Abstract
Background:
Over the past decade, there has been increased utilization of medical cannabis (MC) in the United States. Few studies have described sociodemographic and clinical factors associated with MC use after certification and more specifically, factors associated with use of MC products with different cannabinoid profiles.
Methods:
We conducted a longitudinal cohort study of adults (N=225) with chronic or severe pain on opioids who were newly certified for MC in New York State and enrolled in the study between November 2018 and January 2022. We collected data over participants' first 3 months in the study, from web-based assessment of MC use every 2 weeks (unit of analysis). We used generalized estimating equation models to examine associations of sociodemographic and clinical factors with (1) MC use (vs. no MC use) and (2) use of MC products with different cannabinoid profiles.
Results:
On average, 29% of the participants used predominantly high delta-9-tetrahydrocannabinol (THC) MC products within the first 3 months of follow-up, 30% used other MC products, and 41% did not use MC products. Non-Hispanic White race, pain at multiple sites, and past 30-day sedative use were associated with a higher likelihood of MC use (vs. no MC use). Current tobacco use, unregulated cannabis use, and enrollment in the study during the COVID-19 pandemic were associated with a lower likelihood of MC use (vs. no MC use). Among participants reporting MC use, female gender and older age were associated with a lower likelihood of using predominantly high-THC MC products (vs. other MC products).
Conclusion:
White individuals were more likely to use MC after certification, which may be owing to access and cost issues. The findings that sedative use was associated with greater MC use, but tobacco and unregulated cannabis were associated with less MC use, may imply synergism and substitution that warrant further research. From the policy perspective, additional measures are needed to ensure equitable availability of and access to MC. Health practitioners should check patients' history and current use of sedative, tobacco, and unregulated cannabis before providing an MC recommendation and counsel patients on safe cannabis use.
Keywords: medical cannabis, THC, CBD, demographic characteristics, chronic pain, insomnia, mental health symptoms, opioid use, unregulated cannabis use, substance use, COVID-19 pandemic
Introduction
Thirty-seven states have legalized medical cannabis (MC) use in the United States (National Conference of State Legislatures website). With its legalization in many states, MC is increasingly being used to treat a range of symptoms, such as chronic pain,1–7 insomnia,8,9 and anxiety and depressive symptoms.4,5,10 There is supporting evidence for the therapeutic use of MC11 and ∼25% of U.S. adults report ever having used cannabis for medical purposes.12
Chronic or severe pain is by far the most prevalent patient-reported qualifying condition for MC use.1–7 Evidence suggests that MC use is associated with reduced pain11 and reduced opioid analgesic use.13 However, there is limited information on factors associated with MC use after certification among individuals with chronic or severe pain on opioids,3,5,6,14 and even less is known about how these factors are associated with use of MC products with varying cannabinoid content (delta-9-tetrahydrocannabinol [THC] and cannabidiol [CBD]).3,15 A better understanding of factors associated with MC use after certification is needed to guide clinicians, policy makers, and researchers in predicting patterns of use and in considering interventions.16 Understanding MC use after certification will also help to generate hypotheses about who is likely to find MC helpful and use which types of MC.
MC products have varying concentrations of THC and CBD. They are categorized as high-THC, high-CBD, or equal ratio of THC and CBD.15 THC is the main psychoactive compound in cannabis. CBD is considered to be nonpsychoactive, analgesic, and anxiolytic.17 In New York State (NY), decisions made about which cannabinoid ratio to purchase are guided by certifying provider recommendations, dispensary pharmacists, patient preference, and access.18 The choices that patients make when purchasing MC products have important clinical implications that could impact effectiveness, side effects, medication interactions, and comorbidities.
Among participants in a longitudinal cohort study of people with chronic or severe pain on opioids who were recently certified for MC in NY, this study aimed to examine sociodemographic and clinical factors associated with MC use during their first 3 months in the study, more specifically, (1) any MC use (vs. no MC use), and (2) use of MC product with different cannabinoid profiles (i.e., high THC products vs. other MC products). Based on findings from other investigators, several domains of factors that are associated with MC use were included in this study: (1) sociodemographic factors such as race/ethnicity and socioeconomic status (SES)19; (2) pain and other physical health symptoms1–6; (3) mental health symptoms4,5,10; (3) medication and substance use, such as opioids use,13 sedative use,20 cigarette smoking,21 and unregulated cannabis use6; and (4) COVID-19 pandemic.22
Methods
Overview
For the present analysis, we utilized data from the Medical Marijuana and Opioids (MEMO) Study,23 an 18-month longitudinal cohort study examining how MC use impacts opioid dose in adults with chronic or severe pain on opioids who were recently certified for MC in NY. Inclusion criteria were as follows: (1) >18 years old, (2) fluency in English or Spanish, (3) new certification for MC within 90 days, (4) no MC use in the 6 months before certification, (5) MC qualifying condition of “chronic pain,” or “pain that degrades health and functional capability as an alternative to opioid use” or qualifying complication of “severe or chronic pain resulting in substantial limitation of function” and (6) use of prescribed or illicit opioids within 30 days. Patients with qualifying conditions for MC that were likely to cause unique pain syndromes (cancer, epilepsy, multiple sclerosis, spinal cord injury, amyotrophic lateral sclerosis, Parkinson's disease, inflammatory bowel disease, Huntington's disease) were excluded.
The procedures used in the MEMO study were approved by the Montefiore Medical Center/Albert Einstein College of Medicine institutional review board (Protocol No.: 2017-7857). Additional information regarding the study design, inclusion/exclusion criteria, recruitment, and data collection are available in earlier publications.23,24
Setting
MC became legally available to NY in July 2014. During the study period, the indications for MC in NY were among the strictest in the country.25,26 Until changes were implemented in January 2022, all patients were required to have at least one severe or life-threatening condition (e.g., cancer, HIV, AIDS, or chronic pain), accompanied by at least one associated or complicating condition (e.g., nausea, cachexia, or severe pain) from a limited list of preapproved illnesses and conditions.25 MC delivery methods in NY during the study period included vape cartridge/pen, tablet/capsule/lozenges, oil, oral spray, and oral powder, with combustible “whole flower” becoming available starting on October 26, 2021.27
Data sources and collection
Participants completed study visits quarterly. Questions focused on demographic characteristics, pain, physical health, mental health, medication, and substance use. In response to the COVID-19 pandemic, all study visits were transitioned to remote phone or video visits. The participants also completed brief (2–5 min) personalized, web-based questionnaires every 2 weeks to report how they used MC, including the number of days of the preceding 14 days they used MC and types of MC products (high THC vs. THC: CBD balanced vs. high CBD).
Outcome measures
To examine MC use after certification, we used data from web-based questionnaires over participants' first 3 months in the MEMO Study (seven web-based questionnaires). The distribution of the number of days of the preceding 14 days they used MC was highly skewed, to the extent that a large number of observations were at the most extreme scores on the distribution (i.e., either 0 or 14 days). Such bimodal distribution indicated the presence of two groups, that is, MC use and no MC use.
We therefore defined two dependent variables: (1) MC use (vs. no MC use); and (2) use of predominantly high-THC products (vs. use of other MC products). In each 2-week period, we defined MC use as at least 1 day of reporting MC use. Among participants reporting MC use in any 2-week period, we examined use of MC products. We classified MC products with a THC:CBD ratio ≥2:1 as “high-THC,” CBD:THC ratio ≥2:1 as “high-CBD,” and THC:CBD ratio of 1:1 as “THC-CBD balanced.” High THC use was defined as using high-THC products on more days during a 2-week period than the number of days using other MC products.
Independent variables
Demographic characteristics measured at the baseline visit included gender, age, and race/ethnicity (four categories: Hispanic of any race, Non-Hispanic White, Non-Hispanic Black, and Non-Hispanic other race). SES variables included employment status (not employed vs. employed), educational level (12 years of education or less vs. more than 12 years), poverty (personal annual income lower than the Federal Poverty Level vs. at the Federal Poverty Level or higher), and health insurance status (not having private insurance vs. private insurance).
At baseline we measured pain (Brief Pain Inventory: severity, 4 items, range 0–10, Cronbach's α=0.85; interference, 7 items, range 0–10, Cronbach's α=0.86),28 pain catastrophizing (Pain Catastrophizing Scale: 13 items, range 0–52, moderate/severe ≥30, Cronbach's α=0.93),29 and pain at multiple sites (self-reported whole body pain or pain at two or more body sites, head, neck, back, limb, abdomen, or chest, etc.). Other physical health measures included insomnia symptoms (Insomnia Severity Index: 7 items, range 0–28, moderate/severe ≥15, Cronbach's α=0.87),30 and health-related quality of life (EuroQol 5-Dimension Scale: 5 items, Cronbach's α=0.70).31 The Cronbach's alphas reported in this article were based on the present data.
We also measured mental health symptoms at baseline, including: anxiety symptoms (General Anxiety Disorder—7: 7 items, range 0–21, moderate/severe ≥10, Cronbach's α=0.87),32 depressive symptoms (Patient Health Questionnaire—9: 9 items, range 0–27, moderate/severe ≥10, Cronbach's α=0.84),33 post-traumatic stress disorder (PTSD) symptoms (PTSD Questionnaire—Civilian Version: 6 items, range 6–30, high ≥14, Cronbach's α=0.83),34 and attention deficit hyperactivity disorder (ADHD) symptoms (Adult ADHD Self-Report Screening Scale for DSM-5: 6 items, range 0–25, high ≥14, Cronbach's α=0.70).35
We measured the following medication and substance use in the past 30 days at baseline: (1) daily prescription opioid use (yes/no), (2) use of nonprescribed prescription opioids (yes/no), (3) prescribed or nonprescribed sedative use (yes/no, Addiction Severity Index [ASI]),36 (4) cocaine use (yes/no, ASI),36 (5) current tobacco use (yes/no),37 and (6) problematic alcohol use (Alcohol Use Disorders Identification Test: 10 items, range 0–40, problematic use ≥8).38 In addition, unregulated cannabis use (yes/no) in each 2-week period was included as a time-varying independent variable.
NY was an early epicenter of the COVID-19 pandemic in the United States. To measure the association between the COVID-19 pandemic and MC use, we included an indicator variable for the COVID-19 pandemic (yes/no). We considered a baseline visit date on or after March 16, 2020 as the pandemic period.39
Data analysis
The unit of analysis was participants' assessments for each 2-week period, which allowed us to explicitly model individual change in MC use across time and include time-varying independent variables such as unregulated cannabis use. To account for hierarchical data (repeated measures within participants), we conducted generalized estimating equation (GEE) models with compound symmetry covariance structure. Parameter estimates from the GEE are less sensitive to the specification of covariance structure, which is usually unknown.40 First, we conducted a series of bivariate GEE regressions to explore the associations between each of the key independent variables and each of the dependent variables, that is, use of any MC products (vs. no MC use) and use of predominantly high-THC MC products (vs. use of other MC products).
Because the relationship between MC use and time (measured by week) is nonlinear, we included the number of weeks from the baseline and its quadratic term in each model (i.e., a second-order polynomial model41). Second, we conducted multiple GEE analyses that included all the independent variables that were associated with the dependent variable at the p<0.10 level in the bivariate analyses. To reduce multicollinearity and overfitting issues, we began with a saturated model and eliminated variables stepwisely with partial p>0.10. Third, because associations between baseline measures and later MC uses may depend upon participants' concurrent unregulated cannabis use, we conducted interaction analyses by including unregulated cannabis use and its interaction terms with other independent variables in the multiple GEE models. All analyses mentioned above were conducted using SAS 9.4.42
Results
Sample characteristics
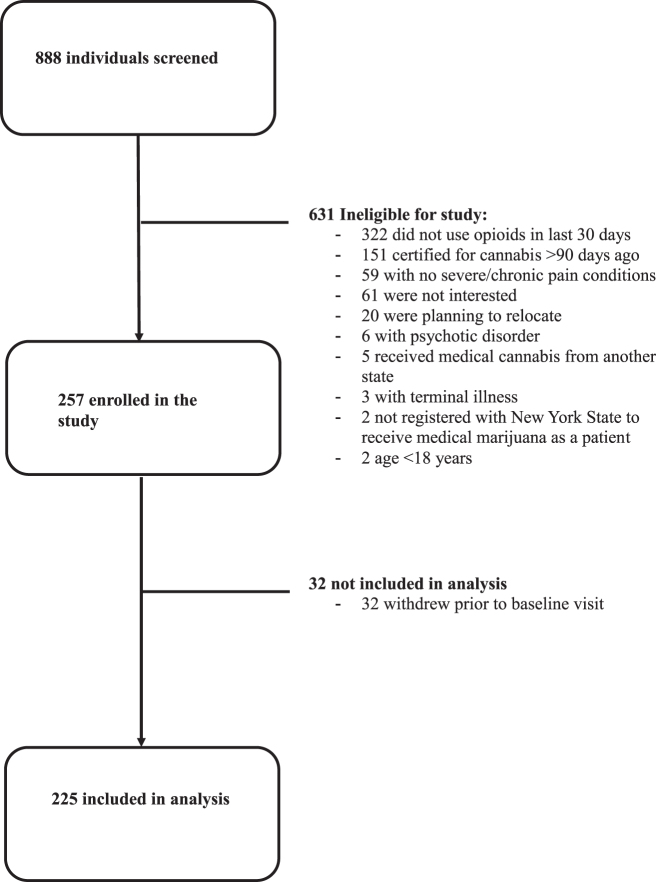
Of 888 individuals screened, 225 enrolled in the MEMO Study between November 2018 and January 2022 and were included in the present analysis (Fig. 1). Table 1 presents percentage or mean and standard deviation (SD) of the key independent variables at baseline. The mean age was 54 years (SD=13), 122 (54%) were women, 59 (26%) were Hispanic, 79 (35%) were Non-Hispanic White, 72 (32%) were Non-Hispanic Black, and 15 (7%) were Non-Hispanic Other. Most were not employed (78%), had an annual income below the Federal Poverty Level (56%), and did not have private insurance (79%). All participants had pain (mean pain severity score=6.6, SD=1.8). The most common pain locations were neck or back (76%) and limb (79%). Seventy-nine percent had pain at multiple body sites.
FIG. 1.
Study flowchart.
Table 1.
Participant Baseline Characteristics (N=225)
| N (%) or Mean (SD) | |
|---|---|
| Sociodemographic characteristics | |
| Female, n (%) | 122 (54.2) |
| Age (years), mean (SD) | 54.1 (13.0) |
| Race/ethnicity | |
| White, non-Hispanic, n (%) | 79 (35.1) |
| Black or African American, non-Hispanic, n (%) | 72 (32.0) |
| Hispanic or Latinx, n (%) | 59 (26.2) |
| Other, non-Hispanic, n (%) | 15 (6.7) |
| Socioeconomic status | |
| Less than 12 years of education, n (%) | 35 (15.6) |
| Not employed, n (%) | 176 (78.2) |
| Poverty,a n (%) | 127 (56.4) |
| Not having private insurance, n (%) | 177 (78.7) |
| Pain/physical health | |
| Pain severity (BPI—severity), mean (SD) | 6.6 (1.8) |
| Pain interference (BPI—interference), mean (SD) | 6.8 (2.1) |
| Moderate/severe pain catastrophizing symptoms,b n (%) | 101 (44.9) |
| Locations of body pain | |
| Head pain, n (%) | 60 (26.7) |
| Neck or back pain, n (%) | 170 (75.6) |
| Limb pain, n (%) | 177 (78.7) |
| Abdomen or chest pain, n (%) | 51 (22.7) |
| Whole body pain, n (%) | 38 (16.9) |
| Other pain, n (%) | 9 (4.0) |
| Pain at multiple body sites,c n (%) | 178 (79.1) |
| Moderate/severe insomnia,d n (%) | 86 (38.2) |
| Health-related quality of life (EuroQol 5-Dimension Scale), mean (SD) | 0.57 (0.18) |
| Mental health symptoms | |
| Moderate/severe anxiety symptoms,e n (%) | 83 (36.9) |
| Moderate/severe depressive symptoms,f n (%) | 103 (45.8) |
| Moderate/severe post-traumatic stress disorder symptoms,g n (%) | 85 (37.8) |
| Moderate/severe attention deficit hyperactivity disorder symptoms,h n (%) | 91 (40.4) |
| Opioid/unregulated cannabis/other substance use | |
| Daily prescription opioid use, n (%) | 118 (52.4) |
| Non-prescribed prescription opioid use, n (%) | 12 (5.3) |
| Unregulated cannabis use,i n (%) | 64 (28.4) |
| Sedative use, n (%) | 57 (25.3) |
| Cocaine use, n (%) | 5 (2.2) |
| Tobacco use, n (%) | 75 (33.3) |
| Hazardous alcohol,j n (%) | 9 (4.0) |
| Contextual factors | |
| COVID-19 pandemic at baseline,k n (%) | 161 (71.6) |
Annual income lower than Federal Poverty Level in the United States.
Score of ≥30 on the Pain Catastrophizing Scale.
Whole body pain or pain at two or more body sites.
Score of ≥15 on the Insomnia Severity Index.
Score of ≥10 on the General Anxiety Disorder-7 scale.
Score of ≥10 on the Patient Health Questionnaire-9 scale.
Score of ≥14 on the Post-Traumatic Stress Disorder Questionnaire–Civilian Version.
Score of ≥14 on the Adult Attention-Deficit Hyperactivity Disorder Self-Report Screening Scale for DSM-5.
Use of unregulated cannabis in the past 14 days.
Score of ≥8 on the Alcohol Use Disorder Identification Test.
Baseline visit date on or after March 16, 2020.
BPI, Brief Pain Inventory; SD, standard deviation.
Overall, psychiatric symptoms were common, with 83 (37%), 103 (46%), 85 (38%), and 91 (40%) reporting moderate/severe anxiety symptoms, depressive symptoms, PTSD symptoms, and ADHD symptoms, respectively. At baseline, 118 (52%) used prescription opioids daily, 64 (28%) reported unregulated cannabis use, 57 (25%) used sedatives, and 75 (33%) used tobacco. In addition, most of the participants (72%) enrolled in the study after the onset of the COVID-19 pandemic.
Patterns of MC use
Among 225 participants, 140 (62.2%) completed the first seven web-based surveys, with 177 (78.7%) completing at least six surveys. These resulted in a total of 1,377 completed surveys by week 12 (on average, six completed surveys per participant). Among these 1,377 two-week periods, 399 (29%) used predominantly high-THC MC products during a 14-day period, 418 (30%) used other MC products (high-CBD or THC:CBD balanced products), and 560 (41%) indicated no MC use. Among those who reported MC use, 21% also reported unregulated cannabis use during the same 2-week period; among those who reported no MC use, 44% reported unregulated cannabis use.
During the 12-week period, the percentage of predominantly high-THC MC product use increased from 22% at baseline (week 0) to 33% by week 12 and the percentage of other MC product use was relatively stable, fluctuating close to 30%. In contrast, the percentage of no MC use was 51% at baseline (week 0) and decreased to 37% by week 12 (Fig. 2). Figure 3 presents the patterns of MC use on an individual basis, which shows that >50% of the participants used MC all the time or most of the time, 28% never initiated, 12% waited 2–4 weeks before initiating, 6% initiated then quit, and 4% used MC occasionally (i.e., MC use reported only once in the seven 2-week periods).
FIG. 2.
Percentage of varied medical cannabis use and no use over time (week 0 [baseline]–week 12).
FIG. 3.
Patterns of medical cannabis use during the first 3 months (12 weeks) in the study.
Predictors of MC use versus no use
Table 2 presents the results of bivariate GEE analyses. Table 3 shows factors independently associated with MC use (vs. no use) in multiple GEE analyses. Non-Hispanic White race (adjusted odds ratio [AOR]=6.08, 95% CI=3.47–10.65, p < 0.001), pain at multiple body sites (AOR=2.10, 95% CI=1.09–4.03, p=0.026), and past 30-day sedative use (AOR=1.93, 95% CI=1.08–3.45, p=0.026) were associated with a higher likelihood of MC use (vs. no MC use). Current tobacco use (AOR=0.55, 95% CI=0.32–0.92, p=0.023), unregulated cannabis use (AOR=0.46, 95% CI=0.27–0.78, p=0.004), and enrollment in the study during the COVID-19 pandemic (AOR=0.30, 95% CI=0.16–0.55, p < 0.001) were associated with a lower likelihood of MC use (vs. no MC use).
Table 2.
Bivariate Generalized Estimating Equation Models Assessing Factor Associated with Medical Cannabis Use
| Participant characteristics |
MC use vs. no MC use (N=225)
a
|
Use of predominantly high-THC MC products vs. use of other MC products (N=163)
b
|
||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-Value | Odds ratio (95% CI) | p-Value | |
| Demographic characteristics | ||||
| Female (vs. male or other) | 0.97 (0.61–1.52) | 0.885 | 0.44 (0.26–0.76) | 0.003 |
| Age (in years) | 0.99 (0.98–1.01) | 0.636 | 0.98 (0.96–0.99) | 0.026 |
| Non-Hispanic White (vs. Hispanic, non-Hispanic Black, or non-Hispanic other races) | 8.65 (5.03–14.86) | <0.001 | 1.36 (0.79–2.34) | 0.268 |
| Socioeconomic status | ||||
| Less than 12 years of education (vs. 12 years or higher than 12 years of education) | 0.32 (0.17–0.63) | <0.001 | 1.26 (0.49–3.24) | 0.629 |
| Not employed (vs. employed) | 0.45 (0.24–0.82) | 0.009 | 1.27 (0.65–2.49) | 0.476 |
| Poverty (vs. annual income higher than the Federal Poverty Level in the United States)c | 0.34 (0.21–0.55) | <0.001 | 1.10 (0.64–1.89) | 0.733 |
| Not having private insurance (vs. private insurance) | 0.25 (0.13–0.47) | <0.001 | 1.07 (0.56–2.04) | 0.828 |
| Pain/physical health | ||||
| Pain severity (per 1 unit of increase) | 0.79 (0.68–0.90) | <0.001 | 1.00 (0.86–1.17) | 0.988 |
| Pain interference (per 1 unit of increase) | 0.96 (0.85–1.07) | 0.432 | 0.98 (0.86–1.11) | 0.723 |
| Moderate/severe pain catastrophizing symptoms (vs. minor or no pain catastrophizing symptoms)d | 0.60 (0.38–0.95) | 0.028 | 0.85 (0.49–1.48) | 0.575 |
| Pain at multiple body sites (vs. single site)e | 3.11 (1.74–5.57) | <0.001 | 0.86 (0.39–1.87) | 0.696 |
| Moderate/severe insomnia (vs. minor or no insomnia)f | 1.01 (0.64–1.60) | 0.952 | 1.43 (0.83–2.47) | 0.197 |
| Health-related quality of life (EuroQol 5-Dimension Scale) (per 1 unit of increase) | 1.38 (0.40–4.77) | 0.607 | 1.37 (0.32–5.91) | 0.669 |
| Mental health symptoms | ||||
| Moderate/severe anxiety symptoms (vs. minor or no anxiety symptoms)g | 0.93 (0.58–1.48) | 0.751 | 1.46 (0.84–2.53) | 0.183 |
| Moderate/severe depressive symptoms (vs. minor or no depressive symptoms)h | 0.66 (0.42–1.03) | 0.069 | 1.55 (0.90–2.66) | 0.115 |
| Moderate/severe PTSD symptoms (vs. minor or no PTSD symptoms)i | 0.99 (0.62–1.57) | 0.968 | 1.57 (0.91–2.70) | 0.102 |
| Moderate/severe ADHD symptoms (vs. minor or no ADHD symptoms)j | 1.29 (0.82–2.05) | 0.275 | 1.37 (0.80–2.36) | 0.250 |
| Opioid/other substance use | ||||
| Daily prescription opioid use (vs. less frequent than daily use or no use) | 0.57 (0.36–0.91) | 0.017 | 0.80 (0.46–1.36) | 0.406 |
| Nonprescribed prescription opioid use (vs. no use) | 1.30 (0.53–3.18) | 0.571 | 2.03 (0.65–6.40) | 0.226 |
| Unregulated cannabis use (vs. no unregulated cannabis use) | 0.44 (0.27–0.71) | <0.001 | 1.15 (0.59–2.25) | 0.68 |
| Sedative use (vs. no use) | 2.01 (1.21–3.35) | 0.007 | 1.39 (0.78–2.46) | 0.265 |
| Tobacco use (vs. no use) | 0.50 (0.31–0.81) | 0.005 | 1.28 (0.72–2.26) | 0.398 |
| Contextual factors | ||||
| COVID-19 pandemic (vs. before the COVID-19 pandemic)k | 0.21 (0.12–0.37) | <0.001 | 1.53 (0.87–2.71) | 0.142 |
Number of 2-week data points=1,377.
Number of 2-week data points=817.
Annual income lower than Federal Poverty Level in the United States.
Score of ≥30 on the Pain Catastrophizing Scale.
Whole body pain or pain at two or more body sites.
Score of ≥15 on the Insomnia Severity Index.
Score of ≥10 on the General Anxiety Disorder-7 scale.
Score of ≥10 on the Patient Health Questionnaire-9 scale.
Score of ≥14 on the Post-Traumatic Stress Disorder Questionnaire—Civilian Version.
Score of ≥14 on the Adult Attention-Deficit Hyperactivity Disorder Self-Report Screening Scale for DSM-5.
Baseline visit date on or after March 16, 2020.
ADHD, attention deficit hyperactivity disorder; MC, medical cannabis; PTSD, post-traumatic stress disorder.
Table 3.
Multiple Generalized Estimating Equation Model: Factors Associated with Medical Cannabis Use Versus No Medical Cannabis Use (N=225)
| Independent variables | Adjusted odds ratio (95% CI) | p-Value |
|---|---|---|
| Non-Hispanic White (vs. Hispanic, non-Hispanic Black, or non-Hispanic other races) | 6.08 (3.47–10.65) | <0.001 |
| Less than 12 years of education (vs. 12 years or higher than 12 years of education) | 0.48 (0.23–1.02) | 0.057 |
| Pain at multiple body sites (vs. single site)a | 2.10 (1.09–4.03) | 0.026 |
| Moderate/severe depressive symptoms (vs. minor or no depressive symptoms)b | 0.60 (0.36–1.003) | 0.052 |
| Sedative use (vs. no use) | 1.93 (1.08–3.45) | 0.026 |
| Tobacco use (vs. no use) | 0.55 (0.32–0.92) | 0.023 |
| Unregulated cannabis use (vs. no unregulated cannabis use) | 0.46 (0.27–0.78) | 0.004 |
| COVID-19 pandemic (vs. before the COVID-19 pandemic)c | 0.30 (0.16–0.55) | <0.001 |
| Time (in weeks) | 1.19 (1.09–1.29) | <0.001 |
| Time (in weeks) squared | 0.99 (0.985–0.998) | 0.008 |
Number of 2-week data points=1,377.
Whole body pain or pain at two or more body sites.
Score of ≥10 on the Patient Health Questionnaire-9 scale.
Baseline visit date on or after March 16, 2020.
In addition, curvilinear time effects on MC use were supported by the data. The likelihood of MC use increased more steeply early in the follow-up period (week: AOR=1.19, 95% CI=1.09–1.29, p < 0.001), but that increase then slowed down (week squared: AOR=0.99, 95% CI=0.985–0.998, p=0.008).
Predictors of using predominantly high-THC MC products versus other MC products
Few factors were associated with using predominantly high-THC products (vs. using other MC products) in bivariate GEE analyses (Table 2). In multiple GEE models (Table 4), female gender (AOR=0.44, 95% CI=0.25–0.75, p=0.003) and older age (AOR=0.98, 95% CI=0.96–0.99, p=0.018) were associated with a lower likelihood of using predominantly high-THC MC products.
Table 4.
Multiple Generalized Estimating Equation Model: Factors Associated with Use of Predominantly High-THC Medical Cannabis Products Versus Use of Other Medical Cannabis Products (N=163)
| Independent variables | Adjusted odds ratio (95% CI) | p-Value |
|---|---|---|
| Female (vs. male or other) | 0.44 (0.25–0.75) | 0.003 |
| Age (in years) | 0.98 (0.96–0.99) | 0.018 |
Number of 2-week data points=817.
THC, delta-9-tetrahydrocannabinol.
Results of interaction analyses
No interactive effects between unregulated cannabis use and other factors on later use of any MC products (vs. no MC use) or on using predominantly high-THC products (vs. other MC products) were statistically significant (p>0.05) (data available upon request).
Discussion
Among a cohort of participants newly certified to receive MC who had chronic or severe pain, we found that (1) during the first 3 months, a considerable proportion of patients did not use MC. The majority of “no MC use” time points were from those who never initiated; (2) Non-Hispanic White race, pain at multiple sites, and past 30-day sedative use were associated with a higher likelihood of MC use (vs. no MC use), whereas current tobacco use, unregulated cannabis use, and enrollment in the study during the COVID-19 pandemic were associated with a lower likelihood of MC use (vs. no MC use); (3) among participants reporting MC use, female gender and older age were associated with a lower likelihood of using predominantly high-THC MC products (vs. other MC products).
This study contributes to the research literature in three important ways. First, in contrast to earlier studies,3,10,14,43,44 which were mostly cross-sectional, this study included prospective data from seven web-based surveys over 3 months, and thus provides a detailed/nuanced understanding of patterns of MC use. Second, we focused not only on any MC use (vs. no MC use), but also examined the use of MC products with different contents. Third, we investigated the associations between a broad spectrum of factors and MC use.
Factors related to MC use versus no MC use
Our results indicate that although all participants overcame the first barrier of being certified for MC, participants of Black/African-American race and Hispanic/Latino ethnicity had a lower likelihood of using MC compared with White participants. Our findings were in accord with Cunningham et al.,19 who used data from the NY Medical Marijuana Program and identified these sociodemographic factors as barriers to MC use. These findings may be owing to racial and SES disparities in MC access, such as the geographic distribution of MC dispensaries in NY19 and high out-of-pocket cost.45,46
The leading reason for MC use in studies of several different populations is chronic pain.1–6 In this study, we found that pain at multiple sites remained statistically significantly associated with MC use when we controlled for other factors in the multiple analyses. Patients with multisite pain are more likely to have refractory chronic pain symptoms43,47 and may seek MC for relief.
With regards to medications at baseline, we found that sedative use in the past 30 days at baseline was associated with a higher likelihood of MC use. This could be because of shared motivation/indication to use a sedative and/or MC to manage symptoms of anxiety or insomnia.48 Cannabis use for insomnia symptoms was common in several analyses,49,50 with nearly half of all participants using MC for insomnia in one cohort.51 This finding could raise a concern about co-use, as patients who use sedatives and MC together could experience negative side effects such as excess daytime drowsiness.8,20 However, participants may have been “substituting” MC for sedatives. For example, Purcell et al.20 found that, in a cohort of patients who use benzodiazepines for insomnia and who start MC, benzodiazepine use reduced over time.
Simultaneous use of cannabis and tobacco is a common practice, particularly among people who use unregulated cannabis.52 In this study, however, people who did not use tobacco were more likely to use MC than people who use tobacco. Only a minority (15.6%) of the participants had access to combustible MC products (available in NY after October 26, 202127) during their first 3 months in the study. People who use tobacco may be less likely to try the noncombustible MC formulations if they prefer mixing tobacco with their cannabis. Other investigators have suggested that some individuals may intentionally substitute MC for tobacco.21 More long-term data can help us gain insight into the motivations of MC use among people who use tobacco.
Research has consistently found that previous experience with unregulated cannabis use was positively associated with MC use,6 which may suggest people substitute MC for unregulated cannabis. However, less is known whether people simultaneously use both MC and unregulated cannabis. In this study, we found that participants who still used unregulated cannabis after MC certification were less likely to simultaneously use MC. These people may find unregulated cannabis cheaper or easier to access. In a related vein, it is challenging to completely disentangle medical versus nonmedical use. People who use cannabis for medical reasons could also use it for nonmedical reasons. We are conducting analyses of qualitative interviews with participants of the MEMO study to better understand participant's motivations and better understand these relationships.
We found participants were significantly less likely to use MC during the COVID-19 pandemic, controlling for other factors. NY enforced social distancing, quarantine, shelter-in-place, and vaccine mandate policies during different stages of the pandemic. Our findings differ from a study that examined the impact of the COVID-19 pandemic on MC use in other settings by Boehnke et al.,22 who reported that 25% of the people using MC decreased their use, whereas 35% increased use and 40% had no change. Our findings suggests that regulations related to COVID-19 in New York may have affected access to MC among patients certified to receive MC.53,54 Participants may also have been scared to go out, may use unregulated cannabis, and may not have money to spend during the COVID-19 pandemic.
Factors related to using predominantly high-THC MC products (vs. other products)
Relatively fewer factors were associated with using predominantly high-THC products (vs. using other MC products). We found that age and gender were associated with using predominantly high-THC MC products (vs. other products). An increase in age was associated with a lower likelihood of using predominantly high-THC MC products. This finding is likely to be explained by the greater safety profile of lower THC products17 and it is consistent with that of Kaufmann et al.,3 who reported that older adults were more likely to use products with a lower THC:CBD ratio. In accord with Kalaba and Ware,55 we found that women were less likely to use predominantly high-THC MC products. The reason remains unclear. Although it is plausible that, like older patients, female patients may choose products with lower risk of intoxication (i.e., lower in THC), further research is needed.
Limitations
Our study has several limitations. First, the measurements of MC use every 2 weeks are self-reported data and thus are subject to recall bias. However, because 2 weeks is a relatively short time, recall bias may be less problematic in our study than in other longitudinal studies that have longer recall periods. Second, we included participants in the New York City area with chronic or severe pain who used opioids, and thus the findings may not be generalizable to the whole population of people who use MC in NY, the United States, or elsewhere. Third, we did not include assessments of participants' expectancies related to MC use (or no use) and to MC product types, which may have impacts on the patterns of use. Fourth, on March 31, 2021, NY legalized adult-use cannabis by passing the Marijuana Regulation & Taxation Act. Thus, our findings may not reflect the landscape of expanding cannabis legalization moving forward. Future studies are needed to assess how these policy changes may affect MC use behaviors.
Conclusions
We showed that a considerable proportion of patients who were newly certified to receive MC were not using MC at all and never initiated MC use. Non-Hispanic White individuals were more likely to use MC. These findings suggest that Non-Hispanic White patients certified for MC have greater access to purchasing MC. From the policy perspective, additional measures are needed to ensure equitable availability of and access to regulated cannabis including MC, which is safer than unregulated cannabis.25,56,57 This could take the form of more clinicians evaluating patients for MC certification in communities of color and dispensaries, as well as reductions in cost of cannabis use for MC.
The findings that sedative use was associated with greater MC use, but cigarette smoking and concurrent unregulated cannabis use were associated with less MC use, may imply synergism and substitution that warrant further research. Health practitioners should check patients' history and current use of sedatives, tobacco, and unregulated cannabis before providing an MC recommendation and counsel patients on safe cannabis use. Clinicians may need to devise strategies to help patients transition from unregulated cannabis to MC. This could include assistance with tobacco cessation or specific dosing regimens to limit THC withdrawal symptoms. The pattern in our study that men and younger individuals used high THC products is likely multifactorial. More research is needed to better understand the barriers and facilitators of MC use and the benefits and harms of different MC products.
Abbreviations Used
- ADHD
attention-deficit hyperactivity disorder
- AOR
adjusted odds ratio
- ASI
Addiction Severity Index
- CBD
cannabidiol
- GEE
generalized estimating equation
- MC
medical cannabis
- MEMO
Medical Marijuana and Opioids
- NY
New York State
- PTSD
post-traumatic stress disorder
- SD
standard deviation
- SES
socioeconomic status
- THC
delta-9-tetrahydrocannabinol
Authors' Contributions
J.H.A., J.L.S., C.O.C., C.Z., and D.E.S. had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the analyses. Study concept and design: J.H.A., J.L.S., C.O.C., C.Z., and D.E.S. Acquisition, analysis, or interpretation of data: J.H.A., J.L.S., C.O.C., C.Z., D.E.S. and J.R. Drafting of the article: C.Z., J.H.A., and D.E.S. Critical revision of the article for important intellectual content: all authors. Statistical analyses: C.Z. Study supervision: J.H.A., C.O.C., J.L.S., C.Z., and D.E.S.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study is supported by the National Institutes of Health (R01DA044171, K23MH114752, K24DA036955, K24DA046309, P30AI124414) and is registered at clinicaltrials.gov (NCT03268551).
Cite this article as: Zhang C, Slawek DE, Ross J, Zolotov Y, Castillo F, Levin FR, Sohler NL, Minami H, Cunningham CO, Starrels JL, Arnsten JH (2023) Factors associated with medical cannabis use after certification: a three-month longitudinal study, Cannabis and Cannabinoid Research X:X, 1–11, DOI: 10.1089/can.2022.0248.
References
- 1. Abuhasira R, Schleider LB, Mechoulam R, et al. Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. Eur J Intern Med. 2018;49:44–50. [DOI] [PubMed] [Google Scholar]
- 2. Boehnke KF, Gangopadhyay S, Clauw DJ, et al. Qualifying conditions of medical cannabis license holders in the United States. Health Aff (Millwood). 2019;38:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaufmann CN, Kim A, Miyoshi M, et al. Patterns of medical cannabis use among older adults from a cannabis dispensary in New York state. Cannabis Cannabinoid Res. 2022;7:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181–192. [DOI] [PubMed] [Google Scholar]
- 5. Mahabir VK, Merchant JJ, Smith C, et al. Medical cannabis use in the United States: a retrospective database study. J Cannabis Res. 2020;2:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park JY, Wu LT. Prevalence, reasons, perceived effects, and correlates of medical marijuana use: a review. Drug Alcohol Depend. 2017;177:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456–2473. [DOI] [PubMed] [Google Scholar]
- 8. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19:1–12. [DOI] [PubMed] [Google Scholar]
- 9. Gates PJ, Albertella L, Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep Med Rev. 2014;18:477–487. [DOI] [PubMed] [Google Scholar]
- 10. Sexton M, Cuttler C, Finnell JS, et al. A cross-sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 2016;1:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: an Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. National Academies Press (U.S.): Washington, DC, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK423845/ (accessed July 7, 2022). [PubMed] [Google Scholar]
- 12. Leung J, Chan G, Stjepanović D, et al. Prevalence and self-reported reasons of cannabis use for medical purposes in USA and Canada. Psychopharmacology (Berl). 2022;239:1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain. 2016;17:739–744. [DOI] [PubMed] [Google Scholar]
- 14. Reiman A. Medical cannabis patients: patient profiles and health care utilization patterns. Complement Health Pract Rev. 2007;12:31–50. [Google Scholar]
- 15. Spindle TR, Bonn-Miller MO, Vandrey R. Changing landscape of cannabis: novel products, formulations, and methods of administration. Curr Opin Psychol. 2019;30:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radhakrishnan R, Ranganathan M, D’ Souza DC. Medical marijuana: what physicians need to know. J Clin Psychiatry. 2019;80:18ac12537. [DOI] [PubMed] [Google Scholar]
- 17. Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem. 2010;17:1360–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slawek D, Arnsten JH, Whitley SD, et al. Therapeutic Use of Medical Cannabis in New York State [Internet]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK577724/ (accessed July 7, 2022). [PubMed]
- 19. Cunningham CO, Zhang C, Hollins M, et al. Availability of medical cannabis services by racial, social, and geographic characteristics of neighborhoods in New York: a cross-sectional study. BMC Public Health. 2022;22:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Purcell C, Davis A, Moolman N, et al. Reduction of benzodiazepine use in patients prescribed medical cannabis. Cannabis Cannabinoid Res. 2019;4:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lucas P, Baron EP, Jikomes N. Medical cannabis patterns of use and substitution for opioids & other pharmaceutical drugs, alcohol, tobacco, and illicit substances; results from a cross-sectional survey of authorized patients. Harm Reduct J. 2019;16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boehnke KF, McAfee J, Ackerman JM, et al. Medication and substance use increases among people using cannabis medically during the COVID-19 pandemic. Int J Drug Policy. 2021;92:103053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cunningham CO, Starrels JL, Zhang C, et al. Medical Marijuana and Opioids (MEMO) Study: protocol of a longitudinal cohort study to examine if medical cannabis reduces opioid use among adults with chronic pain. BMJ Open. 2020;10:e043400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ross J, Slawek DE, Zhang C, et al. First-year trajectories of medical cannabis use among adults taking opioids for chronic pain: an observational cohort study. Pain Med. 2021;22:3080–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richard EL, Althouse AD, Arnsten JH, et al. How medical are states' medical cannabis policies?: proposing a standardized scale. Int J Drug Policy. 2021;94:103202. [DOI] [PubMed] [Google Scholar]
- 26. Williams AR, Olfson M, Kim JH, et al. Older, less regulated medical marijuana programs have much greater enrollment rates than newer “medicalized” programs. Health Aff. 2016;35:480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. New York State Department of Health. New York State Medical Marijuana Program. Available online: https://cannabis.ny.gov/registered-organizations (accessed December 15, 2022).
- 28. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24:733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 30. Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Obradovic M, Lal A, Liedgens H. Validity and responsiveness of EuroQol-5 dimension (EQ-5D) versus Short Form-6 dimension (SF-6D) questionnaire in chronic pain. Health Qual Life Outcomes. 2013;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 33. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lang AJ, Stein MB. An abbreviated PTSD checklist for use as a screening instrument in primary care. Behav Res Ther. 2005;43:585–594. [DOI] [PubMed] [Google Scholar]
- 35. Ustun B, Adler LA, Rudin C, et al. The World Health Organization Adult Attention-Deficit/Hyperactivity Disorder Self-Report Screening Scale for DSM-5. JAMA Psychiatry. 2017;74:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. [DOI] [PubMed] [Google Scholar]
- 37. U.S. Department of Commerce. National Cancer Institute and Food and Drug Administration co-sponsored Tobacco Use Supplement to the Current Population Survey. 2016. Available online: https://cancercontrol.cancer.gov/brp/tcrb/tus-cps (accessed March 9, 2023).
- 38. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 39. Richter F, Strasser AS, Suarez-Farinas M, et al. Neonatal outcomes during the COVID-19 pandemic in New York City. Pediatr Res. 2022;91:477–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 41. Cohen J. Partialed products are interactions; partialed powers are curve components. Psychol Bull. 1978;85:858–866. [Google Scholar]
- 42. SAS Institute. Base SAS 9.4 procedures guide: statistical procedures. Cary, North Carolina: SAS Institute; 2017. [Google Scholar]
- 43. Carter GT, Sullivan MD, ZumBrunnen C, et al. Characteristics of patients with chronic pain accessing treatment with medical cannabis in Washington State. J Opioid Manag. 2009;5:257–286. [DOI] [PubMed] [Google Scholar]
- 44. Compton WM, Han B, Hughes A, et al. Use of marijuana for medical purposes among adults in the United States. JAMA. 2017;317:209–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Casarett D, Abrams DI. Why insurance companies should pay for medical cannabis. Am J Bioeth. 2019;19:8–10. [DOI] [PubMed] [Google Scholar]
- 46. Valencia CI, Asaolu IO, Ehiri JE, et al. Structural barriers in access to medical marijuana in the USA—a systematic review protocol. Syst Rev. 2017;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shirvalkar P, Veuthey TL, Dawes HE, et al. Closed-loop deep brain stimulation for refractory chronic pain. Front Comput Neurosci. 2018;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kang H, Hunniecutt J, Quintero Silva L, et al. Biopsychosocial factors and health outcomes associated with cannabis, opioids and benzodiazepines use among older veterans. Am J Drug Alcohol Abuse. 2021;47:497–507. [DOI] [PubMed] [Google Scholar]
- 49. Bohnert KM, Bonar EE, Arnedt JT, et al. Utility of the comprehensive marijuana motives questionnaire among medical cannabis patients. Addict Behav. 2018;76:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cranford JA, Arnedt JT, Conroy DA, et al. Prevalence and correlates of sleep-related problems in adults receiving medical cannabis for chronic pain. Drug Alcohol Depend. 2017;180:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bonn-Miller MO, Babson KA, Vandrey R. Using cannabis to help you sleep: heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend. 2014;136:162–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vidot DC, Islam JY, Camacho-Rivera M, et al. The COVID-19 cannabis health study: results from an epidemiologic assessment of adults who use cannabis for medicinal reasons in the United States. J Addict Dis. 2020;39:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Y, Ibañez GE, Vaddiparti K, et al. Change in marijuana use and its associated factors among persons living with HIV (PLWH) during the COVID-19 pandemic: findings from a prospective cohort. Drug Alcohol Depend. 2021;225:108770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kalaba M, Ware MA. Cannabinoid profiles in medical cannabis users: effects of age, gender, symptoms, and duration of use. Cannabis Cannabinoid Res. 2022;7:840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McPartland JM, McKernan KJ. Contaminants of concern in cannabis: microbes, heavy metals and pesticides. In: Cannabis sativa L.-Botany and Biotechnology. New York, NY: Springer, Cham, 2017. (pp. 457–474). [Google Scholar]
- 57. Vujanovic V, Korber DR, Vujanovic S, et al. Scientific prospects for cannabis-microbiome research to ensure quality and safety of products. Microorganisms. 2020;8:290. [DOI] [PMC free article] [PubMed] [Google Scholar]