Highlights
-
•
Disruptions in aMTG-Broca’s connectivity is a shared feature across PPA variants.
-
•
All variants showed breakdown in aMTG connectivity.
-
•
lvPPA showed extensive connectivity disruptions between aMTG and all other networks.
-
•
aMTG may be an important hub.
Keywords: Resting state fMRI, Functional connectivity, Logopenic, Non-fluent, Semantic, PPA
Abstract
Primary progressive aphasia (PPA) variants present with distinct disruptions in speech-language functions with little known about the interplay between affected and spared regions within the speech-language network and their interaction with other functional networks.
The Neurodegenerative Research Group, Mayo Clinic, recruited 123 patients with PPA (55 logopenic (lvPPA), 44 non-fluent (nfvPPA) and 24 semantic (svPPA)) who were matched to 60 healthy controls. We investigated functional connectivity disruptions between regions within the left-speech-language network (Broca, Wernicke, anterior middle temporal gyrus (aMTG), supplementary motor area (SMA), planum temporale (PT) and parietal operculum (PO)), and disruptions to other networks (visual association, dorsal-attention, frontoparietal and default mode networks (DMN)).
Within the speech-language network, multivariate linear regression models showed reduced aMTG-Broca connectivity in all variants, with lvPPA and nfvPPA findings remaining significant after Bonferroni correction. Additional loss in Wernicke-Broca connectivity in nfvPPA, Wernicke-PT connectivity in lvPPA and greater aMTG-PT connectivity in svPPA were also noted. Between-network connectivity findings in all variants showed reduced aMTG-DMN and increased aMTG-dorsal-attention connectivity, with additional disruptions between aMTG-visual association in both lvPPA and svPPA, aMTG-frontoparietal in lvPPA, and Wernicke-DMN breakdown in svPPA.
These findings suggest that aMTG connectivity breakdown is a shared feature in all PPA variants, with lvPPA showing more extensive connectivity disruptions with other networks.
1. Introduction
Primary progressive aphasia (PPA) is an umbrella term for a group of neurodegenerative syndromes characterized by progressive language deficits (Gorno-Tempini et al., 2011, Mesulam, 2001). Three different clinical presentations of PPA have been described and these include the logopenic variant of PPA (lvPPA), the semantic variant of PPA (svPPA), and the non-fluent variant of PPA (nfvPPA) (Gorno-Tempini et al., 2011).These syndromes are associated with atrophy in the left hemisphere, particularly involving the temporal, frontal, and parietal cortices (Botha et al., 2015, Gorno-Tempini et al., 2004, Mesulam, 2001, Whitwell et al., 2015b), which in turn affects connectivity of certain brain networks (Bonakdarpour et al., 2019). The language network, in particular, involves several regions including what is often referred to as Broca’s area (inferior frontal pars opercularis and triangularis), Wernicke’s area (posterior superior temporal gyrus) (Bonakdarpour et al., 2019) and the anterior middle temporal gyrus (aMTG) (Battistella et al., 2019).
lvPPA is characterized by primary language deficits such as anomia, impaired sentence repetition and phonological errors, with preserved single word comprehension, grammar (Tetzloff et al., 2018a, Tetzloff et al., 2018b), semantic knowledge (Lee et al., 2011) and motor speech (Rohrer et al., 2010). As the disease progresses lvPPA patients also develop impaired sentence comprehension (Gorno-Tempini et al., 2008). On neuroimaging, lvPPA is characterized by left lateral temporal and inferior parietal atrophy and hypometabolism on [18F] fluorodeoxyglucose PET (Gorno-Tempini et al., 2004, Madhavan et al., 2013, Whitwell et al., 2015b). svPPA is characterized by progressive and multimodal semantic knowledge loss, along with deficits in confrontational naming, object recognition and single-word comprehension. Single-word repetition, speech fluency and motor speech are usually spared in svPPA (Gorno-Tempini et al., 2011, Hodges et al., 1992, Hurley et al., 2012). Patterns of atrophy and hypometabolism in svPPA are observed in the anterior temporal lobes, with greater damage primarily on the left hemisphere (Botha et al., 2015, Gorno-Tempini et al., 2004, Rabinovici et al., 2008). nfvPPA is characterized by the hallmark features of agrammatism with and without apraxia of speech (Gorno-Tempini et al., 2011, Ogar et al., 2007). nfvPPA is also characterized by difficulty in verb naming, and syntactically demanding comprehension tasks (Thompson and Mack, 2014). In nfvPPA there is significant left posterior frontal (Broca’s area) (Caso et al., 2014, Routier et al., 2018) and premotor (Gorno-Tempini et al., 2004, Mandelli et al., 2016) atrophy and hypometabolism (Whitwell et al., 2013).
Previous studies have shown that functional connectivity is disrupted in PPA. Our studies on lvPPA showed significant reductions in within network connectivity in the working memory (Whitwell et al., 2015a), default mode network (DMN), and language networks (Singh et al., 2023). Studies of svPPA showed extensive dysfunction in connectivity from the anterior temporal lobes (Agosta et al., 2014, Guo et al., 2013), specifically reduction within the semantic and language networks (Battistella et al., 2019), along with alterations in between-network connectivity with the DMN, dorsal attention, and visual association networks (Popal et al., 2020a). In nfvPPA there is dysfunction in the inferior frontal gyrus connectivity (Sintini et al., 2022, Tao et al., 2020), specifically affecting the speech production network (Mandelli et al., 2018).
These studies have paved the way for investigation of network reorganization in within and between network connectivity in PPA. However, so far there are no reports on the interplay between affected and spared regions within the speech-language network and their interaction with other functional networks. Therefore, the primary aim of this study was to assess the within and between-network connectivity of the speech-language network in all three PPA variants and evaluate how it differs across the variants and controls. We hypothesize that based on the clinical symptoms seen in these PPA variants, the within-network connectivity of the speech-language network would be reduced. Specifically, based on the imaging patterns and the clinical profiles we hypothesize reduction in the intrahemispheric connectivity between the inferior frontal, middle temporal, and superior temporal gyri in both lvPPA and nfvPPA, with reduced connectivity in the anterior temporal lobe in svPPA. In addition to disruptions within the speech-language network, we also hypothesize disrupted connectivity between the speech-language network and other networks, particularly with the DMN. As a secondary aim, we looked at relationship between the connections with the strongest group differences within the speech-language network and language measures across all PPA variants. We hypothesize that connectivity between inferior frontal and temporal gyri will correlate with repetition and grammar in lvPPA and nfvPPA respectively and the connectivity within the temporal gyri will correlate with word knowledge in svPPA.
2. Methods
2.1. Patients
The PPA cohort consisted of 123 patients that fulfilled clinical diagnostic criteria for lvPPA (n = 55), svPPA (n = 24) or nfvPPA (n = 44) (Gorno-Tempini et al., 2011) recruited by the Neurodegenerative Research group (NRG) from the Department of Neurology, Mayo Clinic, Rochester, MN, between October 25, 2010 and June 07, 2021. All patients underwent extensive neurological evaluations by one of three behavioral neurologists (KAJ, HB or JGR), neuropsychological testing overseen by a neuropsychologist (MMM), and a speech and language battery performed by one of three board certified speech-language pathologists (JRD, HMC or RLU). All patients also completed a structural MRI that included a resting state functional MRI (rsfMRI) protocol and a Pittsburgh Compound B (PiB) positron emission tomography (PET) scan to assess for beta-amyloid deposition. PiB positivity was based on established cut-offs (Jack et al., 2019). All clinical diagnoses were rendered blinded to imaging results. A cohort of 60 cognitively normal healthy amyloid-negative individuals (CU) were recruited by the Mayo Clinic Study of Aging (MCSA) between March 18, 2010, and June 15, 2017, underwent an identical neuroimaging protocol, and served as the controls for this study.
2.2. Patient consent and protocols
The study was approved by the Mayo Clinic IRB. All patients gave written informed consent to participate in this study.
2.3. Clinical testing
The neurological evaluations of the PPA patients included the Montreal Cognitive Assessment (MoCA) for assessing general cognitive function (Nasreddine et al., 2005). The speech and language assessments included the Western Aphasia Battery-Revised (WAB) (Kertesz, 2007), specifically the fluency subscore to assess deficits in fluency, the Aphasia Quotient (WAB-AQ) subtest to measure global language ability and aphasia severity, and the repetition subset of the WAB to assess sentence repetition subtest (Kertesz, 2007); the word-word version of the Pyramids and Palm Trees (PPT) to assess word knowledge (Howard and Patterson, 1992); the Apraxia of Speech Rating Scale (ASRS) to rate the severity of apraxia of speech characteristics (Duffy et al., 2023); Famous faces test to assess face recognition (Josephs et al., 2012) and the Northwestern anagram test (NAT) for assessing sentence production (Weintraub et al., 2009). The NAT was only performed in nfvPPA and svPPA patients. Agrammatism was judged through conversation, writing output, NAT and WAB fluency scores by one of three board certified speech-language pathologists (JRD, HMC or RLU) during the patient visit. The neuropsychological evaluation included the 15-item Boston Naming Test (BNT) for assessing confrontation naming (Lansing et al., 1999), and letter and animal fluency tests for assessing lexical and category fluency performance, respectively (Scheffel et al., 2021),
2.4. Image acquisition
All patients underwent scanning with a 3 T volumetric MRI on GE scanners (GE Healthcare, Milwaukee, Wisconsin) at Mayo Clinic, Rochester, MN that included a magnetization prepared rapid gradient echo (MPRAGE) sequence (TR/TE/T1 = 2300/3/900 ms; 26 cm field of view, slice thickness = 1.2 mm, in plane resolution = 1 mm) (Jack et al., 2008), and rsfMRI scanning using gradient echo planer imaging (TR/TE = 2.9/30 ms; slice thickness = 3.3 mm, in plane resolution = 3.3 mm and 160 volumes). Participants were instructed to keep their eyes open during the scan (Sintini et al., 2021). All images met fMRI protocol standards, motion parameters (scans exceeding 1.5 mm translation or 1.5° rotation were excluded) and quality control measures, with a quality control analyst rating between 1 and 3 (on a scale of 1 to 4 with 1 being high quality and 4 being lowest quality).
2.5. Image processing
Voxel-level comparisons of volume provided reference maps illustrating patterns of atrophy across all PPA variants compared to controls. All MPRAGE scans were spatially normalized to the Mayo Clinic Adult Lifespan Template (MCALT) template, segmented using unified segmentation (Ashburner and Friston, 2005) and smoothed at 6 mm full width at half maximum. Patterns of gray-matter volume loss were assessed at the voxel-level using SPM12, adjusted for age and sex. These models were corrected for family wise error (FWE) at q < 0.001. Results were spatially normalized to the Montreal Neurological Institute template for visualizing and generating images using BrainNet viewer (Xia et al., 2013).
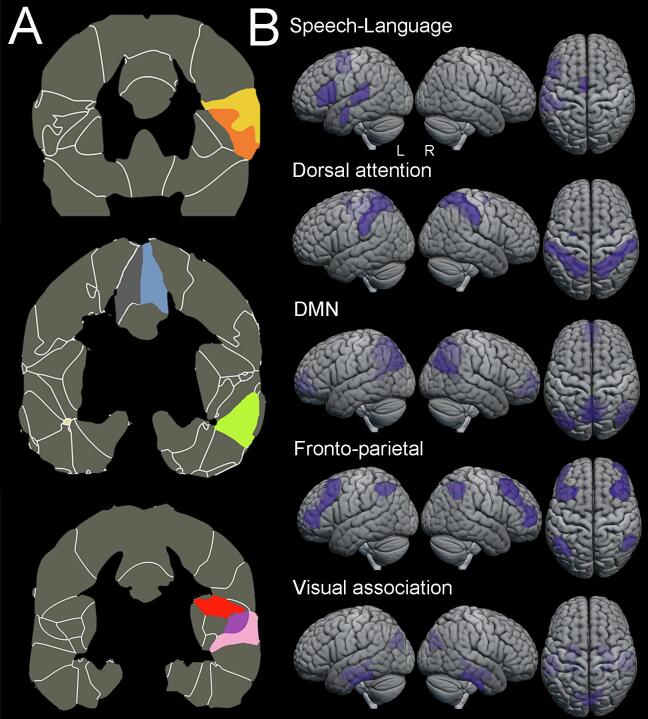
All fMRI images were preprocessed using CONN functional connectivity toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012) (www.nitrc.org/projects/conn). The preprocessing discarded the first 10 volumes to generate a steady state magnetization, slice time correction, re-alignment (motion estimation and correction), with outlier detection, segmentation and direct normalization to MNI template space, smoothing with a gaussian kernel of 6 mm full width, half maximum, nuisance regression for white matter, cerebrospinal fluid (CSF) signal, denoised for six head motion parameters with their first and second order derivatives (Power et al., 2015), and bandpass filtered in the 0.01–0.1 Hz frequency to reduce low frequency drift and noise effects (Lowe et al., 1998). Of note, there was no difference across the three groups in the mean framewise displacement (p = 0.84) and mean motion (p = 0.80) for the fMRI scans. After preprocessing, the functional images were parcellated into cortical and subcortical areas using the Harvard-Oxford atlas (Desikan et al., 2006) and CONN’s network parcellations atlas which was generated using independent component analysis (ICA) on the HCP dataset (N = 497) (Whitfield-Gabrieli and Nieto-Castanon, 2012) in MNI space. The mean blood-oxygen-level-dependent (BOLD) time series within each region of interest (ROI) of the atlas were extracted. Pearson’s R correlation coefficients were calculated across all ROIs and were transformed to Fisher’s R-to-Z transformations. Network (ROI-to-ROI) level connectivity maps were generated for the speech language network (left-sided assessment only) and its connectivity to five other networks (bilateral assessment) was evaluated, namely the DMN, visual association, dorsal attention and frontoparietal networks. The DMN, dorsal attention and frontoparietal networks were defined using CONN’s network parcellations atlas, while the speech-language and visual association networks were defined using speech-language or visual association specific ROIs from the Harvard-Oxford atlas, where the functional connectivity of a network was generated by averaging the signal across multiple regions of interest within that network. Network visualizations are shown in Fig. 1. All images were generated using MRIcroGL (https://www.nitrc.org/projects/mricrogl/).
Fig. 1.
Network visualization. A. Location of the regions of interest that make up the speech-language network (left sided only). Each region of interest is highlighted in different colours. Yellow denotes the inferior frontal cortex par operculum and orange denotes the inferior frontal cortex par triangularis, together both regions make up the Broca’s area. Blue denotes the supplementary motor area, green denotes the anterior middle temporal gyrus, red denotes the parietal operculum, purple denotes the planum temporale and pink denotes the posterior superior temporal gyrus (i.e., Wernicke’s area). B. Network visualization for all five networks, including the speech-language (left sided only), dorsal attention, default mode network (DMN), fronto-parietal and visual association networks. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For the speech-language network, ROIs were placed only in the left hemisphere, specifically Broca’s area (inferior frontal cortex including the pars triangularis and operculum), Wernicke’s area (posterior superior temporal gyrus) (Bonakdarpour et al., 2019), aMTG (Battistella et al., 2019), supplementary motor area (SMA) (Hertrich et al., 2016), planum temporale (PT) and parietal operculum (PO) (Battistella et al., 2020). Broca’s and Wernicke’s area were included as the epicenters of the classic language network (Mesulam, 2005). The extended speech-language network included the aMTG, often damaged in svPPA and located near the anterior temporal lobe, the epicenter of atrophy in svPPA (Battistella et al., 2020, Battistella et al., 2019), the planum temporale and the parietal operculum i.e., the temporoparietal junction which is often damaged in lvPPA (Battistella et al., 2020) and SMA, which does not belong to a major language area but plays an important role in speech programming (Hertrich et al., 2016). For the visual association network ROIs were placed in cuneus, posterior inferior temporal gyrus, amygdala, and hippocampus.
These networks were chosen based on previously reported literature, specifically reports on disrupted connectivity in the DMN, language (Singh et al., 2023), dorsal attention, visual association (Popal et al., 2020a), fronto-parietal networks (Gao and Lin, 2012) and more importantly the clinical and neuroimaging features of PPA (Bonakdarpour et al., 2019).
Regional gray matter volumes were extracted from CONN’s network parcellations atlas and the Harvard-Oxford atlas to allow us to account for volume loss (i.e., atrophy) in the analysis. Associations were only evaluated with regions that survived Bonferroni correction for multiple comparisons in the connectivity analysis. Regional gray-matter volumes of aMTG, Broca’s area and PT were extracted from the Harvard-Oxford atlas and composite gray matter volumes for DMN, dorsal attention and visual association networks were extracted from CONN’s network parcellations atlas.
2.6. Statistical analysis
The CONN functional connectivity toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012) (www.nitrc.org/projects/conn) was used to generate network-level maps for each network within each group. Group-level average connectivity maps (resulting from a one-sample t-test) were corrected for false discovery rate (FDR) at p < 0.001, and the group comparison difference maps (resulting from a two-sample t-test) were FDR corrected at p < 0.05, with cluster size correction for FDR at p < 0.05 for both.
Within- and between-network connectivity estimates were extracted using the CONN functional connectivity toolbox. For investigating the connectivity disruptions within the speech-language network, multivariate linear regression models assessed the relationship between the groups of interest (lvPPA, svPPA, nfvPPA, and CU) for all the speech-language network ROIs, while adjusting for age and sex effects. After fitting the models, we adjusted estimates for group contrasts with and without correction for multiple comparisons using Bonferroni. For the between-network analysis, the methodology using multivariate linear regression models with and without correction for multiple comparisons using Bonferroni described in the within the speech-language network analysis was then repeated to assess relationships between the speech-language network (left-sided assessment only) and all other networks (bilateral assessment) in the groups of interest (lvPPA, svPPA, nfvPPA, and CU), while similarly accounting for age and sex effects.
We also investigated the relationship between significant findings from the connectivity analysis within the speech-language network and language measures across all PPA variants. Linear regression models were fit to predict the language measures as outcome using the functional connectivity findings, while adjusting for age and sex effects.
The influence of atrophy on functional connectivity findings were also investigated. Linear regression models assessed the influence of regional gray matter volume loss (i.e., atrophy) by predicting within- or between-network functional connectivity as outcome between the groups of interest (lvPPA, svPPA, nfvPPA, and CU), while adjusting for total intracranial volume (TIV), age and sex effects.
2.7. Data availability
The data that supports the findings of this study will be available from the corresponding author on request.
3. Results
3.1. Patient demographics and characteristics
The demographic and clinical features of the cohorts are shown in Table 1. The three PPA variants did not differ on sex, age of onset, age at scan, or disease duration. There was a significant difference in education, with nfvPPA having slightly fewer years of education than lvPPA. All lvPPA patients in this study showed evidence of beta-amyloid deposition, with 22 % svPPA and 26 % nfvPPA patients showing evidence of beta-amyloid deposition on PET.
Table 1.
Participant’s demographics and disease characteristics.
| Disease cohort (N = 123) |
||||||
|---|---|---|---|---|---|---|
| lvPPA (N = 55) | nfvPPA (N = 44) | svPPA (N = 24) | P-value lvPPA vs nfvPPA |
P-value lvPPA vs svPPA |
P-value nfvPPA vs svPPA |
|
| Female, n (%) | 29 (53 %) | 23 (52 %) | 13 (54 %) | >0.99 | >0.99 | >0.99 |
| Education, yr | 16 (14, 16) | 14 (12, 16) | 16 (14, 16) | 0.039 | 0.671 | 0.203 |
| Age at onset, yr | 65 (59.5, 70.5) | 65 (59, 70) | 62 (56, 66) | 0.931 | 0.052 | 0.074 |
| Age at scan, yr | 68 (62, 73) | 69 (61.8, 73.3) | 64 (57.8, 69) | 0.807 | 0.112 | 0.074 |
| Disease duration, yr | 3 (1.95, 4.25) | 3.3 (2.3, 4.7) | 3.1 (2.02, 5.1) | 0.274 | 0.434 | 0.911 |
| Presence of apraxia of speech, n (%) | 0 (Absent) | 36 (81 %) | 0 (Absent) | − | − | − |
| PiB positivity | 100 % | 26 % | 22 % | − | − | − |
| MoCA (30) | 20 (17.5, 22) | 24 (21, 26) | 20.5 (16.8, 22) | <0.0001 | 0.813 | 0.0007 |
| BNT (15) | 9 (4.3, 12) | 13 (10, 14) | 1 (0, 4.25) | 0.0002 | <0.0001 | <0.0001 |
| WAB repetition (10) | 8.4 (7.4, 9) | 9 (7.9, 9.4) | 9 (8.1, 9.4) | 0.025 | 0.005 | 0.549 |
| Letter Fluency | 23 (13, 31.5) | 18 (10, 22) | 24 (15, 33) | 0.015 | 0.764 | 0.021 |
| WAB Animal Fluency | 11 (7.3, 15) | 11 (8.8, 15) | 8.5 (4.8, 12.5) | 0.910 | 0.076 | 0.070 |
| WAB fluency (10) | 9 (8, 9) | 6 (5, 9) | 9 (9, 10) | 0.010 | 0.010 | <0.0001 |
| WAB AQ (100) | 87.6 (83, 91) | 86 (81.2, 92.6) | 84.8 (79.2, 92) | 0.953 | 0.819 | 0.856 |
| NAT (10) | − | 8 (5.7, 9) | 8.5 (7.7, 9.2) | − | − | 0.203 |
| Famous faces, recognition (10) | 10 (9, 10) | 10 (9.8, 10) | 9 (6.8, 10) | 0.588 | 0.003 | 0.001 |
| ASRS total-3 (52) | 3 (0, 5) | 14 (8, 21.5) | 0 (0, 1) | <0.0001 | 0.0002 | <0.0001 |
| PPT word-word (52) | 49 (47, 50.8) | 50 (48.8, 51) | 40.5 (34, 47) | 0.098 | <0.0001 | <0.0001 |
Data shown are n (%) or median (first and third quartiles). For continuous variables, p-values are from Mann-Whitney test. For categorical variables, p-values are from Fisher's Exact test. Key; MoCA, Montreal Cognitive Assessment; BNT, 15-item Boston Naming Test; WAB, Western Aphasia Battery; AQ, Aphasia Quotient; ASRS, Apraxia of Speech Rating Scale; PPT, Pyramids and Palm Trees.
Apraxia of speech was noted in 81 % of the nfvPPA cohort. On clinical testing, the nfvPPA group mean on the MoCA was broadly normal, while the mean for both the lvPPA and svPPA groups was mildly impaired and significantly lower than nfvPPA. The svPPA group performed worse on the BNT and PPT word-word test compared to the other two PPA variants. The nfvPPA group performed worse on WAB fluency and letter fluency, and the ASRS, compared to the other variants. The lvPPA group performed worse on WAB repetition task compared to the other two variants, and worse on the BNT compared to nfvPPA.
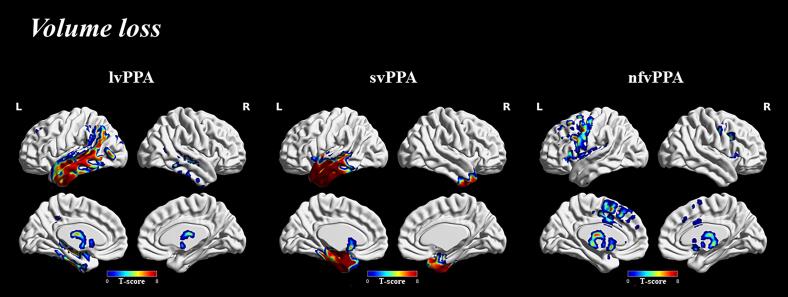
Atrophy patterns in all PPA variants were illustrated using voxel-level comparisons. On comparison to CU, all PPA variants showed predominant left hemisphere involvement with lvPPA showing temporal and inferior parietal volume loss, svPPA showing anterior temporal volume loss and nfvPPA showing posterior frontal volume loss (Fig. 2).
Fig. 2.
Volume loss across all PPA variants. Group level difference maps corrected for family wise error (FWE) at q < 0.001 for volume loss comparing all PPA variants to CU. All images were generated using BrainNet Viewer.
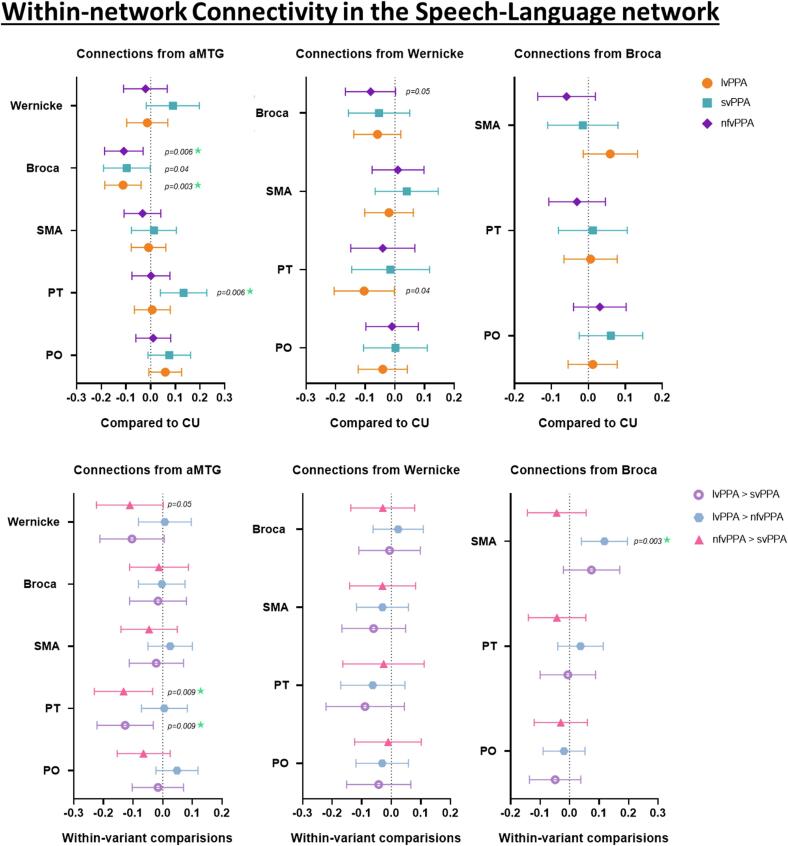
3.2. Within-network connectivity in the speech-language network
Group differences in within-network connectivity for the speech-language network from the multivariate linear regression models are shown in Fig. 3. Reduced connectivity between aMTG and Broca’s area was observed in all PPA variants compared to CU, with findings for lvPPA and nfvPPA surviving correction for multiple comparisons. svPPA also showed increased aMTG-PT connectivity compared to CU, which survived corrections for multiple comparisons. Reduced connectivity between the Wernicke’s area and PT in lvPPA and between Wernicke’s area and Broca’s area in nfvPPA was noted compared to CU (Fig. 3).
Fig. 3.
Within-network functional connectivity in the speech language network. These forest plots compare within-network connectivity from the aMTG, Wernicke’s and Broca’s area across the regions of interest within the speech-language network in all PPA variants compared to CU and from each other. The plot shows estimates (median) and 95 % confidence interval. If the confidence interval does not touch zero, the difference is considered significant.
On exploring comparisons between the PPA variants, nfvPPA showed a decrease in Broca-SMA connectivity when compared to lvPPA, which survived correction for multiple comparisons. Increased aMTG-PT connectivity was noted in svPPA compared to both lvPPA and nfvPPA, with both findings surviving correction for multiple comparisons. svPPA also showed an increase in connectivity between aMTG and Wernicke’s area when compared to nfvPPA (Fig. 3).
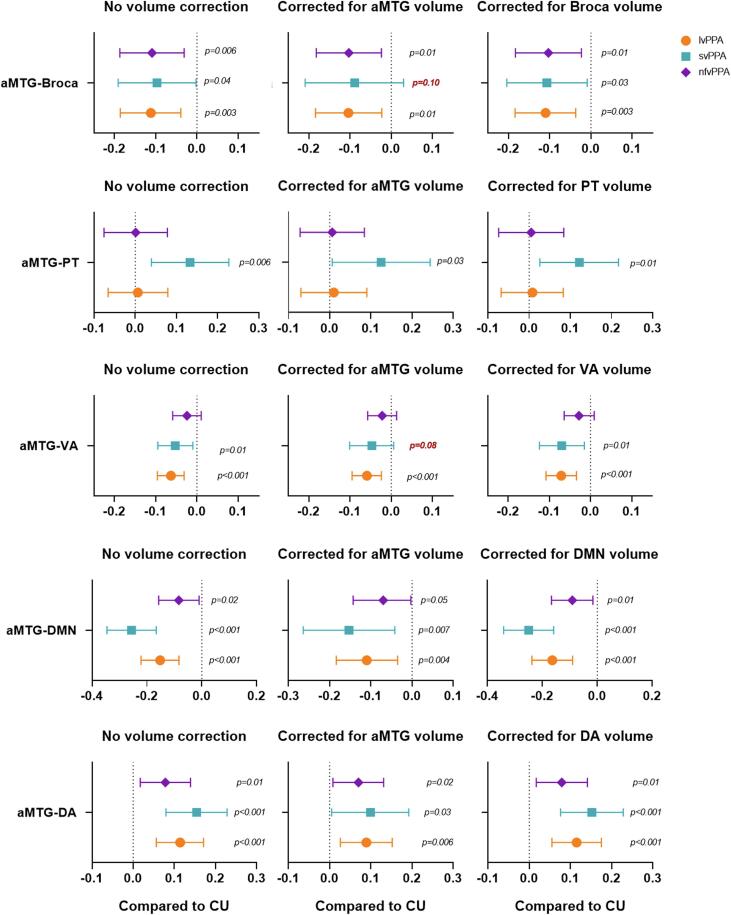
Volume loss adjustment showed that the aMTG-Broca connectivity finding remained significant for all PPA variants despite correction for the effect of volume of Broca’s area, while lvPPA and nfvPPA findings remained significant after adjusting for volume of aMTG. Likewise, the aMTG-PT finding in svPPA also remained significant after adjusting for the effect of aMTG and PT volumes (Supp Fig. 1).
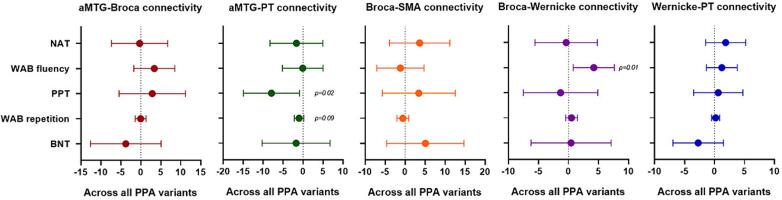
Within the speech-language networks, aMTG-PT connectivity showed a positive association with sentence repetition and word knowledge measured using the WAB repetition and PPT word-word tests respectively. While Broca-Wernicke connectivity showed a positive association with fluency measured using the WAB fluency. However, no significant association were noted between the language measures and aMTG-Broca, Broca-SMA and Wernicke-PT connectivity (Fig. 4).
Fig. 4.
Relationship between connectivity findings and language measures. These plots show the relationship between the connections with the strongest group differences within the speech-language network and language measures across all PPA variants. The plot shows estimates (median) and 95 % confidence interval. If the confidence interval does not touch zero, the difference is considered significant.
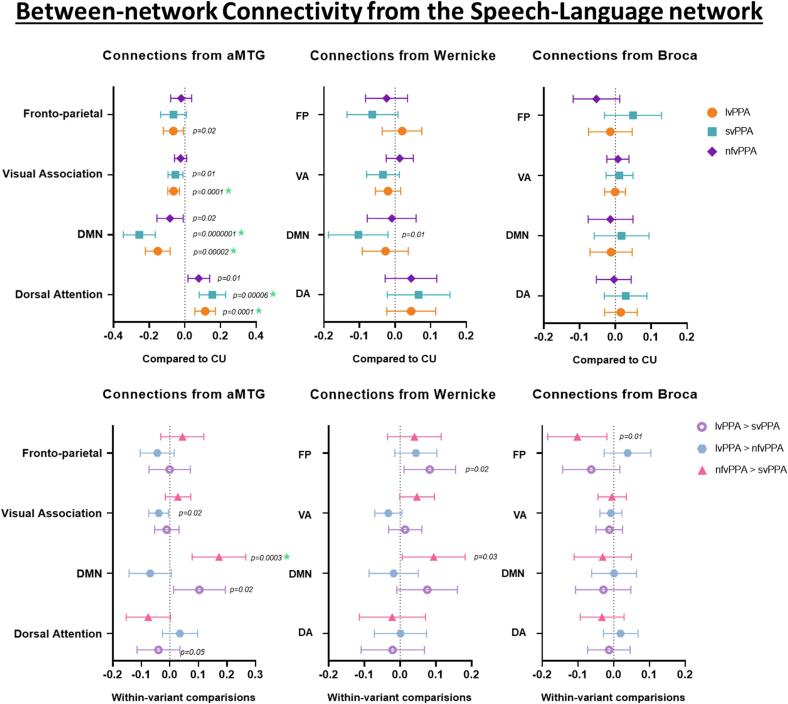
3.3. Between-network connectivity from the speech-language network
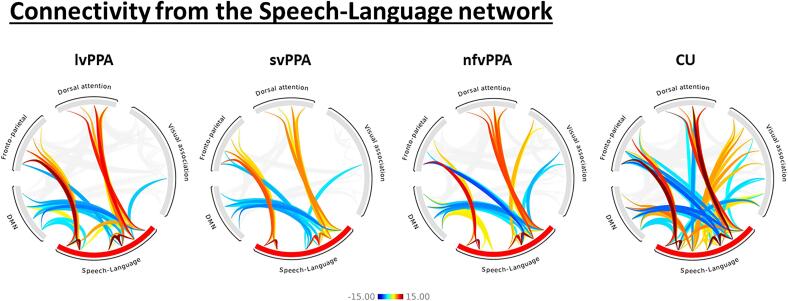
Group differences in connectivity between the speech-language network ROIs and other brain networks from the multivariate linear regression models are shown in Fig. 5. Disrupted aMTG connectivity, specifically reductions in aMTG-DMN connectivity, with an increase in aMTG-dorsal attention network connectivity, was observed in all three PPA variants compared to CU, with findings for lvPPA and svPPA surviving correction for multiple comparisons. Reduced connectivity between aMTG and visual association network was also noted in both lvPPA and svPPA compared to CU, with findings for lvPPA surviving correction for multiple comparisons. lvPPA also showed reduced aMTG-frontoparietal network connectivity compared to CU. Wernicke-DMN connectivity was also reduced in svPPA compared to CU (Fig. 5). The network-level analysis (Fig. 6) also showed comparable findings, with reduced speech-language-DMN and speech-language-visual association network connectivity and an increase in speech-language-dorsal attention network connectivity in all three PPA variants.
Fig. 5.
Between-network functional connectivity in the speech language network. These forest plots compare within-network connectivity from the aMTG, Wernicke’s and Broca’s area across the regions of interest from the speech-language network to other networks in all PPA variants compared to CU and from each other. The plot shows estimates (median) and 95 % confidence interval. If the confidence interval does not touch zero, the difference is considered significant. Abbreviations: FP, Fronto-parietal; DMN, default mode network; VA, Visual association and DA, Dorsal attention.
Fig. 6.
Within and between-network functional connectivity. Group level (p < 0.001, false discovery rate (FDR); cluster threshold = p < 0.05, FDR) functional connectivity patterns within the speech-language network and from the speech-language networks in the PPA variants and CU. The plot shows T-scores generated from one-sample t-tests.
On exploring comparisons between the PPA variants, svPPA showed reduced aMTG-DMN connectivity compared to both lvPPA and nfvPPA, with findings compared to nfvPPA surviving correction for multiple comparisons. Reduced aMTG-visual association network connectivity was also noted in lvPPA, and increased aMTG-dorsal attention network connectivity was observed in svPPA when compared to nfvPPA. Increased Wernicke-frontoparietal network connectivity in lvPPA compared to svPPA, reduced Wernicke-DMN connectivity in svPPA compared to nfvPPA and reduced Broca-frontoparietal connectivity in nfvPPA compared to svPPA were also noted (Fig. 5).
Volume loss adjustment showed that the aMTG-visual association finding in lvPPA and svPPA remained significant after adjusting for the effect of visual association network volumes, while lvPPA findings remained significant after adjusting for volume of aMTG (Supp Fig. 1). Likewise, both aMTG-DMN and aMTG-dorsal attention findings also remained significant in all PPA variants after adjusting for the effect of aMTG, DMN and dorsal attention network volumes (Supp Fig. 1).
4. Discussion
This study investigated functional connectivity across the three well-recognized PPA variants. We found disruptions in the within-network connectivity of the speech-language network, specifically reduced connectivity between aMTG and Broca’s area in all three PPA variants, as well as reduced connectivity from Broca’s area to Wernicke’s area and the SMA in nfvPPA, and reduced connectivity between Wernicke’s area and planum temporale in lvPPA. Disruptions in connectivity between the aMTG and other brain networks were also observed in all three PPA variants, with lvPPA showing disrupted connectivity with all other networks assessed in this study. Importantly, the findings remained after correcting for volume, suggesting these patterns of disrupted connectivity are not confounded by atrophy and may reflect altered connectivity in the remaining tissue.
Our findings showed a significant breakdown of within-network connectivity of the speech-language network in all three PPA variants, which is consistent with previous literature showing evidence of language network disruption in lvPPA (Singh et al., 2023, Whitwell et al., 2015b), svPPA (Battistella et al., 2019) and nfvPPA (Pascual et al., 2020). We further extend these findings by exploring the interplay between the affected and spared regions within the speech-language network and highlight a particularly important finding, a shared feature of reduced aMTG-Broca connectivity in all three PPA variants. Reductions in connectivity between these regions is consistent with the fact that the aMTG is typically involved in svPPA and Broca’s area is typically involved in nfvPPA, and previous studies have observed degeneration in white matter tracts running from these regions in these PPA variants (Mahoney et al., 2013, Schwindt et al., 2013, Valls Carbo et al., 2022). A similar reduction in connectivity between the middle temporal gyrus and the inferior frontal gyrus (Broca’s area) in all three PPA variants has also been previously reported (Bonakdarpour et al., 2019).
The significance of the breakdown in aMTG-to-Broca’s area connectivity to language function across the PPA variants is unclear. We did not find any associations between the strength of this connection and severity of agrammatism, fluency, word knowledge, repetition, or naming, suggesting it is not specific to any one feature of language. It is also not specific to one neuropathology, given that each of the PPA variants is associated with abnormal deposition of different proteins (Mesulam et al., 2014, Spinelli et al., 2017). It is possible that the underlying mechanism causing disruption in this connection differs across PPA variants. In svPPA, the aMTG is the likely epicenter of the disease and this region undergoes severe atrophy (Collins et al., 2017) which would disrupt connectivity (Battistella et al., 2019). Indeed, when we account for volume of the aMTG, the breakdown in aMTG-Broca’s area connectivity is weakened, becoming only a trend. This suggests that atrophy may have been an important variable and that it may be difficult to dissociate aMTG connectivity disruptions from atrophy in this region. In nfvPPA, the disease epicenters are likely Broca’s area and the SMA (Sintini et al., 2022). We did not observe any volume loss in the aMTG in our group analysis, although the temporal lobe can become atrophic as the disease spreads (Tetzloff et al., 2018a, Tetzloff et al., 2018b). Hence, it is possible that breakdowns in connectivity between Broca’s area and the aMTG may proceed neurodegeneration and be an early marker of involvement of this connection. In lvPPA, the aMTG can be atrophic, although this region is likely not the disease epicenter (Sintini et al., 2023). It is possible in this situation either that degeneration of the grey and white matter in the temporal lobes may disrupt the aMTG connections, or that reduced connectivity proceeds degeneration of the aMTG and/or Broca’s area (Lehmann et al., 2013, Whitwell et al., 2015b). Future studies that question the role of aMTG as an important region in the language network in addition to the Broca’s and Wernicke’s area, and that clarify the role of reduced connectivity from this region in mechanisms of disease spread, are required.
Variant specific disruptions in connectivity within the speech-language network were also observed. In lvPPA, reduced Wernicke-PT connectivity was observed compared to CU. This may be consistent with the presence of naming difficulties (Gorno-Tempini et al., 2004), letter fluency, (Riello et al., 2021, Schmidt et al., 2019) and phonological processing deficits (Buchsbaum et al., 2011) in these patients. Breakdown in connectivity between Wernicke’s and PT may also be related to auditory processing deficits (Binder et al., 1996, Ocklenburg et al., 2018). It is important to note that this connectivity breakdown matches well with the disruption seen in the temporoparietal circuitry (Gorno-Tempini et al., 2004) and atrophy seen in Wernicke’s area (Lehmann et al., 2013) and planum temporale (Gorno-Tempini et al., 2004) in lvPPA. In svPPA, we found an increase in aMTG-PT connectivity compared to CU, lvPPA and nfvPPA, and aMTG-Wernicke connectivity compared to nfvPPA. The reason that connectivity from the aMTG to the PT and Wernicke’s would be increased, while connectivity to Broca’s area is decreased, is unclear. One could hypothesize that the increase in connectivity to PT and Wernicke’s may be related to atrophy or the possibility of dysfunction in the remaining neurons and synapses, which may be triggering a compensatory effect. While the decrease in connectivity to Broca’s area may be influenced by aMTG atrophy, which is in line with our results. An association was observed between aMTG-PT connectivity and sentence repetition and word knowledge scores, whereby worse clinical performance on both tests were associated with lower connectivity. Additionally, the aMTG-PT finding in svPPA remained significant after adjusting for the effect of aMTG and PT volumes. In nfvPPA, a significant reduction in Wernicke-Broca connectivity was noted compared to CU. This would be consistent and matches well with the frontal and temporal atrophy seen in patients (Grossman et al., 2013, Tetzloff et al., 2019). This finding would also be consistent with the presence of letter fluency (Riello et al., 2021) and agrammatic difficulties in nfvPPA arising from damage to Broca’s area (Whitwell et al., 2013). Further, a decrease in Broca-SMA connectivity compared to lvPPA was also noted, which fits with the selective atrophy seen in the Broca’s area and the premotor regions in nfvPPA (Lee et al., 2011) and reduced structural connectivity in the frontal aslant tract (Catani et al., 2013, Valls Carbo et al., 2022) in these patients.
Another important finding was the disrupted connectivity between aMTG and several other brain networks across the PPA variants. The temporal lobe, particularly the anterior temporal lobe, is home to connections from many networks including the language, DMN and visual association networks (Guo et al., 2013, Hurley et al., 2015, Pascual et al., 2015). Since several networks traverse through this region, we theorize the possibility of it being an important hub in the speech-language network. Therefore, disruptions within temporal regions may compromise activity of key nodes within other networks. Overall, lvPPA showed the greatest disruption in connectivity between aMTG and all other networks. This may be due to Alzheimer’s disease (AD) pathology in these patients, since it is known that disease mechanisms underlying AD have a widespread influence throughout the brain and affect connectivity of several different networks (Singh et al., 2023). Although some of the other variants showed presence of amyloid, it is likely that the amyloid represents low-intermediate ADNC as a secondary pathology (Bergeron et al., 2018, Josephs et al., 2021, Santos-Santos et al., 2018) and hence may not have such a strong influence on connectivity. However, all three PPA variants showed reduced aMTG-DMN connectivity, along with an increase in aMTG-dorsal attention network connectivity compared to CU, which is in line with the reciprocal balance i.e., activation of one coincides with the deactivation of the other network (Fox et al., 2005, Spreng et al., 2016), seen between the DMN and dorsal attention networks. Furthermore, disruptions in DMN and dorsal attention connectivity have been previously reported in both lvPPA (Putcha et al., 2022, Singh et al., 2023) and svPPA (Popal et al., 2020b). svPPA showed lower DMN disruption compared to both lvPPA and nfvPPA and greater dorsal attention disruption compared to nfvPPA, which may reflect the severe involvement of the aMTG in svPPA patients and matches well with the activation and deactivation patterns of both networks. In nfvPPA, the DMN is generally considered to be spared but here we noted disruptions in DMN connectivity compared to CU, albeit comparatively lower than that seen in both lvPPA and svPPA. Furthermore, both aMTG-DMN and aMTG-dorsal attention findings remained significant in all PPA variants after adjusting for the effect of aMTG, DMN and dorsal attention network volumes. In svPPA, reduction in Wernicke-DMN connectivity compared to CU and nfvPPA was also noted, but the disruption in aMTG-DMN connectivity in these patients was much greater.
The dorsal attention network is activated when engaging in tasks requiring visual attention (Corbetta, 1998, Sestieri et al., 2010). svPPA patients frequently present with visual attention changes, specifically in terms of paying special focus to certain visual stimuli (Miller et al., 2000, Viskontas et al., 2011). svPPA patients have not only shown an intensified focus on certain visual stimuli but may also present with an increase in visual expression i.e., musical and artistic abilities to the point of compulsion or obsession (Miller et al., 2000). nfvPPA patients on rare occasions have also presented with enhanced artistic abilities, creativity, abstract visualization and vibrant colour preference (Mell et al., 2003, Seeley et al., 2008). Although, visual attention is generally considered to be spared in lvPPA (Foxe et al., 2016), there is one report that has shown the presence of these enhanced artistic abilities in lvPPA (Papadopoulou et al., 2023) as well. Together these findings may suggest that emergence of a new ability is a compensation to breakdown in connectivity in another area. We hypothesize that an increase in aMTG-dorsal attention connectivity may have an influence on these artistic abilities and visual attention in these patients; and in fact, there is a possibility that multiple networks working in concert to guide visual attention, perception, and integration. Specifically, the dorsal attention network working in concert with the DMN, which is responsible for integrating visual information (Buckner et al., 2008, Vessel et al., 2019) and the visual association network, which facilitates visual processing and memory formation (Rosen et al., 2018) may be creating these behaviors. Furthermore, this matches well with the rare occurrence of artistic abilities, generally spared visual attention, and lower disruption in visual association seen in lvPPA compared to nfvPPA. One can also hypothesize that reduced in aMTG-DMN connectivity in all PPA variants and reduced aMTG-visual association connectivity in both lvPPA and svPPA compared to CU, may cause disruption in the balance of the dorsal attention network producing an intensified focus on the visual environment without processing and integrating any visual information. Furthermore, the aMTG-visual association finding in lvPPA and svPPA remained significant after adjusting for the effect of visual association network volumes, while lvPPA findings remained significant after adjusting for volume of aMTG (Supp Fig. 1). This suggests that in svPPA, it may be difficult to dissociate aMTG connectivity disruptions from atrophy in this region.
In lvPPA, reduced connectivity between aMTG and frontoparietal network compared to CU was also noted. This is an important network for governing the reciprocal balance between DMN and dorsal attention networks. Studies have shown that the frontoparietal network is anatomically in a position of advantage for integrating information from both these networks (Gao and Lin, 2012, Sridharan et al., 2008) and potentially serving as a gate-keeper in modulating goal-directed attention and cognition (Grady et al., 2016, Spreng et al., 2013, Vincent et al., 2008). These findings therefore highlight the disruption in both upstream and downstream connectivity in lvPPA. Variant specific comparisons showed increase in Wernicke-frontoparietal network connectivity in lvPPA compared to svPPA and reduced Broca-frontoparietal connectivity in nfvPPA compared to svPPA, but these between network connections were not abnormal in these variants when compared to CU.
Strengths of this study include the large PPA cohort with comprehensive clinical evaluations, consistent image acquisition and processing, along with the fact that all models controlled for differences in age and sex. One potential confounder in our analyses could be atrophy in our speech and language ROIs. Another consideration is that the nfvPPA cohort included patients with agrammatic aphasia only and patients with both agrammatic aphasia and apraxia of speech. Further studies will be needed to understand how the connectivity disruptions observed in these patients relate to these different clinical symptoms. We acknowledge that there are several approaches one can select to analyze rsfMRI data. In this study we have used not only used ICA but have also manually defined networks based on specific ROIs, which may be considered a limitation as some patients have different network patterns than the priori assumptions. Additionally, we lack the power to assess the impact of amyloid on connectivity. Studies with longitudinal data will be needed to assess how these network changes evolve over time. Furthermore, studies assessing the impact of amyloid in svPPA and nfvPPA patients will be needed.
5. Conclusion
Our results characterize the disruptions in functional connectivity in all three PPA variants. Breakdown in aMTG connectivity both within the speech-language network and across other brain networks was a shared feature of all PPA variants, with lvPPA showing more extensive connectivity disruptions with other networks. Together these findings suggest brain network reorganization following the impairment of the speech-language network and specifically highlight the possibility of aMTG as an important region in the speech-language network.
CRediT authorship contribution statement
Neha Singh-Reilly: Writing – review & editing, Writing – original draft, Visualization, Formal analysis, Conceptualization. Hugo Botha: Writing – review & editing, Data curation. Joseph R. Duffy: Writing – review & editing, Data curation. Heather M. Clark: Writing – review & editing, Data curation. Rene L. Utianski: Writing – review & editing, Data curation. Mary M. Machulda: Writing – review & editing, Data curation. Jonathan Graff-Radford: Writing – review & editing, Data curation. Christopher G. Schwarz: Writing – review & editing, Data curation. Ronald C. Petersen: Writing – review & editing, Data curation. Val J. Lowe: Writing – review & editing, Data curation. Clifford R. Jack: . Keith A. Josephs: Writing – review & editing, Supervision, Funding acquisition, Data curation, Conceptualization. Jennifer L. Whitwell: Writing – review & editing, Supervision, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Singh has no disclosures to report. Drs. Whitwell, Botha, Utianski, Clark, Duffy, Machulda, Schwarz, Lowe and Josephs reported receiving research funding from the NIH. Dr. Graff-Radford reported receiving research support from the NIH and DSMB for StrokeNET. He is an investigator in a trial sponsored by USC and EISAI. Dr. Jack receives no personal compensation from any commercial entity and has no conflicts. He receives research support from NIH, the GHR foundation and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic.
Acknowledgement
We thank the patients and their families for their commitment.
Funding
This study was funded by the National Institutes of Health, United States grants R01-AG50603, R01-DC10367, R01-DC12519, and R01-DC14942. The funding bodies had no role in the study design, data collection, analysis, interpretation, writing of the manuscript or in the decision to submit an article for publication.
Author contributions
NAS, JLW and KAJ contributed to concept and design of the study; NAS, HB, JRD, HMC, RLU, MMM, JGR, CGS, RCP, VJL, CRJ, JLW, and KAJ contributed to the acquisition and analysis of data; NAS, JLW, and KAJ contributed to drafting the text and preparing the tables and figures. All authors revised the text for intellectual content.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2024.103639.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Data availability
Data will be made available on request.
References
- Agosta F., Galantucci S., Valsasina P., Canu E., Meani A., Marcone A., Filippi M. Disrupted brain connectome in semantic variant of primary progressive aphasia. Neurobiol. Aging. 2014;35(11):2646–2655. doi: 10.1016/j.neurobiolaging.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Battistella G., Henry M., Gesierich B., Wilson S.M., Borghesani V., Shwe W., Gorno-Tempini M.L. Differential intrinsic functional connectivity changes in semantic variant primary progressive aphasia. Neuroimage Clin. 2019;22 doi: 10.1016/j.nicl.2019.101797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G., Borghesani V., Henry M., Shwe W., Lauricella M., Miller Z., Gorno-Tempini M.L. Task-free functional language networks: reproducibility and clinical application. J. Neurosci. 2020;40(6):1311–1320. doi: 10.1523/JNEUROSCI.1485-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron D., Gorno-Tempini M.L., Rabinovici G.D., Santos-Santos M.A., Seeley W., Miller B.L., Ossenkoppele R. Prevalence of amyloid-beta pathology in distinct variants of primary progressive aphasia. Ann. Neurol. 2018;84(5):729–740. doi: 10.1002/ana.25333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Frost J.A., Hammeke T.A., Rao S.M., Cox R.W. Function of the left planum temporale in auditory and linguistic processing. Brain. 1996;119(Pt 4):1239–1247. doi: 10.1093/brain/119.4.1239. [DOI] [PubMed] [Google Scholar]
- Bonakdarpour B., Hurley R.S., Wang A.R., Fereira H.R., Basu A., Chatrathi A., Mesulam M.M. Perturbations of language network connectivity in primary progressive aphasia. Cortex. 2019;121:468–480. doi: 10.1016/j.cortex.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H., Duffy J.R., Whitwell J.L., Strand E.A., Machulda M.M., Schwarz C.G., Josephs K.A. Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex. 2015;69:220–236. doi: 10.1016/j.cortex.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum B.R., Baldo J., Okada K., Berman K.F., Dronkers N., D'Esposito M., Hickok G. Conduction aphasia, sensory-motor integration, and phonological short-term memory - an aggregate analysis of lesion and fMRI data. Brain Lang. 2011;119(3):119–128. doi: 10.1016/j.bandl.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network - Anatomy, function, and relevance to disease. Year Cogn. Neurosci. 2008;2008(1124):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Caso F., Mandelli M.L., Henry M., Gesierich B., Bettcher B.M., Ogar J., Gorno-Tempini M.L. In vivo signatures of nonfluent/agrammatic primary progressive aphasia caused by FTLD pathology. Neurology. 2014;82(3):239–247. doi: 10.1212/WNL.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Mesulam M.M., Jakobsen E., Malik F., Martersteck A., Wieneke C., Rogalski E. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136(Pt 8):2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.A., Montal V., Hochberg D., Quimby M., Mandelli M.L., Makris N., Dickerson B.C. Focal temporal pole atrophy and network degeneration in semantic variant primary progressive aphasia. Brain. 2017;140(2):457–471. doi: 10.1093/brain/aww313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proc. Natl. Acad. Sci. USA. 1998;95(3):831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Duffy J.R., Martin P.R., Clark H.M., Utianski R.L., Strand E.A., Whitwell J.L., Josephs K.A. The apraxia of speech rating scale: reliability, validity, and utility. Am. J. Speech Lang. Pathol. 2023;32(2):469–491. doi: 10.1044/2022_AJSLP-22-00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe D., Leyton C.E., Hodges J.R., Burrell J.R., Irish M., Piguet O. The neural correlates of auditory and visuospatial span in logopenic progressive aphasia and Alzheimer's disease. Cortex. 2016;83:39–50. doi: 10.1016/j.cortex.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Gao W., Lin W.L. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum. Brain Mapp. 2012;33(1):192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Dronkers N.F., Rankin K.P., Ogar J.M., Phengrasamy L., Rosen H.J., Miller B.L. Cognition and anatomy in three variants of primary progressive aphasia. Ann. Neurol. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Brambati S.M., Ginex V., Ogar J., Dronkers N.F., Marcone A., Miller B.L. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71(16):1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C., Sarraf S., Saverino C., Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol. Aging. 2016;41:159–172. doi: 10.1016/j.neurobiolaging.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Grossman M., Powers J., Ash S., McMillan C., Burkholder L., Irwin D., Trojanowski J.Q. Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain Lang. 2013;127(2):106–120. doi: 10.1016/j.bandl.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C.C., Gorno-Tempini M.L., Gesierich B., Henry M., Trujillo A., Shany-Ur T., Seeley W.W. Anterior temporal lobe degeneration produces widespread network-driven dysfunction. Brain. 2013;136(Pt 10):2979–2991. doi: 10.1093/brain/awt222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertrich I., Dietrich S., Ackermann H. The role of the supplementary motor area for speech and language processing. Neurosci. Biobehav. Rev. 2016;68:602–610. doi: 10.1016/j.neubiorev.2016.06.030. [DOI] [PubMed] [Google Scholar]
- Hodges J.R., Patterson K., Oxbury S., Funnell E. Semantic dementia - progressive fluent aphasia with temporal-lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Howard D., Patterson K. Thames Valley Test Company; 1992. The Pyramids and Palm Trees test: A test of semantic access from words and picture. [Google Scholar]
- Hurley R.S., Paller K.A., Rogalski E.J., Mesulam M.M. Neural mechanisms of object naming and word comprehension in primary progressive aphasia. J. Neurosci. 2012;32(14):4848–4855. doi: 10.1523/JNEUROSCI.5984-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley R.S., Bonakdarpour B., Wang X., Mesulam M.M. Asymmetric connectivity between the anterior temporal lobe and the language network. J. Cogn. Neurosci. 2015;27(3):464–473. doi: 10.1162/jocn_a_00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Lowe V.J., Senjem M.L., Weigand S.D., Kemp B.J., Shiung M.M., Petersen R.C. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131(Pt 3):665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Wiste H.J., Therneau T.M., Weigand S.D., Knopman D.S., Mielke M.M., Petersen R.C. Associations of Amyloid, Tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. J. Am. Med. Assoc. 2019;321(23):2316–2325. doi: 10.1001/jama.2019.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K.A., Duffy J.R., Strand E.A., Machulda M.M., Senjem M.L., Master A.V., Whitwell J.L. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(Pt 5):1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K.A., Duffy J.R., Clark H.M., Utianski R.L., Strand E.A., Machulda M.M., Whitwell J.L. A molecular pathology, neurobiology, biochemical, genetic and neuroimaging study of progressive apraxia of speech. Nat. Commun. 2021;12(1):3452. doi: 10.1038/s41467-021-23687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. PsychCorp; San Antonio, Tx: 2007. Western Aphasia Battery (Revised) [Google Scholar]
- Lansing A.E., Ivnik R.J., Cullum C.M., Randolph C. An empirically derived short form of the Boston naming test. Arch. Clin. Neuropsychol. 1999;14(6):481–487. [PubMed] [Google Scholar]
- Lee S.E., Rabinovici G.D., Mayo M.C., Wilson S.M., Seeley W.W., DeArmond S.J., Miller B.L. Clinicopathological correlations in corticobasal degeneration. Ann. Neurol. 2011;70(2):327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M., Madison C.M., Ghosh P.M., Seeley W.W., Mormino E., Greicius M.D., Rabinovici G.D. Intrinsic connectivity networks in healthy subjects explain clinical variability in Alzheimer's disease. PNAS. 2013;110(28):11606–11611. doi: 10.1073/pnas.1221536110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M.J., Mock B.J., Sorenson J.A. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7(2):119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Madhavan A., Whitwell J.L., Weigand S.D., Duffy J.R., Strand E.A., Machulda M.M., Josephs K.A. FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer's type. PLoS One. 2013;8(4):e62471. doi: 10.1371/journal.pone.0062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney C.J., Malone I.B., Ridgway G.R., Buckley A.H., Downey L.E., Golden H.L., Warren J.D. White matter tract signatures of the progressive aphasias. Neurobiol. Aging. 2013;34(6):1687–1699. doi: 10.1016/j.neurobiolaging.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli M.L., Vilaplana E., Brown J.A., Hubbard H.I., Binney R.J., Attygalle S., Gorno-Tempini M.L. Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain. 2016;139(Pt 10):2778–2791. doi: 10.1093/brain/aww195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli M.L., Welch A.E., Vilaplana E., Watson C., Battistella G., Brown J.A., Gorno-Tempini M.L. Altered topology of the functional speech production network in non-fluent/agrammatic variant of PPA. Cortex. 2018;108:252–264. doi: 10.1016/j.cortex.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mell J.C., Howard S.M., Miller B.L. Art and the brain - The influence of frontotemporal dementia on an accomplished artist. Neurology. 2003;60(10):1707–1710. doi: 10.1212/01.Wnl.0000064164.02891.12. [DOI] [PubMed] [Google Scholar]
- Mesulam M.M. Primary progressive aphasia. Ann. Neurol. 2001;49(4):425–432. doi: 10.1002/ana.91. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Imaging connectivity in the human cerebral cortex: the next frontier? Ann. Neurol. 2005;57(1):5–7. doi: 10.1002/ana.20368. [DOI] [PubMed] [Google Scholar]
- Mesulam M.M., Weintraub S., Rogalski E.J., Wieneke C., Geula C., Bigio E.H. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137(Pt 4):1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.L., Boone K., Cummings J.L., Read S.L., Mishkin F. Functional correlates of musical and visual ability in frontotemporal dementia. Br. J. Psychiatry. 2000;176:458–463. doi: 10.1192/bjp.176.5.458. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Ocklenburg S., Friedrich P., Fraenz C., Schluter C., Beste C., Gunturkun O., Genc E. Neurite architecture of the planum temporale predicts neurophysiological processing of auditory speech. Sci. Adv. 2018;4(7) doi: 10.1126/sciadv.aar6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogar J.M., Dronkers N.F., Brambati S.M., Miller B.L., Gorno-Tempini M.L. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis. Assoc. Disord. 2007;21(4):S23–S30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- Papadopoulou V., Chatzidimitriou E., Konstantinopoulou E., Parissis D., Ioannidis P. Emergence of artistic talent in logopenic variant of primary progressive aphasia: a case report. Neurol. Sci. 2023 doi: 10.1007/s10072-023-06647-6. [DOI] [PubMed] [Google Scholar]
- Pascual B., Masdeu J.C., Hollenbeck M., Makris N., Insausti R., Ding S.L., Dickerson B.C. Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb. Cortex. 2015;25(3):680–702. doi: 10.1093/cercor/bht260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual B., Funk Q., Zanotti-Fregonara P., Pal N., Rockers E., Yu M., Masdeu J.C. Multimodal (18)F-AV-1451 and MRI findings in nonfluent variant of primary progressive aphasia: possible insights on nodal propagation of Tau protein across the syntactic network. J. Nucl. Med. 2020;61(2):263–269. doi: 10.2967/jnumed.118.225508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popal H., Quimby M., Hochberg D., Dickerson B.C., Collins J.A. Altered functional connectivity of cortical networks in semantic variant Primary Progressive Aphasia. Neuroimage Clin. 2020;28 doi: 10.1016/j.nicl.2020.102494. doi:10.1016/j.nicl.2020.102494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popal H., Quimby M., Hochberg D., Dickerson B.C., Collins J.A. Altered functional connectivity of cortical networks in semantic variant Primary Progressive Aphasia. Neuroimage-Clinical. 2020;28 doi: 10.1016/j.nicl.2020.102494. ARTN 102494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha D., Eckbo R., Katsumi Y., Dickerson B.C., Touroutoglou A., Collins J.A. Tau and the fractionated default mode network in atypical Alzheimer's disease. Brain Commun. 2022;4(2) doi: 10.1093/braincomms/fcac055. fcac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici G.D., Jagust W.J., Furst A.J., Ogar J.M., Racine C.A., Mormino E.C., Gorno-Tempini M.L. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann. Neurol. 2008;64(4):388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riello M., Frangakis C.E., Ficek B., Webster K.T., Desmond J.E., Faria A.V., Tsapkini K. Neural correlates of letter and semantic fluency in primary progressive aphasia. Brain Sci. 2021;12(1) doi: 10.3390/brainsci12010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J.D., Ridgway G.R., Crutch S.J., Hailstone J., Goll J.C., Clarkson M.J., Warren J.D. Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage. 2010;49(1):984–993. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M.L., Sheridan M.A., Sambrook K.A., Peverill M.R., Meltzoff A.N., McLaughlin K.A. The role of visual association cortex in associative memory formation across development. J. Cogn. Neurosci. 2018;30(3):365–380. doi: 10.1162/jocn_a_01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routier A., Habert M.O., Bertrand A., Kas A., Sundqvist M., Mertz J., Teichmann M. Structural, microstructural, and metabolic alterations in primary progressive aphasia variants. Front. Neurol. 2018;9:766. doi: 10.3389/fneur.2018.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Santos M.A., Rabinovici G.D., Iaccarino L., Ayakta N., Tammewar G., Lobach I., Gorno-Tempini M.L. Rates of amyloid imaging positivity in patients with primary progressive aphasia. JAMA Neurol. 2018;75(3):342–352. doi: 10.1001/jamaneurol.2017.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffel L., Duffy J.R., Strand E.A., Josephs K.A. Word fluency test performance in primary progressive aphasia and primary progressive apraxia of speech. Am. J. Speech Lang. Pathol. 2021;30(6):2635–2642. doi: 10.1044/2021_AJSLP-21-00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C.S.M., Nitschke K., Bormann T., Romer P., Kummerer D., Martin M., Kaller C.P. Dissociating frontal and temporal correlates of phonological and semantic fluency in a large sample of left hemisphere stroke patients. Neuroimage Clin. 2019;23 doi: 10.1016/j.nicl.2019.101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt G.C., Graham N.L., Rochon E., Tang-Wai D.F., Lobaugh N.J., Chow T.W., Black S.E. Whole-brain white matter disruption in semantic and nonfluent variants of primary progressive aphasia. Hum. Brain Mapp. 2013;34(4):973–984. doi: 10.1002/hbm.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Matthews B.R., Crawford R.K., Gorno-Tempini M.L., Foti D., Mackenzie I.R., Miller B.L. Unravelling Bolero: progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;131:39–49. doi: 10.1093/brain/awm270. [DOI] [PubMed] [Google Scholar]
- Sestieri C., Shulman G.L., Corbetta M. Attention to memory and the environment: functional specialization and dynamic competition in human posterior parietal cortex. J. Neurosci. 2010;30(25):8445–8456. doi: 10.1523/Jneurosci.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N.A., Martin P.R., Graff-Radford J., Sintini I., Machulda M.M., Duffy J.R., Whitwell J.L. Altered within- and between-network functional connectivity in atypical Alzheimer’s disease. Brain Commun. 2023;1–16 doi: 10.1093/braincomms/fcad184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintini I., Graff-Radford J., Jones D.T., Botha H., Martin P.R., Machulda M.M., Whitwell J.L. Tau and amyloid relationships with resting-state functional connectivity in atypical Alzheimer's disease. Cereb. Cortex. 2021;31(3):1693–1706. doi: 10.1093/cercor/bhaa319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintini I., Duffy J.R., Clark H.M., Utianski R.L., Botha H., Machulda M.M., Whitwell J.L. Functional connectivity to the premotor cortex maps onto longitudinal brain neurodegeneration in progressive apraxia of speech. Neurobiol. Aging. 2022;120:105–116. doi: 10.1016/j.neurobiolaging.2022.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintini I., Graff-Radford J., Schwarz C.G., Machulda M.M., Singh N.A., Carlos A.F., Whitwell J.L. Longitudinal rates of atrophy and tau accumulation differ between the visual and language variants of atypical Alzheimer's disease. Alzheimers Dement. 2023;19(10):4396–4406. doi: 10.1002/alz.13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli E.G., Mandelli M.L., Miller Z.A., Santos-Santos M.A., Wilson S.M., Agosta F., Gorno-Tempini M.L. Typical and atypical pathology in primary progressive aphasia variants. Ann. Neurol. 2017;81(3):430–443. doi: 10.1002/ana.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Sepulcre J., Turner G.R., Stevens W.D., Schacter D.L. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J. Cogn. Neurosci. 2013;25(1):74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Stevens W.D., Viviano J.D., Schacter D.L. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol. Aging. 2016;45:149–160. doi: 10.1016/j.neurobiolaging.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceed. Natl. Acad. Sci. USA. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Ficek B., Rapp B., Tsapkini K. Different patterns of functional network reorganization across the variants of primary progressive aphasia: a graph-theoretic analysis. Neurobiol. Aging. 2020;96:184–196. doi: 10.1016/j.neurobiolaging.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzloff K.A., Duffy J.R., Clark H.M., Strand E.A., Machulda M.M., Schwarz C.G., Whitwell J.L. Longitudinal structural and molecular neuroimaging in agrammatic primary progressive aphasia. Brain. 2018;141(1):302–317. doi: 10.1093/brain/awx293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzloff K.A., Whitwell J.L., Utianski R.L., Duffy J.R., Clark H.M., Machulda M.M., Josephs K.A. Quantitative assessment of grammar in amyloid-negative logopenic aphasia. Brain Lang. 2018;186:26–31. doi: 10.1016/j.bandl.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzloff K.A., Duffy J.R., Clark H.M., Utianski R.L., Strand E.A., Machulda M.M., Whitwell J.L. Progressive agrammatic aphasia without apraxia of speech as a distinct syndrome. Brain. 2019;142:2466–2482. doi: 10.1093/brain/awz157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C.K., Mack J.E. Grammatical Impairments in PPA. Aphasiology. 2014;28(8–9):1018–1037. doi: 10.1080/02687038.2014.912744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls Carbo A., Reid R.I., Tosakulwong N., Weigand S.D., Duffy J.R., Clark H.M., Whitwell J.L. Tractography of supplementary motor area projections in progressive speech apraxia and aphasia. Neuroimage Clin. 2022;34 doi: 10.1016/j.nicl.2022.102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessel E.A., Isik A.I., Belfi A.M., Stahl J.L., Starr G.G. The default-mode network represents aesthetic appeal that generalizes across visual domains. Proceed. Natl. Acad. Sci. USA. 2019;116(38):19155–19164. doi: 10.1073/pnas.1902650116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas I.V., Boxer A.L., Fesenko J., Matlin A., Heuer H.W., Mirsky J., Miller B.L. Visual search patterns in semantic dementia show paradoxical facilitation of binding processes. Neuropsychologia. 2011;49(3):468–478. doi: 10.1016/j.neuropsychologia.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S., Mesulam M.M., Wieneke C., Rademaker A., Rogalski E.J., Thompson C.K. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am. J. Alzheimers Dis. Other Demen. 2009;24(5):408–416. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Whitwell J.L., Duffy J.R., Strand E.A., Xia R., Mandrekar J., Machulda M.M., Josephs K.A. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang. 2013;125(3):245–252. doi: 10.1016/j.bandl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Duffy J.R., Strand E.A., Machulda M.M., Senjem M.L., Schwarz C.G., Josephs K.A. Clinical and neuroimaging biomarkers of amyloid-negative logopenic primary progressive aphasia. Brain Lang. 2015;142:45–53. doi: 10.1016/j.bandl.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Jones D.T., Duffy J.R., Strand E.A., Machulda M.M., Przybelski S.A., Josephs K.A. Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer's dementia. Neurobiol. Aging. 2015;36(3):1245–1252. doi: 10.1016/j.neurobiolaging.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study will be available from the corresponding author on request.
Data will be made available on request.