Abstract
Purpose
The main purpose of treatment of advanced ocular surface and periocular malignant tumors is to eradicate the tumor while trying to preserve visual function and aesthetics. Our purpose is to describe the outcome of a retrospective case series of 10 patients with advanced ocular surface and periocular tumors treated surgically in first instance and then with postoperative interventional radiotherapy (IRT/Brachiterapy).
Materials and Methods
We describe the clinicopathological features, treatments and outcome, in a retrospective case series of 10 patients with advanced tumors involving ocular surface (staging ≥ T2) and eyelids (staging ≥ T3), with involvement of periocular and/or orbit tissues. Patients were first surgically treated, most of them with incomplete excision, and then underwent a post-operative interventional radiotherapy (IRT/Brachytherapy) as an alternative to more invasive and disfiguring surgical retreatment. Tumor location, risk factors, staging, histological features, and follow-up timing were analyzed.
Results
Three patients had advanced eyelid basal cell carcinomas, 2 patients were diagnosed with eyelid and conjunctival squamous cell carcinomas, 3 as sebaceous carcinomas, and 2 as primary conjunctival melanomas. The mean follow-up time from IRT to last clinical follow-up was 58.6 weeks, range 28.4–168 (median 43.65, IQR 28.9–72.9). Two patients - one with ocular surface SCC, the other with conjunctival melanoma - had a local recurrence 23.4 and 40,9 weeks after IRT, respectively. An overview of the current knowledge on adjuvant or post-operative IRT is also provided.
Conclusions
IRT can be considered an effective therapeutic option to avoid more invasive surgical retreatment in advanced tumors involving eyelids and ocular surface.
Keywords: Eyelid tumors, ocular surface tumors, eyelid tumors AJCC staging system, eyelid tumors histological risk, brachytherapy, interventional radiotherapy
Introduction
Malignant tumors of the ocular surface and periocular region, with extension to periocular tissues and orbit, are neoplasms with several clinicopathological subtypes that often require a multidisciplinary management.
Ocular Surface Squamous Neoplasia (OSSN) is a spectrum of malignancies involving an abnormal ingrowth of dysplastic squamous epithelial cells on the ocular surface, ranging from mild epithelial dysplastic changes (CIN – Conjunctival Intraepithelial Neoplasia, where the corneal basement membrane is spared) to more severe invasive Squamous Cells Carcinoma (SCC, a malignant lesion where dysplastic epithelial cells have penetrated the corneal basement membrane).1–6
Malignant conjunctival melanoma, instead, arise from the mucous membrane of the eye and can be distinguished in primary (when arising de-novo, without a preexisting lesion) or secondary (usually arising from a primary acquired melanosis – PAM – with atypia or, less commonly, from the malignant transformation of a conjunctival nevus). Regarding the periocular skin region, Basal Cell Carcinoma (BCC) is the most common malignancy involving the eyelids (accounting for approximately 90% of cutaneous eyelid tumors, 50% of which arising from the lower eyelid), 7 followed by Squamous Cell Carcinoma (SCC) and sebaceous carcinoma (SC). 8
Cutaneous melanoma and Merkel cells carcinoma are much less common but have a significantly worse prognosis due to a relatively high rate of distant metastasis and death. 9
The main purpose of treatment is to eradicate the tumor while trying to preserve visual function and aesthetic appearance. Unfortunately, when these tumors invade the periocular region and orbital tissues, orbital exenteration is often the treatment of choice.6,10,11
Depending on the anatomical extension and histological subtype, primary surgery or chemotherapy, or extensive and complex surgery, is generally required as the first approach; nevertheless, surgical intervention on advanced tumors can be challenging, due to detrimental functional and aesthetic sequelae. 12
In recent years, the improvement of surgical devices (microscopes and 3D assisted surgery), supported by adjuvant treatments (such as radiotherapy and/or chemotherapy), has provided new therapeutic strategies with better prognostic outcomes, and a control rate of approximately 90% of these tumors has been achieved. 13
External Radiotherapy (ERT) can be delivered in combination with surgery, both for neoadjuvant and/or adjuvant purposes. However, undesirable side effects of ERT may result in aesthetic or functional impairment, such as eyelid deformity, dry eye, skin thinning, and even severe ocular surface alterations or visual loss. 14
In contrast with traditional ERT, Interventional Radiotherapy (IRT or Brachytherapy) has the great advantage of delivering a high target dose of radiation limited directly to the primary tumor bulk, thus maximizing radiation absorption within the tumor mass while minimizing the spread of radiations to nearby healthy tissue, due to a rapid fall-off mechanism. 15
Based on the likelihood of tumor recurrence, the recently established IRT approach involves the temporary placement of radioactive sources directly into the tumor bulk (Interstitial IRT), or, when treating superficial skin tumors, placement of radioactive plaques directly over the tumor surface lesion (Contact IRT).
IRT can be delivered by several different means depending on the type of implant, using needles, dedicated applicators, or plaques that can be customized to the individual tumor characteristics. 16
For non-high-risk zones of the face, contact high-dose-rate (HDR) IRT is usually performed 17 ; when dealing instead with high-risk zones (such as the eyelids and ocular surface), it is advisable to proceed with an Interstitial HDR IRT approach. 18
Particularly, as for eyelid tumors, the gold standard remains surgery; therefore, Interstitial HDR IRT could play an important role, especially in the adjuvant setting, when further surgery might be potentially disfiguring. 19
Interstitial HDR treatments using implanted needles and catheters have indeed been shown to lead to positive prognostic outcomes while preserving cosmesis around the periocular region.20,21
An alternative HDR IRT approach, namely Contact instead of Interstitial (as described before), was also suggested by using custom-made surface molds leaned over the eyelid instead of using interstitial catheters; the outcomes reported in a series of 10 patients were satisfactory in terms of local control. 22
The aim of this study is to analyze, in a retrospective case series, the clinicopathological features, different treatment options and outcomes, of 10 patients with advanced ocular surface tumors (≥T2,N0,M0) and periocular/eyelids tumors (≥T3,N0,M0), with variable extension into periocular and/or orbital tissues, treated with surgery albeit mostly incompletely resected and managed with post-operative (adjuvant or salvage) IRT.
Materials and methods
A retrospective analysis of 10 patients with advanced tumors involving ocular surface and periocular tissues referred to the Ocular Oncology Unit of Fondazione Policlinico Universitario A. Gemelli IRCCS and managed at our Interventional Oncology Center (IOC) with Interstitial HDR-IRT, from June 2019 to June 2023, was carried out. All patients have been initially treated through surgical tumor resection with histopathological examination, then underwent treatment with adjuvant IRT. Except for one case, all patients had positive infiltration of one or more margins at histological examination; further surgical retreatment had been declined by patients because of aesthetically unacceptable and functionally disabling sequelae.
Tumor location, individual risk factors, a comprehensive ophthalmological assessment (including visual acuity, visual fields, and intraocular pressure), American Joint Committee on Cancer Classification (AJCC) staging system (including prognostic staging), histological features, post-surgery and post-IRT follow-up timing, and recurrence rate were analyzed.
Cohort
Ten patients suffering from advanced tumors involving primarily ocular and periocular tissues, including eyelids (7 out of 10, 70%) or ocular surface/conjunctival tissues (3 out of 10, 30%) were enrolled in the current study.
All patients showed spreading of the primary tumor to adjacent structures (medial or lateral canthi, inferior or superior tarsus and fornix, orbital tissues).
A written informed consent for data collection and analysis for research purposes was obtained from all patients. The study was carried out in adherence to the Declaration of Helsinki. Patients were identified through our electronic database based on the SKIN-COBRA system, which allows clinicians to retrospectively retrieve anonymized patient data, thus fully respecting the General Data Protection Regulations (GDPR). 23
All patients presented in this report underwent a primary surgical approach. Surgery was performed according to the following procedure: the perceived edges of lesion were marked under microscopic view and an excisional biopsy of the tumor was performed with a 3-mm margin. Sutures of different colors were applied to the margins for orientation. Permanent paraffin sections were obtained for every specimen for postoperative histological evaluation. In most of the cases, including poorly defined clinical margins and large and recurrent tumors, an intraoperative histological frozen section of the margins was obtained. When positive, a further excision from the margin involved was obtained and paraffin sections postoperatively evaluated. For tumors arising from the conjunctiva, the conjunctival tumor was widely excised with margins control and an amniotic membrane graft was placed; regarding extra-conjunctival extension the technique was similar as described for eyelids tumors. Particularly, when considering our two cases of conjunctival melanoma subsequently described, cryotherapy was also performed on the site involved, as this has been shown to reduce the percentage of local recurrence after surgery.
Reconstruction was then carried out in the same surgical setting. Large defects required more complex repair with flaps or grafts. Strict post-surgery and post IRT monitoring (every 3 weeks for 4 months and once every 2 months after that) was performed through ophthalmic clinical examination, photographic imaging, and radiological imaging when needed.
Patients were then evaluated in a dedicated multidisciplinary ocular and periocular tumor board 24 and referred for adjuvant interstitial HDR IRT. The interval between surgery and HDR IRT was decided, based on discussions at our multidisciplinary meetings, after 4 to 6 weeks after surgery on the primary malignancy was performed, to provide the necessary time for the lesions to recover tissue integrity by scarring and wound healing.
Patients were asked to provide informed consent both for the procedure and for the treatment, as well as for the use of personal data for research purposes.
Head-and-neck Magnetic Resonance Imaging (MRI) or PET-CT was obtained to exclude any gross residual tumor, and a pre-implant definition of the ideal number and position of catheters was discussed. In all cases, metallic needles were placed inside the tumor bulk with roughly 10 mm spacing, to provide adequate coverage to the Clinical Target Volume (CTV) and carefully try to avoid any possible damage to the sensitive surrounding structures.
The metallic needles were subsequently substituted with flexible 6 Fr plastic tubes, which were fixed with plastic buttons sutured to the skin.
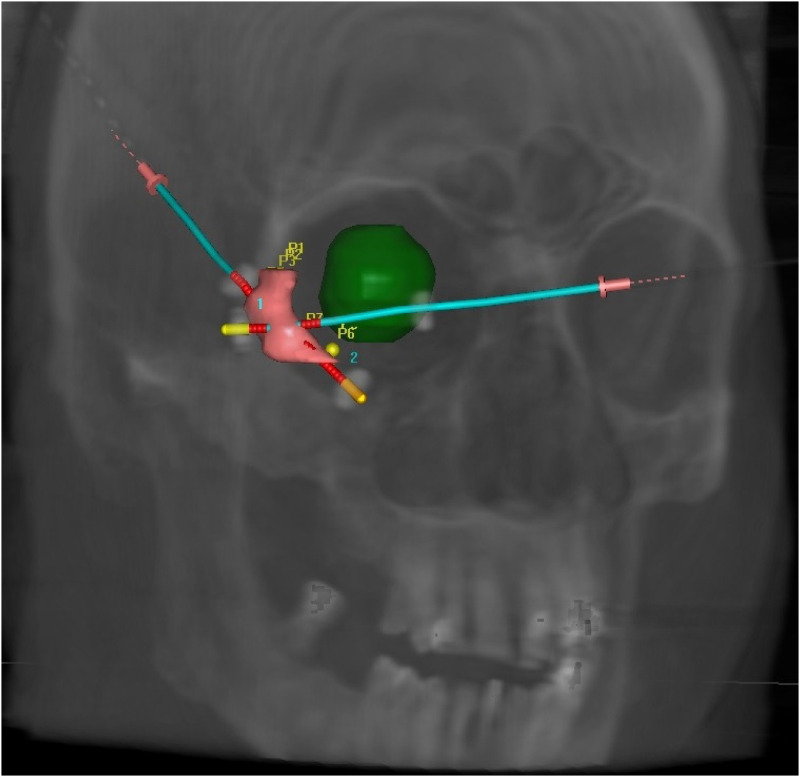
In all cases, Computed Tomography (CT) simulation was performed the day after plastic tube implantation to allow the resolution of swelling related to the interventional procedure, which causes edema due to the interstitial insertion of the needles and the injection of local anesthesia. After CT simulation, the CTV was contoured, and the catheters were digitally reconstructed (Figure 1). The treatment plan was calculated using Oncentra Brachy (Elekta, Stockholm, Sweden) and treatment delivery was performed using a HDR afterloader (Elekta Flexitron).
Figure 1.
Digitally reconstructed image of the right eyelid implant with two catheters placed within the clinical target volume (CTV). The right ocular globe is preserved by the angle chosen for the implant.
The treatment protocol in our cohort, particularly described in 3 selected patients in a dedicated subsequent section of the article was 34 Gy in 10 fractions, 3.4 Gy per fraction, 2 fractions per day (b.i.d.), and for subsequent patients 49 Gy in 14 fractions, 3.5 Gy per fraction, 2 fractions per day (b.i.d.). Catheter removal after treatment completion was performed without complication. The implant procedure included three different phases: pre-planning, implantation, and treatment planning/delivery (Table 1).
Table 1.
Description of steps in interstitial interventional radiotherapy (IRT/brachytherapy) planning and delivery procedure.
| Interstitial Interventional Radiotherapy Planning and delivery | |
|---|---|
| Step 1: Pre-planning |
|
| Step 2: Implant Technique |
|
| Step 3: Treatment planning and delivery |
|
The following data were collected: gender, age, site, eye laterality, risk factors, comprehensive ophthalmic assessment (including visual acuity, visual fields and intraocular pressure), histopathological features, infiltration of surgical margins, AJCC Stage, time from the last surgery to IRT, time from IRT to the last follow-up, recurrence rate (rate at which the disease recurred in the same site as the original tumor).
Results
Ten patients with histologically confirmed malignant tumors involving ocular surface and periocular tissues surgically excised and treated with IRT, from June 2019 to June 2023, were retrospectively enrolled in the current study. The mean age of patients at the time of surgery was 74.93 years (median 77 years; range 68 to 82 years). Five (50%) patients were male and five (50%) were female. The right eye was involved in four patients (40%), the left in 6 patients (60%). All patients were Caucasian. A comprehensive ophthalmological assessment didn’t show significant differences before and after IRT treatment: visual field and intraocular pressure were within normal range in all patients; visual acuity was worse than 0.15 logMAR only in 4 older patients (case #2, case #4, case #6, case #10 in Table 2) due to cortico-nuclear cataracts and was unchanged after treatment.
With regard to risk factors, three patients (30%) reported significant light exposure during their lifetime (due to occupational factors); one patient (10%) suffered from psoriasis, for which prolonged treatment with corticosteroids had been established; one patient (10%) had a high-risk phototype with fair skin and photosensitivity (Fitzpatrick phototype II); three patients (30%) were smokers or former smokers; in four patients (40%) history was unremarkable for risk factors. 25
Upon histopathological examination, 3 out of 10 cases were classified as eyelid BCC (including 2 nodular BCC and 1 basosquamous BCC; 30%), 3 out of 10 cases (30%) cases were diagnosed as invasive SC, 2 out of 10 (20%) as invasive SCC, and 2 out of 10 (20%) as invasive primary conjunctival melanoma.
Patients were treated by G.S. (surgical treatment) and B.F. (IRT).
The mean time from surgery to IRT treatment was 26.61 weeks (median 17.5, IQR 9.7–44.3); the mean post IRT follow-up was 58.6 weeks (median 43,65, IQR 28.9–72.9).
The overall mean CTV was 1.53 cc (range 0.5–3.69 cc) and the median number of catheters used was 3 (range 2–6). Importantly, no interruptions were recorded for any of the patients because compliance to treatment was excellent; therefore, the scheduled fractions were correctly delivered to all patients. As long as side effects are concerned, local hematoma of the periocular region (due to insertion of the catheters) occurred in 3 cases, while radiodermatitis of the eyelid occurred in 2 patients.
Case series
A summary of clinical data and outcomes is shown in Table 2.
Table 2.
Case series with main pathological features, AJCC tumor staging, histologic subtypes, treatment timing and follow-up of 10 patients with different histotypes of eyelid or conjunctival tumors.
| Age- Gender | Risk factors | Sites involved RE: right eye LE: left eye | AJCC Staging | Histology |
Time surgery → to IRT
(weeks) |
Time IRT→ to last follow-up (weeks) | Recurrence | |
|---|---|---|---|---|---|---|---|---|
| Case #1 | 56.4- M | Sun exposure | RE medial canthus positive and caruncle involved |
T4, N0, M0
[IIB] |
BCC | 13.7 | 35.1 | No |
| Case #2 | 83.4 – F | None | LE medial canthus positive and caruncle involved |
T4, N0, M0
[IIB] |
BCC | 12.9 | 68.1 | No |
| Case #3 | 67.1 – M | Fair phototype | LE lower eyelid positive and orbit involved |
T4, N0, M0
[IIB] |
SCC | 42.1 | 87.2 | No |
| Case #4 | 81.3 – F | Sun exposure + smoking | LE upper conjunctiva positive and orbit involved | T3c, N0, M0 | Conjunctival Melanoma | 4 | 168 |
Yes
(40,9 weeks) |
| Case #5 | 67.6 – F | Smoking | RE inferior conjunctiva positive and fornix involved | T3b, N0, M0 | Conjunctival Melanoma | 73 | 43.9 | No |
| Case #6 | 86.3 – M | None | RE upper eyelid positive and medial canthus involved |
T3c, N0, M0
[IIA] |
SC | 33.1 | 54.4 | No |
| Case #7 | 70.5 – M | None | LE inferior conjunctiva positive and lower fornix involved | T2, N0, M0 | SCC | 50.9 | 43.4 |
Yes
(23,4 weeks) |
| Case #8 | 76.3 – F | Sun exposure | LE upper eyelid positive and lateral canthus involved |
T4a, N0, M0
[IIB] |
SC | 21.4 | 28.4 | No |
| Case #9 | 77.7 – M | Smoking | RE lateral canthus positive and eyelids involved |
T3c, N0, M0
[IIA] |
BCC | 3.4 | 29.1 | No |
| Case #10 | 82.7 – F | None | LE upper eyelid positive and upper fornix involved |
T4a, N0, M0
[IIB] |
SC | 11.6 | 28.4 | No |
Cases description
Three cases treated according to the procedure previously described are hereby reported. Particularly, three advanced eyelids tumors (one SC and two BCC) have first been surgically treated with an histopathologically-confirmed incomplete excision and then - after discussion at our Ocular Oncology Multidisciplinary Tumor Board (OOMTB) – underwent an adjuvant or post-operative high dose interventional radiotherapy (IRT – brachytherapy).
No clinical relapse was observed in subsequent follow-ups.
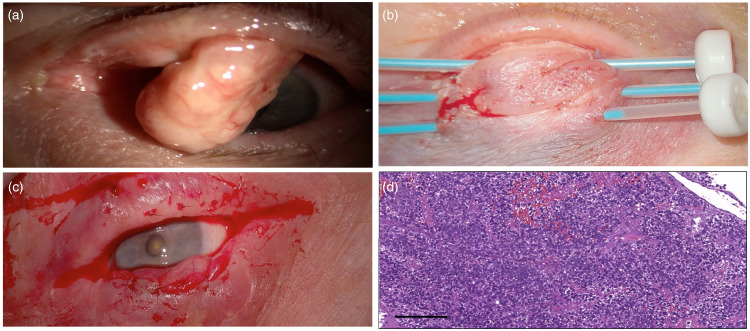
Case 1 (Figure 2, case #10 in Table 2)
An 83-year-old healthy woman, with unremarkable history of systemic disease nor prior treatments, was referred to our Ocular Oncology Unit with a history of 3 months left eye upper eyelid heaviness and swelling (Figure 2 and Table 2). Slit-lamp examination showed a protuberant pedunculated lesion (20 mm × 8 mm) arising from the left upper tarsal plate. The case was discussed at our OOMTB, and a surgical resection was planned. A wide excision was performed with a Cutler-Beard reconstruction. The histopathologic specimen showed an SC with lateral and medial margins involved. The patient, after further OOMTB discussions, was treated with adjuvant IRT 11.6 weeks after surgery and before the second Cutler-Beard surgical step. Three weeks later, the upper eyelid was reconstructed, and multiple incisional control biopsies performed. Histopathological results excluded recurrences and the last clinical follow-up, 10 months post-surgery, was negative
Figure 2.
(a) Preoperative protuberant pedunculated lesion (20 × 8 mm) arising from the left upper tarsal plate; (b) Adjuvant IRT 5 weeks after tumor excision and Cutler-Beard reconstruction; (c) Upper eyelid reconstruction three weeks after IRT; (d) Sebaceous Carcinoma: Histopathological examination showed a proliferation of neoplastic cells with sebaceous differentiation, showing bubbly cytoplasmic vacuolization, pleomorphism, and mitotic activity (hematoxylin and eosin stain, original magnification x10, scale bar 100 µm).
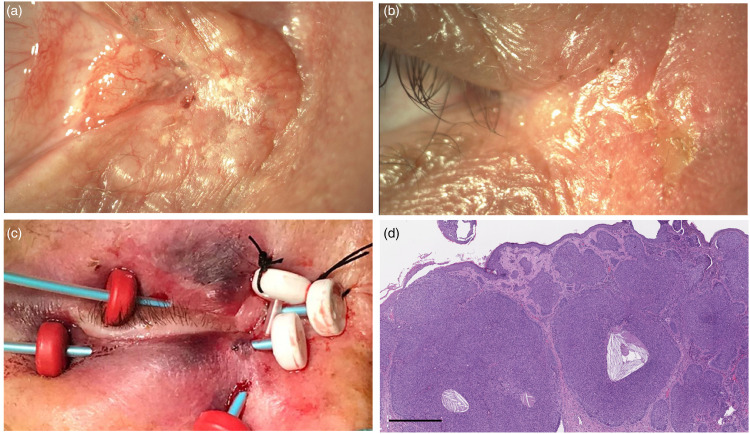
Case 2 (Figure 3, case #1 in Table 2)
A 56-year-old healthy man, with a history of sun exposure, was admitted to our Ocular Oncology Unit with a 12-month history of a right eye lesion on the medial canthus (Figure 3 and Table 2). Eye examination showed right skin medial canthus infiltration with extension toward the upper eyelid and conjunctival injection. A surgical excision was planned with histopathological examination and a skin graft. The histopathological examination showed a nodular BCC with one margin involvement. The patient, after our OOMTB discussion, was treated with adjuvant IRT 13.7 weeks after surgery. No relapse of the disease was evident 9 months after radiotherapy.
Figure 3.
(a) Right medial canthus infiltration with extension toward the upper eyelid; (b) Postoperative medial canthus; (c) Adjuvant IRT 5 weeks after surgery; (d) Basal cell carcinoma, nodular type: histopathological examination showed aggregates of peripherally palisaded basaloid neoplastic cells (hematoxylin and eosin stain, original magnification x4, scale bar 50 µm).
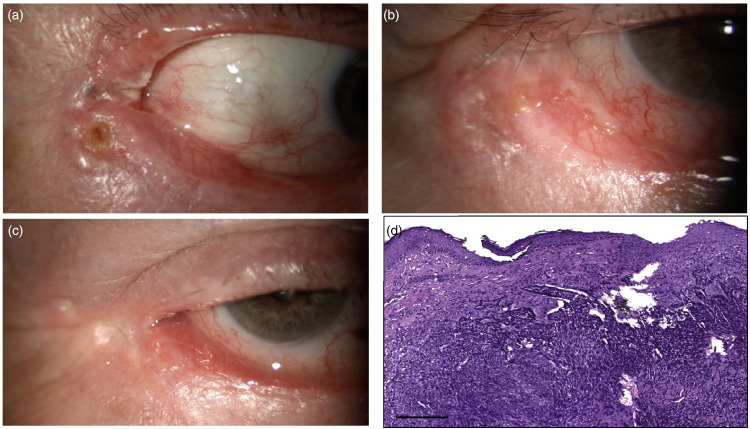
Case 3 (Figure 4, case #9 in Table 2)
A 78-year-old man, healthy, with no history of systemic disease and a previously excised BCC of the lower right eyelid in 2013, was referred to our Ocular Oncology Unit with a suspicion of recurrence with extension to the external canthus and upper eyelid (Figure 4 and Table 2). Slit-lamp examination showed a sclerotic-ulcerative lesion with an extension of 20 mm infiltrating the deep tissues, arising from the lower eyelid, and extending to the lateral canthus and toward the upper eyelid. Following a CT scan, a thickening of the subcutaneous layers of the lower eyelid and external canthus was detected, and on MRI images, a contrast enhancement of antero-lateral and inferior orbit was assessed. The case was discussed at our OOMTB and a wide surgical excision with adjuvant IRT 3.4 weeks after surgery was planned, as shown in Figure 4. At subsequent follow-up visits, the patient suffered from ‘exposure keratitis’ due to the onset of cicatricial ectropion following IRT. Treatment with lubricating eye drops provided rapid resolution of the clinical issue. No relapse of disease was evident 8 months after radiotherapy.
Figure 4.
(a) Sclerotic, ulcerated lesion infiltrating the lower eyelid and extending to the lateral canthus and toward the upper eyelid; (b) Lateral canthus after surgical excision; (c) Lateral canthus 2 weeks after IRT; (d) Basal cell carcinoma, nodular type: Histopathological examination showed neoplastic islands of basaloid neoplastic cells with hyperchromatic nuclei and scant cytoplasm, with peripheral artifactual clefting (hematoxylin and eosin stain, original magnification x10, scale bar 100 µm).
Discussion
Previous studies have shown that the need for exenteration of eyelid BCC may be significantly higher when the lesion involves the medial canthus, margin-controlled excision is not included in the initial management, or pathologic analysis reveals an infiltrative subtype. 10
In our series, 2 out of 3 BCC were recurrent. One BCC showed additional high-risk histopathological features showing a basosquamous pattern with perineural invasion. One SCC showed poor differentiation grade (G3 grading) with high infiltration and deep growth with perineural invasion.
Our cohort also included 3 ocular surface tumors, including 2 conjunctival melanomas and 1 SCC. Two of them (66%) showed local recurrence at 23.4 (SCC) and 40.9 weeks (conjunctival melanoma) after IRT, and in one case (conjunctival melanoma) an orbital exenteration was needed. In a review on eyelid-sparing orbital exenteration, conjunctival and uveal melanomas were the most frequent malignancy of origin. 22
Previous studies have shown that advanced ocular surface tumors were observed in 3–9% of cases, with orbital invasion in 1–6%.1–5 Others reported that, among secondary orbital tumors originating from conjunctival malignancies, 81% of cases were identified as SCC, 19% as malignant melanoma, and 19 out of 21 patients needed exenteration. 6
It should be emphasized that, in advanced ocular and periocular tumors, RT – mostly as ERT – has historically been used in cases having high chances of local recurrence, positive or close margins or perineural invasion, primary treatment of advanced disease or palliative treatment.15,23 The major hindrance in the application of post-operative ERT is the close association of vital structures of the globe and the skin around the eyelids. Radiation-induced ocular morbidity encompasses a spectrum of functional impairment ranging from transient eyelid erythema to complete loss of vision or loss of the globe. 15 For the treatment of eyelid tumors, ERT can present technical concerns and complications due to the thin subcutaneous layer of the eyelid, the relevant curvature of the eye, and the radiation dose reaching underlying structures.
In contrast, IRT is considered an alternative therapeutic option that optimizes the balance between delivering a high radiation dose on the tumor bulk while sparing healthy surrounding tissues. The main reason for this is the rapid dose fall-off for IRT.18,19 IRT can be delivered by superficial (with dedicated commercial or customized applicators) or interstitial applications (with hypodermic needles or catheters) for eyelid tumors. In both cases, IRT allows the placing of applicators in contact with or inside the target, which makes it possible to administer high dosages within the CTV, limiting damage to the surrounding healthy tissues. Moreover, IRT can be performed in unfavorable locations, such as the canthi, and the total dose is usually fractionated to avoid potential toxicity to surrounding tissues.
Usually for conjunctival lesions the adjuvant radiotherapy treatment is performed using a beta-emitter plaque IRT because the depth of prescription and the area to treat are well covered by the radioactive plaque; on the contrary in the case of tarsal lesions interstitial IRT plays a relevant role because it allows to better modulate the dose, sparing the surrounding healthy tissues.
A recent review provided comprehensive information about available evidence in both clinical settings. 16 In Table 3 we present a summary of the main studies available for HDR-IRT. Laskar et al. published a series of 8 patients who were treated in an adjuvant setting with two different fractionation schedules. 26 Mareco et al. reported on 17 patients, 71% of whom were treated with adjuvant intent, either with positive or close margins, using a range of differing fractionation schedules. 27 Cisek et al. published the largest series available so far. They treated 28 patients, of whom only 14% were treated in the adjuvant setting for positive margins after surgery. A brief summary of previous studies on IRT is provided in Table 3. The authors used two different fractionation schedules according to CTV size and other patient-related factors. 28 Unfortunately, due to the retrospective nature of the aforementioned series it is not possible to provide a comprehensive assessment about the side effects, however, no major complications related to the procedure nor significant visus reduction were reported by the authors.
Table 3.
Studies reporting postoperative HDR interventional radiotherapy (brachytherapy) in eyelid cancers.
| HDR-IRT in Eyelid Cancers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Country | n. of patients | Intent: Radical, Adjuvant, or Salvage | Histology | T stage | Fractionation | Total dose | EQD2 (α/β=10) | Follow-up (months) | Outcome |
| Laskar et al. 26 | 2015 | India | 8 | 100% A a | SebC 50% SCC 37.5% BCC 12.5% |
T4: 37.5% Other than T4: 62.5% |
3 Gy B.I.D. for 7 fr 3.5 Gy B.I.D. for 10 fr |
21 Gy 35 Gy |
22.8 Gy 39.4 Gy |
35 | LC 100% |
| Mareco et al. 27 | 2015 | Portugal | 17 | 12% R 71% A b 17% S |
SCC 94% BCC 6% |
Tis: 6% T1: 46% T2a: 18% T2b: 18% T3a: 12% |
median 10 B.I.D. fr (range 9 to 11) |
median 42.8 Gy (range 32 to 50 Gy) | 50.9 Gy | 40 | LC 94.1% |
| Cisek et al. 28 | 2021 | Poland | 28 | 57% R 14% A c 29% S |
BCC 86% SCC 14% |
T1: 82% T2: 18% |
5 Gy B.I.D. for 9 fr 3.5 Gy B.I.D. for 14 fr |
45 Gy 49 Gy |
56.3 Gy 55.1 Gy |
24 | LC 96.5% |
| Cuffaro et al. | 2023 | Italy | 10 | 100% A b | SebC 30% BCC 30% SCC 20% Melanoma 20% |
T2b: 10% T3b-c: 40% T4a: 50% |
3.4 Gy B.I.D. for 10 fr 3.5 Gy B.I.D. for 14 fr |
34 Gy 49 Gy |
38.0 Gy 55.1 Gy |
10 | LC 80% |
aIncludes negative or close margins.
bIncludes close or positive margins.
cIncludes positive margins.
A point to consider when comparing our local control outcomes with other series is that the lower control rate that we obtained could be explained by the fact that we included cases of melanomas (20% of cases), which are typically more radioresistant compared to NMSC.
In our series, we initially treated three patients using a fractionation schedule already in use in our clinical practice for adjuvant interstitial HDR IRT for conjunctival melanoma, which is 34 Gy in 10 fractions, 3.4 Gy per fraction, 2 fractions per day 29 ; after verifying feasibility in terms of the side effects of this approach, also based on the emerging literature evidence, we moved to a fractionation schedule of 49 Gy in 14 fractions, 3.5 Gy per fraction, 2 fractions per day. Regarding available recommendations for HDR interstitial IRT, it is advised that doses between 3 and 5 Gy should be used with two fractions delivered per day (with at least 6 h interval between the two fractions) in no more than 10 days of treatment. 30 As a final consideration, new emerging systemic therapies have been proposed for treatment of advanced eyelid tumors, such as Hedgehog Pathway Inhibitors (eg: Sonidegig, Vismodegib) and PD1 inhibitors (eg: Cemiplimab). Although effective, actual guidelines suggest these therapies only as a salvage treatment for advanced, recurrent and metastatic BCC and/or SCC that are not eligible for surgery and radiotherapy, which are still considered the treatment of choice. The clinical advantage of our proposed technique is undoubtedly to be as conservative as possible in terms of sparing healthy tissue. However, the various subsequent steps, proper tumor staging, proper surgical technique, correct histopathological examination, evaluation in multidisciplinary discussion and IRT, must be appropriately performed in order to achieve the best result. There are several points that still need to be elucidated: first, further studies are required to identify whether specific doses are needed for different histologic subtypes; another important aspect is whether different doses are required for different clinical situations that are currently treated using the same dose (negative margins, microscopic positive margins, and gross disease). Limitations of our study include the retrospective nature and small sample size.
Conclusions
Postoperative IRT can be considered an effective therapeutic option to avoid more invasive surgical retreatment in most cases. Moreover, it has the great advantage, over traditional ERT, of limiting the functional damage to surrounding healthy tissues on the ocular and periocular areas, thus limiting ocular-related impairment, thanks to a rapid fall-off mechanism that hinders the absorption of harmful radiation to nearby healthy structures.
Although limited by the small sample size, our study supports IRT as an alternative and promising therapeutic option in advanced carcinomas involving eyelids and ocular surface or, at least, as a post-operative measure before proceeding with more aggressive surgical retreatment.
Acknowledgements
We greatly appreciate the approval of the patients on collecting data and participating on this study.
Footnotes
Data availability statement: The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request from the SKIN-COBRA database [23].
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Institutional review board statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Catholic University of Sacred Heart [Prot. N. 0012205/15].
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent statement: Written informed consent regarding data collection for observational purpose was obtained from all individual participants enrolled in this study.
ORCID iDs: Fabrizio Piccinni https://orcid.org/0000-0002-9625-8123
Monica Maria Pagliara https://orcid.org/0000-0001-5817-6672
Gustavo Savino https://orcid.org/0000-0002-9993-5986
References
- 1.Arepalli S, Kaliki S, Shields CL, et al. Plaque radiotherapy for scleralinvasive conjunctival squamous cell carcinoma: analysis of 15 eyes. JAMA Ophthalmol 2014; 132: 691–696. [DOI] [PubMed] [Google Scholar]
- 2.Yousef YA, Finger PT. Squamous carcinoma and dysplasia of the conjunctiva and cornea: an analysis of 101 cases. Ophthalmology 2012; 119: 233–240. [DOI] [PubMed] [Google Scholar]
- 3.Erie JC, Campbell RJ, Liesegang TJ. Conjunctival and corneal intraepithelial and invasive neoplasia. Ophthalmology 1986; 93: 176–183. [DOI] [PubMed] [Google Scholar]
- 4.Shields JA, Shields CL, Gunduz K, et al. The 1998 pan American lecture: intraocular invasion of squamous cell carcinoma of the conjunctiva in five patients. Ophthal Plast Reconstr Surg 1999; 15: 153–160. [DOI] [PubMed] [Google Scholar]
- 5.Savino G, Cuffaro G, Maceroni M, et al. Advanced ocular surface squamous cell carcinoma (OSSC): long-term follow-up. Graefes Arch Clin Exp Ophthalmol 2021; 259: 3437–3443. doi: 10.1007/s00417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soysal HG, Ardiç F. Malignant conjunctival tumors invading the orbit. Ophthalmologica 2008; 222: 338–343. doi: 10.1159/000146079 [DOI] [PubMed] [Google Scholar]
- 7.Manghani J, Khan K. A study of role of brachytherapyIR192 in treatment of eyelid tumors. Int J Med Res Rev 2016; 4: 08. doi: 10.17511/ijmrr.2016.i08.07 [DOI] [Google Scholar]
- 8.Petsuksiri J, Frank SJ, Garden AS, et al. Outcomes after radiotherapy for squamous cell carcinoma of the eyelid. Cancer 2008; 112: 111–118. doi: 10.1002/cncr.23143 [DOI] [PubMed] [Google Scholar]
- 9.Brownstein S. Malignant melanoma of the Conjunctiva. Cancer Control 2004; 11: 310–316. doi: 10.1177/107327480401100505 [DOI] [PubMed] [Google Scholar]
- 10.Iuliano A, Strianese D, Uccello G, et al. Risk factors for orbital exenteration in periocular basal cell carcinoma. Am J Ophthalmol 2012 Feb; 153: 238–241.e1. doi: 10.1016/j.ajo.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 11.Madge SN, Khine AA, Thaller VT, et al. Globe-sparing surgery for medial canthal basal cell carcinoma with anterior orbital invasion. Ophthalmology 2010; 117: 2222–2228. doi: 10.1016/j.ophtha.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 12.Guix B, Finestres F, Tello J, et al. Treatment of skin carcinomas of the face by high dose rate brachytherapy and custom made surface molds. Int J Radiat Oncol Biol Phys 2000. doi: 10.1016/S0360-3016(99)00547-7 [DOI] [PubMed] [Google Scholar]
- 13.Malhotra R, Huilgol SC, Huynh NTet al. et al. The Australian mohs database: periocular squamous cell carcinoma. Ophthalmology 2004; 111: 617–623. [DOI] [PubMed] [Google Scholar]
- 14.Barabino S, Raghavan A, Loeffler Jet al. et al. Radiotherapy-induced ocular surface disease. Cornea 2005; 24: 909–914. doi: 10.1097/01.ico.0000154235.64359.d3 [DOI] [PubMed] [Google Scholar]
- 15.Kovács G. Modern head and neck brachytherapy: from radium towards intensity modulated interventional brachytherapy. J Contemp Brachytherapy 2014; 4: 404–416. doi: 10.5114/jcb.2014.47813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pagliara MM, Kakkassery V, Fionda B, et al. Interventional radiotherapy (brachytherapy) in eyelid and ocular surface tumors: a review for treatment of naïve and recurrent malignancies. Neurosignals 2022; 30: 1–10. doi: 10.33594/000000505 [DOI] [PubMed] [Google Scholar]
- 17.Tagliaferri L, Ciardo FG, Fionda B, et al. Non-melanoma skin cancer treated by contact high-dose-rate radiotherapy (brachytherapy): a mono-institutional series and literature review. In Vivo 2021; 35: 2313–2319. doi: 10.21873/invivo.12505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagliaferri L, Giarrizzo I, Fionda B, et al. ORIFICE (Interventional radiotherapy for face aesthetic preservation) study: results of interdisciplinary assessment of interstitial interventional radiotherapy (brachytherapy) for periorificial face cancer. J Pers Med 2022; 12: 1038. doi: 10.3390/jpm12071038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinot JL, Rembielak A, Perez-Calatayud J, et al. GEC ESTRO. GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother Oncol 2018; 126: 377–385. doi: 10.1016/j.radonc.2018.01.013 [DOI] [PubMed] [Google Scholar]
- 20.Krengli M, Masini L, Comoli AM, et al. Interstitial brachytherapy for eyelid carcinoma. Outcome analysis in 60 patients. Strahlenther Onkol 2014; 190: 245–249. doi: 10.1007/s00066-013-0495-y [DOI] [PubMed] [Google Scholar]
- 21.Azad S, Choudhary V. Treatment results of high dose rate interstitial brachytherapy in carcinoma of eyelid. J Cancer Res Ther 2011; 7: 157–161. doi: 10.4103/0973-1482.82922 [DOI] [PubMed] [Google Scholar]
- 22.Vavassori A, Riva G, Durante S, et al. Mould-based surface high-dose-rate brachytherapy for eyelid carcinoma. J Contemp Brachytherapy 2019; 11: 443–448. doi: 10.5114/jcb.2019.88619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancellotta V, Guinot JL, Fionda B, et al. SKIN-COBRA (Consortium for brachytherapy data analysis) ontology: the first step towards interdisciplinary standardized data collection for personalized oncology in skin cancer. J Contemp Brachytherapy 2020; 12: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savino G, Piccinni F, Pagliara MM, et al. Multidisciplinary ocular and periocular cancers meetings: implementation in a tertiary referral center and analysis over a 12-months period. BMC Ophthalmol 2022; 22: 497. doi: 10.1186/s12886-022-02694-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furdova A, Lukacko P. Periocular basal cell carcinoma predictors for recurrence and infiltration of the orbit. J Craniofac Surg 2017; 28: e84–e87. doi: 10.1097/SCS.0000000000003242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laskar SG, Basu T, Chaudhary S, et al. Postoperative interstitial brachytherapy in eyelid cancer: long term results and assessment of cosmesis after interstitial brachytherapy scale. J Contemp Brachytherapy 2014; 4: 350–355. doi: 10.5114/jcb.2014.46693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mareco V, Bujor L, Abrunhosa-Branquinho AN, et al. Interstitial high-dose-rate brachytherapy in eyelid cancer. Brachytherapy 2015; 14: 554–564. doi: 10.1016/j.brachy.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 28.Cisek P, Kieszko D, Bilski M, et al. Interstitial HDR brachytherapy in the treatment of non-melanocytic skin cancers around the eye. Cancers (Basel) 2021; 13: 1425. doi: 10.3390/cancers13061425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagliara MM, Tagliaferri L, Savino G, et al. High-Dose-Rate interstitial brachytherapy (interventional radiotherapy) for conjunctival melanoma with orbital extension. Ocul Oncol Pathol 2021; 7: 199–205. doi: 10.1159/000512344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The GEC ESTRO Handbook of Brachytherapy - ch.29: Skin cancer (2nd edition - 2017)