Abstract
Vision is mediated by light passing through the pupil, which changes in diameter from approximately 2 to 8 mm between bright and dark illumination. With age, mean pupil size declines. In laboratory experiments, factors affecting pupil size can be experimentally controlled. How the pupil reflects the change in retinal input from the visual environment under natural viewing conditions is unclear. We address this question in a field experiment (N = 83, 43 female, 18–87 years) using a custom-made wearable video-based eye tracker with a spectroradiometer measuring near-corneal spectral irradiance. Participants moved in and between indoor and outdoor environments varying in spectrum and engaged in a range of everyday tasks. Our data confirm that light-adapted pupil size is determined by light level, with a better model fit of melanopic over photopic units, and that it decreased with increasing age, yielding steeper slopes at lower light levels. We found no indication that sex, iris colour or reported caffeine consumption affects pupil size. Our exploratory results point to a role of photoreceptor integration in controlling steady-state pupil size. The data provide evidence for considering age in personalized lighting solutions and against the use of photopic illuminance alone to assess the impact of real-world lighting conditions.
Keywords: pupillary light reflex, vision, melanopsin, miosis, field study, ageing
1. Introduction
Human vision depends on light impinging on the retina and exciting the retinal photoreceptors. Serving as the aperture of the visual system, the pupil is able to adjust the amount of incident light by constricting or dilating to diameters ranging from approximately 2 to approximately 8 mm under the brightest and darkest conditions, respectively [1,2]. By this route, the pupil regulates retinal illuminance within a limited range (approx. 16×, or 1.2 log units between smallest and largest pupil) [3]. The pupil is also critical in attaining optimal optical quality of the retinal image [4,5] by balancing necessary retinal illuminance with optical aberrations [6,7] and depth of focus [8]. Hence, pupil size is an essential determinant for visual performance under changing ambient illumination.
1.1. Light-level dependence of pupil size
It is well known that pupil size generally depends on light level. This effect is encoded by the joint action of the five retinal photoreceptor classes in the human retina: rods, three types of cones and melanopsin, which is expressed in the intrinsically photosensitive retinal ganglion cells (ipRGCs). Each photoreceptor class is characterized by a distinct wavelength of maximal sensitivity, but due to their broad spectral tuning, the photoreceptors overlap greatly in their spectral sensitivities [9]. Rods have their peak sensitivity near 495 nm and characteristically show saturation under photopic or ‘daylight’ light levels and are thus mainly responsible for scotopic or ‘night’ vision. The three types of cones controlling photopic vision, namely the long-wavelength-sensitive L-cones, the medium-wavelength-sensitive M-cones and the short-wavelength-sensitive S-cones, peak near 558, 530 and 420 nm, respectively. Melanopsin exhibits its maximum spectral sensitivity near 480 nm. The ipRGCs expressing melanopsin differ markedly from the other photoreceptors in their temporal response to light, showing longer latency and sustained depolarization [10,11]. While the ipRGCs are themselves photosensitive, they also receive synaptic inputs from the cones and rods [10]. All photoreceptors contribute to the control of the pupil, though their spatial distribution and temporal ‘niches’ are different. Converging evidence suggests that in daylight light levels, the mean steady-state pupil size is mainly controlled by the melanopsin-encoded signal [11–16]. For dynamic pupil size responses, the melanopsin-encoded signal seems to have a smaller role in pupil size control with increasing temporal frequency [17,18].
1.2. Age-dependence of pupil size
Pupil size is affected by age. Steady-state pupil size starts to decrease with age after the second decade of life, both in dark [19–21] and light adaptation [13,22–28]. This age effect, also termed ‘senile miosis’, is more pronounced in dimmer light compared with more intense light conditions [13,24,28,29]. For instance, given a luminance of 9 cd m−2, Winn et al. [28] reported an age-related decrease of pupil size of approximately 0.043 mm per year. This amounts to a approximately 1.72 mm difference comparing a typical 60-year-old and 20-year-old person at that light level. A variety of processes have been proposed as possible causes for the age-related functional decrease of the pupil [2] such as structural iris damage like stiffness and muscle degeneration [30,31], sympathetic and parasympathetic deficits [23] as well as deficiency in central inhibition [21]. Notably, there are also various retinal degradation processes associated with ageing such as the decrease in photoreceptor density [32] and delayed photopigment regeneration [33], along with ageing phenomena like lens yellowing [34] that could partly contribute to the functional shrinking of pupil size.
1.3. Pupil size model
Drawing from the extensive literature on stimuli affecting pupil size, Watson and Yellott [35] developed a unified formula for predicting pupil size from luminance, age, number of eyes and field diameter (see figure 1c for example predictions as a function of luminance and age). This formula summarizes the luminance dependence of the pupil from a total of eight extant studies [28,36–42]. However, luminance reflects only a weighted combination of the L- and M-cones. As discussed previously, all photoreceptors participate in controlling pupil size, and therefore, parametrizing retinal intensity as a function of luminance does not predict pupil size well [12,15,43].
Figure 1.
(a) A glass mannequin head with the ambulatory measurement set-up, incorporating an infrared eye tracker, a small-footprint spectrometer, as well as a control computer (Raspberry Pi) and a power bank. (b) Example images under two different illumination conditions varying across three orders of magnitude recorded with our set-up. (c) Predictions of [35] for two observers (20-year-old versus 80-year-old). (d–f) Indoor (d), outdoor (e) and laboratory lighting situations (f).
1.4. Pupil size in naturalistic conditions
Our understanding of the factors influencing pupil size almost exclusively stems from controlled laboratory studies using simplified parametric stimuli and has generated an internally valid body of well-controlled studies. Yet, to predict pupil size in the real world, research needs to be extended to naturalistic environments [44–46]. As pupil size is a crucial factor for visual performance in real life, it is necessary to confirm how it varies in an age- and gender-balanced sample, performing ecologically relevant tasks in everyday-life settings under naturalistic illumination.
To our knowledge, a very limited number of studies have yet investigated pupil size under real-world conditions. A study by Koch et al. [25] simulated viewing conditions of five naturalistic tasks in an indoor laboratory setting (night-time driving, reading dim and bright illumination, distant viewing in direct and indirect sunlight) and confirmed the decrease of pupil size with increased illumination and higher age. Harley and Sliney [47] examined outdoor daylight-adapted pupil sizes of 87 subjects from a military population between 790 and 4250 cd m−2. Crucially, both studies parametrize the lighting conditions in terms of luminance (weighted sum of L- and M-cones). If a spectrum does not change with light level, then the change in (il)luminance as a function of light level will be proportional to the change in melanopic (ir)radiance. However, in the real world, the illuminant and reflectance spectra (and the resultant scene spectra) can be very diverse (the ‘spectral diet’, [48]), due to different sources of illumination (e.g. daylight, incandescent, fluorescent, LED lighting). As a consequence, (il)luminance and melanopic (ir)radiance are not always correlated (see Fig. 2 in [15] for this dissociation under real-world spectra).
Here, we performed a confirmatory analysis of age effects and effects of spectrally weighted illumination on the light-adapted pupil size during real-life indoor and outdoor light and viewing-task conditions in a healthy, age-diverse sample. Our confirmatory hypotheses (CH) were:
-
—
CH1: In real-world conditions, light-adapted pupil size changes as a function of melanopsin sensitivity-weighted retinal 1 illumination, with higher illumination associated with a smaller pupil size.
-
—
CH2: In real-world conditions, melanopsin sensitivity-weighted retinal (see footnote 1) illumination better predicts pupil size than the weighted sum of L- and M-cone-weighted retinal (see footnote 1) illumination (CIE 1931 Y, photopic illuminance).
-
—
CH3: In real-world conditions, light-adapted pupil size changes as a function of age, with higher age associated with a smaller pupil size.
We tested each of these hypotheses using a linear mixed model (LMM) and calculated the total evidence for each of the hypotheses using the Bayes factor (BF) comparing the full model formulated for each hypothesis with the appropriate null model (see Analytic strategy below).
2. Material and methods
2.1. Participant characteristics
We aimed to recruit a total of 96 healthy volunteers (48 female, 48 male) from the community. To achieve a balanced and age-diverse sample, we sought to recruit 16 volunteers in each of the following age groups 2 : 18–24 years, 25–34 years, 35–44 years, 45–54 years, 55–65 years, and greater than 65 years of age. Only participants wearing contact lenses or requiring no vision correction were included as our measurement device was not compatible with wearing glasses. All participants had 20/40 visual acuity or better (assessed by Snellen chart) and normal colour vision (assessed by Hardy–Rand–Rittler (HRR) test). Additionally, participants were screened for relevant medication, disorders (neurological, metabolic), eye conditions, mental health (assessed by the German version of the 12-item General Health Questionnaire (GHQ-12), [49]) and tested for consumption of drugs 3 (urine-based multi-panel drug test, nal von minden; Moers, Germany) and alcohol (saliva-based alcohol test, ultimed; Ahrensburg, Germany). Elderly participants with intraocular lens replacements (IOLs) were allowed to be included in the study, with their lenticular status (i.e. whether they have an IOL) recorded as a binary variable. 4
2.2. Study protocol
Participants performed a range of natural tasks in common everyday lighting conditions as well as a 10 min dark- and a 14 min laboratory-based part during a approximately 1 h ambulatory protocol (approx. 300–360 samples in total) while their approximate corneal spectral irradiance and pupil size were measured. Given the 10 s sampling interval, each task and light condition took at least 60 s to ensure collecting enough valid samples per condition. Details about the tasks and the experimental procedure are given in electronic supplementary material, table S1.
2.2.1. Light settings
During the natural conditions, light sources included fluorescent room lighting, LED lighting (room lighting, laptop screen) and sunlight through the windows as well as outdoors. Participants moved in and between indoor and outdoor environments and engaged in a range of everyday tasks while illuminance ranged from approximately 5 lux to many thousands of lux (upper end depending on weather conditions). Example scenarios are shown in figure 1 e,f.
In the laboratory-based part, the light source was a vertical front panel (220 cm width, 140 cm height) mounted 80 cm above floor level. It consisted of 24 LED panels (RGB + White), each containing 144 LEDs (total of 3456 LEDs; peak emission wavelengths at maximum intensity for RGB primaries: blue, 467 nm; green, 527 nm; red, 630 nm). The panel was covered with a diffuser film. Lighting scenarios were set up using DMXControl (version 2.12.2) software (DMXControl Projects e.V., Berlin, Germany). Participants were seated on a chair in front of the light panel for 14 min (approx. 75 cm distance to the eyes, height varying with participants' height, approximately 90–140 cm) and instructed to gaze at a cross in the middle of the panel. The light set-up comprised four spectrally different 3 min phases of light (appearance: ‘red’, ‘blue’, ‘green’, ‘white’). The four phases were randomized in order and embedded in 20 s dark phases (less than 0.2 lux before and after each light phase). Each 3 min light phase contained three different illuminance levels (10, 100 and 250–1000 lux) in ascending order, each level lasting 1 min. The ‘red’ and ‘blue’ conditions reached their maximum illuminance at 480 and 250 lux, respectively, whereas ‘green’ and ‘white’ reached 1000 lux. In total the light setting added up to 13 min and 40 s duration. The laboratory set-up in shown in figure 1f.
2.2.2. Calibrated spectroradiometric measurements
Irradiance spectrum in the approximate corneal plane was recorded with a calibrated research-grade commercial small-footprint spectroradiometer (STS-VIS, Ocean Insight Inc., Oxford, UK; figure 1a), measuring in the range of 350–800 nm with a wavelength accuracy of ±0.13 nm and optical resolution of 6 nm (100 µm slit). The STS measured with a signal-to-noise ratio of greater than 1500 : 1 and dynamic range of 4600 : 1. Integration times were preset to range from 10 µs to 5 s. Attached to the STS was a direct-attach cosine corrector (CC-3-DA, diameter: 7.14 mm) with 180° field of view.
The spectroradiometer was covered by a customized three-dimensional-printed case (figure 1 a), adjustable in angle and mounted on a wearable, padded headband, which could be adjusted for different head sizes. The mount was centred on the forehead, such that it was approximately conjoint with the corneal plane and in the direction of gaze of the observer. The vertical angle of the spectroradiometer lens was set pointing 15° below the horizontal at default for every participant, in correspondence to typical outdoor gaze angle [50]. Irradiance spectra were measured every 10 s and stored on a custom-made, portable Raspberry Pi-based microcontroller.
2.2.3. Pupil size measurements
An infrared video-based eye tracker (Pupil Labs GmbH, Berlin, Germany; figure 1 a) was used to collect still photographs of the participant's pupil at the same time as the irradiance spectra. These still photographs (figure 1 b) were then subjected to a three-dimensional model estimated during a calibration procedure supplied by the eye tracker manufacturer. Images from the camera were polled using OpenCV and stored on the same microcontroller as the spectroradiometric measurements. The three-dimensional model [51] assumes that the three-dimensional pupil can be modelled as a disc which in each moment is tangent to a rotating eye sphere. Elliptic pupil contours are extracted using a pupil segmentation and the centre of the eye sphere is estimated using a nonlinear optimization procedure (see below under ‘Pupil size estimation’ in the ‘Data analysis’ section). In the 3 min long video-based calibration procedure, the investigator verified that the three-dimensional model could detect pupil size reliably during various head and eye movements and positions.
2.3. Data analysis
2.3.1. Pupil size estimation
Absolute pupil diameter in millimetres was estimated from the raw images using the Pupil Labs software (release version 1.15.71, https://github.com/pupil-labs/pupil/releases). The parameters for the intrinsic three-dimensional model were derived in the calibration stage and then applied on the pupil image sequence, yielding pupil diameter in millimetres.
2.3.2. Processing of spectral data
The spectral data reported by the spectroradiometer were given in irregular wavelength spacing and resampled to 1 nm intervals between 380 and 780 nm (visible range) using energy-preserving interpolation. From the spectral data we calculated the melanopic irradiance and melanopic equivalent daylight illuminance 5 (mEDI) using melanopsin spectral sensitivity curve obtained from the recent CIE S026/E:2018 standard [9] incorporating a previously defined template-derived spectral sensitivity for melanopsin [52]. In addition to the melanopic irradiance and mEDI, we also calculated the photopic illumininance in lux based on the CIE 1924 photopic luminous efficiency curve.
2.3.3. Data quality checks
We implemented the following data quality checks:
-
—
Lack of good-quality fit. The pupil size estimation method was in some cases not able to find a pupil, due to extreme angles of gaze, or partial covering by eye lashes. Any images in which a pupil could not be found or yielded low detection confidence (less than 0.6, as computed by the Pupil Labs software) was simply excluded. In a test run of the experiment, we found that approximately 30% of all images had this problem. This number might seem high at first, but it is important to remember that participants were performing tasks in the real world and therefore blinked and moved their eyes.
-
—
Pupil size screening. Since the natural pupil can only vary in a maximum range between approximately 1 and 9 mm, any estimated pupil size (due to misestimation) outside of this range was excluded.
-
—
Proportion of excluded data. For each participant, the proportion of excluded data points from the entire sample was reported. Initially, we set the following threshold: ‘if less than 50% 6 of data points are included, corresponding to a mere recording time of approximately 30 min, we will exclude that participant altogether.’
-
—
Positive control and quality of pupillometry data. We confirmed that our measurement procedure was sensible and provided upper-bound estimates for data quality by conducting laboratory-based measurements of the dark-adapted pupil size as well as 12 different conditions of light-adapted pupil size early in the protocol (for details see section ‘Light settings’ and figure 1).
2.3.4. Recorded demographic and ancillary information
We recorded ancillary information to describe the sample. Recorded covariates include gender, handedness, body mass index (BMI), vision correction, date and time of experiment, time since waking up, sleep duration, acute and habitual caffeine consumption, chronotype, acute sleepiness, iris colour and weather during the experiment. Except for the last three, these covariates were retrieved via an online questionnaire which was filled out just before and during experiment protocol.
-
—
Iris colour and weather were rated by the experimenter. The former based on a 3-point Likert-scale item using iris colour categories ‘brown’, ‘hazel-green’ and ‘blue’ [53], rated before the beginning of the protocol and the latter on a self-created 4-point Likert-scale item, incorporating the options ‘overcast’, ‘cloudy’, ‘partly cloudy’ and ‘sunny’, rated at the beginning of the outdoor part of the protocol (timestamp 7 in electronic supplementary material, table S1).
-
—
Acute sleepiness was estimated with a German paper-pencil version of the Karolinska sleepiness scale [54], both at the beginning and end of the protocol (timestamp 1 and 9 in electronic supplementary material, table S1). The 9-point Likert-scale item is labelled on all odd steps.
-
—
Both the habitual and acute caffeine consumption questionnaires were developed based on median values of the caffeine content ranges in mg for typical caffeinated beverages and food items [55]. 7 Each questionnaire comprises 18 items (15 on beverages, 2 on chocolate and 1 for caffeine pills). Each item asks the participant to indicate how many portions of the caffeinated item were consumed on a scale from 0 (no) portion to 8 portions. Every item portion matches a specified typical portion size in ml or g and a caffeine content in mg. While the ‘habitual’ questionnaire is asking for intake on an average day, the ‘acute’ questionnaire queries the same items with regards to the last 6 h. For both habitual and acute intake, an absolute sum and a relative sum value of caffeine in mg (divided by weight in kg) were computed for every participant. The two questionnaires were filled out during the protocol as part of a ‘laptop task’ (timestamp 4 in electronic supplementary material, table S1).
-
—
Chronotype was queried with a German translation of the µMCTQ, an ultra-short version of the Munich ChronoType Questionnaire [56]. It comprises six core questions, allowing for a quick and efficient assessment of chronotype. The indicating value for chronotype is computed as the midpoint of sleep on work-free days corrected by potential sleep debt (MSFSC). Sleep duration and time since waking up were assessed from answers to four simple open-ended questions. Participants indicated the times of falling asleep and waking up concerning the night before the experiment as well as the duration of potential naps taken on the day of and before the experiment. Sleep duration was computed by subtracting time of waking up from time of falling asleep and adding nap duration. Time since waking up was computed as the time starting to fill out the questionnaire minus the time of waking up. Both the µMCTQ and the questions concerned with sleep duration and time since waking up were filled out early in the protocol as part of a ‘laptop task’ (timestamp 4 in electronic supplementary material, table S1).
-
—
Gender, handedness, BMI (computed from weight and height) and vision correction (no correction, minus correction or plus correction) were assessed via simple demographic items as part of the online questionnaire, filled out just before the beginning of the protocol. Date and time of the experiment were logged by the start of filling out that online questionnaire.
These parameters were recorded to describe the sample and not used in our confirmatory hypotheses tests. 8
2.3.5. Analytic strategy
We used a linear mixed model framework to examine our three confirmatory hypotheses and examined evidence for each of our three hypotheses using Bayes factor [57]. The general logic in this approach is that a ‘full’ model containing a factor under consideration is compared with a ‘null’ model missing that factor using the likelihood ratio between the two models. If the factor affects the variable in question (pupil size), this is reflected in higher Bayes factors. We considered a BF of 10 as ‘strong’ evidence, following standard categorizations [58]: 1 < BF < 3, anecdotal evidence; 3 < BF < 10, moderate; 10 < BF < 30, strong evidence; BF > 30, very strong evidence; and BF > 100, decisive evidence. 9 We assessed three confirmatory hypotheses independently and converted the Bayes factors obtained to this scale of evidence. The analysed relationship between pupil size and light refers to simultaneously taken samples of those variables.
Our first confirmatory hypothesis (CH1) tested whether melanopsin sensitivity-weighted irradiance predicted our pupil size. The formulation of the full model in Wilkinson–Rogers notation [59] was as follows: 10
This full model was compared with the null model not containing melanopsin irradiance,
The second confirmatory hypothesis tested if melanopsin sensitivity-weighted retinal 11 illumination better predicted pupil size than the weighted sum of L- and M-cone-weighted retinal 12 illumination (CIE 1931 Y, photopic illuminance). We expressed this full model as 13
Here, the null model was
Our third confirmatory hypothesis tested if pupil size changes as a function of age, with higher age associated with a smaller pupil size. We expressed the full model as
Here, the null model was
2.3.6. Power analysis
There were no prior data allowing us to conduct a meaningful power analysis. The largest (to our knowledge) study examining the relationship between pupil size and illuminance [28] only used one spectral stimulus (scaled to different illuminances). We considered the question of power as follows. In hypothesis CH1, we aimed to confirm the light-level dependence of pupil size. Unless a participant had an undetected neurological or retinal condition leading to immobility or paradoxical behaviour of the pupil, the light-level effect would be present in all individuals. Therefore, the effect was nearly guaranteed to exist in a given individual. In hypothesis CH2, we examined whether melanopic irradiance was a better predictor for pupil size than photopic illuminance. From our previous work, we knew that the spectral sensitivity of pupil size was best described using melanopsin rather than photopic luminosity [15]. In the laboratory part of the experiment, we near-recreated these conditions using narrow-band LED lights. Regarding hypothesis CH3, we were confident in the study's power to detect effects of age on pupil size in an age-diverse sample (N = 96). Very stable effects of age on pupil size were shown in a comparable sample (N = 91, [28]). Our sample size (96 individuals) was governed by our resource limitations.
2.3.7. Data collection termination rules
Data were collected until our resource limit (96 participants and study personnel hours) was reached.
2.4. Known limitations of this registered report
We acknowledge that our study is limited with respect to following aspects:
-
—
Other, small-scale determinants of pupil size were ignored. In this work, we were agnostic to other determinants of pupil size, such as fatigue [60], attentional processes [61,62], recognition [63], high-level image content [64], target detection [65], mental effort [63,66], arousal [67] and substance use [68]. These top-down effects are expected to be transient and relatively small in effect compared with the light and age effect [2,35]. In addition, due to the near triad comprising convergence eye movements and ocular accommodation, viewing distance affects pupil size due to a shared neural pathway [1].
-
—
Estimation of retinal illuminance in natural vision. In this study, we were using an irradiance sensor near the corneal plane, placed on the forehead and adjusted to typical outdoor gaze angle (15° below the horizontal, [50]). Given the geometry of the sensor and human head, this was the closest we could get to measuring corneal illuminance. It was impossible to estimate retinal illuminance using sensors in the real world under freely changing geometries and gaze positions.
-
—
Light history. In the present study, we were omitting effects of prior light exposure (e.g. before the experiment) and light sequence effects during the protocol on pupil size. The protocol procedure of the field experiment was held constant across participants (except for the randomized light phases in the laboratory-based part of the experiment).
-
—
Omitting scene radiance. In the present study we did not measure spectral radiance but spectral irradiance. By doing this, we neglected effects of variation in light level across the field of view in different light scenes. Given the limitations of the real-world setting, the technical properties and calibration of the available instruments, collecting real-world radiance data and artificially limiting the angular acceptance of our spectroradiometer was not feasible in the present study.
3. Results
We carried out the experiment according to the Stage 1 protocol, which was accepted in principle (https://osf.io/zrksf/). All LMM analyses were performed in R (v. 4.3.1) [69] using the BayesFactor package (v. 0.9.12–4.5) [57]. The ‘ggplot2’ package (v. 3.4.3) [70] was used for data visualization. More details are available in the electronic supplementary material.
In the following, we first report participant characteristics and quality check analyses. We then present the results of our confirmatory hypotheses using a final sample of 83 participants and log10-transformed light data. For transparency and completeness, we also report the results of our hypothesis tests in the linear, non-transformed light data despite the violation of linear assumptions both in the full (n = 83, 75% data loss threshold) and in the reduced sample of 63 participants (50% data loss threshold). Finally, we summarize our post hoc analyses.
3.1. Participant recruitment
Details of the recruitment process are presented in the study flow diagram (electronic supplementary material, figure S1). Following our recruitment plan of the approved Stage 1 registration, data were collected until we reached our resource limit. Due to difficulties filling the older age groups during the COVID-19 pandemic, we later opened recruitment to more than 16 per age group to achieve a large enough total sample. In total, n = 113 volunteers were invited to the health and eligibility screening. Seven did not meet the inclusion criteria—five because of the visual tests and two for health reasons and medication with potential influence on pupil regulation. The remaining 106 subjects were allocated to the protocol. While n = 87 completed the trials without problems with the equipment, data from n = 19 volunteers' trials were invalid due to technical problems (cable issue, camera slippage or software/format errors) and had to be excluded. A further four participants were then removed before the final analysis because more than 75% 14 of their pupil data were of insufficient quality (see Data quality checks), leaving n = 83 for the final analysis. Given the resource limitations mentioned above, this sample remained as the final sample for statistical analysis.
3.2. Participant characteristics
Table 1 presents sample characteristics based on self-report separately for participants who were included (n = 83), excluded after the study (n = 23), and excluded before the study (n = 7). Among the 83 volunteers included in the final analyses (43 female, 40 male; M age = 35.70, s.d. age = 17.16; M BMI = 22.96, s.d. BMI = 3.47), age groups were not balanced as planned initially (16 per group), and the final sample was skewed towards younger age, with 30 included volunteers aged 18–24 years and five included volunteers over 64. This is also depicted in figure 2, where the included sample is stratified by sex. Notably, the volunteers excluded before the trial were clearly older on average and with higher BMI (M age = 52.00, s.d. age = 21.54, M BMI = 26.39, s.d. BMI = 5.79).
Table 1.
Participant characteristics. Sample properties for participants that were included (n = 83), excluded after the trial (n = 23) and excluded before the trial (n = 7). Age, sex, visual aid status and body mass index (BMI) were based on self-report. Iris colour and weather during the trial were rated by the experimenter. The season was derived from the date of testing. Numerical variables are given as ‘mean (standard deviation)’, categorical variables are given as ‘count (%)’, missing values (‘0 (NA%)’) result from participants being excluded before those items were surveyed.
| variable | included, n = 83a | excluded after trial, n = 23a | excluded before trial, n = 7a |
|---|---|---|---|
| age [years] | 35.70 (17.16) | 37.87 (20.13) | 52.00 (21.54) |
| age group [years] | |||
| 18–24 | 30 (36%) | 9 (39%) | 0 (0%) |
| 25–34 | 18 (22%) | 5 (22%) | 2 (29%) |
| 35–44 | 13 (16%) | 0 (0%) | 1 (14%) |
| 45–54 | 9 (11%) | 3 (13%) | 0 (0%) |
| 55–64 | 8 (9.6%) | 5 (22%) | 1 (14%) |
| >64 | 5 (6.0%) | 1 (4.3%) | 3 (43%) |
| sex (assigned at birth) | |||
| female | 43 (52%) | 17 (74%) | 3 (43%) |
| male | 40 (48%) | 6 (26%) | 4 (57%) |
| uses visual aid | |||
| no | 57 (69%) | 16 (70%) | 3 (43%) |
| yes—correction for myopia | 18 (22%) | 5 (22%) | 2 (29%) |
| yes—correction for hyperopia | 8 (9.6%) | 2 (8.7%) | 2 (29%) |
| wearing contact lenses during trial | 16 (19%) | 5 (22%) | 2 (29%) |
| body mass index (BMI) [kg m−²] | 22.96 (3.47) | 22.07 (3.93) | 26.39 (5.79) |
| iris colour (experimenter-rated) | |||
| blue | 25 (30%) | 9 (39%) | 0 (NA%) |
| hazel/green | 17 (20%) | 8 (35%) | 0 (NA%) |
| brown | 41 (49%) | 6 (26%) | 0 (NA%) |
| weather during trial (experimenter-rated) | |||
| light rain | 9 (11%) | 0 (0%) | 0 (NA%) |
| very cloudy | 16 (19%) | 7 (30%) | 0 (NA%) |
| cloudy | 14 (17%) | 1 (4.3%) | 0 (NA%) |
| somewhat cloudy | 11 (13%) | 5 (22%) | 0 (NA%) |
| sunny | 33 (40%) | 10 (43%) | 0 (NA%) |
| season | |||
| spring | 7 (8.4%) | 5 (22%) | 1 (14%) |
| summer | 40 (48%) | 9 (39%) | 3 (43%) |
| autumn | 16 (19%) | 5 (22%) | 0 (0%) |
| winter | 20 (24%) | 4 (17%) | 3 (43%) |
aMean (s.d.); n (%).
Figure 2.
Included sample (n = 83) stratified for age groups and sex. Green bars on the left depict female, and violet bars on the right depict male participants. The sample was skewed towards younger age groups, especially for female participants.
A total of 40 included participants took part in the experiment during summer, 20 during winter, 16 during autumn and 7 during spring. Weather conditions varied between light rain (11%), very cloudy (19%), cloudy (17%), somewhat cloudy (13%) and sunny (40%). Differences in weather conditions during the trial were associated with different light-level distributions. Figure 3 depicts the density of light level (mEDI) across the whole trial (upper panel) and stratified across the weather conditions (lower panel). Notably, in sunny weather, there was a higher probability density of mEDI values over 1000 lx than in all other weather conditions. Additional participant information like chronotype, sleep duration, start time of the experiment and acute and habitual caffeine consumption are given in electronic supplementary material, table S2. Electronic supplementary material, figure S2 depicts the distribution of all alpha-opic EDIs and photopic illuminance across the trial.
Figure 3.
Density of mEDI across all observations in the field conditions (n = 83, top panel) and stratified across the weather conditions (bottom panel). The dotted and solid vertical grey lines indicate intensities of 250 and 1000 lx mEDI, respectively. Bin breaks in the histogram (top panel) correspond to half log10-unit steps of mEDI. Box plots in the density violin plots (bottom panel) include the median (black square) along with the interquartile range (IQR, black rectangle) under each weather condition. The ‘whiskers’ mark the values below the first and beyond the fourth quartile. Sunny weather was associated with a larger proportion of greater than 1000 lx mEDI values.
3.3. Data quality checks
The quality checks specified earlier in this report were applied and further supplemented by a check of linear assumptions for the hypothesized mixed linear models.
3.3.1. Proportion of excluded data
Out of 32 810 total observations, 43.37% yielded low pupil detection confidence (less than 0.6), 0.16% exceeded maximum pupil range (less than 1 or greater than 9 mm), and 1.41% had saturated spectroradiometer samples (after quick changes from dark to bright). Electronic supplementary material, table S3 shows the proportion of excluded data points for each participant.
As our dataset revealed a higher proportion of pupil data exclusion than expected from the pilot runs, a threshold of 50% data loss per subject would have amounted to excluding an additional n = 24 (out of n = 87) participants from the final analysis (cf. Figure 4). Thus, to address high participant attrition, we decided to additionally determine a second adjusted threshold of ‘acceptable’ data loss proportion per participant after Stage 1 in principle acceptance (IPA) in a data-driven analysis of a ‘scree-like’ plot. The criterion was to find the location beyond the 50% threshold, where increasing the threshold in 5% steps would lead to a minimally increased number of excluded participants. As depicted in figure 4, this procedure resulted in adopting a second data loss threshold of 75% excluded data points per participant, with n = 4 (out of n = 87) exceeding the new threshold with values of 81.60%, 85.48%, 91.67% and 95.65%.
Figure 4.
Excluded participants as a function of possible data loss thresholds (black line). The initial data loss threshold (50%) is marked by the dashed blue vertical line, yielding n = 24 excluded participants. The adjusted threshold (dashed magenta line) was placed at the location over 50%, which marks the smallest increase of excluded participants (+0, from 75 to 80%) across possible thresholds in 5% steps, yielding n = 4 excluded participants and retaining n = 83.
In the remaining dataset of n = 83 (all conditions), a total of 17 199 observations with good-quality pupil and light data were retained. The full dataset is separated into ‘field data’ (10 082 valid observations), ‘dark data’ (2871 valid pupil observations), and ‘lab data’ (3698 valid observations). There were also residual ‘transition samples’ (548 valid observations) taken between the laboratory and field conditions that were not used in the analyses because they could not be clearly assigned to either condition.
Data loss per included participant ranges from 10.82% to 70.64% as listed in electronic supplementary material, table S3. Furthermore, electronic supplementary material, tables S3 and S4 display a summary of minimum, median and maximum values for mEDI and pupil size, separated for field and positive control data (laboratory and dark adaptation conditions), respectively. Electronic supplementary material, tables S5–S9 each give a summary of cone- and rhodopic EDI and illuminance. Only observations from the field condition with retained pupil and light data were used for confirmatory hypothesis testing.
3.3.2. Linear regression assumptions
We discovered a peculiarity in the originally formulated linear mixed model analyses after receiving Stage 1 IPA and collecting data. When predicting pupil size with linear mEDI/melanopic irradiance and photopic illuminance data, these variables violate the assumptions of linear regression models, specifically the assumptions for linearity, homogeneity of variance and collinearity (see electronic supplementary material, figures S3 and S4B, C and F). When light data were log10-transformed prior to inclusion in the linear models, the requirements were approximately met (see electronic supplementary material, figures S5 and S6), suggesting transformation. We additionally tested whether the transformation improved the fit in the LMM, comparing the model with log10-transformation against a null model with linear light data in the same procedure as the hypothesis tests in the pre-registered analysis. As shown in electronic supplementary material, table S10, the resulting likelihood ratio clearly favours the model with log10-transformed light data (log10(BF10) = 996.339 ± 3.55% proportional error (pe)), corresponding to ‘decisive evidence’ in favour of a log10-transformation. For completeness and transparency, we report the results of our hypothesis tests both with transformed and non-transformed data.
3.3.3. Positive control data
Two conditions were included in the experiment as a manipulation control: first, a 10 min dark adaptation to test whether the age effect is generally present; second, a laboratory light condition in which different light colours allow stronger dissociation between photopic illuminance and mEDI values (see Light settings), to confirm that mEDI determines pupil size in the light-adapted pupil and is superior as a predictor compared with photopic illuminance. Notably, the spectral sources in the laboratory were of the same size and geometry.
The recorded light intensities from the laboratory condition, contrasted with the field conditions, are shown in figure 5. In comparison with the extremely high positive correlation between photopic illuminance and mEDI under uncontrolled field conditions (r = 0.999, see figure 5 b), the laboratory condition yielded a clearly lower positive correlation (r = 0.310, see figure 5 a). We tested confirmatory hypotheses CH1 and CH2 with the linear models specified in stage 1 of this report with data from these low-correlation laboratory conditions. Both hypotheses are supported by decisive evidence (log10(BF10) > 2). This is the case for the full sample (n = 83, 75% threshold) as well as the reduced sample (n = 63, 50% threshold) and with log10-transformed as well as linear light data (see rows 1–4 of electronic supplementary material, tables S11, S12, respectively).
Figure 5.
Relationship between melanopic and photopic light units contrasted between laboratory conditions (a) and field conditions (b). Pearson correlation coefficients quantify the association. The laboratory data clearly show a dissociation between the light level units, whereas, under field conditions, these units are highly correlated.
Testing the age effect under dark conditions, we compared a model including age as a predictor versus the null model without it. The third rows of electronic supplementary material, tables S11 and S12, present the tested models and results in the full sample (n = 83, 75% threshold) and reduced sample (n = 63, 50% threshold), respectively. Both provide decisive evidence (log10(BF10) > 2) for age as an influencing factor on pupil size during dark adaptation.
3.4. Confirmatory analyses
3.4.1. Pupillary light response
Hypothesis CH1 proposed that pupil size is dependent on the (melanopsin sensitivity-weighted) light level under naturalistic steady-state conditions. Our results from the LMM analysis (n = 83, 75% threshold) provide decisive evidence (log10(BF10) > 2) for that hypothesis, both when using the log10-transformed mEDI data (cf. Table 2, row 1) and the linear mEDI data in the full model (cf. electronic supplementary material, table S13, row 1). Notably, the likelihood ratio turns out to be magnitudes higher after log10-transformation compared with without it (log10(BF10) = 1288.015 ± 2.20% pe versus log10(BF10) = 291.664 ± 2.78% pe, respectively).
Table 2.
Confirmatory hypothesis tests (n = 83, log10-transformed). The tested models are given in Wilkinson–Rogers' notation (column 2). The likelihood ratios between the tested models are given as log10(Bayes factors) rounded to three decimals (column 3), along with the proportional error in per cent (pe %; column 4), while the interpretation of the likelihood ratios follows the standard categorizations [58]. Here, the sample from the adjusted data loss threshold (75%, n = 83) and log10-transformed light data from the field condition were used. For the initial data loss threshold with reduced sample (50%, n = 63) and non-transformed light data, see results displayed in electronic supplementary material, tables S13–S15. Abbreviations: CH = confirmatory hypothesis; = melanopic equivalent daylight illuminance; = photopic illuminance; BF10 = Bayes factor comparing H1 with H0; % pe = proportional error in per cent.
| hypothesis | H1/H0 model comparison [Wilkinson–Rogers’ notation] | log10(BF10) | % pe | interpretation |
|---|---|---|---|---|
| CH1 | 1288.015 | ±2.2% | decisive evidence for CH1 versus null model | |
| CH2 | 21.855 | ±2.13% | decisive evidence for CH2 versus null model | |
| CH3 | 4.665 | ±2.17% | decisive evidence for CH3 versus null model |
Considering the reduced dataset (n = 63, 50% threshold), the hypotheses tests produce qualitatively similar results, yielding decisive evidence for the full model and CH1 (log10(BF10) > 2), again for both the log10-transformed and linear data (cf. electronic supplementary material, table S14, row 1 and table S15, row 1). Likelihood ratios are larger in the full sample (see above) compared with in the reduced sample (log10(BF10) = 1076.224 ± 2.24% pe and log10(BF10) = 242.197 ± 2.24% pe, respectively).
3.4.2. Predictor performance of mEDI versus illuminance
In hypothesis CH2, we tested whether mEDI was a better linear predictor of steady-state pupil size than photopic illuminance. The LMM results (n = 83, 75% threshold) support this hypothesis with decisive evidence (log10(BF10) > 2), both using the log10-transformed light data (table 2, row 2) and the linear mEDI data (see electronic supplementary material, table S13, row 2). Once more, the likelihood ratio turns out to be several orders of magnitude higher after log10-transformation than without (log10(BF10) = 21.855 ± 2.13% pe versus log10(BF10) = 10.349 ± 3.91% pe, respectively).
Looking at the reduced dataset (n = 63, 50% threshold), the hypothesis tests yield qualitatively equivalent results, providing decisive evidence for the full model of CH2 (log10(BF10) > 2), again for both the log10-transformed and linear data (see electronic supplementary material, table S14, row 2 and table S15, row 2). The Bayes factors are again larger in the full sample (see above) than in the reduced sample (log10(BF10) = 19.844 ± 2.06% pe and log10(BF10) = 6.525 ± 1.93% pe, respectively).
3.4.3. Age effect in the light-adapted pupil
Hypothesis CH3 stated that light-adapted pupil size decreases (on average) with increasing age, a phenomenon also termed ‘senile miosis’. Results from the LMM (n = 83, 75% threshold) support the notion that age is a significant factor for pupil size with decisive evidence (log10(BF10) > 2). This is the case when including log10-transformed mEDI data (table 2, row 3) or linear mEDI data in the models (see electronic supplementary material, table S13, row 3). The likelihood ratio comparing the full and null models, including the log10-transformed data, was approximately 20 times, or 1.3 log10-units, higher than the BF of the model comparison without transformation (log10(BF10) = 4.665 ± 2.17% pe versus log10(BF10) = 3.348 ± 2.66% pe, respectively).
In the reduced dataset (n = 63, 50% threshold), the hypothesis test with the full model of CH3, including age and the log10-transformed mEDI data, leads to the same interpretation: decisive evidence in favour of CH3 (log10(BF10) = 2.096 ± 2.12% pe; see electronic supplementary material, table S14, row 3). However, the likelihood ratio for the full model of CH3, including age and the linear mEDI data, falls below 100 (log10(BF10) = 1.146 ± 1.89% pe, see electronic supplementary material, table S1, row 3) and is therefore interpreted as ‘strong evidence’ (log10(BF10) > 1) for CH3 instead.
3.5. Post hoc analyses
As an extension of our confirmatory hypotheses, we visualized the direction and magnitude of the tested age effects on pupil size, inspired by the work of Winn et al. [28]. We further performed explorative analyses testing sex and iris colour, and reported acute and habitual caffeine consumption (relative to body weight) as potential influence factors on light-adapted pupil size under real-world conditions. Finally, we exploratively compared the LMM model fit of six different light level metrics as single and as pairwise predictors implemented in same model structure for estimating pupil size. These analyses were not included in our Stage 1 submission.
3.5.1. Visualizing the age effect
Providing typical case data, figure 6 depicts pupil size data from a young (18 years of age) and an old participant (87 years of age), plotted against mEDI, including dark adaptation and field data from the experiment (see pupil size and illuminance in electronic supplementary material, figure S7). As can be seen from the maximum to minimum range (given by the coloured horizontal lines) and from the regression equation, the older participant has a clearly smaller baseline pupil diameter (β 0 = 3.83 mm) and magnitude of pupillary light response (β 1 = −0.463 mm per log-unit mEDI) compared with the young subject (versus β 0 = 6.28 mm and β 1 = −0.998 mm per log-unit mEDI). Interestingly, the variation in pupil size within similar light intensities decreases markedly towards the high mEDI intensities.
Figure 6.
Age comparison of typical pupil size data as a function of melanopic equivalent daylight illuminance (mEDI). The scatterplots show data from the field and dark conditions of a young (18 years of age, left panel) and old participant (87 years of age, right panel). The regression line and equation demonstrate the linear relationship between log10-transformed mEDI values in lux and pupil diameter in mm. The coloured horizontal lines mark each subject's minimum and maximum pupil diameter values. A reduced pupil dilation in the lower light intensities is visible for the older subject.
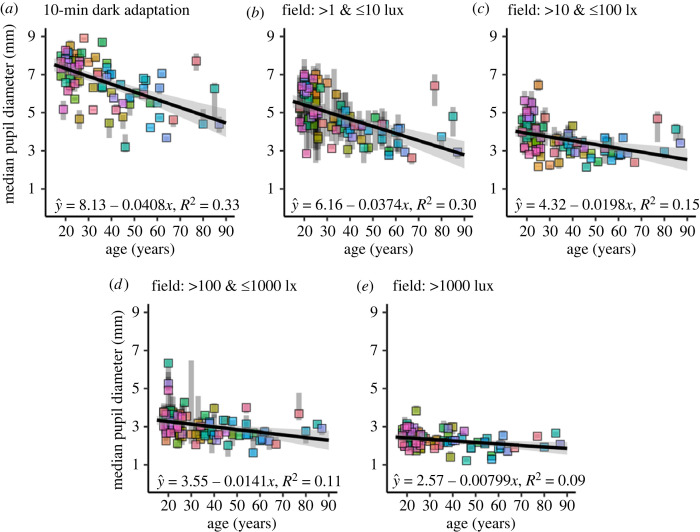
Inspired by Winn et al. (cf. Fig. 2 in [28]), figure 7 visualizes the direction and magnitude of the age effect across the full sample (n = 83) under different light condition clusters given in mEDI. Coloured squares depict each subject's median pupil size values under the respective condition plotted against age in years along with the interquartile range (see grey opaque bars). The linear regression lines are fitted to the median values, while the 95% confidence limits are indicated by the grey shade around the line.
Figure 7.
(a–e) Median values of pupil size per subject (n = 83) as a function of age during different light conditions. (a) In the 10 min laboratory-based dark adaptation condition. (b) In field conditions between 1 and 10 lx mEDI. (c) In field conditions between 10 and 100 lx mEDI. (d) In field conditions between 100 and 1000 lx mEDI. (e) In field conditions over 1000 lx mEDI. The coloured squares display the median values per subject along with the interquartile range (IQR, opaque grey bars). The equations and regression lines, including the 95% confidence intervals (grey line shade), demonstrate the linear relationship between age in years and median pupil diameter in mm across the different clusters of light intensities during the experiment. Field data (b–e) were included in the confirmatory hypothesis analysis, while dark data (a) was only used in the positive control analysis. The effect of ageing is reduced as the light level increases.
Figure 7 a shows the age effect during the 10 min in-laboratory dark adaptation (data not used in the confirmatory analyses), while panels (b–e) depict the age effect under field conditions in log10-unit steps. In all light conditions, we see an apparent reduction of pupil size with increasing age (on average), consistent with the beta values in the regression equations given in each panel. Interestingly, the slopes and intercepts of the age effect decrease with increasing light level (mEDI) across all light level clusters, as shown in figure 8 a,b, along with the 95% confidence intervals given as error bars.
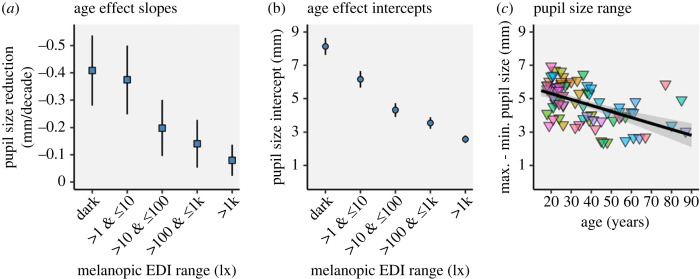
Figure 8.
Slopes (a) and intercepts (b) of the linear regressions for median pupil size as a function of age (in decades), plotted per light level cluster. Error bars give the 95% confidence intervals of the parameters. The slopes and intercepts of the age effect and their 95% confidence intervals continuously decrease with increasing light level (cf. figure 7). (c) Pupil size range (maximum − minimum diameter in mm) as a function of age in years. The equation and regression line, including the 95% confidence interval (grey line shade), demonstrate the linear relationship between age in years and pupil size range in mm.
The magnitudes of the age effect (summarized in figure 8 a) are highest in the dark-adapted pupil with pupil size reduction of β 1 = −0.408 mm per decade (CI95% = [−0.538, −0.279]). Under field conditions between 1 and 10 lx mEDI, age-driven pupil size reduction amounts to β 1 = −0.374 mm per decade (CI95% = [−0.500, −0.248]), between 10 and 100 lx mEDI, it amounts to β 1 = −0.198 mm per decade (CI95% = [−0.301, −0.095]), and between 100 and 1000 lx mEDI the age effect results in a reduction of β 1 = −0.141 mm per decade (CI95% = [−0.228, −0.053]). Finally, in the brightest light conditions (greater than 1000 lx mEDI), the age effect estimation is smallest with β 1 = −0.080 mm per decade (CI95% = [−0.137, −0.023]).
The intercepts of the age effect (figure 8 b) range from fully dilated pupils at β 0 = 8.13 mm in darkness (CI95% = [7.62, 8.64]) to fully constricted pupils at β 0 = 2.57 mm (CI95% = [2.35, 2.80]) under bright light field conditions (greater than 1000 lx mEDI). For the intermediate steps, field conditions between 1 and 10 lx mEDI yield a pupil size intercept of β 0 = 6.16 mm (CI95% = [5.66, 6.66]), field conditions between 10 and 100 lx mEDI, yield β 0 = 4.32 mm (CI95% = [3.91, 4.72]), while field conditions between 100 and 1000 lx mEDI results in β 0 = 3.55 (CI95% = [3.20, 3.90]).
However, we find considerable inter-individual variability in pupil size, even among participants of similar age, at all mEDI levels. With increasing light level, this variability is reduced, showing a close resemblance to the laboratory data of Winn and colleagues [28]. It is also worth noting that the individuals' interquartile ranges of pupil size are lower during controlled 10 min in-laboratory dark adaption but, under field conditions, decrease as the light level increases (cf. IQR in figure 7 a,b,e).
Figure 8 c depicts the maximum to minimum pupil size range in mm plotted against age in years across all subjects. Equivalent to pupil size under different light intensities (figure 7), there is an apparent negative linear effect on pupil size range with β 1 = −0.356 mm per decade (CI95% = [−0.482, −0.230]) and an intercept of β 0 = 6.01 mm (CI95% = [5.51, 6.51]).
3.5.2. Exploratory hypotheses
According to the same LMM procedure as in the confirmatory analyses (see Analytic strategy under Material and methods), we tested sex, iris colour and caffeine consumption (habitual and acute) as potential factors influencing light-adapted pupil size under natural conditions in the full sample (n = 83, 75% threshold). We derived the following exploratory hypotheses:
-
—
EH1: Sex differences in light-adapted pupil size are present under real-world conditions.
-
—
EH2: Light-adapted pupil size varies as a function of iris colour under real-world conditions.
-
—
EH3: Light-adapted pupil size varies as a function of habitual caffeine consumption (relative to body weight) under real-world conditions.
-
—
EH4: Light-adapted pupil size varies as a function of acute caffeine consumption (relative to body weight) under real-world conditions.
As summarized in table 3, along with the corresponding model comparisons in Wilkinson–Rogers’ notation [59], none of the additional factors tested for influence on light-adapted pupil size proved to be supported by evidence from our sample. We found strong evidence in support of the null models (log10(BF10) < −1) for sex and iris colour, suggesting that there are no sex differences in light-adapted pupil size under real-world conditions (log10(BF10) = −1.156 ± 1.44% pe, table 3, row 1) and that pupil size does not vary as a function of iris colour (log10(BF10) = −1.764 ± 2.86% pe, table 3, row 2). We also observed moderate evidence in support of the null models (log10(BF10) < −0.477) for habitual and acute caffeine consumption per body weight (mg kg−1), suggesting that neither habitual (log10(BF10) = −0.883 ± 2.09% pe, table 3, row 3) nor acute caffeine consumption within the last six hours (log10(BF10) = −0.756 ± 2.10% pe, table 3, row 4) had a significant effect on pupil size in our sample under real-world conditions.
Table 3.
Exploratory hypothesis tests (n = 83, log10-transformed). The tested models are given in Wilkinson–Rogers' notation (column 2). The Likelihood ratios between the tested models are given as log10(Bayes factors) rounded to three decimal points (column 3), along with the proportional error in per cent (% pe; column 4), while the interpretation of the likelihood ratios follows the conventional categories [58]. Here, the sample from the adjusted data loss threshold (75%, n = 83) and log10-transformed light data from the field condition were used. Abbreviations: EH = exploratory hypothesis; = melanopic equivalent daylight illuminance; BF10 = Bayes factor comparing H1 with H0; % pe = proportional error in per cent.
| hypothesis | H1/H0 model comparison [Wilkinson–Rogers' notation] | log10(BF10) | % pe | interpretation |
|---|---|---|---|---|
| EH1 | −1.156 | ±1.44% | strong evidence for the null model versus EH1 | |
| EH2 | −1.764 | ±2.86% | strong evidence for the null model versus EH2 | |
| EH3 | −0.883 | ±2.09% | moderate evidence for the null model versus EH3 | |
| EH4 | −0.756 | ±2.10% | moderate evidence for the null model versus EH4 |
3.5.3. Exploring different light level measures as predictors for pupil size
Using the same LMM structure as before, we compared the model fit of six different light level quantifications—the five alpha-opic EDIs and illuminance—as ‘naive’ single and as pairwise predictors in all 15 combinations, implemented in the same linear mixed model structure for estimating steady-state pupil size under natural conditions (n = 83). No hypotheses were specified for this exploratory analysis. Results are listed as matrices in electronic supplementary material, table S16 for the single predictors, and in electronic supplementary material, table 17 for the pairwise combined light level predictors. Note that BF01 values are not the exact inverse of BF10 due to the random (Monte Carlo) sampling used in Bayes factor analysis.
Summarizing all single-measure comparisons, S-cone-opic EDI showed the best model fit, followed by mEDI. The comparison between S-cone-opic EDI versus mEDI yielded log10(BF10) = 3.164 ± 2.19% pe, in favour of S-cone-opic EDI (see electronic supplementary material, table S16, row 2, column 1).
Summarizing all pairwise measure comparisons, the combination of mEDI plus rhodopic EDI showed the best model fit, followed by S-cone-opic EDI plus rhodopic EDI. The comparison between mEDI plus rhodopic EDI versus S-cone-opic EDI plus rhodopic EDI yielded log10(BF10) = 5.191 ± 40.16% pe (see electronic supplementary material, table S17, row 4, column 8), in favour of mEDI plus rhodopic EDI.
Testing the best fitted pairwise measure model (mEDI plus rhodopic EDI) against the best single measure model (S-cone-opic EDI), resulted in log10(BF10) = 4.604 ± 40.17% pe in favour of mEDI plus rhodopic EDI.
4. Discussion
Here, we conducted a real-world experimental study using a novel ambulatory set-up, similar to [71], to perform confirmatory analyses of age effects and effects of spectrally weighted illumination on light-adapted pupil size. To this end, we measured light-adapted pupil size and spectral irradiance during ecologically relevant tasks in everyday indoor and outdoor environments under naturalistic lighting conditions in a healthy, age-diverse and gender-balanced sample.
4.1. Positive control data
Our laboratory data analyses as positive control indicated that our measurements were valid and that our confirmatory hypotheses were supported by decisive evidence, consistent with prior in-laboratory work [11–14,19–21].
However, despite our prior test runs, we underestimated data loss due to the many possible interference factors when performing ecologically relevant tasks in dynamic real-world conditions. Comparable magnitudes of pupil data loss have also been reported in other outdoor eye-tracking studies due to light reflections and squinting [72]. Therefore, we adjusted our initial data loss threshold to avoid high participant attrition. In our confirmatory analyses, we consistently reported both the full and a reduced dataset, showing that while the interpretation did not change, the likelihood ratios in support of the hypotheses were higher in the larger sample.
4.2. Log10-transformation of light level
To deal with the unanticipated violation of linear regression assumptions when including the light data as predictors, we log10-transformed them, which resolved the issue. However, this step was not planned in our Stage 1 registered report, which led us to always report both transformed and untransformed data in our confirmatory analyses for full transparency.
Besides statistical reasons, predicting pupil size employing a log10-transformed light unit also conceptually makes sense. Light exposure under naturalistic conditions spans several orders of magnitude (up to a factor of 1010) [3,73]. It has been suggested that the visual system performs some type of logarithmic compression, not least as it enables scaling of signals according to their proportions rather than absolute scale owing to the difference in solar irradiance [74,75]. The melanopsin-encoded component of ipRGCs appears to operate in a log-linear fashion, thereby encoding irradiance across several orders of magnitude [76,77]. This is consistent with the finding in our dataset that the log10-transformation clearly improves the pupil size prediction when compared with the linear scaled data.
4.3. Pupillary light response in the field
Decisive evidence from our field data confirms the presence of the pupillary light response under natural conditions (CH1). We expected this effect to be present in all participants unless they had an unrecognized medical condition.
We found distinct dose-response curves for the case data presented in figure 6, with smaller pupil sizes observed at brighter conditions. The resulting curves closely resemble the model predictions based on laboratory luminance data shown in figure 1 c [35]. Interestingly, the variation in pupil size within similar light intensities decreases markedly towards the high mEDI intensities. This finding may reflect that the pupillary light response dominates pupil regulation at high light intensities, whereas, at lower intensities, there is room for variation due to the other effects on pupil size, such as accommodation or emotional and cognitive responses. However, it could also be argued that variability is simply reduced at high light levels when pupil size is at its minimum, suggesting a floor effect. It is also possible that there is a greater involvement of the faster cone signals in pupil regulation at indoor light levels, resulting in more variation in our steady-state pupil size measurements.
4.4. Predictor performance of mEDI versus illuminance
Despite the extremely high correlation between photopic illuminance and mEDI under our field conditions, the Bayes factor analysis provided ‘decisive evidence’ in favour of the melanopsin-sensitivity weighted measures over photopic illuminance for linear predictions of the light-adapted pupil size under our natural light and task conditions (CH2). This effect was robust across the reduced and full dataset and log10-transformed and linear light data and is in line with evidence from several laboratory studies suggesting that mean steady-state pupil size under photopic conditions is largely controlled by the melanopsin-encoded signal [11–16].
These results suggest that the application of melanopsin-weighted measures (e.g. mEDI, melanopic irradiance) could be beneficial for predicting pupil size in the field compared with photopic illuminance. Importantly, this evidence does not tell us why mEDI is a superior linear predictor of pupil size in our data. It may be that some other distributional or statistical properties of mEDI favour it over illuminance in our mixed linear models.
4.5. Age effect on pupil size
In line with previous laboratory findings [19–28], our data confirmed that steady-state pupil size decreases with age, even under uncontrolled real-world conditions. In addition, our exploratory results replicate that the effect is stronger in dim compared with bright light conditions [13,24,28,29,78]. Perhaps the most compelling findings are the consistent linear regression parameters between our data and the laboratory data presented by Winn et al. in 1994 ([28], fig. 2). Both studies indicate that under dim light conditions, ageing reduces pupil size by about 0.4 mm per decade, starting at approximately 8 mm intercept. Surprisingly, the clustered field data also closely match the effect magnitudes in the laboratory, as reported by Winn and colleagues. It is important to note, however, that the stimuli used in [28] were circular 10° fields of homogeneous luminance.
In line with this pattern, our data extend to even brighter daylight conditions, where the age effect is even more reduced, and pupils are probably fully constricted. Lastly, just like in the findings of Winn and colleagues, our data also show ‘a substantial amount of interindividual variation in pupil size for subjects of similar age’ (cf. [28, p. 1134]) and decreasing variation with increasing brightness.
In summary, age reduces pupil size in the real world, especially at lower light intensities, albeit with large inter-individual variability. This provides further support for considering age-related differences in lighting solutions for visual and non-visual optimization, as retinal irradiance varies as a function of the pupil area, and thus smaller pupil sizes result in reduced retinal illumination. A study by Giménez et al. [79] suggests that light-induced melatonin reduction in healthy young people might adapt to long-term reductions in short-wavelength light through filters. Whether this is the case in an older population experiencing symptoms of the ageing eye, such as lens yellowing, photoreceptor loss and pupil size reduction, remains an important research question. Prior evidence suggests that there are some compensatory mechanisms at play in ageing [80].
4.6. Other potential factors affecting pupil size
Derived from the literature, sex, iris colour and caffeine consumption were investigated as potential influencing factors. Previously, Abokyi et al. [68] found that acute caffeine intake of 250 mg significantly increased pupil diameter from 30 to 90 min after ingestion. By contrast, our data show no evidence that caffeine intake ‘within the last six hours' or habitual caffeine consumption affect light-adapted pupil size. This discrepancy may be due to the inaccuracy of the used subjective reports as well as the imprecise report of timing and dosage of caffeine consumption in our study.
Our evidence against the occurrence of sex differences contrasts with the work of Harley & Sliney [47], who found that female subjects had significantly larger pupil sizes (0.30 mm on average) than male subjects under outdoor conditions. On the other hand, our results are in line with Winn et al. [28], who found no sex differences in their sample. The body of evidence for sex differences in pupil size therefore remains inconclusive. However, the lack of evidence in our data for iris colour as an influence factor for pupil size is consistent with both reports [28,47].
4.7. Exploring different light level measures as predictors for pupil size
In the original study design, our a priori objective, derived from the literature, was to compare the linear steady-state prediction of pupil size only for melanopic irradiance versus photopic illuminance. However, as research has shown that all photoreceptors contribute to pupil control under appropriate stimulus conditions that isolate the different photoreceptor contributions [15,81], we further investigated whether other metrics could adequately predict steady-state pupil size under our field conditions in our linear mixed models. Surprisingly, in the single metric comparison, we found stronger evidence for a model incorporating S-cone-opic EDI compared with mEDI, which showed the second-best model fit. This is unexpected as S-cones have been shown to contribute to pupil size in a paradoxical manner when selectively activated [18,82]. On the other hand, the combination of mEDI plus rhodopic EDI showed the best model fit when the metrics were included pairwise.
It is important to note that in the current study all photoreceptors are activated non-selectively. Furthermore, these results do not provide any insight into why certain metrics yielded better mixed linear model fits over others in predicting pupil size in our real-world data. For instance, it may be that certain distributional or statistical properties are involved and that carry-over effects from previous light samples influence this. It is also important to note that the low temporal resolution of our data does not allow us to make inferences about pupil dynamics below the 10 s transients.
We suggest that future work with higher temporal resolution real-world data should consider more complex integration models for predicting pupil responses from spectral data (e.g. using the Quick pooling model, [14]). Recently, a promising non-parametric deep learning-based approach has been proposed from laboratory work [83].
4.8. Further limitations
In addition to the list of study restrictions acknowledged in the Stage 1 registered report (see Material and methods), our study is limited in the following ways:
-
—
Time-resolution. Given our temporal sampling resolution (10 s intervals), we may not have been able to capture some of the faster dynamics of pupil size, possibly stemming from cone signalling pathways [17,18]. A finer time resolution would allow a more detailed analysis of the fast response aspects of pupil size regulation and the velocity of adaptation.
-
—
Exhaustive light scenarios. We have not sampled all possible light scenarios and have limited our design to those that balance feasibility with full generalizability. As the actual statistical properties of the human spectral diet are unknown, this generalizability remains a construct.
-
—
Overestimation of retinal illumination. During outdoor light exposure, the recorded spectral irradiance measurements on the forehead probably overestimate the light level and short-wavelength content of the light in relation to retinal illumination, as they recorded overhead light from above 45° field of view which rarely enters the eyes under typical outdoor gaze conditions [9]. However, despite their limitations, hemispherical cosine-corrected sensors are commonly used as a standard for quantifying the non-visual effects of light on humans [84], thus enabling comparability with existing data, including pupil size (e.g. [85]).
-
—
Carry-over effects. We acknowledged the omission of light history effects in Stage 1 and further found high autocorrelations values for pupil size and mEDI in our field data (see electronic supplementary material, figure S8). It is thus plausible that our data include carry-over effects so that long-term pupil effects are mixed into the instantaneous measurements. However, this is a feature of naturalistic exposures in the real world. A clean but non-naturalistic design could employ counterbalanced sequences of light levels.
5. Conclusion
While acknowledging the limitations and high data loss, this study is the first to the authors' knowledge to investigate the pupillary light response under naturalistic light and task conditions in a large cross-sectional approach, enabled by a fully wearable measurement set-up. Under these dynamic real-world conditions, the study sheds light on the factors influencing human pupil physiology in a sex-balanced, age-diverse sample. Using a Bayesian approach, we replicated previous laboratory findings on senile miosis and the dose-response relationship between pupil size and light level. We also found evidence for a better linear prediction of steady-state pupil size using melanopsin-weighted measures compared with the traditionally used photopic illuminance. Yet, our exploratory post hoc comparisons suggested that all photoreceptors may contribute to steady-state pupil size, including surprising evidence favouring S-cone EDI as a single and melanopic plus rhodopic EDI as paired linear predictors. Finally, our post hoc analyses showed moderate to strong evidence that iris colour, reported caffeine consumption and sex did not substantially influence pupil size under these settings. Taken together, the data provide a case for considering age in personalized lighting solutions and against the use of photopic illuminance alone to assess real-world lighting conditions for visual and non-visual functions.
Acknowledgements
We thank Cielle Hawrylenko, Majlinda Maliqi, and Sebastian Saraceno for their assistance in the data collection, Dr Brian Cardini for his assistance with the demographic analysis, Dr Johannes Zauner for his helpful comments on the analysis code, and the anonymous reviewers for their comments in the Stage 1 and Stage 2 submissions.
Footnotes
Note, that we measured spectral irradiance on the forehead as a proxy for retinal illumination—not retinal or corneal illumination directly, as specified under ‘Known limitations of this registered report’ and ‘Calibrated spectroradiometric measurements’ in the Material and methods section.
Due to difficulties in filling the older age groups during the COVID pandemic, we later opened recruitment to more than 16 per age group to achieve a large enough total sample. In total, n = 113 people were invited to the health and eligibility screening of whom n = 106 were assigned to participate in the experimental protocol (see electronic supplementary material, figure S1).
The following drugs were tested with the urine-based test: amphetamines, benzodiazepines, cocaine, morphines/opiates, methadone and tetrahydrocannabinol (THC).
No participants with IOLs were recruited in this study.
After Stage 1 in principle acceptance (IPA): we decided to adopt the more widely established melanopsin sensitivity-weighted light level unit melanopic equivalent daylight illuminance (melanopic EDI or mEDI) instead of melanopic irradiance. This does not affect any of our test results, as the two measures are simply a linear transformation of one another (mEDI = melanopic irradiance
∗ 1.32621318911359).
After Stage 1 IPA: our dataset revealed a higher proportion of pupil data exclusion than expected from the pilot runs. Thus, we decided to additionally use an adjusted threshold of 'acceptable' data loss proportion per participant to address high participant attrition. The second data loss threshold was determined in a data-driven approach (see Results under Data quality checks, Proportion of excluded data), resulting in a 75% threshold. For completeness and transparency, we report the results of our hypothesis tests in the reduced sample (n = 63, 14354 valid observations, 50% threshold) and full sample (n = 83, 17 199 valid observations, 75% threshold).
Correction: an unrelated and wrongfully cited study by Yung et al. (1990) in Stage 1 was replaced with the correct reference to Snel & Lorist [55] in Stage 2.
Iris colour, biological sex and caffeine consumption per body weight were included in the exploratory post hoc analysis.
We followed the standard categorization of Bayes factor strength of evidence according to Jeffreys [58] but omitted to note the interpretation for BF10 > 100 (‘decisive evidence’). Due to large resulting Bayes factors, we reported BF10 as log10(BF10). Accordingly, the categorization corresponds to 0 < log10(BF10) < 0.477, anecdotal evidence; 0.477 < log10(BF10) < 1, moderate evidence; 1 < log10(BF10) < 1.477, strong evidence; log10(BF10) > 1.477, very strong evidence; and log10(BF10) > 2, decisive evidence for H1. The inverse is expressed as negative log10(BF).
See footnote 13.
See footnote 1.
See footnote 1.
During analysis (after Stage 1 IPA and data collection), we realized a peculiarity in our originally formulated linear mixed model analyses. When predicting pupil size with linear light level data (mEDI/melanopic irradiance and photopic illuminance), these variables violate the assumptions of linear regression models, specifically the assumptions for linearity, homogeneity of variance and collinearity (see electronic supplementary material, figure S3 and S4B, C and F). However, when log10-transforming the light data before including them in the linear models, the assumptions were approximately met (cf. electronic supplementary material, figure S5 and S6). Following these results, we log10-transformed our light data (more details see Linear regression assumptions under Results). For completeness and transparency, we report the results of our hypothesis tests with the log10-transformed and linear, non-transformed data. The two alternative methods produce qualitatively similar results, though Bayes factors are larger when employing the log10-transformation.
See footnote 6.
Ethics
Ethical approval has been granted from the cantonal ethics commission (Ethikkommission Nordwest- und Zentralschweiz, project ID 2019-01832, see Supporting materials on FigShare). All current mandatory measures to prevent the spread of COVID-19 in Switzerland were implemented during data collection.
Data accessibility
All code and data are publicly available as part of this publication. Code, raw and processed data (except code to drive the acquisition device, which is proprietary) are available on GitHub (https://github.com/tscnlab/LazarEtAl_RSocOpenSci_2024) under the MIT license. Pre-processed data (https://doi.org/10.6084/m9.figshare.24230848.v3) and supporting material (https://doi.org/10.6084/m9.figshare.24230890.v1) are available on FigShare under the CC-BY 4.0 license. The in-principle accepted Stage 1 protocol can be found here: https://osf.io/zrksf/.
Additional information, figures and tables are provided in the electronic supplementary material [86].
The analysis code has been checked independently through CODECHECK [87], yielding Certificate #2024-001 [88].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
R.L.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing—original draft, writing—review and editing; J.D.: investigation; A.-S.F.: investigation; A.M.: investigation; M.S.: conceptualization, data curation, funding acquisition, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
During parts of this work, M.S. was supported by a Sir Henry Wellcome Trust Fellowship (Wellcome Trust 204686/Z/16/Z) and a Junior Research Fellowship from Linacre College, University of Oxford. R.L. is funded by the European Training Network LIGHTCAP (project number 860613) under the Marie Skłodowska-Curie actions framework H2020-MSCA-ITN-2019. This study was funded by an investigator-initiated research project supported by Ocean Insight.
References
- 1. McDougal DH, Gamlin PD. 2015. Autonomic control of the eye. Comp. Physiol. 5 , 439-473. ( 10.1002/cphy.c140014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loewenfeld IE, Lowenstein O. 1999. The pupil: anatomy, physiology, and clinical applications. Boston, MA: Butterworth-Heinemann. [Google Scholar]
- 3. Barlow HB. 1972. Dark and light adaptation: psychophysics. In Visual psychophysics. Handbook of sensory physiology, vol 7 / 4 (eds Jameson D, Hurvich LM), pp. 1-28. Berlin, Germany: Springer. [Google Scholar]
- 4. Campbell FW, Gregory AH. 1960. Effect of size of pupil on visual acuity. Nature 187 , 1121-1123. ( 10.1038/1871121c0) [DOI] [PubMed] [Google Scholar]
- 5. Westheimer G. 1964. Pupil size and visual resolution. Vision Res. 4 , 39-45. ( 10.1016/0042-6989(64)90030-6) [DOI] [PubMed] [Google Scholar]
- 6. Liang J, Williams DR. 1997. Aberrations and retinal image quality of the normal human eye. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 14 , 2873-2883. ( 10.1364/josaa.14.002873) [DOI] [PubMed] [Google Scholar]
- 7. Schwiegerling J. 2000. Theoretical limits to visual performance. Surv. Ophthalmol. 45 , 139-146. ( 10.1016/s0039-6257(00)00145-4) [DOI] [PubMed] [Google Scholar]
- 8. Wang B, Ciuffreda KJ. 2006. Depth-of-focus of the human eye: theory and clinical implications. Surv. Ophthalmol. 51 , 75-85. ( 10.1016/j.survophthal.2005.11.003) [DOI] [PubMed] [Google Scholar]
- 9. CIE. 2018. CIE S 026/E:2018: CIE system for metrology of optical radiation for ipRGC-influenced responses to light. Vienna, Austria: CIE Central Bureau. [Google Scholar]
- 10. Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. 2005. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433 , 749-754. ( 10.1038/nature03387) [DOI] [PubMed] [Google Scholar]
- 11. Gooley JJ, et al. 2012. Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J. Neurosci. 32 , 14 242-14 253. ( 10.1523/JNEUROSCI.1321-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bouma H. 1962. Size of the static pupil as a function of wavelength and luminosity of the light incident on the human eye. Nature 193 , 690-691. ( 10.1038/193690a0) [DOI] [PubMed] [Google Scholar]
- 13. Daneault V, et al. 2012. Does pupil constriction under blue and green monochromatic light exposure change with age? J. Biol. Rhythms 27 , 257-264. ( 10.1177/0748730412441172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McDougal DH, Gamlin PD. 2010. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 50 , 72-87. ( 10.1016/j.visres.2009.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spitschan M. 2019. Photoreceptor inputs to pupil control. J. Vis. 19 , 5. ( 10.1167/19.9.5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsujimura S, Ukai K, Ohama D, Nuruki A, Yunokuchi K. 2010. Contribution of human melanopsin retinal ganglion cells to steady-state pupil responses. Proc. R. Soc. B 277 , 2485-2492. ( 10.1098/rspb.2010.0330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barrionuevo PA, Nicandro N, McAnany JJ, Zele AJ, Gamlin P, Cao D. 2014. Assessing rod, cone, and melanopsin contributions to human pupil flicker responses. Invest. Ophthalmol. Vis. Sci. 55 , 719-727. ( 10.1167/iovs.13-13252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spitschan M, Jain S, Brainard DH, Aguirre GK. 2014. Opponent melanopsin and S-cone signals in the human pupillary light response. Proc. Natl Acad. Sci. USA 111 , 15 568-15 572. ( 10.1073/pnas.1400942111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kadlecova V, Peleska M, Vasko A. 1958. Dependence on age of the diameter of the pupil in the dark. Nature. 182 , 1520-1521. ( 10.1038/1821520a0) [DOI] [PubMed] [Google Scholar]
- 20. Seitz R. 1957. [Dilation of the pupil in dark adaptation dependent on age]. Klin Monbl Augenheilkd Augenarztl Fortbild. 131 , 48-56. [PubMed] [Google Scholar]
- 21. Loewenfeld IE. 1979. Pupillary changes related to age. In Topics in neuro-ophthalmology (ed. Thompson HS). Baltimore, MD: Williams & Wilkins. [Google Scholar]
- 22. Birren JE, Casperson RC, Botwinick J. 1950. Age changes in pupil size. J. Gerontol. 5 , 216-221. ( 10.1093/geronj/5.3.216) [DOI] [PubMed] [Google Scholar]
- 23. Bitsios P, Prettyman R, Szabadi E. 1996. Changes in autonomic function with age: a study of pupillary kinetics in healthy young and old people. Age Ageing. 25 , 432-438. ( 10.1093/ageing/25.6.432) [DOI] [PubMed] [Google Scholar]
- 24. Guillon M, Dumbleton K, Theodoratos P, Gobbe M, Wooley CB, Moody K. 2016. The effects of age, refractive status, and luminance on pupil size. Optom. Vis. Sci. 93 , 1093-1100. ( 10.1097/OPX.0000000000000893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koch DD, Samuelson SW, Haft EA, Merin LM. 1991. Pupillary size and responsiveness: implications for selection of a bifocal intraocular lens. Ophthalmology. 98 , 1030-1035. [PubMed] [Google Scholar]
- 26. Korczyn AD, Laor N, Nemet P. 1976. Sympathetic pupillary tone in old age. Arch. Ophthalmol. 94 , 1905-1906. ( 10.1001/archopht.1976.03910040615006) [DOI] [PubMed] [Google Scholar]
- 27. Lobato-Rincon LL, Cabanillas-Campos Mdel C, Bonnin-Arias C, Chamorro-Gutierrez E, Murciano-Cespedosa A, Sanchez-Ramos Roda C. 2014. Pupillary behavior in relation to wavelength and age. Front. Hum. Neurosci. 8 , 221. ( 10.3389/fnhum.2014.00221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winn B, Whitaker D, Elliott DB, Phillips NJ. 1994. Factors affecting light-adapted pupil size in normal human subjects. Invest. Ophthalmol. Vis. Sci. 35 , 1132-1137. [PubMed] [Google Scholar]
- 29. Mathur A, Gehrmann J, Atchison DA. 2014. Influences of luminance and accommodation stimuli on pupil size and pupil center location. Invest. Ophthalmol. Vis. Sci. 55 , 2166-2172. ( 10.1167/iovs.13-13492) [DOI] [PubMed] [Google Scholar]
- 30. Wyatt HJ. 1995. The form of the human pupil. Vision Res. 35 , 2021-2036. ( 10.1016/0042-6989(94)00268-q) [DOI] [PubMed] [Google Scholar]
- 31. Meller J. 1904. Über hyaline Degeneration des Pupillarrandes [About hyaline degeneration of the pupillary border]. Albrecht von Græfe's Archiv für Ophthalmologie 59 , 221-228. ( 10.1007/bf01995294) [DOI] [Google Scholar]
- 32. Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M. 1995. Retinal photoreceptor density decreases with age. Ophthalmology. 102 , 1853-1859. ( 10.1016/s0161-6420(95)30784-1) [DOI] [PubMed] [Google Scholar]
- 33. Jackson GR, Owsley C, McGwin G. 1999. Aging and dark adaptation. Vision Res. 39 , 3975-3982. ( 10.1016/s0042-6989(99)00092-9) [DOI] [PubMed] [Google Scholar]
- 34. Pokorny J, Smith VC, Lutze M. 1987. Aging of the human lens. Appl. Opt. 26 , 1437-1440. ( 10.1364/AO.26.001437) [DOI] [PubMed] [Google Scholar]
- 35. Watson AB, Yellott JI. 2012. A unified formula for light-adapted pupil size. J. Vis. 12 , 12. ( 10.1167/12.10.12) [DOI] [PubMed] [Google Scholar]
- 36. Barten PGJ. 1999. Contrast sensitivity of the human eye and its effects on image quality. Bellingham, WA: SPIE Optical Engineering Press. [Google Scholar]
- 37. Blackie C. 1999. An extension of an accommodation and convergence model of emmetropization to include the effects of illumination intensity. Ophthal. Physiol. Opt. 19 , 112-125. ( 10.1016/s0275-5408(98)00077-5) [DOI] [PubMed] [Google Scholar]
- 38. Crawford BH. 1936. The dependence of pupil size upon external light stimulus under static and variable conditions. Proc. R. Soc. Lond. B 121 , 376-395. ( 10.1098/rspb.1936.0072) [DOI] [Google Scholar]
- 39. de Groot SG, Gebhard JW. 1952. Pupil size as determined by adapting luminance. J. Opt. Soc. Am. 42 , 492-495. ( 10.1364/josa.42.000492) [DOI] [PubMed] [Google Scholar]
- 40. Holladay LL. 1926. The fundamentals of glare and visibility. J. Opt. Soc. Am. 12 , 271-319. ( 10.1364/josa.12.000271) [DOI] [Google Scholar]
- 41. Moon P, Spencer DE. 1944. On the Stiles-Crawford effect. J. Opt. Soc. Am. 34 , 319-329. ( 10.1364/josa.34.000319) [DOI] [Google Scholar]
- 42. Stanley P. 1995. The effect of field of view size on steady-state pupil diameter. Ophthal. Physiol. Opt. 15 , 601-603. ( 10.1016/0275-5408(94)00019-v) [DOI] [PubMed] [Google Scholar]
- 43. Berman SM, Fein G, Jewett DL, Saika G, Ashford F. 1992. Spectral determinants of steady-state pupil size with full field of view. J. Illum. Eng. Soc. 21 , 3-13. ( 10.1080/00994480.1992.10747995) [DOI] [Google Scholar]
- 44. Geisler WS, Ringach D. 2009. Natural systems analysis: introduction. Vis. Neurosci. 26 , 1-3. [DOI] [PubMed] [Google Scholar]
- 45. Felsen G, Dan Y. 2005. A natural approach to studying vision. Nat. Neurosci. 8 , 1643-1646. ( 10.1038/nn1608) [DOI] [PubMed] [Google Scholar]
- 46. Simoncelli EP, Olshausen BA. 2001. Natural image statistics and neural representation. Annu. Rev. Neurosci. 24 , 1193-1216. ( 10.1146/annurev.neuro.24.1.1193) [DOI] [PubMed] [Google Scholar]
- 47. Harley SK, Sliney DH. 2018. Pupil size in outdoor environments. Health Phys. 115 , 354-359. ( 10.1097/HP.0000000000000887) [DOI] [PubMed] [Google Scholar]
- 48. Webler FS, Spitschan M, Foster RG, Andersen M, Peirson SN. 2019. What is the ‘spectral diet’ of humans? Curr. Opin Behav. Sci. 30 , 80-86. ( 10.1016/j.cobeha.2019.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Linden M, Maier W, Achberger M, Herr R, Helmchen H, Benkert O. 1996. [Psychiatric diseases and their treatment in general practice in Germany. Results of a World Health Organization (WHO) study]. Nervenarzt. 67 , 205-215. [PubMed] [Google Scholar]
- 50. Deaver DM, Davis J, Sliney DH. 1995. Vertical visual fields-of-view in outdoor daylight. Lasers Light Ophthalmol. 7 , 121-125. [Google Scholar]
- 51. Świrski L, Dodgson NA. 2013. A fully-automatic, temporal approach to single camera, glint-free 3D eye model fitting [Abstract] . In Proc. of ECEM 2013, Lund, Sweden . [Google Scholar]
- 52. Lucas RJ, et al. 2014. Measuring and using light in the melanopsin age. Trends Neurosci. 37 , 1-9. ( 10.1016/j.tins.2013.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muinos Diaz Y, Saornil MA, Almaraz A, Munoz-Moreno MF, Garcia C, Sanz R. 2009. Iris color: validation of a new classification and distribution in a Spanish population-based sample. Eur. J. Ophthalmol. 19 , 686-689. ( 10.1177/112067210901900427) [DOI] [PubMed] [Google Scholar]
- 54. Akerstedt T, Gillberg M. 1990. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 52 , 29-37. ( 10.3109/00207459008994241) [DOI] [PubMed] [Google Scholar]
- 55. Snel J, Lorist MM. 2011. Effects of caffeine on sleep and cognition. Prog. Brain Res. 190 , 105-117. [DOI] [PubMed] [Google Scholar]
- 56. Ghotbi N, et al. 2020. The microMCTQ: an ultra-short version of the Munich ChronoType Questionnaire. J. Biol. Rhythms. 35 , 98-110. ( 10.1177/0748730419886986) [DOI] [PubMed] [Google Scholar]
- 57. Morey RD, Rouder JR. 2018. BayesFactor: computation of Bayes factors for common designs (R package version 0.9.12-4.2). See https://cran.r-project.org/web/packages/BayesFactor/index.html (accessed 3 July 2023).
- 58. Jeffreys H. 1961. The theory of probability. Oxford, UK: Oxford University Press. [Google Scholar]
- 59. Wilkinson GN, Rogers CE. 1973. Symbolic description of factorial models for analysis of variance. Appl. Stat. 22 , 392-399. ( 10.2307/2346786) [DOI] [Google Scholar]
- 60. Wilhelm B, Wilhelm H, Ludtke H, Streicher P, Adler M. 1998. Pupillographic assessment of sleepiness in sleep-deprived healthy subjects. Sleep 21 , 258-265. [PubMed] [Google Scholar]
- 61. Binda P, Pereverzeva M, Murray SO. 2013. Attention to bright surfaces enhances the pupillary light reflex. J. Neurosci. 33 , 2199-2204. ( 10.1523/JNEUROSCI.3440-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoeks B, Levelt WJM. 1993. Pupillary dilation as a measure of attention: a quantitative system analysis. Beh. Res. Meth. Instr. Comp. 25 , 16-26. ( 10.3758/bf03204445) [DOI] [Google Scholar]
- 63. Heaver B, Hutton SB. 2011. Keeping an eye on the truth? Pupil size changes associated with recognition memory. Memory 19 , 398-405. ( 10.1080/09658211.2011.575788) [DOI] [PubMed] [Google Scholar]
- 64. Binda P, Pereverzeva M, Murray SO. 2013. Pupil constrictions to photographs of the sun. J. Vis. 13 , 8. ( 10.1167/13.6.8) [DOI] [PubMed] [Google Scholar]
- 65. Privitera CM, Renninger LW, Carney T, Klein S, Aguilar M. 2010. Pupil dilation during visual target detection. J. Vis. 10 , 3. ( 10.1167/10.10.3) [DOI] [PubMed] [Google Scholar]
- 66. Alnaes D, Sneve MH, Espeseth T, Endestad T, van de Pavert SH, Laeng B. 2014. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J. Vis. 14 , 1. ( 10.1167/14.4.1) [DOI] [PubMed] [Google Scholar]
- 67. Bradley MM, Miccoli L, Escrig MA, Lang PJ. 2008. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 45 , 602-607. ( 10.1111/j.1469-8986.2008.00654.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Abokyi S, Owusu-Mensah J, Osei KA. 2017. Caffeine intake is associated with pupil dilation and enhanced accommodation. Eye (Lond). 31 , 615-619. ( 10.1038/eye.2016.288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. R CoreTeam. 2023. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 70. Wickham H. 2016. Ggplot2: elegant graphics for data analysis, 3.3.2 edn. New York, NY: Springer. [Google Scholar]
- 71. Trottier T. 2020. The human light diet. In U.S. Department of Energy, Light & Physiology Interest Group meeting, Virtual (Zoom), 31 March. [Google Scholar]
- 72. Evans KM, Jacobs RA, Tarduno JA, Pelz JB. 2012. Collecting and analyzing eye-tracking data in outdoor environments. J. Eye Mov. Res. 5 , 6. ( 10.16910/jemr.5.2.6) [DOI] [Google Scholar]
- 73. Rushton WAH. 1965. The Ferrier lecture, 1962: visual adaptation. Proc. R. Soc. Lond. Series B 162 , 20-46. [DOI] [PubMed] [Google Scholar]
- 74. Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 75. Spitschan M, Aguirre GK, Brainard DH, Sweeney AM. 2016. Variation of outdoor illumination as a function of solar elevation and light pollution. Sci. Rep. 6 , 26756. ( 10.1038/srep26756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Do MTH. 2019. Melanopsin and the intrinsically photosensitive retinal ganglion cells: biophysics to behavior. Neuron 104 , 205-226. ( 10.1016/j.neuron.2019.07.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu A, et al. 2023. Encoding of environmental illumination by primate melanopsin neurons. Science 379 , 376-381. ( 10.1126/science.ade2024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Heine C, Yazdani F, Wilhelm H. 2013. Pupillenweite in Alltagssituationen [Pupillary diameter in every day situations]. Klin. Monatsbl. Augenheilkd. 230 , 1114-1118. ( 10.1055/s-0033-1350927) [DOI] [PubMed] [Google Scholar]
- 79. Giménez MC, Beersma DGM, Bollen P, van der Linden ML, Gordijn MCM. 2014. Effects of a chronic reduction of short-wavelength light input on melatonin and sleep patterns in humans: evidence for adaptation. Chronobiol. Int. 31 , 690-697. ( 10.3109/07420528.2014.893242) [DOI] [PubMed] [Google Scholar]
- 80. Najjar RP, Chiquet C, Teikari P, Cornut P-L, Claustrat B, Denis P, Cooper HM, Gronfier C. 2014. Aging of non-visual spectral sensitivity to light in humans: compensatory mechanisms? PLoS ONE. 9 , e85837. ( 10.1371/journal.pone.0085837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Spitschan M, Woelders T. 2018. The method of silent substitution for examining melanopsin contributions to pupil control. Front Neurol. 9 , 941. ( 10.3389/fneur.2018.00941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Woelders T, Leenheers T, Gordijn MCM, Hut RA, Beersma DGM, Wams EJ. 2018. Melanopsin- and L-cone-induced pupil constriction is inhibited by S- and M-cones in humans. Proc. Natl Acad. Sci. USA 115 , 792-797. ( 10.1073/pnas.1716281115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zandi B, Khanh TQ. 2021. Deep learning-based pupil model predicts time and spectral dependent light responses. Sci. Rep. 11 , 841. ( 10.1038/s41598-020-79908-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brown TM, et al. 2022. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS Biol. 20 , e3001571. ( 10.1371/journal.pbio.3001571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. de Zeeuw J, Papakonstantinou A, Nowozin C, Stotz S, Zaleska M, Hadel S, Bes F, Munch M, Kunz D. 2019. Living in biological darkness: objective sleepiness and the pupillary light responses are affected by different metameric lighting conditions during daytime. J. Biol. Rhythms. 34 , 410-431. ( 10.1177/0748730419847845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lazar R, Degen J, Fiechter A-S, Monticelli A, Spitschan M. 2024. Regulation of pupil size in natural vision across the human lifespan. Figshare . ( 10.6084/m9.figshare.c.7124923) [DOI] [PMC free article] [PubMed]
- 87. Eglen S, Nüst D. 2019. CODECHECK: An open-science initiative to facilitate the sharing of computer programs and results presented in scientific publications. Septentrio Conference Series 1 . ( 10.7557/5.4910) [DOI]
- 88. Eglen S. 2024. CODECHECK certificate 2024-001. Codecheck. ( 10.5281/zenodo.10823246) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lazar R, Degen J, Fiechter A-S, Monticelli A, Spitschan M. 2024. Regulation of pupil size in natural vision across the human lifespan. Figshare . ( 10.6084/m9.figshare.c.7124923) [DOI] [PMC free article] [PubMed]
- Eglen S, Nüst D. 2019. CODECHECK: An open-science initiative to facilitate the sharing of computer programs and results presented in scientific publications. Septentrio Conference Series 1 . ( 10.7557/5.4910) [DOI]
- Eglen S. 2024. CODECHECK certificate 2024-001. Codecheck. ( 10.5281/zenodo.10823246) [DOI]
Data Availability Statement
All code and data are publicly available as part of this publication. Code, raw and processed data (except code to drive the acquisition device, which is proprietary) are available on GitHub (https://github.com/tscnlab/LazarEtAl_RSocOpenSci_2024) under the MIT license. Pre-processed data (https://doi.org/10.6084/m9.figshare.24230848.v3) and supporting material (https://doi.org/10.6084/m9.figshare.24230890.v1) are available on FigShare under the CC-BY 4.0 license. The in-principle accepted Stage 1 protocol can be found here: https://osf.io/zrksf/.
Additional information, figures and tables are provided in the electronic supplementary material [86].
The analysis code has been checked independently through CODECHECK [87], yielding Certificate #2024-001 [88].