.; Shin E.; Jang J.-S.; Kim I.-D. Galvanic replacement reaction in perovskite oxide for superior chemiresistors. J. Mater. Chem. A 2022, 10 (43), 23282–23293. 10.1039/D2TA05338A. [DOI] [Google Scholar]
- Shin H.; Kim D.-H.; Jung W.; Jang J.-S.; Kim Y. H.; Lee Y.; Chang K.; Lee J.; Park J.; Namkoong K.; Kim I.-D. Surface activity-tuned metal oxide chemiresistor: toward direct and quantitative halitosis diagnosis. ACS Nano 2021, 15 (9), 14207–14217. 10.1021/acsnano.1c01350. [DOI] [PubMed] [Google Scholar]
- Kang J.-Y.; Jang J.-S.; Koo W.-T.; Seo J.; Choi Y.; Kim M.-H.; Kim D.-H.; Cho H.-J.; Jung W.; Kim I.-D. Perovskite La0.75Sr0.25Cr0.5Mn0.5O3−δ sensitized SnO2 fiber-in-tube scaffold: Highly selective and sensitive formaldehyde sensing. J. Mater. Chem. A 2018, 6, 10543–10551. 10.1039/C8TA02725K. [DOI] [Google Scholar]
- Kim D.-H.; Kim J. K.; Oh D.; Park S.; Kim Y. B.; Ko J.; Jung W.; Kim I.-D. Ex-Solution Hybrids Functionalized on Oxide Nanofibers for Highly Active and Durable Catalytic Materials. ACS Nano 2023, 17, 5842–5851. 10.1021/acsnano.2c12580. [DOI] [PubMed] [Google Scholar]
- Baek J. W.; Kim I.-D. Advances in Perovskite Oxides for Chemiresistive Sensors. Acc. Mater. Res. 2023, 4, 1108–1120. 10.1021/accountsmr.3c00206. [DOI] [Google Scholar]
- Zhou K.; Yang Z.; Yang S. Highly reducible CeO2 nanotubes. Chem. Mater. 2007, 19, 1215–1217. 10.1021/cm062886x. [DOI] [Google Scholar]
- Tok A. I. Y.; Boey F. Y. C.; Dong Z.; Sun X. Hydrothermal synthesis of CeO2 nano-particles. J. Mater. Process. Technol. 2007, 190, 217–222. 10.1016/j.jmatprotec.2007.02.042. [DOI] [Google Scholar]
- Mai H.-X.; Sun L.-D.; Zhang Y.-W.; Si R.; Feng W.; Zhang H.-P.; Liu H.-C.; Yan C.-H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. 10.1021/jp055584b. [DOI] [PubMed] [Google Scholar]
- Sahoo T. R.; Armandi M.; Arletti R.; Piumetti M.; Bensaid S.; Manzoli M.; Panda S. R.; Bonelli B. Pure and Fe-doped CeO2 nanoparticles obtained by microwave assisted combustion synthesis: Physico-chemical properties ruling their catalytic activity towards CO oxidation and soot combustion. Appl. Catal., B 2017, 211, 31–45. 10.1016/j.apcatb.2017.04.032. [DOI] [Google Scholar]
- Kang W.; Ozgur D. O.; Varma A. Solution combustion synthesis of high surface area CeO2 nanopowders for catalytic applications: reaction mechanism and properties. ACS Appl. Nano Mater. 2018, 1, 675–685. 10.1021/acsanm.7b00154. [DOI] [Google Scholar]
- Yang W.; Li C.; Wang H.; Li X.; Zhang W.; Li H. Cobalt doped ceria for abundant storage of surface active oxygen and efficient elemental mercury oxidation in coal combustion flue gas. Appl. Catal., B 2018, 239, 233–244. 10.1016/j.apcatb.2018.08.014. [DOI] [Google Scholar]
- Basina G.; Polychronopoulou K.; Zedan A. F.; Dimos K.; Katsiotis M. S.; Fotopoulos A. P.; Ismail I.; Tzitzios V. Ultrasmall metal-doped CeO2 nanoparticles for low-temperature CO oxidation. ACS Appl. Nano Mater. 2020, 3, 10805–10813. 10.1021/acsanm.0c02090. [DOI] [Google Scholar]
- Carvalho J.; Rocha L.; Renzetti R.; Procopio A.; Mastelaro V. R.; Simões A.; Ponce M.; Macchi C.; Somoza A.; Aldao C.; Longo E.; et al. High-performance CeO2: Co nanostructures for the elimination of accidental poisoning caused by CO intoxication. Open Ceram. 2022, 12, 100298. 10.1016/j.oceram.2022.100298. [DOI] [Google Scholar]
- Tiwari A.; Bhosle V. M.; Ramachandran S.; Sudhakar N.; Narayan J.; Budak S.; Gupta A. Ferromagnetism in Co doped CeO2: Observation of a giant magnetic moment with a high Curie temperature. Appl. Phys. Lett. 2006, 88, 142511. 10.1063/1.2193431. [DOI] [Google Scholar]
- Mohanapriya S.; Priyadharshini P.; Shobika P.; Ponnar M.; Pushpanathan K. Effect of Co dopant on structural, optical, and magnetic properties of CeO2 quantum dots. J. Aust. Ceram. Soc. 2023, 59, 459–480. 10.1007/s41779-023-00834-6. [DOI] [Google Scholar]
- Al-Hashem M.; Akbar S.; Morris P. Role of oxygen vacancies in nanostructured metal-oxide gas sensors: a review. Sens. Actuators, B 2019, 301, 126845. 10.1016/j.snb.2019.126845. [DOI] [Google Scholar]
- Kutin Y.; Cox N.; Lubitz W.; Schnegg A.; Rüdiger O. In situ EPR characterization of a cobalt oxide water oxidation catalyst at neutral pH. Catalysts 2019, 9, 926. 10.3390/catal9110926. [DOI] [Google Scholar]
- Khan M. E.; Khan M. M.; Cho M. H. Ce3+-ionSurface Oxygen Vacancy, and Visible Light-induced Photocatalytic Dye Degradation and Photocapacitive Performance of CeO2-Graphene Nanostructures. Sci. Rep. 2017, 7 (1), 5928. 10.1038/s41598-017-06139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Wang Y.; Lu X.; Guo K.; Xu C. Increased Oxygen Vacancies in CeO2 for Improved Electrocatalytic Nitrogen Reduction Performance. Inorg. Chem. 2022, 61, 17242–17247. 10.1021/acs.inorgchem.2c02834. [DOI] [PubMed] [Google Scholar]
- Zimou J.; Nouneh K.; Hsissou R.; El-Habib A.; El Gana L.; Talbi A.; Beraich M.; Lotfi N.; Addou M. Structural, morphological, optical, and electrochemical properties of Co-doped CeO2 thin films. Mater. Sci. Semicond. 2021, 135, 106049. 10.1016/j.mssp.2021.106049. [DOI] [Google Scholar]
- Venkataswamy P.; Jampaiah D.; Kandjani A. E.; Sabri Y. M.; Reddy B. M.; Vithal M. Transition (Mn, Fe) and rare earth (La, Pr) metal doped ceria solid solutions for high performance photocatalysis: Effect of metal doping on catalytic activity. Res. Chem. Intermed. 2018, 44, 2523–2543. 10.1007/s11164-017-3244-5. [DOI] [Google Scholar]
- Slavinskaya E.; Zadesenets A.; Stonkus O.; Stadnichenko A.; Shchukarev A.; Shubin Y. V.; Korenev S.; Boronin A. Thermal activation of Pd/CeO2-SnO2 catalysts for low-temperature CO oxidation. Appl. Catal., B 2020, 277, 119275. 10.1016/j.apcatb.2020.119275. [DOI] [Google Scholar]
- Ahn H.-J.; Choi H.-C.; Park K.-W.; Kim S.-B.; Sung Y.-E. Investigation of the structural and electrochemical properties of size-controlled SnO2 nanoparticles. J. Phys. Chem. B 2004, 108, 9815–9820. 10.1021/jp035769n. [DOI] [Google Scholar]
- Wang D.; Wan K.; Zhang M.; Li H.; Wang P.; Wang X.; Yang J. Constructing hierarchical SnO2 nanofiber/nanosheets for efficient formaldehyde detection. Sens. Actuators, B 2019, 283, 714–723. 10.1016/j.snb.2018.11.125. [DOI] [Google Scholar]
- Korotcenkov G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R Rep. 2008, 61, 1–39. 10.1016/j.mser.2008.02.001. [DOI] [Google Scholar]
- Hori A.; Suijo K.; Kondo T.; Hotta N. Breath isoprene excretion during rest and low-intensity cycling exercise is associated with skeletal muscle mass in healthy human subjects. J. Breath Res. 2021, 15, 016009. 10.1088/1752-7163/abbf39. [DOI] [PubMed] [Google Scholar]
- Karl T.; Prazeller P.; Mayr D.; Jordan A.; Rieder J.; Fall R.; Lindinger W. Human breath isoprene and its relation to blood cholesterol levels: new measurements and modeling. J. Appl. Physiol. 2001, 91, 762–770. 10.1152/jappl.2001.91.2.762. [DOI] [PubMed] [Google Scholar]
- Gurlo A.; Riedel R. In situ and operando spectroscopy for assessing mechanisms of gas sensing. Angew. Chem., Int. Ed. 2007, 46, 3826–3848. 10.1002/anie.200602597. [DOI] [PubMed] [Google Scholar]
- Han B.; Wang J.; Yang W.; Chen X.; Wang H.; Chen J.; Zhang C.; Sun J.; Wei X. Hydrothermal synthesis of flower-like In2O3 as a chemiresistive isoprene sensor for breath analysis. Sens. Actuators, B 2020, 309, 127788. 10.1016/j.snb.2020.127788. [DOI] [Google Scholar]
- Wu X.; Wang H.; Wang J.; Chen J.; Shi L.; Han B.; Tian X. Hydrothermal synthesis of flower-like Cr2O3-doped In2O3 nanorods clusters for ultra-low isoprene detection. Colloids Surf. A: physicochem. Eng. Asp. 2021, 620, 126606. 10.1016/j.colsurfa.2021.126606. [DOI] [Google Scholar]
- Han B.; Wang H.; Yang W.; Wang J.; Wei X. Hierarchical Pt-decorated In2O3 microspheres with highly enhanced isoprene sensing properties. Ceram. Int. 2021, 47, 9477–9485. 10.1016/j.ceramint.2020.12.081. [DOI] [Google Scholar]
- Park Y.; Yoo R.; Ryull Park S.; Lee J. H.; Jung H.; Lee H.-S.; Lee W. Highly sensitive and selective isoprene sensing performance of ZnO quantum dots for a breath analyzer. Sens. Actuators, B 2019, 290, 258–266. 10.1016/j.snb.2019.03.118. [DOI] [Google Scholar]
- van den Broek J.; Guntner A. T.; Pratsinis S. E. Highly selective and rapid breath isoprene sensing enabled by activated alumina filter. ACS Sens 2018, 3, 677–683. 10.1021/acssensors.7b00976. [DOI] [PubMed] [Google Scholar]
- Güntner A. T.; Pineau N. J.; Chie D.; Krumeich F.; Pratsinis S. E. Selective sensing of isoprene by Ti-doped ZnO for breath diagnostics. J. Mater. Chem. B 2016, 4, 5358–5366. 10.1039/C6TB01335J. [DOI] [PubMed] [Google Scholar]
- Teleki A.; Pratsinis S. E.; Kalyanasundaram K.; Gouma P. Sensing of organic vapors by flame-made TiO2 nanoparticles. Sens. Actuators, B 2006, 119, 683–690. 10.1016/j.snb.2006.01.027. [DOI] [Google Scholar]
- Xing X.; Du L.; Wang C.; Feng D.; Liu G.; Yang D. ZIF-8 micro-polyhedron MOF-transformed ZnO/ZnFe2O4 nanosheets for highly selective detection of ppb-level isoprene. Sens. Actuators, B 2022, 372, 132669. 10.1016/j.snb.2022.132669. [DOI] [Google Scholar]
- Guo X. H.; Mao C. C.; Zhang J.; Huang J.; Wang W. N.; Deng Y. H.; Wang Y. Y.; Cao Y.; Huang W. X.; Yu S. H. Cobalt-Doping-Induced Synthesis of Ceria Nanodisks and Their Significantly Enhanced Catalytic Activity. Small 2012, 8, 1515. 10.1002/smll.201102179. [DOI] [PubMed] [Google Scholar]
- Gurlo A. Interplay between O2 and SnO2: oxygen ionosorption and spectroscopic evidence for adsorbed oxygen. ChemPhyschem 2006, 7, 2041–2052. 10.1002/cphc.200600292. [DOI] [PubMed] [Google Scholar]
- Sopiha K. V.; Malyi O. I.; Persson C.; Wu P. Chemistry of oxygen ionosorption on SnO2 surfaces. ACS Appl. Mater. Interfaces 2021, 13, 33664–33676. 10.1021/acsami.1c08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresse G.; Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. 10.1016/0927-0256(96)00008-0. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. 10.1103/PhysRevB.47.558. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. 10.1103/PhysRevB.59.1758. [DOI] [Google Scholar]
- Grimme S.; Antony J.; Ehrlich S.; Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- Jain A.; Ong S. P.; Hautier G.; Chen W.; Richards W. D.; Dacek S.; Cholia S.; Gunter D.; Skinner D.; Ceder G.; Persson K. A. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1 (1), 011002. 10.1063/1.4812323. [DOI] [Google Scholar]
- Conesa J. Computer modeling of surfaces and defects on cerium dioxide. Surf. Sci. 1995, 339, 337–352. 10.1016/0039-6028(95)00595-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







