Abstract
European catfish is a large-bodied apex predator, a key species in native areas, but invasive in others where it negatively impacts local aquatic fauna necessitates catfish regulation. However, traditional ichthyological methods face challenges in capturing it. The study presents a detailed description of the efficient long-line method, refined through 48 sampling campaigns across twelve European water bodies. This method proves cost-effective and technically undemanding, requiring an average of 5.6 bait fish to catch one European catfish per day. The long-lines outperform other techniques, with the highest Biomass per unit effort (BPUE) of 6.205 kg of catfish per man-hour and minimal by-catch (0.276 kg per man-hour). In contrast, fyke nets, the second most efficient method, achieve a BPUE of 0.621 kg of catfish per man-hour with 3.953 kg of by-catch per man-hour. To optimize long-line catches, a 15 m distance between branch lines and regular relocation is recommended. Live fish is the most effective bait with no significant differences observed among species. However, earthworms, a less controversial alternative, are also efficient, especially for smaller catfish. Our recapture approach using various ichthyological methods revealed no hook avoidance behavior by catfish after a previous catch or avoidance by a certain part of the population. The long-line method is suitable for population regulation, scientific research, and conservation efforts and is the most effective means of capturing live European catfish.
Keywords: Bait, Ichthyological method, Large-bodied predator, Non-native area, Predation pressure
1. Introduction
European catfish (Silurus glanis) is one of the largest freshwater fish species worldwide, excluding anadromous species [1]. It can reach a total length (TL) of over 2.7 m and a body mass of up to 130 kg [2]. Furthermore, it serves as the main freshwater apex predator in European freshwater ecosystems [3]. Over the past half-century, the European catfish has effectively spread from east to west across nearly the entire Europe, including the British Isles [4]. As of at least 2014, the species has also been discovered in Portugal [5], marking the westernmost limit of its distribution on the European continent. Northern Scandinavia remains the only area of continental Europe without its presence [2]. Additionally, the species is currently found, albeit likely to a limited extent, on other continents, specifically South America, Africa and Asia [6,7]. It should be emphasized that the invasive spread is not solely due to its generalist behavior [3], but is especially driven by its appeal to humans [7]. The European catfish primarily spread to new localities to a significant extent through both controlled and illegal activities of recreational anglers, rather than natural overcoming of waterway barriers [7,8]. Anglers highly prize the thrill of big game fishing for European catfish [8]. The initial phase of its range expansion in Europe was often associated with major rivers with high connectivity [9]. However, in the second phase, the European catfish is observed spreading into already populated areas to new water bodies not connected to the large rivers, such as pre-alpine lakes [[10], [11], [12]]. Notably, large lakes in southwest Europe like Lake Maggiore and Lake Bourget are already fully invaded by European catfish, and favorable temperature conditions allow them to prey year-round [[11], [12], [13]].
Due to its dietary plasticity [3], the European catfish thrives in various habitats including littoral and pelagic zones as well as deep water environments [11]. European catfish have been observed at depth exceeding 60 m and their diet often includes species found in deep waters, such as whitefish (Coregonus sp.) and burbot (Lota lota) [10,11,13]. Initially, it appeared that the European catfish did not have a significant impact on fish communities in newly invaded areas [14]. However, it has become evident that the impact on local biodiversity is indeed significant [3]. This potential predation pressure can affect endemic species that have not evolved to coexist with such a large apex predator [9]. This phenomenon has already been observed, for instance, in the lower Po River, leading to the local extinction of native cyprinids [15]. In the near future, this ecological problem is expected to become especially relevant in areas with high endemism, such as the Iberian Peninsula [9], and even some pre-alpine lakes [10,13]. Furthermore, certain species are notably more sensitive to catfish predation, as demonstrated by the experimental study [16]. Due to its remarkable dietary plasticity, European catfish can quickly switch its focus to ecologically and economically important anadromous species that migrate to rivers in large numbers for short-term reproduction, such as Altantic salmon (Salmo salar) [17], allis shad (Alosa alosa) [18,19], and even sea lamprey (Petromyzon marinus; [20]. The intense predation by European catfish on these anadromous species is noteworthy, and similarly intense predation occurs on common freshwater species, such as common roach (Rutilus rutilus), during their reproduction period [3]. Moreover, European catfish can impact reproduction by consuming fish eggs, such as egg strands of Eurasian perch (Perca fluviatilis) [21]. The phenomenon of impacting species reproduction is evidently not limited to fish. At Sebino Peat Bog Nature Reserve, Italy, severe predation by European catfish on waterfowl chicks was documented during the growth period [22]. The impact of invasive European catfish can thus have more farther-reaching consequences than initial observations might suggest. More recently, European catfish has also colonized the Guadalquivir River delta within the Doñana National Park (C. F. Delgado, pers. comm.). Doñana NP represents one of the most important waterfowl habitats in Europe [23], and the impact of European catfish on the waterfowl communities there is yet to be evaluated. However, stable isotope analysis from Camargue Regional Natural Park, a similarly characterized protected area in southern France, revealed a considerable impact of catfish on the local waterfowl communities [24]. The broad dietary spectrum of European catfish also includes aquatic insects, bivalves, crustaceans, amphibians, birds and even mammals [3,11,13].
The negative impact of the European catfish on fauna in non-native localities is on the increase, driven by the species expanding distribution and favorable global climate changes [2,9]. Several international research projects, aiming to find ways to mitigate the uncontrolled growth of European catfish populations in non-native areas, have been in progress; and more are likely to follow [25]. Through a comparison of various available approaches, long-lines appear to be the most efficient method for capturing and thus reducing the European catfish population [26]. The method has been proposed as an efficient tool for both the regulating the European catfish population and capturing a sufficient number of individuals for research purposes [7]. Notably, the method is novel, at least in the freshwater fish research in Europe, and has seen recent improvements to enhance its catch efficiency.
This study aims to introduce the method of long-lines and its enhancements to facilitate its efficient use in the ongoing [25] and future projects focused on regulating the European catfish population, as the number of such projects has been increasing in response to growing awareness of the problems caused by the species spread and invasive behavior. The aims of the study are i) to provide a detailed description of the method making it accessible to scientists, conservationists and others without training, and ii) to determine the optimal parameters for the method, such as the length of the long-line, distances between branch lines, and choice of bait fish species. The method offers flexibility, and we hypothesized that a shorter long-line (60 m) with branch lines placed 5 m apart would yield higher catch efficiency compared to a longer one (235 m) with branch lines placed 15 m apart. This hypothesis is based on the idea that a higher concentration of baits in a smaller space might resemble a shoal, creating more water ripples, a key factor when European catfish hunt for prey [27]. Additionally, we hypothesized that relocating the long-line to a new location within the water body daily could enhance efficiency, assuming that a significant catch is achieved during the first night. Further, iii) we conducted experiments with different types of baits, aiming to identify significant differences in catch efficiency associated with each bait. Additionally, iv) we tested the selectivity of the long-lines on subgroups of populations with a certain pattern of behavior [28] and assessed its effectiveness in preventing fish from avoiding the hook after a previous catch [29]. Lastly, v) we compared the effectiveness of the long-lines with other fishing methods.
2. Methods
2.1. Long-lines
Animals were treated in accordance with the Experimental Animal Welfare Commission under the Ministry of Agriculture of the Czech Republic guidelines (Ref. No. 4253/2019-MZE-17214). The work was approved by the Ethics Committee of the Czech Academy of Sciences. No endangered or protected species were involved.
Long-lines or ‘hook-lines' have been widely used to catch large predatory fish in the marine environment [30,31]. In freshwater environment, it is currently the most commonly used method for monitoring European catfish [7,11,32]. The method can be applied in any standing or slowly flowing waters with a depth exceeding 2 m. We do not recommend using it in shallower depths because when the depth significantly exceeds the length of the expected catch, catfish often become entangled with the leader in the mother line. The long-lines can be deployed in both inshore and offshore (open water) areas. The assembly is flexible, allowing for adjustments in both its length and the number of applied branch lines (snoods). The advantages of long-lines include simple and cost-effective equipment, ease of use and handling, and low damage and resulting mortality of caught individuals (≪ 5 % [8]). The method can be effectively employed in a wide range of environments and tailored to the specific needs of aquatic bodies, making it efficient for reducing the European catfish population to harmless levels [7].
However, there are some disadvantages associated with long-lines, including the need to have baits available. Long-lines also rely on the feeding activity of European catfish, which typically decreases when water temperatures drop below 12 °C and ceases completely below 7 °C [[33], [34], [35]]. This method is primarily intended for catching adult European catfish individuals with a size exceeding 60 cm TL. The selectivity is primarily ensured by using larger baits (preferably >20 cm TL, ideally 25–35 cm TL).
2.1.1. Basic equipment
The long-line is illustrated in Fig. 1. The length of the line can vary, however, for standardization purposes, two basic lengths were used in this study: 60 m and 235 m. The main float line had a diameter of 5 mm. The shorter version (60 m) and the longer version (235 m) of the long-line were equipped with 10 and 15 branch lines, respectively. Three main buoys were positioned at both ends and in the middle of the main line. Anchoring ropes (Ø 10 mm), from 3.5 to X m long (depending on the depth of the locality), with weights (32 kg each) were attached to the buoys to secure the main line in place. Auxiliary buoys (empty 1.5 L plastic bottle or 1.5–2 dm3 polystyrene float) were placed every 5 m (for the 60 m line) or 15 m (for the 235 m line) between the main buoys. These auxiliary buoys mark the location of the branch lines and maintain the bait in the correct position. Each hanging branch line was 2.5 m long and consisted of two parts, i) a 2 m long fishing-line with a maximum load of 50–100 kg, and ii) a more durable 0.5 m long fishing-line with a maximum load of 100 kg or more (Fig. 2a). A swivel was positioned between these two parts to prevent twisting and a 150 g sinker lead was attached to the branch line to maintain the appropriate depth. Rubber shocks were placed both below and above the sinker lead. The lower rubber shock was free-flowing and sliding to protect the swivel knot. In contrast, a small piece of wood was inserted into the middle hole of the upper rubber shock to prevent the sinker lead from moving freely along the branch line. At the end of the branch line, there was a multi-hook system with one single baited hook and one treble hook for catching the European catfish (Fig. 2a). The branch lines and auxiliary buoys were connected to the main line using long-line fishing clips (see Fig. 2b for details).
Fig. 1.
Schematic of the long-line (shorter version, 60 m) a fishing method for European catfish sampling. In certain situations, the anchor rope can also be secured to a shoreline object, such as a tree [36].
Fig. 2.
a) A detail of the branch line (snood) with individual parts described. b) A detail of the branch line and auxiliary buoy connection to the main line using long-line fishing clips.
The bait fish, measuring 180–300 mm TL, was placed on the single hook. When the European catfish attempts to seize the bait, it is captured by the treble-hook suspended below the baited hook [3,7,20]. The appropriate size for the treble-hooks is generally 2–2/0 depending on the size of the bait and the mean size of the European catfish being targeted. Sizes 1/0 for the single hook and 1 for the treble-hook have prove to be optimal in most scenarios [26].
2.1.2. Additional equipment
A sampling boat, a punt, with a load capacity of 800 kg and a length of 5 m was used. It was equipped with oars for easy control when near the long-line. An outboard engine, with at least 15 HP, is recommended for faster transport between the long-lines. It is advisable to have a depth sounder and a GPS device to record the exact position of the long-lines. Two separate tanks were required: for bait fish and for captured European catfish, both with aeration equipment. The separation helps minimize the risk of entanglement or injury. The equipment for working with European catfish included leather gloves, a landing net, a soft pad, rubber gloves, a fish measure, scales (for up to 100 kg or more), a large carrier bag for weighing the largest fish, and catch record cards.
2.1.3. Sampling time, season and location selection
The long-lines were kept deployed continuously for a full day (24 h) and checked regularly three times daily: at or after sunset, in the morning after dawn, and once during the day. The season significantly affects the catchability of European catfish [8]. As mentioned earlier, the method is less efficient during winter due to reduced feeding activity. In central Europe, the most productive season was June–July when water temperatures ranged 19.1–20.2 °C [8].
It is recommended that the long-line be positioned at a distance >20 m from the shore. However, installation on very steep shores with slopes >30° is not advisable, as there is a risk of the anchoring weights shifting from their intended position. In such cases, an alternative approach may involve removing the anchor and securing the anchor line to the shore, typically to trees [36]. Most water bodies are characterized by their heterogeneous environments, making the choice of long-line placement crucial. Based on our experience, the most efficient areas were those with gentler slopes and extensive shallow littoral zones extending into the water body, areas with numerous underwater obstacles (resting spots) or the mouths of side bays. If maximizing catch efficiency is the primary goal, long-lines should be deployed in these areas. However, if the project's objective is to collect reliable population data, particularly for research purposes, it is advisable to distribute long-lines evenly throughout the entire water body [26].
2.1.4. Mounting of the long-lines
Prior to deployment, it was necessary to have essential preparations as follows. Wind the main line onto a suitable spool. Arrange the branch lines side by side on a wooden board for easy access. Equip the main buoys with large carabiners, and load the auxiliary buoys (empty plastic bottles or floats) with carabiners into a plastic container for convenience. Ensure that the anchoring lines have loops at both ends, allowing them to be attached with the weight on one side and the buoy on the other (Fig. 1). Fill the tank for bait fish on the boat with water.
The long-lines were then deployed from the boat. One person uncoiled the pre-prepared branch lines from the wooden board and handed them to a team member responsible for baiting the branch lines. Baiting was done by inserting the single hook into the dorsal part of the bait fish and placing a 1 × 1 cm safety rubber stop on the hook to prevent the bait from slipping. The third team member attached the branch line with the bait to the main line using a long-line fishing clip. Adjacent to the branch line, the auxiliary buoy was also affixed using a long-line fishing clip (Fig. 2b). Meanwhile, the fourth team member ensured the safe movement of the boat. It is possible to pull on the line to reach the next auxiliary buoy. However, in windy weather, approaching the auxiliary buoys one by one using oars was necessary. Once all the branch lines with baits were deployed, the entire system was tightened, when necessary, either at the end or the beginning of the long-line. The anchoring weight was pulled into the boat, and the entire system was stretched by moving the boat backward. A team of four handled three long-lines on a daily basis, which seems to be the optimal number.
2.1.5. Checking of the branch line and collecting the catch
Inspection was conducted in a similar manner to attaching the branch lines, involving the process of pulling out the branch lines one by one and moving the boat from the beginning to the end of the long-line. If a tangled branch line was encountered, it was untangled, or the entire branch line was replaced with a new one (extra branch lines readily available on the wooden board). When a European catfish was found on a branch line (often signaled by the movement of the auxiliary buoy), the line was pulled close to the boat. A large landing net was used to carefully retrieve the catfish. In the case of larger individuals, the branch line was more prone to breaking, so caution was necessary. When the catfish vigorously pulled on the line, it was better to release it temporarily, wait until the catfish calmed down and the tension subsided and then the catfish was removed from the water quickly. Subsequently, the catfish was placed on a wet rubber mat to prevent injury to its delicate skin, that also served as a weighing bag. Prior to biological inspection, each individual was anesthetized by immersion in a solution containing clove oil at a concentration of 0.04 mL/L. The anesthetic's effect was allowed to manifest until the fish exhibited a loss of reflex reactivity characterized by slow and irregular opercular movements, accompanied by an absence of reflexes and reactivity. The hook in the catfish's mouth was carefully removed using pliers.
2.2. Evaluating the selectivity of long-lines and its comparison with alternative methods
-
i)
Continuous boat electrofishing along the shoreline was used during all long-line campaigns serving both as a voluntary sampling method and as a means of obtaining bait fish. Fish were collected using an electrofishing boat (electrofisher EL 65 II GL DC, Hans Grassel, Schönau am Königsee, Germany, 13 kW, 300/600 V; Fig. 3a) with an approximately 6-m-wide electric field to which fish are attracted and immobilized and they were subsequently retrieved into an aerated vat. Additionally, during mentioned biomanipulation campaign at Žlutice reservoir, predatory fish, including European catfish, were caught as by-catch during the campaign. All individuals were measured, weighed, their PIT-tags were checked, after which they were released back into the water body. The frequency distribution of tagged fish originally captured by long-lines was subsequently used to estimate the potential selectivity of long-lines on a subgroup of catfish populations exhibiting specific behavioral patterns.
-
ii)
Rimov fyke nets (Pokorny-site.cz; a mesh size of 20 mm, input frame dimensions of 1.2 × 1.2 m, body length of 8 m, wing dimensions of 1.2 × 12 m; Fig. 3b) were used for an extensive biomanipulation campaign at Žlutice reservoir during springs 2020–2023 where primarily planktivorous fish were captured. These fyke nets were deployed in groups of three in the shallows and in the tributaries of the reservoir where fish spawning was anticipated.
-
iii)
Multimesh gillnets were used at Most, Milada, Římov, Žlutice, Vrchlice, Klíčava, and Lipno concurrently with the long-line campaigns, specifically the most commonly used benthic (net = 45 m2, referred to as B12) and pelagic multimesh gillnets (90 m2, P12) encompassing twelve mesh sizes according to the EU norm (5, 6.25, 8, 10, 12.5, 15.5, 19.5, 24, 29, 35, 43 and 55 mm [37]). Further, the large-mesh gillnets were used with mesh sizes 70, 90, 110 and 135 mm (knot-to-knot, 10 m panels), both benthic (net = 60 m2, B4) and pelagic (net = 120 m2, P4) [38].
-
iv)
Atypical gillnets, used by commercial fishermen in marine environments or large rivers (mesh sizes of 105-, 90-, and 70-mm knot-to-knot. Each size comprised a continuous panel of 40 m in length, 6 m in height, and covering an area of 240 m2). These gillnets were employed at Římov and Žlutice in 2023. The installation of the gillnets followed the recommendations of Filipe Ribeiro and Victor Frossard. They were positioned in the littoral zone, with their height exceeding the water depth, reaching depths of up to 5 m. The manner in which the net is stretched can be crucial in capturing larger catfish.
Fig. 3.
a) Schematic of an electrofishing boat (Electrofisher EL 65 II GL DC, Hans Grassel, Schönau am Königsee, Germany, 13 kW, 300/600 V) used for electrofishing operations. b) Schematic of the Rimov fyke net (Pokorny-site.cz). Both methods were employed for comparison with the long-lines.
The catch efficiency of the methods was measured using Catch per Unit Effort (CPUE; number of catfish per man-hour) and Biomass per Unit Effort (BPUE; kg of catfish per man-hour). The man-hour accounts for the time spent on installation, uninstallation, retrieval, and catch processing which may vary among tested methods. The time required for the boat to reach the fishing gear was not included. Further, CPUE and BPUE were measured for the by-catch, which includes all other species caught using each method, except for the European catfish.
2.3. Study sites
The long-lines were tested from 2013 to 2023 at eight study sites in the Czech Republic and at four study sites in Portugal. The sites were selected to cover a variety of water body types. In Czechia, we sampled two post-mining lakes, Milada (50°39′19.51″N 13°56′14.01″E) and Most (50°32′26.48″N 13°38′49.38″E) both of which are oligotrophic and have a littoral zone full of vegetation up to a depth of 10 m [[39], [40], [41]]. Both lakes are relatively deep with Milada having a uniform bottom relief and Milada having a complex bottom relief of Most lake [3]. The six water reservoirs in Czechia varied considerably: mesotrophic Lipno (49°55′43.21″N 15°13′32.49″E) and Klíčava (50°03′55.98″N 13°55′56.74″E), eutrophic Žlutice (50°05′22.80″N 13°07′36.96″E), Římov (48°50′52.03″N 14°29′13.60″E) and Hubenov (49°23′3.70″N 15°29′07.55″E), and hypertrophic Vrchlice reservoir (49°55′41.95″N 15°13′28.71″E). Vegetation is abundant at Klíčava, Žlutice and Vrchlice, whereas it is sparse at the other reservoirs. See Draštík et al. [42] and Vejřík et al. [3,41] for detailed description of the Czech reservoirs and lakes. Portuguese research took place in the hypertrophic Belver reservoir (39°28′46.97″N 7°59′43.71″W) and on the Tagus River, specifically two lotic sites in Aquapoli (39°26′53.54″N 8°11′55.24″W) and Constância (39°28′21.47″N 8°19′11.68″W), and one estuary Port da Palha (39° 3′19.49″N 8°47′57.97″W). However, only dead bait fish was used at Portuguese sites. The parameters of the sites varied considerably, see Table 1.
Table 1.
Basic characteristics of the 12 study sites. Lotic study sites are in gray. The European catfish population estimates refer to the individuals >60 cm *Values by Vejřík et al. [8]; **Values by Vejřík et al. [67]; × – study sites with only one sampling campaign so far, i.e., with no recaptures, or data that is not available for the lotic sites.
A total of 48 campaigns and 177 fishing days were carried out with a total of 6155 snood-days (Table 2). At the most frequently visited study sites (Milada, Most, Žlutice, Římov and Klíčava) all European catfish individuals were tagged with a passive integrated transponder tag (PIT-tag, Oregon RFID, fullduplex; length 12 mm; diameter 2.15 mm; mass 0.11 g; 11 784/11785 ISO compatible) that was inserted into the dorsal muscle, specifically positioned 5 cm behind the dorsal fin. Thus, it was also possible to determine the number of recaptures.
Table 2.
Summary of the number of days, campaigns and snood-days (catching on one snood/branch line during 24 h) spent at the study sites, the number of catches, recaptures and by-catches. Further, the mean total length (TL) and mass (M) of the caught European catfish, and mean number of catches per snood-day using the long-lines. × – study sites with only one sampling campaign so far, i.e., with no recaptures. Lotic sites and estuary are in gray. At these sites, only dead fish were used as bait. Thus, sum of catches per snood-day was counted separately.
2.4. Statistics
A Bayesian linear mixed-effects model (Wald χ2 test) was used to test the catch efficiency (number and biomass of catfish) of shorter (60 m) and longer (235 m) long-lines, catch efficiency of the first and second day in one place, and catch efficiency at various locations at Římov, Žlutice and Klíčava reservoirs in 2018 and 2019. At these sites, long-lines were used at six different locations in 2018 which were repeated the following year. To improve the data normality, the number and biomass of catfish (biomass expressed as a mean body mass, M) were log10-transformed with the addition of a constant of 0.1 to eliminate the zero values prior to the analyses. The identity of the study site and the effect of the catching season were set as parameters with random effects.
A goodness of fit test (χ2 statistic) was used to compare various baits: i) bait fish species (rudd (Scardinius erythrophthalmus), roach and Eurasian perch), ii) live vs. dead bait fish of the mentioned species, iii) dead fish vs. chicken wings (bought at a supermarket) were tested at Milada and Most lakes in 2013 and 2014. Further, iv) two invasive species as a bait (a juvenile European catfish vs. red swamp crayfish (Procambarus clarkii)) was tested at Belver reservoir in 2022, and v) live fish vs. cluster of eight earthworms (Lumbricus terrestris) was tested at the Hubenov reservoir in 2023. A goodness of fit test was also used to compare hook avoidance behavior in catfish after a previous catch and potential avoidance by a certain part of the catfish population towards the long-lines. This analysis was based on the representation of recaptured individuals and newly caught individuals using long-lines, electrofishing methods, and fyke nets at Žlutice reservoir in 2020–2022.
A one-way repeated measures analysis of variance (ANOVA) with multiple comparisons (Tukey Unequal N HSD test) was used to compare the catch efficiency of various methods based on CPUE and BPUE of both catfish and by-catch (other fish species). The analyses were performed using R 4.0.0 [43] and the blme package [44].
3. Results
3.1. Efficiency of long-lines
Over a total 177 fishing days at twelve study sites, 1043 European catfish individuals were caught (mean TL = 107.3 cm, mean M = 11.1 kg). On average, 0.18 European catfish (min. 0.093; max. 0.247) were caught per snood-day, which refers to catching one European catfish on 5.6 snoods (branch line; ±8.2 SD)) over 24 h (Table 2). A total of 208 individuals were recaptured at sites with tagged European catfish (Table 2). Furthermore, we recorded a total of 164 by-catches, which accounted for 14 % of all catches. Specifically, Northern pike (Esox lucius) constituted 97 % of all by-catches, while the remainder included pikeperch (Sander lucioperca), asp (Leuciscus aspius), barbel (Luciobarbus sp.) and Eurasian perch >40 cm TL. However, aside from Northern pike, all other species were rarely caught on long-lines and generally comprised less than 2 % of the total catch (Table 2).
Significantly more European catfish were caught on the first day compared to the second day at the same location. On the first day, an average of 1.92 European catfish were caught using 10 baits, while only 1.46 were caught on the second day (χ2 1 = 9.98, p < 0.01). Despite the significant decrease in the number of catches on the second day at the same location, larger individuals were caught. The mean body masses of European catfish caught on the first and second days were 8.6 kg and 12.5 kg, respectively. This result was statistically significant (χ2 1 = 6.74, p < 0.01).
The difference in catch efficiency between the shorter (60 m) and longer version (235 m) of the long-line was not statistically significant (χ2 1 = 2.92, p = 0.09). The catch efficiency was 0.138 and 0.154 individuals per one snood-day on the short and long version, respectively. A statistically significant difference was found in the mean size of the caught European catfish, with an average of 8.6 kg on the long version and 6.2 kg on the short version (χ2 1 = 9.98, p < 0.01). Furthermore, statistically significant differences were found in both the number of individuals caught per day (χ2 26 = 51.10, p < 0.01) and the size of individuals (χ2 26 = 53.59, p < 0.01) depending on the location of the long-line.
3.2. Testing various types of bait
In terms of the baits, live bait fish were the most efficient. The length range of the bait fish relative to the captured catfish varied significantly, from 9.1 % to 56.8 % of the catfish TL. On average, it represented 23.3 % ± 9.3 SD of the catfish TL. A significant difference was found comparing the live and dead bait fish (χ2 1 = 4.17, p = 0.04), when 17 and 7 catfish individuals were caught on live and dead bait fish, respectively, using 72 replications (snood days) for each bait (Fig. 4a). When using three different species of bait fish, 20, 16 and 15 catfish individuals were caught on rudd, perch and roach, respectively. However, the differences between the species were not significant (χ2 2 = 0.82, p = 0.66), using 85 replications for each bait (Fig. 4b). A significant difference in catch efficiency was found comparing the dead fish and chicken wings (χ2 1 = 6.4, p = 0.01), when 9 and 1 catfish individuals were caught on dead fish and chicken wings, respectively, using 20 replications for each bait (Fig. 4c). When juvenile catfish alternated red swamp crayfish as bait on the long-lines tested at Belver reservoir, the difference was even more significant (χ2 1 = 8.3, p = 0.004), where 11 and only 1 catfish individual were caught on the juvenile catfish and the crayfish, respectively, using 30 replications for each bait (Fig. 4d). The only reasonable alternative to live fish seems to be using earthworms as bait. When live fish alternated a cluster of eight earthworms as bait tested at Hubenov reservoir, the difference was not significant (χ2 1 = 0.08, p = 0.78), where 6 and 7 catfish individuals were caught on the live fish and a cluster of eight earthvorms, respectively, using 30 replications for each bait (Fig. 4e). Although the cluster of eight earthworms appears to be even slightly more efficient than live fish, the mean size of caught individuals was significantly smaller (mean 4.3 kg ± 1.1 SD) compared to individuals caught on live fish (mean 12.7 kg ± 10.3 SD; χ2 1 = 4.15, p = 0.04).
Fig. 4.
Catch efficiency with different types of baits. Various types of baits were placed on the long-line alternately in a ratio of 1: 1 or 1: 1: 1 in equal numbers. a) Live and dead bait fish (rudd), brown: live bait fish, gray: dead bait fish. b) Comparison of three bait fish species and the relative number of catches per species, blue: Eurasian perch (Perca fluviatilis), green: rudd (Scardinius erythrophthalmus), orange: roach (Rutilus rutilus). c) Dead bait fish (rudd) and chicken wing, gray: dead fish, yellow: chicken wing. d) Juvenile European catfish and red swamp crayfish, purple: juvenile catfish, green: red swamp crayfish. e) Live bait fish and cluster of eight earthworms (Lumbricus terrestris), orange: live bait fish, blue: earthworms.
3.3. Testing hook avoidance behavior by catfish after a previous catch
The analysis of catfish catch records at the Žlutice reservoir involved three distinct techniques: long-lines, electrofishing, and fyke nets. These methods were used to monitor the percentage of individuals that had been previously caught on long-lines, tagged, and subsequently released. On average, the representation of tagged individuals caught by long-lines, electrofishing and fyke nets were 34.4, 31.4 and 37.5 %, respectively (Fig. 5, Table 3). The comparison of the percentage representation of tagged individuals in long-line catches with the other methods showed significant differences in only two of the three monitored years: in 2021, the representation of tagged catfish was significantly lower in electrofishing compared to long-line catches, and in 2022, the representation was higher for fyke nets than for long-lines (Table 2). In other cases, the differences in the representation of tagged fish in catches were inconclusive. Overall, the percentage representation of tagged fish in catches using each method did not exhibit any clear repeating trend over the years (Fig. 5).
Fig. 5.
Percentage representation of recaptured catfish (those tagged during previous long-line fishing) in the total catch of catfish using long-lines, electrofishing, and fyke nets for the years 2020, 2021, and 2022. Additionally, it provides the average mass of all caught individuals, with individuals smaller than 60 cm excluded from the analysis.
Table 3.
Catfish catches by long-lines, electrofishing, and fyke nets in 2020, 2021, and 2022. It includes data on untagged and recaptured catfish (ind. tagged during the previous long-line catch), the percentage of recaptured individuals, mean total length (TL) and mass (M), and the exclusion of catfish under 60 cm. The table also presents χ2 test results, evaluating recaptured catfish representation in individual years between long-lines and other methods.
| Method | Year | No. of catch | Tagged ind. | Recaptures | Recaptures (%) | Mean TL (cm) | Mean M (kg) | Excluded small ind. | Statistics χ2 1/p |
|---|---|---|---|---|---|---|---|---|---|
| Long-lines |
2020 | 27 | 19 | 8 | 29.6 | 94 | 7.3 | 0 | |
| 2021 | 44 | 27 | 17 | 38.6 | 93 | 5.98 | 0 | ||
| 2022 | 20 | 13 | 7 | 35 | 99 | 7.93 | 0 | ||
| Mean |
30.3 |
19.7 |
10.7 |
34.4 |
95.3 |
7.07 |
0 |
||
| Electro fishing |
2020 | 8 | 5 | 3 | 37.5 | 74 | 3.46 | 3 | 2.99/0.08 |
| 2021 | 30 | 22 | 8 | 26.7 | 89.6 | 6.5 | 10 | 5.98/0.02 | |
| 2022 | 3 | 2 | 1 | 30 | 86 | 4.97 | 0 | 1.1/0.30 | |
| Mean |
13.7 |
9,7 |
4 |
31.4 |
83.2 |
4.97 |
4.3 |
||
| Fyke nets | 2020 | 9 | 7 | 2 | 22.5 | 91 | 6.1 | 0 | 2.63/0.11 |
| 2021 | 28 | 18 | 10 | 35.7 | 81.3 | 4.01 | 0 | 0.36/0.55 | |
| 2022 | 11 | 5 | 6 | 54.6 | 95 | 6.3 | 1 | 16.7/0.001 | |
| Mean | 16 | 10 | 6 | 37.5 | 89.1 | 5.47 | 0.3 |
3.4. Efficiency of long-lines compared to other ichthyological methods
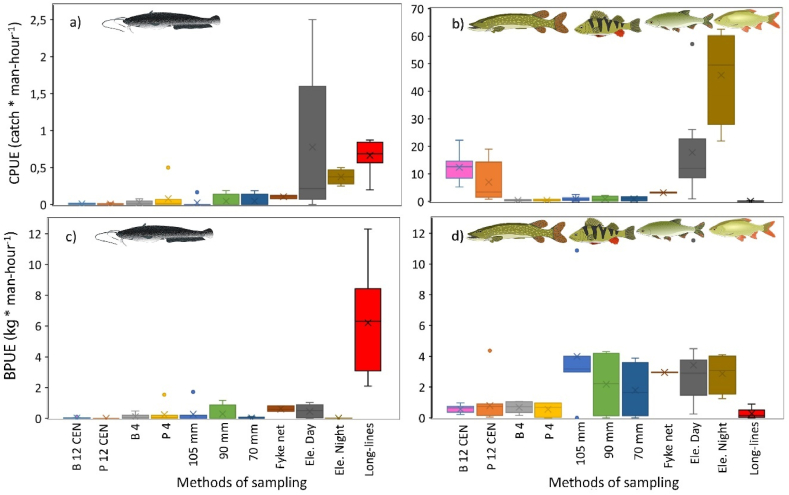
CPUE differed significantly among the methods (ANOVA: F10,80 = 7.43, p < 0.001). The CPUE for different types of gillnets was relatively low, ranging from 0.009 to 0.081 catfish per man-hour (Fig. 6a). Slightly higher efficiency was observed for fyke nets, with an average CPUE of 0.107 catfish per man-hour. Electrofishing demonstrated high values, both during the day (0.777 catfish per man-hour) and at night (0.375 catfish per man-hour). Day electrofishing was significantly more efficient than all types of gillnets (Table S1). High CPUE was recorded for long-lines, with an average value of 0.665 catfish per man-hour (Fig. 6a). Long-lines were significantly more efficient than the gillnets B12; P12; B4; P4 and 105 mm (Table S1).
Fig. 6.
Comparison of the efficiency of fishing methods in terms of CPUE (Catch per Unit Effort, i.e., per 1 man-hour) and BPUE (Biomass per Unit Effort, i.e., per 1 man-hour) for catfish (a, c) and other fish species categorized as by-catch (b, d). The analysis encompasses seven different fishing nets (B 12 CEN, P12 CEN, B4, P4, 105 mm, 90 mm, 70 mm), electrofishing (day and night), fyke nets, and long-lines. The data is presented using box and whisker plots, which illustrate the mean ( × ), upper and lower quartiles (boxes), median values (line within the boxes), maximum and minimum values (whiskers), and outliers (circles).
CPUE of by-catch (i.e., all other fish species) per one man-hour differed significantly among the methods (ANOVA: F10,80 = 19.64, p < 0.001). In contrast, the CPUE of by-catch was significantly higher for electrofishing, averaging 17.784 and 45.865 catch per man-hour during the day and and night, respectively. The day electrofishing CPUE of by-catch was significantly higher than that for long-lines and gillnets B4; P4; 105 mm; 90 mm and 70 mm (Table S1). The night electrofishing CPUE of by-catch was significantly higher than that for all other tested methods, including the day electrofishing. For gillnets, the CPUE of by-catch ranged 12.343–0.408 catch per man-hour, and for fyke nets, it averaged 3.177 catch per man-hour. The long-line CPUE of by-catch was notably low, with a mean value of only 0.098 catch per man-hour, which was significantly lower that that for electrofishing and the B12 gillnets (Fig. 6b).
BPUE also differed significantly among the methods (ANOVA: F10,80 = 25.03, p < 0.001). BPUE for different types of gillnets ranged from 0.002 to 0.274 kg of catfish per man-hour (Fig. 6c). Fyke nets, on average, achieved relatively high values of 0.621 kg of catfish per man-hour. On the other hand, the electrofishing BPUE was relatively low, averaging 0.452 kg of catfish per man-hour during the day (mean mass of caught individual 1.67 kg) and even lower at 0.013 kg of catfish per man-hour during the night (mean mass of caught individual only 0.037 kg). Notably higher values were recorded for the long-lines, where the average BPUE was 6.205 kg of catfish per man-hour (Fig. 6c), and the mean mass of caught catfish was 9.411 kg. The BPUE was significantly higher only for long-lines compared to all other methods (Table S1.)
BPUE of by-catch also differed significantly among the methods (ANOVA: F10,80 = 5.63, p < 0.001). The BPUE of by-catch using gillnets ranged from 0.555 to 3.995 kg per man-hour., specifically the atypical gillnets with diameters of 105, 90, and 70 mm achieved high values in this regard (Fig. 6d; Table S1). Fyke nets achieved in average 3.953 kg per man-hour, and relatively similar values were recorded for electrofishing during the day and night, with averages of 3.428 and 2.878 kg per man-hour, respectively. However, the BPUE of by-catch using long-lines was low, with an average value of 0.276 kg per man-hour (Fig. 6d; Table S1).
4. Discussion
The high catch efficiency and selectivity of long-lines, in general, result from the behavior of European catfish. European catfish inhabit a wide range of aquatic environments, including littoral, epipelagic, and other pelagic zones, and can even be found at depths of up to 60 m [13]. However, European catfish primarily spend the majority of their hunting and movement time in the epipelagic habitat while resting in the littoral zone [8,45]. In contrast, other predators, such as Northern pike or pikeperch, primarily inhabit deeper regions of meso-to bathypelagic habitats [45,46], with Northern pike also occupying littoral habitats [46]. Consequently, long-lines can serve as a suitable selective method for European catfish when placed in epipelagic habitats, setting them apart from other predatory species. Pike was the most common by-catch on the long-lines, particularly at areas with a large pike population exceeding 85 cm TL. The by-catch of Northern pike accounted for up to 20 %, reaching an extreme of 31 % at Milada lake, where the pike population is unusually high [3,46]. The estimated size of the large pike population (based on recaptures) at Milada lake was around 220 individuals, ranging 60–120 cm in size, with a mean mass of 8.9 kg [46]. This translates to a total biomass of 1958 kg in the lake (ind. >60 cm TL, excluding smaller ind.). When scaled to the lake's area, it corresponds to a biomass of 7.83 kg/ha. The biomass of large pike in this context is approximately 22 % greater than the biomass of large catfish (6.1 kg/ha). However, such areas are sparse. Pikes with a size of 60–120 cm are rare at localities with a strong population of catfish, or are completely absent. On average, the by-catch constitutes around 14 %, and this ratio can be further reduced by using bait fish >30 cm TL. Other predatory fish species did not significantly exceed 0.5 % of the catch. At Portuguese sites, there were only two instances of by-catch involving other species: one native barbel (Luciobarbus sp.) and one invasive pikeperch, which is highly abundant in these areas [47,48].
The method's selectivity in freshwater environments offers a significant advantage, unlike in marine environments where long-line fishing poses an existential threat to various species. Many marine creatures, including sharks, sea turtles, seabirds, and marine mammals, suffer as by-catch [31,49]. In freshwater settings, no non-fish species by-catch was recorded. When it comes to fish by-catch, only large predatory fish species were observed, with no non-predatory fish. This stands in stark contrast to other ichthyological methods, such as active and passive net methods and electrofishing, which tend to produce substantial fish by-catch (as supported by our results [26]). Furthermore, the use of gillnets, a frequently employed passive fishing gear, can result in a significant underestimation of European catfish populations [[50], [51], [52]]. The commonly used benthic and pelagic multimesh gillnets, encompassing twelve mesh sizes following EU norms (ranging from 5 mm to 55 mm [37]), are found to be inadequate. Even large-mesh gillnets with mesh sizes of 70, 90, 110, and 135 mm (knot-to-knot, 10 m panels) appear to be insufficient for capturing European catfish effectively [38]. Based on our observations, it appears that the efficiency will increase only slightly. Even the use of atypical gillnets (commonly employed by commercial fishermen in marine environments) with 105-, 90-, and 70-mm knot-to-knot mesh size has not proven to be a very effective method for catching catfish. These gillnets consist of continuous panels, each 40 m in length and 6 m in height, and they also result in a significant number of by-catch. Additionally, our experience indicates that larger catfish individuals significantly prolong the handling time (in hours), as these fish are vital and possess powerful eel-like tails that can move and entangle the net during extended exposure. When trapped overnight, even a 1-m-long fish could completely destroy up to 10 m2 of gillnet. The poor catch efficiency of these nets can be attributed to the European catfish's large cylindrical body shape (resembling a frog-like head), slimy scale-less skin, distinct ability for reverse swimming, and its unconventional ecology and behavior [8,53]. Fyke nets seem to offer a relatively acceptable method for capturing European catfish in terms of CPUE and BPUE. However, even this method results in a significant number of by-catch. Electrofishing appears to be an efficient but non-selective method for capturing catfish, as Carol suggests [54]. Daněk et al. [55] and Guilleault et al. [14] have also considered electrofishing to be the most efficient method. Slavík and Horký [56] have used this method successfully to capture catfish in rivers in the Czech Republic. Thus, electrofishing is generally considered a useful method and is utilized accordingly. However, it does have several disadvantages. It is not efficient at large and deep (>1.5 m) water bodies, such as canyon-shaped reservoirs, deep lakes, or gravel pits, due to the limited reach of the electric current [57]. However, in our experience, this method is particularly effective for significantly smaller (juvenile) individuals and can, therefore, be considered a suitable supplementary method for hunting or reducing the numbers of small individuals. Large individuals are also less attracted by galvanotaxis and can escape when the electrofishing system approaches. They are not drawn to the anodes but tend to sink to great depths from which it is nearly impossible to fish them out, or they may become stuck in obstacles. Capturing and retrieving them, especially getting them onto the boat, is especially challenging. In our experience, long-lines are remarkably more effective, particularly with regard to BPUE, compared to all other tested methods. The catch efficiency at all study sites was consistently high.
Despite the significant differences in the characteristics of the study sites, the effectiveness of the long-lines was very similar. For instance, at Žlutice reservoir, a shallow eutrophic location with the littoral zone rich in vegetation, an average of 5 branch lines were required to catch one European catfish per day. On the other hand, at characteristically different Římov reservoir, with its deep canyon shape and absence of vegetated littoral areas, an average of 6.8 branch lines were needed to catch one European catfish per day. When averaged across nine study sites, it required only 5.6 branch lines to catch one European catfish per day. This allows us to estimate the number of branch lines needed to catch a desired quantity of catfish. The effort required to reduce the entire population at a specific location is discussed by Vejřík et al. [8]. The lower efficiency of the long-lines at three locations on the Tagus River in Portugal, where it took 14.4 branch lines per day to catch one catfish, was likely due to using only dead baits. Our observations suggest that dead baits are only about 1/4 as effective as live baits. Another important factor to consider is that the catfish invasion in these areas occurred relatively recently [5,58], and, therefore, the population of this long-lived fish may not be fully established yet [2,9]. The pronounced preference of indigenous catfish for benthic prey, primarily crayfish, may exert a notable influence [58]. Nonetheless, the potential impact of distinct and insufficiently researched environmental factors such as altitude, latitude, mean temperature, or flow cannot be discounted.
According to Vejřík et al. [8], the majority of European catfish at Czech study sites were primarily caught at night, accounting for over 81 % of catches. In contrast, 75 % of European catfish at Portuguese study sites were caught during the day. Hence, it is essential to consider the variable diurnal behavior of European catfish in different locations and to use the long-lines during both day and night. Seasonal factors [8] and latitudinal aspects also play a significant role.
European catfish primarily locate their prey through water ripples created by the movement of the prey, and possibly, by the scent of the prey [27]. Therefore, our hypothesis was based on the assumption that a shorter version (60 m) of the long-line would yield more catches per one branch line than a longer one (235 m), as baits concentrated in a smaller area would generate more pronounced water ripples and a higher concentration of scent. Although the difference was statistically insignificant, it was slightly more efficient to place branch lines further apart (15 m compared to 5 m). This suggests that an expanded distribution of branch lines, along with coverage over a greater area, enhances the likelihood of a European catfish encountering bait. Consequently, a long-line configuration featuring a 15 m spacing between branch lines proves more efficient compared to a 5 m spacing, particularly when the branch lines are concentrated within a confined area.
The hypothesis that catch efficiency is higher during the first day at a particular location compared to the second day has been confirmed. However, the catch rate remained sufficient during the second day, and the average size of European catfish was larger compared to the first day. This phenomenon is likely caused by the faster metabolism of smaller individuals, which compels them to hunt for prey more actively [2,9], making them more vulnerable to capture at the specific location. Therefore, it is recommended to move the long-line daily if maximum catch efficiency is desired. Furthermore, relocating the long-line to a new site at least a few hundred meters away is advisable because European catfish, in their pursuit of prey, can cover distances ranging from hundreds to thousands of meters per day [45].
The optimal bait size is observed to be approximately 23 % of the desired TL of catch. With a basic understanding of the local catfish population dynamics, selecting the optimal bait size becomes straightforward. In the absence of such knowledge, it is recommended to employ a variety of baits spanning a wide range of sizes. When live fish alternated with dead fish on the long-line, 71 % of catches came from live bait fish. However, it is worth noting that the use of live fish as bait is a controversial issue. While it is common in most parts of the world [59], some European countries, like Germany, Austria, Scotland, Ireland (in freshwater), and Switzerland, have banned it for animal welfare reasons [60]. The debate centers on whether fish can feel pain. Several studies [61] suggest that fish may not have the same nociception range as humans and other mammals, and they are unlikely to experience pain. In the European Union (EU), live-bait fishing was debated, and as of January 10, 2023, it is not banned. EU citizens made the request, but Commission Delegated Regulation (EU) 2020/9907 does not prohibit the use of live bait for fishing. In most EU countries and worldwide, the use of live bait fish remains a personal choice. If live bait fish is allowed by local legislation or personal preference, it is the most effective option; otherwise, catches with dead bait fish are expected to be significantly lower, around one-quarter of those using live bait fish. Earthworms serve as a good alternative bait, with catch rates comparable to or even higher than live fish. However, it appears smaller catfish prefer earthworms, while larger ones may ignore them. In terms of fish species preferences, there was no significant difference, with rudd being the most caught species (39 %) and roach the least (30 %). This preference does not align with their natural diet, where roach predominate [3] and is likely influenced by the behavior of the species in their natural aquatic environment, a behavior that may not be evident when using fish as bait. As a result, it is possible to use any type of fish of a suitable size, provided they are resilient enough to survive on the hook (fragile fish such as bleak (Alburnus alburnus) should be avoided). In the case of Most lake with a significant portion of waterfowl in the catfish diet consists [3], a chicken wing from supermarkets as an alternative bait was tested. However, it proved to be less efficient than dead bait fish. Thus, when using a motionless bait, European catfish clearly prefers the fish possibly due to the smell being the second most important factor in prey detection [27]. Interestingly, red swamp crayfish, a significant part of the European catfish's diet in the Tagus River basin (>50 % [58]), was found to be inefficient for bait. In contrast to their dietary preferences, only 8 % of catches were realized on red swamp crayfish, whereas 92 % on juvenile European catfish as a bait, which is the minor prey of adult European catfish at the study site (3.6 % [58]).
Some studies on various fish species have shown that certain behavioral traits can affect the likelihood of being caught with lures or bait. Fast explorers are more susceptible to capture compared to their more cautious counterparts [28,62]. Additionally, fish with larger home ranges have a higher capture probability [63]. Once caught by angling and released, fish can remember the experience and may exhibit hook avoidance [29,64]. Fish can forget threatening stimuli, with forgetting times ranging from 8 to 55 days without reinforcement [[64], [65], [66]]. However, some species, like the common carp (Cyprinus carpio), can retain this memory for up to six months [29]. This information can help target specific behavioral traits in the catfish population, or conversely, avoid using bait that has previously been used on catfish that were caught before. We aimed to investigate the two phenomena considering the frequency of tagged catfish (individuals being released after previously caught on the long-lines and tagged) caught again by three different methods. The analysis of these data did not reveal a clear trend indicating that the long-lines underestimated the portion of the population more easily caught by other fishing methods. To our knowledge, it remains uncertain whether there is a specific group of catfish individuals that are less likely to be caught on the long-lines. However, it is evident that using alternative catching methods is unlikely to resolve this issue. Regarding the problem of hook avoidance after being caught, our observations suggest that catfish may avoid the hook for several days. Out of a thousand individuals caught, we had only four cases of recapture during a four-day campaign. However, based on the frequency of recaptures using long-lines, electrofishing, and fyke nets, it appears that catfish reliably forget this experience after a few months. In conclusion, it does not seem that the long-lines significantly select a specific catfish subpopulation compared to other methods, nor does the probability of recapturing an individual drop significantly after it has been previously caught on bait.
This study provides a detailed analysis of the most successful method for capturing adult European catfish, a challenging fish predator [26]. It describes and evaluates the various characteristics and features that enhance the catch efficiency. European catfish have become invasive in many areas, posing a significant threat to native aquatic fauna [2]. As such, this method serves as an ideal tool for regulating, reducing, and potentially eradicating European catfish populations in non-native environments [8]. The method is highly effective for capturing adult individuals, which are the primary threat to aquatic ecosystems. Additionally, it addresses individuals involved in reproductive cycles, helping to control and reduce catfish numbers and biomass in these areas. For reducing juvenile catfish, electrofishing proves to be an effective complementary method. Electrofishing can be used in conjunction with the long-lines, not only for targeting smaller individuals but also for obtaining bait fish, whether alive or dead. Using bait fish from the local catch site reduces the risk of introducing foreign pathogens to different areas [59]. In native habitats where European catfish presence is desired, the long-lines can be employed to capture a sufficient number of individuals for scientific or conservation purposes.
Data availability
All data to support the conclusions have been provided as the Supplementary material.
CRediT authorship contribution statement
Lukáš Vejřík: Conceptualization, Investigation, Methodology, Statistics, Visualization, Writing original draft, Resources. Ivana Vejříková: Investigation, Writing original draft. Petr Blabolil: Investigation, Statistics, Writing—review & editing. Daniel Bartoň: Investigation, Writing—review & editing. Zuzana Sajdlová: Investigation, Visualization, Writing—review & editing. Luboš Kočvara: Investigation, Writing—review & editing. Jiří Peterka: Investigation, Resources, Writing—review & editing. Milan Muška: Investigation, Writing—review & editing. Jindřich Duras: Resources, Writing—review & editing. Tomáš Jůza: Investigation, Writing—review & editing. Filipe Ribeiro: Investigation, Resources, Writing—review & editing. Rui Rivaes: Investigation. Diogo Ribeiro: Investigation. Beatriz Castro: Investigation. Mafalda Moncada: Investigation. Martin Čech: Investigation, Resources, Writing—review & editing.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, IV used ChatGPT 3.5 in order to improve the language of 813 certain sections of the text. After using this tool/service, the author reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the Fundação para a Ciência e a Tecnologia through the projects PTDC/ASP-PES/4181/2021 – Megapredator, UIDP/04292/2020 (doi.org/10.54499/UIDP/04292/2020), UIDB/04292/2020 (doi.org/10.54499/UIDB/04292/2020), awarded to MARE and through project LA/P/0069/2020 (doi.org/10.54499/LA/P/0069/2020) granted to the Associate Laboratory ARNET. Further the study was supported by the European Commission within the program of the LIFE21-NAT/IT/PREDATOR (project No. 101074458 – Life Predator), by the European Union within ESIF in frame of Operational Programme Research, Development and Education (project no. CZ.02.1.01/0.0/0.0/16_025/0007417), by the Applied Research Program of the Ministry of Agriculture – project” Methodology of predatory fish quantification in drinking-water reservoirs to optimize the management of aquatic ecosystems” (No. QK1920011), by the Czech National Agency of Agricultural Research (No. QK22020134 Innovative fisheries management of a large reservoir), and by the Czech Academy of Sciences within the program of the Strategy AV 21 (project No. RP21 – Land conservation and restoration).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34125.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Boulêtreau S., Santoul F. The end of the mythical giant catfish. Ecosphere. 2016;7 [Google Scholar]
- 2.Cucherousset J., Horký P., Slavík O., Ovidio M., Arlinghaus R., Bouletreau S., Britton R., Garcia-Berthou E., Santoul F. Ecology, behaviour and management of the European catfish. Rev. Fish Biol. Fish. 2018;28:177–190. [Google Scholar]
- 3.Vejřík L., Vejříková I., Blabolil P., Eloranta A.P., Kočvara L., Peterka J., Sajdlová Z., Chung S.H.T., Šmejkal M., Kiljunen M., Čech M. European catfish (Silurus glanis) as a freshwater apex predator drives ecosystem via its diet adaptability. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-16169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britton J.R., Cucherousset J., Davies G.D., Godard M.J., Copp G.H. Non-native fishes and climate change: predicting species responses to warming temperatures in a temperate region. Freshw. Biol. 2010;55:1130–1141. [Google Scholar]
- 5.Gkenas C., Gago J., Mesquita N., Alves M.J., Ribeiro F. First record of Silurus glanis Linnaeus, 1758 in Portugal (Iberian Peninsula) J. Appl. Ichthyol. 2015;31:756–758. [Google Scholar]
- 6.Cunico A.M., Vitule J.R.S. First records of the European catfish, Silurus glanis Linnaeus, 1758 in the Americas (Brazil) Bioinvasions Rec. 2014;3:117–122. [Google Scholar]
- 7.Vejřík L., Vejříková I., Peterka J., Sajdlová Z., Čech M. In: Advances in Animal Science and Zoology. Jenkins Owen P., editor. Nova Science Pub.; 2019. Area of catfish occurrence and risks connected with introductions to new localities; pp. 135–142. ISBN: 978-1-53616-048-2. [Google Scholar]
- 8.Vejřík L., Vejříková I., Kočvara L., Blabolil P., Peterka J., Sajdlová Z., Jůza T., Šmejkal M., Kolařík T., Kubečka J., Bartoň D., Čech M. The pros and cons of the invasive freshwater apex predator, European catfish Silurus glanis, and powerful angling technique for its population control. J. Environ. Manag. 2019;241:374–382. doi: 10.1016/j.jenvman.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Copp G.H., Britton R., Cucherousset J., García-Berthou E., Kirk R., Beeler E., Skaténas S. Voracious invader or benign feline? A review of the environmental biology of European catfish Silurus glanis in its native and introduced ranges. Fish Fish. 2009;10:252–282. [Google Scholar]
- 10.De Santis V., Volta P. Spoiled for choice during cold season? Habitat use and potential impacts of the invasive Silurus glanis L. in a deep, large, and oligotrophic lake (Lake Maggiore, North Italy) Water. 2021;13:2549. [Google Scholar]
- 11.Vagnon C., Bazin S., Cattanéo F., Goulon C., Guillard J., Frossard V. The opportunistic trophic behaviour of the European catfish (Silurus glanis) in a recently colonised large peri-alpine lake. Ecol. Freshw. Fish. 2022;31:650–661. [Google Scholar]
- 12.Westrelin S., Boulêtreau S., Santoul F. European catfish Silurus glanis behaviour in response to a strong summer hypoxic event in a shallow lake. Aquat. Ecol. 2022;56:1127–1142. [Google Scholar]
- 13.Antognazza C.M., Costantini T., Campagnolo M., Zaccara S. One year monitoring of ecological interaction of Silurus glanis in a novel invaded oligotrophic deep lake (Lake Maggiore) Water. 2022;14:105. [Google Scholar]
- 14.Guillerault N., Delmotte S., Bouletreau S., Lauzeral C., Poulet N., Santoul F. Does the non-native European catfish Silurus glanis threaten French river fish populations? Freshw. Biol. 2015;60:922–928. [Google Scholar]
- 15.Castaldelli G., Pluchinotta A., Milardi M., Lanzoni M., Giari L., Rossi R., Fano E.A. Introduction of exotic fish species and decline of native species in the lower Po basin, north-eastern Italy. Aquat. Conserv. Mar. Freshw. Ecosyst. 2013;23:405–417. [Google Scholar]
- 16.Šmejkal M., Ricard D., Sajdlová Z., Čech M., Vejřík L., Blabolil P., Vejříková I., Prchalová M., Vašek M., Souza A.T., Brönmark C., Peterka J. Can species-specific prey responses to chemical cues explain prey susceptibility to predation? Ecol. Evol. 2018;8:4544–4551. doi: 10.1002/ece3.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulêtreau S., Gaillagot A., Carry L., Tétard S., De Oliveira E., Santoul F. Adult Atlantic salmon have a new freshwater predator. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillerault N., Boulêtreau S., Santoul F. Predation of European catfish on anadromous fish species in an anthropised area. Mar. Freshw. Res. 2018;70:682–686. [Google Scholar]
- 19.Boulêtreau S., Fauvel T., Laventure M., Delacour R., Bouyssonnié W., Améraz F., Santoul F. ‘The giants’ feast’’: predation of the large introduced European catfish on spawning migrating allis shads. Aquat. Ecol. 2021;55:75–83. [Google Scholar]
- 20.Boulêtreau S., Carry L., Meyer E., Filloux D., Menchi O., Mataix V., Santoul F. High predation of native sea lamprey during spawning migration. Sci. Rep. 2020;10:6122. doi: 10.1038/s41598-020-62916-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vejřík L., Vejříková I., Kočvara L., Sajdlová Z., Chung S.H.T., Šmejkal M., Peterka J., Čech M. Thirty-year-old paradigm about unpalatable perch egg strands disclaimed by the freshwater top-predator, the European catfish (Silurus glanis) PLoS One. 2017;12 doi: 10.1371/journal.pone.0169000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milardi M., Green A.J., Mancini M., Trotti P., Kiljunen M., Torniainen J., Castaldelli G. Invasive catfish in northern Italy and their impacts on waterbirds. NeoBiota. 2022;72:109–128. [Google Scholar]
- 23.Guadiamar S.W. Spain and its implications for long-term contaminant fluxes to the Doñana wetlands. Sci. Total Environ. 2008;394:144–161. doi: 10.1016/j.scitotenv.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Syväranta J., Cucherousset J., Kopp D., Crivelli A., Céréghino R., Santoul F. Dietary breadth and trophic position of introduced European catfsh Silurus glanis in the River Tarn (Garonne River basin), southwest France. Aquat. Biol. 2010;8:137–144. [Google Scholar]
- 25.V. De Santis, E. Eckert, D. Fontaneto, F. Ribeiro, F. Magalhães, J. Martelo, L. Vejřík, M. Čech, S. Brignone, P. Volta, (Accepted) LIFE PREDATOR: PREvent, Detect, combAT the Spread of SiluRus glanis in South European Lakes to Protect Biodiversity. NeoBiota.
- 26.Vejřík L., Vejříková I., Peterka J., Čech M. In: Advances in Animal Science and Zoology. Jenkins Owen P., editor. Nova Science Pub.; 2019. Methods for capturing catfish and potential regulation of catfish population; pp. 135–142. ISBN: 978-1-53616-048-2. [Google Scholar]
- 27.Pohlmann K., Atema J.W., Breithaupt T. The importance of the lateral line in nocturnal predation of piscivorous catfish. J. Exp. Biol. 2004;207:2971–2978. doi: 10.1242/jeb.01129. [DOI] [PubMed] [Google Scholar]
- 28.Alós J., Palmer M., Rosselló R., Arlinghaus R. Fast and behavior-selective exploitation of a marine fish targeted by anglers. Sci. Rep. 2016;6 doi: 10.1038/srep38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czapla P., Wallerius M.L., Monk C.T., Cooke S.J., Arlinghaus R. Reexamining one-trial learning in common carp (Cyprinus carpio) through private and social cues: No evidence for hook avoidance lasting more than seven months Fish. Res. 2023;259 [Google Scholar]
- 30.Mytilineou C., Smith C., Anastasopoulou A., Papadopoulou K., Christidis G., Bekas P., Kavadas S., Dokos J. New cold-water coral occurrences in the Eastern Ionian Sea: results from experimental long line fishing. Deep-Sea Res. II: Top. Stud. Oceanogr. 2014;99:146–157. [Google Scholar]
- 31.Petrossian G.A., Rolf A., De By R.A., Clarke R.V. Illegal long-line fishing and albatross extinction risk. Oryx. 2018;52:336–345. [Google Scholar]
- 32.Alp A., Kara C., Buyukcapar H.M. Reproductive biology in a native European catfish, Silurus glanis L., 1758, population in Menzelet Reservoir. Turkish J. Vet. Anim. 2003;28:613–622. [Google Scholar]
- 33.Abdullayev M.A., Khakberdiyev B., Urchinov D. Biology of the European catfish (Silurus glanis) from lakes in the lower reaches of the Zarafshan river and Khorezm Province. J. Ichthyol. 1978;17:487–491. [Google Scholar]
- 34.Omarov O.P., Popova O.A. Feeding behavior of pike, Esox lucius, and catfish, Silurus glanis, in the Arakum reservoirs of Dagestan. J. Ichthyol. 1985;25:25–36. [Google Scholar]
- 35.Monk C.T., Chéret B., Czapla P., Hühn D., Klefoth T., Eschbach E., Hagemann R., Arlinghaus R. Behavioural and fitness effects of translocation to a novel environment: whole-lake experiments in two aquatic top predators. J. Anim. Ecol. 2020;89:2325–2344. doi: 10.1111/1365-2656.13298. [DOI] [PubMed] [Google Scholar]
- 36.Reis İ., Cerim H. Method and technical characteristics of traditional river longline from Lower Sakarya River Fishery, Turkey. J. Limnol. Fish. Res. 2020;6:164–168. [Google Scholar]
- 37.CEN . European Committee for Standardization; Brussels: 2015. Water Quality - Sampling of Fish with Multimesh Gillnets. EN 14757. [Google Scholar]
- 38.Šmejkal M., Ricard D., Prchalová M., Říha M., Muška M., Blabolil P., Čech M., Vašek M., Jůza T., Monteoliva Herreras A., Encina L., Peterka J., Kubečka J. Biomass and abundance biases in European standard gillnet sampling. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vejříková I., Vejřík L., Syväranta J., Kiljunen M., Čech M., Blabolil P., Vašek M., Sajdlová Z., Chung S., Šmejkal M., Frouzová J., Peterka J. Distribution of herbivorous fish is frozen by low temperature. Sci. Rep. 2016;6 doi: 10.1038/srep39600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vejříková I., Vejřík L., Čech M., Říha M., Peterka J. Succession of submerged vegetation in a hydrologically reclaimed opencast mine during first ten years. Restor. Ecol. 2022;30 [Google Scholar]
- 41.Vejřík L., Vejříková I., Blabolil P., Sajdlová Z., Kočvara L., Kolařík T., Bartoň D., Jůza T., Šmejkal M., Peterka J., Čech M. Trophic position of the species and site trophic state affect diet niche and individual specialization: from apex predator to herbivore. Biology. 2023;12:1113. doi: 10.3390/biology12081113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Draštík V., Kubečka J., Čech M., Frouzová J., Řiha M., Jůza T., Tušer M., Jarolím O., Prchalová M., Peterka J., Vašek M., Kratochvíl M., Matěna J., Mrkvička T. Hydroacoustic estimates of fish stocks in temperate reservoirs: day or night surveys? Aquat. Living Resour. 2009;22:69–77. [Google Scholar]
- 43.R Core Team, R . The R Foundation for Statistical Computing; Vienna, Austria: 2020. A Language and Environment for Statistical Computing. [Google Scholar]
- 44.Chung Y., Rabe-Hesketh S., Dorie V., Gelman A., Liu J. “A nondegenerate penalized likelihood estimator for variance parameters in multilevel models. Psychometrika. 2013;78:685–709. doi: 10.1007/s11336-013-9328-2. [DOI] [PubMed] [Google Scholar]
- 45.Říha M., Rabaneda-Bueno R., Jarić I., Souza A.T., Vejřík L., Draštík V., Blabolil P., Holubová M., Jůza T., Gjelland K.Ø., Rychtecký P., Sajdlová Z., Kočvara L., Tušer M., Prchalová M., Seďa J., Peterka J. Seasonal habitat use of three predatory fishes in a freshwater ecosystem. Hydrobiologia. 2022;849:3351–3371. [Google Scholar]
- 46.Říha M., Gjelland K.Ø., Děd V., Eloranta A.P., Rabaneda-Bueno R., Baktoft H., Vejřík L., Vejříková I., Draštík V., Šmejkal M., Holubová M., Jůza T., Rosten C., Sajdlová Z., Økland F., Peterka J. Contrasting structural complexity differentiate hunting strategy in an ambush apex predator. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-96908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gago J., Neves A., Gkenas C., Ribeiro D., Ribeiro F. Condition and size of the non‐native pikeperch Sander lucioperca (Linnaeus, 1758) in Portuguese river basins. Ecol. Evol. 2021;11:5065–5074. doi: 10.1002/ece3.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribeiro D., Gkenas C., Gago J., Ribeiro F. Variation in diet patterns of the invasive top predator Sander lucioperca (Linnaeus, 1758) across Portuguese basins. Water. 2021;13:2053. [Google Scholar]
- 49.Werner T.B., Northridge S., Press K.M., Young N. Mitigating bycatch and depredation of marine mammals in longline fisheries. ICES J. Mar. Sci. 2015;72:1576–1586. [Google Scholar]
- 50.Blabolil P., Boukal D.S., Ricard D., Kubečka J., Říha M., Vašek M., Prchalová M., Čech M., Frouzová J., Jůza T., Muška M., Tušer M., Draštík V., Šmejkal M., Vejřík L., Peterka J. Optimal gillnet sampling design for the estimation of fish community indicators in heterogeneous freshwater ecosystems. Ecol. Indicat. 2017;77:368–376. [Google Scholar]
- 51.Blabolil P., Čech M., Draštík V., Holubová M., Kočvara L., Kubečka J., Muška M., Prchalová M., Říha M., Sajdlová Z., Šmejkal M., Tušer M., Vašek M., Vejřík L., Vejříková I., Peterka J., Jůza T. Less is more - basic quantitative indices for fish can be achieved with reduced gillnet sampling. Fish. Res. 2021;240 [Google Scholar]
- 52.Jůza T., Blabolil P., Čech M., Draštík V., Frouzová J., Sajdlová Z., Holubová M., Kočvara L., Kolařík T., Moraes K.R., Muška M., Souza T., Vašek M., Říha M., Tušer M., Šmejkal M., Peterka J., Prchalová M., Kubečka J. Fish stock mass reduction is indicated in standard abundance and biomass estimates from gillnets and hydroacoustics. Fish. Res. 2022;253 [Google Scholar]
- 53.Slavík O. CZ. Czech University of Life Sciences; Prague: 2013. (Behaviour of European Catfish in Natural Conditions and Aquaculture (Habilitation Thesis)). [Google Scholar]
- 54.Carol J. 2007. Ecology of invasive (Silurus glanis) in Catalan reservoirs. Ph.D. Thesis. Universitat de Girona, Spain. [Google Scholar]
- 55.Daněk T., Kalous L., Petrtýl M., Horký P. Move or die: change in European catfish (Silurus glanis L.) behaviour caused by oxygen deficiency. Knowl. Manag. Aquat. Ecosyst. 2014;414:1–11. [Google Scholar]
- 56.Slavík O., Horký P. Diel dualism in the energy consumption of the European catfish Silurus glanis. J. Fish. Biol. 2012;81:2223–2234. doi: 10.1111/j.1095-8649.2012.03436.x. [DOI] [PubMed] [Google Scholar]
- 57.Zalewski M., Cowx I.G. In: Fishing with Electricity – Application in Freshwater Fisheries Management. Cowx I.G., Lamarque P., editors. Blackwell; Oxford, UK: 1989. Factors affecting the efficiency of electrofishing; pp. 89–110. [Google Scholar]
- 58.Ferreira M., Gago J., Ribeiro F. Diet of European catfish in a newly invaded region. Fishes. 2019;4:58. [Google Scholar]
- 59.McEachran M.C., Mladonicky J., Picasso-Risso C., Drake D.A.R., Phelps N.P.D. Release of live baitfish by recreational anglers drives fish pathogen introduction risk. Prev. Vet. Med. 2023;217 doi: 10.1016/j.prevetmed.2023.105960. [DOI] [PubMed] [Google Scholar]
- 60.Ferter K., Steven J.C., Humborstad O.-B., Nilsson J., Arlinghaus R. In: The Welfare of Fish, Animal Welfare 20. Kristiansen T.S., et al., editors. Springer; 2020. Fish welfare in recreational fishing. [Google Scholar]
- 61.Rose J.D., Arlinghaus R., Cooke S.J., Diggles B.K., Sawynok W., Stevens E.D., Wynne C.D.L. Can fish really feel pain? Fish Fish. 2014;15:97–133. [Google Scholar]
- 62.Härkönen L., Hyvärinen P., Niemelä P.T., Vainikka A. Behavioural variation in Eurasian perch populations with respect to relative catchability. Acta Ethol. 2016;19:21–31. [Google Scholar]
- 63.Alós J., Palmer M., Arlinghaus R. Consistent selection towards low activity phenotypes when catchability depends on encounters among human predators and fish. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Croy M.I., Hughes R.N. The role of learning and memory in the feeding behaviour of the fifteen-spined stickleback, Spinachia spinachia L. Anim. Behav. 1991;41:149–159. [Google Scholar]
- 65.Kimber J.A., Sims D.W., Bellamy P.H., Gill A.B. Elasmobranch cognitive ability: using electroreceptive foraging behaviour to demonstrate learning, habituation and memory in a benthic shark. Anim. Cognit. 2014;17:55–65. doi: 10.1007/s10071-013-0637-8. [DOI] [PubMed] [Google Scholar]
- 66.Zion B., Barki A., Grinshpon J., Rosenfeld L., Karplus I. Retention of acoustic conditioning in St Peter's fish Sarotherodon galilaeus. J. Fish. Biol. 2011;78:838–847. doi: 10.1111/j.1095-8649.2010.02899.x. [DOI] [PubMed] [Google Scholar]
- 67.Vejřík L., Vejříková I., Blabolil P. The use of long lines to determine the population structure of European catfish. Biology Centre of the Czech Academy of Sciences, v. v. i., Institute of Hydrobiology. 2020:26. IN CZECH. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data to support the conclusions have been provided as the Supplementary material.