Abstract
Recently, there has been an increasing demand for medicinal plants to control diseases for good health and well-being, as primary health facilities are inadequate in certain populations to cure infections. Since synthetic medicines are toxic to humans and other animals, the present research is thus focused on using traditional medicine for treating various ailments as they are harmless. Based on the above facts, the current study was conducted to assay the antimicrobial, anti-diabetic, anti-cholinesterase, anti-oxidant, anti-quorum sensing, and anti-antibiotic resistance modifying effect of extracts of Cyperus esculentus. This study found 37 and 30 chemicals in butanol and dichloromethane (DCM) extracts using a gas chromatograph mass spectrophotometer (GC-MS). Most active compounds identified were benzofuran, 2,3-dihydro-, 1,2,3-benzenetriol, 3-bornanone, oxime and oleic acid by extracts of butanol whereas dichloromethane extracted three major active compounds (2,3-dihydro-3,5-dihydroxy-, 4H-pyran-4-one 3-deoxy-d-mannoic lactone and 5-hydroxymethylfurfural). Both dichloromethane and butanol extracts showed the highest antimicrobial activity. Compared to aqueous extracts, dichloromethane, and butanol showed excellent anti-diabetic anti-cholinesterase activities and inhibited virulence factors regulated by quorum sensing (QS). Anti-oxidants increased in solvent extracts (DCM and butanol) compared to aqueous extracts. Results of scanning electron microscope (SEM) and Fourier Transmission Infrared (FTIR) indicated damage to the cell membrane of S. aureus by the formation of pits and breakage in functional groups exposed to the extracts of butanol and dichloromethane compared to aqueous extracts. The above results confirmed that C. esculentus can be an alternative medicine for treating diseases.
Keywords: Antibacterial activity, Anti-diabetic effect, Antifungal activity, Cell damage, Cell-to-cell communication, Cyperus esculentus, Secondary metabolites
1. Introduction
Recently, there has been an increasing demand for medicinal plants to control infectious diseases like abscesses, bloodstream infections, pneumonia, urinary tract infections, aspergillosis, etc., as primary health facilities are inadequate in specific populations [1]. Diseases caused by pathogens expose people to risk, especially older people, children, pregnant women, and those with weak immune systems [2]. Medicinal plants vary in their potency for curing diseases, and their specificity as antimicrobial agents can be ascertained if these plants are tested and compared. The use of plants in curing diseases has led to a discipline called traditional medicine [3]. Conventional medicine, used in treating many ailments, is used in the form of flowers, seeds, leaves, roots, rhizomes, and bark of trees [3]. The most important antimicrobial drugs in clinical usage are naturally derived substances obtained from medicinal plants.
Different compounds found in medicinal herbs possess pharmacological properties to control specific diseases [4]. The diseases mentioned above can be controlled by the metabolites present in medicinal plants [5]. Plant-based metabolites have shown antimicrobial, anti-oxidant, and anti-diabetic activities, as the current medicinal plant used in this study has shown antimicrobial, anti-oxidant, and anti-diabetic activities. Medicinal plants are known for the presence of active ingredients, which include organic acids, volatile oil, phenols, alkaloids, cryogenics, glycosides, terpenoids, anthraquinone, terpenoids, β-sitosterol, cardiac glycosides, δ 5 –avenasterol, flavonoids, steroids. These active compounds possess antibacterial, anti-oxidant, and antitumor agents [6]. These ingredients are effective sweeteners, anti-infectious agents, and anti-bacterials [6]. It is also reported that herbs can control heart disease and thrombosis, improve blood circulation, and lower the risk of colon cancer [7]. Metabolites present in medicinal crops also act as anti-mutagenic, anti-inflammatory, anti-carcinogenic, and antitumor agents for curing ailments like cancer without showing any toxicity to humans or animals [8]. Medicinal plants have also been reported to soothe the liver, aerate and strengthen the spleen and stomach, and prevent many diseases (e.g., obesity, diabetes, gastrointestinal diseases, etc.) [7]. Due to these properties, medicinal plants can be used as an alternative medicine for controlling diseases.
Developing nations use plants (medicinal) as a folk medicine to treat various ailments [9,10]. Cancer in some regions of North Africa is only being treated by medicinal plants because of less income and the unavailability of medical facilities [[10], [11], [12]].
Cyperus esculentus L. (Cyperaceae) is cultivated in Africa, the Middle East, Southern Europe, Madagascar, and India [13]. Seeds, rhizomes, and tubers are responsible for reproducing tiger nuts, a perennial or annual plant that grows up to a length of 90 cm [13]. Tiger nut is cultivated in Nigeria in a small area, especially on river banks. It is called by different names in different parts of the country, such as ‘aya’ in Hausa, and ‘ofio’ in Yoruba [14].
C. esculentus, a tuber plant, is converted into beverages or eaten raw [3]. It comprises lipids, starch, and fiber (in high concentration). Tubers of this plant are reported to control heart disease thrombosis and aid in blood circulation [15] and colon cancer. The tuber of this plant is composed of starch, sugar, protein, fat, phosphorus, potassium, and C and E vitamins [16], can control diabetes, and can help with weight loss [17]. Plants can control blood pressure, promote immune response, and maintain a healthy digestive system. Tiger nut is widely used for animal (feed) and human consumption. In Spain, these tubers mainly make a milk-like beverage called ‘horchata de chufa.’ This beverage (tiger nut milk) is a non-alcoholic refreshing drink with a dairy appearance and is usually consumed in the summertime. Muslims originally made this beverage, and now it is widespread in Spain; the ‘horchata’ industry is of considerable economic importance.
Due to the presence of disease-controlling properties, this study was designed with the following objectives: (1) identify compounds and assess anti-diabetic, antimicrobial, anti-oxidant, and anti-cholinesterase properties of extracts of C. esculentus (2) determine anti-quorum sensing and antibiotic modifying effect of the extracts of C. esculentus (3) Assess morphology, elemental accumulation and functional groups of S. aureus exposed to the extracts of C. esculentus.
2. Results
2.1. Determination of phytochemicals in Cyperus esculentus
Results in Table 1 indicated that varied phytochemicals were extracted from aqueous, dichloromethane (DCM), and butanol. DCM produced the highest total phenols (39.7 mg/g), and alkaloids (13.6 mg/g) were the second best. Among solvents, DCM was followed by butanol (BUT), whereas aqueous extracts were the least used for extracting phytochemicals.
Table 1.
Quantitative phytochemicals identified in aqueous, butanol, and dichloromethane extracts of C. esculentus extracts.
| Solvent Extractions | Phytochemicals (mg/g) |
|||
|---|---|---|---|---|
| Tanin | Alkaloid | Total Phenol | Total Flavonoid | |
| Aqueous | 5.6 ± 0.8 | 4.1 ± 0.40 | 10.8 ± 0.90 | 2.3 ± 0.40 |
| Butanol | 11.2 ± 1.3 | 9.5 ± 0.70 | 27.1 ± 2.40 | 5.4 ± 0.50 |
| Dichloromethane | 12.4 ± 1.2 | 13.6 ± 1.2 | 39.7 ± 3.40 | 6.6 ± 0.60 |
Values are the average of triplicates. ± = Standard deviation. Butanol extracted the maximum quantity of phytochemicals.
2.2. Identification of compounds using gas Chromatograph-Mass spectrophotometer (GC-MS)
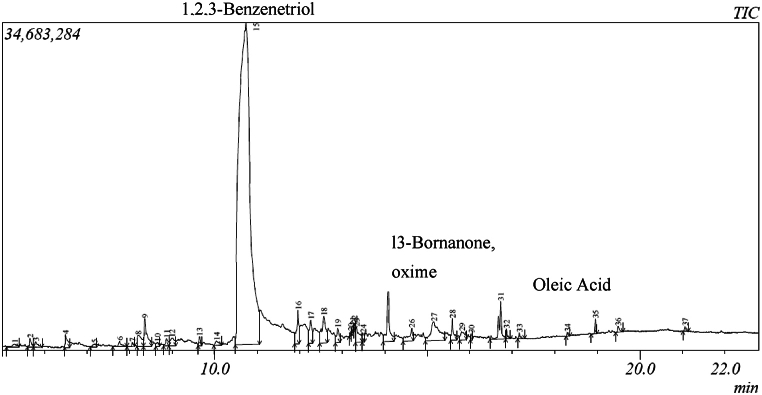
Thirty-seven chemicals were recognized in butanol extracts of C. esculentus, whereas dichloromethane (DCM) extracts extracted 30 compounds using GC-MS. Active compounds identified by GC-MS divulged the existence of benzofuran, 2,3-dihydro-, 1,2,3-benzenetriol, 3-bornanone, oxime, and oleic acid by the extracts of butanol (Table 2 and Fig. 1) whereas extracts of DCM, extracted three major active plant-chemicals (4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-, 5-hydroxymethylfurfural and 3-deoxy-d-mannoic lactone) (Table 3 and Fig. 2).
Table 2.
Chemicals found in the butanolic extract of C. esculentus using GCMS.
| Peak | Compound | Retention Time (min) | Retention Indices (Literature) | Area (%) | Molecular Formula | Molecular Weight (g/ml) |
|---|---|---|---|---|---|---|
| 2 | Pantolactone | 5.658 | 1883 | 0.36 | C6H10O3 | 130.14 |
| 4 | Levoglucosenone | 6.491 | 1404 | 0.70 | C6H6O3 | 126.11 |
| 6 | Benzoic acid | 7.762 | 1554.3 | 0.37 | C7H6O2 | 122.12 |
| 8 | Catechol | 8.201 | 1961 | 0.99 | C6H6O2 | 110.1 |
| 9 | Benzofuran, 2,3-dihydro- | 8.355 | 1152.7 | 1.56 | C8H8O | 120.15 |
| 15 | 1,2,3-Benzenetriol | 10.739 | 1385.7 | 73.69 | C6H6O | 126.11 |
| 17 | 1-Ethynyl-3,5-dimethyladamantane | 12.257 | 1259 | 1.21 | C14H20 | 188.30 |
| 23 | Ethanol, 1-(1-cyclohexenyl)- | 13.332 | 1336.6 | 1.11 | C8 H16 O | 126.2 |
| 25 | 3-Bornanone, oxime | 14.082 | 1135.1 | 2.82 | C10H17NO | 167.25 |
| 26 | Spiro[5.5]undeca-1,7-diene | 14.630 | 1516 | 1.01 | C11H16 | 148.24 |
| 28 | n-Hexadecanoic acid | 15.589 | 1996 | 0.84 | C21H46O2Si2 | 256.43 |
| 29 | Semiarbazide, 4-(1,7,7-trimethylbicyclo[2.2.1] | 15.811 | 1944.7 | 0.82 | C11H19N3O | 209.29 |
| 31 | Oleic Acid | 16.725 | 2165.16 | 1.77 | C18H34O | 282.47 |
1,2,3-Benzenetriol; l3-Bornanone, oxime, and Oleic Acid are the most active compounds identified in the butanol extract.
Fig. 1.
The spectrum of chemical compounds identified in the butanol extract of C.esculentus.
Table 3.
Compounds identified in dichloromethane extract of C. esculentus by gas chromatograph mass spectrophotometer. 5-Hydroxymethylfurfural; 2,3-dihydro-3, 5-dihydroxy-;3-Deoxy-d-mannoic lactone are the most active compounds identified.
| Peak | Compound Name | Retention Time (min) | Retention Indices (Literature) | Area (%) | Molecular Formula | Molecular Weight (g/mol) | |
|---|---|---|---|---|---|---|---|
| 1 | 2-Nonanol | 5.062 | 1117 | 2.11 | C9H20O | 144.25 | |
| 2 | 3-(methoxymethoxy)-1-Octene | 5.537 | 770 | 5.17 | C10H20O3 | 188.26 | |
| 3 | 3-methyl-2-Heptanol | 5.627 | 1076.7 | 4.26 | C8H18O | 130.23 | |
| 6 | 4-hydroxy-3-methyl-2-Butanone | 7.357 | 684.3 | 2.54 | C5H10O2 | 102.13 | |
| 8 | 2,3-dihydro-3,5-dihydroxy- | 7.946 | 1119 | 9.86 | C5H6O4 | 130.1 | |
| 11 | 5-Hydroxymethylfurfural | 8.891 | 799 | 22.47 | C6H6O3 | 126.11 | |
| 17 | 1,2,4-Benzenetriol | 11.176 | 1385.2 | 2.19 | C6H6O3 | 126.11 | |
| 19 | 8-Methyl-6-nonenoic acid | 11.972 | 1372.8 | 2.18 | C10H18O2 | 170.25 | |
| 20 | alpha.-d-Glucopyranoside, O-.alpha.-d-glucopyranosyl | 12.507 | 1455 | 1.39 | C18H32O16 | 342.3 | |
| 21 | 3-Deoxy-d-mannoic lactone | 14.449 | 1323.1 | 28.83 | C6H10O5 | 162.14 | |
| 23 | n-Hexadecanoic acid | 15.596 | 1996 | 1.49 | C21H46O2Si2 | 386.8 | |
| 25 | alpha.-d-Glucopyranoside, O-.alpha.-d-glucopyranosyl | 16.164 | 1926 | 2.45 | C18H32O16 | 342.3 | |
Fig. 2.
The spectrum of chemical compounds identified in the extract of dichloromethane of C. esculentus.
2.3. Minimum inhibitory concentration (MIC) assay
MIC of C. esculentus extracts varied among different microorganisms (Table 4). The most effective extract was DCM, followed by butanol, whereas aqueous extract was the least effective (Table 4). DCM extract manifested an MIC of 25 mg/mL to S. aureus, 35 mg/mL to P. aeruginosa, 30 mg/mL to C. albicans and 35 mg/mL to A. flavus. Similarly, butanol manifested a MIC of 90 mg/mL for Staphylococcus aureus, 100 mg/mL for P. aeruginosa, 65 mg/mL for C. albicans, and 85 mg/mL for A. flavus.
Table 4.
MIC of C. esculentus to bacterial and fungal cultures (mg/mL).
| Microbial Strains | Aqueous | Butanol | Dichloromethane | Penicillin G | Ketoconazole |
|---|---|---|---|---|---|
| Staphylococcus aureus | 135b ± 9.7 | 90c± 7.4 | 25a ± 1.2 | 12.5a ±1.1 | ND |
| Pseudomonas aeruginosa | 145c ± 10.5 | 100d ± 8.3 | 35c ± 2.4 | 12.5a ± 1.2 | ND |
| Candida albicans | 130a ± 12.1 | 65a ± 5.3 | 30b ± 2.6 | ND | 10a ± 0.8 |
| Aspergillus flavus | 145c ± 12.7 | 85b ± 7.1 | 35c ± 2.8 | ND | 12b ± 0.9 |
Values are the mean of triplicates. ± = Standard deviation. N.D = Not detected. Different alphabets indicated values that significantly differ (p < 0.05) from every treatment by Duncan's multiple range test. Butanol and dichloromethane as controls did not divulge antimicrobial properties.
Water extract was the least effective among all the extracts. It was found to have a MIC of 135, 130, 145, and 145 mg/mL for S. aureus, P. aeruginosa, C. albicans, and A. flavus, respectively. Antibacterial and antifungal controls were penicillin G and ketoconazole, respectively. Penicillin G showed a MIC of 12.5 mg/mL for P. aeruginosa and S. aureus, whereas ketoconazole manifests a MIC of 10 and 12 mg/mL for C. albicans and A. flavus, respectively (Table 4).
2.4. Antimicrobial activity of Cyperus esculentus
2.4.1. Open well diffusion technique
Antibacterial and antifungal activities of the extracts of C. esculentus varied and are presented in Table 5. Dichloromethane was the most effective solvent, showing antimicrobial activities, followed by butanol, whereas aqueous extract was the least effective. Dichloromethane was more toxic to P. aeruginosa (27 mm zone) than S. aureus (20 mm zone), whereas, among fungal strains, it showed more inhibitory effect to A. flavus (25 mm zone) compared to C. albicans (22 mm zone) (Table 5).
Table 5.
Antimicrobial activities of C. esculentus extracts against some organisms.
| Organisms | Zone of Inhibition (mm) |
||
|---|---|---|---|
| Aqueous | Butanol | Dichloromethane | |
| Staphylococcus aureus | 09a ± 0.9 | 15a ± 0.6 | 20c ± 0.6 |
| Pseudomonas aeruginosa | 13c ±0.8 | 22c ±0.7 | 27d ± 0.8 |
| Candida albicans | 11b ± 0.7 | 16a ±0.6 | 22b ± 0.5 |
| Aspergillus flavus | 14d ± 0.6 | 19b ± 0.6 | 25c ±0.6 |
Values are the mean of triplicates. ± = Standard deviation. Different alphabets indicated values that significantly differ (p < 0.05) from every treatment by Duncan's multiple range test. Butanol and dichloromethane as controls did not divulge antimicrobial properties.
2.5. Anti-diabetic activities of the extracts of C. esculentus
2.5.1. Repressive property of α--amylase
The inhibitory influence of various extracts of C. esculentus on α-amylase is shown in Table 6. Among extracts, dichloromethane showed maximum inhibitory effect compared to butanol and aqueous extract. As the dosage of extracts increased, α-amylase inhibition also increased. Dichloromethane at 1 mg/mL showed an inhibition of 84.93 % in α-amylase compared to 0.03125 mg/mL of dichloromethane, which showed an inhibition of 58.96 %. Similarly, butanol at 1 mg/mL inhibited 78.20 % of α-amylase activity compared to 0.03125 mg/mL of dichloromethane, which showed an inhibition of 55.35 %. In contrast, aqueous extract showed the least inhibition of α-amylase compared to butanol and dichloromethane. Dichloromethane and butanol divulged half maximal inhibitory concentration (IC50) values of 0.60 and 0.50 mg/mL, respectively, compared to aqueous extract, which showed an IC50 value of 0.20 mg/mL (Table 6).
Table 6.
Repressive properties of various extracts of C. esculentus on α-amylase property.
| Sample | Dose (mg/mL) | Percentage (%) Inhibition | IC50 (mg/mL) ± SEM |
|---|---|---|---|
| Aqueous | 0.03125 | 47.80d ± 0.09 | 0.20 ± 0.01 |
| 0.0625 | 50.94c ± 0.30 | ||
| 0.125 | 51.92c ± 0.15 | ||
| 0.25 | 54.29b ± 0.20 | ||
| 0.5 | 55.48b ± 0.22 | ||
| 1.0 | 61.42a ± 0.39 | ||
| Butanol | 0.03125 | 55.35e ± 0.19 | 0.50 ± 0.00 |
| 0.0625 | 60.70d ± 0.19 | ||
| 0.125 | 63.75d ± 0.07 | ||
| 0.25 | 67.74c ± 0.15 | ||
| 0.5 | 72.08b ± 0.19 | ||
| 1.0 | 78.20a ± 0.81 | ||
| Dichloromethane | 0.03125 | 58.96f ± 0.00 | 0.60 ± 0.01 |
| 0.0625 | 64.43e ± 1.20 | ||
| 0.125 | 67.70d ± 1.50 | ||
| 0.25 | 71.65c ± 2.01 | ||
| 0.5 | 76.44b ± 0.81 | ||
| 1.0 | 84.93a ± 1.01 |
Values are the average of triplicates. ± = Standard deviation. Different alphabets indicated values that significantly differ (p < 0.05) from every treatment by Duncan's multiple range test. Butanol and dichloromethane as controls did not divulge antimicrobial properties.
2.5.2. α- glucosidase inhibitory activity
Extracts of C. esculentus (both dichloromethane and butanol) significantly inhibited α-glucosidase activity compared to aqueous extract (Table 7). Among the three extracts tested, dichloromethane was the most effective, followed by butanol, whereas aqueous extract was the least effective. As the concentration of the extracts increased, α-glucosidase inhibition also increased. Dichloromethane at 1 mg/mL inhibited 87.13 % α-glucosidase activity compared to 0.03125 mg/mL of dichloromethane, which showed an inhibition of 64.62 %. Similarly, butanol at 1 mg/mL showed an inhibition of 84.92 % in α-glucosidase activity compared to 0.03125 mg/mL of dichloromethane, which showed an inhibition of 62.56 %. In contrast, aqueous extract showed the least inhibition of α-glucosidase compared to butanol and dichloromethane. Dichloromethane and butanol showed an IC50 value of 0.65 and 0.60 mg/mL, respectively, compared to aqueous extract, which showed an IC50 value of 0.30 mg/mL (Table 7).
Table 7.
In vitro inhibitory activities of C. esculentus extracts on α-glucosidase.
| Sample | Dose (mg/mL) | Percentage (%) Inhibition | IC50 (mg/mL) ± SEM |
|---|---|---|---|
| Aqueous | 0.03125 | 50.87c ± 0.8 | 0.30 ± 0.0 |
| 0.0625 | 52.26b ± 0.5 | ||
| 0.125 | 53.43b ± 0.4 | ||
| 0.25 | 54.19a ± 0.4 | ||
| 0.5 | 55.85a ± 0.6 | ||
| 1.0 | 56.61a ± 0.5 | ||
| Butanol | 0.03125 | 62.56e ± 0.7 | 0.60 ± 0.03 |
| 0.0625 | 68.54d ± 0.9 | ||
| 0.125 | 70.29d ± 1.1 | ||
| 0.25 | 77.17c ± 1.2 | ||
| 0.5 | 81.10b ± 0.38 | ||
| 1.0 | 84.92a ± 2.50 | ||
| Dichloromethane | 0.03125 | 64.62f ± 5.90 | 0.65 ± 0.06 |
| 0.0625 | 70.67e ± 5.04 | ||
| 0.125 | 73.72d ± 4.31 | ||
| 0.25 | 79.09c ± 1.42 | ||
| 0.5 | 83.42b ± 1.55 | ||
| 1.0 | 87.13a ± 0.42 |
Values are the average of triplicates. ± = Standard deviation. Different alphabets indicated values that significantly differ (p < 0.05) from every treatment by Duncan's multiple range test.
2.6. In vitro anti-cholinesterase assays
2.6.1. Acetylcholinesterase activity
The inhibitory effect of various extracts of C. esculentus on acetylcholinesterase is shown in Table 8. Among all the extracts, dichloromethane was the most effective inhibitory extract against acetylcholinesterase. This was followed by butanol, whereas aqueous extract was the least inhibitory to the enzyme activity. As the concentration of the extracts increased, acetylcholinesterase inhibition also increased. Dichloromethane at 1 mg/mL inhibited 73.03 % of acetylcholinesterase activity compared to 0.03125 mg/mL of dichloromethane, which showed an inhibition of 20.67 %.
Table 8.
Inhibitory Acetylcholinesterase activities of C. esculentus extracts.
| Sample | Concentration (mg/mL) | Inhibition (%) | IC50 (mg/mL) ± SEM |
|---|---|---|---|
| Aqueous | 0.03125 | 6.32d ± 0.71 | 0.29 ± 0.27 |
| 0.0625 | 8.43d ± 1.38 | ||
| 0.125 | 12.11c ± 0.30 | ||
| 0.25 | 15.54b ± 2.13 | ||
| 0.5 | 19.41a ± 1.71 | ||
| 1.0 | 22.07a ± 0.62 | ||
| Butanol | 0.03125 | 16.55f ± 0.09 | 0.70 ± 0.10 |
| 0.0625 | 25.42e ± 0.17 | ||
| 0.125 | 37.65d ± 0.11 | ||
| 0.25 | 41.09c ± 0.43 | ||
| 0.5 | 50.41b ± 0.24 | ||
| 1.0 | 66.78a ± 1.42 | ||
| Dichloromethane | 0.03125 | 20.67f ± 0.22 | 0.80 ± 0.01 |
| 0.0625 | 31.90e ± 1.52 | ||
| 0.125 | 42.11d ± 0.74 | ||
| 0.25 | 47.60c ± 0.43 | ||
| 0.5 | 58.53b ± 1.30 | ||
| 1.0 | 73.03a ± 0.02 |
Values are the average of triplicates. ± = Standard deviation. Different alphabets indicate values that significantly differ (p < 0.05) from every treatment by Duncan's multiple range test. Butanol and dichloromethane as controls did not show any anti-cholinesterase activity.
Similarly, butanol at 1 mg/mL inhibited 66.78 % of acetylcholinesterase activity compared to 0.03125 mg/mL of dichloromethane, which showed inhibition of 16.55 %. In contrast, aqueous extract showed less inhibition to acetylcholinesterase than butanol and dichloromethane. Dichloromethane and butanol showed an IC50 value of 0.80 and 0.70 mg/mL, respectively, compared to aqueous extract, which showed an IC50 value of 0.29 mg/mL (Table 8).
2.6.2. Butyryl cholinesterase activity
Cyperus esculentus extracts (dichloromethane and butanol) significantly inhibited butyrylcholinesterase activity compared to aqueous extract (Table 9). Among the three extracts tested, dichloromethane was the most effective extract, followed by butanol, whereas aqueous extract was the least inhibitory. As the concentration of the extracts increased, butyrylcholinesterase inhibition also increased. Dichloromethane at 1 mg/mL inhibited 76.3 % of butyrylcholinesterase activity compared to 0.03125 mg/mL of dichloromethane, which showed an inhibition of 55.8 %. Likewise, butanol at 1 mg/mL inhibited 70.1 % of butyrylcholinesterase activity compared to 0.03125 mg/mL of dichloromethane, which showed inhibition of 53.5 %. In contrast, the aqueous extract showed the least inhibition of butyrylcholinesterase compared to butanol and dichloromethane. Dichloromethane and butanol showed IC50 values of 0.70 and 0.60 mg/mL, respectively, compared to control (aqueous) (0.20 mg/mL) (Table 9).
Table 9.
Butyrylcholinesterase activities of C. esculentus extracts.
| Sample | Concentration (mg/mL) | Inhibition (%) | IC50 (mg/mL) ± SEM |
|---|---|---|---|
| Aqueous | 0.03125 | 51.3d ± 2.9 | 0.20 ± 0.02 |
| 0.0625 | 53.9c ± 3.0 | ||
| 0.125 | 54.9c ± 3.2 | ||
| 0.25 | 56.7c ± 3.2 | ||
| 0.5 | 61.7b ± 4.1 | ||
| 1.0 | 65.1a ± 4.3 | ||
| Butanol | 0.03125 | 53.5d ± 5.2 | 0.60 ± 0.04 |
| 0.0625 | 55.9c ± 4.8 | ||
| 0.125 | 57.1c ± 4.9 | ||
| 0.25 | 64.9b ± 4.8 | ||
| 0.5 | 68.1a ± 4.3 | ||
| 1.0 | 70.1a ± 0.2 | ||
| Dichloromethane | 0.03125 | 55.8e ± 4.8 | 0.70 ± 0.16 |
| 0.0625 | 57.2e ± 4.9 | ||
| 0.125 | 60.7d ± 6.8 | ||
| 0.25 | 67.4c ± 6.9 | ||
| 0.5 | 72.9b ± 7.1 | ||
| 1.0 | 76.3a ± 7.3 |
Values are the average of triplicates. ± = Standard deviation. Different alphabets indicate values that significantly differ (p < 0.05) from every treatment by Duncan's multiple range test.
2.7. Anti-antibiotic resistance of C. esculentus
Interactions between various extracts and penicillin G against S. aureus are divulged in Table 10. Anti-antibiotic resistance is presented as synergistic or additive by the fractional inhibitory concentration (FIC) index value. If the FIC value is > 0.5—it will be regarded as symbiotic (synergistic), 0.5 < FIC< 1—cumulative (additive), and if it is 1 < FIC < 4—indifferent and FIC 4.0—adversely (antagonistic). Dichloromethane and butanol were synergistic to S. aureus, with an FIC of 0.45 and 0.44, respectively, while water divulged additive properties to S. aureus with an FIC value of 0.70 (Table 10).
Table 10.
Interactions between different solvent fractions and penicillin G on a methicillin-resistant S. aureus.
| Fractions | FIC A | FIC B | FIC | Interpretation |
|---|---|---|---|---|
| Aqueous | 0.6d ± 0.001 | 0.10a ± 0.003 | 0.70b ± 0.003 | Additive |
| Butanol | 0.25b ± 0.03 | 0.20b ± 0.001 | 0.45a ± 0.003 | Symbiotic |
| Dichloromethane | 0.22a ± 0.010 | 0.23c ± 0.002 | 0.45a ± 0.002 | Symbiotic |
Values are the average of triplicates. ± = Standard deviation. Different alphabets indicate values that significantly differ (p < 0.05) from every treatment by Duncan's multiple range test.
2.8. Anti-oxidant activity of Cyperus esculentus
Anti-oxidant activity (Ferric reducing and total anti-oxidant) of C. esculentus solvents varies from one extract to another extract (Table 11). Among various solvents, dichloromethane was the most superior solvent in extracting anti-oxidants. This was followed by butanol, whereas aqueous extract was the least effective. Dichloromethane extracted 75.9 mg AAE/mL of ferric-reducing anti-oxidant and 60.6 mg AAE/mL of total anti-oxidant activity at 1 mg/mL of plant extract. In contrast, butanol extracted 70.1 mg AAE/mL of ferric-reducing anti-oxidant and 52.5 mg AAE/mL of total anti-oxidant activity at 1 mg/mL of plant extract (Table 11).
Table 11.
Cyperus esculentus extracts affect anti-oxidants (total and ferric reducing).
| Fractions | Ferric Reducing Power (mgAAE/mL) | Total anti-oxidants (mgAAE/m) |
|---|---|---|
| Aqueous | 61.8a ± 5.8 | 31.5a ± 2.7 |
| Butanol | 70.1d ± 6.4 | 52.5d ± 4.6 |
| Dichloromethane | 75.9e ± 6.9 | 60.6e ± 5.6 |
Values are the average of triplicates. ± = Standard deviation. Different alphabets indicate values that significantly differ (p < 0.05) from every treatment by Duncan's multiple range test. Butanol and dichloromethane as controls were negative for anti-oxidant production.
2.9. Antivirulence effects of Cyperus esculentus
All concentrations of butanol and dichloromethane extracts of C. esculentus showed the maximum inhibitory effect on the production of pyocyanin, total protease, and chitinase by P. aeruginosa compared to aqueous extract (Table 12). 1 mg/mL concentration of C. esculentus extracts of aqueous, butanol and dichloromethane maximally impacted production of pyocyanin, total protease, and chitinase which was followed by 0.5 mg/mL whereas 0.125 mg/mL was the least effective concentration impacting the production of pyocyanin, total protease and chitinase. Among the extracts, dichloromethane showed the maximum inhibitory effect on quorum sensing regulated pathogenic factors such as pyocyanin, total protease, and chitinase, followed by butanol. In contrast, aqueous extract showed the least inhibitory effect. Dichloromethane at 1 mg/mL showed maximum inhibition to pyocyanin (113 μg/mL), which was followed by butanol (156 μg/mL), whereas aqueous extract was least in inhibiting pyocyanin production (277 μg/mL). Among all the concentrations, 0.125 mg/mL showed the least inhibitory effect on pyocyanin production. Similarly, dichloromethane at 1 mg/mL also showed a maximum inhibitory effect on the secretion of total protease (0.17 absorbance) and chitinase (0.084 absorbance) compared to aqueous extract, which showed the least inhibition in the production of total protease (0.89 absorbance) and chitinin (0.273 absorbance). Total protease and chitinase production were also least affected by 0.125 mg/mL of the extract of dichloromethane and butanol (Table 12).
Table 12.
Effect of C. esculentus extracts on quorum-sensing regulated pathogenic activity of P. aeruginosa.
| Sample | Concentration (mg/mL) | Pyocyanin production (Μg/mL) | Protease activity (OD at 400 nm) | Chitinase activity (OD at 570 nm) |
|---|---|---|---|---|
| Aqueous | 0.125 | 277a ± 22.2 | 0.89a ± 0.47 | 0.273 a ± 0.13 |
| 0.25 | 245b ± 19.5 | 0.74b ± 0.38 | 0.232b ± 0.11 | |
| 0.5 | 205d ± 10.2 | 0.54c ± 0.22 | 0.207c ± 0.0.09 | |
| 1.0 | 178d ± 6.9 | 0.39d ± 0.18 | 0.163d ± 0.07 | |
| Butanol | 0.125 | 241a ± 23.5 | 0.74a ± 0.33 | 0.267a ± 0.13 |
| 0.25 | 202b ± 13.4 | 0.63b ± 0.25 | 0.238b ± 0.11 | |
| 0.5 | 174c ± 9.8 | 0.46c ± 0.21 | 0.202c ± 0.08 | |
| 1.0 | 156d ± 8.3 | 0.28d ± 0.17 | 0.136d ± 0.07 | |
| Dichloromethane | 0.125 | 197a ± 8.2 | 0.61a ± 0.37 | 0.237a ± 0.12 |
| 0.25 | 176b ± 7.4 | 0.49b ± 0.29 | 0.197b ± 0.10 | |
| 0.5 | 137c ± 6.7 | 0.31c ± 0.19 | 0.103c ± 0.08 | |
| 1.0 | 113d ± 6.1 | 0.17d ± 0.12 | 0.084d ± 0.06 |
Values are the average of triplicates. ± = Standard deviation. Different alphabets indicate values that significantly differ (p < 0.05) from every treatment by Duncan's multiple range test. Butanol and dichloromethane as controls did not show anti-quorum sensing activity.
2.10. Surface morphology and elemental uptake by S.aureus using scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) spectroscopy analysis
Staphylococcus aureus morphology was observed under exposure to aqueous, butanol, and dichloromethane using SEM (Fig. 3a). SEM micrograph of S. aureus exposed to aqueous extract showed smooth images without pit formation, whereas S. aureus exposed to butanol and dichloromethane extracts showed deep localized pit formation as indicated by red arrows (Fig. 3a), indicating damage to the cell wall of the S. aureus. Elemental (nutrient) uptake by the S. aureus exposed to aqueous, butanol, and dichloromethane is also shown in the energy-dispersive X-ray microanalysis spectra (Fig. 3b).
Fig. 3.
a. Scanning electron micrograph pictures of S. aureus exposed to aqueous (A), butanol (B), and dichloromethane (C) extracts. Arrows indicate damage to S. aureus due to butanol and dichloromethane extracts compared to aqueous extract. b.Energy dispersive X-ray microanalysis spectra (D–F) of S. aureus exposed to aqueous (D), butanol (E) and dichloromethane (F) extracts.
2.11. Fourier transmission infra red (FTIR) spectra analysis
FTIR spectra analysis of S. aureus exposed to aqueous, butanol, and dichloromethane extracts is shown in Fig. 4A–C. The visible change in the bands can be seen in the butanol and dichloromethane-exposed S. aureus (Fig. 4B and C). Bands at 3761, 3439, 2926.25, and 1731.55 corresponded to –OH, –OH, C–H, and C]O in S. aureus exposed to butanol. Bands at 3758, 3433, 2926, and 2360.56 corresponded to –OH, –OH, C–H, and C]O]C in S. aureus exposed to dichloromethane extract, respectively.
Fig. 4.
FTIR spectra of S. aureus exposed to aqueous (A), butanol (B) and dichloromethane (C) extracts.
New functional groups can be seen in the S. aureus exposed to butanol and dichloromethane extracts, which are absent in the S. aureus exposed to aqueous extract (Fig. 4A–C). Production of new functional groups is due to the damage in functional groups exposed to butanol and dichloromethane, indicating the antimicrobial activity of the extracts. Bands at 1640.80, 1513.75, 1428.66, 1378.59, 1329, 1257, 1039, 593 and 459 belonged to C]C, N–O, N–O, C–H, O–H, C–O, C–N, C–Br and Zn–O respectively when S. aureus was exposed to butanol whereas bands at 1730.37, 1641, 1515.16, 1425.66, 1381.20, 1328.66, 1255.88, 1037.17, 594 and 457 belonged to C]O, C]C, N–O, O–H, S]O, O–H, C–O, S]O, C–Br and Zn–O respectively when S. aureus exposed to dichloromethane. Formation of new bands in S. aureus exposed to butanol and dichloromethane extracts, which are absent in cells exposed to aqueous extracts, indicating damage to the cell (Fig. 4A–C).
3. Discussion
From time immorally, different countries' societies use other plants to control various diseases [18]. Microbial antibiotic resistance has led scientists to look for new antibacterial agents [18]. Medicinal herbs are essential in searching for and progressing phytomedicine to treat ailments properly [4]. Plants possessing anti-diabetic, anticancer, anti-inflammatory, and antimicrobial capacities can help treat such diseases. Chemical compounds such as n-hexadecanoic acid have shown antibacterial and ant-fungal activity, oleic acid as an anti-oxidant, anti-breast cancer, and octadecatrienoic acid as a dietary nutrient and anti-inflammatory. In contrast, non-decanoic acid is a dietary nutrient and anti-inflammatory biomarker for prostate cancer [19].
Gas chromatography (GC) is an important technique to observe active compounds in plants. Over the last decade, GC-MS has become essential for identifying plant and non-plant-based compounds in various specimens [20]. This study analyzed different solvent extracts of dichloromethane and butanol for active compounds using GC-MS. GC-MS analysis of dichloromethane and butanol extracts divulged different chemicals that possessed retention times having different values. Most prominent compounds were found to be benzofuran, 2,3-dihydro-, 1,2,3-benzenetriol, 3-bornanone, oxime and oleic acid by the extracts of butanol whereas extracts of DCM extracted three major active chemicals such as 2,3-dihydro-3,5-dihydroxy-, 4H-pyran-4-one, 5-hydroxymethylfurfural and 3-deoxy-d-mannoic lactone. As revealed by GC-MS, several chemicals were found in X. Americana L [21,22]. These compounds can treat inflammation, diabetes, and cholesterol. They also possess anti-oxidant and cancer-controlling properties [23]. The above biological properties are due to phytochemicals present in C. esculentus [24]. Phenolic compounds reported in acacia species and salad burnet were in high concentration. They are reported for their strong anti-oxidant activity, as phenolics are good anti-oxidants [2,6].
The first line of treatment for S. aureus infection is vancomycin, but these bacterial strains are reported for increasing MIC values. If these strains possess a MIC value greater than one mg/L, they can pose a risk to the animals, mainly because they can damage the kidneys; thus, vancomycin cannot be used to treat animals with infection of S. aureus [25]. In this regard, using medicinal plants to treat infections caused by S. aureus is better. The antimicrobial effect of C. esculentus extracts was potentially effective for all organisms (bacteria and fungi). Dichloromethane extract showed the best effect against all test isolates and showed a maximum zone of inhibition against microorganisms. The above properties can result from phytochemicals present in the plant extract [24]. Phenolic compounds in salad burnet (Sanguisorba minor L.), bitter Kola (Garcinia kola L.), and kolanut (Cola acuminate L.) are reported to show the antimicrobial effect on pathogens as this plant is enriched with different types of phenolics in high concentrations [2,26]. Efficient extraction of phytochemicals due to the use of solvents could have added antimicrobial properties [27]. It is reported that phytochemicals such as saponins, flavonoids, and steroids/triterpenoids have shown anti-microbial activity [28]. The mechanism of antimicrobial action of chemical compounds present in medicinal plants may be due to their action on bacterial membranes, which results in the perturbation of the membrane. This action will result in the leakage of cytoplasmic material, which is considered indicative of gross and irreversible damage to the cytoplasmic membrane [23]. A study by Oladipipo et al. [29] (2016) for an LD50 test was conducted in which the effect of C. esculentus was seen, concentrations of 100–5000 mg/kg body weight were given to the Wistar rats, and these concentrations did not show any mortality but concentrations beyond 1000 mg/kg body weight showed decreased body weight but decrease was not significant, it means that animals can tolerate high concentrations which is beyond 1000 mg/kg body weight and if such concentrations are given to animals for controlling microbial infections will not have any side effect on the experimental animals.
Plant-based drugs and plant substances possessing no side effects and less toxicity are the best options for controlling diseases of humans around the world [24]. Phytochemicals from natural sources can be used as drugs. Diabetes treatment with medicinal plants is successful despite the availability of synthetic drugs in the market. Phytochemicals such as phenolics, alkaloids, xanthophylls, tannins, etc., are the most prominent chemicals used to control diabetes [7]. Lycopene is reported to control many diseases, including diabetes. In the present study, the dichloromethane and butanol extracts of C. esculentus showed excellent anti-diabetic activities (alpha-glucosidase and alpha-amylase suppression) at all concentrations of C. esculentus. Control of the above enzymes can significantly control postprandial hyperglycemia and thus can effectively manage diabetes mellitus, especially type 2 diabetes mellitus. Banerjee et al. [30] reported suppressing enzymes like alpha-glucosidase and alpha-amylase can control hyperglycemia.
The anti-cholinesterase activities of C. esculentus extracts were determined by investigating the acetylcholinesterase and butylcholinesterase inhibitory reaction against cholinesterase. Among the different extracts, dichloromethane showed the most potent inhibitory activity against cholinesterase (acetyl and butyl) with the best IC50 values. To control Alzheimer's disease, it is necessary to inhibit the production of cholinesterase, which can be controlled by using natural compounds in medicinal plants possessing anti-cholinesterase activity [2]. Current experiments agree with the study of Ortega et al. [31] and Obuotor et al. [22]. Anti-cholinesterase activities of C. esculentus extracts could be due to secondary metabolites in C. esculentus plants, which contain chemical compounds such as flavonoids, phenolic acids, and tannins [32].
FIC of penicillin and C. esculentus extracts to S. aureus are presented in Table 10. Dichloromethane and butanol exhibited synergistic effects against S. aureus, whereas aqueous extract showed an additive effect. This study's values are similar to Heinrich et al.'s findings [33]. The mechanism of action of plant extracts to inhibit beta-lactam (methicillin) is unknown [32]. The authors found that the synergistic interaction of penicillin and C. esculentus could be due to an alteration in bacterial membrane function due to penicillin. Biosynthesis of peptidoglycan in bacteria is inhibited at the last stage [32] due to a change in binding protein PBP2a [34].
The present study revealed that dichloromethane and butanol extracts showed the highest anti-oxidant activities (total anti-oxidant and Ferric Reducing Anti-oxidant Power). Anti-oxidants produced by medicinal herbs act as defense mechanisms against reactive species because of cells' normal activity (aerobic) [27].
Phenolic compounds and flavonoids are a class of anti-oxidant agents that act as free radical scavengers and are considered a major group of compounds that contribute to the anti-oxidant activities of plant materials because of their neutralizing ability on free radicals due to their hydroxyl groups [35]. The present study confirmed that C. esculentus is rich in phenolic and flavonoid compounds, which may be the reason for enhancing anti-oxidant activity.
The quorum sensing mechanism controls several microbes interactions, including pathogenicity and microbial competence. Virulence processes such as biofilm formation, the release of toxins, elastase, rhamnolipid, pyocyanin, total protease, chitinase, sporulation, swarming mobility, etc, are the processes involved in the pathogenicity of different microbes. All these processes are mediated via quorum sensing, so it becomes necessary to control quorum sensing by natural compounds found in traditional medicine to neutralize pathogenicity [2].
Control of quorum-sensing activity through plant extracts is an important strategy for controlling pathogenicity in P. aeruginosa. In this study, the hypothesis was made to control the pathogenic activity such as pyocyanin, total protease, and chitinase controlled by quorum sensing using different concentrations of C. esculentus extracts. The experiments of this study confirmed that the C. esculentus extracts are important for controlling virulence factors. There are reports about plants showing good anti-QS activity, including M. indica L [36]. Pyocyanin, total protease, and chitinase are quorum-sensing regulated virulence activity produced by P. aeruginosa, and the pathogenesis of P. aeruginosa has been described by various scientists for cystic fibrosis, host cell protein damage, and tissue invasion [1]. In this study, pyocyanin, total protease, and chitinase levels in P. aeruginosa culture treated with extracts significantly reduced compared to aqueous extract-treated culture, and the release of virulent factors controlled by signaling molecules reduced as the concentration of extracts increased. Similarly, Terminalia chebula L. extracts also reduced virulence factors [37].
SAM images of butanol and dichloromethane extracts showed damage to the cell wall of S. aureus with the formation of pits compared to aqueous extract, which showed smooth images and bio-film formation [38]. The formation of pits by butanol and dichloromethane to S. aureus may be due to cell wall damage. Pit formation and breakage in the cell wall of S. aureus may also be due to damage to the cell membrane as a result of the hydrolysis of the peptidoglycan layer of the cell, which results in the leakage of the internal contents of the cell, thus will result in the death of the cell. Damage to the bacteria's cell wall may result in the formation of various dimmers in the gene's nucleotide sequence, resulting in point mutation and altering the cell. EDX spectra showed the accumulation of different elements by S. aureus exposed to other plants. Similar results of damage to the cell wall of P. aeruginosa were reported for methanolic extracts of Mangifera indica L. compared to control [36].
FTIR spectra of aqueous, butanol, and dichloromethane extracts showed the formation of different functional groups. Many new functional groups are found in S. aureus exposed to butanol and dichloromethane compared to S. aureus exposed to aqueous extract where these new functional groups are absent, indicating damage to S. aureus thus confirming the antibacterial activity of butanol and dichloromethane. The authors found no report where plant extracts could damage functional groups of S. aureus by forming new functional groups. This is the first report showing damage to the functional groups of pathogenic bacteria. The change in functional groups of S. aureus exposed to plant extracts is due to the breakdown of the bonds between different functional groups, creating new functional groups. Breaking functional groups will damage the bacteria's cell wall, which may change the reading frame of the nucleotide, thus resulting in the cell's death.
4. Materials and methods
4.1. Microorganisms and seed collection
Cyperus esculentus seeds obtained from Kuto market, Abeokuta, Ogun State, were identified at the herbarium of a Nigerian University Obafemi Awolowo Ife with an identification voucher (IFE–17819). Pathogenic microorganisms were obtained from the College of Health Diagnostic Centre, Ogun State.
4.2. Seed extracts preparation
Seeds were grind into power to obtain aqueous butanol (CAS71363, Merck, USA) and dichloromethane (CAS75-092, Merck) extracts. Such extracts were obtained by adding 30 g of the powder into water, butanol, and dichloromethane. Such extracts were filtered using Whatman No. 1 filter paper, and a rotary evaporator was used to concentrate them.
4.3. Identification of active compounds in Cyperus esculentus
Chemical compounds of C. esculentus plants were identified by gas chromatograph mass spectrophotometer (GC-MS) attached to CPSIL5-CB capillary column (CPSIL5-CB) (Model, GC-MS-5975C, Shimadzu, Japan). A temperature of 80 and 250 °C was maintained for the oven ramped at 10 °C/min. Compounds in C. esculentus were analyzed by the system agilent (GC-MS-5975C), which contains a mass spectrometer with an autosampler and a gas chromatograph. GC-MS worked using varian column Agilent JSW HP- 5 MS (300 m × 0.32 mm × 0.25 μM), 1 μL of hexane extracted powder was injected into the column using carrier gas helium, which flowed at 1 mL/min. The operation was performed at 50 °C and held for 5 min, which was enhanced to 80 °C @ 5 °C per minute for 9 min 250 °C and 280 °C were the injector and ion temperatures, respectively, and the injection was duplicated for the GC-MS analysis. Sample compound mass spectrum analysis was performed using 7 cV electron ionization, 230 °C ion source temperature, and 250 °C interface temperature. The best performance of the detector was obtained between 45 and 450 amu, 0.5 s interval, and 60 min of running time. The calibration was done using an external calibration standard.
4.4. Recognition of compounds
Comparison of data obtained in this study by GC-MS and that of the library of the National Institute of Standard Technology (NIST) and Wiley data were used to recognize active chemical constituents found in C. esculentus extracts.
4.5. Determination of resistance of microorganisms to Cyperus esculentus extracts
Resistance of bacterial (S. aureus and P. aeruginosa) and fungal (C. albicans and A. flavus) strains to minimum inhibitory concentrations (MIC) of C. esculentus extracts were determined in nutrient agar plates incubated with 108 cells/ml at 35 ± 2 °C for 24 h as per the method described by Wani et al. [39]. Minimum Inhibitory concentration (MIC) is the maximum concentration that inhibits bacterial and fungal growth.
4.6. Antimicrobial activity, Cyperus esculentus
Antifungal and antibacterial properties of butanol (CAS71363, Merck, Germany), dichloromethane (CAS75-092, Merck, Germany), and aqueous extracts were assayed after making wells with sterile cork borer 7.0 mm diameter using open well diffusion method [36]. Bacterial inoculated (having turbidity of 0.5 McFarland with a concentration of 108 cells/ml of S. aureus and P.aeruginosa) nutrient plates containing wells were filled with 0.05 mL of butanol, dichloromethane, and aqueous extracts incubated for a period of 48 h at 35 ± 2 °C. Antifungal properties against (C. albicans and A. flavus) were performed on Sabouraud dextrose agar (SDA) (M1371-500G, Hi-Media, Mumbai, India) plates at 30 °C for 72 h. Butanol and dichloromethane alone acted as controls. All bacterial and fungal strains used in this study were wild-type.
4.7. Anti-diabetic potentials of Cyperus esculentus extracts
4.7.1. Alpha-amylase inhibitory assay
200 μL porcine pancreatic amylase was added to different concentrations of C. esculentus extracts, which were incubated at 35 ± 2 °C for 20 min. The above solution was added to 100 μL (1 %) starch solution and were incubated for 10 min at 37 °C. The reaction of the above solution was stopped only by adding a volume of 200 μL DNSA after keeping it in the boiling water bath for 5 min. After dilution of the reaction mixture with a volume of 2.2 mL of water, the absorbance of the inhibition of the enzyme activity was recorded at a wavelength of 540 nm [30,40].
4.7.2. Alpha-glucosidase inhibitory assay
Alpha-glucosidase, as influenced by different concentrations of plant extracts, was assayed as per the method mentioned by Banerjee et al. [30]. One mg of -glucosidase was dissolved in 100 mL phosphate buffer (pH 6.8). To different concentrations of C. esculentus extracts, 200 μL of glucosidase was added, which was incubated for 20 min at a temperature of 35 ± 2 °C; the solution was further added with 100 μL 3 mM -nitrophenyl -Dglucopyranoside (p-NPG) and incubated for 10 min at 35 ± 2 °C. The addition of 2 mL Na2CO3 0.1 M terminated the reaction mixture; after termination, the solution was read for glucosidase activity at 405 nm by measuring the quantity of -nitrophenol released from p-NPG. Acarbose acted as a control for both amylase and -glucosidase inhibition.
4.8. Cholinesterase assay
Acetylcholinesterase (AChE) and butylcholinesterase (BuCHE) inhibiting activities of butanol and dichloromethane were determined using a modified method of Ellman et al. [21] as described by Obuotor et al. [22]. The reaction assay mixture consisted of 2000 mL 100 mM phosphate butter pH8.0 (M20971-500G, Hi-Media, India), 100 mL of test sample stock solution in methanol (a final concentration of 42.5 μg/mL), 100 mL of enzyme AChE or BuCHE (CS0003, Sigma Aldrich, USA) solution at a final concentration of 0.03 U/mL and 0.01 μ/mL respectively, 100 μL of DTNB (0.3 mM, 22582, Thermo Fisher, USA) prepared in 100 M phosphate buffer pH 7.0 containing 120 mM sodium bicarbonate (CAS 497-198, Merck). The reaction mixture was vortexed and pre-incubated in a water bath at 37 °C for 30 min. The reaction began by adding ATCI or BTCI (100 μL) at a final amount of 0.5 mM (negative control). Methanol was used for the inhibitor solution, and absorbance of the solution was recorded at λmax 412 nm. 42.5 μg/mL was the final amount of the sample. Eserine (−) physiotigmine) was used as a positive control at the same concentration. Inhibition % was determined using the formula –
a = ΔA/minute of control; b = ΔA/minute of treatment solution; ΔA = Absorbance change.
4.9. Anti-antibiotic resistance of the extracts of Cyperus esculentus
The association between C. esculentus and antibiotics was assayed by fractional inhibitory concentrations (FIC) [41]. FIC is calculated as: Minimum inhibitory dose of sample A assayed in combination/MIC of specimen A determined alone + Minimum inhibitory concentration of sample B assayed in combination/MIC of specimen B assayed alone. A = penicillin G and B]C. esculentus. Penicillin G and extracts of C. esculentus extract were assayed by mixing both of them.
4.10. Anti-oxidant property of Cyperus esculentus
4.10.1. Ferric Reducing and total anti-oxidant property
Anti-oxidant properties (Ferric Reducing) were assayed in various doses of butanol and dichloromethane [41].
4.10.2. Determination of plant extract anti-oxidant capacity
Cyperus esculentus anti-oxidant (total) capacity was assayed by phosphomolybadate [42].
4.11. Quorum sensing (QS) regulated virulence factors, C. esculentus extracts
4.11.1. Pyocyanin assay
Pyocyanin production was calculated as described by Essar et al. [42]. Five mL of P. aeruginosa culture was treated with different concentrations of C. esculentus extracts. Chloroform (3 mL) (CAS67-663, Merck, Germany) was used to extract a mixture of bacterial culture and plant extracts, which was again extracted with 0.2 M HCl (1 mL) (101514, Merck), and a wavelength of 520 nm was used to determine the absorbance of reagent.
4.11.2. Total protease activity
Protease activity was determined in supernatants (cell-free) of P. aeruginosa amended with different doses of C. esculentus [36]. One hundred fifty mL of P. aeruginosa supernatants were amended to a solution containing (1 mL of azocasein (0.3 %) (A2765-25G, Merck Millipore) in 0.05 M Tris–hydrochloric acid (1185-531, Sigma Aldrich, USA) and calcium chloride (0.5 mM, 7.5 pH) (10043524, Merck). The above mixture was kept for 15 min at 37 °C, and trichloroacetic acid (l0%, 0.5 mL) (76039, Merck, Germany) was amended to solution to stop the response of the process. A wavelength of 400 nm was used to calculate protease activity after centrifugation.
4.11.3. Chitinase assay
The effect of plant extracts on chitinase produced by P. aeruginosa was assayed as per the method described by Husain et al. [43]. P. aeruginosa supernatant (sterilized and filtered) was blended in a buffer solution having a ratio of 2:1 [0.1 M sodium citrate buffer, (P4809, Sigma Aldrich), 4.8 pH in chitin azure (C3020, Sigma Aldrich, USA), 0.5 mg/mL]. The above reagents were kept at 37 °C for seven days, and 570 nm was used to read absorbance.
4.12. Cell morphology and elemental analysis of S. aureus using scanning electron microscope
The effect of aqueous, butanol, and dichloromethane on the morphology of S. aureus was observed under a scanning electron microscope (SEM) (Talos F200i, Thermo Fisher, USA). The effect of aqueous, butanol, and dichloromethane on nutrient uptake analysis by S. aureus was confirmed by EDX (Genesis/Veritas Series, EmCrafts Co. Ltd., USA) spectroscopy.
4.13. FTIR spectra analysis for detection of functional group analysis in S. aureus
Functional group analysis in S. aureus exposed to aqueous, butanol, and dichloromethane was observed by FTIR (icolet iS50, Thermo Fisher).
4.14. Statistical analysis
Duncan's Multiple range test was used to measure the significance of three variants.
5. Conclusions
Current experiments divulged various chemicals in C. esculentus. Cyperus esculentus extracts, especially dichloromethane and butanol, showed potent anti-microbial activity against different microorganisms. C. esculentus extracts showed an anti-diabetic, anti-cholinesterase, anti-oxidant, antibiotic modifying effect, and anti-quorum sensing activity. SEM and FTIR results showed damage to the cell wall of S. aureus and the breakdown of functional groups with the formation of new functional groups, respectively, when bacteria were exposed to butanol and dichloromethane extracts, thus confirming the plant extracts' antimicrobial activities. C. esculentus extracts showed damage to the cell wall of S. aureus as observed in terms of pit formation and breakage in the cell membrane; it also showed a decrease in quorum sensing regulated virulence factors, which suggested the use of C. esculentus for controlling infections such as abscesses, furuncles, cellulitis, bloodstream infections, pneumonia caused due to pathogens which will ultimately save human beings from the sufferings caused by the pathogens. Thus, the present study confirmed C. esculentus as an alternative medicine against pathogenic microorganisms and other diseases.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Parvaze Ahmad Wani: Writing – original draft, Methodology, Formal analysis, Conceptualization. Lawal Aolat Omobolanle: Methodology, Investigation, Formal analysis, Conceptualization. Burhan Hamid: Methodology, Investigation, Formal analysis. Raheem Aishat Fayokemi: Methodology, Formal analysis. Kahkashan Perveen: Writing – original draft, Resources, Methodology, Data curation, Conceptualization. Najat A. Bukhari: Visualization, Validation, Methodology, Investigation. R.Z. Sayyed: Writing – review & editing, Writing – original draft, Methodology, Data curation, Conceptualization. Andrea Mastinu: Writing – review & editing, Supervision, Funding acquisition, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the support provided by Researchers Supporting Project Number RSP2024R358, King Saud University, Riyadh, Saudi Arabia and Crescent University management for the support in terms of providing different facilities.
Contributor Information
Parvaze Ahmad Wani, Email: wani1889@rediffmail.com.
Lawal Aolat Omobolanle, Email: lawal@gmail.com.
Raheem Aishat Fayokemi, Email: raheemaishat1983@gmail.com.
Kahkashan Perveen, Email: kperveen@ksu.edu.sa.
Najat A. Bukhari, Email: najatab@ksu.edu.sa.
R.Z. Sayyed, Email: sayyedrz@gmail.com.
Andrea Mastinu, Email: andrea.mastinu@unibs.it.
References
- 1.Shanmugam S., Bhavani P. Studies on the comparison of phytochemical constituents and antimicrobial activity of Curcuma longa varieties. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:573–581. [Google Scholar]
- 2.Haouam C., Boudiba S., Tamfu A.N., Kucukaydin S., Hanini K., Zohra H.F., Hioun S., Botezatu A.D., Ceylan Ö., Boudiba L., et al. Assessment of chemical composition and in vitro anti-oxidant, anti-diabetic, anti-cholinesterase, and microbial virulence-quenching effects of Salad burnet (Sanguisorba minor L.) Harvested from Algeria. Plants. 2023;12:4134. doi: 10.3390/plants12244134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adelaja B.A., Adebayo O.S., Adejoro M.A., Nwanguma E.I. Innovative food trends: species. Food Technol. 2008;43:102–106. [Google Scholar]
- 4.Mehtab P., Ali M.M., Mahbbob A., Faheem A., Pedro S.P.S., Manuela R.S. Two new phenolic compounds from Ficus rumphii and their antiproliferative activity. J. Nat. Prod. Res. 2014;28:646–652. doi: 10.1080/14786419.2014.891201. [DOI] [PubMed] [Google Scholar]
- 5.Kaur R., Kapoor K., Kaur H. Plants as a source of anticancer agents. J. Nat. Prod. Plant Resour. 2011;1:119–124. [Google Scholar]
- 6.Alain K.Y., Tamfu A.N., Kucukaydin S., Ceylan O., Pascal A.D.C., Félicien A.…Dinica R.M. Phenolic profiles, anti-oxidant, antiquorum sensing, antibiofilm, and enzyme inhibitory activities of selected Acacia species collected from Benin. Lebensm. Wiss. Technol. 2022;171 [Google Scholar]
- 7.Zhang S., Li P., Wei Z., Cheng Y., Liu J., Yang Y., Wang Y., Mu Z. Cyperus, Cyperus esculentus L. A review of its compositions, medical efficacy, antibacterial activity, and allelopathic potentials. Plants. 2022;11:1127. doi: 10.3390/plants11091127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harun-ur-Rashid M.D., Gafur M.A., Sadik M.G., Rahman M.A.A. Biological activities of a new acrylamide derivative from Ipomoea turpethum. Pakistan J. Biol. Sci. 2002;5:968–969. [Google Scholar]
- 9.Ouelbani R., Bensari S., Mouas T.N., Khelifi D. Ethnobotanical investigations on plants used in folk medicine in the regions of Constantine and Mila (North-East of Algeria) J. Ethnopharmacol. 2016;194:196–218. doi: 10.1016/j.jep.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Najjaa H., Arfa A., Ben Mathe A., Neffati M. vol. 3. Springer; Berlin/Heidelberg, Germany: 2017. (Medicinal and Aromatic Plants of the World–Africa). [Google Scholar]
- 11.Kabbaj F.Z., Meddah B., Cherrah Y., El M., Faouzi A. Ethnopharmacological profile of traditional plants used in Morocco by cancer patients as herbal therapeutics. Phytopharmacology. 2012;2:243–256. [Google Scholar]
- 12.Tlili H., Hanen N., Arfa A.B., Neffati M., Boubakri A., Buonocore D., Dossena M., Verri M., Doria E. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0213049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renne I.J., Tracy B.F. Disturbance persistence in managed grasslands: shifts in aboveground community structure and the weed seed bank. Plant Ecol. 2006;190:71–80. [Google Scholar]
- 14.Odoemelam S.A. Chemical composition and functional properties of conophur nut (Tetracarpidium Conophorum) flour. Int. J. Food Sci. Technol. 2003;38:719–734. [Google Scholar]
- 15.Chukwuma E.C., Soladoye M.O., Amusa N.A., Raji-Esan S.O., Taiwo A.A. Ethnobotanical survey of anticancer plants in Ogun State, Nigeria. Ann. Biol. Res. 2010;1:261–273. [Google Scholar]
- 16.Belewu M.A., Belewu K.Y. Comparative physico-chemical evaluation of tiger nut, soybean and coconut milk sources. Int. J. Agric. Biol. 2007;9:785–787. [Google Scholar]
- 17.Omenka C.A., Osuoha J.O. Antimicrobial potency of grapefruit seed extract on five selected pathogens. Nig. J. Microbiol. 2000;14:39–42. [Google Scholar]
- 18.Nickavar B., Abolhasani L., Izadpanah H. α-Amylase inhibitory activities of six salvia species. Iran. J. Pharm. Res. (IJPR) 2008;7:297–303. [Google Scholar]
- 19.Shettar A.K., Sateesh M.K., Kaliwal B.B., Vedamurthy A.B. In vitro anti-diabetic activities and GC-MS phytochemical analysis of Ximenia americana extracts. South Afr. J. Bot. 2017;111:202–211. [Google Scholar]
- 20.Li Y., Wen S., Kota B.P., Peng G., Li G.Q., Yamahara J., Roufogalis B.D. Punica granatum flower extract, a potent α-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats. J. Ethnopharmacol. 2005;99:239–244. doi: 10.1016/j.jep.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Ellman G.L., Courtney D.K., Andres V.J., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Act. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 22.Obuotor E.M., Elufioye T.O., Sennuga A.T., Agdedahunsi J.M., Adesanya S.A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some selected Nigerian medicinal plants. Rev. Bras. Farm. 2010;20:472–477. [Google Scholar]
- 23.Chovanova R., Maria M., Vaverkova S. In vitro antibacterial and antibiotic resistance modifying effect of bioactive plant extract on methicillin-resistant Staphylococcus epidermis. J. Microbiol. 2013;2013 doi: 10.1155/2013/760969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of anti-oxidant power: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 25.Magreault S., Jauréguy F., Carbonnelle E., Zahar J.R. When and how to use MIC in clinical practice? Antibiotiques. 2022;11:1748. doi: 10.3390/antibiotics11121748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boudiba S., Kucukaydin S., Tamfu A.N., Blaise K., Munvera A.M., Arab Y., et al. HPLC-DAD phenolic composition, antioxidant, anticholinesterase, antidiabetic and anti-quorum sensing properties of bitter kola (Garcinia kola) and kolanut (cola acuminata) Pharmacogn. Res. 2023;15(2):373–383. [Google Scholar]
- 27.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of anti-oxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 28.Garmana A.U., Sukandar E.Y., Fidrianny I. Activity of several plant extracts against drug-sensitive and drug-resistant microbes. Procedia Chem. 2014;13:164–169. [Google Scholar]
- 29.Oladipipo A.E., Saheed S., Abraham B.F. Four weeks daily dose oral administration assessment of Cyperus esculentusL. Aqueous extract on key metabolic markers of Wistar rats. Pharmacologia. 2016;7(2–3):125–136. [Google Scholar]
- 30.Banerjee A., Maji B., Mukherjee S., Chaudhuri K., Seal T. In Vitro Anti-diabetic and anti-oxidant activities of methanol extract of. Tinospora Sinensis. J. Appl. Biol. Biotechnol. 2017;5:61–67. [Google Scholar]
- 31.Ortega M.G., Agnese A.M., Cabera J.L. Anti-cholinesterase activity in an alkaloid extract of Huperzia Saururus. Phytomedicine. 2004;11:539–543. doi: 10.1016/j.phymed.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Tlili H., Hanen N., Arfa A.B., Neffati M., Boubakri A., Buonocore D., Dossena M., Verri M., Doria E. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinrich M., Barnes J., Gibbons S., Williamson E.M. first ed. Elsevier: Amsterdam, The Netherlands; Hungary: 2008. Fundamentals of Pharmacognosy and Phytotherapy. [Google Scholar]
- 34.Praveen K.P., Kumaravel S., Lalitha C. Screening of anti-oxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biochem. Res. 2010;4:191–195. [Google Scholar]
- 35.Sree N.V., Sri P.U., Aswani Kumar Y.V.V., Rama Rao N. In-vitro anti-oxidant and antimicrobial activities of some medicinal plants grown in western Ghats of India. IOSR J. Pharm. 2013;4:25–33. [Google Scholar]
- 36.Husain F.M., Ahmad I., Asif M., Tahseen Q. Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J. Bio. Sci. 2013;38:835–844. doi: 10.1007/s12038-013-9385-9. [DOI] [PubMed] [Google Scholar]
- 37.Kessler E., Safrin M., Olson J.C., Ohman D.E. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J. Biol. Chem. 1993;268:7503–7508. [PubMed] [Google Scholar]
- 38.Singh A.V., Bansod G., Mahajan M., Dietrich P., Singh S.P., Rav K., Thissen A., Bharde A.M., Rothenstein D., Kulkarni S., et al. Digital transformation in toxicology: improving communication and efficiency in risk assessment. ACS Omega. 2023;8:21377–21390. doi: 10.1021/acsomega.3c00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wani P.A., Tolu A.M., Wahid S. Anti-oxidant, antimicrobial and antibiotic resistance modifying effect of Heliotropium indicum. Biocatal. Agric. Biotechnol. 2018;15:113–118. [Google Scholar]
- 40.Eisenberg D.M., Davis R.B., Ettner S.L., Appel S., Wilkey S., Van R.M., Kessler R.C. Trends in alternative medicine use in the United States. Results of a follow-up national survey. JAMA. 1997;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 41.Khurm M., Chaudhry B.A., Uzair M., Janbaz K.H. Antimicrobial, cytotoxic, phytotoxic and anti-oxidant potential of Heliotropium strigosum. Willd. Medicines. 2016;3:20. doi: 10.3390/medicines3030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Essar D.W., Eberly L., Hadero A., Crawford I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Husain F.M., Ahmad I., Al-thubiani A.S., Abulreesh H.H., Al Hazza I.M., Aqil F. Leaf extracts of Mangifera indica L. inhibit quorum sensing–Regulated production of virulence factors and biofilm in test bacteria. Front. Microbiol. 2017;8:727. doi: 10.3389/fmicb.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.