Abstract
Background
Pemigatinib is an oral, potent, selective fibroblast growth factor receptor (FGFR) 1-3 inhibitor. FIGHT-101, a three-part, open-label, first-in-human, phase I/II study (NCT02393248), evaluated pemigatinib in patients with advanced solid tumors. In parts 1 and 2, pemigatinib monotherapy had a manageable safety profile and antitumor activity in FGFR-altered tumors. Part 3 (pemigatinib combination therapies) results are presented here.
Patients and methods
Patients received 9, 13.5, or 20 mg oral once-daily pemigatinib on continuous or intermittent schedules with gemcitabine and cisplatin (pemi/gem/cis), docetaxel (pemi/doc), trastuzumab (pemi/tras), pembrolizumab (pemi/pembro), or retifanlimab (pemi/reti) irrespective of whether the tumor was confirmed as FGFR altered. Primary endpoints were safety and pharmacodynamics. Secondary endpoints were investigator-assessed tumor objective response rates (ORRs) and pharmacokinetics (PK).
Results
Of 65 enrolled patients (pemi/gem/cis, n = 8; pemi/doc, n = 7; pemi/tras, n = 6; pemi/pembro, n = 26; pemi/reti, n = 18), all discontinued. Treatment-emergent adverse events (TEAEs) were generally consistent with individual drug AEs. Serious and grade ≥3 TEAEs occurred in 0%-85.7% and 33.3%-100.0% of patients across treatment groups, respectively. All pemigatinib combinations demonstrated steady-state PK comparable to monotherapy. Pharmacodynamic effects in all pemigatinib combinations, except pemi/gem/cis, were consistent with monotherapy. Less inhibition of FGFR2α phosphorylation was observed with this combination. ORRs (95% confidence interval) were 37.5% [8.5% to 75.5% (pemi/gem/cis)], 14.3% [0.4% to 57.9% (pemi/doc)], 0% (pemi/tras), 26.9% [11.6% to 47.8% (pemi/pembro)], and 11.1% [1.4% to 34.7% (pemi/reti)]. All groups had instances of tumor shrinkage. ORRs in assessable patients with FGFR rearrangements and mutations were 50% and 33%, respectively.
Conclusions
Pemigatinib combination therapy showed no unexpected toxicities. PK and pharmacodynamics were mostly consistent with pemigatinib monotherapy. Pemi/gem/cis (37.5%) and pemi/pembro (26.9%) had the highest ORR; most responders had FGFR alterations.
Key words: solid tumor, phase I/II, clinical trial, fibroblast growth factor receptor, pemigatinib, targeted therapy
Highlights
-
•
Pemigatinib combined with chemotherapy or immunotherapy was evaluated in solid tumors in this open-label, phase I/II study.
-
•
Low treatment discontinuation rates suggest that AEs were manageable in pemigatinib combination therapies.
-
•
Pharmacodynamics of pemigatinib combinations were consistent with monotherapy in all regimens except for pemi/gem/cis.
-
•
Most objective responses to pemigatinib occurred in patients with FGFR alterations.
Introduction
Fibroblast growth factor receptors (FGFRs) are involved in myriad cellular functions, including those regulating cell survival and proliferation, and are expressed in multiple tissues.1 Somatic FGF/FGFR alterations, including amplifications, rearrangements, and activating mutations, can lead to dysregulated FGFR signaling and tumorigenesis.2 Solid tumor malignancies with the highest prevalence of FGF/FGFR alterations are urothelial carcinoma and cholangiocarcinoma (CCA); other solid tumors that frequently harbor FGF/FGFR alterations are breast cancer, gynecologic cancers, head and neck cancer, and non-small-cell lung cancer (NSCLC).3 Targeted inhibition of FGFR signaling has been shown to be a therapeutic option for many solid tumors.4, 5, 6, 7, 8 Chemotherapies and immunotherapies have demonstrated antitumor activity as monotherapies in some cancers; however, combination therapy often results in improved outcomes.9,10 Combining targeted therapies tailored to a patient’s tumor molecular profile, such as FGFR inhibitors for patients with FGF/FGFR alterations, with existing treatments that have different mechanisms of action may improve clinical outcomes with advanced solid tumors.11
Pemigatinib is a potent, selective, oral FGFR1-3 inhibitor approved by multiple regulatory authorities for the treatment of adults with previously treated, unresectable, locally advanced or metastatic CCA with FGFR2 fusions/rearrangements12, 13, 14, 15, 16 and, additionally in the United States, for treatment of adults with relapsed or refractory myeloid/lymphoid neoplasms (MLNs) with FGFR1 rearrangement.12 Pemigatinib demonstrated antitumor activity in FIGHT-101 (NCT02393248), a three-part first-in-human, phase I/II study in patients with and without FGF/FGFR alterations and advanced malignancies. Of 128 patients enrolled in parts 1 or 2, which evaluated pemigatinib monotherapy in dose-escalation and -expansion phases, 12 (9.4%) had partial responses (PRs). Objective response rates (ORRs) were highest among patients with FGFR rearrangements (25.0%) and mutations (23.1%).5
Here we report the results from part 3 of FIGHT-101, where the primary objectives were to evaluate the safety, tolerability, and pharmacodynamics, and secondary objectives were to evaluate the pharmacokinetics (PK) and preliminary efficacy of pemigatinib in combination with chemotherapy and immunotherapy in patients with advanced solid tumor malignancies.
Patients and methods
Study design
This three-part, open-label, dose-escalation, first-in-human, phase I/II study was conducted in Denmark and the United States. Part 3 consisted of dose-escalation and dose-expansion phases (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103625). The recommended phase II dose (RP2D) of pemigatinib in combination with chemotherapy and immunotherapy was determined in the dose-escalation phase. Initially, at least three patients were enrolled in each treatment group for dose-escalation. If no dose-limiting toxicities (DLTs) were observed, then enrollment of the corresponding dose expansion was initiated. If one DLT was observed, then at least six patients were enrolled in the dose-escalation treatment group. If DLTs were observed in two or more patients in a three- or six-patient group, then the dose of pemigatinib was reduced by 25%-50%. Dose assessment and de-escalation could be repeated once more.
The maximum tolerated dose (MTD) was defined as the highest dose in each combination at which ≤0/3 or 1/6 patients experienced DLTs. The combination RP2D was to be a dose less than or equal to the MTD/pharmacologically active dose, dependent on emerging pharmacodynamics, PK, and safety data and was potentially specific to the different combination therapies. FIGHT-101 was carried out in accordance with the Declaration of Helsinki and International Council for Harmonisation guidelines for Good Clinical Practice. The institutional review board of each study center approved the protocol. All patients provided written informed consent.
Patients
Eligibility criteria have been published previously.5 Briefly, patients ≥18 years old with solid tumors with disease progression after ≥1 line of prior therapy treatable with gemcitabine + cisplatin, docetaxel, trastuzumab, or anti-programmed cell death protein 1 (PD-1) monoclonal antibodies were eligible. Documentation of FGF/FGFR alterations was required for patients enrolling in the dose-expansion phase. Additional part 3 key eligibility criteria included life expectancy >12 weeks, Eastern Cooperative Oncology Group performance status ≤2, and willingness to avoid pregnancy or fathering children. Patients could only enroll if the prescribed combination therapy was considered a relevant therapy for their diagnosis or if no further effective standard anticancer therapy was available.
Key exclusion criteria included selective FGFR inhibitor treatment ≤6 months before the first dose of pemigatinib, history of calcium and phosphate homeostasis disorder or systemic mineral imbalance with ectopic calcification of tissues, history or evidence of ectopic mineralization or calcification, or current evidence of clinically significant corneal disorder or keratopathy. Prior radiotherapy within 2 weeks of study treatment was not permitted. Laboratory parameters leading to patient ineligibility were hemoglobin ≤9.0 g/dl, platelet count ≤75 × 109/l, absolute neutrophil count ≤1.5 × 109/l, creatinine clearance ≤40 ml/min or <30 ml/min for urothelial carcinoma, international normalized ratio or prothrombin time >1.5 × upper limit of normal (ULN) unless on warfarin, activated partial thromboplastin time >1.5 × ULN, serum calcium outside the normal range, serum phosphorus exceeding ULN, and parathyroid hormone >1.5 × ULN. Patients with total bilirubin ≥1.5 × ULN, aspartate aminotransferase and alanine aminotransferase ≥3 × ULN, and alkaline phosphatase ≥2.5 × ULN were also excluded unless these values were associated with the patient’s primary cancer or metastases and they had sponsor approval.
Treatments
Patients self-administered pemigatinib on 21-day cycles at a starting oral dose of 9, 13.5, or 20 mg once daily (q.d.) either on a continuous (CD) or an intermittent dosing (ID; 2 weeks on/1 week off) schedule. Patients received pemigatinib in combination with gemcitabine and cisplatin (pemi/gem/cis), docetaxel (pemi/doc), trastuzumab (pemi/tras), pembrolizumab (pemi/pembro), or retifanlimab (pemi/reti). Combination drugs were administered as follows: intravenous (i.v.) gemcitabine starting at 1000 mg/m2 on day 1 and 8 of each 21-day cycle and i.v. cisplatin starting at 70 mg/m2 every 3 weeks (q3w); i.v. docetaxel starting at 75 mg/m2 q3w; i.v. trastuzumab at an initial dose of 8 mg/kg followed by 6 mg/kg infusion q3w; i.v. pembrolizumab 200 mg q3w; and i.v. retifanlimab 500 mg every 4 weeks. To manage toxicity, dose adjustments could be made to all combination therapies except retifanlimab, and combination therapy could be interrupted or discontinued. Investigators were permitted to administer pemigatinib while interrupting treatment with the other drug(s). Patients continued treatment until disease progression, unacceptable toxicity, withdrawal of consent, or physician decision.
Endpoints and assessments
The primary endpoints included safety and tolerability assessments of pemigatinib in combination regimens, evaluated from the frequency, duration, and severity of adverse events (AEs); results of physical examinations; and changes in vital signs, electrocardiograms, and laboratory tests. AE severity was based on National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 and was assessed at screening, during treatment, at end of treatment, and during follow-up. Another primary endpoint was the effect of pemigatinib in combination regimens on pharmacodynamics assessed using an ex vivo pharmacodynamic analysis, where phosphorylated FGFR2α was used as a surrogate pharmacodynamic marker for pemigatinib biologic activity. Plasma samples collected on days 1 and 15 of cycle 1 were added to exogenous KATOIII cells, and inhibition of FGFR2α phosphorylation was measured.5
Secondary endpoints were investigator-assessed tumor response rates in patients with measurable disease as per RECIST v1.1 and PK parameters of pemigatinib in combination regimens. Disease was assessed by computed tomography or other suitable method at screening and every three cycles thereafter. Predose plasma samples for PK analysis were obtained on days 1, 2, 8, 14, 15, and 16 of cycle 1. Postdose plasma samples were obtained on days 1 and 14 of cycle 1 at 0.5, 1 ± 0.25, 2 ± 0.25, 4 ± 0.25, 6 ± 0.5, and 8 ± 0.5 hours after pemigatinib administration.
Statistical analyses
Enrollment of 3-6 and ≈24 patients per combination group was planned for the dose-escalation and -expansion phases, respectively, to enable >90% probability of detecting ≥4 responders in the expansion group, assuming a 30% ORR.
Safety and efficacy populations consisted of patients who received ≥1 dose of pemigatinib. PK and pharmacodynamic populations consisted of all patients with PK and pharmacodynamic data, respectively.
Clinical safety data were summarized for the safety population using descriptive statistics. ORR was calculated as the percentage of patients with complete response or PR as the best overall response. PK parameters were calculated using standard noncompartmental methods and summarized descriptively for the PK population as previously described.5
Results
Patients
Overall, 65 patients were enrolled in part 3 of FIGHT-101 between 27 February 2015 and 19 February 2019. Combination treatment groups were pemi/gem/cis: 8 patients (pemigatinib 9 mg ID, n = 1; pemigatinib 13.5 mg ID, n = 7); pemi/doc: 7 patients (all pemigatinib 13.5 mg ID); pemi/tras: 6 patients (all pemigatinib 13.5 mg ID); pemi/pembro: 26 patients (pemigatinib 9 mg ID, n = 3; pemigatinib 13.5 mg ID, n = 14; pemigatinib 13.5 mg CD, n = 9); and pemi/reti: 18 patients (pemigatinib 9 mg CD, n = 7; pemigatinib 13.5 mg CD, n = 9; pemigatinib 20 mg CD, n = 2).
Across treatment groups, median age was 45.0-66.0 years, 34.6%-72.2% were women, and 75.0%-100.0% were white (Table 1). Overall, 5 (7.7%) patients had FGFR rearrangements, 11 (16.9%) had FGFR mutations, 10 (15.4%) had FGFR amplifications, 2 (3.1%) had FGF mutations, 11 (16.9%) had FGF amplifications, and 35 (53.8%) had no documented FGF/FGFR alterations. The most common tumor types across treatment groups were urothelial tract/bladder cancer (n = 10), pancreatic cancer (n = 8), breast cancer (n = 8), and NSCLC (n = 6). All patients discontinued the study; the most common reason was disease progression (range across treatment groups, 37.5%-83.3%). One patient receiving pemi (13.5 mg ID)/pembro discontinued FIGHT-101 to receive pemigatinib in the FIGHT-801 rollover study (NCT04949191).
Table 1.
Patient baseline demographics and clinical characteristics
| Pemi/gem/cis |
Pemi/doc |
Pemi/tras |
Pemi/pembro |
Pemi/reti |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 mg ID (n = 1) | 13.5 mg ID (n = 7) | Total (n = 8) | 13.5 mg ID (n = 7) | 13.5 mg ID (n = 6) | 9 mg ID (n = 3) | 13.5 mg ID (n = 14) | 13.5 mg CD (n = 9) | Total (n = 26) | 9 mg CD (n = 7) | 13.5 mg CD (n = 9) | 20 mg CD (n = 2) | Total (n = 18) | |
| Age, median (range), years | 61.0 (61.0-61.0) | 58.0 (37.0-64.0) | 59.0 (37.0-64.0) | 63.0 (45.0-73.0) | 45.0 (33.0-71.0) | 52.0 (41.0-72.0) | 67.5 (40.0-79.0) | 61.0 (48.0-77.0) | 65.5 (40.0-79.0) | 66.0 (42.0-73.0) | 65.0 (55.0-83.0) | 68.0 (63.0-73.0) | 66.0 (42.0-83.0) |
| ≥65 years, n (%) | 0 | 0 | 0 | 2 (28.6) | 1 (16.7) | 1 (33.3) | 9 (64.3) | 4 (44.4) | 14 (53.8) | 5 (71.4) | 5 (55.6) | 1 (50.0) | 11 (61.1) |
| Women, n (%) | 0 | 3 (42.9) | 3 (37.5) | 3 (42.9) | 4 (66.7) | 1 (33.3) | 5 (35.7) | 3 (33.3) | 9 (34.6) | 7 (100.0) | 5 (55.6) | 1 (50.0) | 13 (72.2) |
| Race, n (%) | |||||||||||||
| White | 1 (100.0) | 5 (71.4) | 6 (75.0) | 6 (85.7) | 6 (100.0) | 2 (66.7) | 10 (71.4) | 9 (100.0) | 21 (80.8) | 7 (100.0) | 9 (100.0) | 1 (50.0) | 17 (94.4) |
| Black | 0 | 1 (14.3) | 1 (12.5) | 1 (14.3) | 0 | 1 (33.3) | 2 (14.3) | 0 | 3 (11.5) | 0 | 0 | 1 (50.0) | 1 (5.6) |
| Asian | 0 | 1 (14.3) | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 2 (14.3) | 0 | 2 (7.7) | 0 | 0 | 0 | 0 |
| ECOG PS, n (%) | |||||||||||||
| 0 | 0 | 1 (14.3) | 1 (12.5) | 0 | 3 (50.0) | 0 | 2 (14.3) | 3 (33.3) | 5 (19.2) | 0 | 0 | 0 | 0 |
| 1 | 1 (100.0) | 6 (85.7) | 7 (87.5) | 7 (100.0) | 3 (50.0) | 3 (100.0) | 12 (85.7) | 6 (66.7) | 21 (80.8) | 6 (85.7) | 9 (100.0) | 2 (100.0) | 17 (94.4) |
| ≥2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (14.3) | 0 | 0 | 1 (5.6) |
| FGF/FGFR alteration, n (%) | |||||||||||||
| Not assessed/missing | 1 (100.0) | 2 (28.6) | 3 (37.5) | 1 (14.3) | 0 | 0 | 2 (14.3) | 2 (22.2) | 4 (15.3) | 1 (14.3) | 1 (11.1) | 0 | 2 (11.2) |
| No alteration detected | 0 | 3 (42.9) | 3 (37.5) | 3 (42.9) | 3 (50.0) | 3 (100.0) | 4 (28.6) | 0 | 7 (26.9) | 4 (57.2) | 3 (33.3) | 1 (50.0) | 9 (50.4) |
| FGFR rearrangement | 0 | 1 (14.3) | 1 (12.5) | 0 | 0 | 0 | 1 (7.1) | 1 (11.1)a | 2 (7.7) | 0 | 2 (22.2) | 0 | 2 (11.2) |
| FGFR mutation | 0 | 1 (14.3) | 1 (12.5) | 0 | 0 | 0 | 6 (42.9) | 3 (33.3) | 9 (34.6) | 0 | 1 (11.1) | 0 | 1 (5.6) |
| FGFR amplification | 0 | 1 (14.3) | 1 (12.5) | 1 (14.3) | 2 (33.3) | 0 | 2 (14.3) | 1 (11.1) | 3 (11.5) | 1 (14.3) | 2 (22.2) | 0 | 3 (16.7) |
| FGF mutation | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 2 (22.2) | 2 (7.7) | 0 | 0 | 0 | 0 |
| FGF amplification | 0 | 1 (14.3) | 1 (12.5) | 2 (25.0) | 2 (33.3) | 0 | 1 (7.1) | 1 (11.1) | 2 (7.7) | 1 (14.3) | 3 (33.3) | 0 | 4 (22.2) |
| Patients with prior therapy, n (%) | 1 (100.0) | 7 (100.0) | 8 (100.0) | 7 (100.0) | 6 (100.0) | 3 (100.0) | 13 (100.0) | 9 (100.0) | 25 (100.0) | 7 (100.0) | 9 (100.0) | 2 (100.0) | 18 (100.0) |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 0 | 1 (3.8) | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 1 (14.3) | 0 | 0 | 3 (21.4) | 4 (44.4) | 7 (26.9) | 0 | 2 (22.2) | 1 (50.0) | 3 (16.7) |
| 2 | 1 (100.0) | 2 (28.6) | 3 (37.5) | 4 (57.1) | 1 (16.7) | 0 | 5 (35.7) | 3 (33.3) | 8 (30.8) | 2 (28.6) | 1 (11.1) | 1 (50.0) | 4 (22.2) |
| ≥3 | 0 | 5 (71.4) | 5 (62.5) | 2 (28.6) | 5 (83.3) | 3 (100.0) | 5 (35.7) | 2 (22.2) | 10 (38.5) | 5 (71.4) | 6 (66.7) | 0 | 11 (61.1) |
| Tumor type, n (%) | |||||||||||||
| Gynecologic | 0 | 1 (14.3) | 1 (12.5) | 2 (28.6) | 0 | 0 | 1 (7.1) | 1 (11.1) | 2 (7.7) | 4 (57.1) | 1 (11.1) | 0 | 5 (27.8) |
| Lower GI | 0 | 1 (14.3) | 1 (12.5) | 0 | 0 | 0 | 1 (7.1) | 0 | 1 (3.8) | 0 | 0 | 0 | 0 |
| Lung | 0 | 0 | 0 | 0 | 0 | 0 | 4 (28.6) | 2 (22.2) | 6 (23.1) | 1 (14.3) | 1 (11.1) | 0 | 2 (11.1) |
| Upper GI | 0 | 0 | 0 | 0 | 2 (33.3) | 0 | 1 (7.1) | 1 (11.1) | 2 (7.7) | 1 (14.3) | 0 | 0 | 1 (5.6) |
| Breast cancer | 0 | 1 (14.3) | 1 (12.5) | 1 (14.3) | 4 (66.7) | 0 | 0 | 1 (11.1) | 1 (3.8) | 1 (14.3) | 0 | 0 | 1 (5.6) |
| CCA | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) | 0 | 2 (22.2) | 1 (50.0) | 3 (16.7) |
| Head and neck cancer | 0 | 0 | 0 | 1 (14.3) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (11.1) | 0 | 1 (5.6) |
| Pancreatic cancer | 1 (100.0) | 2 (28.6) | 3 (37.5) | 0 | 0 | 1 (33.3) | 1 (7.1) | 0 | 2 (7.7) | 0 | 2 (22.2) | 1 (50.0) | 3 (16.7) |
| Sarcoma | 0 | 0 | 0 | 0 | 0 | 0 | 2 (14.3) | 0 | 2 (7.7) | 0 | 0 | 0 | 0 |
| Testicular cancer | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (7.1) | 0 | 2 (7.7) | 0 | 0 | 0 | 0 |
| Urothelial tract/bladder cancer | 0 | 2 (28.6) | 2 (25.0) | 1 (14.3) | 0 | 0 | 3 (21.4) | 2 (22.2) | 5 (19.2) | 0 | 2 (22.2) | 0 | 2 (11.1) |
| Other | 0 | 0 | 0 | 2 (28.6) | 0 | 0 | 0 | 2 (22.2) | 2 (7.7) | 0 | 0 | 0 | 0 |
Gynecologic includes cervical, endometrial, ovarian, and uterine cancers. Lower GI includes anal and colon cancers. Lung includes NSCLC, mesothelioma, and small-cell lung cancer. Upper GI includes esophageal, gastric, and GE/GE junction cancers. Other includes tumor types with n = 1, including prostate cancer, renal cell carcinoma, thyroid cancer, and unspecified tumor.
CCA, cholangiocarcinoma; CD, continuous dosing; ECOG PS, Eastern Cooperative Oncology Group performance status; FGF, fibroblast growth factor; FGFR, FGF receptor; GE, gastroesophageal; GI, gastrointestinal; ID, intermittent dosing; NSCLC, non-small-cell lung cancer; pemi/doc; pemigatinib + docetaxel; pemi/gem/cis, pemigatinib + gemcitabine + cisplatin; pemi/pembro, pemigatinib + pembrolizumab; pemi/reti, pemigatinib + retifanlimab; pemi/tras, pemigatinib + trastuzumab.
Patient had both an FGFR3 fusion and an FGFR1 amplification.
Safety
Median (range) treatment duration was 2.99 (0.16-11.04) months for pemi/gem/cis, 4.6 (1.15-38.60) months for pemi/doc, 2.17 (1.77-8.05) months for pemi/tras, 1.95 (0.20-53.13) months for pemi/pembro, and 2.04 (0.46-11.04) months for pemi/reti. Additional treatment duration details are presented in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103625. All patients experienced treatment-emergent AEs (TEAEs), with serious and grade ≥3 TEAEs occurring in 0%-85.7% and 33.3%-100.0% across treatment groups, respectively (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103625).
Pemi/gem/cis
Seven (87.5%) and eight (100.0%) patients experienced pemigatinib- and gem/cis-related TEAEs, respectively. Anemia (n = 7, 100.0%) was the most common TEAE in the 13.5-mg ID group (Table 2; Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103625). Dose interruptions and reductions occurred in seven (87.5%) patients and one (12.5%) patient, respectively. Of TEAEs that led to pemigatinib discontinuation, febrile neutropenia was reported in one patient in the 9-mg ID group, and disease progression and acute kidney injury were reported in one patient in the 13.5-mg ID group. One patient experienced the fatal TEAE of disease progression.
Table 2.
Summary of TEAEs observed in ≥30% of patients treated with pemigatinib in combination therapy groups
| TEAE, n (%) | Pemi/gem/cis total (n = 8) | Pemi (13.5 mg ID)/doc (n = 7) |
Pemi (13.5 mg ID)/tras (n = 6) |
Pemi/pembro total (n = 26) | Pemi/reti total (n = 18) |
|---|---|---|---|---|---|
| Diarrhea | 4 (50.0) | 6 (85.7) | 3 (50.0) | 12 (46.2) | 7 (38.9) |
| Hyperphosphatemia | 4 (50.0) | 6 (85.7) | 5 (83.3) | 19 (73.1) | 11 (61.1) |
| Alopecia | — | 3 (42.9) | 5 (83.3) | 11 (42.3) | 6 (33.3) |
| Anemia | 8 (100.0) | 4 (57.1) | 2 (33.3) | 12 (46.2) | — |
| Dry mouth | 3 (37.5) | — | 4 (66.7) | 9 (34.6) | 9 (50.0) |
| Fatigue | 5 (62.5) | 6 (85.7) | 2 (33.3) | — | 11 (61.1) |
| Stomatitis | 3 (37.5) | — | 2 (33.3) | 9 (34.6) | 12 (66.7) |
| Constipation | 5 (62.5) | 3 (42.9) | 2 (33.3) | — | — |
| Dehydration | 3 (37.5) | 5 (71.4) | — | — | 8 (44.4) |
| Nausea | 5 (62.5) | 4 (57.1) | 2 (33.3) | — | — |
| Alanine aminotransferase increased | 4 (50.0) | — | — | 8 (30.8) | — |
| Aspartate aminotransferase increased | 4 (50.0) | — | — | 8 (30.8) | — |
| Decreased appetite | — | — | 3 (50.0) | 11 (42.3) | — |
| Dry eye | — | — | 2 (33.3) | 8 (30.8) | — |
| Neutropenia | 4 (50.0) | 3 (42.9) | — | — | — |
| Taste disorder | — | 3 (42.9) | 2 (33.3) | — | — |
| Vomiting | 3 (37.5) | 3 (42.9) | — | — | — |
| Blood creatinine increased | 5 (62.5) | — | — | — | — |
| Cough | — | — | 4 (66.7) | — | — |
| Dizziness | 3 (37.5) | — | — | — | — |
| Dysgeusia | — | 3 (42.9) | — | — | — |
| Dyspepsia | — | — | 2 (33.3) | — | — |
| Dyspnea | — | — | 2 (33.3) | — | — |
| Epistaxis | — | — | 2 (33.3) | — | — |
| Headache | — | — | 2 (33.3) | — | — |
| Hypokalemia | 3 (37.5) | — | — | — | — |
| Hypomagnesemia | — | — | — | — | 6 (33.3) |
| Hyponatremia | 4 (50.0) | — | — | — | — |
| Pyrexia | — | — | 2 (33.3) | — | — |
| Sinusitis | — | — | 3 (50.0) | — | — |
| Thrombocytopenia | 5 (62.5) | — | — | — | — |
| Upper respiratory tract infection | — | — | 2 (33.3) | — | — |
| Weight decreased | — | 3 (42.9) | — | — | — |
| White blood cell count decreased | 4 (50.0) | — | — | — | — |
Patients were counted once under each MedDRA preferred term. Dashes represent TEAEs either not occurring or reported at a frequency <30% of patients in the combination therapy group.
ID, intermittent dosing; MedDRA, Medical Dictionary for Regulatory Activities; pemi/doc, pemigatinib + docetaxel; pemi/gem/cis, pemigatinib + gemcitabine + cisplatin; pemi/pembro, pemigatinib + pembrolizumab; pemi/reti, pemigatinib + retifanlimab; pemi/tras, pemigatinib + trastuzumab; TEAE, treatment-emergent adverse event.
Pemi/doc
All pemi/doc-treated patients experienced pemigatinib-related TEAEs, with 71.4% reporting TEAEs attributed to docetaxel. The most frequent TEAEs were diarrhea, fatigue, and hyperphosphatemia (n = 6, 85.7% each; Table 2). Three (42.9%) patients interrupted pemigatinib due to TEAEs; no patients had dose reductions. One patient reported peripheral neuropathy leading to pemigatinib discontinuation. Another patient experienced the fatal TEAE of bacterial meningitis.
Pemi/tras
Five (83.3%) and three (50.0%) patients experienced pemigatinib- and trastuzumab-related TEAEs, respectively. Hyperphosphatemia and alopecia occurred most frequently (n = 5, 83.3% each; Table 2). No TEAEs leading to pemigatinib interruption, dose reduction, or discontinuation or fatal TEAEs occurred in this group.
Pemi/pembro
TEAEs attributed to pemigatinib and pembrolizumab were reported in 23 (88.5%) and 18 (69.2%) patients, respectively (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103625). The most common TEAE in the 13.5-mg ID group was hyperphosphatemia (n = 11, 78.6%), with hyperphosphatemia, alopecia, and dry mouth occurring most frequently in the 13.5-mg CD group (n = 5 each, 55.6%; Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.103625). Dose interruptions and reductions because of TEAEs occurred in 15 (57.7%) and two (7.7%) patients, respectively. Aspartate aminotransferase and alcohol poisoning led to pemigatinib discontinuation in the 13.5-mg ID and CD groups, respectively. Two fatal TEAEs occurred in this treatment group: completed suicide (9 mg ID) and alcohol poisoning (13.5 mg CD).
Pemi/reti
Most patients experienced pemigatinib-related (n = 17, 94.4%) and retifanlimab-related (n = 16, 88.9%) TEAEs (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103625). Stomatitis was the most frequent TEAE in the 13.5-mg CD group (n = 7, 77.8%; Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.103625). Sixteen (88.9%) patients experienced TEAEs leading to dose interruption; no dose reductions occurred. TEAEs leading to pemigatinib discontinuation in the 13.5-mg CD group were diarrhea, dehydration, muscular weakness, and pneumonitis, with inappropriate antidiuretic hormone secretion and headache also reported in the pemigatinib 9-mg CD group. The fatal TEAE of disease progression occurred in one patient treated with 13.5 mg CD.
Response to treatment
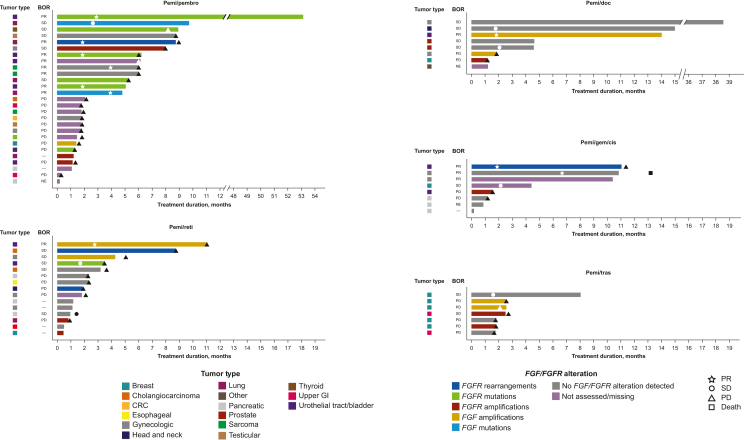
ORR [95% confidence interval (CI)] based on investigator-assessed confirmed tumor responses and duration of response (DOR) are shown in Supplementary Tables S6 and S7, available at https://doi.org/10.1016/j.esmoop.2024.103625, and Figure 1, respectively. In the pemi/gem/cis group, ORR (95% CI) was 37.5% (8.5% to 75.5%) with three PRs. All three responders received pemi (13.5 mg ID)/gem/cis. One responder each had anal cancer (DOR 5.8 months) and ovarian cancer (DOR 6.6 months) with no detectable FGF/FGFR alterations. The third responder had urothelial cancer with an FGFR3–TACC3 fusion (DOR 9.5 months).
Figure 1.
Swimmer plots of treatment duration, investigator-assessed BOR, and tumor type by patient.
BOR, best overall response; CRC, colorectal cancer; FGF, fibroblast growth factor; FGFR, FGF receptor; GE, gastroesophageal; GI, gastrointestinal; NE, not evaluable; PD, progressive disease; pemi/doc; pemigatinib + docetaxel; pemi/gem/cis, pemigatinib + gemcitabine + cisplatin; pemi/pembro, pemigatinib + pembrolizumab; pemi/reti, pemigatinib + retifanlimab; pemi/tras, pemigatinib + trastuzumab; PR, partial response; SD, stable disease.
One patient in the pemi/doc group had a PR, with an ORR (95% CI) of 14.3% (0.4% to 57.9%). The responding patient had urothelial tract/bladder cancer with an FGF10 amplification (DOR 12.5 months).
In the pemi/pembro group, ORR (95% CI) was 26.9% (11.6% to 47.8%), with seven patients experiencing PR. Four pemi (13.5 mg ID)/pembro-treated patients achieved PR: one patient each with urothelial tract/bladder cancer with an FGFR3 p.S249C mutation (DOR 50.3 months), NSCLC with an FGFR2 truncation (DOR 7.1 months), urothelial cancer with an FGFR1 p.M532T mutation (DOR 6.5 months), and sarcoma with no detectable FGF/FGFR alteration (DOR 2.1 months). Three pemi (13.5 mg CD)/pembro-treated patients experienced PR: one patient with mesothelioma with an FGF3 variant of unknown significance (p.G34W; DOR 4.9 months) and two patients with urothelial cancer, of whom one was not assessed for an alteration (DOR 10.4 months) and one had an FGFR3 p.Y373C mutation (DOR 4.2 months).
Two pemi/reti-treated patients had PRs, resulting in an ORR (95% CI) of 11.1% (1.4% to 34.7%). Responders received pemi (13.5 mg CD)/reti, one patient each with urothelial cancer with FGF3/4/19 amplification (DOR 8.3 months) and ovarian cancer with no detectable FGF/FGFR alterations (DOR 4.2 months). No patients in the pemi/tras group responded to treatment.
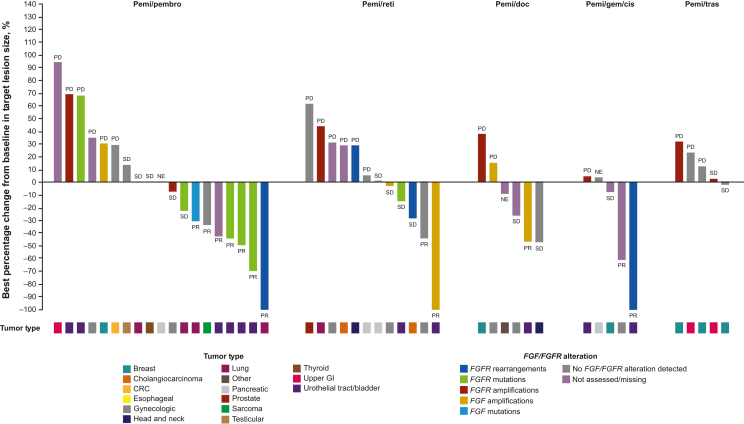
Overall, 22 (33.8%) patients experienced reductions from baseline in target lesion size (Figure 2). The greatest tumor shrinkage generally occurred in patients with FGFR alterations. ORR in assessable patients with FGFR rearrangements and mutations was 50% and 33%, respectively. ORR in patients in whom no FGF/FGFR alteration was detected was 12% (Supplementary Table S7, available at https://doi.org/10.1016/j.esmoop.2024.103625).
Figure 2.
Best percentage change from baseline in target lesion size based on investigator assessment.
BOR noted above or below bars for each patient. BOR, best overall response; CRC, colorectal cancer; FGF, fibroblast growth factor; FGFR, FGF receptor; GE, gastroesophageal; GI, gastrointestinal; NE, not evaluable; PD, progressive disease; pemi/doc; pemigatinib + docetaxel; pemi/gem/cis, pemigatinib + gemcitabine + cisplatin; pemi/pembro, pemigatinib + pembrolizumab; pemi/reti, pemigatinib + retifanlimab; pemi/tras, pemigatinib + trastuzumab; PR, partial response; SD, stable disease.
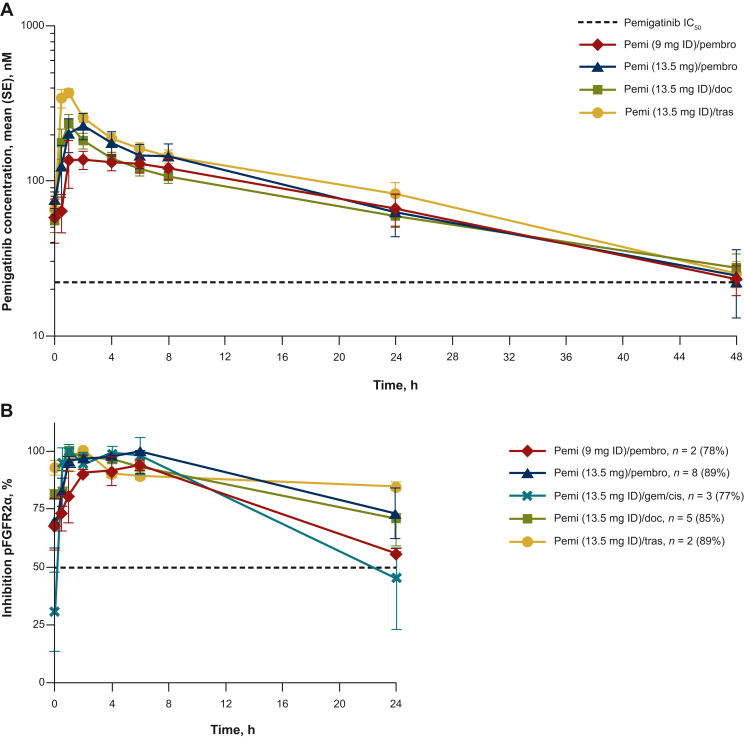
PK and pharmacodynamics
Steady-state pemigatinib concentration data were available for 31 patients treated with pemigatinib 9 mg or 13.5 mg ID or CD in combination therapy. All pemigatinib combinations tested demonstrated steady-state PK parameters comparable to those of pemigatinib monotherapy (Figure 3A; Supplementary Table S8, available at https://doi.org/10.1016/j.esmoop.2024.103625). In ex vivo experiments, using phosphorylated FGFR2α as a surrogate pharmacodynamic marker for pemigatinib biologic activity, inhibition of FGFR2α phosphorylation at trough of steady state exceeded 50% in patients treated with pemi/doc, pemi/tras, and pemi/pembro at both the 9-mg and 13.5-mg doses of pemigatinib, although not in patients treated with pemi (13.5 mg ID)/gem/cis (inhibition, 31%). Mean steady-state inhibition of FGFR2α phosphorylation at both 9-mg and 13.5-mg pemigatinib doses was similar between combination therapy groups tested over the dosing interval (Figure 3B).
Figure 3.
Pemigatinib concentration-time curve and pFGFR2α inhibition. (A) Steady-state pemigatinib concentration–time curve and (B) pFGFR2α inhibition at steady statea in combination therapy regimens. (A) The IC50 (22.6 nM) shown is based on in vivo experiments. (B) The pemigatinib dose was withheld on day 14 due to neutropenia in the patient receiving pemigatinib 13.5 mg + gemcitabine and cisplatin. Data shown are mean (SE) inhibition. aDefined as cycle 1 day 14, 0-24 h.
IC50, half-maximal inhibitory concentration; pFGFR2α, phosphorylated fibroblast growth factor receptor 2α; pemi/doc, pemigatinib + docetaxel; pemi/gem/cis, pemigatinib + gemcitabine + cisplatin; pemi/pembro, pemigatinib + pembrolizumab; pemi/tras, pemigatinib + trastuzumab; SE, standard error.
Discussion
FIGHT-101 was a three-part, first-in-human, phase I/II study evaluating pemigatinib in patients with advanced solid tumors. The study design of FIGHT-101 allowed the safety, preliminary efficacy, and PK/pharmacodynamic profiles of pemigatinib combination therapy to be evaluated in the context of monotherapy in similar patient populations.
Overall, safety data from combination therapy cohorts showed no unexpected toxicities compared with pemigatinib monotherapy. In parts 1 and 2, hyperphosphatemia (75.0%), fatigue (39.1%), dry mouth (38.3%), stomatitis (34.4%), diarrhea (32.0%), and alopecia (31.3%) occurred in ≥30% of patients receiving pemigatinib monotherapy.5 In part 3, rates of hyperphosphatemia in the pemi/doc, pemi/tras, and pemi/pembro groups were generally consistent with monotherapy, with lower rates of hyperphosphatemia observed in the pemi/gem/cis (50.0%) and pemi/reti (61.1%) treatment groups. Rates of fatigue were considerably higher in the pemi/gem/cis, pemi/doc, and pemi/reti groups compared with pemigatinib monotherapy; incidence of fatigue was similar to monotherapy in the pemi/tras (33.3%) and pemi/pembro (26.9%) groups. Dry mouth frequency in the pemi/gem/cis and pemi/pembro groups was similar to monotherapy, whereas dry mouth occurred more frequently in the pemi/tras and pemi/reti groups. Stomatitis rates in combination therapies were consistent with pemigatinib monotherapy, except in the pemi/doc (28.6%) and pemi/reti (66.7%) groups. The incidence of diarrhea was higher in all combination therapy groups versus monotherapy. The highest rate of alopecia was reported in the pemi/tras group (83.3%), with rates marginally higher than monotherapy in the pemi/pembro, pemi/doc, and pemi/reti groups and lowest in the pemi/gem/cis group (12.5%). Among the two groups with the most patients, the percentage of patients experiencing serious or grade ≥3 AEs appeared to be lower in the pemi/pembro group than in the pemi/reti group. However, TEAE incidence within each combination group should be interpreted with caution because cohorts included few patients, and different pemigatinib doses were assessed.
Rates of pemigatinib discontinuation and interruption were only marginally higher in patients treated with combination therapies versus monotherapy, suggesting that AEs were manageable in all combination regimens tested. In parts 1 and 2, 10.2% of patients overall discontinued pemigatinib monotherapy because of TEAEs5; the rate was 13.8% in patients treated with combination therapies in part 3. Pemigatinib interruption was used to manage TEAEs in 51.6% of patients in parts 1 and 2,5 compared with 63.1% in part 3. Dose reductions were less common in parts 1 and 2 than in part 3 (4.6% versus 10.9%).5 Overall, AEs were manageable for 13.5-mg ID and CD doses, which are the approved pemigatinib doses for the treatment of previously treated, unresectable or metastatic CCA with FGFR2 fusions/rearrangements and relapsed or refractory MLNs with FGFR1 rearrangements,12 respectively, in combination with the standard doses of chemotherapy and immunotherapy used in the clinic.
Consistent with the efficacy of pemigatinib monotherapy in patients with advanced solid tumors demonstrated in parts 1 and 2 of FIGHT-101 and in other studies,4,17,18 all combination therapy groups in part 3 had responders except the pemi/tras group. Over half (53.8%) of responders had urothelial tract/bladder cancer; of these, all patients had FGF/FGFR alterations. This histology was overrepresented in the study due to the inclusion criteria, as appropriate treatments for advanced bladder cancer include both chemotherapy and checkpoint inhibitor immunotherapy.19 Urothelial tract/bladder cancer histology was also enriched among patients with the longest durations of treatment. The patient with the longest DOR (50.3 months) had urothelial tract/bladder cancer with an FGFR3 p.S249C mutation and was treated with pemi (13.5 mg ID)/pembro. We also identified a patient with NSCLC with an FGFR2 truncation recently determined to be actionable.20 This patient experienced PR when treated with pemi (13.5 mg CD)/pembro. Limitations of efficacy assessments in this study included small sample sizes in the combination treatment groups and the diversity of tumor types, FGF/FGFR alterations, and type and number of prior therapies of patients enrolled.
In part 3 of FIGHT-101, a partially molecularly selected patient population with diverse alterations was assessed for efficacy. Although genomic analysis of co-alterations was not carried out, the presence of pathogenic co-alterations or other molecular features [e.g. PD-(L)1 expression] may have impacted the efficacy of the combination therapies in part 3. In a recent study, three patients with FGF/FGFR-altered advanced solid tumors with pathogenic co-alterations in cell cycle genes received the multikinase inhibitor lenvatinib, which inhibits FGFRs as well as other kinases,21 in combination with the cyclin-dependent kinase 4/6 inhibitor palbociclib, resulting in stable disease (SD) for ≥6 months or PR. A fourth patient with an FGFR1-amplified gastrointestinal stromal tumor and co-alterations in ARID1A among other pathogenic co-alterations was treated with lenvatinib and pembrolizumab, resulting in SD ongoing for >12 months.22 In contrast, a phase II clinical study evaluating the FGFR inhibitor infigratinib combined with alpelisib in patients with FGFR- and phosphatidylinositol 3-kinase catalytic subunit α-mutated solid tumors did not demonstrate improved efficacy.23 Other clinical trials evaluating FGFR inhibitors in combination with chemotherapy or endocrine therapies are ongoing.24,25
The application of checkpoint inhibitors to metastatic urothelial tract/bladder cancer has recently changed the standard of care.26 Although there is some evidence for poorer responses to checkpoint inhibitor therapy in patients with FGFR3-altered urothelial tract/bladder cancer,27, 28, 29, 30 preclinical experiments suggest that the combination of FGFR inhibitors and checkpoint inhibition may improve clinical outcomes through modulation of the tumor microenvironment.31 Additional clinical studies of combined FGFR and checkpoint inhibition for patients with solid tumors harboring FGF/FGFR alterations are under way.32, 33, 34, 35, 36
Conclusions
Safety data from patients with advanced solid tumors treated with pemigatinib in combination with chemotherapy or immunotherapy showed no unexpected toxicities compared with monotherapy. Pemigatinib combination therapies most commonly showed antitumor activity in patients with FGFR alterations. The PK and pharmacodynamic profiles of pemigatinib in combination groups were generally consistent with monotherapy. The 13.5-mg pemigatinib q.d. dose, administered intermittently or continuously, was selected as the RP2D for further clinical development as monotherapy or combination therapy. Future studies assessing factors that predict which patients may benefit the most from combination therapies are warranted.
Acknowledgements
Writing assistance was provided by Erin McClure, PhD, an employee of ICON (Blue Bell, PA, USA), and was funded by Incyte Corporation (Wilmington, DE, USA).
Funding
This work was supported by Incyte Corporation (no grant number). The sponsor participated in the study design, in the collection, analysis, and interpretation of data, and in the writing of the report. All authors, including authors employed by the sponsor, were responsible for the decision to submit the article for publication.
Disclosure
VS reports scientific advisory board participation for Relay Therapeutics, Incyte, Novartis, Eli Lilly/Loxo Oncology, Roche, Pfizer, Jazz Pharmaceuticals, Bayer, AbbVie, Regeneron, Novartis, Clinical Care Communications, Invited Speaker. KPP reports consulting or advisory roles for Basilea and Turning Point Therapeutics and research funding paid to the institution from 3D Medicines, AbbVie, ADC Therapeutics, Amgen, Anheart Therapeutics, Bayer, Calithera Biosciences, Daiichi Sankyo, EMD Serono, F-star, Incyte, Jounce Therapeutics, Lilly, Linnaeus Therapeutics, MabSpace Biosciences, MedImmune, Merck, Mersana, Mirati Therapeutics, Peloton Therapeutics, Pfizer, Regeneron Pharmaceuticals, Inc., Syros Pharmaceuticals, Tempest Therapeutics, and Treadwell Therapeutics. DM received consulting fees from AbbVie, Arcus, Lilly, and Mirati. Research grants or funds were received from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Epicentrx, Incyte, Lilly, Merck, Novartis, Pfizer, Roche, Surface, and Y-mabs. NBM received research funding paid directly to the institution from Actuate Therapeutics, Amphivena Therapeutics, Aravive Inc., AstraZeneca, BioMed Valley Discoveries, Compass Therapeutics, Erytech Pharma, Genentech, Incyte, Leap Therapeutics, Merck Sharp and Dohme, Mereo BioPharma Group, NuCana, Repare Therapeutics, and Syros Pharmaceuticals. IS received research funding paid directly to the institution from Alligator Bioscience, AstraZeneca, Bristol Myers Squibb, Cantargia AB, Genentech, Genmab, Incyte, Loxo/Bayer, Loxo/Lilly, MSD, Novartis, Orion, Roche, Pfizer, Puma Biotechnology, and Symphogen; and support for attending meetings and/or travel expenses from AstraZeneca, Incyte, Merck, and Pfizer. MLV and CT are employees and shareholders of Incyte. IMS was an employee of Incyte at the time of the study. MG is a speaker for Guardant and a consultant for Cellularity, Merck, and Sanofi. All other authors have declared no conflicts of interest.
Data Sharing
Incyte Corporation (Wilmington, DE, USA) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except phase I studies) for which the product and indication have been approved on or after 1 January 2020 in at least one major market (e.g. US, EU, JPN). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960.
Supplementary data
References
- 1.Xie Y., Su N., Yang J., et al. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020;5(1):181. doi: 10.1038/s41392-020-00222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babina I.S., Turner N.C. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17(5):318–332. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 3.Murugesan K., Necchi A., Burn T.C., et al. Pan-tumor landscape of fibroblast growth factor receptor 1-4 genomic alterations. ESMO Open. 2022;7(6) doi: 10.1016/j.esmoop.2022.100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abou-Alfa G.K., Sahai V., Hollebecque A., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subbiah V., Iannotti N.O., Gutierrez M., et al. FIGHT-101, a first-in-human study of potent and selective FGFR 1-3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann Oncol. 2022;33(5):522–533. doi: 10.1016/j.annonc.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loriot Y., Schuler M.H., Iyer G., et al. Tumor agnostic efficacy and safety of erdafitinib in patients (pts) with advanced solid tumors with prespecified fibroblast growth factor receptor alterations (FGFRalt) in RAGNAR: interim analysis (IA) results. J Clin Oncol. 2022;40(suppl 16):3007. [Google Scholar]
- 7.Meric-Bernstam F., Bahleda R., Hierro C., et al. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose-expansion study. Cancer Discov. 2022;12(2):402–415. doi: 10.1158/2159-8290.CD-21-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pant S., Schuler M.H., Iyer G., et al. Tumor agnostic efficacy and safety of erdafitinib (erda) in patients (pts) with advanced solid tumors with prespecified FGFR alterations (FGFRalt): RAGNAR primary analysis. J Clin Oncol. 2023;41(suppl 16):3121. [Google Scholar]
- 9.Bayat Mokhtari R., Homayouni T.S., Baluch N., et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viscardi G., Tralongo A.C., Massari F., et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175–185. doi: 10.1016/j.ejca.2022.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Luo H., Zhang T., Cheng P., et al. Therapeutic implications of fibroblast growth factor receptor inhibitors in a combination regimen for solid tumors. Oncol Lett. 2020;20(3):2525–2536. doi: 10.3892/ol.2020.11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.PEMAZYRE® (pemigatinib) Incyte Corporation; Wilmington, DE: 2022. Full prescribing information. [Google Scholar]
- 13.PEMAZYRE (pemigatinib) Incyte Biosciences Distribution; Amsterdam, the Netherlands: 2022. Summary of product characteristics. [Google Scholar]
- 14.PEMAZYRE® (pemigatinib) product monograph. Wilmington, DE. Incyte Corporation; 2022. [Google Scholar]
- 15.Innovent Biologics, Inc. The National Medical Products Administration approves PEMAZYRE® (pemigatinib) for the treatment of adults with locally advanced or metastatic cholangiocarcinoma with a FGFR2 fusion or rearrangement that have progressed after at least one prior line of systemic therapy. https://www1.hkexnews.hk/listedco/listconews/sehk/2022/0406/2022040600309.pdf 2022. Available at.
- 16.Incyte Corporation Incyte announces approval of Pemazyre® (pemigatinib) in Japan for the treatment of patients with unresectable biliary tract cancer (BTC) with a fibroblast growth factor receptor 2 (FGFR2) fusion gene, worsening after cancer chemotherapy. https://s21.q4cdn.com/114423841/files/doc_news/Incyte-Announces-Approval-of-Pemazyre-pemigatinib-in-Japan-for-the-Treatment-of-Patients-with-Unresectable-Biliary-Tract-Cancer-BTC-w-QKMHN.pdf 2021. Available at.
- 17.Rodon J., Damian S., Furqan M., et al. Abstract CT016: Clinical and translational findings of pemigatinib in previously treated solid tumors with activating FGFR1-3 alterations in the FIGHT-207 study. Cancer Res. 2023;83(suppl 8):CT016. [Google Scholar]
- 18.Necchi A., Pouessel D., Leibowitz-Amit R., et al. Paper presented at European Society for Medical Oncology; Munich, Germany: October 19-23, 2018. Interim results of FIGHT-201, a phase 2, open-label, multicenter study of INCB054828 dosed intermittently in patients with metastatic or surgically unresectable urothelial carcinoma (UC) harboring fibroblast growth factor (FGF)/FGF receptor (FGFR) genetic alterations. [Google Scholar]
- 19.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer. Version 1.2023. 2022. All rights reserved. https://www.nccn.org/guidelines/category_1 Available at.
- 20.Zingg D., Bhin J., Yemelyanenko J., et al. Truncated FGFR2 is a clinically actionable oncogene in multiple cancers. Nature. 2022;608(7923):609–617. doi: 10.1038/s41586-022-05066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LENVIMA® (lenvatinib). Full prescribing information. Nutley, NJ: Eisai . Inc.; 2022. [Google Scholar]
- 22.Uehara Y., Ikeda S., Kim K.H., et al. Targeting the FGF/FGFR axis and its co-alteration allies. ESMO Open. 2022;7(6) doi: 10.1016/j.esmoop.2022.100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyman D.M., Tran B., Paz-Ares L., et al. Combined PIK3CA and FGFR inhibition with alpelisib and infigratinib in patients with PIK3CA-mutant solid tumors, with or without FGFR alterations. JCO Precis Oncol. 2019;3:1–13. doi: 10.1200/PO.19.00221. [DOI] [PubMed] [Google Scholar]
- 24.Rodon J., O’Neil B., Wacheck V., Liu M., Rosen L.S. 1198TiP A phase Ib/II open-label, nonrandomized study of FGFR inhibitor futibatinib in combination with MEK inhibitor binimetinib in patients with advanced KRAS-mutant cancer. Ann Oncol. 2022;33(suppl 7):S1096. [Google Scholar]
- 25.Damodaran S., Unni N., Giridhar K.V., et al. Abstract P1-18-35: Futibatinib in combination with fulvestrant in patients with metastatic breast cancer (MBC) harboring high-level FGFR1 amplification: preliminary data from a phase 2 study. Cancer Res. 2022;82(suppl 4) P1-18-35. [Google Scholar]
- 26.Bellmunt J., Valderrama B.P., Puente J., et al. Recent therapeutic advances in urothelial carcinoma: a paradigm shift in disease management. Crit Rev Oncol Hematol. 2022;174 doi: 10.1016/j.critrevonc.2022.103683. [DOI] [PubMed] [Google Scholar]
- 27.Rezazadeh A., Loriot Y., Papantoniou D., et al. 757P An observational study of outcomes of patients (pts) with advanced urothelial carcinoma (UC) after anti-programmed death-(ligand) 1 (PD-[L]1) therapy by fibroblast growth factor receptor gene alteration (FGFRa) status. Ann Oncol. 2020;31(suppl 4):S586–S587. doi: 10.1016/j.euros.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg J.E., Hoffman-Censits J., Powles T., et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santiago-Walker A.E., Chen F., Loriot Y., et al. Predictive value of fibroblast growth factor receptor (FGFR) mutations and gene fusions on anti-PD-(L)1 treatment outcomes in patients (pts) with advanced urothelial cancer (UC) J Clin Oncol. 2019;37(suppl 7):419. [Google Scholar]
- 30.Sharma P., Retz M., Siefker-Radtke A., et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 31.Benjamin D.J., Mar N., Rezazadeh Kalebasty A. Immunotherapy with checkpoint inhibitors in FGFR-altered urothelial carcinoma. Clin Med Insights Oncol. 2022;16 doi: 10.1177/11795549221126252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan R., Li L., Li X., et al. Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumor microenvironment. Mol Cancer. 2023;22(1):60. doi: 10.1186/s12943-023-01761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koshkin V.S., Sonpavde G.P., Hwang C., et al. Futibatinib plus pembrolizumab in patients (pts) with advanced or metastatic urothelial carcinoma (mUC): preliminary safety results from a phase 2 study. J Clin Oncol. 2022;40(suppl 6):501. [Google Scholar]
- 34.Muro K., Kato K., Chin K., et al. 1241P Phase Ib study of futibatinib plus pembrolizumab in patients with advanced or metastatic solid tumors: tolerability results and antitumor activity in esophageal carcinoma. Ann Oncol. 2022;33 [Google Scholar]
- 35.ClinicalTrials.gov Phase Ib Trial of Infigratinib in Combination With Atezolizumab and Bevacizumab for the Second-Line Treatment of Advanced Cholangiocarcinoma With FGFR2 Fusion/Amplification. Last update: 2023. https://clinicaltrials.gov/ct2/show/NCT05510427 Available at.
- 36.ClinicalTrials.gov. HMPL-453 (FGFR Inhibitor) in Combination With Chemotherapy or Anti-PD-1 Antibody in Advanced Solid Tumors. Available at https://clinicaltrials.gov/ct2/show/NCT05173142. Accessed January 3, 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.