Abstract
Rationale & Objective
Membranous nephropathy (MN), recognized as an autoimmune kidney disease, responds well to anti-CD20 monoclonal antibodies. Obinutuzumab, a type Ⅱ humanized anti-CD20 and immunoglobulin G1 Fc-optimized monoclonal antibody, when compared with rituximab, has demonstrated superior efficacy in B-cell leukemia and lymphoma, especially in rituximab-resistant cases. However, the efficacy and safety of obinutuzumab in MN remain unclear.
Study Design
A case series study.
Setting & Participants
A total of 18 patients were diagnosed with MN and had received obinutuzumab at our center without secondary MN, undergoing dialysis, having a history of kidney transplantation, or infections requiring treatment.
Exposure
Obinutuzumab treatment.
Outcomes
Primary outcomes included remission rate, time to first remission, and first relapse-free survival time during the follow-up period.
Analytical Approach
Survival analysis was performed with Cox proportional hazards models, log-rank test, and Kaplan–Meier survival analysis.
Results
Patients with MN (median age of 52.5 years, 83.3% males) received an average dose of 2.1 ± 0.8 g of obinutuzumab during a median follow-up period of 13.6 months. During the follow-up, 17 patients (94.4%) achieved remission, with 12 patients (66.7%) achieving partial remission, and 5 patients (27.8%) achieving complete remission. The median time to first remission and first relapse-free survival time was 2.7 (1.0-6.1) months and 9.8 (2.6-11.2) months, respectively. Of 12 patients with previous rituximab treatment, all achieved remission successfully, with 8 (66.7%) achieving partial remission and 4 (33.3%) achieving complete remission. Adverse events were mostly mild, and no severe treatment-related adverse events were observed.
Limitations
Limited or missing data; risks of selection bias; or recall bias; underestimated first relapse-free survival time because of a limited follow-up period; unmonitored counts of CD19+ B-cells and other lymphocyte subsets.
Conclusions
Obinutuzumab demonstrated promising efficacy and safety in inducing remission in MN, particularly in patients with an unsatisfactory response to rituximab.
Index Words: Obinutuzumab, membranous nephropathy, rituximab, remission
Plain Language Summary
Membranous nephropathy (MN), an autoimmune kidney disease, usually responds favorably to rituximab, a chimeric anti-CD20 monoclonal antibody. Nevertheless, certain patients exhibit inadequate responses to rituximab. Obinutuzumab, a novel humanized anti-CD20 monoclonal antibody, has shown enhanced efficacy in cases where rituximab fails to address B-cell leukemias and lymphomas. However, its efficacy and safety in MN treatment remain uncertain. A case series involving 18 patients treated with obinutuzumab at our center demonstrated promising results, suggesting favorable efficacy and safety in inducing and maintaining remission, particularly among patients who did not respond well to rituximab previously. These findings signify a potential alternative for MN treatment, though further research is needed to confirm them.
Membranous nephropathy (MN), the leading cause of primary nephrotic syndrome in adults, is recognized as an autoimmune disease with immune complex deposition along the subepithelial region of the glomerular basement membrane. Identified target antigens, such as phospholipase A2 receptor (PLA2R), thrombospondin type 1 domain-containing 7A (THSD7A), and other emerging new antigens1 have had a profound effect on the management of MN, providing supporting evidence for B-cell depletion therapy.
Rituximab, a type Ⅰ chimeric anti-CD20 monoclonal antibody, was proven not inferior to cyclosporine in inducing complete or partial remission of proteinuria at 12 months and superior to cyclosporine in maintaining remission of proteinuria up to 24 months.2 However, the overall response rate with rituximab at 12 months ranges between 60% and 70% in different studies, with ∼30% to 40% of patients not responding to rituximab treatment.3 Obinutuzumab, a type Ⅱ humanized anti-CD20 and immunoglobulin G1 Fc-optimized monoclonal antibody, demonstrating enhanced antibody-dependent cell-mediated cytotoxicity and direct nonapoptotic cell death but reduced complement-dependent cytotoxicity, has been shown to induce faster and more lasting B-cell depletion and then improved remission rates in B-cell leukemia and lymphoma than the equivalent dose of rituximab and remains effective in rituximab-resistant cases.4,5
However, there are limited studies regarding the efficacy and safety of obinutuzumab in addressing MN, especially in patients who had previously received rituximab treatment but showed an unsatisfactory response. In this study, we aimed to evaluate the efficacy and safety of obinutuzumab in treating MN and attempted to investigate the potential benefits for patients with inadequate responses to rituximab.
Methods
Study Design and Patients
We retrospectively screened patients with refractory MN at our center and enrolled 18 patients with refractory MN who received the initial dose of obinutuzumab from January 2022 to September 2022. Refractory MN was defined by the continued presence of high or unchanged levels of anti-PLA2R antibodies following one line of immunosuppressive therapy, given at an adequate dosage and duration. In addition, patients experiencing persistent nephrotic syndrome with low serum albumin levels during a follow-up period exceeding 6 months were also categorized as having refractory MN.6 Exclusion criteria included MN secondary to drugs, infections, cancers, or autoimmune diseases. Patients treated with dialysis or with a history of kidney transplantation were also excluded. Moreover, patients with active or chronic infections requiring treatment, positive serology for human immunodeficiency virus, seropositivity for hepatitis B surface antigen or hepatitis B core antibody, or seropositivity for hepatitis C were excluded from the study. All patients were prestudied before obinutuzumab and followed up through the electronic medical record system and by phone interview.
Demographic data included gender, age at disease onset, and age at obinutuzumab treatment. Serum anti-PLA2R antibodies, urine protein creatinine ratio (uPCR), serum albumin, and serum creatinine were recorded as laboratory data. All demographic and laboratory data were collected through the electronic medical record system. Remission and relapse during follow-up and drugs administered before and after obinutuzumab, especially rituximab administration, were recorded as clinical data and obtained from both the electronic medical record system and continuous interviews conducted by the telephone.
For patients with previous rituximab, the full dose of rituximab was 375 mg/m2 of body surface area. For 1 course of rituximab, we define that the patient receives doses of rituximab at 1-2 week intervals to achieve B-cell depletion (BCD). When the patient exhibits notable increases in serum anti-PLA2R antibody titers or shows recoveries in peripheral CD19+ B-cell counts, another course of rituximab will be added. Courses of previous rituximab were collected as clinical data.
The titers of serum anti-PLA2R antibodies were analyzed by indirect immunofluorescence assay with a minimum detectable level of 2 RU/ml. The count of B lymphocytes (CD19+) was analyzed after obinutuzumab by flow cytometry. The study conformed to the Declaration of Helsinki and was approved by the ethics committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. 2020571). Written informed consent was obtained from all patients.
Therapy
Patients initially received obinutuzumab at a full dose of 1000 mg. After 2 weeks following the first dose, the counts of lymphocytes in the peripheral blood, particularly CD19+ B-cells, were analyzed. The initial round of obinutuzumab ended if BCD, defined as the peripheral CD19+ B-cells of 0 cells/μL, was achieved. Otherwise, additional doses of 1 g would be added every 2 weeks until achieving BCD.
Outcomes
The primary outcome included the remission rate, the time to first remission after the first dose of obinutuzumab, and the first relapse-free survival time during the follow-up period. Complete remission (CR) was defined as a urinary protein excretion of <0.3 g/d or uPCR < 0.3 g/g, accompanied by a normal serum albumin level and a normal serum creatinine level. Partial remission (PR) was defined as a urinary protein excretion of ≥ 0.3 g/d and < 3.5 g/d, or uPCR ≥ 0.3 g/g and < 3.5 g/g, with a 50% or greater reduction from peak values accompanied by an improvement or normalization of the serum albumin level and a stable serum creatinine. Recurrence of urinary protein excretion at ≥ 3.5 g/d, uPCR at ≥ 3.5 g/g, or proteinuria at 3+ by urine dipstick after remission was considered as a relapse. The first relapse-free survival time was defined as the time from the first remission to the first relapse after the first obinutuzumab dose. The secondary outcomes included changes in the uPCR, serum albumin, serum creatinine, and anti-PLA2R antibody after obinutuzumab in all patients or between specific subgroups. Combined drugs after obinutuzumab therapy and adverse events were also prespecified as secondary outcomes.
Statistical Analysis
Quantitative data conforming to a normal distribution were presented as mean ± standard deviation, whereas non-normally distributed data were described as median and interquartile range (IQR). Categorical data were reported with counts and percentages. A P-value below 0.05 was considered statistically significant. Survival analysis was conducted with Cox proportional hazards models, log-rank test, and Kaplan–Meier survival analysis in R version 4.2.2. The R packages "survival," "survminer," and "ggplot2" were employed. Statistical analysis was performed by SPSS version 26.0 (IBM). All figures except Kaplan–Meier curves were generated using GraphPad Prism 9. Data in figures except for Kaplan–Meier curves were all presented as medians.
Results
Patient Population and Therapy
A total of 18 Chinese patients who received the first dose of obinutuzumab from January 2022 to September 2022 were included in this study. Among these patients, 16 patients (88.9%) were diagnosed with MN by kidney biopsy. The median ages at disease onset and initial obinutuzumab treatment were 50.8 (45.4-55.3) and 52.5 (45.7-56.1) years, respectively.
Concerning anti-PLA2R antibodies or PLA2R antigens, 14 patients (77.8%) had presented serum anti-PLA2R antibody titers of at least 50 RU/mL during the entire disease duration, verifying that they were serum anti-PLA2R antibody-associated MN. One patient without detectable serum anti-PLA2R antibody was found with glomerular PLA2R and IgG4 deposition intensities scoring +++ (strong) on a kidney biopsy. These 15 patients were classified into the PLA2R-associated subgroup as they exhibited positive serum anti-PLA2R antibodies or immunofluorescence staining for PLA2R antigen on kidney biopsy. The remaining 3 patients exhibited serum anti-PLA2R antibody titers below the detectable line (2 RU/mL) and a negative histology associated with PLA2R, identifying them as non-PLA2R-associated. Notably, the 2 patients who did not undergo biopsy demonstrated a high titer of serum anti-PLA2R antibody exceeding 500 RU/mL and presented with nephrotic syndrome, suggestive of MN.
Regarding previous administration of rituximab, 12 patients (66.7%) received rituximab therapy previously, with a median course of 2.0 (1.3-4.8). Among these patients, except for 1 patient who switched to obinutuzumab because of a notable infusion reaction to rituximab, 11 patients shifted to obinutuzumab owing to the unsatisfactory response or insufficient maintenance of efficacy with rituximab. Detailed demographic and clinical characteristics were presented in Table 1.
Table 1.
Baseline Demographic, Clinical, and Biologic Characteristics of the Patients
| Overall Cohort (N = 18) | |
|---|---|
| Demographics | |
| Ethnicity | All Han Chinese |
| Age (y) at obinutuzumab, median (IQR) | 52.5 (45.7-56.1) |
| Male, n (%) | 15 (83.3%) |
| Disease history | |
| Age (y) at disease onset, median (IQR) | 50.8 (45.4-55.3) |
| Duration of disease, mo, median (IQR) | 11.6 (4.2-22.5) |
| Laboratory values before obinutuzumab | |
| Urine protein creatinine ratio, g/g, median (IQR) | 3.5 (1.9-7.1) |
| Serum albumin, g/L, mean ± SD | 27.2 ± 8.2 |
| Serum creatinine, μmol/L, mean ± SD | 86.2 ± 21.8 |
| Serum anti-PLA2R antibody exceeding 50 RU/mL at disease onset, n (%) | 14 (77.8%) |
| Serum anti-PLA2R antibody titer at the commencement of obinutuzumab, RU/mL, median (IQR) | 16.5 (6.1-170.3) |
| Serum anti-PLA2R negative but antigen positive on biopsy, n (%) | 1 (5.6%) |
| Previous therapy | |
| Rituximab, n (%) | 12 (66.7%) |
| Cumulative rituximab courses, median (IQR) | 2.0 (1.3-4.8) |
| Cyclophosphamide, n (%) | 8 (44.4%) |
| Prednisone, n (%) | 14 (77.8%) |
| Mycophenolate, n (%) | 6 (33.3%) |
| CNI (tacrolimus or cyclosporine), n (%) | 6 (33.3%) |
| ARB, n (%) | 7 (38.9%) |
Abbreviations: ARB, angiotensin II receptor blocker; CNI, calcineurin inhibitor; IQR, interquartile range; SD, standard deviation.
Primary Outcome
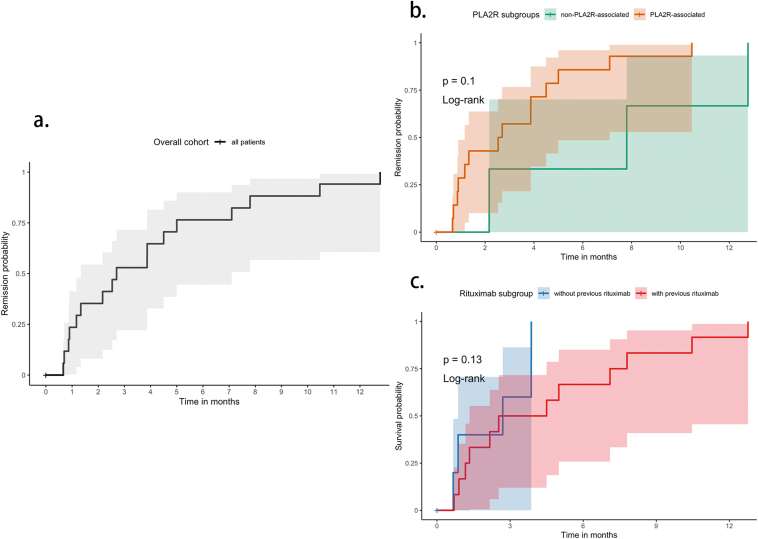
For the initial round to achieve BCD, the average dose of obinutuzumab was 1.0 (1.0-2.0) g, with 13 patients receiving 1 dose and 5 patients receiving 2 doses. During a median follow-up period of 13.6 months, an average obinutuzumab dose of 2.1 ± 0.8 g was administered. Among these 18 patients, 3 patients received only 1 dose of obinutuzumab treatment during the follow-up period, whereas 11 patients received 2 doses, 3 patients received 3 doses, and 1 patient received 4 doses of obinutuzumab. By the end of the follow-up, 12 patients (66.7%) achieved PR and 5 patients (27.8%) achieved CR. One patient failed to achieve remission during the follow-up; yet his serum anti-PLA2R antibody dropped from 572 to 152 RU/mL at 3 months. He changed his regime; thereafter, we lost the opportunity to further observe his remission under obinutuzumab. The Kaplan–Meier curves of the overall cohort and different subgroups were shown in Fig 1. The 6-month and 12-month remission rates were 13 (72.2%) and 16 (88.9%), respectively.
Figure 1.
(A) Probability of achieving remission (either partial remission or complete remission) after obinutuzumab in all patients (n = 18). (B) Probability of achieving remission after obinutuzumab in PLA2R subgroups. “PLA2R-associated” (n = 15) was defined as either serum anti-PLA2R antibody titers exceeding 50 RU/mL or positive immunofluorescence staining for PLA2R antigen on kidney biopsy. (C) Probability of achieving remission after obinutuzumab in previous-rituximab subgroups. “With previous rituximab” (n = 12) was defined as having received at least 1 course of rituximab before the initial infusion of obinutuzumab.
Of the 12 patients who received rituximab previously, all patients achieved remission successfully, including 8 patients (66.7%) achieving PR and 4 patients (33.3%) achieving CR. Primary outcomes between the subgroups with and without previous rituximab treatment are shown in Table 2. Among the 17 patients who attained remission during the follow-up, the median time to first remission (either PR or CR) after the initial dose of obinutuzumab was 2.7 (1.0-6.1) months. For the 5 patients who achieved their best remission of CR, the median time to CR after the initial dose of obinutuzumab was 9.4 (4.4-14.2) months. Among the 17 patients who achieved remission, only 1 experienced a relapse at 9 months after achieving PR, whereas the remaining patients maintained their remission status until the end of the follow-up. Over the median follow-up period of 13.6 months, the median first relapse-free survival time was 9.8 (2.6-11.2) months. Detailed information of primary outcomes is presented in Table 3.
Table 2.
Comparison of Primary Outcomes between Subgroups with and without Previous Rituximab Treatment
| Primary Outcomes | With Previous RTX (n = 12) | Without Previous RTX (n = 6) | P |
|---|---|---|---|
| Total obinutuzumab dose during follow-up, g, median (IQR) | 2.0 (1.3-3.0) | 2.0 (2.0-2.0) | 0.82 |
| Time to first remission, mo, median (IQR) | 3.5 (1.2-7.6) | 2.7 (1.3-3.9) | 0.44 |
| First relapse-free survival time during follow-up, mo, median (IQR) | 10.2 (2.3-11.3) | 9.0 (3.9-11.0) | 0.80 |
| Remission rate during follow-up, n (%)a | 12 (100%) | 5 (83.3%) | 0.33 |
| Partial remission rate during follow-up, n (%) | 8 (66.7%) | 4 (66.7%) | > 0.99 |
| Complete remission rate during follow-up, n (%) | 4 (33.3%) | 1 (16.7%) | 0.62 |
| Remission rate in 6 mo, n (%) | 8 (66.7%) | 5 (83.3%) | 0.62 |
| Remission rate in 12 mo, n (%) | 11 (91.7%) | 5 (83.3%) | > 0.99 |
Abbreviations: IQR, interquartile range; RTX, rituximab.
Includes those who achieved either partial remission or complete remission during the follow-up period.
Table 3.
Primary Outcomes of the Patients Received Obinutuzumab Therapy
| Primary Outcomes | Overall Cohort (N = 18) |
|---|---|
| Total follow-up period, mo, median (IQR) | 13.6 (11.9-14.9) |
| Total obinutuzumab dose during follow-up, g, mean ± SD | 2.1 ± 0.8 |
| Time to first remission, mo, median (IQR) | 2.7 (1.0-6.1) |
| First relapse-free survival time during follow-up, mo, median (IQR) | 9.8 (2.6-11.2) |
| Remission rate during follow-up, n (%)a | 17 (94.4%) |
| Partial remission rate during follow-up, n (%) | 12 (66.7%) |
| Complete remission rate during follow-up, n (%) | 5 (27.8%) |
| Remission rate in 6 mo, n (%) | 13 (72.2%) |
| Remission rate in 12 mo, n (%) | 16 (88.9%) |
Abbreviations: IQR, interquartile range; SD, standard deviation.
Includes those who achieved either partial remission or complete remission during the follow-up period.
Secondary outcome: urine protein creatinine ratio, serum albumin, and serum creatinine
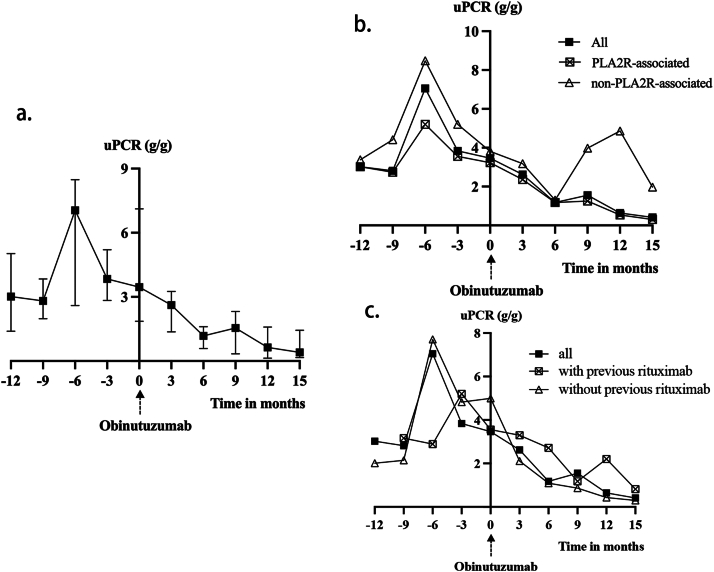
The uPCR decreased from a median baseline of 3.4 (1.9-7.1) g/g to 1.2 (0.6-1.6) g/g and 0.6 (0.1-1.6) g/g at 6 and 12 months after the initial dose of obinutuzumab, respectively, and were all statistically significant when compared with the baseline levels (P = 0.001 and 0.003, respectively). The trend of the uPCR is shown in Fig 2.
Figure 2.
(A) Change of urine protein creatinine ratio (uPCR) after obinutuzumab in all patients (n = 18). (B) Change of uPCR after obinutuzumab in PLA2R subgroups. “PLA2R-associated” (n = 15) was defined as either serum anti-PLA2R antibody titers exceeding 50 RU/mL or positive immunofluorescence staining for PLA2R antigen on kidney biopsy. (C) Change of uPCR after obinutuzumab in previous-rituximab subgroups. “With previous rituximab” (n = 12) was defined as having received at least one course of rituximab before the initial infusion of obinutuzumab.
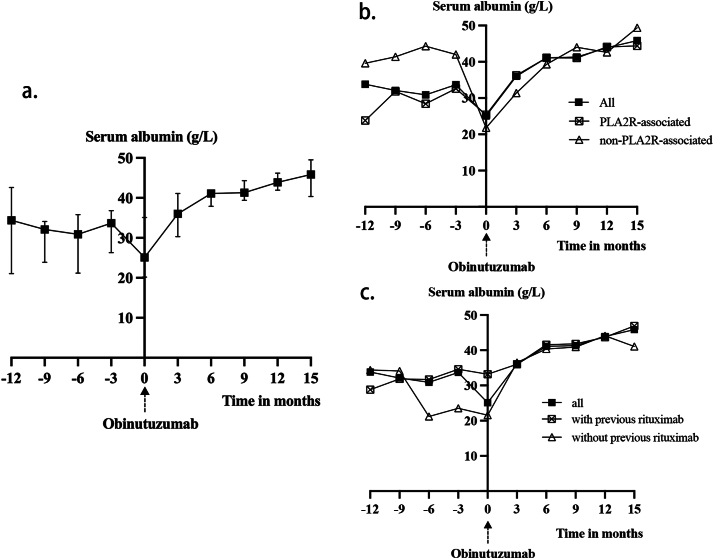
The average serum albumin levels improved significantly from a baseline level of 27.2 ± 8.2 g/L to 40.3 ± 3.8 g/L and 43.8 ± 2.9 g/L at 6 and 12 months after the initial dose of obinutuzumab, respectively (all P values < 0.001). The trend of serum albumin is shown in Fig 3.
Figure 3.
(A) Change of serum albumin level after obinutuzumab in all patients (n = 18). (B) Change of serum albumin level after obinutuzumab in PLA2R subgroups. “PLA2R-associated” (n = 15) was defined as either serum anti-PLA2R antibody titers exceeding 50 RU/mL or positive immunofluorescence staining for PLA2R antigen on kidney biopsy. (C) Change of serum albumin level after obinutuzumab in previous-rituximab subgroups. “With previous rituximab” (n = 12) was defined as having received at least 1 course of rituximab before the initial infusion of obinutuzumab.
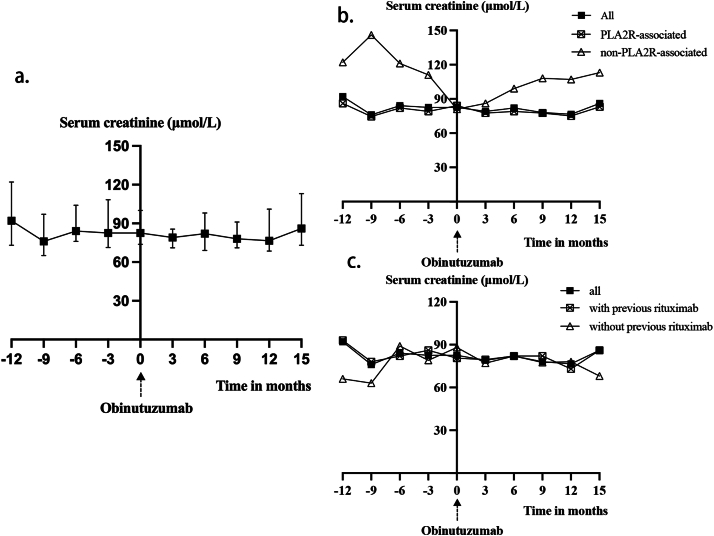
The serum creatinine levels of all patients remained stable during the follow-up period, indicating that the kidney function remained stable after the administration of obinutuzumab. The trend of serum creatinine is shown in Fig 4.
Figure 4.
(A) Change of serum creatinine level after obinutuzumab in all patients (n = 18). (B) Change of serum creatinine level after obinutuzumab in PLA2R subgroups. “PLA2R-associated” (n = 15) was defined as either serum anti-PLA2R antibody titers exceeding 50 RU/mL or positive immunofluorescence staining for PLA2R antigen on kidney biopsy. (C) Change of serum creatinine level after obinutuzumab in previous-rituximab subgroups. “With previous rituximab” (n = 12) was defined as having received at least 1 course of rituximab before the initial infusion of obinutuzumab.
Serum Anti-PLA2R Antibodies
Among the 14 patients with positive serum anti-PLA2R antibodies, only 10 patients presented with an anti-PLA2R titer greater than 2 RU/mL (a titer less than 2 RU/mL was considered negative by our immunology laboratory) at the commencement of obinutuzumab because of the previous immunosuppressive medications. Changes in serum anti-PLA2R antibodies are shown in Table S1.
Peripheral Lymphocyte Subsets
Significant changes in the lymphocyte subsets were noted following obinutuzumab treatment. Notably, decreases were observed not only in the CD19+ B-cell count (72.0 [10.0-227.5] vs 0.0 [0.0-0.0]; P < 0.001) but also in the total lymphocyte count (1,817.8 ± 546.9 vs 1,385.6 ± 575.1; P = 0.006), total T-cell count (1,390.3 ± 430.6 vs 1,134.2 ± 500.5; P = 0.04), and CD4+ T-cell count (842.5 [634.5-1,163.3] vs 594.5 [446.5-769.5]; P = 0.04). However, no significant differences were observed in the CD8+ T-cell count, CD4+ T-cell count/CD8+ T-cell count ratio, or natural killer cell count (all P > 0.05). Detailed information is presented in Table 4.
Table 4.
Changes in Peripheral Lymphocyte Subsets Before and After Obinutuzumab Treatment
| Lymphocyte Subsets | At Baseline | At First BCD | P |
|---|---|---|---|
| Total CD45+ lymphocyte count, 1 cell/μL, mean ± SD | 1,817.8 ± 546.9 | 1,385.6 ± 575.1 | 0.006a |
| Total T-cell count, 1 cell/μL, mean ± SD | 1,390.3 ± 430.6 | 1,134.2 ± 500.5 | 0.04a |
| CD4+ T-cell count, 1 cell/μL, median (IQR) | 842.5 (634.5-1,163.3) | 594.5 (446.5-769.5) | 0.04a |
| CD8+ T-cell count, 1 cell/μL, mean ± SD | 469.6 ± 172.9 | 429.7 ± 198.8 | 0.25 |
| CD4+ T-cell count/CD8+ T-cell count ratio, median (IQR) | 1.8 (1.3-2.7) | 1.4 (1.1-2.1) | 0.42 |
| CD56+CD16+ NK-cell count, 1 cell/μL, median (IQR) | 182.5 (94.8-451.3) | 207.0 (75.5-317.3) | 0.35 |
| CD19+ B-cell count, 1 cell/μL, median (IQR) | 72.0 (10.0-227.5) | 0.0 (0.0-0.0) | <0.001a |
Abbreviations: IQR, interquartile range; SD, standard deviation; BCD, B-cell depletion; NK, natural killer.
Significant P values.
Combined Treatment
After the first dose of obinutuzumab treatment, 16 patients (88.9%) withdrew all immunosuppressive medications successfully during the follow-up. In 1 patient, tacrolimus was continued for 8 months with slow tapering and ceased after another dose of obinutuzumab. A patient who showed a poor response to obinutuzumab was treated with cyclophosphamide and prednisone and failed to achieve remission by the end of the follow-up.
Safety
During the follow-up period, no severe treatment-related adverse events were observed during or shortly after obinutuzumab treatments. Adverse events were observed in 5 patients (27.8%), which included infection (n = 3) and itching (n = 2). At 4 and 8 months after the last dose of obinutuzumab, 2 patients developed viral pneumonia caused by coronavirus, requiring hospitalization, but were successfully treated and cured. One patient exhibited mild inflammation in the lungs during a physical examination conducted 3 months after receiving obinutuzumab. Although viral pneumonia was considered, the patient remained asymptomatic and did not require hospitalization or medication. Itching was experienced by 2 patients during the early stage of the obinutuzumab infusion and occurred every time they received the infusion. However, neither of them developed any accompanying rashes. No anaphylactic shock, sepsis, malignancy, or death was observed.
Discussion
The emergence of novel anti-CD20 monoclonal antibodies raises our interest in exploring their potential role in the treatment of MN, an autoimmune disease characterized by the presence of nephritogenic antigens. In our single-center study, 17 patients (94.4%) with MN achieved either partial or CR with obinutuzumab, showing efficacy even in 11 patients (61.1%) with unsatisfactory responses to previous rituximab treatments.
The remission rate in 6 months in our study was 72.2%, slightly higher than the 6-month remission rate of 60% in the study conducted by Sethi et al7 and the 12-month remission rate was 88.9%, which closely aligned with that of the 90% observed previously. In MENTOR Trial, 35% and 60% of patients with MN achieved remission at 6 months and 12 months after rituximab treatment, lower percentages than those observed in our study.2 Moreover, Ruggenenti et al8 reported a median time to remission of 7.1 months with rituximab treatment, which was much longer than the 2.7 months observed in our study, indicating that obinutuzumab appeared to induce a faster remission in MN. In other sporadic case reports, obinutuzumab had demonstrated promising efficacy in complex, refractory MN, including rituximab-resistant MN, IgG4-associated MN, and MN cases with severe chronic kidney disease.9, 10, 11, 12 Our findings aligned with previous studies on rituximab-resistant patients, indicating that all 11 patients achieved remission, with 4 of them attaining CR by the end of the follow-up.
In our study, obinutuzumab demonstrated good tolerability, and no serious adverse events were reported among the patients. For the patient who experienced a severe infusion reaction to previous rituximab, obinutuzumab was well tolerated, indicating a lower immunogenicity of obinutuzumab as a humanized monoclonal antibody.
With the expanding utilization of rituximab in the management of MN, the treatment failure of rituximab prompted various speculations, such as concerns about anti-rituximab antibody development, rituximab underdosing because of urinary excretion, and incomplete depletion of B-cells in lymphoid organs.13,14
Obinutuzumab, as a humanized antibody, may hold the potential for enhanced efficacy by minimizing the risk of inadequate activity resulting from the production of anti-antibodies, which was supported by the fact that anti-rituximab antibodies neutralized the activity of rituximab in 80% of the cases but exhibited cross-reactivity with obinutuzumab in only 20% of the patients.13 However, Angeletti et al15 found that the presence of anti-rituximab antibodies did not correlate with clinical relapse or B-cell reconstitution in steroid-dependent nephrotic syndrome. Considering that the rate of rituximab resistance was much lower than the 80% rate of anti-rituximab development, factors other than anti-rituximab antibodies may account for rituximab resistance in MN. For rituximab underdosing, increased clearance, a shortened half-life, and lower serum concentrations of rituximab were observed in MN with heavy proteinuria, suggesting higher doses or dosing frequencies may increase the serum concentration and optimize the efficacy of rituximab. In our studies, the median course of previous rituximab treatment was 2.0 (1.3-4.8), comparable to the average dose of 2.1 ± 0.8 for obinutuzumab. However, despite the similar molecular weights of rituximab and obinutuzumab (145 kDa versus 146.1 kDa),16, 17, 18 obinutuzumab led to a significantly different remission outcome in MN. Therefore, we hypothesized that the superior efficacy of obinutuzumab observed in MN could be attributed to the deeper depletion of B-cells when compared with rituximab.
The mechanism by which anti-CD20 antibodies induce B-cell death mainly involves 4 pathways: direct cell death, antibody-dependent cytotoxicity, complement-dependent cytotoxicity, and antibody-dependent phagocytosis.14,18 In contrast to significant complement-dependent cytotoxicity and antibody-dependent cytotoxicity induced by rituximab, obinutuzumab induced minimal complement-dependent cytotoxicity but elicited greater direct cell death as a type II antibody, hence improving the antibody-dependent cytotoxicity and antibody-dependent phagocytosis contributed from the afucosylated Fc portion.14,18 This may minimize the risk of limited efficacy in BCD by activating complement-dependent cytotoxicity due to the expression of complement resistance factors and depletion of complement proteins on target cells.19,20 Despite the difference in the pattern of cell death, type II antibodies also exhibit dissimilarities with type I antibodies in their ability to be internalized after interaction with FcγRIIb expressed on B-cells, which demonstrated 5 times potency in depleting B-cells resulting from reduced macrophage recruitment and CD20/anti-CD20 antibody complex degradation, shortening half-life of anti-CD20 antibodies.21 The factors mentioned above may account for the superior efficacy of obinutuzumab when compared with rituximab observed in this study, but further research is necessary to confirm this efficacy and uncover the underlying mechanisms.
Our study had several limitations, as with many retrospective studies, there were limited or missing data and risks of selection bias or recall bias. To address these limitations, we applied clear inclusion criteria, conducted follow-ups of each patient with adequate observation time, and maintained comprehensive records within the electronic medical record system. In addition, the first relapse-free survival time might have been underestimated because 16 patients remained in remission by the end of the follow-up. Regarding that patient who did not achieve remission, it may be too early to regard him as a nonresponder considering the significant decrease in his serum PLA2R antibody titers. The failure to reach remission might be attributed to insufficient time for BCD and follow-up. Consequently, the efficacy might be underestimated, and we could anticipate promising results with additional cumulative doses and an extended follow-up period. Furthermore, because of limitations in observational studies and clinical practice, regular monitoring of CD19+ B-cells and other lymphocyte subsets was not conducted after obinutuzumab administration. Consequently, the investigation into the correlation between BCD or reconstitution and clinical outcomes remained constrained.
In conclusion, obinutuzumab demonstrated promising efficacy and safety in inducing remission in MN, especially in patients with an unsatisfactory response to rituximab. Further prospective studies and randomized controlled trials are required to confirm these findings.
Article Information
Authors’ Full Names and Academic Degrees
Yuxin Lin, MM, Quan Han, MD, Liangliang Chen, MM, Yaomin Wang, MM, Pingping Ren, MD, Guangjun Liu, MM, Lan Lan, MD, Xin Lei, MD, Jianghua Chen, MM, Fei Han, MD
Authors’ contributions
YL, QH, and FH were involved in the study design, conduct of the study, data collection, and interpretation. LC, YW, PR, GL, LL, XL, and JC were involved in patient follow-up. Each author contributed important intellectual content during article drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The study was supported by the Primary Research and Development Plan of Zhejiang Province (2020C03034) to Fei Han.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received August 15, 2023. Evaluated by 3 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form January 26, 2024.
Footnotes
Complete author and article information provided before references.
Table S1: Changes of Serum Anti-PLAZ2R Antibody Titers (RU/mL).
Supplementary Materials
Table S1.
References
- 1.Sethi S. New ”antigens” in membranous nephropathy. J Am Soc Nephrol. 2021;32(2):268–278. doi: 10.1681/asn.2020071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fervenza F.C., Appel G.B., Barbour S.J., et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381(1):36–46. doi: 10.1056/NEJMoa1814427. [DOI] [PubMed] [Google Scholar]
- 3.Gauckler P., Shin J.I., Alberici F., et al. Rituximab in membranous nephropathy. Kidney Int Rep. 2021;6(4):881–893. doi: 10.1016/j.ekir.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Illidge T., Klein C., Sehn L.H., Davies A., Salles G., Cartron G. Obinutuzumab in hematologic malignancies: lessons learned to date. Cancer Treat Rev. 2015;41(9):784–792. doi: 10.1016/j.ctrv.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Cartron G., Watier H. Obinutuzumab: what is there to learn from clinical trials? Blood. 2017;130(5):581–589. doi: 10.1182/blood-2017-03-771832. [DOI] [PubMed] [Google Scholar]
- 6.KDIGO 2021 Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4s):S1–s276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Sethi S., Kumar S., Lim K., Jordan S.C. Obinutuzumab is effective for the treatment of refractory membranous nephropathy. Kidney Int Rep. 2020;5(9):1515–1518. doi: 10.1016/j.ekir.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruggenenti P., Cravedi P., Chianca A., et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23(8):1416–1425. doi: 10.1681/asn.2012020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klomjit N., Fervenza F.C., Zand L. Successful treatment of patients with refractory PLA2R-associated membranous nephropathy with obinutuzumab: a report of 3 cases. Am J Kidney Dis. 2020;76(6):883–888. doi: 10.1053/j.ajkd.2020.02.444. [DOI] [PubMed] [Google Scholar]
- 10.Hudson R., Rawlings C., Mon S.Y., Jefferis J., John G.T. Treatment resistant M-type phospholipase A2 receptor associated membranous nephropathy responds to obinutuzumab: a report of two cases. BMC Nephrol. 2022;23(1):134. doi: 10.1186/s12882-022-02761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginthör N.E., Artinger K., Pollheimer M.J., Stradner M.H., Eller K. Membranous nephropathy associated with immunoglobulin G4-related disease successfully treated with obinutuzumab. Clin Kidney J. 2022;15(3):564–566. doi: 10.1093/ckj/sfab250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naik S., Shukla S., Av N., et al. Obinutuzumab in refractory phospholipase A2 receptor-associated membranous nephropathy with severe CKD. Kidney Int Rep. 2023;8(4):942–943. doi: 10.1016/j.ekir.2023.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teisseyre M., Boyer-Suavet S., Crémoni M., Brglez V., Esnault V., Seitz-Polski B. Analysis and management of rituximab resistance in PLA2R1-associated membranous nephropathy. Kidney Int Rep. 2021;6(4):1183–1188. doi: 10.1016/j.ekir.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng L., Xu G. Update on the application of monoclonal antibody therapy in primary membranous nephropathy. Drugs. 2023;83(6):507–530. doi: 10.1007/s40265-023-01855-y. [DOI] [PubMed] [Google Scholar]
- 15.Angeletti A., Bruschi M., Colucci M., et al. Circulating anti-rituximab antibodies do not affect response to rituximab in steroid-dependent nephrotic syndrome. Kidney Int Rep. 2022;7(11):2509–2512. doi: 10.1016/j.ekir.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pescovitz M.D. Rituximab, an anti-CD20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6(5 Pt 1):859–866. doi: 10.1111/j.1600-6143.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- 17.Said R., Tsimberidou A.M. Obinutuzumab for the treatment of chronic lymphocytic leukemia and other B-cell lymphoproliferative disorders. Expert Opin Biol Ther. 2017;17(11):1463–1470. doi: 10.1080/14712598.2017.1377178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman C.L., Sehn L.H. A tale of two antibodies: obinutuzumab versus rituximab. Br J Haematol. 2018;182(1):29–45. doi: 10.1111/bjh.15232. [DOI] [PubMed] [Google Scholar]
- 19.Cartron G., Watier H., Golay J., Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104(9):2635–2642. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 20.Mössner E., Brünker P., Moser S., et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beers S.A., French R.R., Chan H.T.C., et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood. 2010;115(25):5191–5201. doi: 10.1182/blood-2010-01-263533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.