Abstract
Pigmented paravenous chorioretinal atrophy (PPCRA) is an uncommon form of chorioretinal atrophy characterized by perivenous aggregations of pigment clumps associated with peripapillary and radial zones of retinal pigment epithelial atrophy that are distributed along the retinal veins. Most patients are asymptomatic, and evidence suggest that PPCRA is slowly progressing. Unless macular involvement is present, the majority of patients usually retain a normal visual function. Our ability to diagnose PPCRA has recently improved thanks to multimodal imaging, especially with the advent of ultra-widefield (UWF) imaging. Blood tests and functional and genetic testing can help with the correct differential diagnosis of pseudo-PPCRA or other disorders with similar characteristics. Although the cause of PPCRA is unknown, it is possible that it has a genetic basis. In this review we provide a summary of the multimodal imaging characteristics of PPCRA, and discuss its possible pathogenesis, based on the genes that have been associated with this disease.
Keywords: Pigmented paravenous chorioretinal atrophy, inherited retinal disease, retina, multimodal imaging, ultra-widefield
Introduction
Pigmented paravenous chorioretinal atrophy (PPCRA; OMIM #172870) is an uncommon form of chorioretinal atrophy characterized by perivenous aggregations of pigment clumps associated with peripapillary and radial zones of retinal pigment epithelial atrophy that are distributed along the retinal veins. 1 Its first description dates back to 1937 when Brown TH described a case of chorioretinal atrophy in which the atrophic areas were confined to the immediate proximity of the retinal veins, and called it “retinochoroiditis radiata”. 2
It has since been known also as pseudoretinitis pigmentosa following measles, chorioretinitis striata, congenital pigmentation of the retina, melanosis of the retina, paravenous retinal degeneration, pigmented paravenous chorioretinal degeneration, pigmented paravenous retinochoroidal atrophy (PPRCA) and PPCRA. 3 The last two names are those generally accepted for the disease. Many case reports and some case series have been published since its original description.1,4–8
Patients affected by PPCRA are frequently asymptomatic, and the diagnosis is established on the basis of its distinctive fundus appearance, which is usually discovered during a routine ophthalmic examination. The natural history of the disease is either non-progressive or slowly progressive. It has frequently been reported to be bilateral and symmetric. The etiology of the disease is unknown, and it occurs sporadically in the majority of PPCRA patients. 3
Nonetheless, in some cases, family transmission was observed, leading to the hypothesis of a genetic origin.9–16 Inflammatory and infectious etiologies have also been proposed, since cases of PPCRA that developed after various condition, such as uveitis, Behcet disease, congenital syphilis, measles and rubeola have been described.17–21
The recent literature, however, agrees that these changes are associated to retinal vein vasculopathy related by various inflammatory causes and should be classified as pseudo-PPCRA.1,3
Clinical findings
The mean age at presentation in a retrospective case series of 23 patients was 35 years, ranging from 10 to 67 years. 8 A considerable proportion (varying from 36% to 57% in different studies) of PPCRA patients are asymptomatic.1,8 When present, the most common symptoms are a mild visual loss, reduction of the peripheral visual field and nyctalopia.1,3,8 Visual acuity in PPCRA is generally unaffected or minimally decreased. Nevertheless, some patients also exhibit macular involvement, with severe visual acuity deterioration. 1 Nyctalopia was previously considered to be uncommon, but it has recently been reported in 28% of PPCRA symptomatic patients. 1 However, some patients complaining impaired night vision, poor dark adaptation or night blindness actually have retinitis pigmentosa (RP) or pseudo-PPCRA instead of PPCRA. 3 Other symptoms reported from PPCRA patients include: perception of a shadow in front of the eye, diminished peripheral vision, mild pain and photopsia, involuntary closure of the upper eyelid, headaches and halos around lights.22–24 Nonetheless, it remains uncertain if these observations have an actual causal relation with the disease entity itself.5,25
Associated ocular findings
A bilateral symmetrical accumulation of pigment and chorioretinal atrophy scattered along the retinal veins is the typical funduscopic appearance of PPCRA. Yet, this condition exhibits variable expressivity, and not every patient displays the characteristic fundus features. 1 Chorioretinal atrophy in PPCRA manifests as contiguous yellowish or grayish lesions around the retinal vessels, coexisting with missing or depigmented retinal pigment epithelium (RPE), that arborize and spread into the peripheral retina. The pigmentary alterations range from typical bone corpuscle pigmentation to fine pigmentary alterations to coarse pigment clumping. 1
PPCRA has been classified into three categories based on the distribution of pigmentary changes:
- The “paravenous” type is distinguished by characteristic chorioretinal atrophy with pigment clumping that is geographically connected. 1
- The “focal” type is characterized by isolated chorioretinal atrophy with pigment clumping.1,26
- The “confluent” type exhibits chorioretinal atrophy with bone spicules that extensively merge with one another and it may be more easily misdiagnosed as RP. 1
Another approach to categorize the PPCRA is by severity 3 :
- In the mild form, there are only a few scattered patches with minor evidence of chorioretinal atrophy and paravenous pigmentation.
- In intermediate cases, bone spicule pigment accumulates around the majority of the veins, resulting in minimal or regional chorioretinal atrophy.
- Severe cases have a peripapillary and posterior pole pigmentary sheen, as well as diffuse patches of chorioretinal atrophy next to widespread and dense paravenous pigment deposit.
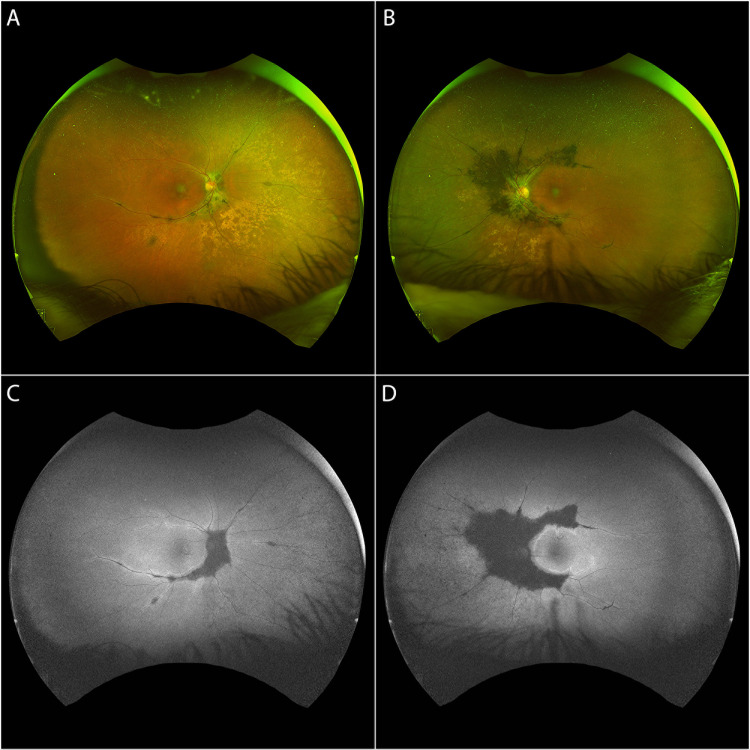
The funduscopic appearance of PPCRA can be characterized by a marked asymmetry (Figure 1), therefore two eyes from the same patient may fall in different categories, either by distribution and severity.1,13,27
Figure 1.
Ultra-widefield (UWF) fundus photograph (A, B) and UWF fundus autofluorescence (C, D) of a patient with asymmetric pigmented paravenous chorioretinal atrophy.
It has been reported that in cases of PPCRA progression, the grayish lesions and RPE atrophy gradually expand, and the pigment clumping becomes darker and more extensive, possibly accompanied by further constriction of the peripheral visual fields.1,3 Moreover, due to the progressive RPE atrophy, the choroidal vessels within the atrophic lesions become increasingly prominent. 3
The involvement of the macula is an important factor that may aggravate the disease, leading to severe visual impairment.1,28 In a prior study, chorioretinal atrophy affecting the fovea was found to be present in only a small proportion of patients.1,29 On optical coherence tomography (OCT) images, macular involvement is usually seen as extensive damage of the outer retina and RPE. 1
Contrarily to RP, the caliber of the retinal vessels is usually normal, however attenuated retinal arteries and arteriolar attenuation with sheathing have been occasionally reported.3,5,14,21,30 Similarly, the optic disc is normal in most cases, with no waxy pallor or frank optic nerve atrophy. 3 Gliotic disc or peripapillary gliosis has been described, and optic disc drusen may be present in patients with typical PPCRA.3,14,31
We provide a list of many other less common associated ocular findings in Table 1.
Table 1.
Unusual ocular findings in pigmented paravenous chorioretinal atrophy.
| Macular findings | |
| Cystoid macular edema | 32–34 |
| Star-shaped exudate | 21 |
| Macular wrinkling | 11 |
| Epiretinal membrane (with and without foveoschisis) | 1,5 |
| Macular pigmentary alterations (stripling, degeneration, depigmentation) | 15,23,32,35 |
| Lamellar and full-thickness macular holes | 36,37 |
| RPE atrophy | 38,39 |
| Excavated macula | 38 |
| Macular coloboma | 7 |
| Other fundus abnormalities | |
| Contralateral retinitis pigmentosa | 40–42 |
| Abnormal retinal sheen | 14 |
| Crystal deposition in the peripheral retina | 24 |
| Vitreous abnormalities | 9,14 |
| Vascular abnormalities (e.g., peripheral changes, microangiopathy) | 3,39,40 |
| Other ocular associations | |
| Angle-closure glaucoma and congenital glaucoma | 38 |
| Nystagmus | 9,14 |
| Ambliopia | 24,25 |
| Different kinds of anisometropia | 11,14,16,19,24,31 |
| Eso- and exotropia | 5,14,43 |
Systemic associations
Many inflammatory and infectious causes have been linked to the disease, including uveitis, Vogt-Koyanagi-Harada disease, Behcet's disease, congenital syphilis, measles, rubeola, tuberculosis, and Neurofibromatosis Type 1.17–21,40–42 However, there has been no evidence linking the retinal findings in PPCRA to any specific systemic disorder.
Diagnosis
Patients are typically asymptomatic, and the diagnosis is established primarily on its distinctive appearance on fundus examination.1,3 However, PPCRA can manifest in a variety of ways, and multimodal imaging can aid with diagnosis, classification, and patient follow-up by providing insights into its clinical characteristics. 1 Visual field testing and electrophysiology are two other common diagnostic criteria for PPCRA. In the forthcoming chapters, we will provide an overview of the multimodal imaging features, functional evaluations, and electrophysiological findings of PPCRA that may aid in its diagnosis.
Functional and electrophysiological exams
Visual field and microperimetry
Most PPCRA patients exhibit functional changes in the visual field in accordance to the anatomical location of their pigmentary changes, with peripheral constriction, enlarged blind spot, geographic scotoma, central, temporal, scattered scotomas or no detectable changes being possible.1,3,5,9,35 Over time, some patients experience a progression of visual field defects, while others remain stable.1,8
In a study conducted by Fleckenstein et al., areas without obvious funduscopic changes that were demarcated by an hyperautofluorescent border on FAF showed a reduced retinal sensitivity with microperimetry. 44
Electrophisiology and dark adaptometry
Electrophysiological findings are extremely variable and aspecific, ranging from normal to completely extinguished electroretinograms (ERGs), and do not always match the extent of funduscopic lesions or patients’ referred symptoms. 3 The most common finding is a reduction of the B-wave amplitude, followed by A-wave reduced amplitude and delayed latency.1,10–13 A selective reduction in either scotopic or photopic response is also possible, reflecting an heterogeneous impairment of either rods or cones.14,21 Generalized on-bipolar cell dysfunction of both rod and cone systems, with preservation of photoreceptor function bilaterally is also possible. 8
Similarly to fundus findings, electrophysiological recordings are often asymmetric.1,8,45 On the other hand, cases with symmetrical ERG responses but asymmetrical fundus changes have been described. 14 Pattern ERG and visual evoked potentials are also aspecific: the former may demonstrate macular disfunction, indicating ganglion cell involvement, with poor concordance with the full-field ERG, whereas the latter may exhibit normal to significantly aberrant responses.8,32,46,47 Additionally, on multifocal ERG reduced responses, especially in the paracentral area, have been reported. 8
Electrooculography (EOG) may display normal, subnormal or significantly reduced Arden ratio.3,8,38,48 In a recent study, EOG findings were considerably proportionate with scotopic ERG findings, which, together with the observation that many cases of unilateral PPCRA have been described, suggests a generalized disfunction of the photoreceptor/RPE interface. 8 Finally, in some patients dark adaptation is delayed with an increased rod-treshold. 23
Multimodal imaging in PPCRA
Color fundus photograph
Fundus examination in PPCRA reveals perivenous aggregations of pigment clumps associated with areas of RPE atrophy radiating from the peripapillary region with a typical distribution along the retinal veins. 49 As previously mentioned, three funduscopic patterns have been described in PPCRA: the classical “perivenous” distribution, a focal pattern and PPCRA with a confluent disposition of the typical lesions.1,8 Ultra-widefield (UWF) imaging is particularly useful in these patients, because of the peripheral extension of the disease.50,51 Nonetheless, standard fundus photograph is a valuable technique to visualize less common cases with optic disc pallor, arteriolar attenuation or patchy macular atrophy. 51
Dye angiography imaging
On fluorescein angiography (FA), areas of hyperpigmentation and pigment clumping, corresponding to blocked hypofluorescence, are surrounded by chorioretinal atrophy, which is hyperfluorescent due to window defect.1,45 Usually, there is no fluorescein leakage. However, Prithvi et al. recently described a case of PPCRA with peripheral capillary non-perfusion, microaneurysms and vascular leakage imaged with ultra-widefield FA. In addition, Tandon et al. reported an atypical case of PPCRA, associated with Coat's like response.40,52
Indocyanine angiography (ICG) shows hypofluorescence along the retinal vessels in all phases, revealing a damage of the choriocapillaris, which partly extends over the areas that are hyperfluorescent on FA.1,35,45
Fundus autofluorescence
Fundus autofluorescence (FAF) is an extremely useful tool for diagnosing PPCRA, especially when ultra- widefield (UWF) technology is available. 50
On blue-light FAF imaging, PPCRA is characterized by continuous decreased FAF signal along the large retinal veins distributed in a geographic fashion, surrounded by linear hyperautofluorescence of variable thickness extending to the periphery.1,8 In the peripheral retina the areas of hyper-/hypoautofluorescence may expand, assuming a fin-like shape, and sometimes patchy areas of atrophy are present. 5 Shona et al. subclassified patients with the perivenous pattern on the basis of their lesions showing the typical hyper-/hypoautofluorescence FAF signal (type 1a) or a predominantly increased FAF (type 1b) 8 ; the latter might only show subtle changes on fundus examination.53,54 The focal (or type 2) pattern consists in focal discontinued areas of decreased FAF signal, whereas the confluent (or type 3) pattern is characterized by coalescing areas of hypoautofluorescence extending beyond the perivascular area.1,8 In a fraction of the patients a hyperautofluorescent ring at the posterior pole can be found, which has been reported to be present in almost a quarter of the cases. 1
Areas of decreased FAF indicates zones of chorioretinal atrophy, whereas the surrounding increased FAF represents a “junctional zone” between affected and healthy retina, where the outer retina is thinner but the RPE is still present (Figure 2).50,55,56
Figure 2.
(A) Fundus autofluorescence and optical coherence tomography (OCT) of the left eye from the same patient from figure 1. The hyperautofluorescent border corresponds to an area of retinal thinning, with underlying intact RPE (green arrowheads). Moreover, an epiretinal membrane is observable. (B) Magnification of the green boxes showing the junctional zone between damaged and healthy retina.
Fundus abnormalities in FAF extend beyond those visible with FA and ICG, making the former a valuable non-invasive examination in patients diagnosed with PPCRA. 1 Moreover, the borders of the increased FAF signal sharply demarcate the area of reduced retinal sensitivity on microperimetry. 44
Optical coherence tomography
When macular involvement is present, OCT shows severe disruption of the RPE and outer retinal layers, leading to outer retinal thinning. In particular, all layers from the external limiting membrane to the interdigitation zone and RPE are affected.1,8 Outer retinal atrophy is usually associated with choroidal thinning, which may sometimes be the only pathological modification. 1
With this technique, other macular comorbidities have been reported, such as epiretinal membrane, cystoid macular edema, lamellar and full-thickness macular hole, RPE atrophy and macular coloboma.5,28,30,36,57
OCT of the affected paravenous regions show variable degree of outer retinal layers thinning, focal or generalized choroidal thinning and RPE atrophic changes, together with areas of pigment clumping visible as intraretinal hyperreflective plaques with posterior hypotransmission.1,8 In particular, areas characterized by a reduction of the outer retinal thickness without RPE atrophy correspond to the junctional zones in FAF.50,55,56 As for the macula, some cases exhibit significant choroidal thinning even in the absence of RPE atrophy. 1
Optical coherence tomography angiography
OCTA findings have been explored seldom in PPCRA, with just few studies and case reports available.1,45,58–60 Existing literature suggest that OCTA is relatively normal in the earlier stages of the disease, with preserved retinal capillary plexa and choriocapillaris.45,58 In more advanced stages, the most relevant finding is a prominent choriocapillaris impairment, in form of flow voids increase.1,60,61
Battaglia Parodi et al. recently found that, compared to healthy subjects, vessel density is reduced not only in the choriocapillaris, but also at the level of the deep capillary plexus, regardless of the presence of chorioretinal atrophy (Figure 3). 59 Finally, in a recent case report Fallon et al. utilized a combination of OCTA and en face OCT to visualize the morphology of posterior hyalocites in a case of asymmetric PPCRA. 62 In more detail, hyalocites in the most advanced eye displayed a ramified appearance, suggestive of a “quiescent” stage, whereas those of the less advanced eye had an ameboid, “activated” shape. Their findings support the hypothesis that inflammation is a key factor in the pathophysiology of PPCRA.
Figure 3.
(A) Fundus autofluorescence of the right eye of a patient affected by pigmented paravenous chorioretinal atrophy displaying major involvement of the inferior retina. Green arrowheads: the line of increased autofluorescence delineates a region with diminished autofluorescence, corresponding to a decrease in vessel density for both superficial (B) and deep (C) capillary plexa on OCTA. Meanwhile, a more diffuse (D) choriocapillaris impairment is apparent.
Differential diagnosis
The PPCRA differential diagnoses comprise chorioretinal degeneration and inflammatory diseases that cause chorioretinal atrophy, including: pericentral, sectorial, and typical RP, helicoid peripapillary chorioretinal atrophy, serpiginous choroidopathy, autoimmune retinopathy, hydroxychloroquine retinopathy, sarcoidosis, gyrate atrophy, choroideremia, tuberculous choroiditis, toxoplasmosis, cone dystrophy, syphilis and angioid streaks. 3
Because PPCRA is primarily diagnosed based on fundus appearance, the “confluent” variety is more prone to be misdiagnosed as RP. 1 As a consequence, RP must be ruled out, and this can be achieved with the following criteria: history of night blindness, nonrecordable ERG, and characteristic perimetric defect. 1
Furthermore, visualizing the typical appearance of PPCRA lesions along the retinal veins extending from the disc to the periphery is key in the diagnostic process. As a result, UWF imaging is a critical tool in distinguishing PPCRA from RP. The FA and ICG are useful in distinguishing PPCRA from retinal vasculitis caused by infectious or inflammatory factors. 1
Finally, genetic testing could support a correct differential diagnosis if pathogenetic variants known to cause RP are discovered. 1
Depending on the presentation, laboratory studies should be aimed at narrowing the differential diagnosis, and given the overlap with inflammatory degenerative diseases, a workup can include: comprehensive metabolic panel, complete and differential blood cell counts, erythrocyte sedimentation rate, C-reactive protein, antinuclear antibody, serum protein electrophoresis, chest x-ray, venereal disease research laboratory test or rapid plasma regain test, serum antibody tests for herpes simplex virus I and II, herpes zoster virus, cytomegalovirus, measles, tuberculin skin test and serological tests for toxoplasmosis or rheumatoid arthritis. 3
Familial cases and genetics
PPCRA has been reported to be most frequently sporadic, although some familiar cases have been reported, with many inheritance patterns being hypothesized.3,9–16,43,63
Noble et al. reported the case of three siblings, all of whom had early (or maybe congenital) onset PPCRA, without a clear inheritance pattern. 9 Traversi and colleagues described a case of unilateral RP in a woman and PPCRA in her daughter and son. 16 In another report, a father and his son were affected by PPCRA; the authors hypothesized a Y-chromosome-mediated male-to-male transmission. 10 Traboulsi et al. described a family with a pedigree compatible with X-linked inheritance. 14 Interestingly, many authors described familial cases with a more severe phenotype in males than in females.12,13,15 Finally, some authors described cases of monozygotic twins that were discordant for PPCRA, leading to the hypothesis that in some cases, even without any inflammatory cause, this disease can lack a clear mendelian inheritance pattern and may represent an acquired disorder.11,47 To sum up, there is currently no compelling evidence to support the notion that PPCRA is a genetic disorder or to determine its mode of transmission. Additionally, the etiology of the disease remains controversial. 1
To this date, mutations in only four genes have been associated with PPCRA.12,25,63,64
McKay et al. first reported the c.619G→A heterozygous mutation in the CRB1 gene in association with a family with PPCRA. 12 In particular, six out of the seven probands examined carried signs of the disease and the inheritance pattern was autosomal dominant. The c.619G→A mutation was responsible for an amino acid change from valine to methionine at codon 162 (p.Val162Met) within the fourth EGF-like domain. 12 CRB1 encodes for the Crumbs homologue 1 protein, whose biallelic mutations in humans have been associated with severe forms of autosomal recessive RP, RP with Coats’-like vasculopathy, Leber congenital amaurosis (LCA), and a retinal phenotype characterized by retinal thickening due to immature retinal lamination.65–68 It is a transmembrane protein expressed in the human retina and brain composed by 19 epidermal growth factor (EGF)-like domains, three laminin A globular-like domains and a 37-amino-acid cytoplasmatic tail, which plays major roles in the regulation of epithelial apical-basal polarity, suppression of the light-induced photoreceptor degeneration and probably also in laminar development.12,69 The authors hypothesized that the possible cause of PPCRA was a dominant negative effect of the Val162Met amino acid. 12
In another report from Oh and colleagues, two brothers harboring the c.119G > A (p.Arg40Gln) pathogenic missense variant of the CRX (cone-rod homeobox) gene had ocular manifestations phenocopying PPCRA and a history of nyctalopia and progressive worsening of visual acuity. 63 The locus involved in the specific variant carried by the two brothers is key for binding DNA and has been implicated in a broad spectrum of CRX-associated diseases, such as cone-rod and rod-cone dystrophies, macular dystrophies and LCA.70,71 CRX is a transcription factor involved in photoreceptors development and differentiation which is co-expressed with other transcription factors including NRL and NR2E3, capable of causing photoreceptor diseases when mutated.72,73 In their work, Oh and colleagues suggested that genetic modifiers co-expressed with CRX, such as NR2E3 could have influenced the resulting phenotype of their patients. 63
Shah et al. described the case of a woman with a distant family history of RP with a phenotype suggestive of PPCRA carrying the c.2551G > A (p.Glu851Lys) heterozygous pathological variant of the HK1 gene. 25 The same variant had been previously described in a six-generation family with adRP. 74 The HK1 gene encodes for the Hexokinase 1 protein, which is an ubiquitarian enzyme catalyzing the first step of glycolysis. 75 In their work, Shah and colleagues hypothesized that the Glu851Lys variant might affect a HK1 function that is exclusive to the retina, not specifically involved with its enzymatic activity, which is a mechanism that has been already described in adRP due to IMPHD1. 76
Finally, Bianco et al. described the case of a PPCRA male patient with a 30-year history of nyctalopia and progressive central vision blurring. 64 Their patient carried a dominant frameshift variant of the RPGRIP1 gene, the c.631del (p.Ser211Valfs*64), which encodes for a protein product that interacts with the retinitis pigmentosa GTPase regulator (RPGR) protein, a gene that causes X-Linked RP. 77 The RPGRIP1 has also been associated with LCA and cone-rod dystrophies.78,79 In their work, Bianco et al. suggest that the haploinsufficiency of the RPGRIP1 gene may be the cause of the phenotype of their patient, with an adult-onset slow-progressing retinal disease. 64 Of note, Liu et al. almost contemporarily described the case of a 2-year-old boy affected by PPCRA carrying two compound heterozygous variant in the same gene, namely the c.2592T > G (p,Tyr864*) and c.154C > T (p.Arg52*) variants. 80 Their patient showed a recessive mode of inheritance, but they did not further speculate on the possible disease mechanisms.
Only few studies have investigated the specific mutations behind PPCRA phenotypes, however this knowledge is essential to achieve better understanding of this disease. Notably, all of the mutations that have been associated with PPCRA are also known to cause other forms of rod-cone dystrophies.12,25,63,64
Remarkably, CRB1, HK1, CRX and RPGRIP1 are all highly expressed in the photoreceptors of the human retina, and capable of determining RP,12,25,63,64,72 which indicates that PPCRA may start at this level, while the choroid is affected only later over the course of the disease.
However, the phenotypic, and recently genotypic, variability of PPCRA leaves open questions on the underlying causes of the disease, suggesting that its phenotype could be the result of many interacting factors. Recently, a study conducted on mice models found out that two genetic modifiers, Arhgef12 and Prkci, play a key role in shaping the phenotype of CRB1-associated retinopathy. 81 Similar interations may also take place in the human retina, thus explaining part of the clinical variability of PPCRA.
Etiology and pathogenesis of PPCRA: updated scenario
The pathophysiology of PPCRA is currently under debate and many etiologies have been proposed. After its first description, many associations with inflammatory diseases have been reported, leading to the hypothesis that PPCRA could be a post-inflammatory disease.2,8 Indeed, cases of PPCRA following episodes of optic nerve edema or optic neuritis, and a previous history of infectious or immune-mediated diseases have been described.3,8,19 By contrast, the description of several cases of familiar PPCRA, and, more recently the characterization of four genes associated with this disease, suggest a genetic etiology.9,10,12–14,43,63,64,74
The primary site of damage in PPCRA is still unclear and contradictory evidence on this topic have been published up to this date.1,8,55,56,59 Previous angiographic studies found evidence of choroidal and choriocapillaris sparing, comparing PPCRA to RP and hypothesizing that alterations in the RPE occurred before vascular impairment of the choroid.3,10 Conversely, Barteselli et al. first reported the case of a 25-year-old male affected by early PPCRA showing focal choroidal thinning, without overlying RPE atrophy have been described. 55 Subsequently, the same alterations were found in a large case series by Lee et al, therefore supporting the theory that choroidopathy precedes RPE damage in PPCRA. 1 An alternative mechanism has been proposed by Shona et al., that suggested a generalized photoreceptor/RPE interface dysfunction rather than a primary or selective RPE dysfunction. 8
In a recent OCTA study, Battaglia Parodi et al. found that choriocapillaris impairment is present even without RPE atrophy. 59 However, they also reported a prevalent impairment in the deep vascular complex, together with a generalized thinning of the retinal layers in patients with atrophic changes and thinning confined to the inner retina and outer nuclear layer in patients without atrophy. 59 Inner retinal thinning on OCT had already been described, and is also consistent with the finding of GCL impairment, detectable with pattern ERG in cases of macular involvement.8,82 Linek et al. conducted a study on histological samples of dog retinas with a phenotype resembling those of human PPCRA. 83 In their study, the perivenous retinal atrophy was characterized by a complete loss or marked reduction of the outer and inner retinal layers, a partially disrupted RPE cell layer, dilated vessels of the inner plexiform layer, unaffected choroid and an abrupt transition to normal retina. 83 Moreover, regions of retinal thinning above intact RPE have been shown to correspond to junctional areas of hyperautofluorescence on FAF, implicating that retinal thinning occurs before RPE atrophy.50,56
In summary, many authors speculated on PPCRA etiology and pathogenesis, proposing a broad spectrum of underlying mechanisms at the basis of this disease. On the basis of previously described multimodal imaging, functional, histopathological and genetic findings, we support the model of a photoreceptor/RPE dysfunction, with the photoreceptors being the primary site of damage. Sustained by the expression of the genes involved and the familial clusters of PPCRA described, we hypothesize a primary disfunction of the photoreceptors, leading to retinal thinning, RPE metabolic stress and reduced oxygen request, which corresponds to the junctional areas hyperautofluorescence, retinal thinning and intact RPE described by Fleckenstein in 2009. 44 Subsequentely, RPE atrophic changes lead to the distinctive PPCRA lesions, whose perivenous localization remains unclear. The heterogeneous presentations and transmissions patterns may be influenced by complex gene or gene-environment interactions, which are yet to be studied.
Conclusions
PPCRA is a rare disease with varying expressivity that is usually discovered as an incidental finding during a routine clinical examination. Indeed, most patients are asymptomatic, and evidence suggest that PPCRA is slowly progressing, with the majority retaining a good visual function, unless macular involvement is present. Multimodal imaging has increased our capacity to diagnose PPCRA in recent years, particularly with the introduction of UWF imaging. Functional and genetic testing, in conjunction with blood tests, can aid in the right differential diagnosis of pseudo-PPCRA or other diseases with comparable characteristics. Its cause is unknown, but it is conceivable that PPCRA has a genetic basis. Future natural history research with extensive follow-ups and genetic testing will help us understand this condition better.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alessio Antropoli https://orcid.org/0000-0001-9630-0830
Lorenzo Pili https://orcid.org/0000-0002-0582-3191
Lorenzo Bianco https://orcid.org/0000-0002-4023-5387
Alessandro Berni https://orcid.org/0000-0002-6412-1288
Francesco Bandello https://orcid.org/0000-0003-3238-9682
Declarations
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Financial disclosure
Francesco Bandello consultant for: Alcon (Fort Worth, Texas, USA), Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California, USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, New York, USA), Genentech (San Francisco, California, USA), Hoffmann-La-Roche (Basel, Switzerland), NovagaliPharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA). All other authors have no disclosures to declare.
References
- 1.Lee EK, Lee SY, Oh BL, et al. Pigmented paravenous chorioretinal atrophy: clinical Spectrum and multimodal imaging characteristics. Am J Ophthalmol 2021; 224: 120–132. [DOI] [PubMed] [Google Scholar]
- 2.Brown TH. Retino-choroiditis radiata. Br J Ophthalmol 1937; 21: 645–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H-B, Zhang Y-X. Pigmented paravenous retinochoroidal atrophy (Review). Exp Ther Med 2014; 7: 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noble KG, Carr RE. Pigmented paravenous chorioretinal atrophy. Am J Ophthalmol 1983; 96: 338–344. [DOI] [PubMed] [Google Scholar]
- 5.Johansen J, Lund-Andersen C, Autzen T. Pigmented paravenous chorioretinal atrophy. Acta Ophthalmol (Copenh) 1988; 66: 474–477. doi:10.1111/j.1755-3768.1988.tb04044.x [DOI] [PubMed] [Google Scholar]
- 6.Kükner AS, Yilmaz T, Celebi S, et al. Pigmented paravenous retinochoroidal atrophy. A literature review supported by seven cases. Ophthalmol J Int Ophtalmol Int J Ophthalmol Z Augenheilkd 2003; 217: 436–440. [DOI] [PubMed] [Google Scholar]
- 7.Choi JY, Sandberg MA, Berson EL. Natural course of ocular function in pigmented paravenous retinochoroidal atrophy. Am J Ophthalmol 2006; 141: 763–765. [DOI] [PubMed] [Google Scholar]
- 8.Shona OA, Islam F, Robson AG, et al. Pigmented paravenous chorioretinal atrophy: detailed clinical study of a large cohort. Retina Phila Pa 2019; 39: 514–529. [DOI] [PubMed] [Google Scholar]
- 9.Noble KG. Hereditary pigmented paravenous chorioretinal atrophy. Am J Ophthalmol 1989; 108: 365–369. [DOI] [PubMed] [Google Scholar]
- 10.Skalka HW. Hereditary pigmented paravenous retinochoroidal atrophy. Am J Ophthalmol 1979; 87: 286–291. [DOI] [PubMed] [Google Scholar]
- 11.Small KW, Anderson WB, Jr. Pigmented paravenous retinochoroidal atrophy: discordant expression in monozygotic twins. Arch Ophthalmol 1991; 109: 1408–1410. [DOI] [PubMed] [Google Scholar]
- 12.McKay GJ, Clarke S, Davis JA, et al. Pigmented paravenous chorioretinal atrophy is associated with a mutation within the crumbs homolog 1 (CRB1) gene. Invest Ophthalmol Vis Sci 2005; 46: 322–328. [DOI] [PubMed] [Google Scholar]
- 13.Obata R, Yanagi Y, Iriyama Aet al. et al. A familial case of pigmented paravenous retinochoroidal atrophy with asymmetrical fundus manifestations. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 2006; 244: 874–877. [DOI] [PubMed] [Google Scholar]
- 14.Traboulsi EI., Maumenee IH. Hereditary pigmented paravenous chorioretinal atrophy. Arch Ophthalmol Chic Ill 1960 104, 1636–1640 (1986). [DOI] [PubMed] [Google Scholar]
- 15.Al-Husainy S, Sarodia U, Deane JS. Pigmented paravenous retinochoroidal atrophy: evidence of progression to macular involvement in a family with a 42-year history. Eye 2001; 15: 329–330. [DOI] [PubMed] [Google Scholar]
- 16.Traversi C, Tosi GM, Caporossi A. Unilateral retinitis pigmentosa in a woman and pigmented paravenous chorioretinal atrophy in her daughter and son. Eye 2000; 14: 395–397. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi K, Hara S, Tanifuji Yet al. et al. Inflammatory pigmented paravenous retinochoroidal atrophy. Br J Ophthalmol 1989; 73: 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellner U, Helbig H, Foerster MH. Phenocopies of hereditary retinal degenerations. Ophthalmol Z Dtsch Ophthalmol Ges 1996; 93: 680–687. [DOI] [PubMed] [Google Scholar]
- 19.Hsin-Hsiang C. Retinochoroiditis Radiata*. Am. J. Ophthalmol 1948; 31: 1485–1487. [PubMed] [Google Scholar]
- 20.Peduzzi M, Guerrieri F, Torlai Fet al. et al. Bilateral pigmented paravenous retino-choroidal degeneration following measles. Int Ophthalmol 1984; 7: 11–14. [DOI] [PubMed] [Google Scholar]
- 21.Foxman SG, Heckenlively JR, Sinclair SH. Rubeola retinopathy and pigmented paravenous retinochoroidal atrophy. Am J Ophthalmol 1985; 99: 605–606. [DOI] [PubMed] [Google Scholar]
- 22.Murray AT, Kirkby GR. Pigmented paravenous retinochoroidal atrophy: a literature review supported by a unique case and insight. Eye Lond Engl 2000; 14 Pt 5: 711–716. [DOI] [PubMed] [Google Scholar]
- 23.Pearlman JT, Kamin DF, Kopelow SMet al. et al. Pigmented paravenous retinochorodial atrophy. Am J Ophthalmol 1975; 80: 630–635. [DOI] [PubMed] [Google Scholar]
- 24.Pearlman JT, Heckenlively JR, Bastek JV. Progressive nature of pigmented paravenous retinochoroidal atrophy. Am J Ophthalmol 1978; 85: 215–217. [DOI] [PubMed] [Google Scholar]
- 25.Shah SM, Schimmenti LA, Chiang Jet al. et al. Association of pigmented paravenous retinochoroidal atrophy with a pathogenic variant in the HK1 gene. Retin Cases Brief Rep 2022; 16: 770–774. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad M, Leisy H, Carr REet al. et al. A rare case of unifocal, unilateral pigmented paravenous retinochoroidal atrophy (PPRCA). Am J Ophthalmol Case Rep 2016; 4: 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukkamala L, Yiu G. Asymmetry in pigmented paravenous retinochoroidal atrophy. JAMA Ophthalmol 2020; 138: e190911. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed AS, Rishi P. A rare presentation of pigmented paravenous retinochoroidal atrophy. Oman J Ophthalmol 2015; 8: 47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prieto Del Cura M, Crespo Carballés MJ, Acebes Garcia M, et al. Macular involvement in a pigmented paravenous retinochoroidal atrophy. J Fr Ophtalmol 2020; 43: e73–e76. [DOI] [PubMed] [Google Scholar]
- 30.Chen MS, Yang CH, Huang JS. Bilateral macular coloboma and pigmented paravenous retinochoroidal atrophy. Br J Ophthalmol 1992; 76: 250–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young WO, Small KW. Pigmented paravenous retinochoroidal atrophy (PPRCA) with optic disc drusen. Ophthalmic Paediatr Genet 1993; 14: 23–27. [DOI] [PubMed] [Google Scholar]
- 32.Batioglu F, Atmaca LS, Atilla Het al. et al. Inflammatory pigmented paravenous retinochoroidal atrophy. Eye Lond Engl 2002; 16: 81–84. [DOI] [PubMed] [Google Scholar]
- 33.Falfoul Y, Hassairi A, Chaker Net al. et al. A case of pigmented paravenous retinochoroidal atrophy with cystoid macular edema. J Fr Ophtalmol 2020; 43: 834–836. [DOI] [PubMed] [Google Scholar]
- 34.Figueiredo R, Morais Sarmento T, Garrido Jet al. et al. Pigmented paravenous retinochoroidal atrophy associated with unilateral cystoid macular oedema. BMJ Case Rep 2019; 12: e230633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanagi Y, Okajima O, Mori M. Indocyanine green angiography in pigmented paravenous retinochoroidal atrophy. Acta Ophthalmol Scand 2003; 81: 60–67. [DOI] [PubMed] [Google Scholar]
- 36.Xiang W, Wei Y. Pigmented paravenous chorioretinal atrophy with macular hole. JAMA Ophthalmol 2022; 140: e222437. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh B, Goel N, Batta Set al. et al. SD-OCT in pigmented paravenous retinochoroidal atrophy. Ophthalmic Surg Lasers Imaging Off J Int Soc Imaging Eye 2012; 43: e41–e43. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y, Li J, Yu Let al. et al. Pigmented paravenous retinochoroidal atrophy with acute angle-closure glaucoma and posterior subcapsular cataract: a case report. BMC Ophthalmol 2022; 22: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Limaye SR, Mahmood MA. Retinal microangiopathy in pigmented paravenous chorioretinal atrophy. Br J Ophthalmol 1987; 71: 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramtohul P, Chehaibou I, Bonnin S. Peripheral retinal vascular abnormalities in pigmented paravenous retinochoroidal atrophy. Am J Ophthalmol 2022; 236: e4–e5. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Sanz G, Carreño E, Mall S, et al. Unilateral pigmented paravenous retinochoroidal atrophy associated with presumed ocular Tuberculosis. Ophthalmic Surg Lasers Imaging Retina 2017; 48: 345–349. [DOI] [PubMed] [Google Scholar]
- 42.Karam EZ, Gomez R, Marco HD. Pigmented paravenous retinochoroidal atrophy in neurofibromatosis type 1. Ophthalmol Retina 2018; 2: 683. [DOI] [PubMed] [Google Scholar]
- 43.Bozkurt N, Bavbek T, Kazokoğlu H. Hereditary pigmented paravenous chorioretinal atrophy. Ophthalmic Genet 1998; 19: 99–104. [DOI] [PubMed] [Google Scholar]
- 44.Fleckenstein M, et al. Discrete arcs of increased fundus autofluorescence in retinal dystrophies and functional correlate on microperimetry. Eye Lond Engl 2009; 23: 567–575. [DOI] [PubMed] [Google Scholar]
- 45.Jung I, Lee Y, Kang Set al. et al. Pigmented paravenous retinochoroidal atrophy: a case report supported by multimodal imaging studies. Med Kaunas Lith 2021; 57: 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parafita M, Diaz A, Torrijos IGet al. et al. Pigmented paravenous retinochoroidal atrophy. Optom Vis Sci Off Publ Am Acad Optom 1993; 70: 75–78. [DOI] [PubMed] [Google Scholar]
- 47.Fischer N, Duignan E, Robson AG, et al. Monozygotic twins discordant for asymmetric pigmented paravenous chorioretinal atrophy. Retin Cases Brief Rep 2022; 16: 507–510. [DOI] [PubMed] [Google Scholar]
- 48.Hernández-Da Mota SE, Chacón-Lara A. Bilateral pigmented paravenous chorioretinal atrophy: a case report. Case Rep Ophthalmol 2011; 2: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsang SH, Sharma T. Pigmented paravenous chorioretinal atrophy (PPCRA). Adv Exp Med Biol 2018; 1085: 111–113. doi:10.1007/978-3-319-95046-4_22 [DOI] [PubMed] [Google Scholar]
- 50.Takagi S, Hirami Y, Takahashi M, et al. Use of wide-field Fundus camera, Fundus autofluorescence, and OCT in cases of pigmented paravenous retinochoroidal atrophy. Ophthalmol Retina 2018; 2: 79–81. [DOI] [PubMed] [Google Scholar]
- 51.Kumar V, Kumawat D, Tewari Ret al. et al. Ultra-wide field imaging of pigmented para-venous retino-choroidal atrophy. Eur J Ophthalmol 2019; 29: 444–452. [DOI] [PubMed] [Google Scholar]
- 52.Tandon M, Shukla D, Huda Ret al. et al. Pigmented paravenous chorioretinal atrophy with Coat’s like response. Indian J Ophthalmol 2013; 61: 586–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gasparian S, Sierpina DI. Fundus autofluorescence detects subtle pigmentary alterations in pigmented paravenous retinochoroidal atrophy. Am J Ophthalmol Case Rep 2021; 23: 101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirose T, Miyake Y. Pigmentary paravenous chorioretinal degeneration: fundus appearance and retinal functions. Ann Ophthalmol 1979; 11: 709–718. [PubMed] [Google Scholar]
- 55.Barteselli G. Fundus autofluorescence and optical coherence tomography findings in pigmented paravenous retinochoroidal atrophy. Can J Ophthalmol J Can Ophtalmol 2014; 49: e144–e146. [DOI] [PubMed] [Google Scholar]
- 56.Fleckenstein Met al. Correlation of lines of increased autofluorescence in macular dystrophy and pigmented paravenous retinochoroidal atrophy by optical coherence tomography. Arch Ophthalmol Chic Ill 1960 126, 1461–1463 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Romero R, Castaño A, Moriche M, et al. Pigmented paravenous retinochoroidal atrophy with macular involvement. Arch Soc Esp Oftalmol Engl Ed 2013; 88: 77–79. [DOI] [PubMed] [Google Scholar]
- 58.Ranjan R, Jain MA, Verghese S, et al. Multimodal imaging of pigmented paravenous retinochoroidal atrophy. Eur J Ophthalmol 2022; 32: NP125–NP129. [DOI] [PubMed] [Google Scholar]
- 59.Battaglia Parodi M, Arrigo A, Chowers I, et al. Optical coherence tomography angiography findings in pigmented paravenous chorioretinal atrophy. Retina Phila Pa 2022; 42: 915–922. [DOI] [PubMed] [Google Scholar]
- 60.Shen Y, Xu X, Cao H. Pigmented paravenous retinochoroidal atrophy: a case report. BMC Ophthalmol 2018; 18: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cicinelli MV, Giuffrè C, Rabiolo A, et al. Optical coherence tomography angiography of pigmented paravenous retinochoroidal atrophy. Ophthalmic Surg Lasers Imaging Retina 2018; 49: 381–383. [DOI] [PubMed] [Google Scholar]
- 62.Fallon J, Ahsanuddin S, Otero-Marquez O, et al. Posterior vitreous cortex hyalocytes visualization in asymmetric pigmented paravenous chorioretinal atrophy (PPCRA) using en face OCT. Am J Ophthalmol Case Rep 2023; 30: 101846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh JK, Nuzbrokh Y, Lee W, et al. A mutation in CRX causing pigmented paravenous retinochoroidal atrophy. Eur J Ophthalmol 2022; 32: NP235–NP239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bianco L, Antropoli A, Arrigo A, et al. RPGRIP1 variant associated with pigmented paravenous chorioretinal atrophy. Eur J Ophthalmol 2023; 11206721231155042. doi: 10.1177/11206721231155042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.den Hollander AI, ten Brink JB, de Kok YJM, et al. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat Genet 1999; 23: 217–221. [DOI] [PubMed] [Google Scholar]
- 66.den Hollander AI, Heckenlively JR, van den Born LI, et al. Leber congenital amaurosis and retinitis Pigmentosa with coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet 2001; 69: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobson SGet al. Crumbs homolog 1 (CRB1) mutations result in a thick human retina with abnormal lamination. Hum Mol Genet 2003; 12: 1073–1078. [DOI] [PubMed] [Google Scholar]
- 68.Lotery A. Jet al. Mutations in the CRB1 gene cause Leber congenital amaurosis. Arch Ophthalmol Chic. Ill 1960 119, 415–420 (2001). [DOI] [PubMed] [Google Scholar]
- 69.den Hollander AIet al. CRB1 Has a cytoplasmic domain that is functionally conserved between human and Drosophila. Hum Mol Genet 2001; 10: 2767–2773. [DOI] [PubMed] [Google Scholar]
- 70.Chau KY, Chen S, Zack DJet al. et al. Functional domains of the cone-rod homeobox (CRX) transcription factor. J Biol Chem 2000; 275: 37264–37270. [DOI] [PubMed] [Google Scholar]
- 71.Hull S, Arno G, Plagnol V, et al. The phenotypic variability of retinal dystrophies associated with mutations in CRX, with report of a novel macular dystrophy phenotype. Invest Ophthalmol Vis Sci 2014; 55: 6934–6944. [DOI] [PubMed] [Google Scholar]
- 72.Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell 1997; 91: 531–541. [DOI] [PubMed] [Google Scholar]
- 73.Peng G-H, Ahmad O, Ahmad F, et al. The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum Mol Genet 2005; 14: 747–764. [DOI] [PubMed] [Google Scholar]
- 74.Sullivan LS, Koboldt DC, Bowne SJ, et al. A dominant mutation in hexokinase 1 (HK1) causes retinitis Pigmentosa. Invest Ophthalmol Vis Sci 2014; 55: 7147–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Irwin DM, Tan H. Evolution of glucose utilization: glucokinase and glucokinase regulator protein. Mol Phylogenet Evol 2014; 70: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bowne SJ. Mutations in the inosine monophosphate dehydrogenase 1 gene (IMPDH1) cause the RP10 form of autosomal dominant retinitis pigmentosa. Hum Mol Genet 2002; 11: 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y, Hong DH, Pawlyk B, et al. The retinitis pigmentosa GTPase regulator (RPGR)- interacting protein: subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci USA 2003; 100: 3965–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hameed A. Evidence of RPGRIP1 gene mutations associated with recessive cone-rod dystrophy. J Med Genet 2003; 40: 616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khan AO, Al-Mesfer S, Al-Turkmani S, et al. Genetic analysis of strictly defined Leber congenital amaurosis with (and without) neurodevelopmental delay. Br J Ophthalmol 2014; 98: 1724–1728. [DOI] [PubMed] [Google Scholar]
- 80.Liu Z, Wang H, He X, et al. Identifying two pathogenic variants in a patient with pigmented paravenous retinochoroidal atrophy. Open Life Sci 2023; 18: 20220532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weatherly SM, Collin GB, Charette JR, et al. Identification of Arhgef12 and Prkci as genetic modifiers of retinal dysplasia in the Crb1rd8 mouse model. PLOS Genet 2022; 18: e1009798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Junqueira DLM, Lopes FSS, Biteli LGet al. et al. Pattern of inner retinal layers involvement in pigmented paravenous retinochoroidal atrophy as determined by SD-OCT: case report. Arq Bras Oftalmol 2013; 76: 380–382. [DOI] [PubMed] [Google Scholar]
- 83.Linek J, Gruber AD, Mecklenburg L. Five cases of pigmented paravenous retinochoroidal atrophy in dogs. Vet Ophthalmol 2012; 15: 41–47. [DOI] [PubMed] [Google Scholar]