Abstract
Background:
Normal brain development, mood, and cognitive functions depend on thyroid hormone (TH) action. However, little is known about how TH mediates its actions in the human brain. This is due to limited access to human brains deprived of TH during fetal and early postnatal life, as well as from adults with altered thyroid status. One way to partially bypass these limitations is by using magnetic resonance imaging and spectroscopy, two neuroimaging techniques that provide detailed, noninvasive information on human brain structure and function. Another way is using human-induced pluripotent stem cell (hiPSCs)-derived three-dimensional in vitro systems, known as brain organoids, which allow for the study of fundamental aspects of the early stages of human brain development.
Summary:
This narrative review focuses on neuroimaging and brain organoid studies. Neuroimaging of human brains performed in individuals with different thyroid conditions provides information on the volume, myelination, blood flow, neural activity, and connectivity of different areas. Such studies show that suboptimal thyroid status can impact human brain development and its normal function throughout life. This is true not only for patients with sporadic congenital hypothyroidism, during pregnancy or early after birth, but also for adult patients with hypo- or hyperthyroidism, patients carrying mutations that manifest as impaired sensitivity to TH, and even for normal individuals during aging. Studies using brain organoids generated from hiPSCs of healthy individuals or patients with thyroid genetic conditions provide insights into how TH can impact the early development of the human cerebral cortex.
Conclusions:
The developmental alterations in children born to mothers with different degrees of gestational hypothyroidism or who developed hypothyroidism early in life are remarkable, affecting multiple brain regions and pathways, including the cerebral cortex, hippocampus, cerebellum, interhemispheric and corticospinal tracts, and associative nuclei. The data connecting such changes to poor neurological outcomes in adult patients with hypothyroidism represent an objective link between thyroid-specific functional brain alterations and behavior. Growing brain organoids require TH, which is critical for human neurogenesis and oligodendrogenesis. These models have proven useful in screening drugs with potential therapeutic effects for patients with genetic thyroid diseases.
Keywords: MRI, brain development, congenital hypothyroidism, resistance to thyroid hormone, brain organoids
Introduction
The fact that the human brain responds to thyroid hormone (TH) and that TH is important for normal human brain development are well-established concepts. However, with a few exceptions,1,2 these conclusions are based on observational studies of manifested symptoms of hypothyroidism and hyperthyroidism, as well as the assessment of cognitive outcomes of children born with congenital hypothyroidism.3–7 There is a large volume of literature focused on the brain of animal models (mostly mice and rats), and indeed, these animals with congenital hypothyroidism exhibit many brain alterations, including smaller size, thinner cerebral cortices with decreased blood vessel networks, hypomyelinated interhemispheric commissures, and arrested migration and growth of neural cells.7–13 However, the brain cytoarchitecture differs greatly between humans and mice,14 making these models poorly applicable to humans. Thus, having access to the brains of humans with thyroid dysfunction would be the ultimate resource for scientists who study TH and the brain, but the invasive nature of the available methods limits such an approach.
To overcome this limitation, noninvasive studies can use magnetic resonance imaging (MRI) to visualize the human brain. Routinely acquired anatomical MRIs (Fig. 1) create images of the brain with a resolution of less than 1 mm and can distinguish brain areas with different water content, discriminating between grey and white matter (less water content in the latter15) and even degrees of myelination. Anatomical images are also used to measure the thickness and volume of brain regions, which may be influenced by changes in the number and size of neural cells in these areas16 and other physiological factors.17 MRI diffusion tensor images (DTI) detect changes in the freedom of water movement derived from changes in the brain structure and can produce images reflecting the organization of axonal bundles, as in the white matter tracts.18 The availability of functional imaging techniques (fMRI) makes it possible to measure the energy demand of activated brain areas by detecting changes in the blood’s oxygen levels.19 Moreover, magnetic resonance spectroscopy (MRS) can measure changes in the concentration of critical brain metabolites in vivo, allowing the detection of neuronal damage or demyelination.20
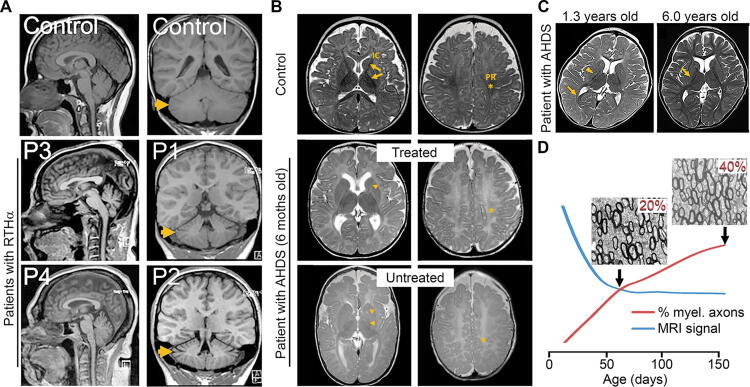
FIG. 1.
Examples of studies using anatomical MRIs. (A). T1-weighted MRIs of patients with RTHα show microcephaly and an increase in the CSF space around the cerebellum and between folia (arrows), denoting reduced cerebellar size (modified from49) (B). T2-weighted MRIs of patients with AHDS obtained at six months of age. Compared to controls, the in-utero-treated patient with AHDS showed mildly delayed myelination (arrowheads and asterisks) that was more severe in the untreated brother. This is evident in the perirolandic area (PR; asterisk) and posterior and anterior internal capsule (IC; arrowheads). The abnormal head shape in the untreated brother suggests severe hypotonia, which may be caused by difficulty moving his head and persistently lying on the temporal side of the head (modified from22) (C). T2-weighted MRIs of a patient with AHDS depicting an area of delayed myelination at 1.3 years (arrowheads) that looks normal at 6 years (arrow; modified from68) (D). Profiles show how the T2-weighted MRI signal (blue line) cannot detect a late-postnatal increase in the percentage of myelinated axons (20% to 40%; red line; modified from9). AHDS, Allan–Herndon–Dudley syndrome; MRIs, magnetic resonance imaging; RTHα, resistance to thyroid hormone alpha.
Unfortunately, neuroimaging studies do not provide direct mechanistic insight into how TH acts in the brain and are insufficient to study the role of TH during the early stages of brain development. To overcome this hurdle, a possible approach is the use of human-induced pluripotent stem cell (hiPSCs)-derived three-dimensional neural cultures, so-called human brain organoids. As the name implies, a human brain organoid is not the same as a human brain, but it can be considered a reductionist model that allows the study of some of the early stages of fetal brain development.
This narrative review presents neuroimaging and brain organoid studies and aims to build a comprehensive catalog relating brain alterations to thyroid conditions and neurological outcomes. The review criteria included a search for original articles published in English until February 2024 using PubMed with the following search terms: “thyroid hormones” and “magnetic resonance imaging or spectroscopy.” The search terms “resistance to thyroid hormone,” “Allan–Herndon–Dudley,” “hypothyroidism,” “hyperthyroidism,” and “organoids” were also used alone or in combination with “brain.” The reference lists of identified papers were also used to identify additional material.
Disruption of TH Action Early in Life
This review intends to distinguish between congenital and adult hypothyroidism. Congenital hypothyroidism can be endemic or sporadic. Endemic is caused by iodine deficiency. In contrast, sporadic hypothyroidism is caused by a genetic abnormality of the fetus, impairing thyroid gland development or hormone biosynthesis. The former is almost always severe because it affects both mother and fetus, while the latter form is usually mild or even asymptomatic because the fetal thyroid gland malfunction is minimized by the placental transfer of TH and prompt postnatal treatment.24,25
Pregnancy and neonatal period
An example of how devastating endemic congenital hypothyroidism can be is illustrated in a unique brain MRI study of three patients with mental deficiency, deaf mutism, and a spastic-rigid motor disorder (aged 33–39) showing alterations in two connected regions: the substantia nigra and globus pallidus,26 which correlate well with their motor disorders. Two additional neuroimaging studies of patients (6–16 years old) with late-treated sporadic congenital hypothyroidism described mild cerebral cortical atrophy in the frontal and parietal lobes27 and elevated choline-containing compounds and N-acetylaspartate in the hippocampus, cerebellum, and temporal lobe, suggesting abnormal myelination and neural function. Remarkably, the level of these metabolites reverted to normal with TH replacement.27
The outcome of a child with sporadic congenital hypothyroidism depends on the severity of the thyroid defect. In severe cases, neurological symptoms may persist even when the diagnosis is made at birth.28 In all cases, time is of the essence, and a delayed diagnosis of sporadic congenital hypothyroidism, even mild forms, may result in neurological abnormalities.29 Alterations in the brains of children with sporadic congenital hypothyroidism who started receiving treatment between the second and fourth weeks of life include reduced function and volume of the hippocampus and disruptive associative processing.30–33 Interestingly, music lessons ameliorated the hippocampal reductions,31 suggesting structural neuroplasticity.34 Sporadic congenital hypothyroidism is also associated with changes in the thickness of cortical areas, leading to altered language, motor, auditory, and visual functions,35 and with structural abnormalities in their white matter tracts—despite timely and adequate treatment.36 Conversely, one study found no alterations in MRI brain examination or myelination in this population.37
Less severe alterations are expected in children born to mothers with poorly controlled gestational hypothyroidism. Both low and high maternal free thyroxine (FT4) levels during pregnancy have been associated with low child’s (∼9 years old) IQ score and reduced total grey matter and cortex volume38 (representative studies associating abnormal thyroid status with MRI brain alterations and neurological outcomes are shown in Table 1). A similar association exists between maternal TSH and their children’s total brain and cortical grey matter volume.45 In another study, the maternal FT4 has also been associated with changes in the male offspring’s (∼26 years old) DTI values in three projecting fibers: (i) the corticospinal tracts (motor control), (ii) the anterior and superior thalamic radiations, (memory and limbic system and sensory and motor information, respectively), and (iii) the forceps minor of the corpus callosum (the biggest interhemispheric commissure of the brain, helping integrate sensory, motor, and cognitive information).39
Table 1.
Representative Studies Associating Abnormal Thyroid Status With MRI Brain Alterations and Neurological Outcomes
| Exposure | Subjects (age in years) | MRI | Brain alterations | Neurological outcome | Ref |
|---|---|---|---|---|---|
| High/low FT4 levels during Pregnancy | 649 child-mother pairs (6–10) |
T1-w | Reduced volume of the cerebral cortex | Reduced IQa | 38 |
| 114 males 172 females (23 ± 33) |
DTI | Only in males: reduced water diffusivity (FA) in the corticospinal tract and forceps minor of the corpus callosum |
No differences in the participants’ IQa | 39 | |
| Poorly controlled gestational hypothyroidism | 22 exposed 22 controls (10 ± 6 years) |
T1-w | Smaller corpus callosum genu and splenium |
Attention difficulties and worse vocabulary performance | 40 |
| 24 exposed 30 controls (9–12 years) |
T1-w | Reduced volume in the hippocampus | Altered functioning and verbal associative memory | 30 | |
| children with sporadic congenital hypothyroidism | 14 exposed 14 controls (13 ± 1 years) |
fMRI | Abnormal hippocampal function | Worse verbal memory processing | 33 |
| Induced subclinical hypothyroidism | 15 exposed (19–61) |
rs-fMRI | Reduced connectivity in the cuneus | Longer reaction times and less accuracy in working memory tasks | 41 |
| Hypothyroidism | 92 exposed 40 controls (26–50) |
T1-w fMRI |
Decreased volume and functional connectivity of the hippocampus | Alterations in cognitive and emotional scale scores | 42 |
| Hypothyroidism | 13 exposed 12 controls (29 ± 7) |
fMRI | Decreased neural activity in the bilateral medial prefrontal cortices, posterior cingulate cortices, and left inferior partial lobule. Regions are part of the default mode network | Poor memory states and slight working memory deficit. | 43 |
| Hyperthyroidism | 28 exposed 28 controls (27–40) |
rs-fMRI | Decreased functional connectivity in the sensorimotor, frontotemporal, and parietal lobes. | Decline in visual retention, object recognition, mental balance, and performance on neuropsychological tests | 44 |
| Patients with RTHβ | 21 RTHβ 21 controls (39 ± 15) |
T1-w DTI |
Reduced water diffusivity (FA) in the corticospinal tract, increased cortical thickness in the parietal cortex, and decreased grey matter volume in the temporal cortex and thalamus | Correlation between MRI alterations and the attention deficit hyperactivity disorder subscales I and II | 21 |
Compared to subjects exposed during pregnancy to FT4 levels within the normal range. T1-w, T1-weighted; rs-fMRI, resting-state functional magnetic resonance imaging; DTI, diffusion tensor images; FA, fractional anisotropy; FT4, free thyroxine.
In other studies of children born to mothers exhibiting elevated TSH values and autoimmune thyroiditis, children (∼10 years old) exhibited abnormal development of the corpus callosum.40 Their anterior corpus callosum, which connects regions involved in attention, was smaller and correlated with increased difficulties in shifting attention. The posterior corpus callosum, which connects areas involved in forming and storing language information (e.g., Wernicke’s area), was larger and was inversely correlated with vocabulary performance. Two follow-up studies demonstrated that in this scenario, maternal TSH values are associated with changes in the thickness of the cerebral cortex within multiple brain regions and reductions in the offspring’s hippocampus. The latter plays a central role in memory, and these patients also obtained lower scores in memory indices.30,46 In addition, DTIs of preterm infants (36–41 weeks) with subclinical hypothyroidism (elevated circulating TSH with FT4 in reference range) showed shorter thalamocortical axon fibers lengths in the bilateral superior temporal and Heschl’s gyrus (auditory processing), lingual gyrus, the calcarine cortex (visual cortex/processing), and the cuneus.47
Impaired sensitivity to thyroid hormone
Defects at different steps along the pathway leading to TH action at the cellular level48 can manifest as impaired sensitivity to TH. A peculiarity is that, albeit specific to some organs and cells, the defect has been present in the fetus since conception. Thus, these defects have the potential to affect the early stages of fetal brain development and cause severe neurological damage.
Resistance to thyroid hormone alpha
Pathogenic mutations in the THRA gene cause resistance to TH alpha (RTHα), and patients exhibit growth retardation with skeletal dysplasia and mild-to-moderate intellectual disability, notably affecting nonverbal IQ and sensorimotor processing. MRI shows reduced cerebellar volume and microencephaly in two adult cases49 (Fig. 1A) and DTI in two young patients (9 and 13 years old), shows a global increase in the mean water diffusivity in white matter tracts, including those connecting the cerebellum to the neostriatum and the cerebral cortex. These patients also exhibit reduced N-acetylaspartate in the frontal white matter and thalamus. There is also one study describing a 2-year-old girl with RTHα presenting neurological manifestations and normal brain MRIs.50 In the same study, a different girl with RTHα who was born prematurely presented altered myelination but no morphological abnormalities. Other cases exhibit general brain atrophy with the widening of the sulci (fissures) and normal ventricles.51
Resistance to thyroid hormone beta
About one-half of subjects with resistance to TH beta (RTHβ; caused by dominant loss-of-function mutations in the THRB gene) have a learning disability, mental retardation (IQ <60) is present in ∼3% of cases,52 and ∼50% are diagnosed with attention deficit hyperactivity disorder.53 Notwithstanding, the course of the condition is variable, and most individuals achieve normal stature and development and lead a normal life at the expense of high TH levels and a slight thyroid gland enlargement. In others, especially those with biallelic mutations, low stature, hyperactivity, and intellectual impairment persist.
There are three MRI studies of the brains of these patients. One scanned 43 subjects with RTHβ54 and described dramatic alterations consisting of extra or missing gyri (the bumps and ridges on the cerebral cortex) in the parietal bank of the Sylvian fissure and multiple Heschl’s transverse gyri. These areas are involved in auditory and language function and, thus, could contribute to the common, albeit variable, language disorders or the much less common hearing loss observed in patients with RTHβ.55 However, these alterations were not present in a second study that scanned 21 patients with RTHβ.21 Instead, they describe alterations in the corticospinal tract, an increased cortical thickness in the bilateral superior parietal cortex, and a decreased grey matter volume in the bilateral inferior temporal cortex and thalamus. These patients obtained higher scores in a self-rating questionnaire for attention deficit hyperactivity disorder, and a certain degree of correlation is described between the MRI alterations and these behavioral results. The third one is a case report of a severe case of RTHβ in a 22-year-old female with MRI evidence of demyelination and bilateral ventricular enlargement,56 alongside neurological symptoms such as deafness, hypotonia, mental retardation, visual impairment, and a history of seizures. The clinical implications of having RTHβ also include negative pregnancy outcomes,24,57 but we do not know if the brains of the progeny of mothers with RTHβ are affected.
Defects in TH cell membrane transporters
Different proteins can carry TH through cell membranes. Among them, the monocarboxylate transporter 8 (MCT8) is a potent and specific TH transporter, particularly important in supplying TH to the human brain. Loss-of-function mutations in the SLC16A2 gene (encoding MCT8) produce TH deprivation in the brain, with a paradoxical increase in TH levels in peripheral tissues. The result is Allan–Herndon–Dudley syndrome (AHDS), and patients present TH abnormalities accompanied by severe and irreversible neurological deficits and hypermetabolism.58–61 Moreover, with advancing age, microcephaly becomes apparent.62
While the brains of patients with AHDS exhibit normal gross anatomy, alterations in neural populations and hypomyelination are well documented in a landmark study of a post-mortem examination of brain sections of a 30-gestational week fetus and an 11-year-old boy with AHDS.2 MRIs from patients with AHDS show hypomyelination during the first years of life (Fig. 1B), sometimes extending into early adulthood.23,63,64 For instance, a report presented a 6-year-old patient with hypomyelinated axonal tracts, including the subcortical U-fibers and periventricular tracts.64 Alterations in the latter are also described in three other studies.65–67 In some cases, however, hypomyelination improves with age2,64,68,69 (Fig. 1C), making it unclear whether hypomyelination in these patients persists into adulthood.70 Deficits in myelination may not be detected in adult patients due to a lack of MRI sensitivity, as illustrated in two preclinical studies (using MRIs much more sensitive than the ones used in clinical settings) in which the MRI signal could not detect a ∼20% increase in the percentage of myelinated axons in two interhemispheric commissures in adult rats (Fig. 1D).8,9 Also, DTI alterations in the cerebral cortex and striatum are described in 11 patients (3–13 years old) with AHDS, and a reduction in the cerebral blood flow in the bilateral frontal cortex and the cerebellum is described in two patients.71 The latter can be partly influenced by an altered blood vessel network.72 In addition, MRS detected an increase in choline and myoinositol and a decrease in N-acetylaspartate levels in the supraventricular grey and white matter of patients with AHDS.73
Another example supporting the clinical relevance of suffering suboptimal TH transport into the brain is the juvenile neurodegeneration and cerebral hypometabolism detected in the case of a 15-year-old patient harboring a missense mutation in the gene codifying for the TH cell membrane organic anion transporter polypeptide 1C1.74 Notwithstanding, the relevance of this transporter for TH uptake by human brain cells is unclear since its expression in the blood-brain barrier is low,75,76 and its role in other expressing neural cells, including astrocytes and tanycytes,77 is undetermined.
Defects in TH metabolism
In the brain, deiodinases activate (type 2 deiodinase [D2]) or inactivate (D3) TH.78 D2 is expressed in astrocytes and tanycytes,79,80 whereas D3 is expressed in neurons.81 Alterations in the function of these enzymes can unbalance TH action in the brain.78 The Thr92Ala-DIO2 single nucleotide polymorphism reduces D2 catalytic activity,82 leading to hypothyroidism in distinct brain areas in mice.83 This defect in the D2 pathway has been linked to a higher proneness to suffering anxiety or depression.84 Still, no MRI alterations have been described in this population.85 Deiodinases are selenoproteins, and the rare selenocysteine amino acid is located in the catalytic active center of the enzymes, making it critical for enzymatic activity. Patients with loss-of-function mutations in the SBP2 gene, an important protein involved in the synthesis of all selenoproteins (including the deiodinases), exhibit severe neurological abnormalities, including impaired mental and motor coordination development and hearing loss;86,87 however, routine MRIs have failed to detect brain alterations in these patients.
Disruption of TH Action in Adult Life
Hypothyroidism
Fortunately, with appropriate treatment, the effects of thyroid dysfunction in the adult brain are notable but reversible, so one would not expect to find severe permanent structural alterations in these patients. According to this hypothesis, subtle alterations in the white matter tracts, including the cingulum, the corpus callosum, and the corticospinal tracts,88–90 have been described in patients who also performed worse in cognitive assessments. Moreover, the concentration of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) is also reduced in the medial prefrontal and posterior cingulate cortices of adult patients with hypothyroidism. Still, the GABA levels normalized when patients received adequate levothyroxine (L-T4) treatment. A reduction in GABA is likely to cause an imbalance between the inhibitory and the excitatory brain networks,91 which may result in many neurological symptoms, including mood alterations. Of note, patients on L-T4 exhibited fewer depressive symptoms and better memory function.92
Similarly, the reduced levels of glutamate and myoinositol in the hippocampus of these patients reverted to normal values after adequate treatment.93 Along these lines, two fMRI studies41,43 detected a reduced connectivity between the cuneus and (i) the cerebellum (ii) the medial prefrontal cortex, and (iii) bilateral angular gyri, and in the posterior cingulate cortices (among other regions). This brain network is important for cognitive and self-awareness processes, and indeed, these alterations were accompanied by slightly longer reaction times and less accuracy in working memory tasks. A decrease in the global brain blood perfusion is also associated with high and low FT4 levels, suggesting that thyroid dysfunction could lead to stroke or dementia through suboptimal brain circulation.94 However, a population study failed to associate TSH values (high/low) with cerebral small vessel disease.95
A few studies found changes in the volume of the brain of patients with hypothyroidism. One described reduced total cerebellar and subcortical volumes of areas that regulate motor, cognitive, and affective function.96 Another two studies describe a volume reduction in different areas of the hippocampus,97 alongside significant alterations in cognitive and emotional scale scores.42 Other brain areas may present decreased grey matter volumes and reduced activity, including the prefrontal and cingulate cortex, precuneus, and insula, which may lead to longer reaction times and lower performance accuracy,98 suggesting impaired attentional/executive function in these patients. A study of patients with newly diagnosed hypothyroidism demonstrates a reduction in the cerebral cortex volumes in five regions, including the middle frontal gyrus and the supplementary motor area;99 other areas also exhibit elevated activity. Similar results were found in patients with subclinical hypothyroidism, presenting reductions in the volume of the cerebral cortex in some areas of the frontal, central, and occipital gyrus100 and elevated activity in some of these areas. In agreement, patients with major depressive disorders comorbid with subclinical hypothyroidism (high TSH vs healthy euthyroid controls)101,102 exhibited reduced grey matter volume in areas including the left rectus and middle frontal gyrus and performed worse than controls in executive function tests.
Hyperthyroidism
Adult hyperthyroidism is mainly caused by autoimmune Grave’s disease, which involves hyperactivity of the thyroid gland, leading to high levels of circulating TH.103,104 Cognitive impairment is common in adult patients with hyperthyroidism.105 Compared to controls, adult patients with hyperthyroidism exhibited grey matter volume reductions in several brain regions, including the hippocampus, the left temporal pole,106 the amygdala,107 and the sulci.108 Differently, one study found no reductions in the hypothalamus volume and limbic structures in these patients.108 DTI parameters are also altered in white matter tracts that connect distal areas of the brain,44,109 and one study shows altered functional connectivity between several brain networks.102 In this study, cognitive functions such as visual retention, recognition of objects, and performance on neuropsychological tests were also reduced. Remarkably, after these patients received an anti-thyroid treatment (carbimazole), they improved the connectivity in the frontoparietal network (other networks remained altered), and some of the memory, executive, visuospatial, and motor functions.110 More studies have described changes in the functional connectivity between different brain regions,111–114 including one that experimentally made the participants thyrotoxic (250 g L-T4 for 8 weeks), which caused increased connectivity between the left temporal pole and different parts of the cerebral cortex.115
A compilation of studies has examined the brains of patients with Grave’s disease presenting ophthalmopathy and found alterations in the volume and connectivity between different brain areas116–120 and the neurovascular system.121 A caveat of these studies is that this autoimmune condition involves various autoantigens and antibodies that can affect these patients’ optic nerves and brains,122 making it difficult to attribute a causal relationship between the neuroimaging alterations and their TH status.
Aging
The prevalence of minor abnormalities in serum TSH and TH concentrations is common among elderly individuals. Cognitive impairments are particularly severe in older patients with hypothyroidism, presenting dementia and confusion in 33% and 18% of the cases.123 Therefore, the possibility of an age-dependent association of thyroid function with brain alterations has been explored. For instance, an association between higher FT4 levels and MRI of ∼4600 participants aged 45 to 90 showed that the total intracranial and total brain and white matter volumes increased in younger individuals but decreased in the older group.124 TSH levels in the high normal range are also associated with cortical atrophy and a higher proportion of infarct-like vascular lesions in male subjects aged 65–83.125 Similarly, in ∼900 subjects aged 60 to 90, higher FT4 and reverse T3 were associated with a reduced hippocampus and amygdala volume.126
Brain Organoids to Study TH Action in the Developing Human Brain
Human brain organoids recapitulate the gene expression programs of the fetal neocortex127 and allow the study of fundamental aspects of its early development (this explains why they are often referred to as cerebral organoids). Although determined empirically, brain organoids seem to mirror the fetal cortex at gestational weeks 6.5–14.128 However, long-term maturation (∼300 days in vitro) allows brain organoids to mature beyond the late mid-fetal stages.129
Common to current hiPSCs-derived brain organoid protocols is that to promote the induction, proliferation, and maturation of the neural cell lineage, brain organoids require the addition of supraphysiological amounts of T3 to the medium.128,130 Even higher doses of T3 for more extended periods are required to generate brain organoids containing robust populations of oligodendrocytes.131,132 The induction cocktail also includes insulin-like growth factor 1 and platelet-derived growth factor-AA, which act downstream of the T3 signaling pathway.133,134 These requirements represent a limitation to studies on the involvement of specific TH transporters in cellular processes. For instance, human oligodendrocytes express MCT8 with no other TH transporter to compensate for MCT8 deficiency,135,136 but with the use of the current protocols, MCT8 mutations do not affect the in vitro differentiation of hiPSCs into oligodendrocytes.137
An array of hiPSCs derived from patients with thyroid genetic conditions, including mutant THRB, THRA, and SLC16A2 (encoding MCT8), are currently available49,138–140 and can be used to generate brain organoids. Such disease-specific brain organoids can then be used to explore mechanistic explanations for the pathophysiology of these disorders. Following this approach, MCT8-deficient brain organoids—from hiPSCs obtained from corresponding patients—have demonstrated that MCT8 mediates the bulk of T3 transport in developing neural cells,141 providing evidence that the role of this transporter in the human brain goes beyond facilitating the passage of TH through the blood-brain barrier.140 This also seems to be the case in neurons, as demonstrated in a mouse study showing that, partly facilitated by MCT8, TH can act in these cells, entering hidden inside endosomes that act akin to a Trojan horse—protecting TH from degradation from the axonal termini to the nucleus.81 Due to the altered T3 transport, MCT8-deficient brain organoids exhibit altered neurogenesis,141 pointing to an MCT8-mediated TH transport into the neural precursor cells (the source of most human cortical neurons142) that can trigger developmental programs in these cells.143 This is supported by the fact that MCT8 is expressed in neural precursor cells in fetal brains and brain organoids.77,144,145 Further evidence comes from a study that used hiPSCs from patients with RTHα showing premature neurogenesis and neural precursor cell depletion.49
Regulation of gene expression is central to our understanding of how TH acts in the developing brain, but most of what we know is inferred from mouse studies.79,146–151 Brain organoids have shown that TH in these models regulates genes critical for cerebral cortex development.141 Many of these genes are altered in MCT8-deficient brain organoids, making the case that TH action could be severely altered during the neurodevelopment of these patients.
In addition, studies on MCT8-deficient brain organoids have validated the TH-analogs 3,5-diiodothyropropionic acid and 3,3’,5-triiodothyroacetic acid as treatments that can elicit similar responses as TH in human MCT8-deficient neural cells.141 These findings are exciting, considering that treating intraamniotically with a high dose of L-T4 a fetus with MCT8-deficiency from gestational age of 18 until birth at 35 weeks22 improved myelination (Fig. 1B) and neuromotor and neurocognitive function. The results from MCT8-deficient brain organoids support the idea that early prenatal treatment with TH analogs that can be available to a fetus when given to the mother152 might further rescue the AHDS phenotype.
Conclusion
Neuroimaging studies strengthen the concept that the human brain responds to TH and that TH is important for human brain development. The alterations in children born to mothers with different degrees of gestational hypothyroidism or who developed hypothyroidism early in life are noteworthy, affecting multiple brain regions and circuits. The cerebral cortex, hippocampus, cerebellum, interhemispheric and corticospinal tracts, and associative nuclei are among the most sensitive ones. The data connecting such changes to poor neurological outcomes in adult patients with hypothyroidism represent an objective link between thyroid-specific functional brain alterations and behavior. Neuroimaging studies prove that alterations in the brain are in play in the syndromes of impaired sensitivity to TH. However, many of the studies reviewed here are based on a small number of subjects (partly due to the rare clinical occurrence of some conditions), and prospective investigations need to include more subjects with detailed thyroid function test monitoring and experimental paradigms to evaluate neurological outcomes.
An approach for studying the mechanisms of TH action in the developing human brain constitutes a “holy grail” for the field. Brain organoids fit this purpose and have highlighted the importance of TH for early human neurogenesis, cerebral cortex maturation, and myelination. Observations in specific brain organoids modeling thyroid genetic diseases carry significant physiological implications and constitute a new groundwork for developing new treatments. Many scientific efforts aim to improve the protocols and experimental designs necessary to level up the current neuroimaging and stem cell technologies. Future advances will transform the way we investigate how TH orchestrates human brain development and regulates its function through life—ultimately helping to improve the treatment options for patients with hypothyroidism or inherited thyroid diseases.
Acknowledgments
The author thanks Profs. Samuel Refetoff and Antonio Bianco for their critical reading of the article, and Deb Werner for her assistance with some aspects of the search strategy.
Author Contribution
F.S.-L. is solely responsible for writing, editing, and approving this review article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
National Institutes of Health grants DK15070 and DK58538 support F.S.-L.
References
- 1. Marcelino CP, McAninch EA, Fernandes GW, et al. Temporal pole responds to subtle changes in local thyroid hormone signaling. J Endocr Soc 2020;4(11):bvaa136; doi: 10.1210/jendso/bvaa136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. López-Espíndola D, Morales-Bastos C, Grijota-Martínez C, et al. Mutations of the thyroid hormone transporter MCT8 cause prenatal brain damage and persistent hypomyelination. J Clin Endocrinol Metab 2014;99(12):E2799–804; doi: 10.1210/jc.2014-2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hegedüs L, Bianco AC, Jonklaas J, et al. Primary hypothyroidism and quality of life. Nat Rev Endocrinol 2022;18(4):230–242; doi: 10.1038/s41574-021-00625-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ettleson MD, Raine A, Batistuzzo A, et al. Brain fog in hypothyroidism: Understanding the patient’s perspective. Endocr Pract 2022;28(3):257–264; doi: 10.1016/j.eprac.2021.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casula S, Ettleson MD, Bianco AC. Are we restoring thyroid hormone signaling in levothyroxine-treated patients with residual symptoms of hypothyroidism? Endocr Pract 2023;29(7):581–588; doi: 10.1016/j.eprac.2023.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joffe RT, Pearce EN, Hennessey JV, et al. Subclinical hypothyroidism, mood, and cognition in older adults: A review. Int J Geriatr Psychiatry 2013;28(2):111–118; doi: 10.1002/gps.3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madeira MD, Pereira A, Cadete-Leite A, et al. Estimates of volumes and pyramidal cell numbers in the prelimbic subarea of the prefrontal cortex in experimental hypothyroid rats. J Anat 1990;171:41–56. [PMC free article] [PubMed] [Google Scholar]
- 8. Salas-Lucia F, Pacheco-Torres J, González-Granero S, et al. Transient hypothyroidism during lactation alters the development of the corpus callosum in rats. An in vivo magnetic resonance image and electron microscopy study. Front Neuroanat 2020;14:33; doi: 10.3389/fnana.2020.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lucia FS, Pacheco-Torres J, González-Granero S, et al. Transient hypothyroidism during lactation arrests myelination in the anterior commissure of rats. A magnetic resonance image and electron microscope study. Front Neuroanat 2018;12:31; doi: 10.3389/fnana.2018.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang L, Cooper-Kuhn CM, Nannmark U, et al. Stimulatory effects of thyroid hormone on brain angiogenesis in vivo and in vitro. J Cereb Blood Flow Metab 2010;30(2):323–335; doi: 10.1038/jcbfm.2009.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishii S, Amano I, Koibuchi N. The role of thyroid hormone in the regulation of cerebellar development. Endocrinol Metab (Seoul) 2021;36(4):703–716; doi: 10.3803/EnM.2021.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bárez-López S, Guadaño-Ferraz A. Thyroid hormone availability and action during brain development in rodents. Front Cell Neurosci 2017;11:240; doi: 10.3389/fncel.2017.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O’Shaughnessy KL, Thomas SE, Spring SR, et al. A transient window of hypothyroidism alters neural progenitor cells and results in abnormal brain development. Sci Rep 2019;9(1):4662; doi: 10.1038/s41598-019-40249-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat Rev Neurosci 2009;10(10):724–735; doi: 10.1038/nrn2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whittall KP, MacKay AL, Graeb DA, et al. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med 1997;37(1):34–43; doi: 10.1002/mrm.1910370107 [DOI] [PubMed] [Google Scholar]
- 16. Asan L, Falfán-Melgoza C, Beretta CA, et al. Cellular correlates of gray matter volume changes in magnetic resonance morphometry identified by two-photon microscopy. Sci Rep 2021;11(1):4234; doi: 10.1038/s41598-021-83491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zahid U, Hedges EP, Dimitrov M, et al. Impact of physiological factors on longitudinal structural MRI measures of the brain. Psychiatry Res Neuroimaging 2022;321:111446; doi: 10.1016/j.pscychresns.2022.111446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soares JM, Marques P, Alves V, et al. A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci 2013;7:31; doi: 10.3389/fnins.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glover GH. Overview of functional magnetic resonance imaging. Neurosurg Clin N Am 2011;22(2):133–139, vii; doi: 10.1016/j.nec.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soares DP, Law M. Magnetic resonance spectroscopy of the brain: Review of metabolites and clinical applications. Clin Radiol 2009;64(1):12–21; doi: 10.1016/j.crad.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 21. Rogge B, Heldmann M, Chatterjee K, et al. Changes in brain structure in subjects with resistance to thyroid hormone due to THRB mutations. Thyroid Res 2023;16(1):34; doi: 10.1186/s13044-023-00176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Refetoff S, Pappa T, Williams MK, et al. Prenatal treatment of thyroid hormone cell membrane transport defect caused by. Thyroid 2021;31(5):713–720; doi: 10.1089/thy.2020.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tonduti D, Vanderver A, Berardinelli A, et al. MCT8 deficiency: Extrapyramidal symptoms and delayed myelination as prominent features. J Child Neurol 2013;28(6):795–800; doi: 10.1177/0883073812450944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salas-Lucia F, Stan MN, James H, et al. Effect of the fetal THRB genotype on the placenta. J Clin Endocrinol Metab 2023;108(10):e944–e948; doi: 10.1210/clinem/dgad243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vulsma T, Gons MH, de Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med 1989;321(1):13–16; doi: 10.1056/NEJM198907063210103 [DOI] [PubMed] [Google Scholar]
- 26. Ma T, Lian ZC, Qi SP, et al. Magnetic resonance imaging of brain and the neuromotor disorder in endemic cretinism. Ann Neurol 1993;34(1):91–94; doi: 10.1002/ana.410340116 [DOI] [PubMed] [Google Scholar]
- 27. Gupta RK, Bhatia V, Poptani H, et al. Brain metabolite changes on in vivo proton magnetic resonance spectroscopy in children with congenital hypothyroidism. J Pediatr 1995;126(3):389–392; doi: 10.1016/s0022-3476(95)70454-x [DOI] [PubMed] [Google Scholar]
- 28. Rovet JF. Congenital hypothyroidism: An analysis of persisting deficits and associated factors. Child Neuropsychol 2002;8(3):150–162; doi: 10.1076/chin.8.3.150.13501 [DOI] [PubMed] [Google Scholar]
- 29. Rovet JF. Congenital hypothyroidism: Long-term outcome. Thyroid 1999;9(7):741–748; doi: 10.1089/thy.1999.9.741 [DOI] [PubMed] [Google Scholar]
- 30. Willoughby KA, McAndrews MP, Rovet JF. Effects of maternal hypothyroidism on offspring hippocampus and memory. Thyroid 2014;24(3):576–584; doi: 10.1089/thy.2013.0215 [DOI] [PubMed] [Google Scholar]
- 31. Zendel BR, Willoughby KA, Rovet JF. Neuroplastic effects of music lessons on hippocampal volume in children with congenital hypothyroidism. Neuroreport 2013;24(17):947–950; doi: 10.1097/WNR.0000000000000031 [DOI] [PubMed] [Google Scholar]
- 32. Wheeler SM, Willoughby KA, McAndrews MP, et al. Hippocampal size and memory functioning in children and adolescents with congenital hypothyroidism. J Clin Endocrinol Metab 2011;96(9):E1427–34; doi: 10.1210/jc.2011-0119 [DOI] [PubMed] [Google Scholar]
- 33. Wheeler SM, McLelland VC, Sheard E, et al. Hippocampal functioning and verbal associative memory in adolescents with congenital hypothyroidism. Front Endocrinol (Lausanne) 2015;6:163; doi: 10.3389/fendo.2015.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Batistuzzo A, de Almeida GG, Brás TS, et al. Multisensory stimulation improves cognition and behavior in adult male rats born to LT4-treated thyroidectomized dams. Endocrinology 2022;163(9); doi: 10.1210/endocr/bqac105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clairman H, Skocic J, Lischinsky JE, et al. Do children with congenital hypothyroidism exhibit abnormal cortical morphology? Pediatr Res 2015;78(3):286–297; doi: 10.1038/pr.2015.93 [DOI] [PubMed] [Google Scholar]
- 36. Perri K, De Mori L, Tortora D, et al. Cognitive and white matter microstructure development in congenital hypothyroidism and familial thyroid disorders. J Clin Endocrinol Metab 2021;106(10):e3990–e4006; doi: 10.1210/clinem/dgab412 [DOI] [PubMed] [Google Scholar]
- 37. Siragusa V, Boffelli S, Weber G, et al. Brain magnetic resonance imaging in congenital hypothyroid infants at diagnosis. Thyroid 1997;7(5):761–764; doi: 10.1089/thy.1997.7.761 [DOI] [PubMed] [Google Scholar]
- 38. Korevaar TI, Muetzel R, Medici M, et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: A population-based prospective cohort study. Lancet Diabetes Endocrinol 2016;4(1):35–43; doi: 10.1016/S2213-8587(15)00327-7 [DOI] [PubMed] [Google Scholar]
- 39. Björnholm L, Orell O, Kerkelä M, et al. Maternal thyroid function during pregnancy and offspring white matter microstructure in early adulthood: A prospective birth cohort study. Thyroid 2023;33(10):1245–1254; doi: 10.1089/thy.2022.0699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Samadi A, Skocic J, Rovet JF. Children born to women treated for hypothyroidism during pregnancy show abnormal corpus callosum development. Thyroid 2015;25(5):494–502; doi: 10.1089/thy.2014.0548 [DOI] [PubMed] [Google Scholar]
- 41. Göbel A, Göttlich M, Heldmann M, et al. Experimentally induced subclinical hypothyroidism causes decreased functional connectivity of the cuneus: A resting state fMRI study. Psychoneuroendocrinology 2019;102:158–163; doi: 10.1016/j.psyneuen.2018.12.012 [DOI] [PubMed] [Google Scholar]
- 42. Zhang T, Zhao L, Chen C, et al. Structural and functional alterations of hippocampal subfields in patients with adult-onset primary hypothyroidism. J Clin Endocrinol Metab 2024; doi: 10.1210/clinem/dgae070 [DOI] [PubMed] [Google Scholar]
- 43. He XS, Ma N, Pan ZL, et al. Functional magnetic resource imaging assessment of altered brain function in hypothyroidism during working memory processing. Eur J Endocrinol 2011;164(6):951–959; doi: 10.1530/EJE-11-0046 [DOI] [PubMed] [Google Scholar]
- 44. Kumar M, Rana P, Modi S, et al. Aberrant intra and inter network resting state functional connectivity in thyrotoxicosis. J Neuroendocrinol 2019;31(2):e12683; doi: 10.1111/jne.12683 [DOI] [PubMed] [Google Scholar]
- 45. Jansen TA, Korevaar TIM, Mulder TA, et al. Maternal thyroid function during pregnancy and child brain morphology: A time window-specific analysis of a prospective cohort. Lancet Diabetes Endocrinol 2019;7(8):629–637; doi: 10.1016/S2213-8587(19)30153-6 [DOI] [PubMed] [Google Scholar]
- 46. Lischinsky JE, Skocic J, Clairman H, et al. Preliminary findings show maternal hypothyroidism may contribute to abnormal cortical morphology in offspring. Front Endocrinol (Lausanne) 2016;7:16; doi: 10.3389/fendo.2016.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jang YH, Kim J, Kim S, et al. Abnormal thalamocortical connectivity of preterm infants with elevated thyroid stimulating hormone identified with diffusion tensor imaging. Sci Rep 2022;12(1):9257; doi: 10.1038/s41598-022-12864-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bianco AC, Dumitrescu A, Gereben B, et al. Paradigms of dynamic control of thyroid hormone signaling. Endocr Rev 2019;40(4):1000–1047; doi: 10.1210/er.2018-00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krieger TG, Moran CM, Frangini A, et al. Mutations in thyroid hormone receptor α1 cause premature neurogenesis and progenitor cell depletion in human cortical development. Proc Natl Acad Sci U S A 2019;116(45):22754–22763; doi: 10.1073/pnas.1908762116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Le Maire A, Bouhours-Nouet N, Soamalala J, et al. Two novel cases of resistance to thyroid hormone due to THRA mutation. Thyroid 2020;30(8):1217–1221; doi: 10.1089/thy.2019.0602 [DOI] [PubMed] [Google Scholar]
- 51. Paisdzior S, Knierim E, Kleinau G, et al. A new mechanism in THRA resistance: The first disease-associated variant leading to an increased inhibitory function of THRA2. Int J Mol Sci 2021;22(10); doi: 10.3390/ijms22105338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev 1993;14(3):348–399; doi: 10.1210/edrv-14-3-348 [DOI] [PubMed] [Google Scholar]
- 53. Hauser P, Zametkin AJ, Martinez P, et al. Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med 1993;328(14):997–1001; doi: 10.1056/NEJM199304083281403 [DOI] [PubMed] [Google Scholar]
- 54. Leonard CM, Martinez P, Weintraub BD, et al. Magnetic resonance imaging of cerebral anomalies in subjects with resistance to thyroid hormone. Am J Med Genet 1995;60(3):238–243; doi: 10.1002/ajmg.1320600314 [DOI] [PubMed] [Google Scholar]
- 55. Mixson AJ, Parrilla R, Ransom SC, et al. Correlations of language abnormalities with localization of mutations in the beta-thyroid hormone receptor in 13 kindreds with generalized resistance to thyroid hormone: Identification of four new mutations. J Clin Endocrinol Metab 1992;75(4):1039–1045; doi: 10.1210/jcem.75.4.1400869 [DOI] [PubMed] [Google Scholar]
- 56. Phillips SA, Rotman-Pikielny P, Lazar J, et al. Extreme thyroid hormone resistance in a patient with a novel truncated TR mutant. J Clin Endocrinol Metab 2001;86(11):5142–5147; doi: 10.1210/jcem.86.11.8051 [DOI] [PubMed] [Google Scholar]
- 57. Anselmo J, Cao D, Karrison T, et al. Fetal loss associated with excess thyroid hormone exposure. Jama 2004;292(6):691–695; doi: 10.1001/jama.292.6.691 [DOI] [PubMed] [Google Scholar]
- 58. Dumitrescu AM, Liao XH, Best TB, et al. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 2004;74(1):168–175; doi: 10.1086/380999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Friesema EC, Grueters A, Biebermann H, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 2004;364(9443):1435–1437; doi: 10.1016/S0140-6736(04)17226-7 [DOI] [PubMed] [Google Scholar]
- 60. Schwartz CE, May MM, Carpenter NJ, et al. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet 2005;77(1):41–53; doi: 10.1086/431313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Groeneweg S, van Geest FS, Abacı A, et al. Disease characteristics of MCT8 deficiency: An international, retrospective, multicentre cohort study. Lancet Diabetes Endocrinol 2020;8(7):594–605; doi: 10.1016/S2213-8587(20)30153-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schwartz CE, Stevenson RE. The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract Res Clin Endocrinol Metab 2007;21(2):307–321; doi: 10.1016/j.beem.2007.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kakinuma H, Itoh M, Takahashi H. A novel mutation in the monocarboxylate transporter 8 gene in a boy with putamen lesions and low free T4 levels in cerebrospinal fluid. J Pediatr 2005;147(4):552–554; doi: 10.1016/j.jpeds.2005.05.012 [DOI] [PubMed] [Google Scholar]
- 64. Matheus MG, Lehman RK, Bonilha L, et al. Redefining the pediatric phenotype of X-linked Monocarboxylate Transporter 8 (MCT8) deficiency: Implications for diagnosis and therapies. J Child Neurol 2015;30(12):1664–1668; doi: 10.1177/0883073815578524 [DOI] [PubMed] [Google Scholar]
- 65. Charzewska A, Wierzba J, Iżycka-Świeszewska E, et al. Hypomyelinating leukodystrophies—a molecular insight into the white matter pathology. Clin Genet 2016;90(4):293–304; doi: 10.1111/cge.12811 [DOI] [PubMed] [Google Scholar]
- 66. Remerand G, Boespflug-Tanguy O, Tonduti D, et al. Expanding the phenotypic spectrum of Allan-Herndon-Dudley syndrome in patients with SLC16A2 mutations. Dev Med Child Neurol 2019;61(12):1439–1447; doi: 10.1111/dmcn.14332 [DOI] [PubMed] [Google Scholar]
- 67. Vaurs-Barrière C, Deville M, Sarret C, et al. Pelizaeus-Merzbacher-Like disease presentation of MCT8 mutated male subjects. Ann Neurol 2009;65(1):114–118; doi: 10.1002/ana.21579 [DOI] [PubMed] [Google Scholar]
- 68. Iwayama H, Tanaka T, Aoyama K, et al. Regional difference in myelination in Monocarboxylate Transporter 8 deficiency: Case reports and literature review of cases in Japan. Front Neurol 2021;12:657820; doi: 10.3389/fneur.2021.657820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Azzolini S, Nosadini M, Balzarin M, et al. Delayed myelination is not a constant feature of Allan-Herndon-Dudley syndrome: Report of a new case and review of the literature. Brain Dev 2014;36(8):716–720; doi: 10.1016/j.braindev.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 70. Vancamp P, Demeneix BA, Remaud S. Monocarboxylate Transporter 8 deficiency: Delayed or permanent hypomyelination? Front Endocrinol (Lausanne) 2020;11:283; doi: 10.3389/fendo.2020.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Goto M, Ito K, Okamoto N, et al. Cerebral blood flow on (99m)Tc ethyl cysteinate dimer SPECT in 2 siblings with monocarboxylate transporter 8 deficiency. Clin Nucl Med 2013;38(6):e276-8–e278; doi: 10.1097/RLU.0b013e31827082d8 [DOI] [PubMed] [Google Scholar]
- 72. Guillén-Yunta M, Valcárcel-Hernández V, García-Aldea Á, et al. Neurovascular unit disruption and blood-brain barrier leakage in MCT8 deficiency. Fluids Barriers CNS 2023;20(1):79; doi: 10.1186/s12987-023-00481-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sijens PE, Rödiger LA, Meiners LC, et al. 1H magnetic resonance spectroscopy in monocarboxylate transporter 8 gene deficiency. J Clin Endocrinol Metab 2008;93(5):1854–1859; doi: 10.1210/jc.2007-2441 [DOI] [PubMed] [Google Scholar]
- 74. Strømme P, Groeneweg S, Lima de Souza EC, et al. Mutated thyroid hormone transporter OATP1C1 associates with severe brain hypometabolism and juvenile neurodegeneration. Thyroid 2018;28(11):1406–1415; doi: 10.1089/thy.2018.0595 [DOI] [PubMed] [Google Scholar]
- 75. Ito K, Uchida Y, Ohtsuki S, et al. Quantitative membrane protein expression at the blood-brain barrier of adult and younger cynomolgus monkeys. J Pharm Sci 2011;100(9):3939–3950; doi: 10.1002/jps.22487 [DOI] [PubMed] [Google Scholar]
- 76. Roberts LM, Woodford K, Zhou M, et al. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 2008;149(12):6251–6261; doi: 10.1210/en.2008-0378 [DOI] [PubMed] [Google Scholar]
- 77. López-Espíndola D, García-Aldea Á, Gómez de la Riva I, et al. Thyroid hormone availability in the human fetal brain: Novel entry pathways and role of radial glia. Brain Struct Funct 2019;224(6):2103–2119; doi: 10.1007/s00429-019-01896-8 [DOI] [PubMed] [Google Scholar]
- 78. Russo SC, Salas-Lucia F, Bianco AC. Deiodinases and the metabolic code for thyroid hormone action. Endocrinology 2021;162(8); doi: 10.1210/endocr/bqab059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bárez-López S, Obregon MJ, Bernal J, et al. Thyroid hormone economy in the perinatal mouse brain: Implications for cerebral cortex development. Cereb Cortex 2018;28(5):1783–1793; doi: 10.1093/cercor/bhx088 [DOI] [PubMed] [Google Scholar]
- 80. Tu HM, Kim SW, Salvatore D, et al. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology 1997;138(8):3359–3368; doi: 10.1210/endo.138.8.5318 [DOI] [PubMed] [Google Scholar]
- 81. Salas-Lucia F, Fekete C, Sinko R, et al. Axonal T3 uptake and transport can trigger thyroid hormone signaling in the brain. Elife 2023;12; doi: 10.7554/eLife.82683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McAninch EA, Jo S, Preite NZ, et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J Clin Endocrinol Metab 2015;100(3):920–933; doi: 10.1210/jc.2014-4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jo S, Fonseca TL, Bocco B, et al. Type 2 deiodinase polymorphism causes ER stress and hypothyroidism in the brain. J Clin Invest 2019;129(1):230–245; doi: 10.1172/jci123176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Salvatore D, Porcelli T, Ettleson MD, et al. The relevance of T. Lancet Diabetes Endocrinol 2022;10(5):366–372; doi: 10.1016/S2213-8587(22)00004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. de Jong FJ, Peeters RP, den Heijer T, et al. The association of polymorphisms in the type 1 and 2 deiodinase genes with circulating thyroid hormone parameters and atrophy of the medial temporal lobe. J Clin Endocrinol Metab 2007;92(2):636–640; doi: 10.1210/jc.2006-1331 [DOI] [PubMed] [Google Scholar]
- 86. Çatli G, Fujisawa H, Kirbiyik Ö, et al. A novel homozygous Selenocysteine Insertion Sequence Binding Protein 2 (SECISBP2, SBP2) gene mutation in a Turkish boy. Thyroid 2018;28(9):1221–1223; doi: 10.1089/thy.2018.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dumitrescu AM, Liao XH, Abdullah MS, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 2005;37(11):1247–1252; doi: 10.1038/ng1654 [DOI] [PubMed] [Google Scholar]
- 88. Gunbey HP, Has AC, Aslan K, et al. Microstructural white matter abnormalities in hypothyroidism evaluation with diffusion tensor imaging tract-based spatial statistical analysis. Radiol Med 2021;126(2):283–290; doi: 10.1007/s11547-020-01234-7 [DOI] [PubMed] [Google Scholar]
- 89. Cao J, Chen C, Zhang T, et al. Segmental abnormalities of white matter microstructure in primary hypothyroidism identified by automated fiber quantification. Neuroendocrinology 2023;113(6):589–605; doi: 10.1159/000529062 [DOI] [PubMed] [Google Scholar]
- 90. Singh S, Trivedi R, Singh K, et al. Diffusion tensor tractography in hypothyroidism and its correlation with memory function. J Neuroendocrinol 2014;26(11):825–833; doi: 10.1111/jne.12193 [DOI] [PubMed] [Google Scholar]
- 91. Navarro D, Alvarado M, Figueroa A, et al. Distribution of GABAergic neurons and VGluT1 and VGAT immunoreactive boutons in the ferret (Mustela putorius) piriform cortex and endopiriform nucleus. Comparison with visual areas 17, 18 and 19. Front Neuroanat 2019;13:54; doi: 10.3389/fnana.2019.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liu B, Wang Z, Lin L, et al. Brain GABA+ changes in primary hypothyroidism patients before and after levothyroxine treatment: A longitudinal magnetic resonance spectroscopy study. Neuroimage Clin 2020;28:102473; doi: 10.1016/j.nicl.2020.102473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Singh S, Rana P, Kumar P, et al. Hippocampal neurometabolite changes in hypothyroidism: An in vivo (1) H magnetic resonance spectroscopy study before and after thyroxine treatment. J Neuroendocrinol 2016;28(9); doi: 10.1111/jne.12399 [DOI] [PubMed] [Google Scholar]
- 94. Fani L, Roa Dueñas O, Bos D, et al. Thyroid status and brain circulation: The Rotterdam study. J Clin Endocrinol Metab 2022;107(3):e1293–e1302; doi: 10.1210/clinem/dgab744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tian Y, Yao D, Jin A, et al. Thyroid function in causal relation to MRI markers of cerebral small vessel disease: A mendelian randomization analysis. J Clin Endocrinol Metab 2023;108(9):2290–2298; doi: 10.1210/clinem/dgad114 [DOI] [PubMed] [Google Scholar]
- 96. Chambers T, Anney R, Taylor PN, et al. Effects of thyroid status on regional brain volumes: A diagnostic and genetic imaging study in UK biobank. J Clin Endocrinol Metab 2021;106(3):688–696; doi: 10.1210/clinem/dgaa903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cooke GE, Mullally S, Correia N, et al. Hippocampal volume is decreased in adults with hypothyroidism. Thyroid 2014;24(3):433–440; doi: 10.1089/thy.2013.0058 [DOI] [PubMed] [Google Scholar]
- 98. Yin J, Xie L, Luo D, et al. Changes of structural and functional attention control networks in subclinical hypothyroidism. Front Behav Neurosci 2021;15:725908; doi: 10.3389/fnbeh.2021.725908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Su W, Zhao L, Bao S, et al. Alterations in gray matter morphology and functional connectivity in adult patients with newly diagnosed untreated hypothyroidism. Thyroid 2023;33(7):791–803; doi: 10.1089/thy.2022.0476 [DOI] [PubMed] [Google Scholar]
- 100. Zhang Y, Yang Y, Tao B, et al. Gray matter and regional brain activity abnormalities in subclinical hypothyroidism. Front Endocrinol (Lausanne) 2021;12:582519; doi: 10.3389/fendo.2021.582519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhao S, Xia Y, Huang Y, et al. The correlation between thyroid function, frontal gray matter, and executive function in patients with major depressive disorder. Front Endocrinol (Lausanne) 2021;12:779693; doi: 10.3389/fendo.2021.779693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhao S, Du Y, Zhang Y, et al. Gray matter reduction is associated with cognitive dysfunction in depressed patients comorbid with subclinical hypothyroidism. Front Aging Neurosci 2023;15:1106792; doi: 10.3389/fnagi.2023.1106792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Seetharaman S, Quintos JB, Salas-Lucia F. Resistance to thyroid hormone beta in a patient born to a mother with undiagnosed grave’s disease. AACE Clin Case Rep 2023;9(3):63–66; doi: 10.1016/j.aace.2023.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jacobson EM, Tomer Y. The genetic basis of thyroid autoimmunity. Thyroid 2007;17(10):949–961; doi: 10.1089/thy.2007.0153 [DOI] [PubMed] [Google Scholar]
- 105. Yudiarto FL, Muliadi L, Moeljanto D, et al. Neuropsychological findings in hyperthyroid patients. Acta Med Indones 2006;38(1):6–10. [PubMed] [Google Scholar]
- 106. Zhang W, Song L, Yin X, et al. Grey matter abnormalities in untreated hyperthyroidism: A voxel-based morphometry study using the DARTEL approach. Eur J Radiol 2014;83(1):e43–e48; doi: 10.1016/j.ejrad.2013.09.019 [DOI] [PubMed] [Google Scholar]
- 107. Holmberg M, Malmgren H, Heckemann RA, et al. A longitudinal study of medial temporal lobe volumes in graves disease. J Clin Endocrinol Metab 2022;107(4):1040–1052; doi: 10.1210/clinem/dgab808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Genç B, Aslan K, Avcı U, et al. Opposing effects of thyroid hormones on hypothalamic subunits and limbic structures in hyperthyroidism patients: A comprehensive volumetric study. J Neuroendocrinol 2024;36(3):e13369; doi: 10.1111/jne.13369 [DOI] [PubMed] [Google Scholar]
- 109. Aslan K, Gunbey HP, Cortcu S, et al. Diffusion tensor imaging in hyperthyroidism: Assessment of microstructural white matter abnormality with a tract-based spatial statistical analysis. Acta Radiol 2020;61(12):1677–1683; doi: 10.1177/0284185120909960 [DOI] [PubMed] [Google Scholar]
- 110. Kumar M, Singh S, Rana P, et al. Brain functional connectivity in patients with hyperthyroidism after anti-thyroid treatment. J Neuroendocrinol 2022;34(1):e13075; doi: 10.1111/jne.13075 [DOI] [PubMed] [Google Scholar]
- 111. Liu B, Wen L, Ran Q, et al. Dysregulation within the salience network and default mode network in hyperthyroid patients: A follow-up resting-state functional MRI study. Brain Imaging Behav 2020;14(1):30–41; doi: 10.1007/s11682-018-9961-6 [DOI] [PubMed] [Google Scholar]
- 112. Zhi M, Hou Z, Zhang Y, et al. Cognitive deficit-related interhemispheric asynchrony within the medial hub of the default mode network aids in classifying the hyperthyroid patients. Neural Plast 2018;2018:9023604; doi: 10.1155/2018/9023604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang W, Liu X, Zhang Y, et al. Disrupted functional connectivity of the hippocampus in patients with hyperthyroidism: Evidence from resting-state fMRI. Eur J Radiol 2014;83(10):1907–1913; doi: 10.1016/j.ejrad.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 114. Khushu S, Kumaran SS, Sekhri T, et al. Cortical activation during finger tapping in thyroid dysfunction: A functional magnetic resonance imaging study. J Biosci 2006;31(5):543–550; doi: 10.1007/BF02708405 [DOI] [PubMed] [Google Scholar]
- 115. Göttlich M, Heldmann M, Göbel A, et al. Experimentally induced thyrotoxicosis leads to increased connectivity in temporal lobe structures: A resting state fMRI study. Psychoneuroendocrinology 2015;56:100–109; doi: 10.1016/j.psyneuen.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 116. Luo L, Gao L, Li D, et al. Depression- and anxiety-associated disrupted brain structural networks revealed by probabilistic tractography in thyroid associated ophthalmopathy. J Affect Disord 2024;347:515–525; doi: 10.1016/j.jad.2023.11.089 [DOI] [PubMed] [Google Scholar]
- 117. Jiang WH, Liu J, Zhou J, et al. Altered dynamic brain activity and functional connectivity in thyroid-associated ophthalmopathy. Hum Brain Mapp 2023;44(16):5346–5356; doi: 10.1002/hbm.26437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wu Q, Hu H, Chen W, et al. Morphological and microstructural brain changes in thyroid-associated ophthalmopathy: A combined voxel-based morphometry and diffusion tensor imaging study. J Endocrinol Invest 2020;43(11):1591–1598; doi: 10.1007/s40618-020-01242-4 [DOI] [PubMed] [Google Scholar]
- 119. Zhou J, Chen W, Wu Q, et al. Reduced cortical complexity in patients with thyroid-associated ophthalmopathy. Brain Imaging Behav 2022;16(5):2133–2140; doi: 10.1007/s11682-022-00683-0 [DOI] [PubMed] [Google Scholar]
- 120. Liu P, Luo B, Feng Y, et al. Aberrant spontaneous brain activity in patients with thyroid-associated ophthalmopathy with and without optic neuropathy: A resting-state functional MRI study. Eur Radiol 2023;33(11):7981–7991; doi: 10.1007/s00330-023-09829-0 [DOI] [PubMed] [Google Scholar]
- 121. Chen W, Hu H, Chen HH, et al. Altered neurovascular coupling in thyroid-associated ophthalmopathy: A combined resting-state fMRI and arterial spin labeling study. J Neurosci Res 2023;101(1):34–47; doi: 10.1002/jnr.25126 [DOI] [PubMed] [Google Scholar]
- 122. Mohyi M, Smith TJ. IGF1 receptor and thyroid-associated ophthalmopathy. J Mol Endocrinol 2018;61(1):T29–T43; doi: 10.1530/JME-17-0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Martin FI, Deam DR. Hyperthyroidism in elderly hospitalised patients. Clinical features and treatment outcomes. Med J Aust 1996;164(4):200–203. [PubMed] [Google Scholar]
- 124. Chaker L, Cremers LGM, Korevaar TIM, et al. Age-dependent association of thyroid function with brain morphology and microstructural organization: Evidence from brain imaging. Neurobiol Aging 2018;61:44–51; doi: 10.1016/j.neurobiolaging.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 125. Reitz C, Kretzschmar K, Roesler A, et al. Relation of plasma thyroid-stimulating hormone levels to vascular lesions and atrophy of the brain in the elderly. Neuroepidemiology 2006;27(2):89–95; doi: 10.1159/000095244 [DOI] [PubMed] [Google Scholar]
- 126. de Jong FJ, den Heijer T, Visser TJ, et al. Thyroid hormones, dementia, and atrophy of the medial temporal lobe. J Clin Endocrinol Metab 2006;91(7):2569–2573; doi: 10.1210/jc.2006-0449 [DOI] [PubMed] [Google Scholar]
- 127. Camp JG, Badsha F, Florio M, et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A 2015;112(51):15672–15677; doi: 10.1073/pnas.1520760112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 2014;9(10):2329–2340; doi: 10.1038/nprot.2014.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gordon A, Yoon SJ, Tran SS, et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat Neurosci 2021;24(3):331–342; doi: 10.1038/s41593-021-00802-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Salas-Lucia F, Bianco AC. T3 levels and thyroid hormone signaling. Front Endocrinol (Lausanne) 2022;13:1044691; doi: 10.3389/fendo.2022.1044691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Madhavan M, Nevin ZS, Shick HE, et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat Methods 2018;15(9):700–706; doi: 10.1038/s41592-018-0081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Marton RM, Miura Y, Sloan SA, et al. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci 2019;22(3):484–491; doi: 10.1038/s41593-018-0316-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang L, Shao YY, Ballock RT. Thyroid hormone-mediated growth and differentiation of growth plate chondrocytes involves IGF-1 modulation of beta-catenin signaling. J Bone Miner Res 2010;25(5):1138–1146; doi: 10.1002/jbmr.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Giordano T, Pan JB, Casuto D, et al. Thyroid hormone regulation of NGF, NT-3 and BDNF RNA in the adult rat brain. Brain Res Mol Brain Res 1992;16(3–4):239–245; doi: 10.1016/0169-328x(92)90231-y [DOI] [PubMed] [Google Scholar]
- 135. Lee JY, Kim MJ, Deliyanti D, et al. Overcoming Monocarboxylate Transporter 8 (MCT8)-deficiency to promote human oligodendrocyte differentiation and myelination. EBioMedicine 2017;25:122–135; doi: 10.1016/j.ebiom.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Vancamp P, Darras VM. From zebrafish to human: A comparative approach to elucidate the role of the thyroid hormone transporter MCT8 during brain development. Gen Comp Endocrinol 2018;265:219–229; doi: 10.1016/j.ygcen.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 137. Vatine GD, Shelest O, Barriga BK, et al. Oligodendrocyte progenitor cell maturation is dependent on dual function of MCT8 in the transport of thyroid hormone across brain barriers and the plasma membrane. Glia 2021;69(9):2146–2159; doi: 10.1002/glia.24014 [DOI] [PubMed] [Google Scholar]
- 138. Ludwik KA, Jahn R, Schörding AK, et al. Generation of THRB-GS(E125G_G126S) and THRB-KO human iPSC lines to study noncanonical thyroid hormone signalling. Stem Cell Res 2024;74:103275; doi: 10.1016/j.scr.2023.103275 [DOI] [PubMed] [Google Scholar]
- 139. Ludwik KA, Opitz R, Jyrch S, et al. Generation of iPSC lines with SLC16A2:G401R or SLC16A2 knock out. Stem Cell Res 2023;73:103256; doi: 10.1016/j.scr.2023.103256 [DOI] [PubMed] [Google Scholar]
- 140. Vatine GD, Al-Ahmad A, Barriga BK, et al. Modeling psychomotor retardation using iPSCs from MCT8-deficient patients indicates a prominent role for the blood-brain barrier. Cell Stem Cell 2017;20(6):831–843.e5; doi: 10.1016/j.stem.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Salas-Lucia F, Escamilla S, Bianco AC, et al. Impaired T3 uptake and action in MCT8-deficient cerebral organoids underlie the Allan-Herndon-Dudley syndrome. JCI Insight 2024;9(7); doi: 10.1172/jci.insight.174645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lewitus E, Kelava I, Huttner WB. Conical expansion of the outer subventricular zone and the role of neocortical folding in evolution and development. Front Hum Neurosci 2013;7:424; doi: 10.3389/fnhum.2013.00424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Diez D, Morte B, Bernal J. Single-cell transcriptome profiling of thyroid hormone effectors in the human fetal neocortex: Expression of SLCO1C1, DIO2, and THRB in specific cell types. Thyroid 2021;31(10):1577–1588; doi: 10.1089/thy.2021.0057 [DOI] [PubMed] [Google Scholar]
- 144. Wilpert NM, Krueger M, Opitz R, et al. Spatiotemporal changes of cerebral Monocarboxylate Transporter 8 expression. Thyroid 2020;30(9):1366–1383; doi: 10.1089/thy.2019.0544 [DOI] [PubMed] [Google Scholar]
- 145. Graffunder AS, Bresser AAJ, Fernandez Vallone V, et al. Spatiotemporal expression of thyroid hormone transporter MCT8 and THRA mRNA in human cerebral organoids recapitulating first trimester cortex development. Sci Rep 2024;14(1):9355; doi: 10.1038/s41598-024-59533-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chatonnet F, Flamant F, Morte B. A temporary compendium of thyroid hormone target genes in brain. Biochim Biophys Acta 2015;1849(2):122–129; doi: 10.1016/j.bbagrm.2014.05.023 [DOI] [PubMed] [Google Scholar]
- 147. Fauquier T, Romero E, Picou F, et al. Severe impairment of cerebellum development in mice expressing a dominant-negative mutation inactivating thyroid hormone receptor alpha1 isoform. Dev Biol 2011;356(2):350–358; doi: 10.1016/j.ydbio.2011.05.657 [DOI] [PubMed] [Google Scholar]
- 148. Bernal J. Thyroid hormone regulated genes in cerebral cortex development. J Endocrinol 2017;232(2):R83–R97; doi: 10.1530/JOE-16-0424 [DOI] [PubMed] [Google Scholar]
- 149. Gil-Ibañez P, García-García F, Dopazo J, et al. Global transcriptome analysis of primary cerebrocortical cells: Identification of genes regulated by triiodothyronine in specific cell types. Cereb Cortex 2017;27(1):706–717; doi: 10.1093/cercor/bhv273 [DOI] [PubMed] [Google Scholar]
- 150. Muñoz A, Rodriguez-Peña A, Perez-Castillo A, et al. Effects of neonatal hypothyroidism on rat brain gene expression. Mol Endocrinol 1991;5(2):273–280; doi: 10.1210/mend-5-2-273 [DOI] [PubMed] [Google Scholar]
- 151. Hernandez A, Morte B, Belinchón MM, et al. Critical role of types 2 and 3 deiodinases in the negative regulation of gene expression by T3in the mouse cerebral cortex. Endocrinology 2012;153(6):2919–2928; doi: 10.1210/en.2011-1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ferrara AM, Liao XH, Gil-Ibáñez P, et al. Placenta passage of the thyroid hormone analog DITPA to male wild-type and Mct8-deficient mice. Endocrinology 2014;155(10):4088–4093; doi: 10.1210/en.2014-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]