Abstract
Background:
Distraction therapy use such as virtual reality is novel in the pediatric orthopedic field. In this study, we use subjective and objective metrics to evaluate virtual reality efficacy to reduce anxiety and pain in a pediatric orthopedic cohort.
Methods:
A prospective randomized controlled trial included patients between age 5 and 17 years, presenting to a tertiary care pediatric orthopedic clinic. Parallel groups underwent orthopedic procedures in clinic, utilizing immersive and interactive virtual reality distraction therapies versus standard of care. Procedures included cast application, cast removal, bone pin removal, and fracture reduction. All preprocedure parameters were similar between the groups. Primary outcome was the difference between maximum procedural heart rate and baseline. Secondary outcomes included Wong Baker FACES Rating Scale (Wong & Baker, 1988, Oklahoma, USA) for pain and Visual Analog Scale scores for anxiety.
Results:
Ninety-five patients (66 M, 29 F) underwent 59 cast removals, 26 cast applications, 7 percutaneous pin removals, and 3 fracture reductions. Average patient age in the virtual reality and control cohorts was 10.1 (5–17) and 10.6 (5–17), respectively. Average change in maximum heart rate in the virtual reality and control groups was 10.6 ± 10.1 versus 18.4 ± 11.0 (p = 0.00048). The virtual reality group demonstrated trends toward lower perceived anxiety (1.7 ± 2.8 versus 2.9 ± 3.6, p = 0.0666) when compared to controls.
Conclusions:
This level 1 study is the first to utilize objective biometric measurements to evaluate use of interactive virtual reality during multiple types of pediatric orthopedic procedures in the clinical setting. The findings suggest that an interactive and immersive virtual reality experience can be effective in reducing pain and anxiety.
Level of evidence:
Level 1, Randomized Controlled Trial.
Keywords: Virtual reality, pediatric orthopedics, fracture, distraction therapy, hardware removal
Introduction
Pediatric patients are exposed to procedures that can result in pain and anxiety. Recently, distraction therapy (DT) techniques have been implemented, including therapeutic play, breathing and music, among others.1 –4 Virtual reality (VR) is a novel modality to implement DT. VR requires a head-mounted display (HMD), which creates an immersive 3D visual and audio environment with which users can interact by using handheld controllers. Because this is a more interactive and immersive environment than simple distractions, VR is thought to be more beneficial than standard DT techniques. Using an HMD blocks visual and audible interaction with the real world, decreasing disruption due to pain or fear. 5
In the pediatric setting, multiple recent meta-analyses have demonstrated that VR DT is effective in reducing both preoperative anxiety and procedural pain and anxiety during burn wound care, chemotherapy, and intravenous port access. In many of these cases, VR was found to be significantly more effective in reducing self-reported pain and anxiety than the standard of care.6,7 Standard of care involved no use of tactile or visual distraction modalities during the procedure.
In the pediatric orthopedic setting, increased fear or anxiety can decrease effectiveness and procedural efficiency while imposing additional medical risks.8,9 Documented post-traumatic stress reactions and anxiety toward subsequent healthcare encounters are additional drawbacks. As a result, multiple types of DT have been utilized to reduce anxiety, particularly for cast removal. Examples include therapeutic play or presence of certified child life specialists.1,2 With technological progression, novel distraction systems have been used for pediatric cast removal, including iPads, video games, and electronic noise cancelling headphones.3,4 A literature review found only one study, which evaluated the use of VR DT during pediatric orthopedic clinical procedures, which demonstrated the effectiveness of this therapy to reduce anxiety and nausea during pediatric cast removal. However, this study was limited in its scope by excluding other orthopedic procedures and did not assess level of pain or heart rate. Of note, 90% of subjects in this trial reported that they would use VR again in future hospital visits. 10
Our study aimed to prospectively determine if pediatric patients undergoing in-office orthopedic procedures can benefit from the implementation of VR for pain and anxiety management. We hypothesized that the use of VR DT during these procedures would reduce perceived pain and anxiety metrics when compared to the control.
Materials and methods
A nonblinded randomized prospective trial was conducted after approval by the Institutional Review Board at the participating medical center (No. 2017.522). The clinical trial was registered at the US National Institutes of Health (details omitted for double-anonymized peer review). Enrollment and data collection took place between July 2019 and March 2020. Inclusion criteria included pediatric patients (ages 5–17 years) who presented to the tertiary care pediatric orthopedic clinic for an in-office procedure after primary orthopedic injury. Exclusion criteria included conditions limiting the subject’s ability to perceive and communicate information (e.g., developmental delay, cerebral palsy, and autism spectrum disorder). Subjects were excluded if limited in ability to use the VR system, unable to sit upright, or bilateral upper extremity injuries preventing the use of the handheld controller. All subjects and legal guardians provided written informed consent.
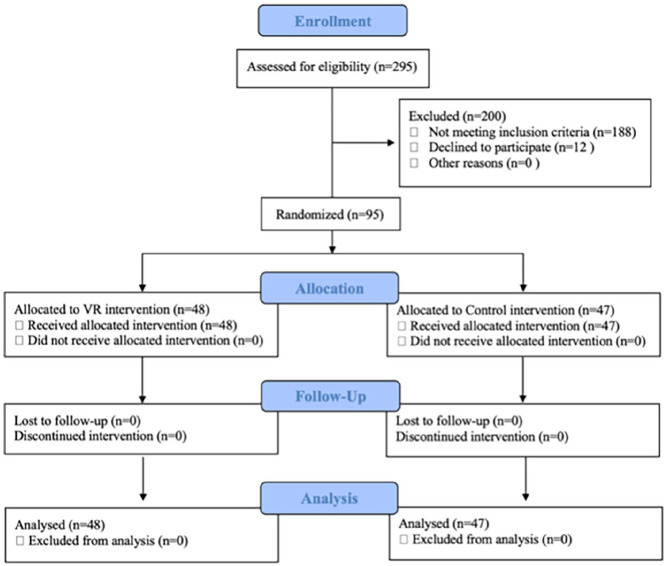
Cohort size determination was based on primary outcome mean difference of 8.0 BPM (beats per minute) with a standard deviation of 10.0. To achieve 80% power (1−β) with α = 0.05, enrollment of 25 patients per arm (50 total) was required to demonstrate statistical and clinical equivalence. The initial study parameters doubled this requirement to include 100 subjects. Each subject was randomly assigned to the VR intervention group (48 subjects) or control group (47 subjects) via a computerized random number generator (Figure 1).
Figure 1.
Consolidated Standards of Reporting Trials diagram showing study enrollment.
Procedures were categorized as “Cast Removal,” “Cast Application,” “Percutaneous Bone Pin Removal,” and “Fracture Reduction.” Cast removals and applications were performed by a certified Orthopedic Technologist employed by our institution. Pin removals and reductions were performed by orthopedic surgery attendings.
The study design was integrated into an existing clinical workflow preventing delays. This was coordinated and executed by an orthopedic research associate. The patient and family were placed in a designated cast room where they were educated and consented while waiting for the provider. Once consented, the cast tech or attending surgeon proceeded with the procedure, while the research associate documented outcome variables. This process did not adversely affect clinic flow. Patients’ heart rate (HR) was continuously monitored with pulse oximetry on the 5th digit of the noninjured upper extremity. Baseline HR was recorded prior to intervention. Maximum heart rate (HR max) was recorded during three distinct periods: 2-min preprocedure period, the intraprocedure period, and a 5-min postprocedure period. An Oculus VR headset system (Oculus, Facebook Technologies, Menlo Park, CA, USA) was used with accompanying wireless remote controller. The VR setup includes the headset, two touch controllers, and charging cord, which costs $399. This was used with the novel rock-skipping VR application (Video 1) developed by Innovation Ochsner (New Orleans, LA, USA). For lower extremity injuries, the dominant upper extremity was used for the controller. The patient could move the controller to simulate a throwing motion, which would initiate a rock being thrown across the virtual lake. The headset included a built-in audio component that played a calming melody, while the patient interacted with in the virtual lake environment. Redundancies were built into the application to allow easy usage with either the dominant or nondominant upper extremity. The procedure began within 1 min of headset placement. No trial period using the VR device was conducted to ensure continued engagement. The VR headset remained in place until procedure completion.
In the control group, standard of care was followed during the 2-min preprocedure period, while HR max was recorded. Standard of care included a parent or guardian present with the patient who could console or distract the subject.
Data collection was kept uniform between both groups. The maximum HR during the intervention was recorded serving as a surrogate for pain and/or perceived anxiety. HR was recorded at conclusion of the 5-min postprocedure period. After the procedure, each subject was asked standardized questions regarding their experience. Questionnaire included “how much did your procedure hurt?” followed by “how nervous or scared were you about the procedure?” Wong Baker FACES Scale was shown to determine the patient’s perceived pain and Visual Analog Scale (VAS) scores on a scale of 1–10 were used for perceived anxiety.11 –13 Postprocedure follow-up instructions were identical between the two groups.
The primary outcome was difference between the procedural HR max and baseline HR max. Secondary outcomes included perceived pain and anxiety scores. Average differences in HR max and average FACES/VAS scores were analyzed with T-tests with two-tail and two-sample unequal variance as the parameters. The cast removal subset was further analyzed as this cohort had an adequate population size (VR group n = 31, control group n = 28) to achieve 80% power. Significance was determined by p < 0.05. Minimum clinically important difference (MCID) was calculated as part of post-hoc analysis to evaluate clinically significant change in HR max, change in VAS anxiety, and FACES pain scores.
Results
Ninety-five patients (66 M, 29 F) were enrolled in the study (48 VR intervention, 47 control) (Table 1).
Table 1.
Demographics and Procedure Frequencies.
| Qualifying Factor | VR group | Control group | Total population |
|---|---|---|---|
| Sex, female (%) | 12 (25.0) | 17 (36.2) | 29 (30.5) |
| Mean age (SD) | 10.1 (3.1) | 10.6 (3.3) | 10.4 (3.2) |
| Cast application (%) | 13 (27.1) | 13 (27.7) | 26 (27.4) |
| Cast removal (%) | 31 (64.6) | 28 (59.6) | 59 (62.1) |
| Percutaneous bone pin removal (%) | 2 (4.2) | 5 (10.6) | 7 (7.4) |
| Fracture reduction (%) | 2 (4.2) | 1 (2.1) | 3 (3.2) |
| Total (%) | 48 (100.0) | 47 (100.0) | 95 (100.0) |
VR: Virtual Reality.
Table 1 outlines patient demographic factors and the procedures that were examined.
The enrollment period ended early due to imposed COVID-19 restrictions and concerns over infection control. A total of 295 patients were assessed and 107 met inclusion criteria; 12 declined to participate. The average patient age was 10.4 ± 3.2 years, 10.1 ± 3.1 (5–17) in the VR cohort and 10.6 ± 3.3 (5–17) in the control cohort. There were 59 patients with cast removals (31 VR, 28 control), 26 with cast applications (13 VR, 13 control), 7 with percutaneous bone pin removal (2 VR, 5 control), and 3 with fracture reductions (2 VR, 1 control). Patients undergoing cast removals involved treatment of both upper and lower extremity fractures—short arm (42%), long arm (26%), ulnar/radial gutter (11%), thumb spica (8%), short leg (8%), and long leg (3%). There was no significant difference between groups in type of cast removed. No analgesic or anxiolytic medications were used for any subject. No complications occurred in either group. No procedures in the VR group were aborted due to technical difficulties or patient noncompliance.
The VR cohort had a lower average difference in HR max (10.6 ± 10.1bpm) compared to the control group (18.4 ± 11.0 bpm), a reduction of 42% with a mean difference of 7.8 bpm (95% confidence interval [CI] 5.5–10.1; p = 0.00048) (Table 2).
Table 2.
Results of VR Versus Control Group.
| Sub-analysis | VR group* | Control group* | Mean difference † | p-Value |
|---|---|---|---|---|
| Change in HR max (bpm) • Cast removal subset |
10.6 ± 10.1 10.5 ± 11.1 |
18.4 ± 11.0 18.5 ± 12.2 |
7.8 (5.5–10.1) 8.0 (4.9–11.1) |
0.00048 0.0107 |
| Perceived anxiety (VAS Score) • Cast removal subset |
1.7 ± 2.8 1.6 ± 2.9 |
2.9 ± 3.6 2.9 ± 3.5 |
1.2 (0.5–1.9) 1.3 (0.5–2.0) |
0.0666 0.1214 |
| Perceived pain (FACES Score) • Cast removal subset |
0.9 ± 2.2 0.5 ± 1.2 |
1.8 ± 2.6 1.8 ± 2.8 |
0.9 (0.4–1.4) 1.3 (0.7–1.9) |
0.0675 0.0229 |
Values are given as the mean and standard deviation.
Values are given as mean difference with the 95% CI in parentheses.
VAS: Visual Analog Scale, VR: Virtual Reality.
Table 2 demonstrates the key study results.
The VR cohort had lower average VAS anxiety scores (1.7 ± 2.8) compared to the control (2.9 ± 3.6), a decrease of 41% with a mean difference of 1.2 (95% CI 0.5–1.9; p = 0.0666). The VR cohort had lower average pain FACES scores (0.9 ± 2.2) compared to the control (1.8 ± 2.6), a decrease of 50% with a mean difference of 0.9 (95% CI 0.4–1.4; p = 0.0675).
In the cast removal subset, the VR group had lower average differences in HR max (10.5 ± 11.1 bpm) versus the control group (18.5 ± 12.2 bpm), a 43% decrease with a mean difference of 8.0 bpm (95% CI 4.9–11.1; p = 0.0107). The VR cohort had lower average perceived anxiety scores (1.6 ± 2.9) compared to the control (2.9 ± 3.5), a decrease of 45% with a mean difference of 1.3 (95% CI 0.5–2.0; p = 0.1214). Lastly, the VR cohort had lower average perceived pain FACES scores (0.5 ± 1.2) compared to the control (1.8 ± 2.8), a decrease of 72% with a mean difference of 1.3 (95% CI 0.7–1.9; p = 0.0229).
Discussion
In-office orthopedic procedures can be both painful and anxiety-inducing, particularly in the pediatric population. Percutaneous bone pin removal is a painful procedure for up to 90% of children and adults, and up to 2% require general anesthesia or a more extensive procedure.14 –16 Multiple studies suggest that painful and anxiety-inducing procedures early in life may lead to increased pain, anxiety, and analgesia requirements with procedures later in life as well as avoidance of medical care.17 –19 To reduce procedural pain and anxiety, benzodiazepines, nitrous oxide gas, ketamine, or even general anesthesia have been used. However, multiple studies have demonstrated that these medications are ineffective at adequately improving patient anxiety and pain or are associated with undesired side effects, with some recommending the use of DT instead.16,20 Benzodiazepines and ketamine require respiratory monitoring at doses necessary for procedure sedation, and the pharmacokinetics of minimal dosing present challenges in optimal timing of administration. Novel technological innovations such as VR have demonstrated effectiveness in improving levels of anxiety, pain, and patient satisfaction for burn care, blood draws, and chemotherapy.4,6
Our study is the first to assess the impact of interactive VR DT during multiple modalities of pediatric outpatient orthopedic procedures. Unlike other VR distraction studies, both subjective and objective metrics of pain and anxiety were used, including HR as a physiological surrogate. 10 The modality for evaluating procedural anxiety is needed to be easy-to-use, quick, and requires no training. These principles were employed so that the study could be implemented into an existing clinical workflow. VAS has been validated in the pediatric population to assess anxiety and demonstrates strong correlation with other validated modalities including the modified Yale Preoperative Anxiety Scale. 21 Two separate studies have also validated the use of VAS in assessing anxiety by demonstrating statistically significant correlation with Spielberger State Trait Anxiety Inventory and Corah’s Dental Anxiety Scale while requiring a fraction of the time.22,23 The desired outcome of DT was to treat the pediatric patient’s perception of impending pain and lower anxiety by removing noxious stimuli. Our interactive VR system is immersive with both visual and audible stimuli. The in-house developed application can also be used without the handheld controller, providing an immersive experience with audio integration.
Increases in HR have a known association with sympathetic response due to fear or anxiety. 24 A smaller magnitude in HR change was observed in the VR group compared to the control group, likely representing lower perceived pain and anxiety. This difference was illustrated in both the total population (p = 0.00048) and the cast removal subset (p = 0.0107). Perceived anxiety and pain were also lower in the VR cohort with score reductions of 41% and 50%, respectively. While these differences were not statistically significant, both trended toward significance. Post-hoc analysis for MCID calculations showed a necessary change of 13.5 bpm between HR max and preprocedure HR to detect a clinical difference. MCID for change in pain and anxiety were 2.7 and 4.0, respectively. The MCID findings suggest that although statistical significance was found for HR change, more data and larger groups are needed to thoroughly examine the effect. In addition, more painful or anxiety-inducing procedures in a larger cohort may create a wider spread in variables in future trials to definitively determine if this trend is significant in the orthopedic population. It is possible that VR DT may subconsciously mitigate anxiety and pain in ways that young patients cannot adequately describe based on the significant changes in HR without significant differences in subjective metrics.
When stratified by procedure type, subjects in the cast removal subset had a reduction in pain seen both subjectively with FACES scores (p = 0.0229) and objectively with change in HR max (p = 0.0107). Perceived anxiety also improved in the VR group by 45% when stratified by cast removal; however, this was not a statistically significant finding (p = 0.1214). A reduction in anxiety during cast removal is consistent with the only other study evaluating the use of VR DT on pediatric cast removal, which found improvement in anxiety versus standard of care (p = 0.01). 10 However, this study by Jivraj et al. 25 used the Children’s Emotional Management Scale designed to measure anxiety during minor medical procedures with both subjective and physiological findings.
A common criticism of VR is its inferiority to standard 2D DT such as television or electronic tablets. Some similar nonorthopedic studies have added a third treatment group with 2D DT, including iPads or standard video games.26,27 Our injuries involved the upper extremities and inhibited the use of an electronic tablet or videogame controller, which typically require two hands. In contrast, the VR system only requires one noninjured hand. Because the VR intervention was implemented for both genders with a wide range of pediatric ages and common procedures, our results demonstrate that VR DT would benefit a variety of patients undergoing in-office orthopedic procedures. Enrolled patients were able to freely interact with the virtual headset environment using their controller without impeding the completion of the procedure. Staff acceptance and barriers to implementation were important factors in the overall success of this project. They additionally represent a key factor in clinic implementation outside of the study context. We found VR technology made a meaningful difference in the care of our patients and the barriers to implantation for clinic staff were minimal. Our patients undergoing clinic procedures have certified child life specialists (CCLSs) available for DT, and thus, the implementation of VR did not present a new logistical challenge. CCLS participate regularly at our institution to minimize psychosocial trauma associated with healthcare and hospitalization while promoting optimal development of infants, children, youth, and family members. The CCLS team was integrated within the pediatric clinic setting and was available at all times for orthopedic patients undergoing procedures, physical examinations, or upon request.
We acknowledge that the level of immersion seen with VR distraction may itself be anxiety-provoking, and patients may prefer to be aware of what is happening in their procedure. This was demonstrated by 12 of 112 (10.7%) eligible subjects who elected not to participate. We were also unable to evaluate the use of VR DT in patients with developmental delay, cerebral palsy and autism spectrum disorder. A further limitation involves subset analysis by procedure type—three of four procedures types did not reach the minimum power necessary for further analysis. Future directions should include larger cohorts to delineate differences between procedure types.
In terms of the control group, other studies have made it clear whether parents or guardians were present as distractors or removed any potential distractor. This study attempted to demonstrate that VR therapy was superior to standard of care, which typically includes parents rather than no distraction at all. Lastly, the study could not be blinded due to the nature of VR. Based on our review of the literature, no other studies evaluating the use of VR DT were blinded.
In conclusion, immersive VR DT is a viable modality to reduce both pain and perceived anxiety during pediatric in-office orthopedic procedures with little to no adverse effects. We anticipate that as VR DT continues to demonstrate efficacy in different medical situations, it will be implemented as a new option for care.
Supplemental Material
Supplemental material, sj-pdf-1-cho-10.1177_18632521241254707 for Virtual reality use in pediatric patients for orthopedic clinical procedures: A randomized prospective trial of efficacy by Bhumit Desai, Nicholas Newcomb, Brielle Plost, Sean Waldron, Korak Sarkar and Lawrence Haber in Journal of Children’s Orthopaedics
Footnotes
Author contributions: Bhumit Desai: contributed to conception or design; contributed to acquisition, analysis, or interpretation; drafted the manuscript; agrees to be accountable for all aspects of work ensuring integrity and accuracy. Nicholas Newcomb: drafted the manuscript; agrees to be accountable for all aspects of work ensuring integrity and accuracy. Brielle Plost: contributed to acquisition, analysis, or interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. Sean Waldron: contributed to acquisition, analysis, or interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. Korak Sarkar: contributed to conception or design; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. Lawrence Haber: contributed to conception or design; contributed to acquisition, analysis, or interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical statement: The Ochsner Medical Center Institutional Review Board approved the study on March 8th, 2018 (No. 2017.522). The clinical trial was registered at the US National Institutes of Health (clinicaltrials.gov, NCT04901793) and a written consent form was furnished to participants at time of study enrollment.
ORCID iD: Bhumit Desai, MD,  https://orcid.org/0000-0002-5456-3939
https://orcid.org/0000-0002-5456-3939
Supplemental material: Supplemental material for this article is available online.
References
- 1. Wong CL, Ip WY, Kwok BMC, et al. Effects of therapeutic play on children undergoing cast-removal procedures: a randomised controlled trial. BMJ Open 2018; 8(7): e021071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schlechter JA, Avik AL, DeMello S. Is there a role for a child life specialist during orthopedic cast room procedures? A prospective-randomized assessment. J Pediatr Orthop B 2017; 26(6): 575–579. [DOI] [PubMed] [Google Scholar]
- 3. Mahan ST, Harris MS, Lierhaus AM, et al. Noise reduction to reduce patient anxiety during cast removal: can we decrease patient anxiety with cast removal by wearing noise reduction headphones during cast saw use? Orthop Nurs 2017; 36(4): 271–278. [DOI] [PubMed] [Google Scholar]
- 4. Ko JS, Whiting Z, Nguyen C, et al. A randomized prospective study of the use of ipads in reducing anxiety during cast room procedures. Iowa Orthop J 2016; 36: 128–132. [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta A, Scott K, Dukewich M. Innovative technology using virtual reality in the treatment of pain: does it reduce pain via distraction, or is there more to it? Pain Med 2018; 19(1): 151–159. [DOI] [PubMed] [Google Scholar]
- 6. Eijlers R, Utens E, Staals LM, et al. Systematic review and meta-analysis of virtual reality in pediatrics: effects on pain and anxiety. Anesth Analg 2019; 129: 1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koo CH, Park JW, Ryu JH, et al. The effect of virtual reality on preoperative anxiety: a meta-analysis of randomized controlled trials. J Clin Med 2020; 9(10): 3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanders MB, Starr AJ, Frawley WH, et al. Posttraumatic stress symptoms in children recovering from minor orthopaedic injury and treatment. J Orthop Trauma 2005; 19(9): 623–628. [DOI] [PubMed] [Google Scholar]
- 9. Katz K, Fogelman R, Attias J, et al. Anxiety reaction in children during removal of their plaster cast with a saw. J Bone Joint Surg Br 2001; 83: 388–390. [DOI] [PubMed] [Google Scholar]
- 10. Jivraj BA, Schaeffer E, Bone JN, et al. The use of virtual reality in reducing anxiety during cast removal: a randomized controlled trial. J Child Orthop 2020; 14: 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abend R, Dan O, Maoz K, et al. Reliability, validity and sensitivity of a computerized visual analog scale measuring state anxiety. J Behav Ther Exp Psychiatry 2014; 45: 447–453. [DOI] [PubMed] [Google Scholar]
- 12. Rossi V, Pourtois G. Transient state-dependent fluctuations in anxiety measured using STAI, POMS, PANAS or VAS: a comparative review. Anxiety Stress Coping 2012; 25: 603–645. [DOI] [PubMed] [Google Scholar]
- 13. von Baeyer CL. Children’s self-reports of pain intensity: scale selection, limitations and interpretation. Pain Res Manag 2006; 11: 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dulai SK, Firth K, Al-Mansoori K, et al. Does topical anesthetic reduce pain during intraosseous pin removal in children? A randomized controlled trial. J Pediatr Orthop 2016; 36: 126–131. [DOI] [PubMed] [Google Scholar]
- 15. Symons S, Persad R, Paterson M. The removal of percutaneous Kirschner wires used in the stabilisation of fractures in children. Acta Orthop Belg 2005; 71: 88–90. [PubMed] [Google Scholar]
- 16. Templeton P, Burton D, Cullen E, et al. Oral midazolam for removal of Kirschner wires in the children’s orthopaedic outpatient department: a randomized controlled trial. J Pediatr Orthop 2010; 30: 130–134. [DOI] [PubMed] [Google Scholar]
- 17. Noel M, Rabbitts JA, Fales J, et al. The influence of pain memories on children’s and adolescents’ post-surgical pain experience: a longitudinal dyadic analysis. Health Psychol 2017; 36: 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taddio A, Katz J, Ilersich AL, et al. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet 1997; 349: 599–603. [DOI] [PubMed] [Google Scholar]
- 19. Weisman SJ, Bernstein B, Schechter NL. Consequences of inadequate analgesia during painful procedures in children. Arch Pediatr Adolesc Med 1998; 152: 147–149. [DOI] [PubMed] [Google Scholar]
- 20. Sorenson SM, Hennrikus W. Pain during office removal of K-wires from the elbow in children. J Pediatr Orthop 2015; 35: 341–344. [DOI] [PubMed] [Google Scholar]
- 21. Berghmans JM, Poley MJ, van der Ende J, et al. A Visual Analog Scale to assess anxiety in children during anesthesia induction (VAS-I): results supporting its validity in a sample of day care surgery patients. Paediatr Anaesth 2017; 27: 955–961. [DOI] [PubMed] [Google Scholar]
- 22. Ducoulombier V, Chiquet R, Graf S, et al. Usefulness of a visual analog scale for measuring anxiety in hospitalized patients experiencing pain: a multicenter cross-sectional study. Pain Manag Nurs 2020; 21: 572–578. [DOI] [PubMed] [Google Scholar]
- 23. Facco E, Stellini E, Bacci C, et al. Validation of visual analogue scale for anxiety (VAS-A) in preanesthesia evaluation. Miinerva Anesesiol 2013; 79: 1389–1395. [PubMed] [Google Scholar]
- 24. Rathmell J, Fields H. Pain: Pathophysiology and Management. 18 ed. New York, NY: McGraw-Hill, 2012. [Google Scholar]
- 25. Li HC, Lopez V. Children’s Emotional Manifestation Scale: development and testing. J Clin Nurs 2005; 14: 223–229. [DOI] [PubMed] [Google Scholar]
- 26. Robertson A, Fick D, Robertson WB, et al. The effect of Virtual Reality in reducing preoperative anxiety in patients prior to arthroscopic knee surgery: a randomised controlled trial. In: Engineers IoEaE, ed., Program Booklet: IEEE 5th International Conference on Serious Games and Applications for Health. Perth, Western Australia, 2017. [Google Scholar]
- 27. Gershon J, Zimand E, Pickering M, et al. A pilot and feasibility study of virtual reality as a distraction for children with cancer. J Am Acad Child Adolesc Psychiatry 2004; 43: 1243–1249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cho-10.1177_18632521241254707 for Virtual reality use in pediatric patients for orthopedic clinical procedures: A randomized prospective trial of efficacy by Bhumit Desai, Nicholas Newcomb, Brielle Plost, Sean Waldron, Korak Sarkar and Lawrence Haber in Journal of Children’s Orthopaedics