Abstract
Studies on the replication of the pestivirus bovine viral diarrhea virus (BVDV) were considerably facilitated by the recent discovery of an autonomous subgenomic BVDV RNA replicon (DI9c). DI9c comprises mainly the untranslated regions of the viral genome and the coding region of the nonstructural proteins NS3, NS4A, NS4B, NS5A, and NS5B. To assess the significance of the NS3-associated nucleoside triphosphatase/helicase activity during RNA replication and to explore other functional features of NS3, we generated a repertoire of DI9c derivatives bearing in-frame mutations in different parts of the NS3 coding unit. Most alterations resulted in deficient replicons, several of which encoded an NS3 protein with an inhibited protease function. Three lesions permitted replication, though at a lower level than that of the wild-type RNA, i.e., replacement of the third position of the DEYH helicase motif II by either T or F and an insertion of four amino acid residues in the C-terminal part of NS3. While polyprotein proteolysis was found to be almost unaffected in these latter replicons, in vitro studies with the purified mutant NS3 proteins revealed a significantly impaired helicase activity for the motif II substitutions. NS3 with a DEFH motif, moreover, showed a significantly lower ATPase activity. In contrast, the C-terminal insertion had no negative impact on the ATPase/RNA helicase activity of NS3. All three mutations affected the synthesis of both replication products—negative-strand intermediate and progeny positive-strand RNA—in a symmetric manner. Unexpectedly, various attempts to rescue or enhance the replication capability of nonfunctional or less functional DI9c NS3 derivatives, respectively, by providing intact NS3 in trans failed. Our experimental data thus demonstrate that the diverse enzymatic activities of the NS3 protein—in particular the ATPase/RNA helicase—play a pivotal role even during early steps of the viral replication pathway. They may further indicate the C-terminal part of NS3 to be an important functional determinant of the RNA replication process.
The genus Pestivirus comprises three viruses, bovine viral diarrhea virus (BVDV), border disease virus, and classical swine fever virus, which represent important disease agents in their respective animal host species (reviewed in reference 45). Together with the flaviviruses and hepaciviruses—the latter term denotes the group of hepatitis C viruses (HCVs)—pestiviruses constitute the family Flaviviridae (11). The positive-strand RNA genome has a length of about 12.5 kb and consists of a single open reading frame (ORF), which is flanked by untranslated regions (UTRs) at the 5′ and 3′ ends. Translation of the ORF is mediated by an internal ribosomal entry site within the 5′ UTR and yields initially a polyprotein, which is co- and posttranslationally cleaved into different viral polypeptides (reviewed in references 36 and 45). The product order along the ORF has been determined as NH2 (Npro, C, Ems, E1, E2, p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B) COOH (36). Npro, a unique feature of pestiviruses, acts as an autoprotease, which releases itself from the precursor (51). Experimental evidence for almost all the structural proteins suggests that they arise from the polyprotein via proteolytic processing by signal peptidases (reviewed in references 36 and 45). A serine protease domain within the N terminus of NS3 catalyzes the majority of cleavages, generating the nonstructural proteins NS3 to NS5B (44, 52, 53).
Toward the understanding of the biochemical functions of pestivirus proteins, in vitro assay systems based on heterologously expressed proteins revealed additional enzymatic activities associated with homologous polypeptide counterparts in each of the three Flaviviridae genera. In accordance with previous predictions (24, 30), the HCV NS5B, the flavivirus NS5, and the pestivirus NS5B proteins were demonstrated to possess RNA-dependent RNA polymerase activity (2, 37, 41, 57). Along the same line, an RNA-stimulated nucleoside triphosphatase (NTPase) activity was established to reside within all Flaviviridae NS3 proteins (38, 40, 49; reviewed in reference 36). The NS3 proteins of BVDV and HCV were shown to exhibit a further predicted activity, i.e., to operate in vitro as an RNA helicase (21, 48). In contrast to the RNA-dependent RNA polymerase, which is expected to play a key role during the viral replication pathway, nothing is yet known about the implication of these latter functions of NS3 in vivo.
The replication strategy of pestiviruses follows a scheme in which many molecular details are so far undefined. Concomitant with the translation and/or proteolysis of the polyprotein, the diverse viral proteins and hypothetical host factors conceivably form ribonucleoprotein complexes with the viral RNA. Those initially catalyze the transcription of the genome into full-length complementary negative-strand copies, which then in turn act as templates for the synthesis of progeny positive-strand viral RNAs (3).
Along with the successful composition of stable cDNA copies of the genome of certain BVDV strains that are capable of generating infectious RNA transcripts in vitro (28, 29, 46), detailed investigations of the viral replication process have recently been initiated—not least to also work out an in vivo model for the related human pathogen HCV. In the course of these studies, a subgenomic BVDV RNA molecule (DI9c) comprising mainly the 5′ and 3′ UTRs and the coding region of NS3 to NS5B turned out to support both steps of the replication pathway upon transfection into host cells (3).
In the work presented here, we combined genetic approaches to the BVDV RNA replicon with in vitro studies on purified NS3 protein to shed light on the actual role of the different functions of this viral protein and its mode of action during RNA replication in vivo.
MATERIALS AND METHODS
Cells and viruses.
Cells, viruses, and culture conditions were described previously (3).
Construction of recombinant plasmids.
Restriction and cloning procedures were performed according to standard protocols. Restriction and modifying enzymes were purchased from Biolabs (Schwalbach, Germany), Pharmacia (Freiburg, Germany), MBI Fermentas (St. Leon-Rot, Germany), and Roche Diagnostics (Mannheim, Germany). Oligonucleotides that were used for primer-directed mutagenesis or for sequencing, the latter 5′ IRD71 labeled, were obtained from MWG Biotech (Eberbach, Germany) (Table 1).
TABLE 1.
Oligonucleotides used for primer-directed mutagenesis and RT-PCR
| Primer | Sequencea (5′→3′) | Nucleotide positions (BVDV CP7) |
|---|---|---|
| B24 | ccggccttcttcgacc | 5613–5629 |
| B26 | gatagaggagataggac | 5882–5898 |
| B33R | ccaacatgttacccttca | 6369–6352 |
| 3′-NS3Not(rev) | tgaatgcggccgctacagtcctaccacttgcttc | 5629–5613 |
| BV-LEYH | catatttcgtctcgagtatcactg | 6113–6136 |
| BV-LEYHrev | gtgatactcgagcagaaatatgtatg | 6134–6109 |
| BV-DETH | ggatgagacacactgtgctactcctg | 6122–6147 |
| BV-DETHrev | ggagtagcacagtgtgtctcatccag | 6145–6120 |
| BV-DEFH | ggatgagtttcactgcgcaactcctgagc | 6122–6150 |
| BV-DEFHrev | caggagttgcgcagtgaaactcatccag | 6147–6120 |
| BV-DEEH | tttctggatgaagagcactgcgctactc | 6117–6144 |
| BV-DEEHrev | gcgcagtgctcttcatccagaaatatg | 6139–6113 |
| BEMLU | acgcgt | |
| SACLU | gacgcgtcagct | |
| ECOLU | aattgacgcgtc | |
| AFLU | ttaacacgcgtg | |
| BALU | gatcggacgcgtc |
The mutated nucleotides are indicated by boldface italics. Novel restriction sites (XhoI, BV-LEYH and BV-LEYHrev; DraIII, BV-DETH and BV-DETHrev; FspI, BV-DEFH and BV-DEFHrev; SapI, BV-DEEH and BV-DEEHrev; and MluI, BEMLU, SACLU, ECOLU, AFLU, and BALU) are underlined.
The full-length BVDV CP7 cDNA clone pA/BVDV/N and the DI9c cDNA construct pA/BVDV/D9 were described previously (29). Substitutions within the DEYH box (corresponding to nucleotides 6123 to 6134 of the full-length BVDV CP7 genome [43]) were introduced into pA/BVDV/D9 by primer-directed mutagenesis. Initially, the 2.85-kb ApaI-SalI fragment of pA/BVDV/D9 was ligated into the corresponding restriction sites of the multicloning site (MCS) of the pBluescript II KS(+) vector (Stratagene, La Jolla, Calif.), yielding construct pAS. To create the LEYH mutation, two PCR fragments were generated with B24/BV-LEYHrev and BV-LEYH/3′-NS3Not as primers and pA/BVDV/D9 as template. Both PCR products were digested with XhoI and ligated, and the ligation product was cut with SacI and BlpI (corresponding to nucleotide positions 5865 and 6663). The resulting 798-bp fragment was introduced into pAS where the wild-type fragment had been previously removed. Finally, the ApaI-SalI fragment from pAS carrying the LEYH mutation was cloned into pA/BVDV/D9. Mutations DETH, DEFH, and DEEH were created by the same procedure with primers BV-DETHrev/BV-DETH, BV-DEFHrev/BV-DEFH, and BV-DEEHrev/BV-DEEH and the restriction enzymes DraIII, FspI, and SapI, respectively (Table 1).
Most NS3 insertion mutants were made by using oligonucleotides BEMLU, SACLU, ECOLU, and BALU, which contain palindromic sequences. Hybridization of oligonucleotides of the same species led to short DNA double-stranded molecules containing cohesive ends and an MluI restriction site. To create mutations RV1693, TRQL1834, and LTRQ1966 (names and numbering refer to the amino acid residues encoded by the insert and the codon of the BVDV CP7 ORF which precedes the insertion; see also Fig. 1), the BEMLU, SACLU, and ECOLU double strands were ligated into pAS that had been cut with HpaI, SacI, and EcoRI (positions 5444, 5865, and 6264), respectively. The 2.85-kb ApaI-SalI fragments including the respective mutations were cloned into pA/BVDV/D9. Insertion mutant NTRV2088 was originated by direct introduction of the AFLU double-stranded oligonucleotide into the AflII site (position 6630) of pA/BVDV/D9. To assemble DRRV2191 and DRRV2249, construct pES was initially generated by inserting the 1.47-kb EcoRI-SalI fragment (nucleotides 6264 to 7734) of pA/BVDV/D9 into the pBluescript II KS(+) MCS. pES was then digested with BclI (position 6941) and BglII (position 7115), and the double-stranded oligonucleotide BALU was introduced via these restriction sites. pES plasmids containing these insertions were cut with BlpI (position 6663) and SalI (position 7734), and the resulting 1.07-kb fragments were inserted into pA/BVDV/D9. Mutant H2099 was generated by digestion of pA/BVDV/D9 with BlpI (position 6663), blunting with T4 DNA polymerase, and religation. Deletion mutant ΔAB was created by cutting pA/BVDV/D9 with AflII (position 6630) and BlpI (position 6663). Blunting and religation resulted in the loss of codons 2090 to 2099 and replacement of lysine 2089 by asparagine. Successful introduction of each mutation was confirmed by dideoxy sequencing. The protease mutant pm1 has been described previously (3).
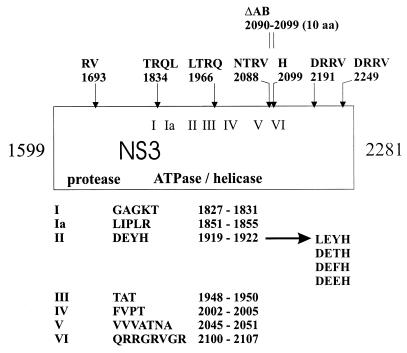
FIG. 1.
Mutagenesis of the BVDV DI9c NS3 coding unit. Mutations were introduced into the DI9c cDNA as described in Materials and Methods. In the schematic drawing of the NS3 protein (corresponding to amino acids 1599 to 2281 of the BVDV CP7 polyprotein [43]), the positions of the insertions are marked by arrows. Names and numbering of the insertions refer to the inserted amino acid residues and the preceding amino acid of the BVDV CP7 polyprotein, respectively. The positions of the 10 deleted amino acids of mutation ΔAB are indicated by vertical lines above. Roman numerals mark the seven conserved amino acid motifs of superfamily II helicases (15). Amino acid sequences and the corresponding BVDV CP7 polyprotein positions of the seven motifs as well as the diverse mutations affecting the NS3 DEYH box (motif II) are shown below. The protease domain comprises the initial approximately 200 amino acids of the NS3 protein (41a).
For in vitro transcription with T7 RNA polymerase (Stratagene), all pA/BVDV/D9 derivatives were linearized with SmaI.
Generation of the radiolabeled RNA probes for RNase protection was described previously (3). Detection of cotransfected pBluescript II KS(+) or unrelated RNA was achieved by transcription of complementary probes comprising 200 or 300 nucleotides, respectively. Removal of the SmaI-SacI fragment from the MCS of pBluescript II SK(+), blunting, and religation led to plasmid pRSK. For the helicase assay (see below), the template strand and the [α-32P]UTP-radiolabeled release strand were transcribed from pBluescript II KS(+) cut with SacI and from pRSK cut with SalI, respectively, by using T3 RNA polymerase.
To obtain a radiolabeled RNA probe (ΔPvu RNA) for the RNA binding assay (see below), plasmid pΔPvu (56) was linearized with SmaI and transcribed with T3 RNA polymerase.
To generate a Sindbis virus-derived RNA replicon encoding BVDV NS3 protein, a PCR product containing the entire NS3 coding unit (nucleotides 5163 to 7211) was cloned into the MCS of pSinRep5 (Invitrogen). The PCR primers contained artificial translation initiation and termination codons. The recombinant pSinRep5 was linearized with PacI and transcribed with SP6 RNA polymerase (Roche Diagnostics).
DNA sequencing.
Dideoxy sequencing of double-stranded DNA was carried out as described in reference 56.
In vitro transcription, purification, and transfection of RNA.
All procedures were essentially performed as described in references 3 and 56.
RT-PCR analysis.
Reverse transcriptase PCR (RT-PCR) with appropriate primers was performed to ensure the stability and identity of the transfected RNA replicon derivatives prior to transfection as well as posttransfection (p.t.), to discriminate RNA amplification of different replicons upon cotransfection (see Fig. 5), and to complement the monitoring of RNA replication by RNase protection. The protocol is described in reference 3.
FIG. 5.
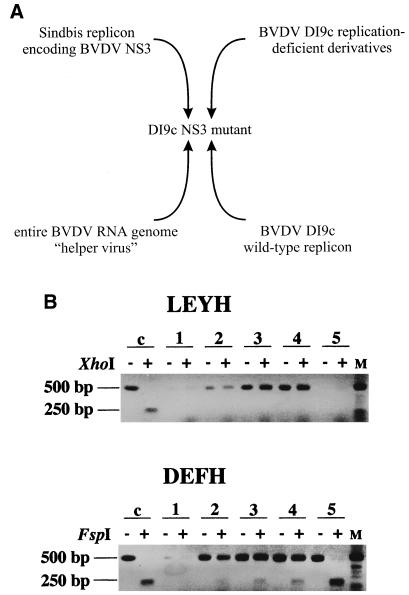
trans complementation experiments. (A) Schematic representation of cotransfection experiments that intended to complement the NS3 function in trans. To rescue or to enhance the replication ability of nonfunctional or less functional DI9c NS3 derivatives, respectively, various RNA molecules that expressed native NS3 were cotransfected together with the mutant DI9c replicon. For a symmetric supplementation of NS3, replication-deficient DI9c derivatives comprising lethal mutations in genetic units of the ORF other than the NS3 coding unit were cotransfected in a range of different concentrations, and analysis of replication was performed 48 h p.t. To provide NS3 in a unidirectional way, the entire BVDV CP7 genome as well as a Sindbis virus replicon (6) encoding BVDV NS3 was cotransfected with the DI9c NS3 derivatives at different concentrations (data not shown), and analysis was carried out at 48 or 14 h p.t., respectively. Moreover, DI9c NS3 derivatives were cotransfected with the wild-type DI9c replicon (see also panel B). (B) RT-PCR analysis of cotransfection experiments, aimed at the complementation of the ATPase/helicase function of NS3 in trans. DI9c LEYH (not replication capable) was cotransfected into BHK-21 cells together with the wild-type replicon with a threefold molar excess of the mutant RNA (upper panel). The same experiment was carried out with DI9c DEFH (replication capable; lower panel). The transfected cells were separated into four aliquots which were harvested 6, 12, 18, and 24 h p.t. To detect simultaneously amplification of both mutant and wild-type replicons, reverse transcription was carried out on the cytoplasmic RNA fractions with B33R (Table 1) as first-strand synthesis primer. In the case of RNA replication, a DNA fragment of about 500 bp was generated by subsequent PCR with primers B33R and B26 (Table 1). Discrimination between wild-type and mutant RNA-derived PCR products was made possible by restriction of the mutant-derived products via the introduced restriction site (LEYH, XhoI; DEFH, FspI), thus resulting in two fragments of about 250 bp. Lanes: c, positive control (PCR performed on pLEYH and pDEFH [top and bottom, respectively]); 1 to 4, RT-PCR products obtained 6, 12, 18, and 24 h, respectively, postcotransfection of mutant and wild-type replicons; 5, RT-PCR products obtained 24 h p.t. of DI9c derivatives LEYH and DEFH (top and bottom, respectively) without cotransfection of DI9c wild-type RNA; M, DNA length marker. Untreated or digested PCR products obtained after addition of the specific restriction endonuclease are indicated by minus or plus signs, respectively.
RNase protection assay.
The protocol to detect the RNA replication products was carried out as described in detail by Yu et al. (56). The procedure to monitor transfected pBluescript II KS(+) plasmid DNA was identical to the protocol used for viral or nonviral RNA detection, except that the Bluescript-specific radiolabeled probe was hybridized to an aliquot corresponding to 1/10 of a total cytoplasmic nucleic acid preparation of 7 × 106 cells. To quantify protected RNA species, the band intensities were determined with a Fuji Bio Imaging analyzer and the corresponding software, TINA 2.09f.
In vitro translation.
BHK-21 S10 extract and BHK-21 cell eukaryotic initiation factor (translation initiation factor) fractions were prepared according to the protocol of Barton and Flanegan (1). Translation of 1 μg of in vitro-transcribed and purified RNA was performed as previously described (1), by using 30% (vol/vol) BHK-21 S10 extract, 10% (vol/vol) BHK-21 cell eukaryotic initiation factors, 40 U of RNaseOut, and 10 to 15 μCi of [35S]methionine in a final volume of 50 μl. The reaction mixture was incubated for 14 h at 30°C. The 35S-labeled proteins were solubilized in standard sample buffer and analyzed by sodium dodecyl sulfate (SDS)–10% polyacrylamide gel electrophoresis (PAGE) (30:0.8) at a constant voltage of 110 V. Gels were fixed in 10% acetic acid–40% methanol, treated with Amplify reagent (Amersham), dried, and exposed to X-ray film (Kodak).
Immunoaffinity purification of BVDV NS3 proteins.
The monoclonal anti-BVDV NS3 antibody (10) was coupled to protein A-Sepharose beads (CL-4B; Sigma) via a rabbit anti-mouse immunoglobulin G (Fc)-specific antibody (Cappel) with dimethylpimelimidate as cross-linking agent. Transfected BHK-21 cells from three 100-mm-diameter dishes (corresponding to approximately 2 × 107 cells) were harvested at 24 h (mock; replicons DI9c wild type, DETH, and DEFH) or 48 h (replicon DI9c DRRV2191) p.t. Six hundred microliters of the cytoplasmic fraction containing 1 mM phenylmethylsulfonyl fluoride was supplemented with 1.2 ml of IBP 150 (20 mM Tris-HCl [pH 8.0], 150 mM KCl, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA) and incubated with 60 μl (bead volume) of immunoaffinity matrix. After incubation at 4°C overnight with continuous rotation, the matrix was washed and the NS3 proteins were eluted with 210 μl of 0.2 M ethanolamine (pH 11.2). The pH was adjusted to 7, and the elution mixture was dialyzed against 25 mM MOPS (morpholinepropanesulfonic acid)-KOH (pH 6.5)–10% glycerol. The dialyzed proteins were quantified by SDS-PAGE and by a protein assay kit (Bio-Rad) and stored at −70°C.
In vitro helicase assay.
The helicase substrate was generated according to the protocol of Warrener and Collett (48). Thirty to ninety nanograms of the immunopurified NS3 proteins was added to a mixture containing 25 mM MOPS-KOH (pH 6.5), 100 μg of bovine serum albumin per ml, 2 mM dithiothreitol (DTT), 3 mM MnCl2, 7.5 U of RNaseOut, and 1,200 to 3,000 cpm of radiolabeled substrate in a total volume of 30 μl. After 15 min of incubation at room temperature, ATP was added to a final concentration of 5 mM. Reaction mixtures were incubated for 30 min at 30°C and terminated by the addition of 5× RNA sample buffer (48). The mixture was electrophoretically separated on 12% polyacrylamide (30:0.8)–1× Tris-borate-EDTA–1% SDS gels at 18 mA of constant current. Gels were dried and exposed on X-ray film. The helicase activity was determined by quantification of the radiolabeled release-strand by using a Fuji Bio Imaging analyzer.
ATPase assay.
Thirty to ninety nanograms of immunopurified NS3 proteins was incubated for 15 min at 37°C with 40 mM MOPS-KOH (pH 6.5)–100 μg of bovine serum albumin per ml–2 mM DTT–2.5 mM MgCl2–5 U of RNaseOut–500 μM ATP–1 μCi of [α-32P]ATP (400 Ci/mmol; Amersham Pharmacia Biotech) with or without 400 μM poly(C) (Sigma) as nucleic acid cofactor in a final volume of 30 μl. The reaction was terminated by the addition of EDTA to a final concentration of 45 mM. [α-32P]ATP hydrolysis was analyzed by thin-layer chromatography as described by Tamura et al. (40) and quantified by using a Fuji Bio Imaging analyzer.
RNA binding assay.
Gel retardation reaction mixtures (30 μl) contained approximately 30 ng of immunopurified NS3 protein, 25 mM MOPS-KOH (pH 6.5), 2 mM DTT, 3 mM MnCl2, 7.5 U of RNaseOut, and 10,000 cpm of [32P]UTP-labeled ΔPvu RNA. After incubation for 30 min at 30°C, samples were adjusted to 8% glycerol, and 17-μl aliquots were electrophoresed in native 6% polyacrylamide (80:1)–0.5× Tris-borate-EDTA–10% glycerol gels at 15 mA of constant current. Labeled RNAs were visualized by autoradiography.
RESULTS
Mutagenesis of the NS3 protein coding unit of BVDV DI9c—effects on RNA replication in vivo.
In the poliovirus system, insertion, deletion, and substitution mutations which were introduced at various positions into the infectious cDNA provided valuable insights into the function of virus-encoded proteins and considerably complemented previous in vitro data on these factors (reviewed in reference 50). A similar experimental strategy was chosen for the BVDV DI9c system. To affect different portions of the NS3 protein, seven linker insertions encoding one to four additional amino acid residues and a deletion of 10 amino acids were introduced at or between unique restriction sites within the NS3 coding unit of the cDNA (see Materials and Methods) (Fig. 1). A second series of mutations was intended to specifically modify the NTPase/RNA helicase activity of NS3. For this purpose, single amino acid residues were substituted within the DEYH sequence (also known as Walker B motif or motif II [see reference (47)]), one of seven conserved motifs that are characteristic for RNA helicases of the DExH type (Fig. 1 and see below).
The respective DI9c derivatives were raised by transcription in vitro and introduced into BHK-21 cells via electroporation. RNA replication was measured at different time points p.t. by three established strategies: RNase protection and RT-PCR for direct monitoring of the in vivo-synthesized replication products and immunofluorescence (IF) analysis of the transfected cells to detect the replication-associated synthesis of NS3 protein via an anti-BVDV NS3 antibody. The results of these assays are summarized in Table 2; RNase protection, RT-PCR, and IF analysis yielded congruent results, irrespective of the time p.t. at which the analysis was performed (some of the data not shown). Accordingly, the deletion mutation and six of seven insertion mutations led to DI9c RNAs which did not replicate, i.e., neither synthesis of both the RNA replication products (Fig. 2A) nor that of NS3 protein could be detected. Likewise, two of the DEYH substitution mutants failed to produce detectable amounts of viral RNA and NS3. However, one insertion mutant and two of the DEYH substitutions turned out to support both replication steps, although to a considerably lower degree than the wild-type replicon (Fig. 2A).
TABLE 2.
Replication ability of BVDV DI9c NS3 mutantsa
| Mutant type and name | Replication abilityb |
|---|---|
| Protease, pm1 | − |
| DEYH box | |
| LEYH | − |
| DETH | + |
| DEFH | + |
| DEEH | − |
| Deletion, ΔAB | − |
| Insertion | |
| RV1693 | − |
| TRQL1834 | − |
| LTRQ1966 | − |
| NTRV2088 | − |
| H2099 | − |
| DRRV2191 | + |
| DRRV2249 | − |
Summary of all experimental results obtained by monitoring the replication ability of each of the diverse DI9c NS3 derivatives. RNase protection (shown in Fig. 2A), RT-PCR, and IF analysis yielded congruent results, independently of the time p.t. at which analysis was performed.
−, replication deficient; +, replication capable.
FIG. 2.
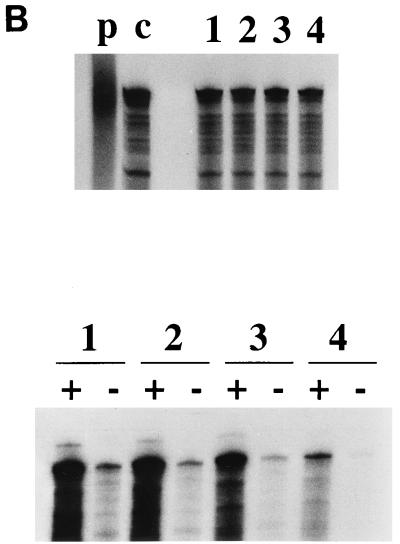
Replication ability of BVDV DI9c NS3 mutants. (A) Monitoring RNA replication by RNase protection. The diverse mutant replicons were transfected into BHK-21 cells, and RNase protection was performed as described in Materials and Methods at 24 h p.t. The autoradiogram shows the protected RNA fragments detected via application of a 32P-labeled antisense probe for detection of positive-strand RNA (+) and a 32P-labeled sense probe for detection of negative-strand RNA (−), which were analyzed on a 5% polyacrylamide–7 M urea gel. Lanes: as and s, input antisense and sense probe, respectively; R, RNase protection of 500 ng of in vitro-transcribed DI9c RNA with antisense probe (positive control); 1, RNase protection performed with antisense and sense probes on BVDV DI9c cDNA (positive control); 2, RNase protection with cytoplasmic RNA derived from cells that were previously transfected with DI9c pm1 (3) (negative control); 3 to 15, identical experiments with cytoplasmic RNA from cells that were previously transfected with the different DI9c derivatives: wild-type DI9c, LEYH, DETH, DEFH, DEEH, ΔAB, RV1693, TRQL1834, LTRQ1966, NTRV2088, H2099, DRRV2191, and DRRV2249, respectively. (B) Quantification of the replication ability of functional BVDV DI9c NS3 mutants. Each of three replication-capable DI9c NS3 derivatives (Table 2 and Fig. 2A) and the wild-type replicon were transfected into BHK-21 cells together with 5 μg of pBluescript II KS(+) DNA. One aliquot of the transfected cells was harvested 12 h p.t. to determine the transfection efficiencies, and the second aliquot was harvested 24 h p.t. to monitor RNA replication. (Top) RNase protection assay with cytoplasmic nucleic acids prepared 12 h p.t. Protected RNA products were obtained by applying a Bluescript-specific radiolabeled probe. Lanes: p, Bluescript-specific probe; c, 500 ng of pBluescript II KS(+) DNA hybridized with the Bluescript-specific probe (positive control); 1 to 4, RNase protection with cytoplasmic nucleic acids derived from cells transfected with wild-type DI9c, DI9c DETH, DI9c DEFH, and DI9c DRRV2191, respectively. (Middle) RNase protection assay with cytoplasmic nucleic acids prepared 24 h p.t. Synthesis of positive- and negative-strand RNA was analyzed and monitored as described for panel A. The order of lanes is the same as described above. (Bottom) Calculation of the replication ability of the three functional DI9c NS3 mutants. The major protected band of each lane was quantified with a phosphorimager (see Materials and Methods). The respective positive-strand RNA signal was normalized to the corresponding plasmd DNA signal. However, variations of transfection efficiency turned out to be negligible (mean deviation, <7%) as assayed in experiments where only pBluescript II KS(+) DNA was transfected (data not shown). The relative replication ability was determined for each mutant with respect to the BVDV wild-type (wt) DI9c (estimated as 100% replication competent) and depicted as a column diagram. Error bars indicate the mean deviations of four independent transcription-transfection experiments performed with each of the functional replicons.
We decided to analyze the replication capability of these latter mutants more accurately. To enable a quantitative comparison of the different DI9c derivatives, the above procedure was modified in such a way that the viral RNAs were cotransfected together with a fixed amount of a certain DNA plasmid or an unrelated RNA (data not shown) into the cells. The transfection efficiency of independent experiments was then calculated by RNase protection of a control-specific 32P-labeled riboprobe, which was performed in parallel with the conventional monitoring of RNA replication (Fig. 2B and Materials and Methods). With respect to the wild-type replicon, the DI9c DETH mutant was thus found to display a replication ability of about 80%; substitution of DEFH for DEYH yielded a more drastic reduction—replicon derivatives encoding this sequence in NS3 produced only about 65% of the wild-type viral RNA level. The most dramatic effect was observed in the behavior of the DI9c DRRV2191 replicon; as shown in Fig. 2, this RNA replicates at barely 10% of the wild-type level.
Interestingly, the ratio of positive-strand RNA to negative-strand RNA was in all cases found not to be significantly affected. At 24 h p.t. but also at earlier or later points, it was observed to range reproducibly at a value indistinguishable from that measured for the wild-type replicon (Fig. 2; see also reference 3). Moreover, as synthesis of negative-strand RNA in all cases was exclusively detectable with the simultaneous generation of positive-strand RNA (Fig. 2), we concluded that the different functions provided by the NS3 unit of BVDV should be involved at an early stage of the RNA replication pathway (see also below).
Effects of mutagenesis on the NS3-mediated proteolysis of the nonstructural polyprotein.
Previous studies indicated an intact protease function and proteolytic processing of the replicon-encoded polyprotein NS3-NS4A-NS4B-NS5A-NS5B as essential prerequisites of the RNA replication pathway (3, 28). Hence, we were interested in estimating the ability of the entire set of DI9c NS3 derivatives to catalyze the proteolysis of the nonstructural polyprotein. Accordingly, each RNA was subjected to an in vitro translation assay by a protocol which with other positive-strand RNA virus systems was proven to allow translation of the viral RNA as well as proteolytic processing of the polyprotein to proceed in vitro (1, 31). In our case, the assay was based on cytoplasmic extracts and translation factors which were prepared from BHK-21 cells, because this cell line was demonstrated to support replication of DI9c efficiently (see above). To visualize the different translation and/or proteolytic processing products, [35S]methionine was included in the reaction mixture. As shown in Fig. 3A, translation of the wild-type DI9c RNA gave rise to a characteristic pattern of labeled protein bands by SDS-PAGE. Comigration experiments with individually translated nonstructural proteins and mutational analysis of the diverse proteolytic cleavage sites revealed these bands to be representing most of the mature nonstructural proteins as well as certain intermediates of the proteolysis (data not shown). The translation assay was hence found suitable for monitoring the NS3-directed cleavages of the DI9c-encoded polyprotein. As expected, efficient release of NS3 in cis as well as trans cleavage of the residual polyprotein was determined to be entirely inhibited in the case of a previously described DI9c derivative (DI9c pm1) encoding an NS3 protein in which the catalytic residue of the serine protease domain, S1752, was replaced by A (3) (Fig. 3). Consistently, insertion of two amino acids (DI9c RV1693 [Fig. 1]) into the protease domain also yielded a drastic decline of proteolysis (Fig. 3A, lane 3). Conversely, the protein cleavage pattern of most of the other DI9c NS3 mutants was found to be virtually unaltered with respect to that of the wild-type replicon. However, in a number of cases, a discernibly lower efficiency of cis and/or trans cleavages was detected, e.g., the DRRV2249 mutation, which presumably affects the NS3-NS4A cleavage (Fig. 3, lane 9). Within the limits of accuracy of our analysis, certain NS3 mutations comprising also those which allow RNA replication to occur were found to exert only a minor effect on the protease activity (Fig. 3A, lanes 8, 12, and 13).
FIG. 3.
Monitoring proteolytic processing of the nonstructural polyprotein of different DI9c NS3 derivatives. (A) In vitro translation-analysis of the proteolytic cleavage products. A portion of the translation reaction mixture was solubilized in protein sample buffer, and the labeled proteins were analyzed by SDS–10% PAGE (see Materials and Methods). Positions of Npro and of some of the mature BVDV nonstructural proteins are indicated. Lanes: M, molecular mass markers; 1, labeled proteins obtained by translation of DI9c pm1 (negative control; see also Results); 2 to 14, labeled proteins obtained by translation of wild-type DI9c RNA (lane 2) and the different DI9c NS3 derivatives: RV1693 (lane 3), TRQL1834 (lane 4), LTRQ1966 (lane 5), NTRV2088 (lane 6), H2099 (lane 7), DRRV2191 (lane 8), DRRV2249 (lane 9), ΔAB (lane 10), LEYH (lane 11), DETH (lane 12), DEFH (lane 13), and DEEH (lane 14). (B) Protease activity; calculation of the NS3-directed cis and trans cleavages of the replicon-encoded polyprotein. Band intensities of Npro, NS3, and NS4B of each translation-processing assay (see panel A) were quantitated with a phosphorimager. Because Npro releases itself autocatalytically from the polyprotein precursor widely independently from downstream mutations, NS3 and NS4B data were normalized to the amount of radioactivity of the respective Npro band. The percentages of cis release of NS3 and of trans cleavage of NS4B were then determined for each mutant by calculating the ratio of NS3mut to NS3wt and NS4Bmut to NS4Bwt, respectively, estimating DI9c wild-type (wt) NS3 as 100% proteolytically active. The results are depicted as a column diagram: 1, RV1693; 2, TRQL1834; 3, LTRQ1966; 4, NTRV2088; 5, H2099; 6, DRRV2191; 7, DRRV2249; 8, ΔAB; 10, LEYH; 11, DETH; 12, DEFH. Error bars indicate the mean deviations of at least three translation-processing assays.
Monitoring the RNA helicase, ATPase, and RNA binding activity of mutant replicon-derived NS3 proteins in vitro.
Next, we wanted to assess whether the diminished replication ability of the DETH, DEFH, and DRRV2191 mutants would coincide with a reduced helicase/ATPase activity of NS3. For this purpose, BHK cells were transfected with the different RNA replicons and—depending on the replicon’s replication capability—harvested at 24 or 48 h p.t., respectively. From the cytoplasmic fraction, we recovered replicon-derived NS3 protein by an immunoaffinity purification procedure (see Materials and Methods). Each NS3 preparation contained at least 90% of the viral protein and was confirmed to be not contaminated with RNases or proteases (Fig. 4A, and some data not shown). Most importantly, the mock control was verified to contain neither a cellular helicase nor ATPase or RNA binding activities (Fig. 4). Using identical quantities of these fractions (Fig. 4A), we initially compared mutant and wild-type NS3 proteins in terms of their helicase activity, i.e., catalysis of strand displacement of a partly double-stranded RNA template (Fig. 4B) under conditions that were previously demonstrated as being suitable to support the BVDV NS3 RNA helicase activity in vitro (48). Each of the three mutant NS3 proteins was determined to promote strand displacement of the helicase substrate, though to rather different degrees: while NS3 proteins with a DETH or DEFH box were found to exhibit a helicase activity corresponding to only about 65 and 75% of the wild-type level, respectively, the C-terminal DRRV insertion turned out to have no effect on the NS3-encoded helicase function in this assay (Fig. 4B).
FIG. 4.
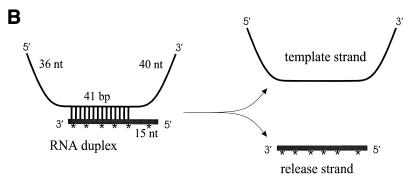
Enzymatic assays with purified wild-type and mutant NS3 proteins. (A) SDS-PAGE analysis. (Left) SDS-PAGE of immunoaffinity-purified NS3 protein. Approximately 0.4 μg of dialyzed protein preparations (see Materials and Methods) was subjected to SDS–10% PAGE and visualized by Coomassie blue staining. Lane 1, NS3 protein purified from DI9c-transfected cells; lane 2, mock control (identical purification procedure performed with mock-transfected cells). Positions and sizes (in kilodaltons) of marker proteins are indicated on the right. (Right) SDS-PAGE (same conditions as above) of identical amounts of purified wild-type and mutant NS3 proteins as calculated with a commercial protein determination kit (see Materials and Methods). According to these calculations, identical amounts of independent protein preparations were applied in the in vitro assays described below. Lanes: M, molecular mass markers; 1, wild-type NS3; 2, NS3 DETH; 3, NS3 DEFH; 4, NS3 DRRV2191; 5, mock control (identical purification procedure performed with mock-transfected cells). (B) RNA helicase assay. (Top) Schematic drawing of the partially double-stranded RNA substrate used in the helicase assay (see Materials and Methods). The lengths of the single- and double-stranded regions are given. nt, nucleotides; ∗, radiolabeled nucleotide. (Middle) Helicase assay. Reactions and analysis were carried out as described in Materials and Methods. Positions of the substrate and the release strand are marked on the right. Lanes: 1, RNA substrate incubated for 2 min without protein at 95°C; 2, control reaction carried out with wild-type NS3 in the absence of ATP; 3 to 7, helicase assay performed with the mock control (see panel A), wild-type NS3, NS3 DETH, NS3 DEFH, and NS3 DRRV2191, respectively. (Bottom) Calculating the helicase activity of the NS3 mutants. Band intensities of the released radiolabeled single strand were quan- tified with a phosphorimager. The relative helicase activity of each NS3 mutant was calculated with respect to the wild-type (wt) NS3 whose helicase activity was set as 100% and depicted as a column diagram. Error bars indicate the mean deviations of five helicase assays performed with wild-type and mutant NS3 proteins which were simultaneously prepared from three independent transfection experiments. (C) ATPase activity. (Top) ATPase assays performed in the absence or in the presence of 400 μM poly(C) nucleotides as effector. The reactions were carried out, and reaction mixtures were analyzed by thin-layer chromatography as described in Materials and Methods. Lanes: 1 and 6, mock control (see above); 2 and 7, wild-type NS3; 3 and 8, NS3 DETH; 4 and 9, NS3 DEFH; 5 and 10, NS3 DRRV2191. (Bottom) Column diagram of ATPase activities of mutant NS3 proteins. The amount of radioactivity contained in each labeled spot was measured with a phosphorimager. Conversion rates were determined as the ratio of the amount of radioactivity in the ADP spot to the entire amount of radioactivity in both ADP and ATP spots. The relative ATPase activity of each NS3 mutant was calculated with respect to the wild-type (wt) NS3 conversion rate, which was set as 100%. Error bars indicate the mean deviations of seven ATPase assays which were performed with wild-type and mutant NS3 proteins simultaneously prepared from three independent transfection experiments. (D) RNA binding assay. The gel retardation reactions were carried out as described in Materials and Methods. Lanes: 1 and 2, control reactions performed with 50 and 20 ng of bovine serum albumin, respectively; 3, mock control (see above); 4, wild-type NS3; 5, NS3 DETH; 6, NS3 DEFH; 7, NS3 DRRV2191.
In the following experiments, each of the viral enzyme preparations was tested for its ability to catalyze the hydrolysis of [α-32P]ATP. In the absense of polynucleotide effector, all mutant forms showed a significantly lower ATPase activity than did the wild-type NS3. The addition of effector raised the activity of NS3 DETH and NS3 DRRV2191 to a level indistinguishable from that of the wild-type protein. In remarkable contrast, the low ATPase activity of NS3 DEFH could not be rescued by the addition of effector (Fig. 4C).
Finally, we monitored the RNA binding activity of the purified wild-type and mutant NS3 proteins by a gel mobility shift assay. As indicated in Fig. 4D, all four NS3 proteins were found to bind unrelated (data not shown) and viral RNA molecules at high efficiency. A slightly lower RNA binding activity was reproducibly observed for the DETH mutant.
Taken together, the above experiments revealed a compelling relationship of a functional helicase and ATPase activity on the one hand and the replication capability of the replicon on the other hand. However, the low replication capability of the DI9c DRRV2191 replicon could not be explained by the in vitro data.
The different functions of NS3 during RNA replication cannot be provided in trans.
We were interested in understanding how the NS3 protein mediates its multiple functions during the viral RNA replication process. To address this issue, we wanted to evaluate the possibility whether addition of intact NS3 in trans would enhance or even rescue the replication capability of the less functional or nonfunctional DI9c NS3 derivatives, respectively. As the conditions of coexpression of NS3 were considered to be critical for a successful complementation, different experimental setups were chosen to supply the protein in trans: as schematized in Fig. 5A, these attempts involved either unidirectional or symmetric supplement of NS3. Unidirectional supplementation should be achieved by cotransfecting the mutant DI9c replicon together with either the DI9c wild-type RNA, an entire BVDV CP7 helper virus, or a Sindbis virus replicon (6) expressing the BVDV NS3 protein. The symmetric attempts involved cotransfection of the DI9c NS3 mutants together with nonviable DI9c derivatives which contained lethal lesions in different genetic units of the ORF, for instance, within the NS4B coding unit. If feasible, each experiment was verified by IF to yield a maximum number of transfected cells and a reasonable amount of coexpressed NS3 protein (data not shown). Replication of the Sindbis virus replicon was confirmed not to interfere with the replication of BVDV DI9c (data not shown). To search for positive complementation or compensation events, specific RNase protection and RT-PCR monitoring procedures were established, which allowed us to discriminate between RNA amplification of the cotransfected helper-expression system and that of the applied DI9c NS3 mutant, respectively (see Materials and Methods and below).
Interestingly, all complementation experiments which were intended to restore the function of the replication-deficient insertion, deletion, and substitution DI9c NS3 mutants yielded a negative result, irrespective of whether we assayed for mutant RNA replication at 24 h or at earlier or later time points p.t. (see below; most of the data not shown). Identical results were obtained for the above-mentioned mutants as well as other DI9c protease mutants (data not shown). Since cotransfection of different deficient RNA molecules never led to the appearance of novel replicating species, RNA recombination events were thus concluded to occur at a subdetectable level in our experimental system (see also below).
These results suggested either that each of the different mutations led to the destruction of cis-acting parts of the NS3 coding region or—more likely—that the various functions of NS3 may operate preferentially in cis (see below). This latter idea was substantiated at least for the ATPase/helicase activity by data obtained during cotransfection experiments with DI9c NS3 derivatives that replicate at different efficiencies (see above). A particular example of such an experiment is presented in Fig. 5B: in this case, two different DEYH mutants were cotransfected together with the wild-type replicon into BHK cells. Since engineering of each of these mutations was accompanied by introduction of an additional restriction site into the DI9c cDNA (see Materials and Methods), screening of the replication of the mutant RNA was accordingly permitted by RT-PCR and subsequent cleavage of the PCR products with the respective restriction enzyme. Cotransfection of wild-type DI9c with the DI9c LEYH derivative, which was demonstrated above to be incapable of RNA replication, yielded only the wild-type RT-PCR product. This result was obtained even if the mutant RNA was originally transfected with a molar excess and irrespective of which time p.t. the analysis was performed (Fig. 5B). Conversely, cotransfection with the functional but less active DEFH mutant yielded a mutant-specific signal. However, although the mutant replicon was again transfected in excess (to rule out a possible ab initio disadvantage of the mutant versus the wild-type RNA), the amount of PCR product reflecting mutant RNA replication turned out to be evidently less than that of the wild-type product (Fig. 5B). Regarding the control experiment in which the DI9c DEFH was transfected alone, cotransfection of wild-type RNA caused an even weaker mutant signal (see Discussion).
Considering the above notion that RNA recombination is negligible, functional DI9c NS3 derivatives were hence concluded to replicate side by side in the transfected cells. The fact that amplification of the less functional replicon was at least not significantly enhanced by the coreplicating wild-type RNA thus strengthened the suspicion that the NS3-encoded ATPase/RNA helicase acts mostly, if not entirely, in cis—e.g., in statu nascendi during translation and proteolytic release. The same may be assumed for the NS3 protease function.
DISCUSSION
Several factors recommend the DI9c replicon as a suitable system to analyze the molecular requirements of the BVDV replication process. (i) DI9c encodes none of the virus structural proteins; hence, RNA replication can be explored independently of RNA packaging and virus maturation. (ii) As a cDNA-derived homolog of a natural defective interfering particle (42), the subgenomic RNA replicates at a significantly higher efficiency than the entire viral genome (3, 15a). Reverse genetics studies thus yielded valuable information on the function of defined cis-acting RNA elements during RNA replication (56). In this work, we took a genetic approach to the NS3 genetic unit of the replicon’s ORF to analyze the role of the different enzymatic activities of the nonstructural protein NS3.
Previous data suggested the serine protease activity of NS3 to be an essential determinant of the RNA replication process (3, 28), a fact that has been plainly confirmed here. Surprisingly, as indicated by the translation data, the efficiency of proteolytic cleavage of the DI9c RNA-encoded polyprotein was affected not only by alterations within the protease domain itself, but also by rather distant and conservative mutations such as the DEEH and LEYH substitutions or insertion of a single amino acid near the helicase motif VI (mutant H2099) (Fig. 3). This suggests a tight intramolecular modulation of the NS3 protease and helicase activities—a hypothesis which is supported by data derived from in vitro studies on heterologously expressed NS3 protein of the pestivirus-related HCV (17, 20, 21, 32, 35, 39). Hence, the lethal effect caused by less conservative insertion-deletion mutations may be attributed to disintegration of the overall conformation of NS3 rather than to inhibition of a single function. For some of the mutant replicons, this fact may be indicated by a lower stability of NS3 and/or a slightly modified proteolysis pattern of the polyprotein (Fig. 3, e.g., lanes 5, 9, and 10).
Certain parts of the NS3 protein, however, emerged as being less stringently involved in this functional framework, i.e., one residue in the DEYH motif and a region near the protein’s C terminus. Considering that the respective mutant RNAs had essentially the same stability as the wild-type RNA (data not shown) and that mutant and wild-type NS3 proteins were shown to behave identically during translation and purification (Fig. 3 and 4A), recovery of these functional replicon derivatives enabled us to correlate in vivo data on the replication capability with the in vitro activity of the different NS3 functions.
The DEYH sequence was chosen as a target for mutagenesis, because it represents one of the best-characterized conserved amino acid sequence motifs classifying the BVDV NS3 protein as a member of the superfamily II of DEAD/DExH box helicases (13–15, 19, 23). Crystallization of the C-terminal region of the NS3 protein of HCV (9, 26, 55) suggested this motif to be part of a domain with a fold similar to ATP transphosphorylases: the N-terminal aspartate is expected to be essentially implicated in binding and orientation of the Mg2+-ATP substrate (5, 54), while the C-terminal histidine appears to be necessary for coupling ATPase activity to polynucleotide binding and/or nucleic acid duplex unwinding activity (5, 7, 16, 18, 34). The third (x) position varies among different helicase molecules (12, 25, 27). Substitution of E for Y led to a nonfunctional BVDV replicon, showing that this position may not be occupied by any residue (see above). Mutation of DEYH to DETH or DEFH turned out to be rather informative, particularly because these mutations had no effect on the proteolysis of the polyprotein (Fig. 3). Thus, exchange of T for Y had a profound inhibitory effect on the helicase activity of NS3 and resulted in a diminished ability of the protein to bind RNA. Substitution of F for Y generated an NS3 protein which exhibited a less disturbed helicase and RNA binding capability on the one hand but a considerably damaged ATPase activity on the other hand (Fig. 4). These data, which are compatible with results obtained during mutagenesis of the HCV NS3 DECH motif (25), allowed a direct tracing back of a lower capacity for RNA replication to a weaker helicase and/or ATPase activity and evidently demonstrated the importance of both activities during the BVDV replication process in vivo.
A common function envisaged for RNA virus helicases is causing RNA strand separation during the different steps of the RNA replication pathway. By RNase protection as well as by RT-PCR (the latter not shown), the negative-strand RNA intermediate could be detected only with the simultaneous synthesis of progeny positive-strand RNA (Fig. 2). This result suggests that the NS3 functions are recruited either in only the first or in both replication steps. Interestingly, the 3′ terminus of the BVDV genome exhibits structural features that ought to fulfill requirements which were shown to be essential for the NS3 helicase activity in vitro (48), i.e., a stable stem-loop structure and non-base-paired residues at the immediate 3′ end (56). Considering that unwinding of RNA duplexes is an early event in BVDV replication, it is tempting to speculate that the genomic 3′ end provides the signal not only to initiate nucleotide polymerization but also to prime strand displacement. Elucidation of the exact biochemical mechanisms underlying the activity of the NS3 NTPase/helicase and its linkage to the RNA polymerization process will be an exciting goal of future research.
In contrast to the DEYH mutations, insertion of four amino acid residues near the C terminus of the BVDV NS3 had a strong negative effect on the replication capability of DI9c without having a severe effect on those activities of NS3 which can be monitored in vitro (Fig. 2 to 4). Two interpretations are conceivable for this result. First, this lesion may disrupt the general functional shape of the NS3 protein (see above) as possibly indicated by a slight inhibition of the trans cleavage efficiency of the polyprotein (Fig. 3). The second explanation concerns the location of this mutation. Like the HCV NS3 helicase (9, 26, 55), the overall shape of the BVDV NS3 helicase may be schematically outlined as a Y (36a). The region affected by the DRRV2191 insertion represents the stem of this Y—the so-called helical domain (9)—which displays an obvious physical distance from the catalytic center of the ATPase/helicase as well as from the NS3-NS4A cleavage site (Fig. 1). This domain may thus represent yet another uncharacterized functional determinant of NS3, which, for example, might serve as an interaction partner of other viral or cellular factors during assembly of the replication complex. Numerous experiments are needed to validate this attractive hypothesis.
The last interesting aspect of this study concerns the unexpected finding that all mutations within the NS3-encoding region of the replicon RNA could not be complemented by providing the viral protein in trans—not even when a helper virus RNA was cotransfected. Not only did other cotransfection experiments confirm this cis dominance, but moreover, replication of different replicon derivatives was found to occur side by side in the cell (Fig. 5B). Several scenarios are imaginable for interpretation of these results. One possibility which cannot be discarded for all mutations yielding replication-defective RNA molecules concerns the destruction of cis-acting RNA signals within the NS3 coding region. However, this cannot entirely explain the phenomenon observed, particularly in view of the fact that the respective mutations mapped at rather different parts of the NS3 coding unit and evidently affected different functional areas of the protein (see above). Along the same line, since all lesions kept the polyprotein reading frame intact—as mirrored by the in vitro translation data—cis dominance of the NS3 mutants cannot be explained by a cis-translation-required region as proposed for a certain part of the poliovirus ORF (33). We thus favor the idea that the stated functions of NS3 are restricted to operating preferentially in cis. They may act transiently or only when nascent or newly synthesized—possibly also in the shape of a polyprotein cleavage intermediate with a distinct and yet unknown function. Alternatively, NS3 might exhibit a restricted ability to diffuse to other templates due to the formation of a closed-up complex or a direct or indirect association with localized structures, e.g., cellular membranes. Given that these structures would be limited in the cell, such a situation would provide an explanation for the observation shown in Fig. 5B indicating competition (interference) between RNAs that replicate at different efficiencies.
In the poliovirus system, genetic complementation studies yielded controversial results, depending on which parts of genetic units of the ORF were affected (4, 8, 22, 33; reviewed in reference 50). This is in keeping with the consideration that the nonstructural ORF of poliovirus as well as of the pestivirus replicon should be regarded as a monocistronic, finely adjusted functional entity during translation, polyprotein processing, and replication. Accordingly, it is difficult to decide whether our cis-dominant mutations undoubtedly identify NS3 as a cis-acting protein. However, the broad spectrum of mutations which in no case could be compensated strongly argues for this assumption.
In summary, our work combining reverse genetics in vivo data with biochemical in vitro approaches presents an important starting point on the way to unraveling the intricate interplay of numerous factors of both viral and host origin that coordinate the BVDV RNA replication pathway.
ACKNOWLEDGMENTS
This study was supported by the SFB 535 Invasionsmechanismen und Replikationsstrategien von Krankheitserregern (C.W.G. and O.I.) from the Deutsche Forschungsgemeinschaft at the Justus-Liebig-Universität Giessen. S.-E.B. was partly supported by the Infektionsforschung-Stipendienprogramm (2131) of the BMBF (Bundesministerium Bildung und Forschung) administrated by the Deutsches Krebsforschungszentrum (DKFZ).
We thank N. Tautz for critical reading of the manuscript and H.-J. Thiel for generous support.
REFERENCES
- 1.Barton D J, Flanegan J B. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J Virol. 1993;67:822–831. doi: 10.1128/jvi.67.2.822-831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens S-E, Tomei L, De Francesco R. Identification and properties of the RNA-dependent RNA polymerase of hepatitis C virus. EMBO J. 1996;15:12–22. [PMC free article] [PubMed] [Google Scholar]
- 3.Behrens S-E, Grassmann C W, Thiel H-J, Meyers G, Tautz N. Characterization of an autonomous subgenomic RNA replicon of a pestivirus. J Virol. 1998;72:2364–2372. doi: 10.1128/jvi.72.3.2364-2372.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein H D, Sarnow P, Baltimore D. Genetic complementation among poliovirus mutants derived from an infectious cDNA clone. J Virol. 1986;60:1040–1049. doi: 10.1128/jvi.60.3.1040-1049.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black M E, Hruby D E. Site-directed mutagenesis of a conserved domain in vaccinia virus thymidine kinase. Evidence for a potential role in magnesium binding. J Biol Chem. 1992;267:6801–6806. [PubMed] [Google Scholar]
- 6.Bredenbeek P, Rice C M. Animal RNA virus expression systems. Semin Virol. 1992;3:297–310. [Google Scholar]
- 7.Brosh R M, Jr, Matson S W. Mutations in motif II of Escherichia coli DNA helicase II render the enzyme nonfunctional in both mismatch repair and excision repair with differential effects on the unwinding reaction. J Bacteriol. 1995;177:5612–5621. doi: 10.1128/jb.177.19.5612-5621.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charini W A, Burns C C, Ehrenfeld E, Semler B L. trans rescue of a mutant poliovirus RNA polymerase function. J Virol. 1991;65:2655–2665. doi: 10.1128/jvi.65.5.2655-2665.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho H-S, Ha N-C, Kang L-W, Chung K M, Back S H, Jang S K, Oh B-H. Crystal structure of the RNA helicase from genotype 1B hepatitis C virus. A feasible mechanism of unwinding duplex RNA. J Biol Chem. 1998;273:15045–15052. doi: 10.1074/jbc.273.24.15045. [DOI] [PubMed] [Google Scholar]
- 10.Corapi W V, Donis R O, Dubovi E J. Characterization of a panel of monoclonal antibodies and their use in the study of the antigenic diversity of bovine viral diarrhea virus. Am J Vet Res. 1990;51:1388–1394. [PubMed] [Google Scholar]
- 11.Francki R I, Fauquet D L, Knudson D L, Brown F. Classification and nomenclature of viruses. Arch Virol Suppl. 1991;2:223–233. [Google Scholar]
- 12.George J W, Brosh R M, Jr, Matson S W. A dominant negative allele of the Escherichia coli uvrD gene encoding DNA helicase II. A biochemical and genetic characterization. J Mol Biol. 1994;235:424–435. doi: 10.1006/jmbi.1994.1003. [DOI] [PubMed] [Google Scholar]
- 13.Gorbalenya A E, Donchenko A P, Koonin V, Blinov V M. A conserved NTP-motif in putative helicases. Nature (London) 1988;333:22. doi: 10.1038/333022a0. [DOI] [PubMed] [Google Scholar]
- 14.Gorbalenya A E, Donchenko A P, Koonin V, Blinov V M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989;17:4713–4729. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorbalenya A E, Koonin E V. Helicases: amino acid sequence comparisons and structure-function relationships. Curr Opin Struct Biol. 1993;3:419–429. [Google Scholar]
- 15a.Grassmann, C. W. Unpublished results.
- 16.Gross C H, Shuman S. Mutational analysis of vaccinia virus nucleoside triphosphate phosphohydrolase II, a DExH box RNA helicase. J Virol. 1995;69:4727–4736. doi: 10.1128/jvi.69.8.4727-4736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwack Y, Kim D W, Han J H, Choe J. Characterization of RNA binding activity and RNA helicase activity of the hepatitis C virus NS3 protein. Biochem Biophys Res Commun. 1996;225:654–659. doi: 10.1006/bbrc.1996.1225. [DOI] [PubMed] [Google Scholar]
- 18.Heilek G M, Peterson M G. A point mutation abolishes the helicase but not the nucleoside triphosphatase activity of hepatitis C virus NS3 protein. J Virol. 1997;71:6264–6266. doi: 10.1128/jvi.71.8.6264-6266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgman T. A new superfamily of replicative proteins. Nature (London) 1988;333:22–23. doi: 10.1038/333022b0. [DOI] [PubMed] [Google Scholar]
- 20.Hong Z, Ferrari E, Wright-Minogue J, Chase R, Risano C, Seelig G, Lee C G, Kwong A D. Enzymatic characterization of hepatitis C virus NS3/4A complexes expressed in mammalian cells by using the herpes simplex virus amplicon system. J Virol. 1996;70:4261–4268. doi: 10.1128/jvi.70.7.4261-4268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin L, Petterson D L. Expression, isolation, and characterization of the hepatitis C virus ATPase/RNA helicase. Arch Biochem Biophys. 1995;323:47–53. doi: 10.1006/abbi.1995.0008. [DOI] [PubMed] [Google Scholar]
- 22.Johnson K L, Sarnow P. Three poliovirus 2B mutants exhibit noncomplementable defects in viral RNA amplification and display dosage-dependent dominance over wild-type poliovirus. J Virol. 1991;65:4341–4349. doi: 10.1128/jvi.65.8.4341-4349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadare G, Haenni A. Virus-encoded helicases. J Virol. 1997;71:2583–2590. doi: 10.1128/jvi.71.4.2583-2590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamer G, Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal, and bacterial viruses. Nucleic Acids Res. 1984;12:7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D W, Kim J, Gwack Y, Han J H, Choe J. Mutational analysis of the hepatitis C virus RNA helicase. J Virol. 1997;71:9400–9409. doi: 10.1128/jvi.71.12.9400-9409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J L, Morgenstern K A, Griffith J P, Dwyer M D, Thomson J A, Murcko M A, Lin C, Caron P R. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: the crystal structure provides insights into the mode of unwinding. Structure. 1998;6:89–100. doi: 10.1016/s0969-2126(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 27.Laín S, Riechmann J L, García J A. RNA helicase: a novel activity associated with a protein encoded by a positive-strand RNA virus. Nucleic Acids Res. 1989;18:7003–7006. doi: 10.1093/nar/18.23.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendez E, Ruggli N, Collett M S, Rice C M. Infectious bovine viral diarrhea virus (strain NADL) RNA from stable cDNA clones: a cellular insert determines NS3 production and viral cytopathogenicity. J Virol. 1998;72:4737–4745. doi: 10.1128/jvi.72.6.4737-4745.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyers G, Tautz N, Becher P, Thiel H-J, Kümmerer B M. Recovery of cytopathogenic and noncytopathogenic bovine viral diarrhea viruses from cDNA constructs. J Virol. 1996;70:8606–8613. doi: 10.1128/jvi.70.12.8606-8613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller R H, Purcell R H. Hepatitis C virus shares amino acid sequence similarity with pestivirus and flavivirus as well as members of two plant virus supergroups. Proc Natl Acad Sci USA. 1990;87:2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molla A, Paul A V, Wimmer E. Cell-free, de novo synthesis of poliovirus. Science. 1991;254:1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- 32.Morgenstern K A, Landro J A, Hsiao K, Lin C, Gu Y, Su M S, Thomson J A. Polynucleotide modulation of the protease, nucleoside triphosphatase, and helicase activities of a hepatitis C virus NS3-NS4A complex isolated from transfected COS cells. J Virol. 1997;71:3767–3775. doi: 10.1128/jvi.71.5.3767-3775.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novak J E, Kirkegaard K. Coupling between genome translation and replication in an RNA-virus. Genes Dev. 1994;8:1726–1737. doi: 10.1101/gad.8.14.1726. [DOI] [PubMed] [Google Scholar]
- 34.Pause A, Sonenberg N. Mutational analysis of a DEAD box RNA helicase: the mammalian translation initiation factor eIF-4A. EMBO J. 1992;11:2643–2654. doi: 10.1002/j.1460-2075.1992.tb05330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preugschat F, Averett D R, Clarke B E, Porter D J T. A steady-state and pre-steady-state kinetic analysis of the NTPase activity associated with the hepatitis C virus NS3 helicase domain. J Biol Chem. 1996;271:24449–24457. doi: 10.1074/jbc.271.40.24449. [DOI] [PubMed] [Google Scholar]
- 36.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Raven Press; 1996. pp. 931–959. [Google Scholar]
- 36a.Sauerborn, M. Personal communication.
- 37.Steffens, S., H.-J. Thiel, and S.-E. Behrens. The RNA-dependent RNA polymerases of different members of the Flaviviridae family exhibit similar properties under in vitro conditions. J. Gen. Virol., in press. [DOI] [PubMed]
- 38.Suzich J A, Tamura J K, Palmer-Hill F, Warrener P, Grakoui A, Rice C M, Feinstone S M, Collett M S. Hepatitis C virus NS3 protein: polynucleotide-stimulated NTPase and comparison with the related pestivirus and flavivirus enzymes. J Virol. 1993;67:6152–6158. doi: 10.1128/jvi.67.10.6152-6158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tai C L, Chi W K, Chen D S, Hwang L H. The helicase activity associated with hepatitis C virus nonstructural protein 3 (NS3) J Virol. 1996;70:8477–8484. doi: 10.1128/jvi.70.12.8477-8484.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamura J K, Warrener P, Collett M S. RNA-stimulated NTPase activity associated with the p80 protein of the pestivirus bovine viral diarrhea virus. Virology. 1993;193:1–10. doi: 10.1006/viro.1993.1097. [DOI] [PubMed] [Google Scholar]
- 41.Tan B-H, Fu J, Sugrue R J, Yap E-H, Chan Y-C, Tan Y H. Recombinant Dengue type 1 virus NS5 protein expressed in E. coli exhibits RNA-dependent RNA polymerase activity. Virology. 1996;216:317–325. doi: 10.1006/viro.1996.0067. [DOI] [PubMed] [Google Scholar]
- 41a.Tautz, N. Unpublished results.
- 42.Tautz N, Thiel H-J, Dubovi E J, Meyers G. Pathogenesis of mucosal disease: a cytopathogenic pestivirus generated by an internal deletion. J Virol. 1994;68:3289–3297. doi: 10.1128/jvi.68.5.3289-3297.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tautz N, Meyers G, Stark R, Dubovi E J, Thiel H-J. Cytopathogenicity of a pestivirus correlates with a 27-nucleotide insertion. J Virol. 1996;70:7851–7858. doi: 10.1128/jvi.70.11.7851-7858.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tautz N, Elbers K, Stoll D, Meyers G, Thiel H-J. Serine protease of pestiviruses: determination of cleavage sites. J Virol. 1997;71:5415–5422. doi: 10.1128/jvi.71.7.5415-5422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thiel H-J, Plagemann P G W, Moennig V. Pestiviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Raven Press; 1996. pp. 1059–1074. [Google Scholar]
- 46.Vassilev V B, Collett M S, Donis R O. Authentic and chimeric full-length genomic cDNA clones of bovine viral diarrhea virus that yield infectious transcripts. J Virol. 1997;71:451–457. doi: 10.1128/jvi.71.1.471-478.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the alpha and beta subunits of ATP synthase, myosin kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warrener P, Collett M S. Pestivirus NS3 (p80) protein possesses RNA helicase activity. J Virol. 1995;69:1720–1726. doi: 10.1128/jvi.69.3.1720-1726.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wengler G, Wengler G. The carboxy-terminal part of the NS3 protein of the West Nile flavivirus can be located as soluble protein after proteolytic cleavage and represents an RNA-stimulated NTPase. Virology. 1991;184:309–319. doi: 10.1016/0042-6822(91)90440-m. [DOI] [PubMed] [Google Scholar]
- 50.Wimmer E, Hellen C U T, Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:453–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 51.Wiskerchen M, Belzer S K, Collett M S. Pestivirus gene expression: the first protein product of the bovine viral diarrhea virus large open reading frame, p20, possesses proteolytic activity. J Virol. 1991;65:4508–4514. doi: 10.1128/jvi.65.8.4508-4514.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiskerchen M, Collett M S. Pestivirus gene expression: protein p80 of bovine viral diarrhea virus is a proteinase involved in polyprotein processing. Virology. 1991;184:341–350. doi: 10.1016/0042-6822(91)90850-b. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Mendez E, Caron P R, Lin C, Murcko M A, Collett M S, Rice C M. Bovine viral diarrhea virus NS3 serine proteinase: polyprotein cleavage sites, cofactor requirements, and molecular model of an enzyme essential for pestivirus replication. J Virol. 1997;71:5312–5322. doi: 10.1128/jvi.71.7.5312-5322.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan H G, Tsai M D. Mechanism of adenylate kinase. Demonstration of a functional relationship between aspartate 93 and Mg2+ by site-directed mutagenesis and proton, phosphorus-31, and magnesium-25 NMR. Biochemistry. 1991;30:5539–5546. doi: 10.1021/bi00236a029. [DOI] [PubMed] [Google Scholar]
- 55.Yao N, Hesson T, Cable M, Hong Z, Kwong A D, Le H V, Weber P C. Structure of the hepatitis C virus RNA helicase domain. Nat Struct Biol. 1997;4:463–467. doi: 10.1038/nsb0697-463. [DOI] [PubMed] [Google Scholar]
- 56.Yu H, Grassmann C W, Behrens S-E. Sequence and structural elements at the 3′ terminus of bovine viral diarrhea virus genomic RNA: functional role during RNA replication. J Virol. 1999;73:3638–3648. doi: 10.1128/jvi.73.5.3638-3648.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong W, Gutshall L L, Del Vecchio A M. Identification and characterization of an RNA-dependent RNA polymerase activity within the nonstructural protein 5B region of bovine viral diarrhea virus. J Virol. 1998;72:9365–9369. doi: 10.1128/jvi.72.11.9365-9369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]