Abstract
Background
In the United States in 2017, there were an estimated 903,745 hospitalizations involving mechanical ventilation (MV). Complications from ventilation can result in longer hospital stays, increased risk of disability, and increased healthcare costs. It has been hypothesized that electrically pacing the diaphragm by phrenic nerve stimulation during mechanical ventilation may minimize or reverse diaphragm dysfunction, resulting in faster weaning.
Methods
The ReInvigorate Trial is a prospective, multicenter, randomized, controlled clinical trial evaluating the safety and efficacy of Stimdia’s pdSTIM System for facilitating weaning from MV. The pdSTIM system employs percutaneously placed multipolar electrodes to stimulate the cervical phrenic nerves and activate contraction of the diaphragm bilaterally. Patients who were on mechanical ventilation for at least 96 h and who failed at least one weaning attempt were considered for enrollment in the study. The primary efficacy endpoint was the time to successful liberation from mechanical ventilation (treatment vs. control). Secondary endpoints will include the rapid shallow breathing index and other physiological and system characteristics. Safety will be summarized for both primary and additional analyses. All endpoints will be evaluated at 30 days or at the time of removal of mechanical ventilation, whichever is first.
Discussion
This pivotal study is being conducted under an investigational device exception with the U.S. Food and Drug Administration. The technology being studied could provide a first-of-kind therapy for difficult-to-wean patients on mechanical ventilation in an intensive care unit setting.
Trial registration
Clinicaltrials.gov, NCT05998018, registered August 2023.
Keywords: Phrenic nerve, Diaphragm stimulation, Mechanical ventilation, Ventilator-induced diaphragm dysfunction, Weaning
Background
In the United States in 2017, there were an estimated 903,745 hospitalizations involving mechanical ventilation [1]. Complications from mechanical ventilation can result in longer hospital stays, increased risk of disability, and increased healthcare costs. The estimated national costs were 27 billion USD, representing 12% of all hospital costs [2]. Over a third of the time in the ICU is spent on mechanical ventilation, and approximately 40% of the time on mechanical ventilation may be spent weaning from ventilation [3]. Additionally, those with prolonged mechanical ventilation are 50% more likely to be discharged to skilled nursing facilities for further care [4].
Many studies have demonstrated that mechanical ventilation has an unloading effect on respiratory muscles that leads to diaphragmatic atrophy and dysfunction (ventilator-induced diaphragm dysfunction [VIDD]). As little as 18 h has resulted in muscle fiber atrophy in both slow and fast muscle fibers associated with oxidative injury and increased muscle proteolysis in an animal model [5]. Sonographic assessments of diaphragm thickness and diaphragm biopsies have shown that a rapid rate of diaphragm atrophy during mechanical ventilation can impair functional recovery and weaning from mechanical ventilation [6]. This effect appears to be most prominent during controlled mechanical ventilation [7], while assisted ventilation can attenuate dysfunction [8].
It has been suggested that electrically pacing the diaphragm during mechanical ventilation may minimize or reverse diaphragm dysfunction, resulting in faster recovery of muscle strength and shorter weaning times [9]. This concept has been demonstrated in a spinal injury patient providing electrical stimulation for as little as 30 min per day [10]. Diaphragm pacing achieved by surgically implanted electrodes on the phrenic nerves, or the diaphragm itself, is currently used in conjunction with mechanical ventilation in patients who require extended diaphragm reconditioning, which is common in spinal cord injury patients [11].
Surgical implantation of electrodes is not practical for short-term stimulation to assist with weaning from mechanical ventilation. Stimdia Medical developed a bilateral percutaneous electrical phrenic nerve stimulation system (pdSTIM™) to temporarily stimulate the phrenic nerves to activate and recondition the diaphragm. Preclinical testing demonstrated that diaphragm strength was maintained in paced animals compared with nonpaced animals [12]. A feasibility study demonstrated that percutaneous placement of multipolar leads could be safely accomplished and effective at stimulating the leads [13] and was effective at increasing diaphragm thickness in mechanically ventilated patients [14].
Device description
The pdSTIM™ System is designed to stimulate the patient’s phrenic nerves to cause contraction of the diaphragm during the inspiratory cycle of mechanical ventilation. The pdSTIM™ System recognizes the onset of inspiration through flow sensing and bilaterally stimulates the phrenic nerves, using multipolar leads placed percutaneously in the patient’s neck above the clavicles. Stimulation ceases when the patient goes into expiration. The pdSTIM System is connected to a patient’s mechanical ventilation circuit, as shown in Fig. 1.
Fig. 1.
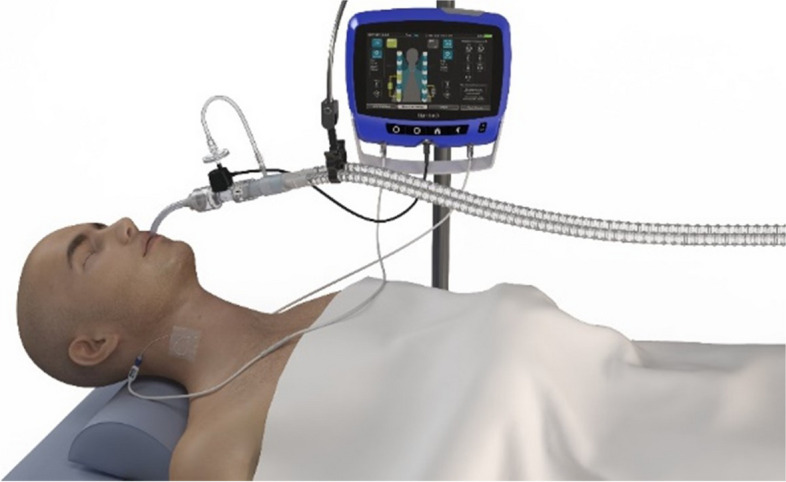
The pdSTIM system is shown with bilateral percutaneous phrenic nerve electrodes and the flow monitoring system attached to the ventilator circuit
Stimdia Medical recently launched a pivotal study, the Randomized Study of the pdSTIM™ System (phrenic nerve to diaphragm STIMULATION), in Patients who were Failure to Wean Mechanically Ventilated Patients (ReInvigorate Study). This study is being conducted under the Food and Drug Administration (FDA) Investigational Device Exemption (IDE). The pdSTIM™ System is not presently available for commercial use and is therefore limited to participants and institutions participating in the study.
The objective of this study was to evaluate the safety and efficacy of the use of the pdSTIM™ System for increasing diaphragm strength and reducing the weaning time of patients on mechanical ventilation compared to those of patients receiving standard of care.
Study design
The ReInvigorate Study is a prospective, multicenter, randomized, controlled clinical trial to evaluate the safety and efficacy of the pdSTIM™ System for facilitating weaning from mechanical ventilation through phrenic nerve stimulation. Potential patients who have received mechanical ventilation for ≥ 96 h and have failed at least one weaning attempt will be considered for enrollment. Those enrolled will be randomized at a 1:1 ratio to the pdSTIM™ System versus standard of care. A maximum of 350 subjects will be randomized in the study. Up to an additional 70 subjects may be enrolled in a nonrandomized roll-in group, resulting in up to 420 subjects in the study. This was an open-label study; neither the study subject nor the investigator was blinded to the treatment arm or study outcomes.
Study endpoints
The study has two primary endpoints and four secondary endpoints. All endpoints and additional objectives will be assessed following completion of the 30-day follow-up visit by all available randomized subjects:
Primary efficacy endpoint: Time to successful weaning from mechanical ventilation for the subjects randomized to treatment compared to subjects randomized to the control arm
Primary safety endpoint: Assessment of serious adverse events (SAEs) for the subjects randomized to treatment compared to subjects randomized to the control arm
Secondary endpoints include the 30-day mortality rate, adverse event rate, and number of days on mechanical ventilation. Additional objectives will summarize the characteristics of the pdSTIM™ system (e.g., lead placement success rate, duration of the procedure, waveforms of flow/pressure, etc.), mean change in the Rapid Shallow Breathing Index (RSBI), number of ICU days, and rate of reintubation following successful weaning for the treatment and control subjects.
Study population
The study will enroll subjects who have at least one failed weaning attempt following a minimum of 96 h on mechanical ventilation via either endotracheal (ET) or tracheostomy tube. Prior to randomization, all study participants will undergo screening (physical examination, medical history, vital signs, etc.) and will be included if they meet all the study inclusion criteria and none of the exclusion criteria.
Up to two nonrandomized roll-in subjects will be allowed at each investigational site prior to randomization. The first roll-in subject will assist with training of the investigator in lead placement in the clinical setting. A second roll-in subject can assist with training of a second investigator or in whom appropriate phrenic nerve stimulation could not be achieved in the first. The sites will be limited to two investigators trained and authorized to place the leads. Roll-in subjects will require the same data collection and follow-up procedures as treatment group subjects, with the data entered into the electronic data capture system.
Inclusion criteria
The study will require that subjects be 22 years of age or older, provide written informed consent (may be completed by their legally authorized representative), be mechanically ventilated via an endotracheal or tracheostomy tube for at least 96 h and have at least one failed weaning attempt, defined as a site-directed spontaneous breathing trial that did not result in liberation from mechanical ventilation.
Exclusion criteria
Patients were excluded if they were ventilated for more than 45 days, had certain preexisting neurological or neuromuscular disorders affecting respiratory muscle function (e.g., spinal cord injury, phrenic nerve paralysis, myasthenia gravis), were at risk of significant hemorrhage, had specific chronic lung diseases or disorders, had been diagnosed and treated for neck cancer within the past 5 years, had prior radiation to the neck, or were at elevated risk of developing or extending infection at the site of lead placement. The full list of study inclusion and exclusion criteria is available on ClinicalTrials.gov (NCT05998018).
Subject management and assessments
After providing informed consent and confirming the enrollment criteria, subjects randomized to the control group will continue to receive site-directed standard of care treatment for patients on mechanical ventilation at their research institution. Roll-in subjects and subjects randomized to the treatment group will undergo pdSTIM™ Therapy in addition to institutional standard of care, twice per day for a duration of 2 h, stimulating every 4th breath during this time. Phrenic nerve stimulation is coordinated via the RespiSync™ algorithm to produce physiologic diaphragmatic movement. The pdSTIM System is agnostic to the type or mode of ventilator. Prior to initiating a stimulation therapy session, a handful of measurements are read from the patient’s ventilator and entered into the pdSTIM console, including FiO2, humidifier type, static compliance, and static resistance. Measured inspired tidal volume (VTI, positive end-expiratory pressure (PEEP), inspiratory time (Ti), and work of breathing (WOB) are confirmed to align with the ventilator settings prior to initiating therapy. Additionally, the pdSTIM console is designed with several alarms, which are displayed both visually and with an audible sound when an alarm condition has occurred. The alarms and settings for the console are provided to sites in the pdSTIM System Operator’s Manual.
All subjects, regardless of treatment group assignment, will undergo study-specified assessments (Table 1).
Table 1.
Summary of procedures and data collection
| Data collection | Baseline within 2 days prior to randomization | Daily while on MV prior to 30-day visit | Every 3 days | Removal of MV if prior to 30-day visit | MV liberation assessment (48 ± 2 h following extubation/removal from mechanical ventilation) | 30-dayb follow-up (± 2 days) | 60-dayb follow-up (± 7 days) |
|---|---|---|---|---|---|---|---|
| Informed consent | X | ||||||
| Medical history | X | ||||||
| Physical exam | X | X | X | X |
X If hospitalized |
X If hospitalized |
|
| Medications | X | X | X | X | X | X | |
| RASS | X | X | X |
X If ventilated |
|||
| RSBI | X | X | X |
X If ventilated |
|||
| Weaning readiness assessment | X | X | X |
X If ventilated |
|||
| SBT | X |
X as indicated |
X |
X as indicated |
|||
| Therapy delivery (roll-in and treatment arm only)c | 2-h sessions 2x/day | ||||||
| pdSTIM system lead removalc |
X If successful |
X If ventilated |
|||||
| Adverse event assessment | Xa | X | X | X | X | X |
RASS Richmond Agitation-Sedation Score, RSBI Rapid shallow breathing index, SBT Spontaneous breathing trial
aAEs will be recorded at baseline only if they occur due to any prerandomization procedures required for purposes of this study protocol that were not standard of care
bFollow-up visits for subjects who have been discharged from the hospital prior to the visit will be conducted with Subject/LAR by phone or other communication
cRoll-in and Treatment group only—leads must be removed no later than 30 days after placement
Weaning readiness assessment
All enrolled subjects regardless of randomized group will be evaluated daily to determine protocol-specific readiness-to-wean criteria while on mechanical ventilation unless clinically contraindicated. The weaning readiness criteria include reversal of the underlying cause or reason for intubation, adequate oxygenation, hemodynamic stability, no administration of neuromuscular blockers, capability of initiating spontaneous breaths, and secretions that are not excessive, with details of the criteria in the clinical investigation plan. A protocol-specific spontaneous breathing trial (SBT) is conducted if the subject passes weaning criteria or, if in the opinion of the investigator, the subject is clinically able to attempt the SBT. Study-specific SBT settings are defined in the clinical investigation plan and generally follow best practices and published literature. In an effort to understand the impact of the therapy on all subjects meeting the study entry criteria, ventilator modes of the subjects are at the discretion of the investigators and will be documented daily and at the time of liberation from mechanical ventilation in the study database. If the SBT was successful, the subject was removed from MV. Subjects will be monitored for 48 h and then assessed to determine whether liberation from MV has been successful. Subjects that do not require reintubation or a return to mechanical ventilation within 48 h are considered successfully liberated from MV. The pdSTIM™ System leads were removed from the treatment group after the subject met the criterion for successful liberation from MV or no later than 30 days after lead insertion.
Data management
The study will utilize an electronic data capture (EDC) system. All subject data will be anonymized and stored securely with limited access by researchers at each site and by Stimdia’s monitors. Queries will be generated for missing or inaccurate data. All data queries will be resolved prior to data export for primary analyses.
Safety and study oversight
The principal investigator at each site will be responsible for overseeing timely and accurate reporting of adverse events, adverse device effects, and device deficiencies. Investigators will report serious adverse events (SAEs) and device deficiencies within 48 h of becoming aware of each event.
The ReInvigorate Study will be supported by an independent Clinical Events Committee (CEC), which will classify and adjudicate adverse events as related/not related to the study device and/or the study procedure. Additionally, an independent data safety and monitoring board (DSMB) will be established to oversee the study progress and review the clinical data and safety parameters. Each committee will hold routine meetings throughout the course of the study and manage their respective responsibilities per a Charter, which will be prepared and agreed upon by the respective committees.
Study monitoring will be conducted per the study monitoring plan. The study monitors will perform source data verification, review all the informed consent forms, ensure timely and appropriate Institutional Review Board (IRB) communication, verify that the documentation is complete and accurate, and ensure that the safety and study deviation reports are complete, timely, and accurate. Training will be undertaken prior to first study subject enrollment, and retraining will take place throughout the study, if warranted. In addition to study protocol training, investigators will receive training in the placement of pdSTIM™ leads.
The study was approved by a central institutional review board (WIRB Copernicus Group (WCG), Work Order Number 1601635). Up to 35 sites will be included in the study. The participating sites may use their local IRB or defer to the central IRB. In either case, site-specific informed consent forms will be prepared, submitted to the IRB, and approved by the IRB prior to use in the study. Each informed consent form will be confirmed to have all the required elements per the US Code of Federal Regulations regarding Protection of Human Subjects (21 CFR Part 50.25) prior to use in the study.
Statistical plan
As an intent-to-treat analysis to represent real-world application of this therapy in critically ill patients, this study is applying the Treatment Strategy in the Estimand framework as outlined in ECH E9 (R1) where occurrence of any intercurrent events is considered irrelevant [15]. The following intercurrent events have been identified:
• Mortality—will be treated with freedom from ventilation status at time of death as a right-censured event to the end of the study period (30 days).
• Study withdrawal by patient or treating physician—will result in continued data collection following removal of the treatment (if in treatment arm).
• Use of respiratory depressant or neuromuscular blocking agents—will result in continued treatment and data collection until end of the treatment period. This event could occur due to unplanned medical or surgical procedures or a change in patient condition.
• Adverse events requiring electrode catheter removal—will result in continued data collection following removal of the catheter. This event could occur for example if a catheter site infection developed.
Statistical analysis
Analysis of the primary endpoint will be based on a log-rank test at a one-sided 0.025 alpha level. The planned sample size is expected to provide 90% power for a range of plausible assumptions for successful weaning (e.g., 50% of control subjects are expected to be successfully weaned by day 30 vs. 65% of treatment subjects, corresponding to a hazard ratio of approximately 1.6). The primary analysis will be by intent-to-treat. A detailed statistical analysis plan (SAP) has been created and will be followed for all primary, secondary, and prespecified additional analyses. The analysis will include appropriate handling of missing data, subgroup analyses, and multiple sensitivity analyses. After the database is locked, the statistical analysis will be completed by a statistician independent of Stimdia. The data from the study will be analyzed once the sample size for the primary efficacy endpoint has been met. There are no planned interim analyses in this study. Data from roll-in subjects will be summarized separately and will not be included in the primary endpoint analysis.
In sensitivity analyses, we will examine the potential impact of mortality on the results. One approach will use the Finkelstein-Schoenfeld approach for analyzing a hierarchical composite of time to mortality and weaning, and another will include subjects who die in the primary analysis but counting their time as 30 days. This latter analysis assumes that subjects who die would not have been successfully weaned during the 30-day follow-up.
Discussion
Weaning from mechanical ventilation can be a challenge for critically ill patients receiving ventilation for durations of several days or more. The concept of ventilator-induced diaphragm dysfunction (VIDD) is a recently recognized cause of diaphragm weakness that can prolong the weaning process, increase the length of ICU stay, and increase the risk of complications from prolonged mechanical ventilation. These patients are at a higher risk of infectious complications [16], ventilator-induced lung injury and further diaphragm dysfunction [16], airway complications [17], and mortality within 1 year [18]. These complications increase the need for transfer to long-term acute care hospitals, each with attendant excess costs.
The concept of ventilation-induced diaphragm dysfunction was first described in an animal model of mechanical ventilation-induced diaphragm unloading for 48 h, leading to atrophy of the diaphragm [19]. Atrophy is the result of increased proteolysis and reduced protein synthesis [5]. The first description in humans involved autopsy specimens from brain-dead organ donors. After 18–69 h of mechanical ventilation, donors showed a greater than 50% reduction in the cross-sectional areas of diaphragm myofibers compared to short-term ventilation in patients undergoing surgical procedures [20]. The findings included increased diaphragmatic proteolysis during inactivity in as little as 18 h of disuse. Subsequent studies have shown that 60 to 80% of mechanically ventilated patients experience clinically significant diaphragm dysfunction [21, 22].
Ventilator-induced diaphragm dysfunction may be attenuated, but not prevented, by maintaining active diaphragm contraction with partial unloading using pressure support ventilation in place of controlled ventilation. In an animal model comparing the two modes, diaphragm proteolysis was demonstrated after 18 h of controlled ventilation and was reduced in the group receiving pressure support ventilation [23].
Pavlovic and Wendt proposed the application of phrenic nerve pacing in humans to prevent respiratory muscle fatigue during mechanical ventilation, translating from existing applications of pacing in patients with high spinal cord injury [9]. Implementation of phrenic nerve pacing via intravascular electrodes incorporated into a central venous catheter placed from the left subclavian vein and crossing the midline was effective at stimulating the phrenic nerves in a porcine model and mitigating the development of ventilator-induced diaphragm dysfunction during controlled mechanical ventilation [24]. This intravascular approach has been introduced into clinical trials as the LungPacer LIVE® catheter (LungPacer Medical, Inc., Burnaby, BC, Canada) [25]. A randomized open-label clinical trial (RESCUE2) of the LIVE® catheter in 112 patients demonstrated an improvement in maximal inspiratory pressure but may have been underpowered to demonstrate a higher incidence of successful weaning [26]. A larger trial (RESCUE3) has been completed (ClinicalTrials.gov NCT03783884). Using a technology related to but different from diaphragm stimulation, Liberate Medical, LLC (Crestwood, KY, USA) has introduced a system for noninvasive synchronous electrical stimulation of the abdominal wall muscles during expiration to assist weaning from mechanical ventilation (ClinicalTrials.gov NCT 05759013).
Although phrenic nerve stimulation has been used for chronic support through surgical approaches, such as cervical or intrathoracic approaches, support for weaning from or supporting patients during mechanical ventilation warrants a nonsurgical approach that can be placed at the bedside. The intravascular approach has been shown to successfully provide diaphragm pacing with the placement of an intravascular catheter but entails the risk of central venous catheterization with adjustment of the insertion depth to fully capture both phrenic nerves and potential interference with existing intravascular devices.
The pdSTIM™ system uses small-diameter (2.6 Fr) bilateral percutaneously placed temporary pacing leads inserted at the bedside. The pdSTIM™ system works independently of ventilator type and mode of ventilation using the proprietary RespiSync™ algorithm. Multiple electrodes on each lead help to ensure successful capture without requiring adjustment of the insertion depth. Like in the intravascular approach, it can be placed at the bedside, but unlike the intravascular approach, it will not interfere with existing intravascular devices.
As with any percutaneous device, there are associated risks. Risks for the pdSTIM™ System have been identified based on similar technologies and procedures. Risks are categorized as insertion, stimulation, and ventilation associated. Subcutaneous insertion across the posterior aspect of the sternocleidomastoid muscle is expected to reduce the risk of internal jugular or carotid artery puncture, but vascular puncture exists and can lead to blood loss, hematoma or pseudoaneurysm formation, and compression of cervical vascular and nerve structures leading to vascular thrombosis and stroke. The insertion site and lead length are expected to maintain the pdSTIM™ introducer needle in the anterior cervical soft tissue, but the risk of pneumothorax is low, although it is expected to be lower than that associated with deeper intravascular insertion. The risk of soft tissue infection exists, but a tunneled subcutaneous location without vascular insertion may reduce the risk of bloodstream infection. A percutaneous rather than an open surgical approach is expected to reduce the risk of surgical incisions and wound infections.
There are risks associated with electrical stimulation that are expected to be similar to those of other phrenic nerve stimulators. Stimulation of cervical muscles can occur, accompanied by associated discomfort due to muscle injury. Tissue injury, phrenic nerve injury, inflammation, arrhythmias, cardiac pacing, and cardiac arrest can result in inadvertent excessive stimulation currents. Overstimulation can overwork and injure the diaphragm or lead to barotrauma or volutrauma. Even without direct injury, the use of phrenic stimulation poses the risk of over- or underventilation, resulting in hypocarbia or hypercarbia, respectively. Hypoventilation could also result in hypoxemia due to its associated risks.
A transcutaneous approach to phrenic nerve stimulation in the neck using electromagnetic stimulation was reported by Charité University in Berlin. A proof-of-concept study demonstrated the ability to generate clinically meaningful tidal volumes through diaphragm contraction [27], and a clinical trial has been undertaken (STIMIT-II, ClinicalTrials.gov NCT05238753). This alternative approach to the minimally invasive percutaneous approach is the use of magnetic fields generated by coils placed on the skin overlying the phrenic nerves. The risks associated with percutaneous access could be eliminated with this system, but other risks related to stimulation would likely remain. However, whether the use of magnetic coils (up to 0.55 T or higher) impacts medical equipment in the vicinity has yet to be determined.
Conclusions
The pdSTIM™ system is the first percutaneous approach for short-term phrenic nerve stimulation intended to promote diaphragm recovery in patients receiving mechanical ventilation in the intensive care unit (ICU) setting. The ReInvigorate Trial is a prospective, multicenter, 1:1 randomized, controlled clinical trial to evaluate the safety and efficacy of the pdSTIM™ system for facilitating weaning in patients ventilated for more than 96 h and failing at least one weaning attempt. The proposed trial design and methods are designed to minimize risk, evaluate safety, and reduce weaning time.
Trial status
Approval to begin the study under an investigational device exception (IDE) from the FDA was obtained by Stimdia Medical in July 2023. The first subject was enrolled on September 29, 2023, and five subjects, including two roll-in subjects, were enrolled as of December 15, 2023. Enrollment is expected to be completed in late 2025.
Authors’ contributions
KS and CMM contributed to the design of the trial, and KS drafted the manuscript. KWC and JE participated in the patient enrollment and contributed to the revisions of the manuscript. SAC provided feedback on the clinical trial design and revised the manuscript.
Funding
This study is sponsored by Stimdia Medical, Inc.
Declarations
Ethics approval and consent to participate
This trial protocol was reviewed and approved by a central institutional review board (WIRB-Copernicus Group, Princeton, NJ, www.wcgclinical.org), Work Order Number 1601635. The study complies with the Declaration of Helsinki and will be conducted with Good Clinical Practice. Written informed consent will be obtained from all participants.
Consent for publication
Not applicable—no identifying images or other personal or clinical details of participants are presented here or will be presented in reports of the trial results. The participant information and informed consent forms are available from the corresponding author upon request.
Competing interests
KS is an employee of Stimdia Medical, Inc., the sponsor of the trial. CMM is an employee of the trial management firm contracted by Stimdia Medical, Inc. SAC receives compensation from Stimdia Medical, Inc., for consulting services related to the clinical trial. KWC and JE have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kempker JA, Abril MK, Chen Y, Kramer MR, Waller LA, Martin GS. The epidemiology of respiratory failure in the United States 2002–2017: a serial cross-sectional study. Crit Care Explor. 2020;2: e0128. 10.1097/CCE.0000000000000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–53. 10.1097/CCM.0b013e3181ef4460 [DOI] [PubMed] [Google Scholar]
- 3.Esteban A, Alía I, Ibañez J, Benito S, Tobin MJ. Modes of mechanical ventilation and weaning. A national survey of Spanish hospitals. The Spanish Lung Failure Collaborative Group. Chest. 1994;106:1188–93. 10.1378/chest.106.4.1188 [DOI] [PubMed] [Google Scholar]
- 4.Zilberberg MD, Shorr AF. Prolonged acute mechanical ventilation and hospital bed utilization in 2020 in the United States: implications for budgets, plant and personnel planning. BMC Health Serv Res. 2008;8:242. 10.1186/1472-6963-8-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med. 2002;166:1369–74. 10.1164/rccm.200202-088OC [DOI] [PubMed] [Google Scholar]
- 6.Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose KM. Diaphragm muscle thinning in patients who are mechanically ventilated. Chest. 2012;142:1455–60. 10.1378/chest.11-1638 [DOI] [PubMed] [Google Scholar]
- 7.Sassoon CS, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol. 2002;92:2585–95. 10.1152/japplphysiol.01213.2001 [DOI] [PubMed] [Google Scholar]
- 8.Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;170:626–32. 10.1164/rccm.200401-042OC [DOI] [PubMed] [Google Scholar]
- 9.Pavlovic D, Wendt M. Diaphragm pacing during prolonged mechanical ventilation of the lungs could prevent from respiratory muscle fatigue. Med Hypotheses. 2003;60:398–403. 10.1016/S0306-9877(02)00413-9 [DOI] [PubMed] [Google Scholar]
- 10.Ayas NT, McCool FD, Gore R, Lieberman SL, Brown R. Prevention of human diaphragm atrophy with short periods of electrical stimulation. Am J Respir Crit Care Med. 1999;159:2018–20. 10.1164/ajrccm.159.6.9806147 [DOI] [PubMed] [Google Scholar]
- 11.Dalal K, Dimarco AF. Diaphragmatic pacing in spinal cord injury. Phys Med Rehabil Clin N Am. 2014;25:619–29 viii. 10.1016/j.pmr.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 12.O'Mahony J, Dingfelder C, Polyakov I, Jocewicz T, Mischke J: [Preprint] Evaluation of the safety of percutaneous electrical stimulation of the phrenic and femoral nerves in a chronic porcine model: a GLP study. In bioRxiv: Cold Spring Harbor Laboratory; 2020.
- 13.O’Rourke J, Sotak M, Curley GF, Doolan A, Henlin T, Mullins G, Tyll T, Omlie W, Ranieri MV. Initial assessment of the percutaneous electrical phrenic nerve stimulation system in patients on mechanical ventilation. Crit Care Med. 2020;48:e362–70. 10.1097/CCM.0000000000004256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sotak M, Roubik K, Henlin T, Tyll T. Phrenic nerve stimulation prevents diaphragm atrophy in patients with respiratory failure on mechanical ventilation. BMC Pulm Med. 2021;21:314. 10.1186/s12890-021-01677-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration: E9(R1) Statistical principles for clinical trials: Addendum: Estimands and sensitivity analysis in clinical trials - guidance for industry. (Department of Health and Human Services ed., Revision 1 edition: Office of Communications, Center for Drug Evaluation and Research 2021.
- 16.Huang HY, Huang CY, Li LF. Prolonged mechanical ventilation: outcomes and management. J Clin Med. 2022;11(9):2451. 10.3390/jcm11092451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung NH, Napolitano LM. Tracheostomy: epidemiology, indications, timing, technique, and outcomes. Respir Care. 2014;59:895–915 discussion 916–899. 10.4187/respcare.02971 [DOI] [PubMed] [Google Scholar]
- 18.Damuth E, Mitchell JA, Bartock JL, Roberts BW, Trzeciak S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:544–53. 10.1016/S2213-2600(15)00150-2 [DOI] [PubMed] [Google Scholar]
- 19.Le Bourdelles G, Viires N, Boczkowski J, Seta N, Pavlovic D, Aubier M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med. 1994;149:1539–44. 10.1164/ajrccm.149.6.8004310 [DOI] [PubMed] [Google Scholar]
- 20.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–35. 10.1056/NEJMoa070447 [DOI] [PubMed] [Google Scholar]
- 21.Demoule A, Molinari N, Jung B, Prodanovic H, Chanques G, Matecki S, Mayaux J, Similowski T, Jaber S. Patterns of diaphragm function in critically ill patients receiving prolonged mechanical ventilation: a prospective longitudinal study. Ann Intensive Care. 2016;6:75. 10.1186/s13613-016-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dres M, Dube BP, Mayaux J, Delemazure J, Reuter D, Brochard L, Similowski T, Demoule A. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med. 2017;195:57–66. 10.1164/rccm.201602-0367OC [DOI] [PubMed] [Google Scholar]
- 23.Futier E, Constantin JM, Combaret L, Mosoni L, Roszyk L, Sapin V, Attaix D, Jung B, Jaber S, Bazin JE. Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm. Crit Care. 2008;12:R116. 10.1186/cc7010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds SC, Meyyappan R, Thakkar V, Tran BD, Nolette MA, Sadarangani G, Sandoval RA, Bruulsema L, Hannigan B, Li JW, et al. Mitigation of ventilator-induced diaphragm atrophy by transvenous phrenic nerve stimulation. Am J Respir Crit Care Med. 2017;195:339–48. 10.1164/rccm.201502-0363OC [DOI] [PubMed] [Google Scholar]
- 25.Evans D, Shure D, Clark L, Criner GJ, Dres M, de Abreu MG, Laghi F, McDonagh D, Petrof B, Nelson T, Similowski T. Temporary transvenous diaphragm pacing vs standard of care for weaning from mechanical ventilation: study protocol for a randomized trial. Trials. 2019;20:60. 10.1186/s13063-018-3171-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dres M, de Abreu MG, Merdji H, Muller-Redetzky H, Dellweg D, Randerath WJ, Mortaza S, Jung B, Bruells C, Moerer O, et al. Randomized clinical study of temporary transvenous phrenic nerve stimulation in difficult-to-wean patients. Am J Respir Crit Care Med. 2022;205:1169–78. 10.1164/rccm.202107-1709OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panelli A, Bartels HG, Krause S, Verfuss MA, Grimm AM, Carbon NM, Grunow JJ, Stutzer D, Niederhauser T, Brochard L, et al. First non-invasive magnetic phrenic nerve and diaphragm stimulation in anaesthetized patients: a proof-of-concept study. Intensive Care Med Exp. 2023;11:20. 10.1186/s40635-023-00506-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


