Abstract
Phosphorus is critical for life and an indispensable element for biologically occurring organic molecules such as DNA, ATP, and phosphoproteomes. Butafosfan is a synthetically produced organic phosphorous (phosphonic acid) compound that contains 17.3% phosphorus. It does not belong to the group of biologically occurring organic phosphorous compounds. Butafosfan has been used in many animal species as a metabolic stimulant interfering with energy metabolism. Vitamin B12 (VB12) is a unique cobalt-containing vitamin. It functions as a cofactor for the enzymes methionine synthase and methyl-malonyl-CoA mutase. It is necessary for the conversion of propionate into succinyl-CoA in ruminants. A combination of butafosfan and VB12 (iBC) has been available for veterinary use since 1958 and the first publication appeared in the 1970s in cows. However, the first molecular biological studies about its mode of action appeared in early 2000s. Recent studies revealed that iBC has significant effects on carbohydrate and lipid metabolism. Investigations between 1970 and 1994 focused mainly on cows at risk of metabolic and reproductive disease in the dry period. Studies as of 2000 investigated its efficacy for the prevention and treatment of subclinical and secondary ketosis, adjunctive/supportive therapy for displaced abomasum operation, milk fever, improvement of postoperative rumen activity, uterus involution, and mastitis in transition dairy cows.
Keywords: butaphoshan, catosal, cattle, cyanocobalamin, metabolic diseases
Introduction
Phosphorus is a ubiquitous element, it is in and around us in the form of many different compounds. The number of known phosphorous compounds probably now exceeds 106 (Corbridge 2013). Phosphorus is physiologically and biochemically important for energy metabolism and storage, cell signalling (protein phosphorylation), DNA and RNA synthesis, and milk production. Lots of cellular activities, including internal/external cellular signalling and differentiation of cells are regulated by the reversible biochemical reactions of protein phosphorylation (phosphoproteomes) controlled by protein kinases and phosphatases (Graves and Krebs 1999; Venerando et al. 2017).
Vitamin B12 (VB12) is widely used in animal pharmaceuticals since its discovery as a factor in pernicious anaemia in 1948 (Shampo et al. 2006). This unique vitamin can be synthetized by certain bacteria and archaea, but not by plants or mammals except by ruminants (Nohr and Biesalski 2016; Combs and McClung 2017; Watanabe and Bito 2018). Due to its role as a cofactor for two of the enzymes responsible for energy metabolism and cell division (McDowell 2000), VB12 is indispensable, especially in dairy cattle and can be supplemented either via feed or parenterally for production purposes (Mahalle et al. 2019; Robinson 2019).
In the transition period (TP), which refers roughly a period of three weeks before and after calving, there is a largely increased requirement for energy and nutrients to support the development of the foetus and milk production in early lactation (Baumgard et al. 2017; Overton et al. 2017; Deniz et al. 2020). Decreased blood and liver concentrations of vitamin B12 (VB12) and inorganic phosphorus requires sufficient support in the early lactation period of transition dairy cows (Girard and Matte 1999; Graulet et al. 2007; Kincaid and Socha 2007; Grunberg et al. 2009; Furll et al. 2010; Fadlalla et al. 2020).
An organic phosphorous (phosphonic acid) compound butaphosphan (butafosfan) was synthetically produced and it is different from naturally occurring organic phosphorous compounds. It is currently labelled as a veterinary tonic and metabolic stimulant for the prevention or treatment of deficiencies of phosphorus in cattle, horses, swine, and poultry (EMEA 1999; EMEA 2000; Rollin et al. 2010; EMA 2014). Butafosfan is classified by the European Medicinal Agency (EMA) in the therapeutic class of mineral supplements and phosphorus sources for food-producing mammals without residues in milk and meat that includes cattle (EMA 2014).
Organic phosphorous compound butafosfan and VB12 were combined as an injectable product (injectable butafosfan/cyanocobalamin: iBC, Catosal®) in 1958 by Bayer Animal Health for veterinary use in cows and other animals. But numerous similar generic compositions are available currently. The rationale for this combination was probably to benefit the efficacy of both active ingredients for metabolic and reproductive challenges in different production stages of animals. Studies in the last 60 years have shown that iBC was widely used for the prevention, treatment and adjunct treatment of metabolic and reproductive diseases in transition dairy cows (Sommer and Starker 1971; Lohr et al. 2006; Deniz et al. 2010a; Furll et al. 2010; Deniz 2011; Szelenyi et al. 2015; Sahal et al. 2016; Gordon et al. 2017a; Gordon et al. 2017b). Published data in dairy cows provide pieces of evidence that iBC was often used around calving (both pre-, peri- and post-calving), but with different dosage regimes, protocol and indications, which differ from EMEA (1999), EMEA (2000) and EMA (2014) reports and label recommendations.
On the other side, there are no published document or comprehensive summary about the mode of action and clinical efficacy of butafosfan and iBC in transition dairy cows. This present review aimed to analyse and discuss critically the peer-reviewed published data generated between 1971 and January 2022 about the biological importance, mode of action and clinical efficacy of butafosfan/phosphorous and VB12 injectable combination in transition dairy cow.
Methodology
Various databases and search engines such as PubMed, ScienceDirect, Web of Science, Publons, Scopus, Google, Research Gate as well as proceedings of World Buiatrics Congress (WBC) and other international cattle congresses were used for the collection of original peer reviewed papers, oral or poster presentations, PhD theses and official institutional reports to create the present review. The present review focused on clinical use and mode of action of butafosfan and iBC in transition dairy cows only. Papers in mice (n = 3), poultry (n = 2), sheep (n = 1), dog (n = 1) and pigs (n = 2) were identified and included in the review as supportive data to discuss the mode of action of butafosfan and iBC.
Used keywords were butaphosphan, butafosfan, phosphorus, vitamin B12, cyanocobalamin, Catosal, transition period in dairy cow. Inclusion criteria of the references: papers in scientific peer reviewed journals, state regulatory reports, instructions and guidelines for the veterinary drugs (EMEA, EMA), commonly accepted official web page reports (NRC), international cattle congresses (e.g., WBC) and PhD thesis from the universities were included in the review. Exclusion criteria: papers published in not regular peer reviewed journals, papers in beef cattle and calves, papers which did not deliver a clear treatment protocol of iBC and butafosfan, reports published by a not official governmental web page and not having binding power were excluded in the review. If PhD theses or international congress papers were published in a scientific peer reviewed journal, they were excluded in the review.
Twenty-three peer-reviewed published papers, five congress papers, three PhD theses, two EMEA and one EMA reports were identified for butafosfan and iBC in dairy cattle. Furthermore, numerous original published papers and textbooks were available about the biological importance and mode of action of phosphorus, VB12 and TP in dairy cattle, than selected papers were listed in the references.
Butafosfan (butaphoshan) and iBC: Chemistry and biological importance
Butafosfan as a phosphonic acid derivative and an organic phosphorous compound has been used in animal health in the form of iBC and the first publications appeared in the 1970s (Sommer and Starker 1971; Sommer et al. 1975a; Sommer et al. 1975b; Zepgi et al. 1976; Wiedenroth 1979). Synthetically produced organic phosphonic acid compound butafosfan contains 17.5% phosphorus, not as phosphate and phosphoric acid. That makes it chemically different (Figure 1) from other phosphorus-containing organic compounds. Butafosfan (synonyms butaphosphan and butaphosphane) does not belong to the naturally occurring phosphorus-containing organic compounds like ATP, DNA, thiamine pyrophosphate, creatine phosphate, glucose-6-phosphate, phosphoenolpyruvate.
Figure 1. Chemistry of butafosfan (butaphosphan).
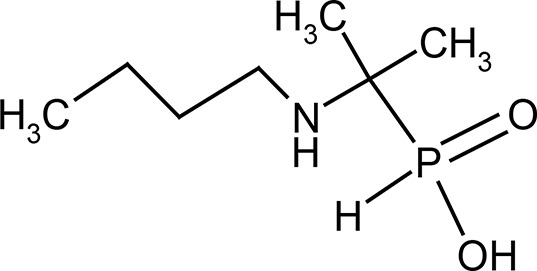
Chemical names: [1-(butylamino)-1-methyl-ethyl]-phosphonic acid phosphonic acid, P-[1-(butylamino)-1-methylethyl]-2-(butylamino)propan-2-yl-hydroxy-oxophosphanium
Molecular formula: C7H18NO2P+
CAS No.: 17316-67-5
Molecular weight: 178.19 g/mol
Melting point: 219 °C
Phosphorous content: 17.3 mg phosphorous/100 mg
The rate of gluconeogenesis and glycolysis is dependent on phosphorus availability in the organism because all intermediary molecules must be phosphorylated (Berg et al. 2006). Especially after calving, when lactation starts stored phosphorus content in the liver drops (Grunberg et al. 2009) thereby increasing the phosphorus requirement for energy metabolism, milk production and milk phosphoproteins (Corbridge 2013; Manuelian et al. 2018). Consequently, there is an enormous requirement for a lactating cow for these minerals, glucose/galactose and amino acids every day. Particularly when the dry dairy cows enter into the lactation stage (Deniz et al. 2020).
Organic phosphorous compound butafosfan is known as an amino phosphonic acid derivative, [1-(n-butylamino)-1-methylethyl]-phosphonic acid (EMEA 1999; EMEA 2000; EMA 2014). Butafosfan (10%) was combined with 0.005% of VB12 in an injectable form (iBC) for veterinary use in animal health in 1958. It is labelled worldwide as a veterinary tonic and metabolic stimulant for animals (Rollin et al. 2010) and classified by EMA (2014) in the therapeutic class of mineral supplements and phosphorus sources for food-producing mammals. EMEA (2000) reported that the major indications were disorders of the metabolism and supportive treatment for infertility, tetany and paresis. However, various field studies were conducted up to now and they indicated miscellaneous field of use other than label claims (Tables 1, 2, 3). Butafosfan does not produce residues in milk and meat that might be risky for human health (EMA 2014). There are two different combinations containing butafosfan with either calcium, magnesium salts or cyanocobalamin registered in the European Union (EMEA 1999) and other parts of the world.
Table 1. Studies as of the year 2000 about the preventive use of iBC at prepartum (PrP), calving (Cal) and postpartum period (PP) in transition dairy cows.
| Reference | Treatment protocol with iBC | Significant effects on blood parameters | General health status and effects on other parameters | |||||||||
| aim | time | volume for 100 kg b.w. or cow | route | number of days | NEFA | BHBA | glucose | P | Ca | |||
| Chalmeh et al. (2020) 1,G | A | PrP | 2 ml | i.v. | 9 | ↓ | ↓ | ↑§ ↓+ | NS | NS | Highest insulin sensitivity observed in 6 ml group. 6 ml group had the lowest NEFA and BHBA levels. Blood glucose (Gu) was higher after calving and before i.v. Gu infusion. | |
| Chalmeh et al. (2020) 1,G | A | PrP | 4 ml | i.v. | 9 | ↓ | ↓ | ↑§ ↓+ | NS | NS | ||
| Chalmeh et al. (2020) 1,G | A | PrP | 6 ml | i.v. | 9 | ↓ | ↓ | ↑§ ↓+ | NS | NS | ||
| Antunes et al. (2019)2,* | B | Cal | 20 ml/cow | i.m. | 5 | NE | NE | NE | NE | ↑++ | Lower blood pH and higher pCO2 in both groups. Glucose level tended to be higher in butafosfan group. | |
| Antunes et al. (2019)2,** | B | Cal | 20 ml/cow | i.m. | 5 | NE | NE | ↑ | NE | ↑++ | ||
| Yildiz (2016) 3 | C | Prp/Cal | 5 ml | s.c. | 3 | NS | ↓ | NS | NS | NS | A 4.3 times lower RP incidence compared to control group. | |
| Pereira et al. (2013b) 4 | B | Cal | 10 ml/cow | i.m. | 4 | ↓ | ↓ | NE | NE | NE | Increased milk production and reduced NEB intensity were observed in both dosages. | |
| Pereira et al. (2013b) 4 | B | Cal | 20 ml/cow | i.m. | 4 | ↓ | ↓ | NE | NE | NE | ||
| Furll et al. (2010) 5 | B | PrP | 10 ml | i.v. | 3 | NE | NE | NE | NE | NE | Rate of puerperal infection was 13%, 24% and 53% in 6 × 10 ml, 3 × 5 ml and control group respectively. 6 × 10 ml group received the fewest intrauterine or injectable antibiotic treatments just after calving. | |
| Furll et al. (2010) 6 | B | PrP | 10 ml | i.v. | 6 | ↓ | ↓ | ↑ | ↑ | NE | ||
| Rollin et al. (2010)7,*** | D | Cal | 25 ml/cow | s.c. | 2 | NS | ↓ | NS | NE | NE | Cows with parity ≥ 3 and long dry periods, high BCS, or retained placenta and dystocia had benefits from the treatment. | |
| Gegenbach (2009) 8 | B | Prp/PP | 20 ml/cow | s.c. | 2 | NE | NE | NE | NE | NE | The milk yield of PP group was higher on PP days 45 and 100. The first artificial insemination was significantly earlier in PP group. | |
| Gegenbach (2009) 9 | B | PP | 20 ml/cow | s.c. | 2 | NE | NE | NE | NE | NE | ||
Aim A = against insulin resistance, glucose infusion was given at PP week 1, 2 and 3 to all; aim B = improvement of metabolic profile and prevention from postpartum metabolic and reproductive diseases; aim C = prevention from the retained placenta; aim D = prevention from SCK; BCS = body condition score; BHBA = beta-hydroxybutyric acid; iBC = contains 100 mg butafosfan and 0.05 mg cyanocobalamin/ml; NE = no significant effect was found; NEB = negative energy balance; NEFA = non-esterified fatty acid; NS = not studied; P = phosphorus; SCK = subclinical ketosis
1iBC was used for 3 consecutive days between PrP days 21–19, 12–10, 3–1 relative to calving (total 9 treatments); 2iBC was used starting at calving for 5 consecutive days; 3iBC was used for 3 consecutive days (before calving and once at calving); 4iBC was used every 5 days from calving up to postpartum day 20 (total 4 treatments); 5iBC was used between PrP days 7 to 3 relative to calving (total 3 consecutive days); 6iBC was used between PrP days 14 to 10 and 7 to 3 relative to calving (total 6 treatments); 7iBC was used as a blind treatment at calving and day after in 4 herds with a total of 1 122 cows; 8iBC was used 10 days before calving and between 2–4 days after calving (total 2 treatments); 9iBC was used between 2–4 and 5–7 days after calving (total 2 treatments); GA generic iBC was used
↓ = decreased; ↑ = increased; §Before i.v. glucose infusion to test insulin resistance at 1st week after parturition; +Significantly decreased after i.v. glucose infusion to test insulin resistance; ++Ionised calcium
*iBC treatment; **Butafosfan 10% solution only (without VB12); ***Effects was observed in mature cows only
Table 2. Recent studies on the use of iBC for the treatment of subclinical ketosis (SCK) in transition dairy cows.
| Reference | SCK* diagnosis | Treatment protocol with iBC | Significant effect on blood parameters | General health status and effects on other parameters | |||||||
| BHBA threshold (mmol/l) | PP time (day) | volume for 100 kg b.w. or cow | route | number of days | NEFA | BHBA | glucose | ||||
| Gordon et al. (2017a) | 1.2 | 3–16 | 25 ml/cow | s.c. | 3 | NS | NE | NE | Ketotic animals with blood glucose levels ≤ 2.2 mmol/l were 2.1 times more likely to be cured if treated with iBC. Animals in LN ≥ 3.0 and with blood glucose < 2.2 mmol/ produced 2.8 kg/day more milk if treated with iBC. | ||
| Gordon et al. (2017b) | 1.2 | 3–16 | 25 ml/cow | s.c. | 3 | NS | NE | NE | Ketotic cows with blood glucose levels < 2.2 mmol/l produced 3.1 kg/day more milk if treated with iBC. | ||
| Sahal et al. (2016) 1 | 1.0 | 7–15 | 10 ml | i.m. | 4 | ↓ | ↓ | NS | Increased milk production, faster uterus involution, shorter days-open period and better conception rate were observed in treatment groups. Serum creatine kinase activity and bilirubin levels were significantly decreased in 10 ml group. Control cows lost more BCS at PP day 60. | ||
| Sahal et al. (2016) 1 | 1.0 | 7–15 | 5 ml | i.m. | 4 | NE | ↓ | NS | |||
| Nuber et al. (2016) (butafosfan 10% group) | 1.2 | 14–28 | 10 ml | i.v. | 3 | NE | ↓ | NE | Plasma glucagon concentrations were high in butafosfan group. Hepatic mRNA abundance of liver X receptor A, a nuclear receptor protein involved in lipid metabolism, was higher in iBC. The mRNA abundance of BHBA-DHG 2 in the liver was higher in iBC group. | ||
| Nuber et al. (2016) (iBC group) | 1.2 | 14–28 | 10 ml | i.v. | 3 | ↓ | ↓ | NE | |||
| Szelenyi et al. (2015) | 1.0 | 0–3 | 20 ml/cow | i.m. | 3 | NS | ↓ | NS | BHBA level of iBC group decreased below the threshold on day 10 after calving. The service period and culling rate in 200 DIM was better in iBC group. Better production parameters was reached using iBC. | ||
| Deniz et al. (2010a) | 0.95–4.0 | 14 | 5 ml | i.m. | 4 | NS | NE | NS | The conception rate (89%), number of inseminations (n = 2.2), days-open period (113 days) of iBC group were significantly better than control (40%, n = 5, 180 days) and generic 3 (44%; n = 4.5; 190 days) groups. Blood BHBA was reduced faster and greater in iBC group than in the control, generics 1, 2 and 3 groups, but the difference was not significant. | ||
| Cuteri et al. (2008) | 200** | – | 25 ml/cow | i.m. | 5 | NS | ↓ | NS | The prevalence of SCK was significantly reduced at day 10 posttreatment. Daily milk production increased significantly by 3.4 kg (calculated) by the iBC treatment. Anorexia decreased significantly at day 5th after iBC treatment. | ||
BCS = body condition score; BHBA-DHG 2 = 3-hydroxybutyrate dehydrogenase 2; BHBA = beta-hydroxybutyric acid; b.w. = live body weight; DHG = dehydrogenase; DIM = days in milk; iBC = contains 100 mg butafosfan and 0.05 mg cyanocobalamin/ml; LN = lactation number; NE = not effective; NEFA = non-esterified fatty acids; NS = not studied; PP = postpartum
1iBC was applied for 4 consecutive days after diagnosis of SCK; *SCK (subclinical ketosis) defined by elevated BHBA in the blood or milk; **200 μmol/l for milk BHBA threshold value, other values in the same row refer to blood BHBA threshold; ↓ = decreased; ↑ = increased
Table 3. Recent studies on the use of iBC for the supportive and adjunct treatments in transition dairy cows.
| Reference | Aim | Treatment protocol with iBC | Significant effect on blood parameters | General health status and effects on other parameters | ||||||||
| time | volume for 100 kg b.w. or cow | route | number of days | NEFA | BHBA | glucose | P | Ca | ||||
| Tabeleao et al. (2016) 1 | A | PP | 10 ml | i.v. | 3 | NE | NS | NE | NS | NS | Significant positive contribution in recovery and in the reduction of milk somatic cell counts in iBC group. | |
| Lohr et al. (2006) 2 | B | PP | 5 ml | i.v. | 3 | NE | ↓ | NS | NS | NS | 65% and 81.6% of iBC group had healthy rumen activity on post-operative days 2 and 3 respectively. It was only 28% and 63.3% in the control group. The decrease of serum BHBA in iBC group was higher than in the control group. | |
| Furll et al. (2006) 3 | B | PP | 5 ml | i.v. | 1 | ↓ | ↓ | NE | NS | NS | Earlier rumination, better DMI and rumen activity in postoperative 8–12 h were observed in iBC group. iBC treated cows had lower serum levels of bilirubin and AST in postoperative 48 and 72 hours. | |
| Delport et al. (2006) 4 | C | PP | 5 ml | i.v. | 1 | NS | ↓ | NS | NS | NE | iBC stabilized better serum calcium levels. A lower incidence of SCK was found in iBC (26.7%) compared to control (42.9%) group. | |
| Deniz et al. (2009b) 5 | D | Cal | 50 ml/cow | i.v. | 2 | NS | NE | ↑ | NS | NS | Higher DMI on PP days 3–17 and better and faster uterus involution on PP days 12–17 (30% completed) and 30–35 (93.5% completed) were observed in iBC group. Control group did not complete uterus involution on PP day 17. Treatment reduced serum GLDH enzyme and cholesterol levels on PP day 12. Time to first service was tendentious reduced (6 days) by the iBC treatment. | |
Aim A = for fast recovery after the antimicrobial treatment in mild and moderate mastitis; aim B = supportive treatment for left abomasal operation to prevent from secondary ketosis; aim C = supportive treatment for milk fever; aim D = supportive and adjunct treatment for the better uterus involution and metabolic profile; BHBA = beta-hydroxybutyric acid; b.w. = live body weight; DMI = dry matter intake; iBC = contains 100 mg butafosfan and 0.05 mg cyanocobalamin/ml; NE = not effective; NEFA = non-esterified fatty acids; NS = not studied; P = phosphorus; PP = postpartum; SCK = subclinical ketosis
1iBC was used every 5 days after antimicrobial therapy (total 3 treatments); 2iBC was used starting at the operation day for 3 consecutive days; 3iBC was used 2 h before the start of operation of left abomasal displacement (plus: 0.9% NaCl 20 l + 40% dextrose + sodiumbicarbonate + oxytetracycline); 4i.v. treatment with electrolytes (calcium and magnesium) at the diagnosis followed by Catosal application 15–30 min later; 550 ml iBC and 1 000 ml infusion of calcium (24%) + magnesium (6%) (at calving and day after); ↓ = decreased; ↑ = increased
In the following reports, butafosfan with or without VB12 combination did not significantly increase phosphorus concentration in the blood of dairy cattle (Rollin et al. 2010; Kreipe et al. 2011; Pereira et al. 2013b; Antunes et al. 2019; Scharen et al. 2021).
In contrast, significantly increased concentrations of blood phosphorus were reported in dairy cattle by Furll et al. (2010); Krdzalic and Curcic (1976), in mice treated with much higher doses of butafosfan (50 mg/kg b.w.) by Weiller et al. (2020) and in ewes by Pereira et al. (2013a). Non-ketotic cows treated with iBC produced 2.8 kg more milk per day (calculated) resulting in lower blood phosphorus concentrations (Kreipe et al. 2011). Increased milk production trend was also reported in SCK treatment by others (Cuteri et al. 2008; Sahal et al. 2016; Gordon et al. 2017a; Gordon et al. 2017b) or in metabolic improvement by Scharen et al. (2021) after iBC treatment, consequently with dose-dependent lower blood phosphorus concentration (Furll et al. 2010; Scharen et al. 2021).
Although butafosfan was included in the mineral supplement class (EMEA 2000; EMA 2014), it is still not clarified how much phosphorus bound in butafosfan is available to animals. That might be possible the effect of butafosfan is simply a matter of phosphorus substitution, but butafosfan is metabolically very stable following injection in cattle, the majority of this active compound is excreted unchanged in the urine (EMEA 1999; EMEA 2000; EMA 2014). Less than 12% of butafosfan was degraded when incubated with liver microsomes from pigs, cattle and rats, and no metabolites were obtained (EMA 2014). Thus, it is more likely that butafosfan acts stand alone in the organism.
Butafosfan: Toxicity
According to EMEA (1999), the acute toxicity of butafosfan is very low with an oral LD50 in mice of around 16 000 mg/kg b.w. The parenteral LD50 in mice for subcutaneous (s.c.) administration is 21 000 mg/kg b.w. and 10 000 mg/kg b.w. for intravenous (i.v.) administration, and greater than 2 500 mg/kg b.w. for intraperitoneal administration. Mutagenicity studies have confirmed that it is not mutagenic. The LD50 value for intramuscular (i.m.) administration is 9 974 mg/kg b.w. in chickens. No evidence of teratogenicity, fetotoxicity, maternal toxicity was observed after oral administration of 50, 250 or 1 000 mg/kg b.w. of butafosfan to pregnant Wistar rats between days 6–19 of gestation. Residue depletion studies were conducted with butafosfan administered intramuscularly at a dose of 10 mg/kg b.w. once daily for five consecutive days in pigs. That revealed there was no risk of consumers being exposed to butafosfan residues at limits above the average daily intake (36 mg/person), and consequently the establishment of numerical maximum residue limits was not necessary to conduct for human health. This conclusion of the residue depletion studies was expanded to all mammalian food producing species (EMA 2014).
Butafosfan: Pharmacokinetic
Butafosfan was eliminated within 12 h via urine (77%) after a single i.v. dose of 5.6 mg/kg b.w. in lactating and nonlactating cattle as a parent compound (EMEA 2000). It is rapidly eliminated from the organism after i.v. application, with a half-life of 116 min in dairy cows (EMEA 1999; EMEA 2000). The pharmacokinetic parameters were generally similar between the i.m. and s.c. routes and showed rapid absorption and elimination (terminal elimination half-life: 3.5–3.7 h) following a single administration of 10 mg/kg b.w. in pigs (EMA 2014). Study results in swine and cattle revealed that the pharmacokinetic pattern, with rapid elimination, is similar after i.v., i.m. and s.c. administration. The pharmacokinetic pattern is expected to be similar across mammalian food producing species (EMA 2014).
Evidences about the mode of action of butafosfan as a single molecule
EMEA (1999) reported that the precise mode of action of butafosfan was unknown. Many of the studies suggest that it is acting pharmacologically as a complete molecule rather than supplying phosphorus to the organism because blood phosphorus concentration was not significantly changed after iBC administrations in cattle (Rollin et al. 2010; Kreipe et al. 2011; Pereira et al. 2013b; Antunes et al. 2019; Scharen et al. 2021). This is in line with the pharmacokinetic data which confirmed that butafosfan was eliminated rapidly and a mean of 77% of butafosfan was recovered in the urine within 12 h (EMEA 2000).
The most recent in vivo biochemical studies conducted on both butafosfan alone or in combination with VB12 (iBC) showed that it interfered with metabolic function and the energy processes in the organism (Hasi et al. 2005a; Furll et al. 2010; Kreipe et al. 2011; Gomes Jose et al. 2012; Nuber et al. 2016; Antunes et al. 2019; Weiller et al. 2020; Scharen et al. 2021). We identified seven studies about the effect of butafosfan as a single compound in mice (Hasi et al. 2004; Hasi et al. 2005a; Weiller et al. 2020), in poultry (Hasi et al. 2005b; Hasi et al. 2005c) and in cattle (Nuber et al. 2016; Antunes et al. 2019).
Butafosfan improved liver and muscle glycogen concentrations and promoted significantly the synthesis of ATP and ADP, but decreased AMP in mice (Hasi et al. 2004). A study investigating the mode of action of butafosfan (Weiller et al. 2020) showed that an injection of butafosfan at 10 × recommended dose rate (50 mg/kg b.w., twice a day, 7 days) in mice fed a hypercaloric diet and then food-restricted, preserved epididymal white adipose tissue mass, reduced fat mobilization, increased blood glucose concentration and the homeostatic model assessment index (insulin resistance index). Mice treated with butafosfan had increased expression of mRNA associated with fatty acid metabolism such as ACOX1 (peroxisomal acyl-coenzyme A oxidase 1), CPTa (carnitine palmitoyltransferase 1A), and ACACa (acetyl-CoA carboxylase alpha) and with glucose metabolism such as IRS2 (insulin receptor substrate 2) and GK (glukokinase). The results indicated that butafosfan significantly reduced fatty acid accumulation in the liver tissues of animals that are diet restricted and that this was associated with increased gene expression. However, serum concentration of phosphorus did not increase despite the high dose rate. Antunes et al. (2019) reported that butafosfan with or without cyanocobalamin increased ionized calcium levels and tended to stabilise blood pH in early postpartum cows, and butafosfan without cyanocobalamin tended to increase blood glucose concentrations. Another study was conducted in dairy cattle with subclinical ketosis and treated with either injectable butafosfan, iBC or saline (Nuber et al. 2016). Results of this study indicated that the application of butafosfan in combination with cyanocobalamin (iBC) had a positive effect on energy/lipid metabolism only. The abundance of mRNA encoding the beta-hydroxybutyrate dehydrogenase 2 in the liver was higher in iBC compared to the butafosfan and control groups. The hepatic abundance of liver X receptor alfa mRNA, a nuclear receptor protein involved in lipid metabolism, was increased with iBC administration. Consequently, plasma non-esterified fatty acids (NEFA) concentration was also significantly reduced with the iBC treatment and moderately reduced in the butafosfan group. However, there was a significant reduction of plasma beta-hydroxybutyric acid (BHBA) in both iBC and butafosfan groups. Butafosfan had positive effects on the macrophage phagocytosis index, CD4+ helper cells counts, CD4+/CD8+ ratio and humoral immunity in mice (Hasi et al. 2005a), enhanced significantly energy metabolism and the ability to withstand stress due to cold, heat and hypoxia in broiler chicks treated preventively with oral butafosfan three days before the onset of stress (Hasi et al. 2005b; Hasi et al. 2005c). Serum alkaline phosphatase, creatinine phosphokinase, and lactate dehydrogenase activities were significantly reduced, but glucose concentration was increased in the butafosfan treated chicks (Hasi et al. 2005b; Hasi et al. 2005c).
If summarised this section in different animal species: the interaction of butafosfan with the carbohydrate and lipid metabolism and immune system prevented an increase of NEFA and BHBA, increased glycogen, ATP, ADP synthesis, modulated immune system, enhanced the ability to withstand cold and heat stress situations while an increase of glucose but not phosphorus observed.
Mode of action of vitamin B12
VB12 is an important active component of iBC. VB12 is an essential cofactor for the activity of enzymes like methyl-malonyl-CoA mutase (MCM) and methionine synthase (MS) (McDowell 2000). MCM, a mitochondrial enzyme, is involved in the conversion of propionate to succinyl-CoA. Succinyl-CoA is an important gluconeogenic substrate that enters the TCA cycle (Krebs cycle) (Kennedy et al. 1992; McDowell 2000) in the liver for the synthesis of NADH + H, FADH2 and ATP to supply energy for the organism. An important energy source, propionic acid produced in the rumen of ruminants can enter the TCA after conversion to succinyl-CoA in the presence of activated MCM enzyme (McDowell 2000). The activity of MS is dependent on VB12 because MS is responsible for the synthesis of methionine via the transfer of a methyl group from 5-methyl-tetrahydrofolate to homocysteine (McDowell 2000). Methionine is considered an important limiting amino acid in milk protein synthesis (NRC 2001) and takes a key role as a methyl donor in the synthesis of S-adenosylmethionine (McDowell 2000). It is also important for choline synthesis and increases milk yield and fat concentration without an effect on the level of milk protein (Wang et al. 2010).
Evidences about the mode of action of iBC
Studies about iBC as combined form of VB12 and butafosfan revealed that it increased the energy parameters such as ATP and ADP in the liver and muscle in cattle (Gomes Jose et al. 2012). They used a single injection of 10 ml/100 kg b.w. of iBC and that resulted in increased liver concentrations of ATP, ADP, AMP and glycogen and cell energy charge and increased energy charge, ATP and ADP in the muscle at 6 h after the injection. This is in line with the study results by Hasi et al. (2004) in mice treated with butafosfan. However, Kreipe et al. (2011) were also able to establish significant effects of iBC on energy metabolism in dairy nonketotic lactating cattle. The cows were treated with iBC at a dose of 10 ml/100 kg b.w. (10 mg butafosfan/kg b.w. + 0.005 mg cyanocobalamin/kg b.w.) as i.v. for 3 days had a significantly decreased abundance of mRNA coding for acyl-coenzyme A synthetase long-chain family member 1 (ACSL1) in the liver. ACSL1 is an enzyme involved in fatty acid β-oxidation in the liver (Kreipe et al. 2011; Huh et al. 2020). ACSL1 plays an important role in lipid metabolism, mainly present in muscle, liver, adipose tissue, and heart and it regulates the composition of unsaturated fatty acids in the skeletal muscle of cattle, which in turn regulates the fatty acid synthesis and the generation of lipid droplets (Zhao et al. 2021).
As presented in Tables 1, 2 and 3, numerous studies have been conducted using iBC at various dose rate in transition dairy cows. Blood concentrations of NEFA and BHBA were significantly reduced if used at a dose of 5–10 ml/100 kg b.w. by multiple i.m. or i.v. administration in subclinical ketosis (SCK) treatment (Table 2). However, no significant effect of iBC on blood BHBA was observed by others (Gordon et al. 2017a; Gordon et al. 2017b) in SCK treatment with a much lower dosage, whereas Rollin et al. (2010) reported a significant reduction of blood BHBA concentration with similar dosage in multiparous dairy cows. Increased blood glucose concentration was reported by Chalmeh et al. (2020), Furll et al. (2010), Deniz et al. (2009b) and Krdzalic and Curcic (1976) after iBC treatment at pre-, peri- and the postpartum period in dairy cow, but there are also studies in those no effect of iBC was observed on blood glucose (Tables 1, 2, 3). Thus, the effect of iBC on blood glucose concentration in transition cows can be multifactorial and needs to be studied in detail. Most recently, Chalmeh et al. (2020) tested three different dosages of a generic iBC against insulin resistance in dairy cows treated in the dry period. Blood glucose concentrations of cows treated with iBC were significantly higher than those in control group postpartum. However, after the study cows received glucose infusion (50% glucose as i.v.) at postpartum week 1, 2 and 3, blood glucose and insulin concentrations were significantly lower in the iBC treatment groups compared to the control group and 6 ml/100 kg b.w. was more effective in preventing insulin resistance. The treatments decreased postpartum blood NEFA and BHBA concentrations, but no effect was observed on milk yield and BCS (Table 1). In addition, Scharen et al. (2021) observed a clear dose-dependent effect of iBC on several acylcarnitines and phosphatidylcholines that indicated a more efficient influx and oxidation of NEFA and consequently an increase in energy supply and more efficient triglyceride (TG) export in the liver of transition dairy cows at postpartum day 7. But, the observation of not increased or partially decreased blood phosphorus concentrations in different metabotypes might be due to higher milk production of treated cows (Scharen et al. 2021) which was also discussed by Furll et al. (2010) and Kreipe et al. (2011).
Moreover, the immunomodulatory effect of iBC was studied in pregnant sows and newborn piglets (Deniz et al. 2010b). The treatment of pregnant sows with iBC at a dosage of 20 ml i.m. at 3, 2 and 1 week before farrowing increased IgG, IgA and IgM in the colostrum of sows and IgA concentration was also higher in the blood of newborn piglets. Thus, the immunomodulatory effect of butafosfan in mice (Hasi et al. 2005a) was confirmed at certain extent in sows treated with iBC and in their newborn piglets. These findings should be repeatable with double-blinded field studies in transition dairy cows. That can be the next study subject.
Furthermore, subcutaneous injection of iBC reduced the stress-induced salivary cortisol concentrations and aggressive behaviour pigs (Van der Staay et al. 2007). The reduction of side effects of dexamethasone administration for 7 days was observed on biochemical and haematological indicators in dogs if they were treated simultaneously with iBC (Deniz et al. 2009a). A dexamethasone dependent decrease in amylase activity recovered faster in iBC treated dogs and the dexamethasone induced increase of liver enzyme gamma glutamine transaminase was kept within the reference range in iBC treated dogs (Deniz et al. 2009a).
To summarise this section in different animal species: the effect of the compound butafosfan was confirmed by dose dependent-treatments of iBC in selected clinical cases, especially on blood NEFA, BHBA, TG, immune indicators, glucose, tissue ATP, ADP and glycogen concentrations, and stress marker cortisone.
Vitamin B12: Chemistry and biological importance
VB12 also known as cobalamin or cyanocobalamin is a water-soluble, complex structured vi-tamin consisting of a porphyrin-like, cobalt-centred, corrin-nucleus (Figure 2). It is essential for lipid metabolism, methionine synthesis, energy provision and cell division in people and animals (McDowell 2000; Nohr and Biesalski 2016; Combs and McClung 2017; Rizzo and Lagana 2020). The central cobalt atom is bound with four coordinating nitrogen atoms forming a tetrapyrrolic corrinic nucleus with a 5,6-dimethylbenzimidazole base (DMB) at the lower side. DMB conformation seems to be crucial for interaction as a cofactor with the final enzymes (Rizzo and Lagana 2020). VB12 is a generic description for all compounds containing a corrin-nucleus with the same biological characteristics as cyanocobalamin (Combs and McClung 2017).
Figure 2. Chemical structure of vitamin B12 and isoforms [adapted from Rizzo and Lagana (2020)].
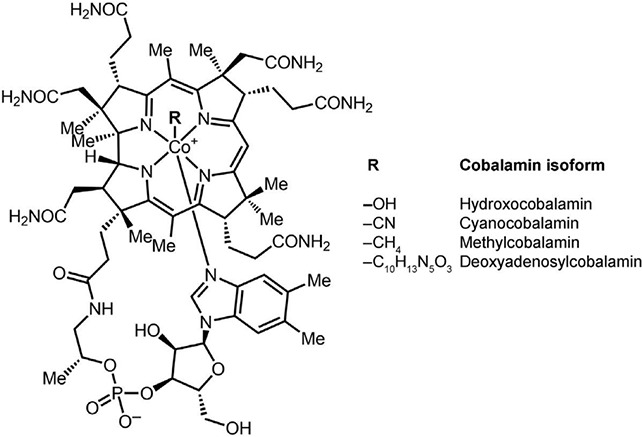
Cyanocobalamin is the first isoform in the group labelled VB12. Six different forms of cobalamin are reported as cyano-, methyl-, 5-deoxyadenosyl-, hydroxy-, aqua- and nitro-cobalamin (Nohr and Biesalski 2016; Combs and McClung 2017). A 5-deoxyadenosyl and methyl group bound to the cobalt atom form the adenosylcobalamin and the methylcobalamin, respectively. These alkyl cobalamin isoforms of VB12 have a biochemical cofactor function (Rizzo and Lagana 2020) for the enzymes.
As only a few species of micro-organism such as some bacteria, blue algae and yeast (Friedrich 1987; Smith 1997) can produce VB12, synthesis is not possible in plants, human and animals other than ruminants. There are both aerobic and anaerobic microbial biosynthetic pathways of VB12 compounds (Watanabe and Bito 2018). Ruminants are dependent on microbial VB12 synthesis in the rumen. Rumen microorganisms, mainly anaerobes or archaebacteria can synthetize VB12 in the presence of cobalt ions (Smith and Loosli 1957; NRC 2001; Gille and Schmid 2015; Watanabe and Bito 2018). Information about the variety of the microorganism species that synthetize VB12 in the rumen is limited. Dryden et al. (1962) were able to identify the VB12 synthesizing bacteria in the rumen that were Selenomonas ruminantium and Peptostreptococcus elsdenii. Species that degrade ruminal fibre seem to be of major importance for the synthesis of VB12 (Swanon et al. 2011).
VB12 production in the rumen depends on the availability of cobalt and the level of fibre in the feed. The requirement of cyanocobalamin for dairy cattle is 0.34 μg/kg to 0.68 μg/kg of live weight (NRC 2001) and 0.2 mg to 0.5 mg for most Holsteins (Rollin et al. 2010). It has been shown that the liver is an essential storage tissue for VB12 in cattle (Millar et al. 1984). VB12 deficiency resulting from Co deficiency, leads to a reduction in the activity of MCM and to hyperhomocysteinaemia, with a consequent rise in circulating levels of methylmalonic acid (MMA). Serum MMA concentrations seem to give the most accurate indication of Co deficiency, but serum VB12 concentrations can be used as a prognostic indicator of Co deficiency (Fisher and MacPerson 1990). Cobalt deficiency resulted in reduced serum and liver VB12 level and an accumulation of homocysteine in the plasma of male Simmental cattle (Stangl et al. 2000). However, dairy cows fed an optimum ration supplemented with additional cobalt or VB12 during the transition period did not have increased blood VB12 concentrations (Weerathilake et al. 2018) and a basal dietary concentration of 0.2 mg cobalt/kg DM was sufficient to meet the requirements of high yielding dairy cows during this time (Weerathilake et al. 2018). VB12 synthesis in the rumen has been shown to increase with cobalt supplementation in growing beef cattle (Stangl et al. 2000). VB12 is excreted via milk especially in early lactation (Kincaid and Socha 2007). A reduction in the blood concentration of VB12 has been observed during and after parturition and during early lactation in dairy cows (Kincaid and Socha 2007; Girard et al. 2009; Furll et al. 2010; Akins et al. 2013; Duplessis et al. 2017; Weerathilake et al. 2018). This is probably due to an increased demand arising from the secretion of VB12 in milk, increased metabolism and reduced dry matter intake (DMI) during the early lactation period (Kincaid and Socha 2007).
Transition dairy cows and clinical effects of iBC
TRANSITION DAIRY COWS: IMPORTANCE OF PHOSPHORUS/BUTAFOSFAN AND VB12
The indispensable importance of phosphorus for living creatures has been well-known since its invention in 1669 (Corbridge 2013; Walsh 2020), as the invention of VB12 in 1948 (Shampo et al. 2006). It is a fact that blood concentrations of VB12 (Girard and Matte 1999; Kincaid and Socha 2007; Furll et al. 2010) and inorganic phosphorus (Furll et al. 2010; Fadlalla et al. 2020) and liver concentration of phosphorus (Grunberg et al. 2009) and VB12 (Graulet et al. 2007) drop in the early lactation period of dairy cows.
There is a large requirement for energy, especially for milk production in early lactation and throughout TP. Ration deficiencies and nonadaptive feeding in the dry-off and TP, fatty liver syndrome, and negative energy balance (NEB) can lead to postpartum metabolic and reproductive disorders and milk production loss (Baumgard et al. 2017; Overton et al. 2017; Deniz et al. 2020). One of the most important metabolic diseases is ketosis and SCK in early lactation of dairy cattle (Overton et al. 2017; Deniz et al. 2020).
The rationale for the indication of iBC based probably on these challenges in TP of dairy cows to benefit the biological functions of two active ingredients. However, can ruminants with normal ruminal function fed an adequate dietary cobalt level still suffer from clinically observable VB12 deficiency? Given that VB12 is stored in the liver and synthetised in the rumen, if the answer is yes, what would be the observed harmful consequences? This is still unclear. The observed physiological reduction of blood VB12 in early lactation is a consequence of the immediate requirement for high milk production because VB12 is excreted via milk (Kincaid and Socha 2007), just like calcium, phosphorus and many other compounds.
Although some authors believe that iBC aids phosphorus for ATP synthesis in liver metabolism (Rollin et al. 2010), and it provides mineral to the organism (EMEA 2000), it is still questionable because majority of injected butafosfan is excreted unchanged via urine and blood phosphorus was not increased after iBC administration in some studies (Rollin et al. 2010; Kreipe et al. 2011; Pereira et al. 2013b; Antunes et al. 2019; Scharen et al. 2021). This might lead to the conclusion that butafosfan itself as stand-alone functions in the metabolic processes.
The milk production per lactation increased significantly from 2 000 kg to 10 300 kg worldwide in the last years due to high demand for milk consumption (Baumgard et al. 2017). This might trigger NEB and consequently postpartum metabolic diseases in overstressed dairy cows and as a consequence, the need for the minerals, tonics and vitamins can increase to support the metabolic functions in TP.
TRANSITION DAIRY COWS: CLINICAL USE OF iBC
Details of recent clinical studies conducted with iBC in transition dairy cattle including dosage, application route, indication and the effects are summarized in Tables 1, 2 and 3. Previously, most of the studies conducted with iBC focused on TP in dairy cattle.
The first papers concerning the use of iBC were about the prevention of postpartum metabolic and reproductive problems in dairy cows and they were published in the 1970s (Sommer and Starker 1971; Flasshoff 1974; Sommer 1975a; Sommer 1975b; Krdzalic and Curcic 1976; Zepgi et al. 1976; Wiedenroth 1979; Palmer 1980; Schuh 1994). But, new studies were published also for preventive use of iBC (Deniz et al. 2009b; Gegenbach 2009; Furll et al. 2010; Pereira et al. 2013b; Yildiz 2016; Antunes et al. 2019; Chalmeh et al. 2020; Scharen et al. 2021). Table 1 presents the recent studies about the preventive use of iBC since 2000.
The treatment and adjunct treatment approaches with iBC appeared first at the beginning of 2000 in dairy cattle (Delport et al. 2006; Furll et al. 2006; Lohr et al. 2006; Deniz et al. 2010a; Rollin et al. 2010; Deniz 2011; Szelenyi et al. 2015; Sahal et al. 2016; Tabeleao et al. 2016; Gordon et al. 2017a; Gordon et al. 2017b). Tables 2 and 3 summarised the most recent studies about the treatment and adjunct treatment approaches with iBC.
EMEA (1999) reported dosage of butafosfan 1 000–2 500 mg/animal by i.v., i.m. or s.c. route, and treatment may be repeated daily if required. However, the maximum recommended dosage was finally 5.6 mg/kg b.w. by i.v. route in lactating dairy cows (EMEA 2000). It was well known that butafosfan is rapidly eliminated from the organism after parenteral administration. Thus, it was also apparent that studies have focused on finding the best treatment protocol for dairy cows to prevent or treat the metabolic and reproductive diseases at prepartum or calving or ill animals in the early postpartum period.
Furthermore, the present review will analyse clinical studies classified in the preventive use, supportive and adjunct treatment use and use for SCK treatment in dairy cattle. Figure 3 presents the different treatment and prevention options with iBC in dairy cattle starting at dry-off and throughout the different stages of the transition period including lactation (an overview summarised from all studies).
Figure 3. Summary of published studies about options for treatment and prevention with a butafosfan and cyanocobalamin (iBC) combination in transition dairy cattle.
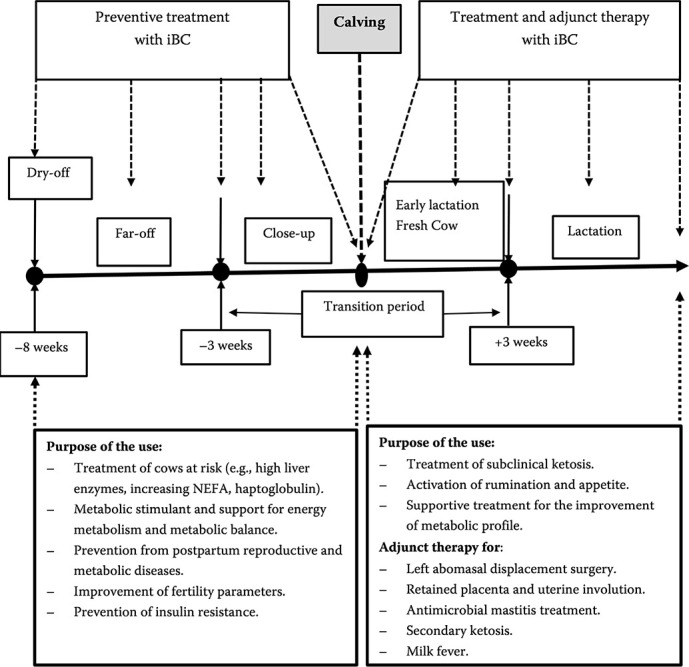
Dashed arrow = application of iBC and purpose of treatment; straight arrow = production stages of a dairy cow
PREVENTIVE USE OF iBC IN TRANSITION DAIRY COWS
The majority of the studies in the 1970s were randomised and controlled studies and administrations of 20–35 ml iBC for 1–4 consecutive days in dry-cows resulted in significantly reduced incidences of parturition syndromes and reproductive disorders. These cows were at risk 7–8 weeks before calving based on the elevated blood liver enzyme activities or cholesterol and treated preventively with iBC.
Sommer and Starker (1971) treated cows at risk for metabolic diseases (cows with elevated blood AST enzyme, cholesterol and glucose concentrations) every 3 days starting at 7 weeks before calving up to day 3 after calving with a total of 4 administrations of iBC (20 ml/cow, s.c.) to prevent from postpartum metabolic and reproduction disease. Improved liver function (reduced serum AST), reduced reproduction problems (metritis, pyometra, hypooestrus, anoestrus, follicular cysts) and the number of uterus treatments in treatment groups compared to an untreated control group. Similarly, Sommer (1975a), Sommer (1975b) treated cows at risk in much larger sample size of control and treatment groups (above 200 cows each) with iBC 8 weeks before calving. However, no exact dosage of iBC was reported in these studies. Assuming that the previous dosage (20 ml/cow s.c.) was used in these studies, a reduction of postpartum disease treatment and parturition syndrome incidence were observed by 89% and 79%, respectively. Incidence of postpartum diseases as such retained placenta, ovarian dysfunction, metritis and mastitis was markedly reduced in treatment groups versus to untreated control group and the success of the first insemination was higher in treatment groups. Flasshoff (1974) treated cows with iBC of 35 ml/cow as s.c. that were at risk in the prepartum period and observed improvement of liver enzymes, decreased incidence of postpartum diseases and increased success rate of the first insemination versus to untreated control group. Zepgi et al. (1976), Wiedenroth (1979) and Palmer (1980) used a similar protocol, but with a slightly different iBC dosage for the prevention programme from postpartum metabolic and reproductive disorders. They observed significantly reduced postpartum metabolic and reproductive problems, increased success of the first insemination and overall reduced treatments of postpartum diseases. Schuh (1994) confirmed in a PhD work the results of the previous studies using the preventive use of iBC at a dosage rate of 3 × 20 ml in cows that were at risk (elevated blood AST and cholesterol) in the prepartum 7 weeks. Reduced incidence of metabolic diseases, culling rate and improved fertility profile (reduced number of sterility treatments and artificial insemination) was observed in cows treated with iBC preventively before calving compared to the placebo-treated control group. No further significant effect of the treatment was found on the blood biochemical indicators (Schuh 1994).
The most recent studies about the preventive use of iBC as of the 2000s were summarised in Table 1. One PhD work was available about this preventive use approach of iBC from Leipzig University/Germany (Gegenbach 2009). A different prevention protocol was implemented in that study in cows that were treated 10 days before calving and 2 to 4 days after calving with iBC 20 ml/cow as s.c., and the second group of cows were treated with the same dosage on 2 to 4 days and 5 to 7 days after calving only. The milk yield of the second group was higher on days 45 and 100 postpartum. The first artificial insemination was significantly earlier in the second group than the control group. But, no further beneficial postpartum clinical and biochemical effects regarding energy metabolism were observed in the iBC treatment groups (Gegenbach 2009). However, this study did not distinguish cows according to the presence of SCK and risk factors, thus the preventive treatment effect on SCK incidence in the farms was not analysed, rather absolute NEFA and BHBA concentrations were analysed. Another study about the preventive use of iBC (Furll et al. 2010) reported that much higher dosage regime given i.v. in cows (group 1: 3 × 10 ml/100 kg b.w. before calving, or group 2: 6 × 10 ml/100 kg b.w. before calving) reduced the rate of puerperal infections and intrauterine or injectable antimicrobial treatments after calving. Serum NEFA and BHBA concentrations were significantly lower in group 2 cows on postpartum day 1. In both treatment groups, serum phosphorus concentration was significantly higher on postpartum day 1, but glucose concentration was higher in group 2 only. But, no effect was observed on serum liver enzymes in contrast to previous studies from 1970s. Chalmeh et al. (2020) reported a new approach using different dosages of generic iBC in cows before calving to prevent insulin resistance. The administration of a generic iBC at 2, 4 or 6 ml/100 kg b.w. dosage as i.v. (3 consecutive days between 21–19, 12–10 and 3–1 before calving) protected from insulin resistance in the postpartum period of cows. The highest insulin sensitivity was observed in the 6 ml group. Blood NEFA and BHBA concentrations were reduced in all dosage groups. Blood glucose and insulin levels were lower in iBC groups after i.v. glucose infusion at postpartum weeks 1, 2 and 3. Scharen et al. (2021) tested two different dosages such as 5 ml/100 kg b.w. and 10 ml/100 kg b.w. applied at 6 time points (7, 6, and 5 days prepartum, and 1, 2, and 3 days postpartum) i.v. in dairy cows. Blood and urine analysis and 3 different liver metabolome characteristics (mild, medium and large) between prepartum and postpartum based on liver biopsy results were investigated. The 10 ml iBC group had a nonsignificant trend for higher milk yield, reduced alkaline phosphatase activity and increased TG concentrations in metobotype A class, but they had lower TG in metabotype B class indicated a better metabolic capacity via iBC treatment. A dose dependent effect of iBC from previous studies (Furll et al. 2010; Sahal et al. 2016; Chalmeh et al. 2020) was confirmed by Scharen et al. (2021). Although no significant beneficial effects were observed on NEFA and BHBA concentrations, a better metabolic profile was apparent in the metabolically challenged animals via iBC treatment and the administrations of an indication-based metaphylactic treatment with iBC was recommended. This was in line with the first studies from 1970s (Sommer and Starker 1971; Sommer 1975a; Sommer 1975b; Zepgi et al. 1976; Wiedenroth 1979) that were conducted studies in cows at risk.
Overall, if looked at the preventive use of iBC since the 1970s and the newest results in Table 1, the outcomes of iBC use in cows at risk in the dry period looked much satisfactory for the reduction of postpartum metabolic disorders, as also stated by Scharen et al. (2021), rather than a blind treatment of all cows without screening for the risk assessment. Furthermore, weekly multiple use and little higher dosage of iBC (6–10 ml/100 kg b.w. given i.m. or i.v.) in the close-up, calving and very early lactation provided benefits for better metabolic profile, general health status, better uterus involution, lower incidence of SCK and insulin resistance.
SUPPORTIVE/ADJUNCT AND THERAPEUTIC USE OF iBC IN TRANSITION DAIRY COWS
The major indication of iBC is disorders of metabolism in young animals, also the supportive treatment of infertility, tetany and paresis as an adjunct to calcium and magnesium therapy in dairy cows (EMEA 1999). This has been confirmed in another report by EMEA in 2000 (EMEA 2000). iBC was classified as vitamin and mineral supplementation, interestingly, clinical therapeutic studies were more focused on the treatment or adjunct therapy of metabolic diseases despite the recommendation of EMEA and EMA.
There are recent studies that investigated the effects of iBC or butafosfan stand-alone on the indicators related to energy metabolism in the liver and muscle (Kreipe et al. 2011; Gomes Jose et al. 2012; Nuber et al. 2016) and clinical aspects in dairy cattle (Delport et al. 2006; Furll et al. 2006; Lohr et al. 2006; Cuteri et al. 2008; Deniz et al. 2010a; Rollin et al. 2010; Szelenyi et al. 2015; Sahal et al. 2016; Tabeleao et al. 2016; Gordon et al. 2017a; Gordon et al. 2017b). The clinical studies as of 2006 focused on the treatment of SCK (Table 2), rapid recovery following displaced abomasum surgery, prevention from secondary ketosis, improvement of metabolic and reproductive functions, as an adjunct therapy for fast recovery from mastitis and milk fever (Table 3).
The most important indicator for the detection of ketosis or SCK in cows is the elevated concentration of BHBA in the blood or milk and NEFA in the blood (Duffield 2000; McArt et al. 2012; Deniz et al. 2020). SCK is well known to harm milk production and postpartum health conditions (McArt et al. 2012; Deniz et al. 2020). The first study on SCK treatment in dairy cow was reported in 1976 by Krdzalic and Curcic (1976). According to this study, treatment of cows with indigestions (n = 128) and SCK diagnosed via milk (35% out of all) with iBC two times 24 h apart reduced SCK incidence from 35% to 18% in 72 h and the percentage of cows with indigestion problem was reduced to 65% and 4% in 48 h and 72 h after the treatment, respectively. Serum concentrations of glucose and phosphorus increased in 48 h and 72 h after the treatment. This pioneer study did not deliver statistical evaluation of the results rather a descriptive statistic was available. Contradictory results were reported about the efficacy of iBC on blood BHBA in SCK positive cows (Table 2). Some studies reported that blood BHBA and NEFA concentrations were decreased after iBC administrations, but some of them did not deliver the same effect on BHBA. However, they reported significantly increased milk yield (Cuteri et al. 2008; Kreipe et al. 2011; Sahal et al. 2016; Gordon et al. 2017a; Gordon et al. 2017b) and shorter days open period, faster uterus involution and fewer retained placenta cases (Deniz et al. 2010a; Sahal et al. 2016) in dairy cows. European studies on SCK treatment defined with a cut-point of roughly 1.0 mmol/l (Szelenyi et al. 2015; Sahal et al. 2016) and 1.2 mmol/l of blood BHBA (Nuber et al. 2016) showed significant effect of iBC, even with normal dosage 5 ml/100 kg b.w. i.v. or i.m. for 3–4 consecutive days or high dosage (10 ml/100 kg b.w.), while studies from US did not produce significant effects of iBC on blood BHBA in SCK positive cows treated with 25 ml/cow s.c. for 3 consecutive days (Gordon et al. 2017a; Gordon et al. 2017b). Exceptionally, Rollin et al. (2010) from the US showed a significant effect of iBC (25 ml/cow s.c. for 2 days) in mature cows (lactation ≥ 3) with positive SCK. Interestingly, treated cows produced more milk than placebo control cows in the US studies. The difference between results might be because of the cut-off point of blood BHBA, which was defined for SCK diagnosis, selection of cows with hypoglycaemia or different administration protocol of iBC given s.c., i.m. or i.v.
Adjunct treatment with iBC (a single administration 5 ml/100 kg b.w. i.v.) for milk fever resulted in better blood calcium profile (but nonsignificant) and lower blood BHBA (Delport et al. 2006). However, increased concentration of blood ionized calcium and lower blood pH were observed by Antunes et al. (2019) after the preventive treatment with iBC for 5 consecutive days (20 ml/cow i.m.) at calving. Lower blood pH, higher PCO2 and higher blood ionised calcium levels in butafosfan and iBC groups revealed that treatments might have activated parathormone via acidifying the blood. Another supportive adjunct treatment with iBC (a single or 3 consecutive day administration of 5 ml/100 kg b.w. i.v.) in the left abomosal displacement (LAD) operation resulted in lower blood BHBA within 2–3 postoperative days (Furll et al. 2006; Lohr et al. 2006). Tabeleao et al. (2016) used iBC as adjunct therapy to the antimicrobial treatment of mastitis at a high dosage (10 ml/100 kg b.w. i.v., 3 applications every 5 days) and that resulted in the lower somatic cell counts and faster recovery from a mild to moderate mastitis.
Overall, multiple application such as 3–5 consecutive days, i.m. or i.v. use and 5–10 ml/100 kg b.w. had a significant reducing effect on blood BHBA in SCK positive cows and an adjunct therapy with iBC accelerated the recovery from LAD operation, mastitis and milk fever diseases. It should be kept in mind that the rate of NEB and the threshold of blood BHBA defined for ketosis and SCK diagnosis are crucial parameters to treat the imbalances in energy metabolism. Thus, they can have a direct effect on the treatment success. Therefore, treatment protocols focused probably more on subclinical and secondary ketosis rather than clinical form of ketosis that might need further sophisticated cure protocol.
Summary and conclusion of all sections
VB12 and phosphorus are two important compounds in TP of dairy cows, especially in the early lactation for metabolic function and milk production. The use of iBC was clinically effective in transition dairy cows depending on the risk. Especially, a significant effect was observed on blood BHBA, NEFA, TG, ionized calcium and ATP, ADP and glycogen levels in the muscle and liver, mRNA abundance of enzymes related to carbohydrate and lipid metabolism in the liver. The effect was more obvious in metabolically stressed animals based on metabolomics analyses. Three studies reported an increase of DMI, rumen activity or appetite after iBC treatment at postpartum.
Four out of seven studies found that iBC was effective to reduce blood BHBA in cows treated for SCK. The dosage, route and frequency of iBC application were different among the studies and a dose dependent effect was apparent; i.m. and i.v. administration, for 3–5 days, 5–10 ml/100 kg b.w. looked to be better in SCK treatment. Blood NEFA concentration was reduced in two studies in which it was used at a dosage of 10 ml/100 kg b.w. i.m. or i.v. for the treatment of SCK in dairy cows. However, the cut-point of BHBA for SCK definition can play an essential role in the treatment protocol. This must be taken in account. A dose-dependent daily milk yield increase and improved metabolic functions were observed in cows at risk.
Three out of seven studies found that iBC increased blood glucose concentrations at postpartum period if used preventively at prepartum or calving. But, the treatment of SCK with iBC did not increase blood glucose concentrations. Increased glycogen, ATP, ADP concentrations in liver and muscle that were observed after butafosfan or iBC treatment in mice and cattle might indicate that iBC may trigger the use and uptake of glucose in the cells. These findings require further investigations in detail.
Despite EMEA and EMA reports in 1999, 2000 and 2014 as supportive therapy and nutritional supplement; iBC was used at prepartum and calving for the improvement of postpartum metabolic and reproductive functions as well as at postpartum for the treatment of SCK and the adjunct/supportive therapy to secondary ketosis associated with LAD, mastitis and milk fever in recently published clinical studies.
Multiple applications preferably i.m. or i.v. as 5–10 ml/100 kg b.w. appeared to be more efficient rather than one single and s.c. treatment, this is probably required because of the rapid elimination of butafosfan and its bioavailability. It was obvious that iBC provides directly butafosfan and VB12 as active ingredients to the organism rather than increasing phosphorus concentration, although some controversial data are available.
Finally, further in vivo studies using radiolabelled iBC and butafosfan should be conducted in transition dairy cows with or without NEB and at risk. The interference on mRNA abundance of carbohydrate and lipid metabolism-related enzymes and metabolites should be investigated in connection with ATP, ADP, acetyl-CoA, glucose, glycogen, acylcarnitines and phosphatidylcholines, BHBA, NEFA, TG and hormone concentrations in the tissues and blood.
Acknowledgement
Authors thank gratefully Ms. Sarah Weston from New Zealand for her valuable contribution for editing the first version of this manuscript in English.
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- Akins MS, Bertics SJ, Socha MT, Shaver RD. Effects of cobalt supplementation and vitamin B12 injections on lactation performance and metabolism of Holstein dairy cows. J Dairy Sci. 2013 Jan 11;96(3):1755-68. [DOI] [PubMed] [Google Scholar]
- Antunes MM, Londero US, Pizoni C, Feijo JO, Prietsch RF, Barbosa SC, Primel EG, Hertzog GI, Pereira RA, Rincon JAA, Pino FABD, Rabassa VR, Correa MN. Hemogasometry, metabolic profile and acute phase proteins in primiparous Holstein cows supplemented with butafosfan associated or not of cyanocobalamin. Ital J Anim Sci. 2019 May 22;18(1):957-62. [Google Scholar]
- Baumgard LH, Collier RJ, Bauman DE. A 100-year review: Regulation of nutrient partitioning to support lactation. J Dairy Sci. 2017 Dec;100(12):10353-66. [DOI] [PubMed] [Google Scholar]
- Berg JM, Tymoczo JL, Stryer L. Glycolysis and gluconeogenesis. In: Berg JM, Tymoczo JL, Stryer L, editors. Biochemistry. New York, USA: W. H. Freeman and Company; 2006. p. 433-74. [Google Scholar]
- Chalmeh A, Pourjafar M, Badiei K, Jalali M, Mazrouei Sebdani M. Intravenous administration of butaphosphan and cyanocobalamin combination to late-pregnant dairy cows reduces their insulin resistance after calving. Biol Trace Elem Res. 2020 Aug;199(6):2191-200. [DOI] [PubMed] [Google Scholar]
- Combs GF, McClung JP. Vitamin B12. In: Combs GF, McClung JP, editors. The vitamins: Fundamental aspects in nutrition and health. 5th ed. London, UK: Academic Press; 2017. p. 431-57. [Google Scholar]
- Corbridge DEC. Phosphorus: Chemistry, biochemistry and technology. 6th ed. FL, US: CRC Press, Boca Raton; 2013. 1473 p. [Google Scholar]
- Cuteri V, Nisoli L, Attili AR, Tejeda AR, Preziuso S, Fruganti A. [Clinical field evaluation of a butafosfan + vitamin B12 compound (Phosphorum B12/Catosal) in the treatment of subclinical ketosis in dairy cows]. Magyar Allat Lapja. 2008;130(Suppl_II):16-7. Hungarian. [Google Scholar]
- Delport PC, Fourie L, Schmidt B. Efficacy and safety of Catosal® in the treatment of parturient paresis in cows. Proceeding of the XXIV World Buiatrics Congress; 2006 Oct 15-19, Nice, France; 2006. 120 p. [Google Scholar]
- Deniz A, Spiecker-Hauser U, Rehagen M. Efficacy of a butafosfan and vitamin B12 combination (Catosal®) on biochemical and hematological blood parameters in dogs treated with dexamethasone. Int J Appl Res Vet M. 2009a;7(3):128-41. [Google Scholar]
- Deniz A, Torralbo, Jose I, Garfia B. [Treatment with butafosfan + vitamin B12 (Catosal®) and calcium + magnesium combinations (Calcio® Inyectable Bayer) in fresh cows very early postpartum provides benefits in terms of uterus involution and metabolic functions]. Proceedings of the XIV Congreso International de Medicina Bovina (ANEMBE); 2009 May 6-8; A Coruña, Spain; 2009b. p. 226-9. Spanish. [Google Scholar]
- Deniz A, Watanapongchati S, Aiumlamai S. Effect of original combination of butafosfan and vitamin B12 and generics from Asia on reproduction parameters in cattle. Proceedings XXVI World Buiatrics Congress; 2010 Nov 14-18; Santiago, Chile; 2010a. 102 p. [Google Scholar]
- Deniz A, Watanapongchat S, Nuntaprasert A. Metaphylaxis with butafosfan and vitamin B12 (Catosal®) in pregnant sows enhanced the immunity of newborn piglets that resulted in improved weaning weight. 21st IPVS Congress; 2010 July 18-21; Vancouver, Canada; 2010b. p. 679. [Google Scholar]
- Deniz A, Aksoy K, Metin M. Transition period and subclinical ketosis in dairy cattle: Association with milk production, metabolic and reproductive diseases and economic aspects. Med Weter. 2020 Sep;76(9):495-502. [Google Scholar]
- Deniz A. Treatment of subclinical ketosis: The silent profit robber. Proceedings of the 21st International Congress of Hungarian Association for Buiatrics; 2011 Oct 11-15; Sümeg, Hungary; 2011. p. 15-7. [Google Scholar]
- Dryden LP, Hartman AM, Bryant MP, Robinson IM, Moore LA. Production of vitamin B12 and vitamin B12 analogues by pure cultures of ruminal bacteria. Nature. 1962 Jul 14;195:201-2. [DOI] [PubMed] [Google Scholar]
- Duffield TF. Subclinical ketosis in lactating dairy cattle. Vet Clin N Am-Food A. 2000 Jul;16(2):231-53. [DOI] [PubMed] [Google Scholar]
- Duplessis M, Lapierre H, Pellerin D, Laforest JP, Girard CL. Effects of intramuscular injections of folic acid, vitamin B12, or both, on lactational performance and energy status of multiparous dairy cows. J Dairy Sci. 2017 Feb;100(5):4051-64. [DOI] [PubMed] [Google Scholar]
- EMA – The European Medicine Agency: Science Medicine Health. Butafosfan (all mammalian food producing species) EMA/CVMP/335153/2013 [Internet]. London, UK: European public MRL assessment report (EPMAR). 2014. [cited 2021 Apr 12]. Available from: https://www.ema.europa.eu/en/documents/mrl-report/butafosfan-all-mammalian-food-producing-species-european-public-maximum-residue-limit-assessment_en.pdf. [Google Scholar]
- EMEA – The European Agency for the Evaluation of Medicinal Products. Butafosfan EMEA/MRL/630/99-Final [Internet]. London, UK: Veterinary Medicine and Information Technology Unit. 1999. [cited 2021 Apr 12]. Available from: https://www.ema.europa.eu/en/documents/mrl-report/butafosfan-summary-report-1-committee-veterinary-medicinal-products_en.pdf. [Google Scholar]
- EMEA – The European Agency for the Evaluation of Medicinal Products. Butafosfan EMEA/MRL/734/00-Final [Internet]. London, UK: Veterinary Medicine and Information Technology Unit. 2000. [cited 2021 Apr 12]. Available from: https://www.ema.europa.eu/en/documents/mrl-report/butafosfan-extension-lactating-cows-summary-report-2-committee-veterinary-medicinal-products_en.pdf. [Google Scholar]
- Fadlalla IMT, Omer SA, Atta M. Determination of some serum macro element minerals levels at different lactation stages of dairy cows and their correlations. Sci Afr. 2020 Jul;8:e00351 [Google Scholar]
- Fisher GE, MacPherson A. Serum vitamin B12 and methylmalonic acid determinations in the diagnosis of cobalt deficiency in pregnant ewes. Br Vet J. 1990 Mar-Apr;146(2):120-8. [DOI] [PubMed] [Google Scholar]
- Flasshoff FH. Clinical and chemical blood serum investigations in cattle and treatment studies with ornithine-aspartate-product HMV 20 and with Catosal for the reduction of fertility and health disorders [PhD thesis]. Hannover, Germany: Tierärztliche Hochschule; 1974. [Google Scholar]
- Friedrich W. Vitamin B12. Handbuch der Vitamine. München, Germany: Urban & Schwarzenberg; 1987. p. 539-95. [Google Scholar]
- Furll M, Deniz A, Westphal B, Illing C, Constable PD. Effect of multiple intravenous injections of butaphosphan and cyanocobalamin on the metabolism of periparturient dairy cows. J Dairy Sci. 2010 Sep;93(3):4155-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furll M, Witteck T, Gengenbach S, Schmidt B. Effects of pre-operative application of butaphosphan and cyanocobalamin on recoalescence clinico-chemical parameters, antioxidative metabolism and postoperative abomasal emptying in cows with abomasal dislocation. Tierarztl Prax. 2006 Jan;34(G):351-6. [Google Scholar]
- Gegenbach S. Wirkungen von Catosal auf den Allgemeinzustand und den Stoffwechsel bei Kuhen nach linksseitiger Dislocatio abomasi sowie bei Kuhen im peripartalen Zeitraum [Effects of Catosal® on general state and metabolism in cows after abomasal displacement to the left and in cows in the peripartal period] [PhD thesis]. Germany: Veterinärmedizinischen Fakultät der Universität Leipzig; 2009. German. [Google Scholar]
- Gille D, Schmid A. Vitamin B12 in meat and dairy products. Nutr Rev. 2015 Feb;73(2):106-15. [DOI] [PubMed] [Google Scholar]
- Girard CL, Matte JJ. Changes in serum concentrations of folates, pyridoxal, pyridoxal-5-phosphate and vitamin B12 during lactation of dairy cows fed dietary supplements of folic acid. Can J Anim Sci. 1999 Mar 1;79(1):107-13. [Google Scholar]
- Girard CL, Santschi DE, Stabler SP, Allen RH. Apparent ruminal synthesis and intestinal disappearance of vitamin B12 and its analogs in dairy cows. J Dairy Sci. 2009 Sep;92(9):4524-9. [DOI] [PubMed] [Google Scholar]
- Gomes Jose M, Videira R, Silva F, Almeida J, Deniz A, Raposa J, Dargent F, Peixoto F, Silva A. Effect of a butaphosphan and cyanocobalamin combination on energetic metabolism assessed by live and muscle biopsies in non-lactating Holstein cows. Proceedings of the XXVII World Buiatrics Congress; 2012 Jun 18-22; Lisbon, Portugal; 2012. 103 p. [Google Scholar]
- Gordon JL, Duffield TF, Herdt TH, Kelton DF, Neuder L, LeBlanc SJ. Effects of a combination butaphosphan and cyanocobalamin product and insulin on ketosis resolution and milk production. J Dairy Sci. 2017a Feb 16;100(4):2954-66. [DOI] [PubMed] [Google Scholar]
- Gordon JL, LeBlanc SJ, Kelton DF, Herdt TH, Neuder L, Duffield TF. Randomized clinical field trial on the effects of butaphosphan cyanocobalamin and propylene glycol on ketosis resolution and milk production. J Dairy Sci. 2017b Mar 3;100(5):3912-21. [DOI] [PubMed] [Google Scholar]
- Graulet B, Matte JJ, Desrochers A, Doepel L, Palin MF, Girard CL. Effects of dietary supplements of folic acid and vitamin B12 on metabolism of dairy cows in early lactation. J Dairy Sci. 2007 Jul;90(7):3442-55. [DOI] [PubMed] [Google Scholar]
- Graves JD, Krebs EG. Protein phosphorylation and signal transduction. Pharmacol Therapeut. 1999 May-Jun;82(2-3):111-21. [DOI] [PubMed] [Google Scholar]
- Grunberg W, Staufenbiel R, Constable PD, Dann HM, Morin DE, Drackley JK. Liver phosphorus content in Holstein-Friesian cows during the transition period. J Dairy Sci. 2009 May;92(5):2106-17. [DOI] [PubMed] [Google Scholar]
- Hasi S, Du X, Zhu B, Jiang J. [Studies on effects of compound butaphosphan solution on endurance capability and energy metabolism in mice]. Acta Vet Et Zoot Sinica. 2004;35(3):290-4. Chinese. [Google Scholar]
- Hasi S, Du XY, Jiang JS, Zhu BL. [Study on immune stimulating properties of compound butafosfan solution in mice]. Chi J Vet Sci Technol. 2005a;35(7):574-8. Chinese. [Google Scholar]
- Hasi S, Jiang JS, Du XY, Zhu B. [Anti-cold stress effects and mechanisms of compound butafosfan solution]. Prog Vet Med. 2005b;26:59-62. Chinese. [Google Scholar]
- Hasi S, Jin-Shu J, Bei-Lei Z, Xiao-Yan D. [Studies on anti-heat stress effects and mechanisms of compound butafosfan solution]. Acta Vet Et Zoot Sinica. 2005c;36:1334-8. Chinese. [Google Scholar]
- Huh JY, Reilly SM, Abu-Odeh M, Murphy AN, Mahata SK, Zhang J, Cho Y, Seo JB, Hung CW, Green CR, Metallo CM, Saltiel AR. TANK-binding kinase 1 regulates the localization of acyl-CoA synthetase ACSL1 to control hepatic fatty acid oxidation. Cell Metab. 2020 Dec 1;32(6):1012-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DG, Blanchflower WJ, Scott JM, Weir DG, Molloy AM, Kennedy S, Young PB. Cobalt-vitamin B-12 deficiency decreases methionine synthase activity and phospholipid methylation in sheep. J Nut. 1992 Jul;122(7):1384-90. [DOI] [PubMed] [Google Scholar]
- Kincaid RL, Socha MT. Effect of cobalt supplementation during late gestation and early lactation on milk and serum measures. J Dairy Sci. 2007 Apr;90(4):1880-6. [DOI] [PubMed] [Google Scholar]
- Krdzalic P, Curcic M. Die Moglichkeit einer Indigestions Therapie bei Kuhen mit Catosal und Methaphylaxe der subklinischen Ketose [The possibility of therapy of indigestions in cows with Catosal and metaphylaxis of subclinical ketosis]. Vet Glas. 1976;8:687-93. German. [Google Scholar]
- Kreipe L, Deniz A, Bruckmaier RM, van Dorland HA. First report about the mode of action of combined butafosfan and cyanocobalamin on hepatic metabolism in nonketotic early lactating cows. J Dairy Sci. 2011 Oct;94(10):4904-14. [DOI] [PubMed] [Google Scholar]
- Lohr B, Brunner B, Jonowitz H, Hummel M, Seeger T, Weber I, Wittek T, Schmidt B, Hellmann K. Klinische Wirksamkeit von Catosal in der Behandlung der Ketose von Kuhen mit Linksseitige Labmagenverlagerung [Efficacy of Catosal® for the treatment of ketosis in cows with left abomasal displacement]. Tieraerztl Umsch. 2006 Apr 1;61(4):187-90. German. [Google Scholar]
- Mahalle N, Bhide V, Greibe E, Heegaard CW, Nexo E, Fedosov SN, Naik S. Comparative bioavailability of synthetic B12 and dietary vitamin B12 present in cow and buffalo milk: A prospective study in lactovegetarian Indians. Nutrients. 2019 Feb 1;11(2):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelian CL, Penasa M, Visentin G, Zidi A, Cassandro M, De Marchi M. Mineral composition of cow milk from multibreed herds. Anim Sci J. 2018 Sep 16;89(11):1622-7. [DOI] [PubMed] [Google Scholar]
- McArt JAA, Nydam DV, Oetzel GR. Epidemiology of subclinical ketosis in early lactation dairy cattle. J Dairy Sci. 2012 Sep;95(9):5056-66. [DOI] [PubMed] [Google Scholar]
- McDowell LR. Vitamins in animal and human nutrition. Ames, IA, USA: Iowa State University Press; 2000. 793 p. [Google Scholar]
- Millar KR, Albyt AT, Bond GC. Measurement of vitamin B12 in the livers and sera of sheep and cattle and an investigation of factors influencing serum vitamin B12 levels in sheep. N Z Vet J. 1984 May;32(5):65-70. [DOI] [PubMed] [Google Scholar]
- Nohr C, Biesalski HK. Vitamin B12. Reference module in food science. Amsterdam, The Netherlands: Elsevier Inc.; 2016. p. 1-4. [Google Scholar]
- NRC – National Research Council. Nutrient requirements of dairy cattle. 7th ed. Washington, DC, USA: National Academy Press; 2001. p. 132-72. [Google Scholar]
- Nuber U, Dorland HAV, Bruckmaier RM. Effects of butafosfan with or without cyanocobalamin on the metabolism of early lactating cows with subclinical ketosis. J Anim Physiol Anim Nutr. 2016 Feb;100(1):146-55. [DOI] [PubMed] [Google Scholar]
- Overton TR, McArt AA, Nydam DV. A 100-year review: Metabolic health indicators and management of dairy cattle. J Dairy Sci. 2017 Dec;100(12):10398-417. [DOI] [PubMed] [Google Scholar]
- Palmer CR. Metaphylaxis in postpartum conditions in dairy cows with butaphosphone: A trial under South African conditions. J S Afr Vet Assoc. 1980 Dec;51(4):239-42. [PubMed] [Google Scholar]
- Pereira RA, Fensterseifera S, Barcelosa VB, Martinsa CF, Schneidera A, Schmitta E, Pfeifera LFM, Pinoa FABD, Correa MN. Metabolic parameters and dry matter intake of ewes treated with butaphosphan and cyanocobalamin in the early postpartum period. Small Rumin Res. 2013a Aug;114(1):140-5. [Google Scholar]
- Pereira RA, Silveira PAS, Montagner P, Schneider A, Schmitt E, Rabassa VR, Pfeifer LFM, Pino FAB, Del Pulga ME, Corre MN. Effect of butaphosphan and cyanocobalamin on postpartum metabolism and milk production in dairy cows. Animal. 2013b Dec;7(7):1143-7. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Lagana AS. A review of vitamin B12. In: Patel VB, editor. Molecular nutrition. Oxford, UK: Elsevier Academic Press; 2020. p. 105-29. [Google Scholar]
- Robinson PH. Vitamin B requirements and duodenal deliveries in lactating dairy cows: Organization of a limited literature. Livest Sci. 2019 Aug;226:48-60. [Google Scholar]
- Rollin E, Berghaus RD, Rapnick P, Godden SM, Overton MW. The effect of injectable butafosfan and cyonacobalamin on postpartum serum beta-hydroxybutyrate, calcium, and phosphorus concentration in dairy cattle. J Dairy Sci. 2010 Mar;93(3):978-87. [DOI] [PubMed] [Google Scholar]
- Sahal M, Deniz A, Vural R, Kuplulu S, Polat I, Colakoglu C, Ocal N, Macun Ceyhun H, Pekcan M, Ocak M. Evaluation of the effect of different doses of butaphosphan and cyanocobalamin combination in dairy cattle with subclinical ketosis. Kafkas Univ Vet Fak Derg. 2016;23(3):349-56. [Google Scholar]
- Scharen M, Snedec T, Riefke B, Slopianka M, Keck M, Gruendemann S, Wichard J, Brunner N, Klein S, Theinert KB, Pietsch F, Leonhardt A, Theile S, Rachidi F, Kaiser A, Koller G, Bannert E, Spilke J, Starke A. Aspects of transition cow metabolomics – Part I: Effects of a metaphylactic butaphosphan and cyanocobalamin treatment on the metabolome in liver, blood, and urine in cows with different liver metabotypes. J Dairy Sci. 2021 Aug;104(8):9205-26. [DOI] [PubMed] [Google Scholar]
- Schuh R. Investigations on the efficacy of butafosfan in the prevention of metabolic disorders in dairy cows in the peri-partal period [PhD thesis]. Giessen, Germany: Justus-Liebig-University; 1994. [Google Scholar]
- Shampo MA, Kyle RA, Steensma DP. William Murphy – Nobel Prize for the treatment of pernicious anemia. Mayo Clin Proc. 2006 June;81(6):726. [DOI] [PubMed] [Google Scholar]
- Smith RM. Cobalt. In: O’Dell BL, Sunde RA, editors. Handbook of nutritionally essential minerals. New York, USA: Marcel Dekker Inc.; 1997. p. 357-87. [Google Scholar]
- Smith SE, Loosli JK. Cobalt and vitamin B12 in ruminant nutrition: A review. J Dairy Sci. 1957 Oct;40(10):1215-27. [Google Scholar]
- Sommer H, Starker DM. Versuche zur Reduzierung der Fruchtbarkeitsstorungen beim Rind durch die Anwendung von Catosal in der Metaphylaxe [Experiments fo the reduction of fertility disturbances in cattle using Catosal during metaphylaxis]. Dtsch Tieraerztl Wschr. 1971;78:593-616. German. [PubMed] [Google Scholar]
- Sommer H. Preventive medicine in dairy cows. Vet Med Rev. 1975a;1-2:42-63. [Google Scholar]
- Sommer H. Untersuchung uber die Metaphylaxe der Sterilitat und puerperaler Erkrankungen [Investigations about the metaphylaxis for the sterility and puerperal diseases]. Prak Tierar. 1975b;8:462-4. German. [Google Scholar]
- Stangl GI, Schwarz FJ, Jahn B, Kirchgessner M. Cobalt-deficiency induced hyperhomocysteineaemia and oxidative status of cattle. Br J Nutr. 2000 Mar 9;83(1):3-6. [DOI] [PubMed] [Google Scholar]
- Swanon S, Koike S, Kobayashi Y. Evidence for the possible involvement of Selenomonas ruminantium in rumen fiber digestion. FEMS Microbiol Lett. 2011;325:170-9. [DOI] [PubMed] [Google Scholar]
- Szelenyi Z, Bujak D, Nagy K, Boldizsar S, Keresztesi Z, Szakallas E, Szenci O. [Treatment of subclinical ketosis in dairy cattle with a product containing cyanocobalamin and butafosfan (Catosal®)]. Magyar Allat Lapja. 2015 Sep;137:515-22. Hungarian. [Google Scholar]
- Tabeleao VC, Pereira RA, Priersch RF, Feijo JO, Bondan C, Mattei P, Schmitt E, Del Pino FAB, Correa MN. [Butafosfan and cyanocobalamin: Indirect effects on the mammary gland of dairy cows after clinical mastitis]. Sci Anim Health. 2016;4(3):238-54. Portuguese. [Google Scholar]
- Van der Staay FJ, De Groot J, Van Reenen C, Hoving-Bolink AH, Schurman T, Schmidt BH. Effects of butafosfan on salivary cortisol and behavioral response to social stress in piglets. J Vet Pharmacol Therap. 2007 Oct;30(5):410-6. [DOI] [PubMed] [Google Scholar]
- Venerando A, Cesaro L, Pinna LA. From phosphoproteins to phosphoproteomes: A historical account. FEBS J. 2017 Jan 12;284:1936-51. [DOI] [PubMed] [Google Scholar]
- Walsh CT. Introduction to phosphorus chemical biology. In: Walsh CT, editor. The chemical biology of phosphorus. 1st ed. UK: Royal Society of Chemistry; 2020. p. 3-26. [Google Scholar]
- Wang C, Liu HY, Wang YM, Yang ZQ, Liu JX, Wu YM, Yan T, Ye HW. Effects of dietary supplementation of methionine and lysine on milk production and nitrogen utilization in dairy cows. J Dairy Sci. 2010 Aug;93(8):3661-70. [DOI] [PubMed] [Google Scholar]
- Watanabe F, Bito T. Vitamin B12 sources and microbial interaction. Exp Biol Med. 2018 Jan;243(2):148-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerathilake WADV, Brassington AH, Williams SJ, Kwong WY, Sinclair LA, Sinclair KD. Added dietary cobalt or vitamin B12, or injecting vitamin B12 does not improve performance or indicators of ketosis in pre- and post-partum Holstein-Friesian dairy cows. Animals. 2018 Apr;13(4):750-9. [DOI] [PubMed] [Google Scholar]
- Weiller MAA, Alvarado-Rincon JA, Jacometo CB, Barros CC, de Souza ICC, Hax LT, da Silva TC, Mattei P, Barbosa AA, Feijo JO, Pereira RA, Brauner CC, Rabassa VR, Del Pino FAB, Correa MN. Butaphosphan effects on glucose metabolism involve insulin signaling and depends on nutritional plan. Nutrients. 2020 Jun 22;12(6):1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenroth H. Die wirtschaftliche Bedeutung einer Vorsorgeuntersuchung bei Milchkuhen [The economic impact of a preventive examination in dairy cows]. Tierarztl Umsch. 1979;34:84-93. German. [Google Scholar]
- Yildiz A. Ineklerde Retensiyo Sekundinarum Proflaksisi Uzerine Butafosfan ve Vitamin B12’nin Birlikte Uygu-lanmasinin Etkisi [The effects of butaphosphan and vitamin B12 combination on the prophlaxis of retained placenta in cows]. Harran Üniv Vet Fak Derg. 2016 Jun 21;5(1):29-33. Turkish. [Google Scholar]
- Zepgi A, Rusch K, Correa J, Villouta G, Concha M, Bobrik J. Metaphylactic study and treatment of metabolic and reproductive disorders in dairy cows during the last three months of pregnancy. Vet Med Rev. 1976;1(76):63-71. [Google Scholar]
- Zhao Z, Bai Y, Tian H, Shi B, Li X, Luo Y, Wang J, Hu J, Raza SHA. Interference with ACSL1 gene in bovine adipocytes: Transcriptome profiling of circRNA related to unsaturated fatty acid production. Genomics. 2021 Nov;113(6):3967-77. [DOI] [PubMed] [Google Scholar]


