Abstract
The existence of an extrahepatic reservoir of hepatitis C virus (HCV) is suggested by differences in quasispecies composition between the liver, peripheral blood mononuclear cells, and serum. We studied HCV RNA compartmentalization in the plasma of nine patients, in CD19+, CD8+, and CD4+ positively selected cells, and also in the negatively selected cell fraction (NF). HCV RNA was detected in all plasma samples, in seven of nine CD19+, three of eight CD8+, and one of nine CD4+ cell samples, and in seven of eight NF cells. Cloning and sequencing of HVR1 in two patients showed a sequence grouping: quasispecies from a given compartment (all studied compartments for one patient and CD8+ and NF for the other) were statistically more genetically like each other than like quasispecies from any other compartment. The characteristics of amino acid and nucleotide substitutions suggested the same structural constraints on HVR1, even in very divergent strains from the cellular compartments, and homogeneous selection pressure on the different compartments. These findings demonstrate the compartmental distribution of HCV quasispecies within peripheral blood cell subsets and have important implications for the study of extrahepatic HCV replication and interaction with the immune system.
Hepatitis C virus (HCV) infection induces chronic hepatitis, cirrhosis, and hepatocellular carcinoma worldwide. At all stages of liver disease, HCV exists as a heterogeneous population of distinct but closely related genomes referred to as quasispecies (26). HCV quasispecies composition is mainly assessed by focusing on a particular hypervariable region (HVR1) 27 amino acids long located at the NH2 terminus of envelope glycoprotein 2 (E2) (11). The HVR1 quasispecies composition undergoes extensive variations during the course of chronic infection, possibly corresponding to the emergence of immune escape mutants. This has been proposed as a pivotal mechanism of HCV persistence (15, 16, 19, 46, 51, 53). Nevertheless, numerous authors have detected HCV RNA in peripheral blood mononuclear cells (PBMC) and the bone marrow cells of chronically infected individuals (23, 24, 32, 34, 56). Interference with cells of the immune system is a known mechanism by which viruses evade the host response and become chronically infective (for a review, see reference 38). The notion of the infection of extrahepatic tissues by HCV is still controversial, however. Indeed, detection of HCV RNA in PBMC could be due to the simple adsorption of viral particles. The specificity and sensitivity of methods used to detect the negatively stranded HCV RNA, the obligatory replication intermediate (30), are also controversial (9, 21, 22, 29, 37, 54). Infection of extrahepatic tissues is supported by the finding that HCV quasispecies composition differs according to the sample type, i.e., liver, serum, or PBMC (2, 8, 25, 35, 37). Some human lymphoid cell lines are susceptible to HCV infection in vitro (12, 31, 47, 48, 49). A few minority HCV strains in the inoculum continued to replicate in such cells, supporting the concept that some quasispecies are preferentially lymphotropic rather than hepatotropic. However, the possibility that the difference observed in quasispecies composition between compartments is due to chance has not been formally studied. Furthermore, this compartmentalization has been described in total PBMC but not in the constituent cell types. We thus studied the distribution of HCV quasispecies in plasma, in peripheral positively selected CD19+, CD8+, and CD4+ cells, and in negatively selected remnant cells.
MATERIALS AND METHODS
Patients.
Nine anti-HCV-positive patients were studied. Six were infected by genotype 1b, two were infected by genotype 4, and one was infected by genotype 2 (InnoLipa II Genotyping Kit; Innogenetics, Gent, Belgium). The estimated duration of the disease ranged from 7 years to more than 30 years. Five patients had compensated cirrhosis, while three had chronic hepatitis at various stages. One patient was tested 6 months after liver transplantation for decompensated cirrhosis. None of the patients had received antiviral therapy, and all were hepatitis B surface antigen negative and anti-human immunodeficiency virus (HIV) negative.
Cell sorting.
Mononuclear cells were obtained from 20 ml of EDTA-treated blood by centrifugation through a Ficoll density gradient. Cells were washed three times, counted, and immediately used for separation and purification following immunomagnetic-positive selection with antibodies directed against specific surface molecules. First, 10 × 106 to 20 × 106 PBMC were suspended in 1 ml of RPMI medium. Then, 25 μl of anti-CD19 coupled to magnetic microbeads (Dynabeads M450 CD19; Dynal, Oslo, Norway) was added. The cell sample was then incubated at 4°C with gentle rotation for 30 min. After magnetic separation, the remaining cells were subjected to CD8- and then CD4-positive sorting with 140 μl of anti-CD8 (Dynabeads M450 CD8; incubation time, 30 min) and 140 μl of anti-CD4 (Dynabeads M450 CD4; incubation time, 60 min). All sorted cells were washed five times in RPMI medium. Dynabeads were next removed by using Detachbeads CD19 or CD4/CD8 (Dynal), according to the manufacturer’s instructions, and then washed. Our positive purification protocol resulted in very pure (>95%) CD19+, CD8+, and CD4+ cell subsets as assessed by flow cytometry with paired and directly labeled monoclonal antibodies (B4-fluorescein isothiocyanate [FITC] for CD19-positive cells, T8-FITC, and T4-FITC) purchased from Coulter (Immunotech, Marseille, France) (data not shown). The negative fraction (NF) collected at the end of the last immunomagnetic selection step contained less than 1% of CD19+, CD8+, or CD4+ cells and up to 80% of CD45+ cells. Further characterization with FITC or phycoerythrin-labeled antibodies (Ortho-Clinical Diagnostics GmbH, Neckargemünd, Germany) showed 10 to 38% CD11c+ cells (mainly monocytes) and 13 to 38% CD16+ cells (mainly natural killer cells). The remaining 10 to 20% of cells were granulocytes (not shown). Cell subsets were stored at −80°C until use.
Extraction of nucleic acids, RT-PCR, and quantification of HCV RNA.
RNA was extracted from 140 μl of plasma by using the QIAmp viral RNA kit (Qiagen GmBH) and from cell subsets by using the RNeasy Minikit (Qiagen). For detection of the HCV genome, one-fifth of the extracted plasma RNA or 1 μg of cellular RNA was subjected to reverse transcriptase PCR (RT-PCR) by using Ready-To-Go RT-PCR beads (Pharmacia Biotech, Uppsala, Sweden). A 3-μl portion of the first PCR product was subjected to a second-round PCR with Ready-To-Go PCR beads according to the manufacturer’s instructions. Outer and inner primers for the 5′ noncoding region were as follows: SF1 (5′-TGCACGGTCTACGAGACCTC-3′) and SR1 (5′-GCCATGGCGTTAGTATGAGT-3′) (outer) and SF2 (5′-GTGCAGCCTCCAGGACCCCC-3′) and SR2 (5′-GGGCACTCGCAAGCACCCTA-3′) (inner).
Outer and inner primers for HVR1, which generate a 307-bp product (+956 to +1262 according to the numbering system of Choo et al. [4]) encompassing the C-terminal E1 transmembrane region and the N-terminal hypervariable E2 region, were described elsewhere (13). To check for the absence of PCR inhibitors, RT-PCR was performed with the following primers spanning exons 3 and 4 of the cyclin A gene (52): He4 (sense) (5′-GCGGAATTCGAGTCACCACATACTATGGAC-3′) and He3 (antisense) (5′-GCGCTGCAGTAACAGCATAGCAGCAGTGC-3′).
The molecular weight of the amplified products is 545 bp for the DNA fragment and 335 bp for the mRNA fragment. Amplification products were revealed by ethidium bromide staining after agarose gel electrophoresis. Plasma HCV RNA was quantified by using the noncompetitive PCR-based Amplicor HCV Monitor assay (Roche Molecular Systems, Branchburg, N.J.) as specified by the manufacturer.
Cloning and sequencing of PCR products.
HCV isolates were amplified from plasma or cell subsets by using the HVR1 nested primer set. PCR products were cloned in the pGEM-T Easy Vector System (Promega Corp., Madison, Wis.) and transformed into Escherichia coli JM109 competent cells (Promega). After overnight incubation at 37°C, insertion was checked by PCR with the HVR1 inner primer pair on white colonies. Each of these PCR products with the correct molecular weight was sequenced bidirectionally and automatically by using the HVR1 inner primer pair on an ABI377 sequencer with the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit with Amplitaq DNA polymerase (FS; Perkin-Elmer/Applied Biosystems, Foster City, Calif.).
Sequence analysis.
Nucleotide and deduced peptide sequences of cloned products were aligned by using the CLUSTALW program, version 1.5 (50). As an index of HVR1 genetic complexity within a given compartment, the normalized Shannon entropy (Sn) (55) was calculated as follows: Sn = −Σi (pi ln pi)/ln N, where pi is the frequency of each sequence and N is the total number of sequences analyzed in each compartment. Sn theoretically varies from 0 (no diversity) to 1 (maximum diversity). Synonymous (dS) and nonsynonymous (dN) distances were calculated with the Jukes-Cantor correction in the Molecular Evolutionary Genetics Analysis (MEGA) package, version 1.01 (18). Statistical comparisons of distances were made by using the t test. Pairwise nucleotide distances were calculated by using the Kimura two-parameter method with a transition-to-transversion ratio of 2 (DNADIST from the Phylogeny inference package (PHYLIP), version 3.2. This matrix was used to determine both evolutionary relationships among sequences and correlation with compartmental distribution. Unrooted phylogenetic trees were constructed with the MEGA package by using the neighbor-joining algorithm, a cluster analysis method that fits sequences with high similarity scores such as HCV quasispecies. The statistical evaluation of the obtained topology was performed with 500 replications of bootstrap sampling. To determine whether sequences from a given compartment shared more genetic identity with each other than with sequences from other compartments, we used the Mantel’s test (43), which compares the Kimura two-parameter distance matrix to a compartment distribution matrix Mc of the same dimensions, where Mc(i, j) = 0 if sequence i is from the same compartment as sequence j and where Mc(i, j) = 1 otherwise. The Pearson correlation coefficient r2 was computed for all pairs excluding the diagonal of both matrices (observed r2). The null distribution was constructed by permuting the rows and columns of the matrix Mc 10,000 times. The number of times, more than 10,000 permutations, where the observed r2 was exceeded gave the exact P value of the correlation observed.
Antigenic analysis.
The antigenic analysis was done blindly with regard to the origin of sequence. Peptide sequences were analyzed by one of us (F.P.) according to their antigenicity, hydrophobicity, and polarity values. The antigenic grouping was performed manually and with Parker’s algorithm (40). Comparisons of antigenic grouping and compartmental distribution were made by using the χ2 or F tests.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the nucleotide sequences reported here are AF163152 to AF163253.
RESULTS
HCV RNA detection in plasma and cell subsets.
HCV RNA sequences were detected in the plasma and in at least one peripheral blood cell subset of the nine patients tested, regardless of the degree of viremia, the disease duration, and the genotype. Overall results are shown in Table 1, along with the respective genotypes and viral loads. Cyclin A mRNA was positive in all but one of the cell samples, rendering the negative result of HCV RNA in the CD8+ subset of patient F noninterpretable. HCV RNA was detected in CD19+ cells from seven of nine patients, in CD8+ cells from three of eight patients, in CD4+ cells from one of nine patients, and in the NF cells from seven of eight patients. Two patients (A and B) had HVR sequences in four compartments: plasma, CD19+, CD8+, and NF cells.
TABLE 1.
Detection of HCV and cyclin RNA in plasma, in CD19+, CD4+, and CD8+ cells, and in the NF cells (CD19−, CD4−, and CD8−) of PBMC
| Patient (genotype)a | HCV load | Plasma
|
CD19+ cells
|
CD4+ cells
|
CD8+ cells
|
NF cells
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5′NC | HVR | Cyclin | 5′NC | HVR | Cyclin | 5′NC | HVR | Cyclin | 5′NC | HVR | Cyclin | 5′NC | HVR | ||
| A (1b) | 480,000 | + | + | + | + | + | + | − | − | + | + | + | + | + | + |
| B (1b) | 999,000 | + | + | + | + | + | + | − | − | + | + | + | + | + | + |
| C (1b) | <1,000 | + | + | + | + | − | + | − | − | + | − | − | + | − | − |
| D (1b) | 11,900 | + | + | + | − | − | + | − | − | + | − | + | NDb | ND | ND |
| E (2) | 1,510,000 | + | + | + | − | + | + | − | − | + | − | − | + | + | + |
| F (1b) | 391,000 | + | + | + | − | − | + | − | − | − | − | − | + | + | − |
| G (1b) | 207,742 | + | + | + | + | + | + | + | − | + | − | − | + | + | + |
| H (4) | 190,601 | + | + | + | + | − | + | − | − | + | − | − | + | + | − |
| I (4) | 13,981 | + | + | + | + | + | + | − | − | + | − | − | + | + | − |
Clinical status: A and B, cirrhosis; C, transplanted with chronic active hepatitis; D, E, and F, cirrhosis and hepatocellular carcinoma; G, H, and I, chronic active hepatitis.
ND, not done.
Analysis of HVR1 cloning and sequencing in two patients.
From the 307-bp PCR product, a final length of 273 bp for patient A and 240 bp for patient B was used to perform genetic analysis.
(i) Genetic diversity.
Totals of 48 and 53 HVR1 clones were obtained from patients A and B, respectively. The number of clones per compartment ranged from 3 to 19. The alignment of the C-terminal part of E1 clearly showed the existence of two main strains, one specific to each patient, thus excluding the possibility of sample carryover. Each patient harbored a mixture of genetically distinct but closely related variants (Table 2): the average within-patient genetic distance was 0.048 ± 0.029 in patient A and 0.057 ± 0.018 in patient B. Both patients’ CD8+ cells harbored fewer and less-divergent variants than their plasma and CD19+ cells. Indeed, the genetic diversity of the E1/E2 region, estimated by calculating the normalized amino acid entropy, ranged from 0.66 (CD8+) to 0.94 (CD19+) in patient A and from 0.48 (CD8+) to 0.98 (plasma) in patient B; the degree of genetic diversity, determined as the average within-compartment genetic distance, was lower in CD8+ than in CD19+ cells both in patient A (0.027 ± 0.021 versus 0.07 ± 0.047; P was not significant) and in patient B (0.009 ± 0.004 versus 0.06 ± 0.011; P < 0.001).
TABLE 2.
Genetic diversity of E1/E2 quasispecies from plasma and CD8+, CD19+, and NF cells in two patients as determined by E1 and HVR1 nucleotide substitution analysis
| Patient and compartment | Na | Normalized amino acid entropy | Mean genetic distanceb | Type of nucleotide substitutionc
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| E1
|
HVR
|
||||||||
| dS | dN | dN/dS | dS | dN | dN/dS | ||||
| Patient A | 48 | 0.74 | 0.048 (0.029) | ||||||
| Plasma | 13 | 0.68 | 0.065 (0.050) | 0.070 (0.026) | 0.016 (0.006) | 0.228* | 0.191 (0.058) | 0.142 (0.032) | 0.743 |
| CD19+ | 16 | 0.94 | 0.070 (0.047) | 0.082 (0.027) | 0.019 (0.007) | 0.232* | 0.296 (0.092) | 0.245 (0.051) | 0.828 |
| CD8+ | 16 | 0.66 | 0.027 (0.021) | 0.061 (0.021) | 0.009 (0.004) | 0.147** | 0.032 (0.014) | 0.029 (0.012) | 0.906 |
| NF | 3 | 0 | 0.030 (0.00) | ||||||
| Patient B | 53 | 0.86 | 0.057 (0.018) | ||||||
| Plasma | 19 | 0.98 | 0.067 (0.006) | 0.021 (0.016) | 0.021 (0.008) | 1 | 0.281 (0.091) | 0.3535 (0.054) | 1.258 |
| CD19+ | 17 | 0.94 | 0.060 (0.011) | 0.024 (0.016) | 0.014 (0.007) | 0.583 | 0.096 (0.04) | 0.2151 (0.039) | 2.240* |
| CD8+ | 6 | 0.48 | 0.009 (0.004) | 0.010 (0.010) | 0.007 (0.005) | 0.67 | 0 | 0.0202 (0.012) | ND |
| NF | 11 | 0.77 | 0.104 (0.051) | 0.083 (0.032) | 0.034 (0.012) | 0.41 | 0.247 (0.089) | 0.2473 (0.089) | 1 |
Number of independent analyzed clones.
The within-sample genetic distances were calculated with the DNADIST module of the PHYLIP package and were based on a Kimura two-parameter matrix with a transition to transversion ratio of 2.
dN and dS, the mean proportion of nonsynonymous substitutions per nonsynonymous site and the synonymous substitutions per synonymous site, were computed by the MEGA package with the Jukes-Cantor correction. ND, not determined. dN and dS were compared by using a t test (∗, P < 0.05; ∗∗, P < 0.01).
(ii) Nucleotide substitutions.
The relative proportion of synonymous (dS) and nonsynonymous (dN) mutations can reveal positive selection pressure operating on a viral population if dN is greater than dS (36). We determined average dN and dS values for E1 and HVR1 sequences obtained from each compartment (Table 2). In a given patient, the sequences from all the compartments yielded dN/dS ratios of similar magnitude, suggesting a relatively homogeneous selective pressure in all the compartments concerned. All HVR1 variants from patient A had dN < dS, whereas those from patient B had dN ≥ dS. In this latter patient, the existence of positive selection pressure on the quasispecies originating from CD19+ cells was demonstrated.
In the absence of a reliable method to assess HCV replication in PBMC, we assumed that only the liver contributed to the plasma viral pool and that cellular sequences originated primarily from plasma. We compared the average proportion of nucleotide substitutions per nonsynonymous sites in sequences derived from CD8+ and CD19+ cells and from plasma. In patient A, greater divergence was observed between the CD19+ compartment and plasma (25 ± 5%) than between the CD8+ compartment and plasma (11 ± 2%; P < 0.01). Conversely, in patient B, greater divergence was observed between the CD8+ compartment and plasma (32 ± 5%) than between the CD19+ compartment and plasma (20 ± 2%; P < 0.05).
(iii) Genetic relationships among compartment quasispecies.
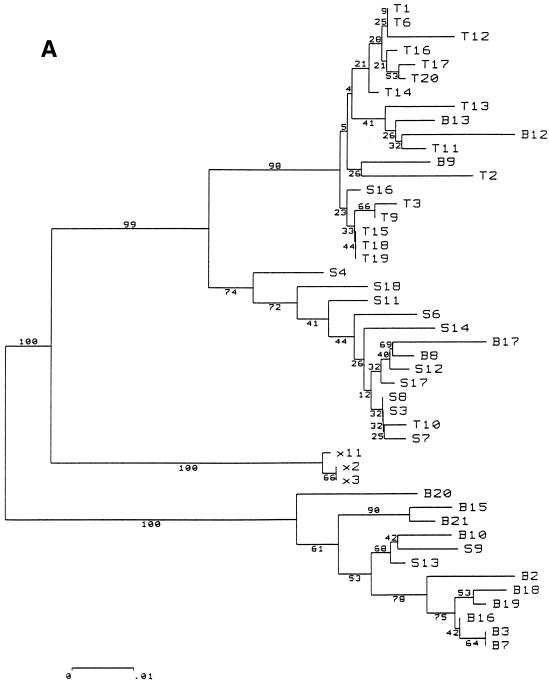
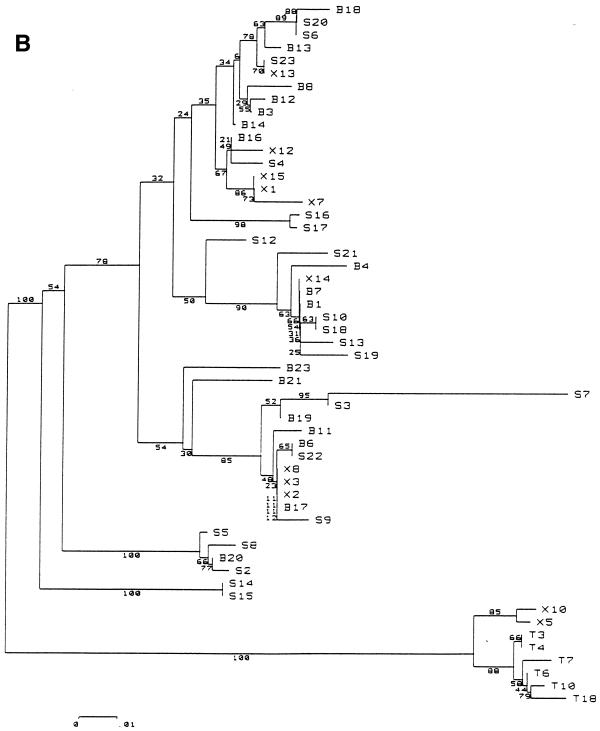
Bootstrapped phylogenetic trees obtained from viral sequences suggested significant phylogenetic grouping according to the compartmental origin of the clones. The strains harbored by CD8+ lymphocytes clustered close to one another in both patients. The CD19+, plasma, and NF variants also clustered in patient A, whereas patient B’s plasma, NF, and CD19+ sequences appeared to be melted (Fig. 1).
FIG. 1.
Phylogenetic tree, patients A (A) and B (B). Abbreviations: T, CD8+ lymphocytes; B, CD19+ lymphocytes; X, negative cell fraction (CD19−, CD8−, and CD4−); S, plasma. The phylogenetic grouping of sequences from all four compartments in patient A and from CD8+ cells in patient B was confirmed by a significant correlation between genetic proximity and compartment appurtenance (P < 0.0001, by Mantel’s test; see Materials and Methods). Variants in NF cells from patient B were not included in the same node of the phylogenetic tree but were overall more genetically identical to each other than to variants from any other compartment (P < 0.0001). These NF cells have at least three phenotypes: monocytes/macrophages, natural killer cells, and granulocytes.
To obtain statistical confirmation of genetic compartmentalization, we used Mantel’s test to search for a relation between pairwise Kimura two-parameter distances and compartment distribution. Significant genetic compartmentalization was observed for CD8+ variants in both patients, since 10,000 random permutations of the Mc matrix did not produce a correlation coefficient higher than that found with the observed distribution (P < 0.0001). Similarly, a significant compartmental structure, with a P value of less than 0.001, was observed for sequences harbored by CD19+ cells in patient A and by NF cells in patients A and B. For patient B, it is noteworthy that sequences from the NF cells were not included in the same node of the phylogenetic tree. These sequences segregated in a few clusters of the tree topology. The high P value of the Mantel’s test demonstrated formally that sequences from NF cells were closer to each other than to any sequences from other compartments. This was not the case for variants in CD19+ cells from patient B (P = 0.07). This statistical analysis of genetic distances unambiguously demonstrated that the compartmental sequence distribution was not due to chance: quasispecies harbored by CD8+ and NF cells, and in at least one patient by CD19+ cells, were significantly different from those detected in plasma.
(iv) Grouping of quasispecies according to amino acid sequences.
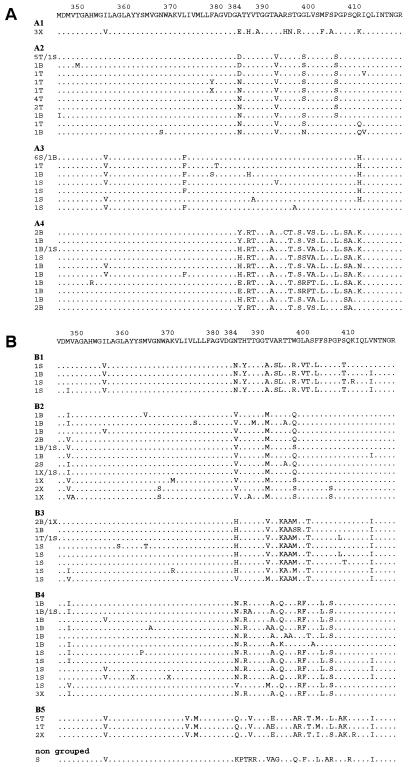
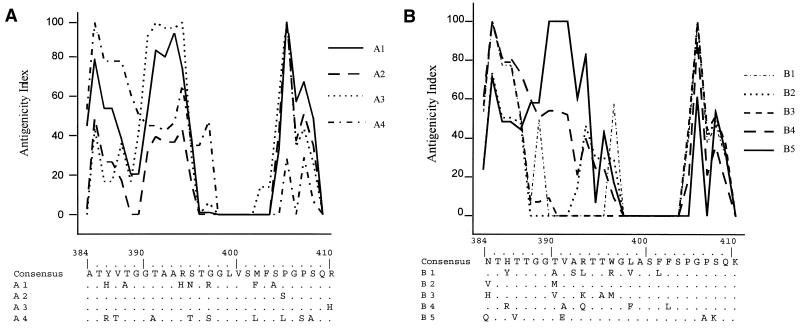
Blinded antigenic grouping of all E1/E2 sequences distinguished two main groups, corresponding to each of the two patients. Further grouping yielded four antigenic groups in patient A and five in patient B (Fig. 2). The F test showed that the global antigenic distribution in patient A was compartment-specific (P = 0.04) (Table 3). Each antigenic group corresponded statistically to a particular quasispecies origin: A1 overlapped all 3 variants from NF cells; A2 overlapped 15 of 16 variants (94%) from CD8+ cells; A3 overlapped 10 of 13 variants (77%) from plasma; and A4 overlapped 11 of 16 variants (69%) from B lymphocytes (P < 0.001). The global antigenic distribution among compartments was not statistically significant in patient B, although B5 contained five of six (83%) variants from CD8+ cells (P < 0.001). These cell-specific variants were rarely found in plasma: 1 of 13 and 2 of 13 plasma variants from patient A resembled the CD8+ and CD19+ variants, respectively, and none of the plasma variants from patient B was homologous to those found in CD8+ cells. Although specific antigenicity profiles were detected in groups A2 and B5 (mainly composed of CD8+ strains), no CD8+ cell-specific signature was detected: A2 and B5 antigenicity profiles differed mainly at the N terminus of HVR1 (Fig. 3).
FIG. 2.
Peptide alignment, patient A (A) and patient B (B). The lefthand column indicates the origin and number of identical clones: T, CD8+ cells; B, CD19+ cells; X, negative fraction cells; S, plasma. A consensus sequence was generated for each patient. Antigenic grouping was done manually and blindly with regard to the origin of the clones.
TABLE 3.
HVR1 quasispecies distribution according to the site of detection and antigenic grouping
| Antigenic groupa(n) | Quasispecies distributionb (n [patient A], n [patient B]d)
|
|||
|---|---|---|---|---|
| Plasma (13, 19) | CD8+ cells (16, 6) | CD19+ cells (16, 17) | NF cells (3, 11) | |
| Patient Ac | ||||
| A1 (3) | 0 | 0 | 0 | 3* |
| A2 (19) | 1 | 15* | 3 | 0 |
| A3 (13) | 10* | 1 | 2 | 0 |
| A4 (13) | 2 | 0 | 11* | 0 |
| Patient B | ||||
| B1 (4) | 3 | 0 | 1 | 0 |
| B2 (16) | 4 | 0 | 7 | 5 |
| B3 (11) | 6 | 1 | 3 | 1 |
| B4 (15) | 6 | 0 | 6 | 3 |
| B5 (7) | 0 | 5* | 0 | 2 |
Antigenic grouping was done blindly with regard to the origin of the clones.
∗, Antigenic groups statistically corresponding to a single compartment (P < 0.001).
The global antigenic distribution was compartment specific in patient A (P = 0.04).
n, number of clones.
FIG. 3.
Antigenic grouping of HVR1 variants from patient A (A) and patient B (B). Antigenic profiles were obtained by using the Parker’s algorithm. HVR1 amino acid sequences composing the signature of antigenic groups are aligned with the consensus sequence.
(v) Amino acid composition of HVR1.
McAllistair et al. (27) recently showed that amino acid replacements in HVR1 were highly constrained at some positions and confined to certain residues with similar characteristics at the majority of variable positions. We observed the same conserved positions in our two patients (positions 2, 6, 20, 23, and 26) in both plasma and cellular variants, a finding suggesting that cellular variants had the same structural constraints as plasma variants in the HVR1. We then matched HVR1 antigenic types against GenBank types by using the BLAST program. For each antigenic group, we entered the consensus sequence with the amino acid residues composing the type signature (Fig. 3). More than 700 hits were obtained for seven of nine type sequences, including the CD8+-specific groups A2 and B5. This indicates that the cellular HVR1 quasispecies did not differ substantially from the plasma variants in terms of their global sequence and structure. Two antigenic groups (A4 and B4) did not match any GenBank HCV sequence. They were composed mainly of variants from CD19+ cells and also of variants from plasma (15 and 30%) and NF cells.
DISCUSSION
This study demonstrates that HCV quasispecies are not randomly distributed among the different cell subsets composing PBMC in chronic HCV carriers but that they display a statistically significant compartmental structure.
Our results are consistent with the detection of HCV sequences, regardless of the infecting genotype and viral load, in B (CD19+) lymphocytes and phagocytic cells (monocytes/macrophages and granulocytes) and the occasional presence of HCV in T (CD3+) lymphocytes (24, 56). Quasispecies harbored by CD8+ lymphocytes are likely to be a minor fraction of the total PBMC variants. Detection of such quasispecies from total PBMC or CD3+ lymphocytes (in which CD4+ cells are in the majority) could thus be impaired. HCV detection was based on an equal number of cells in the different compartments, thereby increasing the probability of discovering such minor strains in minor cell subsets. The observation of two individual cases, both long-term infected by the HCV genotype 1b, may limit the strength of our conclusions on HCV quasispecies compartmentalization. Indeed, genotype 1 is associated with higher detection rates of HCV RNA in PBMC compared to other genotypes (24). Our data confirm by phylogenetic and statistical sequence analysis the previously reported compartmentalization between serum and total PBMC (23, 35, 37). However, further studies are required to assess HCV compartmentalization within lymphoid compartment in patients with different stages of disease and different genotypes. Given the high variability of HCV and the limitations of techniques for quasispecies analysis, the probability that a particular variant would only be detected in a given site is not nil. To overcome such difficulties, we applied Mantel’s test, an approach previously used to demonstrate HIV anatomic compartmentalization (43). The phylogenetic grouping was confirmed by a statistically significant compartmental structure: variants from CD8+ or NF cells were much closer to one another than to those from any other compartment, including plasma. This genetic grouping for NF sequences in patient B was established despite the absence of an obvious phylogenetic structure. Indeed, NF cells are composed of several cellular subsets, and each of them could harbor specific quasispecies clustering at different levels of the tree. Such compartmentalization was also evidenced in CD19+ cells from patient A by both phylogenetic and statistical approaches.
The clear compartmentalization of HCV variants shows that HCV PCR positivity on PBMC was not due to contamination by plasma-derived HCV RNA or to virus carried on the membrane in a nonspecific manner. Despite the very specific antigenicity patterns of CD8+ and, to a lesser extent, CD19+ quasispecies, cellular and plasma variants seemed to have the same structural constraints (27). Moreover, no stop mutations were observed, and most quasispecies found in all the compartments matched GenBank sequences. This suggests that the cellular quasispecies are not defective. The important issue of so-called lymphotropic variants was first raised by Shimizu et al. (47): the same inoculum produced identical variants in cultured T-cell lines and PBMC of an infected chimpanzee. The widespread cellular distribution of CD81, the putative HCV receptor, is consistent with HCV binding to cells other than hepatocytes (41). We failed to reliably detect negative-stranded HCV RNA in the cell subsets tested (data not shown), the specific methods (21) being poorly sensitive (37). Because HCV replication in the lymphoid compartment remains unproven, we must interpret our results according to both possibilities. If HCV productively infects certain peripheral blood cell subsets, the observed divergence would not only be related to the binding of these quasispecies to specific molecules but also to replication, even at a low level.
In both patients, the rate of HCV replication (if any) in CD8+ cells may be low given the low genetic diversity of CD8+ variants and the very low frequency of antigenically homologous variants in plasma. The quantification issue was not addressed in our study. From other studies (24, 56) it appears that the detection of HCV RNA is largely limited to B lymphocytes and monocytes/macrophages. The very low heterogeneity of sequences in CD8+ cells might be related to a very weak viral load. Indeed, quantitative differences among compartments may result in quasispecies complexity differences. However, quantitative factors do not explain the compartment specificity of sequences. In patient A, CD19+ and NF cells also harbored a major antigenic group that was scarce in plasma. If extrahepatic replication does not occur, it is conceivable that so-called lymphotropic variants are ancient serum quasispecies bound to long-lived plasma cells. However, as this process would be continuous, CD8+ or CD19+ cells should also harbor current plasma variants and should not therefore show such a significant compartmental structure. The limited number of analyzed sequences (3 to 19 per compartment) may raise the concern of sampling bias. The cloning approach yields only a partial view of the viral population. In this view, these cellular variants might represent minor plasma quasispecies sharing the ability to bind to CD8+, CD19+, or NF cells through different receptors in a cell-phenotype-specific manner. HVR1 sequences common to PBMC and liver but absent from serum can be detected (37). In the absence of analyzed liver sequences, we cannot exclude that some variants found in CD8+, CD19+, or NF cells may also be found in the liver. Indeed, some PBMC could be infected in the liver and then migrate to the bloodstream. In that case, different cellular subsets could have been infected by specific strains with particular lymphotropism.
The heterogeneity of HVR1 quasispecies has been linked to the presence of antibodies to HVR1 (6, 15, 16, 46), which are found in up to 80% of chronically infected patients (44). Circulating immune complexes are found in most chronically infected patients (5, 10, 14, 33). Moreover, the quasispecies composition differs between the immunoglobulin-free and the immunoglobulin-associated fractions (5, 14, 17, 20). Immune-complexed virions could thus bind to cells bearing immunoglobulin Fc receptors, such as phagocytes, monocytes, B cells, and activated T cells. However, HCV binding via Fc receptors cannot alone explain such a significant compartmental structure in CD8+, CD19+, or NF cells. Alternatively, free virions could bind to anti-HCV membrane antibodies on B cells (B-cell receptors). The evidence of positive selection pressure acting on quasispecies harbored by CD19+ cells in patient B supports antibody-mediated binding to these cells. Compartmentalization of HCV quasispecies in B lymphocytes could thus reflect the viral counterpart of the humoral immune response to HVR1. However, the striking similarity of the dN/dS ratios in each compartment of a given patient and the conservation of invariant sites of HVR1 in all the compartments suggest that common positive and negative selective pressures are exerted on all quasispecies, whatever their cellular origin.
Experimental and clinical studies support the hypothesis that neutralizing antibody response to HVR may be important for virus clearance (7, 57). At the onset of the disease, the quasispecies nature of HCV may lead to a lack or delayed production of neutralizing antibodies to minor variants which become predominant during chronic infection. In addition, though it is generally assumed that HCV elicits a strong humoral immune response, quantitative and qualitative defects of the antibody response may contribute to persistence (3). As we mentioned in the introduction, the infection of immune cells is a well-known mechanism of persistence, especially for noncytopathic viruses. For lymphocytic choriomeningitis virus (40) and hepatitis B virus (1), the lack or delayed production of neutralizing antibodies has been linked to the destruction of virus-specific B cells by virus-specific cytotoxic T cells. The noncytopathic infection of B cells by measles virus aborts humoral immunity (28). Our findings support the hypothesis of a specific interplay between HCV and B lymphocytes. Indeed, whatever the HCV replication status in PBMC, viral proteins are likely processed and presented by class I or class II major histocompatibility complex molecules. Furthermore, the HCV core protein has been shown to interact with the lymphotoxin-β receptor known to play a role in antigen display to B cells. Recently, CD81, which associates with CD21 and CD19 on B cells, has been characterized as a putative receptor for HCV. This suggests for HCV, in addition to the hypervariability advantages, an active strategy to achieve persistent infection.
In conclusion, the compartmental distribution of HCV quasispecies in different PBMC phenotypes is consistent with the existence of distinct viral receptors and, possibly, with the immune presentation of particular viral epitopes, leading to interference with the immune system. Further clinical and experimental studies on nonhepatocytic HCV tropism must take into account the cell phenotype and include the monocyte/macrophage and dendritic cell fractions.
ACKNOWLEDGMENTS
We thank Bruno Falissard for helping us with the mathematical model and Michele Gigou and Agnès Charpentier for technical help.
REFERENCES
- 1.Barnaba V, Franco A, Alberti A, Benvenuto R, Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I-restricted, exogenous antigen-specific T lymphocytes. Nature. 1990;345:258–260. doi: 10.1038/345258a0. [DOI] [PubMed] [Google Scholar]
- 2.Cabot B, Esteban J I, Martell M, Genesca J, Vargas V, Esteban R, Guardia J, Gomez J. Structure of replicating hepatitis C virus (HCV) quasispecies in the liver may not be reflected by analysis of circulating HCV virions. J Virol. 1997;71:1732–1734. doi: 10.1128/jvi.71.2.1732-1734.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M, Sällberg M, Sönnerborg A, Weiland O, Mattsson L, Jin L, Birkett A, Peterson D, Milich D R. Limited humoral immunity in hepatitis C virus infection. Gastroenterology. 1999;116:135–143. doi: 10.1016/s0016-5085(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 4.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradly D W, Houghton M. Isolation of a cDNA clone derived from blood-borne non-A non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 5.Choo S H, So H S, Cho J M, Ryu W S. Association of hepatitis C virus particles with immunoglobulin: a mechanism for persistent infection. J Gen Virol. 1995;76:2337–2341. doi: 10.1099/0022-1317-76-9-2337. [DOI] [PubMed] [Google Scholar]
- 6.Farci P, Alter H J, Wong D C, Miller R H, Govindarajan S, Engle R, Shapiro M, Purcell R H. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci USA. 1994;91:7792–7796. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farci P, Shimoda A, Wong D, Cabezon T, De Giaonnis D, Strazzera A, Shimiza Y, Shapiro M, Alter H J, Purcell R H. Prevention of HCV infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci USA. 1996;93:15394–15399. doi: 10.1073/pnas.93.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii K, Hino K, Okazaki M, Okuda M, Kondoh S, Okita K. Differences in hypervariable region 1 quasispecies of hepatitis C virus between human serum and peripheral blood mononuclear cells. Biochem Biophys Res Commun. 1996;225:771–776. doi: 10.1006/bbrc.1996.1249. [DOI] [PubMed] [Google Scholar]
- 9.Gunji T, Kato N, Hijikata M, Hayashi K, Saitoh S, Shimotohno K. Specific detection of positive and negative stranded hepatitis C viral RNA using chemical RNA modification. Arch Virol. 1994;134:293–302. doi: 10.1007/BF01310568. [DOI] [PubMed] [Google Scholar]
- 10.Hijikata M, Kato N, Ootsuyama Y, Nakagawa M, Ohkoshi S, Shimotohno K. Hypervariable region in the putative glycoprotein of hepatitis C virus. Biochem Biophys Res Commun. 1991;175:220–228. doi: 10.1016/s0006-291x(05)81223-9. [DOI] [PubMed] [Google Scholar]
- 11.Hijikata M, Shimizu Y K, Kato H, Iwamoto A, Shih J W, Alter H J, Purcell R H, Yoshikura H. Equilibrium centrifugation studies of hepatitis C virus: evidence for circulating immune complexes. J Virol. 1993;67:1953–1958. doi: 10.1128/jvi.67.4.1953-1958.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda M, Kato N, Mizutani T, Sugiyama K, Tanaka K, Shimotohno K. Analysis of the cell tropism of HCV by using in vitro HCV-infected human lymphocytes and hepatocytes. J Hepatol. 1997;27:445–454. doi: 10.1016/s0168-8278(97)80347-9. [DOI] [PubMed] [Google Scholar]
- 13.Jung-Hung L, Stripf T, Roth W K, Zeuzem S. Non-isotopic detection of hepatitis C virus quasispecies by single strand conformation polymorphism. J Med Virol. 1997;53:245–251. doi: 10.1002/(sici)1096-9071(199711)53:3<245::aid-jmv11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Kanto T, Hayashi N, Takehara T, Hagiwara H, Mita E, Naito M, Kasahara A, Fusamoto H, Kamada T. Density analysis of hepatitis C virus particle population in the circulation of infected hosts: implications for virus neutralization or persistence. J Hepatol. 1995;22:440–448. doi: 10.1016/0168-8278(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 15.Kato N, Ootsuyama Y, Sekiya H, Ohkoshi S, Nakazawa T, Hijikata M, Shimotohno K. Genetic drift in hypervariable region 1 of the viral genome in persistent hepatitis C virus infection. J Virol. 1994;68:4776–4784. doi: 10.1128/jvi.68.8.4776-4784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato N, Sekiya H, Ootsuyama Y, Nakazawa T, Hijikata M, Ohkoshi S, Shimotohno K. Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol. 1993;67:3923–3930. doi: 10.1128/jvi.67.7.3923-3930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korenaga M, Hino K, Okazaki M, Okuda M, Okita K. Differences in hypervariable region 1 quasispecies between. immune complexed and non-immune complexed hepatitis C virus particles. Biochem Biophys Res Commun. 1997;240:677–682. doi: 10.1006/bbrc.1997.7693. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Tamura K, Nei M. Molecular evolutionary genetics analysis software for microcomputers. Comput Appl Biosci. 1994;10:189–191. doi: 10.1093/bioinformatics/10.2.189. [DOI] [PubMed] [Google Scholar]
- 19.Kurosaki M, Enomoto N, Marumo F, Sato C. Evolution and selection of hepatitis C virus variants in patients with chronic hepatitis C. Virology. 1994;205:161–169. doi: 10.1006/viro.1994.1631. [DOI] [PubMed] [Google Scholar]
- 20.Kurosaki M, Enomoto N, Nouchi T, Sakuma I, Marumo F, Sato C. Fraction-specific populations of the hypervariable region of the hepatitis C virus in a patient with cryoglobulinemia. J Med Virol. 1995;46:403–408. doi: 10.1002/jmv.1890460418. [DOI] [PubMed] [Google Scholar]
- 21.Lanford R E, Chavez D, Chisari F V, Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase PCR. J Virol. 1995;69:8079–8083. doi: 10.1128/jvi.69.12.8079-8083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laskus T, Radkowski M, Wang L F, Cianciara J, Vargas H, Rakela J. Hepatitis C virus negative strand is not detected in peripheral blood mononuclear cells and viral sequences are identical to those in serum: a case against extra-hepatic replication. J Gen Virol. 1997;78:2747–2750. doi: 10.1099/0022-1317-78-11-2747. [DOI] [PubMed] [Google Scholar]
- 23.Lerat H, Berby F, Trabaud M A, Vidalin O, Major M, Trepo C, Inchauspe G. Specific detection of hepatitis C virus minus strand RNA in hematopoietic cells. J Clin Investig. 1996;9:845–851. doi: 10.1172/JCI118485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lerat H, Umin S, Habersetzer F, Berby F, Trabaud M A, Trépo C, Inchauspé G. In vivo tropism of hepatitis C virus genomic sequences in hematopoietic cells: influence of viral load, viral genotype and cell phenotype. Blood. 1998;91:3841–3849. [PubMed] [Google Scholar]
- 25.Maggi F, Fornai C, Vatteroni M L, Giorgi M, Morrica A, Pistello M, Cammarota G, Marchi S, Ciccorossi P, Bionda A, Bendinelli M. Differences in hepatitis C virus: quasispecies composition between liver, peripheral blood mononuclear cells and plasma. J Gen Virol. 1997;78:1521–1525. doi: 10.1099/0022-1317-78-7-1521. [DOI] [PubMed] [Google Scholar]
- 26.Martell M, Esteban J, Ouer J, Genesca J, Weiner A, Esteban R, Guardia J, Gomez J. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J Virol. 1992;66:3225–3229. doi: 10.1128/jvi.66.5.3225-3229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAllistair J, Casino C, Davidson F, Power J, Lawlord E, Lee-Yap P, Simmonds P, Smith D B. Long-term evolution of the hypervariable region of hepatitis C virus in a common-source-infected cohort. J Virol. 1998;72:4893–4905. doi: 10.1128/jvi.72.6.4893-4905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McChesney M B, Fujinami R S, Lampert P W, Oldstone M B. Viruses disrupt functions of human lymphocytes. II. Measles virus suppresses antibody production by acting on B lymphocytes. J Exp Med. 1986;163:1331–1336. [PMC free article] [PubMed] [Google Scholar]
- 29.McGuinness P H, Bishop G A, McCaughan G W, Trowbridge R, Gowans E J. False detection of negative-strand hepatitis C virus RNA. Lancet. 1994;343:551–552. [PubMed] [Google Scholar]
- 30.Miller R H, Purcell R H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as two plant viruses supergroups. Proc Natl Acad Sci USA. 1990;8:2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizutani T, Kato N, Saito S, Ikeda K, Sugiyama K, Shimotohno K. Characterization of hepatitis C virus replication in cloned cells obtained from a human T-cell leukemia virus type 1-infected cell line, MT-2. J Virol. 1996;70:7219–7223. doi: 10.1128/jvi.70.10.7219-7223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moldvay B J, Deny P, Pol S, Brechot C, Lamas E. Detection of hepatitis C virus RNA in peripheral blood mononuclear cells of infected patients by in situ hybridization. Blood. 1994;83:269–273. [PubMed] [Google Scholar]
- 33.Morita T, Hada H, Koide N, Shiraha H, Shinji T, Nakamura M, Ujike K, Wato M, Shimomura H, Tsuji T. Detection of hepatitis C virus RNA in circulating immune-complexes by RT-PCR. Hepatogastroenterology. 1996;43:582–585. [PubMed] [Google Scholar]
- 34.Müller H M, Pfaff E, Goeser T, Kallinwski B, Solbach C, Thielman L. Peripheral blood leukocytes serve as a possible extra-hepatic site for hepatitis C virus replication. J Gen Virol. 1993;74:669–676. doi: 10.1099/0022-1317-74-4-669. [DOI] [PubMed] [Google Scholar]
- 35.Navas S, Martin J, Quiroga J A, Castillo I, Carreno V. Genetic diversity and tissue compartmentalization of the hepatitis C virus genome in blood mononuclear cells, liver and serum from chronic hepatitis C patients. J Virol. 1998;72:1640–1646. doi: 10.1128/jvi.72.2.1640-1646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and non-synonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 37.Okuda M, Hino K, Korenaga M, Yamaguchi Y, Katoh Y, Okita K. Differences in hypervariable region 1 quasispecies of hepatitis C virus in human serum, peripheral blood mononuclear cells and liver. Hepatology. 1999;29:217–222. doi: 10.1002/hep.510290117. [DOI] [PubMed] [Google Scholar]
- 38.Oldstone M B. How viruses escape from cytotoxic T lymphocytes: molecular parameters and players. Virology. 1997;234:179–185. doi: 10.1006/viro.1997.8674. [DOI] [PubMed] [Google Scholar]
- 39.Orita M, Suzuki Y, Sekiya T, Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989;5:874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- 40.Parker J M, Guo D, Hodges R S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986;25:5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- 41.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner A J, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 42.Planz O, Seiler P, Hengartner H, Zinkernagel R M. Specific cytotoxic T cells eliminate cells producing neutralizing antibodies. Nature. 1996;382:726–729. doi: 10.1038/382726a0. [DOI] [PubMed] [Google Scholar]
- 43.Poss M, Rodrigo A G, Gosnik J J, Learn G H, De Vange Panteleef D, Martin H L, Bwayo J, Kreiss J K, Overbaugh J. Evolution of envelope sequences from the genital tract and peripheral blood of women infected with clade A human immunodeficiency virus type 1. J Virol. 1998;72:8240–8251. doi: 10.1128/jvi.72.10.8240-8251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puntoriero G, Meola A, Lahm A, Zucchelli S, Ercole B B, Tafi R, Pezzanera M, Mondelli M J, Cortese R, Tramontano A, Galfre G, Nicosia A. Towards a solution for hepatitis C virus hypervariability: mimotopes of the hypervariable region 1 can induce antibodies cross-reacting with a large number of viral variants. EMBO J. 1998;13:3521–3533. doi: 10.1093/emboj/17.13.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sekiya T. Detection of mutant sequences by single-strand conformation polymorphism analysis. Mutat Res. 1993;288:79–83. doi: 10.1016/0027-5107(93)90209-x. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu Y K, Hijikata M, Iwamoto A, Alter H J, Purcell R H, Yoshikura H. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol. 1994;68:1494–1500. doi: 10.1128/jvi.68.3.1494-1500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu Y K, Igarashi H, Kanematsu T, Fujiwara K, Wong D C, Purcell R H, Yoshikura H. Sequence analysis of the hepatitis C virus genome recovered from serum, liver, and peripheral blood mononuclear cells of infected chimpanzees. J Virol. 1997;71:5769–5773. doi: 10.1128/jvi.71.8.5769-5773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimizu Y K, Iwamoto A, Hijikata M, Purcell R H, Yoshikura H. Evidence for in vitro replication of hepatitis C virus genome in a human T cell line. Proc Natl Acad Sci USA. 1992;89:5477–5481. doi: 10.1073/pnas.89.12.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimizu Y K, Purcell R H, Yoshikura H. Correlation between the infectivity of hepatitis C virus in vivo and its infectivity in vitro. Proc Natl Acad Sci USA. 1993;90:6037–6041. doi: 10.1073/pnas.90.13.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Doorn L J, Capriles I, Maertens G, DeLeys R, Murray K, Kos T, Schellekens H, Quint W. Sequence evolution of the hypervariable region in the putative envelope region E2/NS1 of the hepatitis C virus is correlated with specific humoral immune response. J Virol. 1995;69:773–778. doi: 10.1128/jvi.69.2.773-778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Chenivesse X, Henglein B, Brechot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990;343:555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- 53.Weiner A J, Geysen H M, Christopherson C, Hall J E, Mason T J, Saracco G, Bonino F, Crawford K, Marion C D, Crawford K A, Brunetto M, Barr P J, Miyamura T, McHutchinson J, Houghton M. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc Natl Acad Sci USA. 1992;89:3468–3472. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willems M, Peerlinck K, Moshage H, Deleu I, Van den Eynde C, Vermylen J, Yap S H. Hepatitis C virus RNAs in plasma and in peripheral blood mononuclear cells of hemophiliacs with chronic hepatitis C: evidence for viral replication in peripheral blood mononuclear cells. J Med Virol. 1994;42:272–278. doi: 10.1002/jmv.1890420314. [DOI] [PubMed] [Google Scholar]
- 55.Wolinski S M, Korber B T M, Neuman A U, Daniels M, Kunstman K J, Whetsell A J, Furtado M R, Cao Y, Ho D D, Safrit J T, Koup R A. Adaptive evolution of human immunodeficiency virus type 1 during the natural course of infection. Science. 1996;272:537–542. doi: 10.1126/science.272.5261.537. [DOI] [PubMed] [Google Scholar]
- 56.Zehender G, Meroni L, De Maddalena C, Varchetta S, Monti G, Galli M. Detection of hepatitis C virus RNA in CD19 peripheral blood mononuclear cells of chronically infected patients. J Infect Dis. 1997;176:1209–1214. doi: 10.1086/514114. [DOI] [PubMed] [Google Scholar]
- 57.Zibert A, Meisel H, Kraas W, Schulz A, Jung G, Roggendorf M. Early antibody response against hypervariable region 1 is associated with acute self-limiting infections of hepatitis C virus. Hepatology. 1997;25:1245–1249. doi: 10.1002/hep.510250530. [DOI] [PubMed] [Google Scholar]