Abstract
Purpose
Recently, the detrimental effect of cigarette smoking on muscle metabolism has attracted much attention, but the relationship between cigarette smoking and muscle mass is poorly understood. Thus, this study investigated the association between exposure to cigarette smoke, defined based on serum cotinine, and muscle mass in the US population.
Methods
We utilized National Health and Nutrition Examination Survey (NHANES) data between 2011 and 2018 for analysis. Data on serum cotinine, muscle mass (quantified by appendicular skeletal muscle mass index, ASMI), and covariates were extracted and analyzed. Weighted multivariate linear regression analyses and smooth curve fittings were performed to investigate the association between serum cotinine and ASMI. Subgroup analyses were stratified by gender, race and smoking status. When nonlinearity was detected, the threshold effects were analyzed using a two-piecewise linear regression model.
Results
In total, 8004 participants were included for analysis. The serum level of cotinine was negatively associated with ASMI in the fully adjusted model. Furthermore, comparing participants in the highest vs. the lowest tertile of serum cotinine, we found that ASMI decreased by 0.135 Kg/m2. In subgroup analysis stratified by gender and race, the association between serum cotinine and ASMI remained significant in all genders and races. In addition, the association remained significant among current and former smokers, but not among those who never smoked. Smooth curve fittings showed nonlinear relationships between serum cotinine and ASMI, with the inflection points identified at 356 ng/mL.
Conclusions
Our study revealed that serum cotinine was negatively related to muscle mass. This finding improves our understanding of the deleterious effects of cigarette smoking on muscle mass and highlights the importance of smoking cessation for muscle health.
Keywords: Cigarette smoke, Serum cotinine, Skeletal muscle, NHANES
Introduction
Sarcopenia, an age-associated progressive loss of skeletal muscle mass, is accompanied by decreased muscle strength and function [1]. It has been estimated that sarcopenia will affect more than 500 million older adults by 2050 [2]. Numerous studies reported that sarcopenia significantly increased the risk of falls, disability, frailty, hospitalization, and mortality [3, 4]. Given the serious consequences of sarcopenia, identifying modifiable risk factors has become an urgent public health priority.
Smoking is the single most preventable cause of disease, disability, and death [5]. Studies have demonstrated that active or passive smoking is associated with a variety of diseases, including cancer, cardiovascular, respiratory, and neurological diseases [6, 7]. Recently, the detrimental effects of smoking on muscle metabolism have attracted much attention. Several basic studies have revealed that smoking inhibits the synthesis and promotes the degradation of muscle proteins [8, 9]. Furthermore, some researchers reported that cigarette smoking was associated with reduced muscle strength [10]. However, few studies reported the potential effect of cigarette smoke exposure on muscle mass. Cigarette smoke contains large amounts of harmful compounds, including nicotine, nitrosamines, and heavy metals [11]. Cotinine, a major metabolite of nicotine, is currently used as a reliable biomarker of exposure to cigarette smoke [12]. Thus, we conducted this study to investigate the association between cigarette smoke exposure, defined based on serum cotinine, and muscle mass in the US population.
Materials and methods
Source of data
The NHANES is a national survey conducted by the Centers for Disease Control and Prevention, which monitors the health and nutritional status of children and adults across the US. The National Center for Health Statistics (NCHS) Ethics Review Board approved NHANES protocols, and all participants gave informed consent for using their data for research [13].
Study population
We utilized data from four 2-year cycles of NHANES (2011–2018) for analysis.
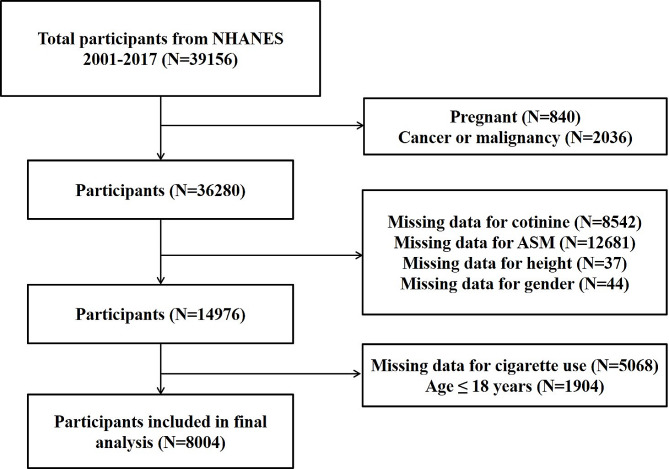
Participants with complete data on serum cotinine, appendicular skeletal muscle mass (ASM), standing height, and cigarette use were enrolled in this study. We excluded participants who were pregnant (n = 840), had cancer (n = 2036), or lacked data for cotinine (n = 8542), ASM (n = 12681), standing height (n = 37), gender (n = 44), and cigarette use (n = 5068), age ≤ 18 years (n = 1904). (The flowchart of the selection processes is shown in Fig. 1.)
Fig. 1.
The flowchart of selection processes
For each included participant, data on serum cotinine, ASM, standing height, cigarette use, and covariates were extracted and analyzed.
Study variables
Serum cotinine
Serum cotinine (ng/mL) was measured by an isotope dilution-high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry (ID HPLC-APCI MS/MS).
Muscle mass
Body composition was evaluated by dual-energy X-ray absorptiometry (DXA) scan (Hologic, Inc., Bedford, Massachusetts), which measured bone and soft tissue for the whole body, including both arms and legs, the truck, and the head. The ASM (Kg) was defined as the sum of the lean soft tissue mass of both arms and legs [14]. We used appendicular skeletal muscle mass index (ASMI, Kg/m2) to further quantify muscle mass. It was calculated by ASM divided by height square (ASM/ht2) [15].
Covariates
Based on the literature, the following variables were selected as covariates in this study [13, 16–18]. Data on demographic variables, including age, gender (male or female), race (Hispanic, Non-Hispanic White, Non-Hispanic Black, and Other Races), and family income to poverty ratio (FIPR), were collected from demographic data. The FIPR was categorized as < 1.3, 1.3–3.5, > 3.5 [15]. White blood cell counts (WBC, 1000 cells/uL) and hemoglobin (g/dL) were measured using the Coulter® DxH 800 analyzer. Glycohemoglobin was measured using G7/G8 Glycohemoglobin analyzer. Serum albumin (g/dL), globulin (g/dL), AST (IU/L), iron (ug/dL), uric acid (mg/dL), and creatinine (mg/dL) were measured using Beckman UniCel® DxC800 Synchron. HDL-cholesterol, LDL-cholesterol, total cholesterol, and triglyceride were measured using Roche/Hitachi Modular P Chemistry Analyzer. Vitamin D was measured using High-Performance Liquid Chromatography-Tandem Mass Spectrometry.
The presence or absence of hypertension and diabetes was defined by participants’ self-reports of their doctor’s diagnosis. Smoking status was collected from Questionnaire Data. Participants who smoked less than 100 cigarettes in their life were defined as never smokers; participants who had smoked at least 100 cigarettes, but did not smoke at the time of survey, were defined as former smokers; and participants who had smoked more than 100 cigarettes and smoked cigarettes at the time of survey were considered current smokers [19].
Statistical analysis
The statistical analyses were conducted using R 3.4.3 (https://www.r-project.org/) and EmpowerStats 2.0 (http://www.empowerstats.com). P < 0.05 was considered statistically significant. The NHANES sample weights were used as recommended by the NCHS. The weighted linear regression model and weighted chi-square test were used to compare the continuous and categorical variables, respectively. Weighted multivariable linear regression analyses were performed to investigate the association between serum cotinine and ASMI. Subgroup analyses stratified by gender, race and smoking status were also performed. The generalized additive models and smooth curve fittings were conducted to explore the nonlinear relationship. When nonlinearity was detected, a two-piecewise linear regression model was performed to analyze the threshold effect.
Results
In total, 8004 participants were enrolled based on the inclusion and exclusion criteria. There were 4750 men and 3254 women, and their mean ages were 38.406 ± 11.909 years and 37.477 ± 11.747 years, respectively. Men were more likely than women to have higher levels of hemoglobin, albumin, AST, iron, uric acid, creatinine, LDL-cholesterol, triglyceride, vitamin D, ASMI, and cotinine, higher rate of current and former smoking, and lower levels of WBC, globulin, and HDL-cholesterol (Table 1).
Table 1.
Baseline characteristics of study participants
| Man | Woman | P value | |
|---|---|---|---|
| n (%) | 4750 (59.35) | 3254 (40.65) | |
| Age (year) | 38.406 ± 11.909 | 37.477 ± 11.747 | 0.00056 |
| WBC (1000 cells/uL) | 7.225 ± 2.121 | 7.411 ± 2.185 | 0.00014 |
| Hemoglobin (g/dL) | 15.227 ± 1.059 | 13.143 ± 1.265 | < 0.00001 |
| Glycohemoglobin (%) | 5.538 ± 0.946 | 5.571 ± 1.011 | 0.13772 |
| Albumin (g/dL) | 4.431 ± 0.316 | 4.181 ± 0.324 | < 0.00001 |
| Globulin (g/dL) | 2.765 ± 0.407 | 2.993 ± 0.429 | < 0.00001 |
| AST (IU/L) | 27.232 ± 18.178 | 22.666 ± 19.254 | < 0.00001 |
| Iron (ug/dL) | 96.360 ± 36.860 | 76.717 ± 37.552 | < 0.00001 |
| Uric acid (mg/dL) | 6.012 ± 1.218 | 4.579 ± 1.122 | < 0.00001 |
| Creatinine (mg/dL) | 0.965 ± 0.315 | 0.726 ± 0.230 | < 0.00001 |
| HDL-Cholesterol (mg/dL) | 47.853 ± 13.463 | 56.558 ± 15.589 | < 0.00001 |
| LDL-Cholesterol (mg/dL) | 114.019 ± 33.994 | 111.350 ± 35.100 | 0.02229 |
| Total Cholesterol (mg/dL) | 189.902 ± 39.890 | 188.798 ± 38.828 | 0.21922 |
| Triglyceride (mg/dL) | 133.673 ± 128.096 | 97.479 ± 71.650 | < 0.00001 |
| Vitamin D (nmol/L) | 63.399 ± 22.933 | 61.204 ± 26.878 | 0.00009 |
| ASMI (Kg/m2) | 8.902 ± 1.375 | 7.087 ± 1.487 | < 0.00001 |
| Cotinine (ng/mL) | 77.197 ± 147.908 | 43.811 ± 104.380 | < 0.00001 |
| Race (%) | < 0.00001 | ||
| Hispanic | 19.419 | 12.428 | |
| White | 59.669 | 63.749 | |
| Black | 10.784 | 14.548 | |
| Other | 10.128 | 9.275 | |
| FIPR (%) | 0.20616 | ||
| < 1.3 | 22.849 | 23.069 | |
| 1.3–3.5 | 35.715 | 33.804 | |
| > 3.5 | 41.436 | 43.127 | |
| Hypertension (%) | 0.12483 | ||
| Yes | 23.860 | 22.385 | |
| No | 76.140 | 77.615 | |
| Diabetes (%) | 0.10610 | ||
| Yes | 5.731 | 6.796 | |
| No | 92.384 | 91.589 | |
| Borderline | 1.885 | 1.615 | |
| Smoking status (%) | < 0.00001 | ||
| Current | 30.306 | 17.831 | |
| Former | 15.353 | 11.996 | |
| Never | 54.341 | 70.173 |
Mean ± SD for continuous variables: P-value was calculated by weighted linear regression model. Percent for categorical variables: P-value was calculated by weighted chi-square test
Association between serum cotinine and muscle mass
Table 2 presents the association between serum cotinine and muscle mass in three multivariate linear regression models. In the unadjusted model, there was no significant association between serum cotinine and muscle mass (model 1: β = 0.000, 95%CI: -0.000 to 0.001). When adjusted for covariates, we observed negative associations between serum cotinine and muscle mass in model 2: β=-0.001, 95%CI: -0.001 to -0.000 and model 3: β=-0.001, 95%CI: -0.001 to -0.001. Furthermore, by comparing participants in the highest vs. the lowest tertile of serum cotinine in model 3, we found that ASMI decreased by 0.135 Kg/m2, P trend = 0.01.
Table 2.
Association between serum cotinine and ASMI.
| Outcome | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| β (95%CI) | P-value | β (95%CI) | P-value | β (95%CI) | P-value | |||
| Cotinine (ng/mL) | 0.000 (-0.000, 0.001) | 0.07990 | -0.001 (-0.001, -0.000) | < 0.00001 | -0.001 (-0.001, -0.001) | < 0.00001 | ||
| Cotinine (tertiles) | ||||||||
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 0.150 (0.060, 0.240) | 0.00107 | 0.158 (0.082, 0.234) | 0.00005 | 0.028 (-0.080, 0.136) | 0.60821 | ||
| Q3 | 0.241 (0.152, 0.330) | < 0.00001 | -0.003 (-0.079, 0.073) | 0.93640 | -0.135 (-0.264, -0.007) | 0.03845 | ||
| P for trend | < 0.001 | 0.02 | 0.01 | |||||
Model 1: no covariates were adjusted. Model 2: age, gender and race were adjusted. Model 3: age, gender, race, FIPR, WBC, hemoglobin, glycohemoglobin, albumin, globulin, AST, iron, uric acid, creatinine, HDL, LDL, total Cholesterol, triglyceride, vitamin D, hypertension, diabetes, and smoking status were adjusted
In subgroup analysis stratified by gender, the association between serum cotinine and ASMI remained significant in both man and woman. In subgroup analysis stratified by race, the association between serum cotinine and ASMI remained significant in all races (Table 3). The association remained significant in current smokers and former smokers, but not in never smokers (Table 4).
Table 3.
Association between serum cotinine and ASMI stratified by gender and race
| Outcome | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| β (95%CI) | P-value | β (95%CI) | P-value | β (95%CI) | P-value | |||
| Gender | ||||||||
| Man | -0.001 (-0.001, -0.000) | < 0.00001 | -0.001 (-0.001, -0.001) | < 0.00001 | -0.001 (-0.001, -0.000) | 0.00026 | ||
| Woman | -0.000 (-0.001, 0.000) | 0.61601 | -0.000 (-0.001, 0.000) | 0.39149 | -0.002 (-0.003, -0.001) | 0.00006 | ||
| Race | ||||||||
| Hispanic | 0.000 (-0.001, 0.001) | 0.88234 | -0.001 (-0.001, 0.000) | 0.09341 | -0.002 (-0.003, -0.001) | 0.00241 | ||
| Non-Hispanic White | 0.000 (-0.000, 0.001) | 0.16893 | -0.001 (-0.001, -0.000) | 0.00182 | -0.001 (-0.001, -0.000) | 0.00532 | ||
| Non-Hispanic Black | 0.000 (-0.000, 0.001) | 0.33647 | -0.001 (-0.002, -0.001) | < 0.00001 | -0.001 (-0.002, -0.000) | 0.03349 | ||
| Other | 0.001 (0.000, 0.002) | 0.01091 | 0.000 (-0.000, 0.001) | 0.70546 | -0.001 (-0.002, -0.000) | 0.00589 | ||
Model 1: no covariates were adjusted. Model 2: age, gender and race were adjusted. Model 3: age, gender, race, FIPR, WBC, hemoglobin, glycohemoglobin, albumin, globulin, AST, iron, uric acid, creatinine, HDL, LDL, total Cholesterol, triglyceride, vitamin D, hypertension, diabetes, and smoking status were adjusted
Table 4.
Association between serum cotinine and ASMI stratified by smoking status
| Outcome | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| β (95%CI) | P-value | β (95%CI) | P-value | β (95%CI) | P-value | |||
| Current smoker | -0.001 (-0.001, -0.000) | 0.01413 | -0.001 (-0.001, -0.000) | 0.00057 | -0.001 (-0.002, -0.000) | 0.00076 | ||
| Former smoker | -0.001 (-0.002, -0.001) | 0.00005 | -0.001 (-0.002, -0.001) | 0.00002 | -0.002 (-0.003, -0.001) | < 0.00001 | ||
| Never smoker | 0.002 (0.001, 0.002) | < 0.00001 | 0.000 (-0.000, 0.001) | 0.36979 | -0.000 (-0.001, 0.001) | 0.82087 | ||
Model 1: no covariates were adjusted. Model 2: age, gender and race were adjusted. Model 3: age, gender, race, FIPR, WBC, hemoglobin, glycohemoglobin, albumin, globulin, AST, iron, uric acid, creatinine, HDL, LDL, total Cholesterol, triglyceride, vitamin D, hypertension, diabetes, and smoking status were adjusted
Threshold effect analysis
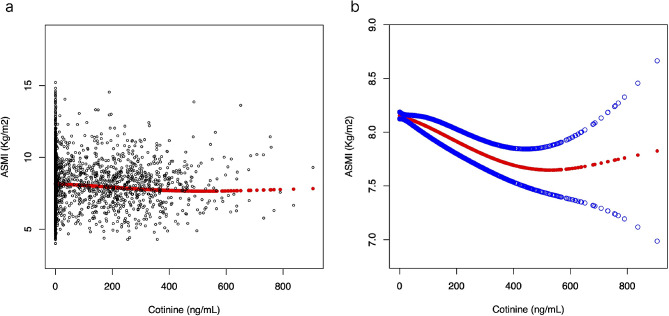
There was a nonlinear relationship between serum cotinine and ASMI (Fig. 2), with the inflection point identified at 356 ng/mL. When serum cotinine levels were < 356 ng/mL, a one-unit increase in serum cotinine level was associated with 0.001 units reduction in ASMI. When serum cotinine levels were > 356 ng/mL, no significant association was observed with ASMI. (Table 5)
Fig. 2.
The relationship between serum cotinine and ASMI. (a) Each black point represents a sample. (b) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit
Table 5.
Threshold effect analysis of serum cotinine on ASMI using two-piecewise linear regression model
| Adjusted β(95%CI) | P-value | |
|---|---|---|
| All participants | ||
| cotinine < 356 ng/mL | -0.001 (-0.002, -0.001) | < 0.0001 |
| cotinine > 356 ng/mL | 0.001 (-0.000, 0.002) | 0.1991 |
| Man | ||
| cotinine < 386 ng/mL | -0.002 (-0.002, -0.001) | < 0.0001 |
| cotinine > 386 ng/mL | 0.002 (-0.001, 0.004) | 0.149 |
| Woman | ||
| cotinine < 92 ng/mL | 0.001 (-0.002, 0.005) | 0.4600 |
| cotinine > 92 ng/mL | -0.003 (-0.004, -0.001) | 0.0002 |
| Non-Hispanic White | ||
| cotinine < 393 ng/mL | -0.001 (-0.002, -0.001) | 0.0004 |
| cotinine > 393 ng/mL | 0.002 (-0.001, 0.005) | 0.1352 |
| Current smoker | ||
| cotinine < 459 ng/mL | -0.001 (-0.002, -0.001) | < 0.0001 |
| cotinine > 459 ng/mL | 0.003 (-0.000, 0.006) | 0.0568 |
Note: age, gender, race, FIPR, WBC, hemoglobin, glycohemoglobin, albumin, globulin, AST, iron, uric acid, creatinine, HDL, LDL, total Cholesterol, triglyceride, vitamin D, hypertension, diabetes, and smoking status were adjusted
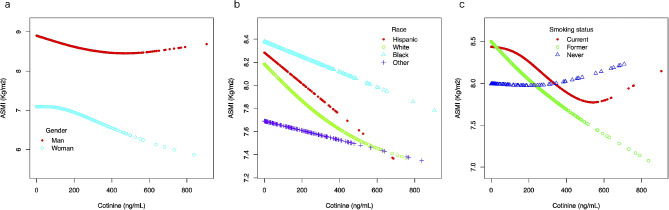
A nonlinear relationship was detected between serum cotinine and ASMI in man and woman. When serum cotinine levels were < 386 ng/mL, a one-unit increase in serum cotinine level was associated with 0.002 units reduction of ASMI in man. When serum cotinine levels were > 386 ng/mL, no significant association was observed with ASMI. When serum cotinine levels were > 92 ng/mL, a one-unit increase in serum cotinine level was associated with 0.003 units reduction of ASMI in woman. When serum cotinine levels were < 92 ng/mL, no significant association was observed with ASMI. (Fig. 3a; Table 5)
Fig. 3.
The subgroup analysis of the relationship between serum cotinine and ASMI. (a) Stratified by gender. (b) Stratified by race. (c) Stratified by smoking status
Similarly, a nonlinear relationship was detected between serum cotinine and ASMI among participants of the Non-Hispanic White race, with the inflection point identified at 393 ng/mL. When serum cotinine levels were < 393 ng/mL, a one-unit increase in serum cotinine level was associated with 0.001 units reduction in ASMI. When serum cotinine levels were > 393 ng/mL, no significant association was observed with ASMI. (Fig. 3b; Table 5)
There was a nonlinear relationship among current smokers, with the inflection point identified at 459 ng/mL. When serum cotinine levels were < 459 ng/mL, a one-unit increase in serum cotinine level was associated with 0.001 units reduction in ASMI. When serum cotinine levels were > 459 ng/mL, no significant association was observed with ASMI. (Fig. 3c; Table 5)
Discussion
With the aging population, the prevalence of sarcopenia continues to rise. A previous study reported that the incidence of sarcopenia varied from 6.1 to 44.3% among the elderly population [20]. To date, there is no effective treatment for sarcopenia; therefore, preventive measures are of great importance [21]. Although several risk factors have been identified, the effect of smoking on muscle mass remains poorly defined. Therefore, we performed this study to explore the role of cigarette smoke exposure on muscle loss. The results demonstrated a negative association between serum cotinine and muscle mass.
Although various tobacco control measures have been implemented, the prevalence of smoking remains considerably high, particularly in low-income and middle-income countries [22, 23]. To make matters worse, the prevalence of smoking among adolescents increased from 8.3% in 2003 to 12.5% in 2013 [22]. Cigarette smoking is closely related to chronic non-communicable diseases, such as chronic obstructive pulmonary diseases, ischemic stroke, ischemic heart disease, diabetes, osteoporosis, and cancers [24, 25]. Besides, accumulating evidence showed a strong link between cigarette smoke exposure and skeletal muscle dysfunction. Hansen et al. reported a significant loss of skeletal muscles (soleus and gastrocnemius) in mice exposed to cigarette smoke compared with sham mice [26]. Similarly, Caron et al. found a 10.8% reduction in gastrocnemius weight in mice exposed to cigarette smoke compared to mice inhaling room air [8]. Other studies also observed a significant muscle type redistribution (shift from type I to type II), and a reduction in muscle capillary to fiber ratio in mice exposed to cigarette smoke [27]. In addition to structural alteration of skeletal muscle, cigarette smoke exposure has deleterious effects on muscle function. Wang et al. found that the average and maximum grip strength were significantly lower in mice exposed to cigarette smoke compared with sham mice [28]. In a cross-sectional study, Carrasco-Rios et al. revealed that participants with the highest quartile of serum cotinine had 1.41 kg reduction in combined grip strength compared with participants with the lowest quartile of serum cotinine [10]. Similar results were also reported by other studies [29, 30]. This study is the largest population-based study that investigated the association between serum cotinine and muscle mass. The results demonstrated a negative association between serum cotinine and muscle mass. Participants with the highest tertile of serum cotinine had 0.135 Kg/m2 reduction in ASMI compared with participants with the lowest tertile of serum cotinine.
Although the mechanisms that lead to muscle dysfunction have not been fully elucidated, multiple factors, such as systemic and local inflammation, oxidative stress, and anti-estrogen effect, may be involved [26, 31, 32]. Animal studies indicated a significantly higher abundance of inflammatory cells, proinflammatory cytokines, and chemokines in mice exposed to cigarette smoke [26, 28]. Human studies further confirmed systemic low-grade inflammation in smokers, evidenced by elevated serum levels of interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) [33, 34]. Several studies demonstrated that exposure to cigarette smoke disrupted the oxidative/antioxidant balance by increasing superoxide radicals and decreasing superoxide dismutase and catalase levels [35]. Additionally, smoking has potential anti-estrogenic effects by increasing estradiol 2-hydroxylation and producing metabolites with minimal estrogenic activity [36]. Moreover, smoking may reduce IGF-1 and vitamin-D levels [26, 37]. The combination of these factors enhances proteolysis, inhibits protein synthesis, and finally reduces muscle mass [38].
Although we cannot effectively prevent the aging process, we can reduce cigarette exposure and maintain serum cotinine at low levels for better muscle health. In vivo study showed that quitting smoking for 60 days in BALB/c mice attenuated the pro-catabolic and anti-anabolic signaling induced by cigarette exposure, suggesting that smoking cessation has beneficial effects on skeletal muscle metabolism [8]. In a twelve-month longitudinal study, the authors reported that adult smokers who abstained from cigarette smoking gained 1.26 kg lean mass and 3.6 kg handgrip strength compared with those who continued smoking [39]. Another study focusing on postmenopausal women found that smoking cessation was associated with increased muscle mass and functional capacity [40]. Consistently, a meta-analysis performed by Aubin et al. revealed that smoking cessation was associated with an average weight gain of 4–5 kg after 12 months of abstinence [41]. Thus, avoiding cigarette smoke exposure may help prevent or delay the progression of age-related muscle loss.
Limitations
There were some limitations to this study. First, since this was a cross-sectional study using NHANES data, the causal relationship could not be verified. Second, although the present study adjusted for several relevant variables, potential residual confounding, such as physical activity, dietary habits, volatile organic compounds exposure, and use of nicotine replacement therapy or corticosteroid agents may have altered our results. Third, the DXA scan was performed only on 8- to 59-years-old participants; therefore, the relationship between serum cotinine and muscle mass among elderly participants could not be explored. Fourth, there are many techniques can be used to evaluate the muscle mass, including DXA, CT, MRI, ultrasound, etc. Compared with CT and MRI, DXA can only estimate the value of muscle mass, but can’t measure the area of the muscle and the rate of intermuscular adipose infiltration. Thus, more studies are needed to confirm these findings and explore the underlying mechanisms.
Conclusion
The present study provides compelling evidence that the serum level of cotinine is negatively associated with muscle mass. This finding improves our understanding of the deleterious effects of cigarette smoke on muscle mass and highlights the importance of smoking cessation for muscle health. Hence, more practical and effective policies are needed to reduce prevalence of smoking.
Acknowledgements
Not applicable.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- ASMI
Appendicular skeletal muscle mass index
- NCHS
National Center for Health Statistics
- ASM
Appendicular skeletal muscle mass
- DXA
Dual-energy x-ray absorptiometry
- FIPR
Family income to poverty ratio
- WBC
White blood cell counts
- IL-1β
Interleukin-1β
- TNF-α
Tumor necrosis factor-α
- CRP
C-reactive protein
Author contributions
Zhi Chen wrote the manuscript, Zhi Chen, Hongxiang Li, Chenyang Song and Jun Sun collected and analyzed the data, Wenge Liu designed this study and revised the manuscript. All authors read and approved the final manuscript.
Funding
There was no funding information to declare.
Data availability
All data was collected in the NHANES database (http://www.cdc.gov/nchs/nhanes/). The data are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The National Center for Health Statistics ethics review board approved all NHANES protocols, and informed consent was obtained from every participant. We confirmed that all methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhi Chen and Hongxiang Li contributed equally to this work.
References
- 1.Yuan S, Larsson SC. Epidemiology of Sarcopenia: prevalence, risk factors, and consequences. Metabolism 2023:155533. [DOI] [PubMed]
- 2.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older people. Age Ageing. 2010;39(4):412–23. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Cao M, Liu M, Liu S, Zhao Z, Chen H. Association between Sarcopenia and cognitive impairment in the older people: a meta-analysis. Eur Geriatr Med. 2022;13(4):771–87. 10.1007/s41999-022-00661-1 [DOI] [PubMed] [Google Scholar]
- 4.Xia L, Zhao R, Wan Q, Wu Y, Zhou Y, Wang Y, Cui Y, Shen X, Wu X. Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med. 2020;9(21):7964–78. 10.1002/cam4.3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soteriades S, Basagiannis A, Soteriades ES, Barbouni A, Mouchtouri V, Rachiotis G, Hadjichristodoulou C. Nicotine dependence and loss of autonomy among Greek adolescent smokers: a Countrywide Cross-sectional Study. Int J Environ Res Public Health 2020, 17(21). [DOI] [PMC free article] [PubMed]
- 6.Sharma R, Rakshit B. Global burden of cancers attributable to tobacco smoking, 1990–2019: an ecological study. Epma j. 2023;14(1):167–82. 10.1007/s13167-022-00308-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groenewald P, Pacella R, Sitas F, Awotiwon OF, Vellios N, Van Rensburg CJ, Manda S, Laubscher R, Nojilana B, Joubert JD, et al. Estimating the changing disease burden attributable to smoking in South Africa for 2000, 2006 and 2012. S Afr Med J. 2022;112(8b):649–61. 10.7196/SAMJ.2022.v112i8b.16492 [DOI] [PubMed] [Google Scholar]
- 8.Caron MA, Morissette MC, Thériault ME, Nikota JK, Stämpfli MR, Debigaré R. Alterations in skeletal muscle cell homeostasis in a mouse model of cigarette smoke exposure. PLoS ONE. 2013;8(6):e66433. 10.1371/journal.pone.0066433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinaldi M, Maes K, De Vleeschauwer S, Thomas D, Verbeken EK, Decramer M, Janssens W, Gayan-Ramirez GN. Long-term nose-only cigarette smoke exposure induces emphysema and mild skeletal muscle dysfunction in mice. Dis Model Mech. 2012;5(3):333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrasco-Rios M, Ortolá R, Rodríguez-Artalejo F, García-Esquinas E. Exposure to secondhand tobacco smoke is associated with reduced muscle strength in US adults. Aging. 2019;11(24):12674–84. 10.18632/aging.102594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alanazi MA, El-Hiti GA, Alturki OA, Alanazi M, Fagehi R, Masmali AM. Assessment of tear osmolarity in smokers using TearLab and I-Pen systems. J Ophthalmol. 2022;2022:9970388. 10.1155/2022/9970388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou W, Chen S, Zhu C, Gu Y, Zhu L, Zhou Z. Associations between smoke exposure and osteoporosis or osteopenia in a US NHANES population of elderly individuals. Front Endocrinol (Lausanne). 2023;14:1074574. 10.3389/fendo.2023.1074574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Song C, Yao Z, Sun J, Liu W. Associations between albumin, globulin, albumin to globulin ratio and muscle mass in adults: results from the national health and nutrition examination survey 2011–2014. BMC Geriatr. 2022;22(1):383. 10.1186/s12877-022-03094-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Shivappa N, Dong X, Ming J, Zhao Q, Xu H, Liang P, Cheng M, Liu J, Sun P, et al. Association between appendicular skeletal muscle index and leukocyte telomere length in adults: a study from National Health and Nutrition Examination Survey (NHANES) 1999–2002. Clin Nutr. 2021;40(5):3470–8. 10.1016/j.clnu.2020.11.031 [DOI] [PubMed] [Google Scholar]
- 15.Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing Sarcopenia. Korean J Intern Med. 2016;31(4):643–50. 10.3904/kjim.2016.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Chen J, Song C, Sun J, Liu W. Association between serum Iron status and muscle Mass in adults: results from NHANES 2015–2018. Front Nutr. 2022;9:941093. 10.3389/fnut.2022.941093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi L, Zhang L, Zhang D, Chen Z. Association between systemic immune-inflammation index and low muscle mass in US adults: a cross-sectional study. BMC Public Health. 2023;23(1):1416. 10.1186/s12889-023-16338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Q, Zhang T, Cheang I, Lu X, Shi M, Zhu X, Liao S, Gao R, Li X, Yao W. Negative association between triglyceride glucose index and BMI-adjusted skeletal muscle mass index in hypertensive adults. BMC Musculoskelet Disord. 2023;24(1):571. 10.1186/s12891-023-06700-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B, Sun Y, Xu G, Snetselaar LG, Ludewig G, Wallace RB, Bao W. Association between body Iron status and leukocyte telomere length, a Biomarker of Biological Aging, in a nationally Representative Sample of US adults. J Acad Nutr Diet. 2019;119(4):617–25. 10.1016/j.jand.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim WS, Cheong CY, Lim JP, Tan MMY, Chia JQ, Malik NA, Tay L. Singapore Clinical Practice guidelines for Sarcopenia: screening, diagnosis, Management and Prevention. J Frailty Aging. 2022;11(4):348–69. [DOI] [PubMed] [Google Scholar]
- 21.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019, 393(10191):2636–2646. [DOI] [PubMed]
- 22.Wang M, Luo X, Xu S, Liu W, Ding F, Zhang X, Wang L, Liu J, Hu J, Wang W. Trends in smoking prevalence and implication for chronic diseases in China: serial national cross-sectional surveys from 2003 to 2013. Lancet Respir Med. 2019;7(1):35–45. 10.1016/S2213-2600(18)30432-6 [DOI] [PubMed] [Google Scholar]
- 23.Malta DC, Silva AGD, Machado ÍE, Sá A, Santos FMD, Prates EJS, Cristo EB. Trends in smoking prevalence in all Brazilian capitals between 2006 and 2017. J Bras Pneumol. 2019;45(5):e20180384. 10.1590/1806-3713/e20180384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Zeng X, Liu J, Liu Y, Liu S, Yin P, Qi J, Zhao Z, Yu S, Hu Y, et al. Can China achieve a one-third reduction in premature mortality from non-communicable diseases by 2030? BMC Med. 2017;15(1):132. 10.1186/s12916-017-0894-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Yoon DH, Kim KN, Shin DA, Ha Y. Association between urine cotinine levels and bone mineral density in lumbar spine and femoral neck among adult males. Spine (Phila Pa 1976). 2014;39(4):311–7. 10.1097/BRS.0000000000000138 [DOI] [PubMed] [Google Scholar]
- 26.Hansen MJ, Chen H, Jones JE, Langenbach SY, Vlahos R, Gualano RC, Morris MJ, Anderson GP. The lung inflammation and skeletal muscle wasting induced by subchronic cigarette smoke exposure are not altered by a high-fat diet in mice. PLoS ONE. 2013;8(11):e80471. 10.1371/journal.pone.0080471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Paepe B, Brusselle GG, Maes T, Creus KK, D’Hose S, D’Haese N, Bracke KR, D’Hulst AI, Joos GF, De Bleecker JL. TNF alpha receptor genotype influences smoking-induced muscle-fibre-type shift and atrophy in mice. Acta Neuropathol. 2008;115(6):675–81. 10.1007/s00401-008-0348-4 [DOI] [PubMed] [Google Scholar]
- 28.Wang L, van Iersel LEJ, Pelgrim CE, Lu J, van Ark I, Leusink-Muis T, Gosker HR, Langen RCJ, Schols A, Argilés JM et al. Effects of Cigarette Smoke on Adipose and Skeletal Muscle Tissue: In Vivo and In Vitro Studies. Cells 2022, 11(18). [DOI] [PMC free article] [PubMed]
- 29.Stenholm S, Tiainen K, Rantanen T, Sainio P, Heliövaara M, Impivaara O, Koskinen S. Long-term determinants of muscle strength decline: prospective evidence from the 22-year Mini-finland follow-up survey. J Am Geriatr Soc. 2012;60(1):77–85. 10.1111/j.1532-5415.2011.03779.x [DOI] [PubMed] [Google Scholar]
- 30.Quan S, Jeong JY, Kim DH. The relationship between smoking, socioeconomic status and grip strength among community-dwelling Elderly men in Korea: Hallym Aging Study. Epidemiol Health. 2013;35:e2013001. 10.4178/epih/e2013001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tankó LB, Christiansen C. An update on the antiestrogenic effect of smoking: a literature review with implications for researchers and practitioners. Menopause. 2004;11(1):104–9. 10.1097/01.GME.0000079740.18541.DB [DOI] [PubMed] [Google Scholar]
- 32.Ardite E, Peinado VI, Rabinovich RA, Fernández-Checa JC, Roca J, Barberà JA. Systemic effects of cigarette smoke exposure in the guinea pig. Respir Med. 2006;100(7):1186–94. 10.1016/j.rmed.2005.10.023 [DOI] [PubMed] [Google Scholar]
- 33.Colsoul ML, Goderniaux N, Onorati S, Dupuis S, Jamart J, Vanpee D, Berlin I, Galanti L. Changes in biomarkers of endothelial function, oxidative stress, inflammation and lipids after smoking cessation: a cohort study. Eur J Clin Invest 2023:e13996. [DOI] [PubMed]
- 34.Taati B, Arazi H, Suzuki K. Oxidative stress and inflammation Induced by Waterpipe Tobacco Smoking despite possible Protective effects of Exercise Training: a review of the literature. Antioxid (Basel) 2020, 9(9). [DOI] [PMC free article] [PubMed]
- 35.Aspera-Werz RH, Ehnert S, Heid D, Zhu S, Chen T, Braun B, Sreekumar V, Arnscheidt C, Nussler AK. Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke. Oxid Med Cell Longev 2018, 2018:3172480. [DOI] [PMC free article] [PubMed]
- 36.Ruan X, Mueck AO. Impact of smoking on estrogenic efficacy. Climacteric. 2015;18(1):38–46. 10.3109/13697137.2014.929106 [DOI] [PubMed] [Google Scholar]
- 37.Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999;53(12):920–6. 10.1038/sj.ejcn.1600870 [DOI] [PubMed] [Google Scholar]
- 38.Degens H, Gayan-Ramirez G, van Hees HW. Smoking-induced skeletal muscle dysfunction: from evidence to mechanisms. Am J Respir Crit Care Med. 2015;191(6):620–5. 10.1164/rccm.201410-1830PP [DOI] [PubMed] [Google Scholar]
- 39.Rom O, Reznick AZ, Keidar Z, Karkabi K, Aizenbud D. Smoking cessation-related weight gain–beneficial effects on muscle mass, strength and bone health. Addiction. 2015;110(2):326–35. 10.1111/add.12761 [DOI] [PubMed] [Google Scholar]
- 40.Kleppinger A, Litt MD, Kenny AM, Oncken CA. Effects of smoking cessation on body composition in postmenopausal women. J Womens Health (Larchmt). 2010;19(9):1651–7. 10.1089/jwh.2009.1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012;345:e4439. 10.1136/bmj.e4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data was collected in the NHANES database (http://www.cdc.gov/nchs/nhanes/). The data are available from the corresponding author on reasonable request.