Abstract
Purpose
Venous leg ulcers (VLUs) are prevalent chronic wounds with limited treatment options. This study aimed to investigate the potential of berberine to enhance endothelial progenitor cell (EPC) function in VLU healing.
Methods
Histopathological changes and inflammatory cytokine levels in a deep venous thrombosis (DVT) mouse model were assessed using HE staining and ELISA assays. A luciferase reporter assay was employed to identify the miR-21-3p and RRAGB targeting relationship. EPC proliferation, migration, and tube formation were evaluated through CCK-8, Transwell, and tubule formation assays, while the mTOR pathway and autophagy-related proteins were analyzed by immunofluorescence staining and western blotting.
Results
Berberine significantly improved EPC functions, such as proliferation, migration, and tube formation in vitro, and enhanced in vivo EPC-mediated wound healing in a DVT mouse model. Furthermore, miR-21-3p was downregulated in EPCs from VLU patients, and its overexpression improved model EPC functions. Mechanistically, RRAGB, which regulates the mTOR pathway, was identified as a potential miR-21-3p target in EPCs. Overexpression of RRAGB inhibited autophagic activity and impaired EPC function.
Conclusion
Berberine shows promise in ameliorating EPC function and promoting wound healing in VLUs. The regulation of the miR-21-3p/RRAGB axis by berberine could offer a promising therapeutic approach for managing VLUs.
Keywords: Endothelial progenitor cells, Berberine, miR-21-3p, RRAGB
1. Introduction
Venous leg ulcers (VLUs) are a prevalent and challenging clinical problem, characterized by non-healing wounds in the lower extremities. They often occur in individuals with chronic venous insufficiency, leading to impaired venous circulation and compromised tissue perfusion [1]. According to statistics, VLU is responsible for roughly 70% of chronic lower limb ulcers and it afflicts about 1% adult population worldwide [[2], [3], [4]]. VLUs can significantly impact patients' quality of life, causing pain, disability, and prolonged healing times. Despite advances in wound care, effective treatment options for VLUs remain limited, highlighting the need for novel therapeutic strategies.
Endothelial progenitor cells (EPCs) play a vital role in angiogenesis and wound healing, as they possess the unique ability to differentiate into mature endothelial cells, promoting the formation of new blood vessels and facilitating tissue repair [5,6]. Studies have indicated that dysfunction and a decrease in circulating EPC numbers are observed in obesity and type 2 diabetes, both of which are major risk factors for VLU [[7], [8], [9]]. Notably, Tecilazich et al. found that EPCs were reduced in diabetic patients at risk for or with active diabetic foot ulcers, suggesting a correlation between reduced EPC and the complete wound healing of foot ulcers [10]. In a recent phase II clinical study, centrifuged adipose tissue containing progenitor cells effectively treated non-healing VLU, confirming the therapeutic potential of EPCs in VLU treatments [11]. Despite these associations, direct evidence concerning EPC function in VLU remains limited. Moreover, the impaired function of EPCs in metabolic diseases suggests that alterations in energy metabolism may regulate EPC behavior during the development of such diseases. Autophagy, a conserved cellular process crucial for maintaining cellular energy balance and recycling biomacromolecules, is dysregulated in numerous metabolic disorders [12]. For instance, EPCs exposed to high glucose exhibit impaired autophagy, while correcting EPCs' autophagic flux proves cytoprotective against hypoxia [13]. However, the precise role of autophagy in EPC function during VLU remains unclear.
Although most VLU patients can get healed after a course of around 3-month treatment, new therapeutic interventions against VLU are needed for a better control of this disease, including the reduction of the severity of wound-associated pain and itching [14]. Berberine, a natural isoquinoline alkaloid derived from various medicinal plants, has shown diverse pharmacological properties, including anti-inflammatory, antioxidant, and anti-microbial effects [15,16]. Recent studies have demonstrated the potential of berberine in promoting wound healing in different contexts [17,18]. However, its specific role in ameliorating EPC function and facilitating wound healing in VLUs has not been extensively investigated. In light of the above, this study aims to explore the therapeutic potential of berberine in improving EPC function and enhancing wound healing in VLUs. Furthermore, we seek to investigate the underlying molecular mechanisms involving autophagy pathway.
2. Materials and methods
2.1. Ethical clearance
Written informed consent was obtained from all patients and the study protocol was approved by the Committee on the Ethics of Animal Experiments and Human Subject Research of Jiangsu Province Hospital of Chinese Medicine (Ethical approval number: 2022NL-196-02; Approval date: November 1, 2022). All animal experiments were conducted in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals.
2.2. Isolation of EPCs from the blood of healthy subjects and VLU patients
Fifty (50) mL of peripheral blood samples were drawn from the healthy subjects and VLU patients at our hospital. Next, the peripheral blood was diluted with equal volume of phosphate buffered saline (PBS; Hyclone, Logan, UT, USA), and then isolated with the Ficoll-Paque PLUS solution (Cat#17-1440-03, GE Healthcare, NY, USA). The buffy coat cells were aspirated and washed twice with PBS, and then centrifuged at 1500 rpm/min for 8 min to obtain the monocytes. Next, the monocytes were resuspended in M199 medium (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (FBS; Sigma, USA) and seeded at a concentration of 1 × 106 cells to a 25 cm2 cell culture flask pre-coated with fibronectin (5 μg/cm2). The culture medium was changed every 3 days before cell density reached to ∼90% confluence. During the culture period, the morphological changes of adherent cells was routinely observed under an inverted phase contrast microscope every day.
2.3. Flow cytometric identification of cell phenotypes
The isolated EPCs were treated with 0.25% trypsin and then suspended in PBS buffer. After passing through a 100-μm cell sieve, the cells were counted, and their concentration was adjusted to 1 × 106 cells/mL. In each flow cytometry tube, the cell solution was combined with 5 μL of flow cytometry grade antibodies, specifically (B303115; BioLegend, USA) for one, CD34 (2172937; Invitrogen, USA) for another, and CD309 antibody (B287854; BioLegend, USA) for the third. Following the incubation, the cells were washed with 10 mL of PBS buffer, centrifuged, and the supernatant was discarded. Subsequently, 200 μL of PBS buffer was added to each tube to resuspend the cells. Finally, 200 μL of sheath fluid was added, thoroughly mixed, and the samples were loaded for detection.
2.4. CCK-8 assay
The proliferation of EPCs was assessed using the Cell Counting Kit-8 assay (Biyuntian, Shanghai, China). Cells were seeded at a density of 1 × 104 cells per well in 96-well plates and cultured for 48 h, respectively. After the designated incubation periods, 10 μl of CCK-8 (Dojindo, Kumamoto, Japan) was added to each well, and the cells were cultured for an additional 2 h. To measure the cell viability, the optical density (OD) values at 450 nm were determined for each well using the Multiskan Go Spectrophotometer (Thermo Fisher Scientific, USA).
2.5. Transwell migration assay
EPCs at a concentration of 1 × 105 cells/ml were seeded in the Transwell upper chamber (Corning, USA) with 200 μL of serum-free medium. Following that, 600 μL of M199 medium containing 10% FBS was added to the lower chamber, and the cells were incubated for 24 h to allow for migration. After the incubation period, the upper chambers were fixed with 4% polymethanol for 30 min and then stained with 0.1% crystal violet for another 30 min. Subsequently, the cells that had successfully migrated through the membrane were visualized and photographed using an optical microscope (Shanghai Leiden Information Technology Co., Ltd., China).
2.6. PCR
MiRNA was extracted and converted into cDNA using the miRneasy Mini kit (Qiagen, USA) and miScript II RT kit (Qiagen), respectively. For total RNA extraction, the TRIzol reagent (Invitrogen) was employed, followed by reverse transcription using the cDNA Synthesis Kit (Invitrogen). Furthermore, qRT-PCR was carried out to assess the levels of miRNA. The mRNA analysis used the SYBR qPCR Master Mix (Vazyme, USA), and qRT-PCR reactions were conducted on the Bio-Rad CFX96 System (Hercules, USA). To normalize miRNA expression levels, U6 was used as reference genes. The 2−ΔΔCt method was employed to calculate the relative expression levels. The primer sequences used are as follows: miR-21-3p, forward 5′-GAATTCGCCTCAAGAGAACAAAGTGGAG-3′ and reverse 5′-AGATCTCCCATGGGGGCTCAGCCCCT-3′; U6, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse 5′-CGCTTCACGAATTTGCGTGTCAT-3′.
2.7. Cell transfection
To achieve RRAGB overexpression, the cDNA of RRAGB was cloned into the mammalian expression vector pcDNA3.1 (Invitrogen, USA) to create the overexpressed vectors (RRAGB-OE). As a negative control (NC), the empty pcDNA3.1 vector was used. To upregulate miR-21-3p expression, miR-21-3p mimic (miR-21-3p mimics) and a corresponding negative control (miR-21-3p NC) were obtained from SwitchGear Genomics (USA). EPCs were transfected with the aforementioned plasmids for 48 h using Lipofectamine 2000 (Invitrogen, USA), following the manufacturer's protocol. The transfection efficiency was assessed 48 h after transfection.
2.8. Western blotting
The total protein content was extracted using RIPA buffer (Millipore, USA) and quantified using the BCA kit (Solarbio, China). The proteins were then separated by 12% SDS-PAGE and transferred to PVDF membranes. Following this, the membranes were blocked with 5% BSA for 1 h at 37 °C. Next, the membranes were incubated overnight at 4 °C with primary antibodies against RRAGB (diluted 1:1000; ab160946, Abcam, USA), mTOR (diluted 1:10000; ab134903, Abcam, USA), p-mTOR (diluted 1:1000; ab109268, Abcam, USA), LC3A/B (diluted 1:1000; ab62721, Abcam, USA), and GAPDH (diluted 1:1000; ab181602, Abcam, USA). After incubation with the primary antibodies, the membranes were further incubated with an HRP-conjugated secondary antibody (1:2000, Cat#: ab6721, Abcam) for 1 h. The protein bands were visualized using an enhanced chemiluminescence (ECL) kit (Shanghai Lianmai Bioengineering Co., Ltd., China) for 5 min. Finally, grayscale analysis and quantification of the protein bands were performed using Image J software (version 1.2; National Institutes of Health, USA).
2.9. Dual-luciferase reporter assay
The miR-21-3p mimics, pmirGLO-RRAGB-3′UTR-WT, and pmirGLO-RRAGB-3′UTR-MUT (Suzhou Zima Gene, China) were transfected into HEK293T cells (Chinese Academy of Sciences, China) and incubated for 24 h before harvesting for the dual luciferase reporter gene assay. To perform the assay, 10 μl of cell lysate from each sample was used to measure the relative luciferase activity, employing the detection kit (Promega E1910). After 48 h of transfection, the detection of luciferase activity was carried out using a luminescence detector (Molecular Devices SpectraMax® i3).
2.10. Immunofluorescence staining assay
After rinsing the EPCs with PBS, they were fixed using 4% paraformaldehyde for 15 min. Subsequently, the cells were treated with 0.1% Triton X-100 for 15 min at room temperature (RT) to enhance permeability. Next, the cells were blocked with 5% BSA at RT for 30 min before being incubated overnight at 4 °C with the primary anti-LC3B antibody (diluted 1:200; ab192890, Abcam, USA). On the following day, a goat anti-rabbit FITC-conjugated secondary antibody (diluted 1:500; Jackson Immunoresearch, USA) was added and allowed to incubate at RT for 1 h. Meanwhile, the nuclei were counter-stained with Hoechst 33342 (C1022, Beyotime, Shanghai, China) for 15 min at RT under protection from light. The relative fluorescence intensity was analyzed using Image J (NIH Image J system, Bethesda, MD, USA).
2.11. Tubule formation assay
Matrigel (BD Biosciences, USA) was dissolved overnight, and the 24-well plates were pre-cooled at 4 °C during the same period. The following day, the Matrigel was spread onto an ice box and added to the pre-cooled 24-well plates at 250 μL per well. As the gel solidified during incubation at 37 °C, a cell suspension was prepared, and its concentration was adjusted to 2 × 105 cells/mL after digestion. The cells were then seeded into each well and placed in an incubator for 6 h. After this period, Calcein AM (0.25 μg/mL; C7600; Solarbio, Beijing, China) was added for staining, and the cells were incubated for an additional 30 min. Subsequently, the calcein staining was observed and captured using a fluorescence microscope (Olympus BX 60, Japan).
2.12. Deep venous thrombosis (DVT) model
Male SPF Kunming mice, six weeks old (n = 3), were acquired from Qinglongshan Animal Center (Nanjing, China). The mice were housed in a controlled environment with constant room temperature and provided with unrestricted access to food and water. For the experiments, the mice were anesthetized through intraperitoneal injection of 3% pentobarbital sodium. Subsequently, the left thighs of the mice were shaved, and they were positioned in a supine posture. A longitudinal incision was made on the inner side of the left groin skin, exposing the iliac femoral vein at a distance of 1.0 cm from the incision site. The femoral vein was temporarily blocked using mosquito clamps for 2–3 s, with sutures placed at its proximal and distal ends. The mosquito clamps were then released, and the incision was sutured. After 24 h from the operation, the mosquito clamp was removed.
2.13. HE staining
The thrombus tissue was fixed in a 4% paraformaldehyde solution at room temperature for 72 h, and subsequently embedded in paraffin. Sections with a thickness of 5 μm were cut from the tissue and subjected to deparaffinization by treating with xylene for 30 min. Then, the sections were treated with ethanol using different concentration gradients before being stained with hematoxylin and eosin at room temperature. After the staining process, the slices were sealed following treatment with xylene. Finally, the samples were observed and photographed under an optical microscope (Nikon, Tokyo, Japan).
2.14. Skin injury mouse model
After a 3-day modeling period, the outer hair of the left thigh of the mice was carefully trimmed and removed under general anesthesia. The mice were then positioned on their stomachs, and the local skin was routinely disinfected. A sterile hole towel was placed, and the modeling site was marked using a 1.5 cm diameter punch. Subsequently, a circular skin defect with a 1.5 cm diameter was created by excising the entire layer of skin at the marked position. In the Model group, mice were injected subcutaneously in the mouse back skin with 100 μL of sterile PBS, while the remaining mice in the other two groups received injections of 5 × 105 EPCs treated with either the vehicle or berberine. The mice's skin lesions and general behaviors were closely monitored, and photographs were taken at 0, 3, 5, and 10 days after the procedure. The wound area was determined using Image J software to calculate the healing rate, which was defined as follows: wound healing rate = (initial area - unhealed area)/initial area × 100%.
2.15. Statistical analysis
The data in this study are presented as mean ± SEM. GraphPad Prism software (version 8.0, GraphPad Software, USA) was utilized for graph generation. Two-Way ANOVA, followed by Tukey's post hoc test, was employed to analyze multiple groups, while a two-tailed unpaired t-test was used for analyzing two groups. Statistical significance was determined by a p-value less than 0.05.
3. Results
Berberine promotes the proliferation, migration and tubular formation of EPCs isolated from VLU patients.
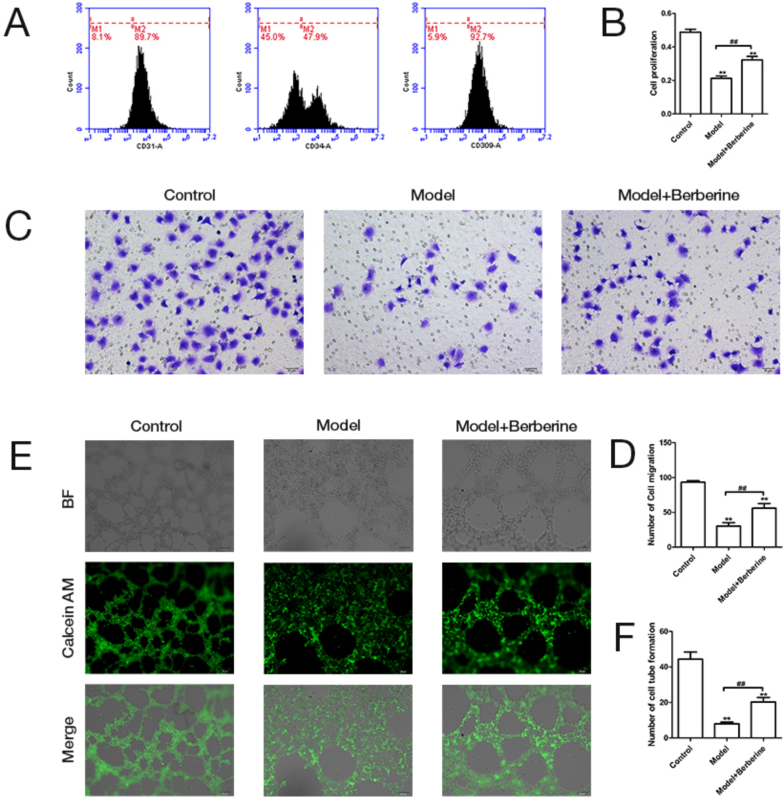
We first isolated and extracted EPCs from peripheral blood of patients with VLU and detected the expression of endothelial marker (CD31, CD34 and CD309) in EPCs by flow cytometry. EPCs were identified by demonstrating the expression of CD31 (89.7%), CD34 (47.9%) and CD309 (92.7%) through the flow cytometry analysis, demonstrating the EPC isolation was successful (Fig. 1A). Subsequently, the proliferation, migration and angiogenesis of these isolated EPCs were detected by CCK-8, Transwell and tubule formation assay. Compared with the healthy subjects, the proliferative activity of EPCs isolated form VLU patients was significantly decreased, but this change could be reversed by berberine treatment (Fig. 1B). Moreover, berberine effectively reduced the low migration activity of EPCs in VLU patients (Fig. 1C & D). Unsurprisingly, berberine also improved the lower capacity of tube formation in EPCs of VLU patients (Fig. 1E & F). On the whole, these results demonstrate that berberine has beneficial effects on the biological functions of EPCs.
Fig. 1.
Berberine promotes the proliferation, migration and tubular formation of EPCs isolated from VLU patients. (A) EPCs cells expressing CD31, CD34 and CD309 were identified using flow cytometric. (B) The proliferative ability of normal EPCs, untreated EPCs isolated from VLU patients and berberine-treated EPCs isolated from VLU patients was analyzed by CCK-8 assay. (C) The representative picture of migration ability was shown in normal EPCs, untreated EPCs isolated from VLU patients and berberine-treated EPCs isolated from VLU patients. (D) The migration ability of normal EPCs, untreated EPCs isolated from VLU patients and berberine-treated EPCs isolated from VLU patients was detected by Transwell migration assay. (E) The representative picture of tube-forming capability was shown in normal EPCs, untreated EPCs isolated from VLU patients and berberine-treated EPCs isolated from VLU patients. (F) The tube-forming capability of normal EPCs, untreated EPCs isolated from VLU patients and berberine-treated EPCs isolated from VLU patients was evaluated by tubule formation assay. ∗∗p < 0.01 vs. Control; ##p < 0.01.
3.1. Berberine accelerates skin wound healing in DVT mouse model
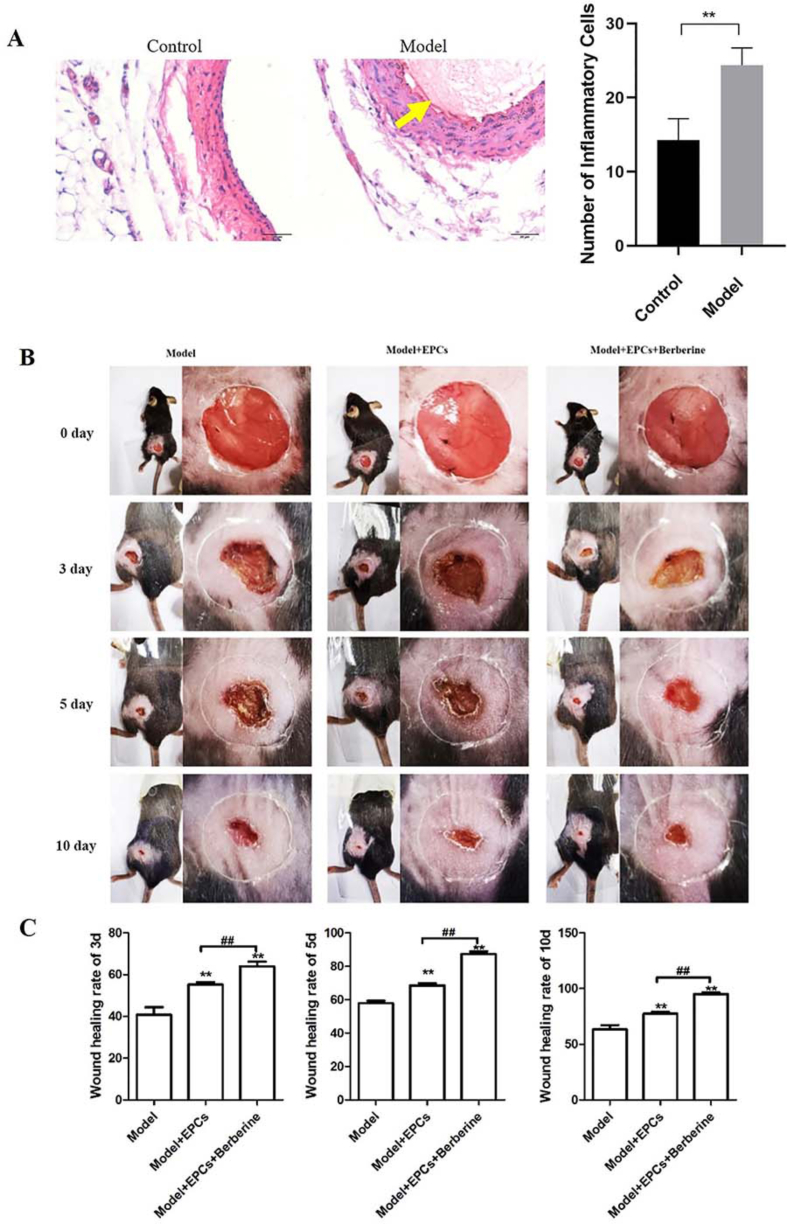
Proper functioning of EPCs is crucial for the maintenance of normal wound healing. To investigate the effect of berberine on wound healing, we generated the DVT mouse model with skin injury in vivo. Results of HE staining and ELISA assays showed that vascular thrombosis and inflammatory cells increased significantly in the Model group compared with the control group (Fig. 2A). In addition, we also observed the wound area of mice in each group at 0, 3, 5 and 10 days after operation. The results showed that the wound area of mice transplanted with EPCs and berberine-treated EPCs recovered faster than that of model mice. Among them, mice transplanted with berberine-treated EPCs recovered most quickly (Fig. 2B). Next, we quantified the wound area for 3, 5, 10 days after operation to evaluate the wound healing rate. The results showed that the wound healing rate of mice injected with EPCs was significantly higher than that of model mice on the 3rd, 5th and 10th days, but it was still significantly lower than that of mice injected with berberine-treated EPCs at each time point (Fig. 2C). At large, these results suggest that EPCs is beneficial to wound healing, while berberine can further promote the improving effects of EPCs on wound healing.
Fig. 2.
Berberine accelerates skin wound healing in DVT mouse model. (A) Thrombosis pathological changes and inflammatory cytokine levels in DVT mouse model was detected by HE staining and ELISA assay, respectively. ∗∗p < 0.01. (B) Representative images of skin wound healing in DVT mice treated or not treated with berberine at the indicated time point (0, 3, 5, 10 days postoperative, respectively). (C). Degree of skin wound healing was quantified in DVT mice treated or not treated with berberine at the indicated time point (0, 3, 5, 10 days postoperative, respectively). ∗∗p < 0.01 vs. Model; ##p < 0.01.
3.2. Berberine improves the impaired autophagic activity in EPCs isolated from VLU patients
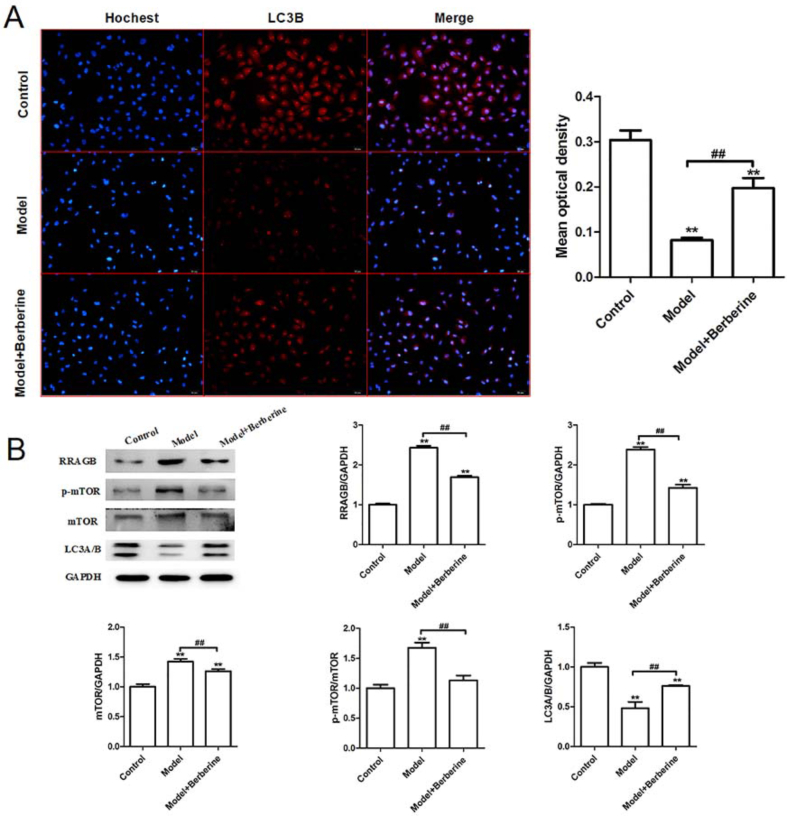
Autophagy is critical for cellular homeostasis and it was reported that autophagy in EPCs could protect cells under hypoxia [13]. Based on the above result, we speculate that berberine may affect the biological function of EPCs by regulating autophagy. To test whether berberine affects autophagy in EPCs, the expression of the autophagy marker LC3B was detected. Immunofluorescence assay showed that the expression of LC3B in the EPCs isolated from VLU patients was significantly reduced, while this tendency was reversed notably by berberine treatment (Fig. 3A). Subsequently, the expression of mTOR pathway-related proteins were detected using western blotting. Compared with the normal, the expression of RRAGB, mTOR and p-mTOR in the EPCs isolated from VLU patients was significantly increased, while the expression of LC3A/B was decreased. Following berberine treatment, the expression of RRAGB, mTOR, p-mTOR and LC3A/B in EPCs of Model group was reversed markedly (Fig. 3B). Collectively, berberine could efficiently promote autophagy in EPCs from VLU patients.
Fig. 3.
Berberine improves the impaired autophagic activity in EPCs isolated from VLU patients. (A) The expression of autophagy-related protein LC3B was detected by immunofluorescence staining in the normal EPCs, untreated EPCs isolated from VLU patients and berberine-treated EPCs isolated from VLU patients. (B) The protein expression of RRAGB, p-mTOR, mTOR and LC3A/B in the normal EPCs, untreated EPCs isolated from VLU patients and berberine-treated EPCs isolated from VLU patients was detected by western blotting. ∗∗p < 0.01 vs. Control; ##p < 0.01.
3.3. MiR-21-3p overexpression promotes the proliferation, migration and tubular formation of EPCs isolated from VLU patients
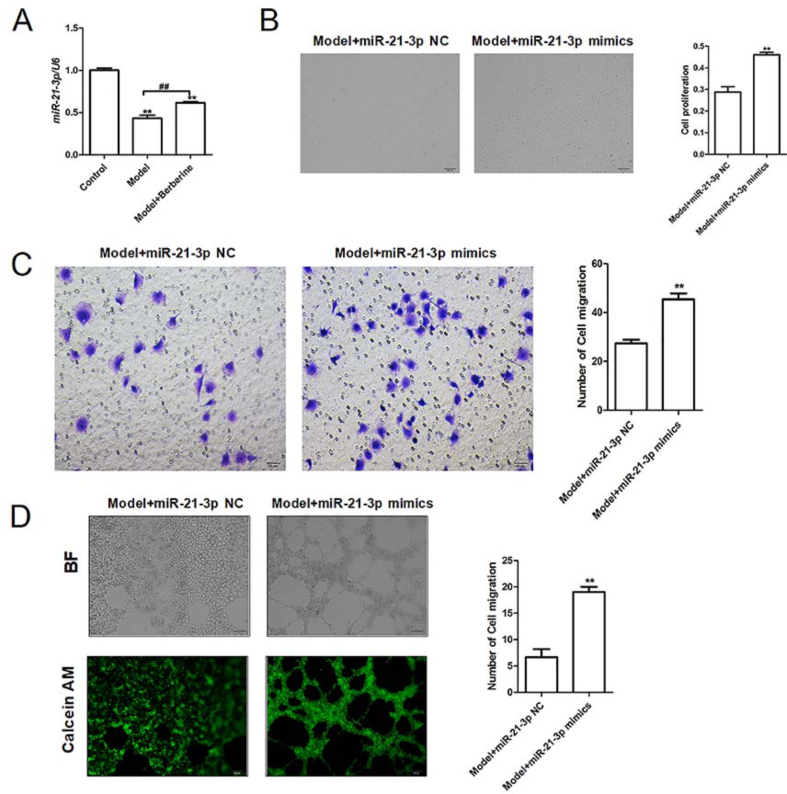
We continued to explore the potential mechanisms of berberine regulating the biological function of EPCs. As miR-21 was reported to regulate EPC proliferation and angiogenesis [19], the expression pattern and biological function of miR-21-3p in EPCs were analyzed. PCR results showed that miR-21-3p expression was significantly decreased in EPCs of Model group, and the low miR-21-3p expression could be alleviated by berberine treatment (Fig. 4A), suggesting that miR-21-3p may involve in the berberine-induced improvement of EPC function. Next, EPCs transfected with miR-21-3p mimics was employed to assess the capacity of EPC proliferation, migration and tube formation. Results indicated that miR-21-3p overexpression enhanced the capacity of EPC proliferation, migration and tube formation compared with the control group (Fig. 4B, C & 4D). In general, these data indicate that berberine may accelerate EPC growth through upregulating miR-21-3p.
Fig. 4.
MiR-21-3p overexpression promotes the proliferation, migration and tubular formation of EPCs isolated from VLU patients. (A) The mRNA expression miR-21-3p in the normal EPCs, untreated EPCs isolated from VLU patients and berberine-treated EPCs isolated from VLU patients was detected by RT-PCR. ∗∗p < 0.01 vs. Control; ##p < 0.01. (B) The proliferative ability of model EPCs transfected with miR-21-3p overexpression plasmid (miR-21-3p mimics) was analyzed by CCK-8 assay. ∗∗p < 0.01 vs. Model + miR-21-3p NC. (C) The migration ability of model EPCs transfected with miR-21-3p mimics was detected by Transwell migration assay. ∗∗p < 0.01 vs. Model + miR-21-3p NC. (D) The tube-forming capability of model EPCs transfected with miR-21-3p mimics was evaluated by tubule formation assay. ∗∗p < 0.01 vs. Model + miR-21-3p NC.
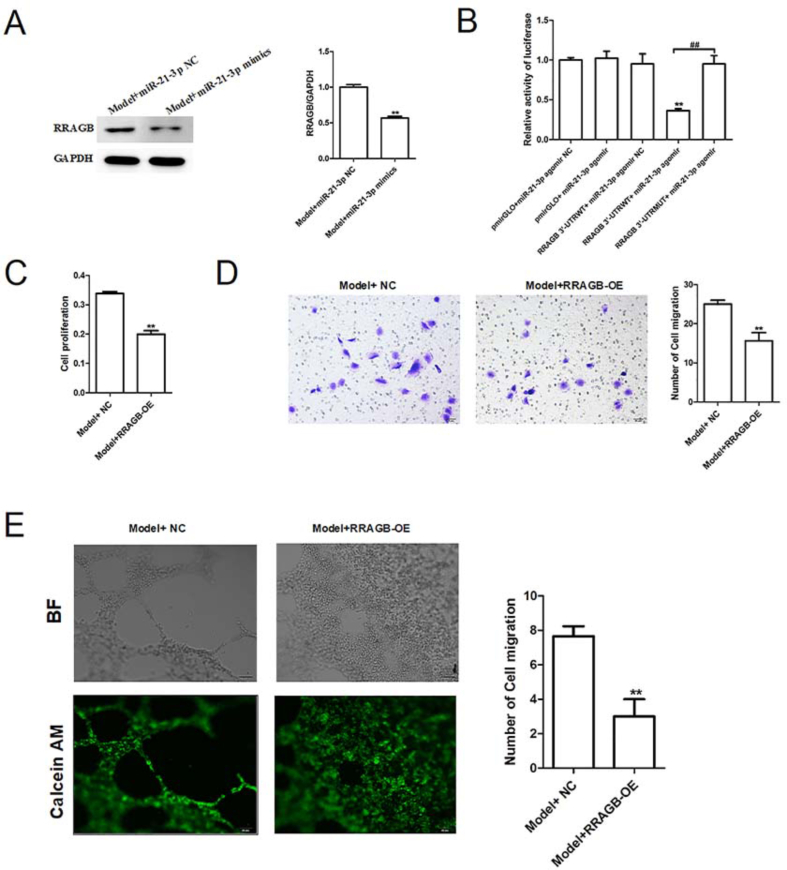
3.4. MiR-21-3p directly targets RRAGB whose overexpression inhibits the proliferation, migration and tubular formation of EPCs isolated from VLU patients
Next, to test whether miR-21-3p regulates EPC function through modulating autophagy pathway, we explored the relationship between miR-21-3p and RRAGB. Western blotting showed that miR-21-3p overexpression led to a dramatic reduction in the protein level of RRAGB in EPCs (Fig. 5A). Moreover, luciferase reporter assay indicated that transfection of miR-21-3p mimics induced a decrease in luciferase activity of wild-type RRAGB luciferase plasmid, whereas did not show any effect on the luciferase activity of mutate-type RRAGB luciferase plasmid (Fig. 5B). These data suggest that miR-21-3p is highly likely to directly bind to the 3′UTR of RRAGB mRNA. To investigate whether RRAGB can directly impact on EPC function, RRAGB overexpression plasmids (RRAGB-OE) were successfully constructed and transfected. Functional experiments showed that overexpression of RRAGB significantly weakened the proliferation, migration and tube formation of EPCs (Fig. 5C, D & 5E). Overall, miR-21-3p promoted the growth of EPCs by downregulating RRAGB expression.
Fig. 5.
MiR-21-3p directly targets RRAGB whose overexpression inhibits the proliferation, migration and tubular formation of EPCs isolated from VLU patients. (A) The protein expression of RRAGB in the model EPCs transfected with miR-21-3p mimics was detected by western blotting. ∗∗p < 0.01 vs. Model + miR-21-3p NC. (B) The binding correlation between miR-21-3p and RRAGB was identified by the luciferase reporter assay. ∗∗p < 0.01 vs. pmirGLO + miR-21-3p agomir NC; ##p < 0.01. (C) The proliferative ability of model EPCs transfected with RRAGB overexpression plasmid (RRAGB-OE) was analyzed by CCK-8 assay. ∗∗p < 0.01 vs. Model + NC. (D) The migration ability of model EPCs transfected with RRAGB-OE was detected by Transwell migration assay. ∗∗p < 0.01 vs. Model + NC. (E) The tube-forming capability of model EPCs transfected with RRAGB-OE was evaluated by tubule formation assay. ∗∗p < 0.01 vs. Model + NC.
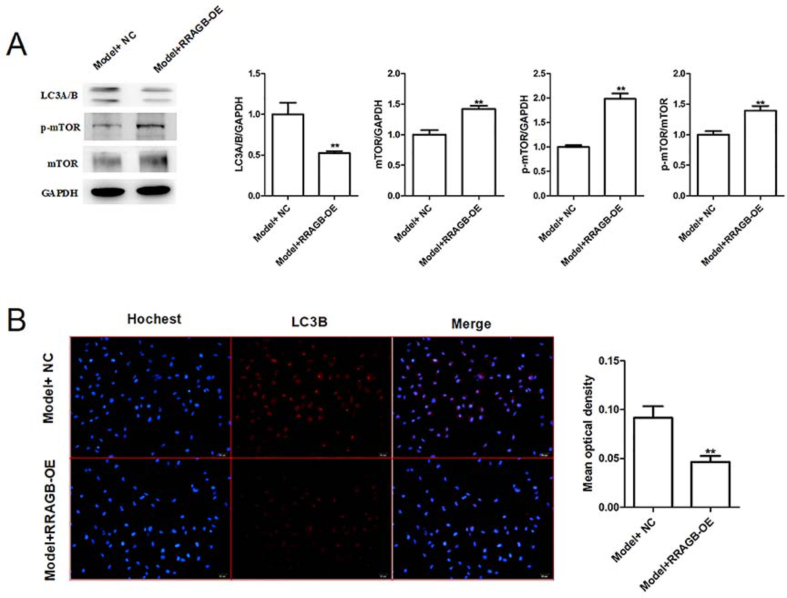
3.5. RRAGB overexpression upregulates the protein expression of mTOR pathway-related proteins (p-mTOR and mTOR) while downregulates the autophagy-related proteins (LC3A/B and LC3B)
Finally, the effects of RRAGB overexpression on the expression of mTOR pathway-related proteins (p-mTOR, mTOR, LC3A/B) were detected by western blotting and immunofluorescence. We found the expression of p-mTOR and mTOR in the EPCs of Model group were increased significantly by RRAGB overexpression, while the expression of LC3A/B was reduced (Fig. 6A). Meanwhile, immunofluorescence assay also showed that RRAGB overexpression impaired the expression of LC3A/B in EPCs (Fig. 6B). Together, these above results suggest that berberine-induced improvement effects on EPC growth is potentially attributed to miR-21-3p/RRAGB axis.
Fig. 6.
RRAGB overexpression upregulates the protein expression of mTOR pathway-related proteins (p-mTOR and mTOR) while downregulates the autophagy-related proteins (LC3A/B and LC3B). (A) The protein expression of LC3A/B, p-mTOR and mTOR in the model EPCs transfected with RRAGB-OE was detected by western blotting. (B) The expression of autophagy-related protein LC3B was detected by immunofluorescence staining in the model EPCs. ∗∗p < 0.01 vs. Model + NC.
4. Discussion
VLUs are a prevalent and challenging health problem, affecting millions of individuals worldwide [1]. The present study investigated the potential therapeutic effects of berberine on VLUs through its impact on EPC function and wound healing of DVT mouse model. The findings revealed that berberine exerts its beneficial effects through the miR-21-3p/RRAGB axis, which is a novel and intriguing mechanism that warrants further investigation.
Berberine, a natural alkaloid derived from various medicinal plants, has drawn significant attention due to its diverse pharmacological properties, including anti-inflammatory, anti-oxidative, and anti-microbial effects [20]. Recently, Panda et al. demonstrated that berberine may have a potential role in wound healing and tissue repair [21]. For instance, berberine nano-colloids have been shown to accelerate wound healing and exert a protective effect in diabetic mice by regulating the Sirt1/NF-κB pathway [17]. Recently, Zhou et al. reported that berberine promoted wound healing in diabetic mice by activating TrxR1/JNK pathway to restore redox homeostasis [18]. However, there have been no studies exploring the potential beneficial effects of berberine on wound injury in patients with VLU. It is reported that EPCs play a crucial role in vascular repair and wound healing processes [22]. Berberine has been demonstrated to reduce TNF-α-induced damage to EPCs through the PI3K/AKT/eNOS pathway [15]. Furthermore, berberine enhanced the normal biological functions of EPCs by increasing the NO content in healthy subjects [16]. However, its specific effects on EPC function and its underlying mechanisms in the context of VLUs have not been thoroughly investigated. In the present study, we found that berberine treatment significantly enhanced EPC function in vitro. By enhancing EPC function, berberine has the potential to promote neovascularization, re-epithelialization, and overall tissue regeneration, which are essential processes for successful wound healing. The in vivo experiments further corroborated the in vitro findings, showing that berberine administration accelerated wound healing in a murine model of VLU. The wounds treated with berberine exhibited reduced wound size, increased vascularization, and improved granulation tissue formation. These results underscore the translational potential of berberine as a therapeutic intervention for VLUs in clinical settings.
To gain the insights into the cellular mechanisms accounting for the beneficial effects of berberine on EPCs, the autophagic activity in EPCs treated with berberine was assessed. Research has shown that autophagy plays a critical role in various stages of wound healing, involving multiple biological processes [23]. For instance, autophagy can facilitate skin vascular regeneration mediated by stromal stem cells in mice by regulating VEGF secretion [24]. Notably, the herbal extract Monotropein has been found to promote wound healing by inhibiting oxidative stress-induced autophagy in EPCs [25]. Based on the aforementioned studies, we hypothesize that berberine may impact wound healing by modulating autophagy in EPCs. Multiple pathways have been reported involve in the regulation of cellular autophagy, such as the ubiquitin-proteasome system, mTOR pathway, and etc [26]. Among them, the mTOR pathway in regulating skin wound healing in mice are widely studied [27]. For example, Acemannan accelerated wound healing in mice through the AKT/mTOR pathway [28]. Ozone oil enhanced fibroblast migration and exacerbated wound healing in mice via the PI3K/Akt/mTOR pathway [29]. In this study, we observed a significant increase in RRAGB, p-mTOR, and mTOR levels in EPCs after treatment with berberine. The restored autophagy by berberine is a putative mechanism for the amelioration of EPC function.
MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression post-transcriptionally [30]. MiRNAs have been recognized as critical modulators of various cellular processes, including those involved in wound healing and angiogenesis [31,32]. MiR-21-3p, a member of the miR-21 family, has been shown to promote angiogenesis and enhance cellular migration, suggesting a potential role in wound healing [33]. Wu et al. demonstrated that miR-21-3p promoted wound healing in diabetic mice by downregulating SPRY1 [34]. Hu et al. found that human umbilical cord blood-derived exosomes enhanced angiogenesis and wound healing through the regulation of miR-21-3p expression [35]. Therefore, understanding the interactions between berberine, EPCs, and miR-21-3p may provide valuable insights into novel therapeutic strategies for VLUs. In this study, berberine promoted miR-21-3p expression in EPCs of the Model group. Overexpression of miR-21-3p significantly enhanced EPC proliferation, migration, and tube formation. It is worth noting that through luciferase reporter experiments, we identified RRAGB as a downstream regulatory gene of miR-21-3p. mTOR, a key regulatory protein in autophagy initiation, consists of two complexes, mTORC1, and mTORC2. Inhibiting mTORC1 activity in the mTOR pathway can effectively promote autophagy levels [36]. RRAGB, as an activator of mTORC1, is known to play a pro-oncogenic role in various tumor progressions [37,38]. However, RRAGB's potential role in wound healing and its regulatory relationship with miR-21-3p have not been explored in the context of VLUs. Our results indicate that RRAGB overexpression inhibited EPCs' growth activity, mediating that RRAGB is a potential direct target of miR-21-3p to mediate impaired autophagy and EPC function observed in VLU.
Although we have demonstrated the involvement of the miR-21-3p/RRAGB axis in impaired EPC function in VLU patients, the current data have limitations in establishing a direct causal role of this axis in mediating the impairment of EPC function. Future studies utilizing mouse models with EPC-specific overexpression of miR-21-3p will provide more direct evidence regarding the effectiveness of restoring miR-21-3p as an approach to improving EPC function. Additionally, the upstream regulators of miR-21-3p expression in EPCs remain unclear. Further molecular investigations into the mediators responsible for miR-21-3p downregulation in EPCs during VLU would offer a better rationale for the development of new pharmacological interventions.
5. Conclusions
Our study demonstrates that berberine enhances the proliferation, migration, and tube formation ability of EPCs from patients with VLU. This effect is likely mediated by an improved autophagic activity through the miR-21-3p/RRAGB axis. These findings provide valuable insights into the novel molecular mechanisms underlying the effects of berberine, offering promising prospects for targeted interventions in chronic wound healing. However, further research is warranted to address remaining questions and pave the way for the clinical application of berberine as a therapeutic agent for VLUs.
Funding
No funding was received.
Data availability
All data generated or analyzed during this study are included in this published article.
Author contributions
JLL, WQ and LYN made substantial contributions to the conception and design of the present study. JLL provided administrative support. JLL, WHJ and LL provided study materials or patients. WQ, LYN, WHJ and LL performed collection and assembly of data, data analysis, and interpretation. WQ and LYN wrote the paper. Final approval of the manuscript was by all authors.
Ethics statement
Written informed consent was obtained from all patients and the study protocol was approved by the Committee on the Ethics of Animal Experiments and Human Subject Research of Jiangsu Province Hospital of Chinese Medicine. All animal experiments were conducted in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals. The ethical approval number for the study is 2022NL-196-02.
Declaration of competing interest
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Nelson E.A., Adderley U. Venous leg ulcers. Clin Evid. 2016;2016 [PMC free article] [PubMed] [Google Scholar]
- 2.Abbade L.P.F., Lastória S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol John Wiley Sons, Ltd. 2005 Jun;44(6):449–456. doi: 10.1111/j.1365-4632.2004.02456.x. [DOI] [PubMed] [Google Scholar]
- 3.Probst S., Weller C.D., Bobbink P., Saini C., Pugliese M., Skinner M.B., et al. Prevalence and incidence of venous leg ulcers-a protocol for a systematic review. Syst Rev BioMed Central. 2021 May 12;10(1):148. doi: 10.1186/s13643-021-01697-3. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins L., Seraj S. Diagnosis and treatment of venous ulcers. Am Fam Physician. Am Fam Physician. 2010 Apr 15;81(8):989–996. [PubMed] [Google Scholar]
- 5.Lin C.-P., Lin F.-Y., Huang P.-H., Chen Y.-L., Chen W.-C., Chen H.-Y., et al. Endothelial progenitor cell dysfunction in cardiovascular diseases: role of reactive oxygen species and inflammation. Biomed Res Int Hindawi. 2013;2013 doi: 10.1155/2013/845037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balaji S., King A., Crombleholme T.M., Keswani S.G. The role of endothelial progenitor cells in postnatal vasculogenesis: implications for therapeutic neovascularization and wound healing. Adv Wound Care (New Rochelle) Mary Ann Liebert, Inc 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA. 2013 Jul;2(6):283–295. doi: 10.1089/wound.2012.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukmawati D., Tanaka R. Introduction to next generation of endothelial progenitor cell therapy: a promise in vascular medicine. Am J Transl Res e-Century Publishing Corporation. 2015;7(3):411–421. [PMC free article] [PubMed] [Google Scholar]
- 8.Tobler K., Freudenthaler A., Baumgartner-Parzer S.M., Wolzt M., Ludvik B., Nansalmaa E., et al. Reduction of both number and proliferative activity of human endothelial progenitor cells in obesity. Int J Obes (Lond) Nature Publishing Group. 2010 Apr;34(4):687–700. doi: 10.1038/ijo.2009.280. [DOI] [PubMed] [Google Scholar]
- 9.Fadini G.P., Miorin M., Facco M., Bonamico S., Baesso I., Grego F., et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005 May 3;45(9):1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 10.Tecilazich F., Dinh T., Pradhan-Nabzdyk L., et al. Role of endothelial progenitor cells and inflammatory cytokines in healing of diabetic foot ulcers. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zollino I., Campioni D., Sibilla M.G., et al. A phase II randomized clinical trial for the treatment of recalcitrant chronic leg ulcers using centrifuged adipose tissue containing progenitor cells. Cytotherapy. 2019;21(2):200–211. doi: 10.1016/j.jcyt.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Ryter S.W., Koo J.K., Choi A.M.K. Molecular regulation of autophagy and its implications for metabolic diseases. Curr Opin Clin Nutr Metab Care. 2014 Jul;17(4):329–337. doi: 10.1097/MCO.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H.-J., Zhang D., Tan Y.-Z., Li T. Autophagy in endothelial progenitor cells is cytoprotective in hypoxic conditions. Am J Physiol, Cell Physiol American Physiological Society Bethesda, MD. 2013 Apr 1;304(7):C617–C626. doi: 10.1152/ajpcell.00296.2012. [DOI] [PubMed] [Google Scholar]
- 14.Marola S., Ferrarese A., Solej M., Enrico S., Nano M., Martino V. Management of venous ulcers: state of the art. Int J Surg. 2016 Sep;33(Suppl 1):S132–S134. doi: 10.1016/j.ijsu.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Xiao M., Men L.N., Xu M.-G., Wang G.B., Lv H.T., Liu C. Berberine protects endothelial progenitor cell from damage of TNF-α via the PI3K/AKT/eNOS signaling pathway. Eur J Pharmacol. 2014 Nov 15;743:11–16. doi: 10.1016/j.ejphar.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Xu M.-G., Wang J.-M., Chen L., Wang Y., Yang Z., Tao J. Berberine-induced upregulation of circulating endothelial progenitor cells is related to nitric oxide production in healthy subjects. Cardiology Karger Publishers. 2009;112(4):279–286. doi: 10.1159/000157336. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P., He L., Zhang J., et al. Preparation of novel berberine nano-colloids for improving wound healing of diabetic rats by acting Sirt1/NF-κB pathway. Colloids Surf B Biointerfaces. 2020;187 doi: 10.1016/j.colsurfb.2019.110647. [DOI] [PubMed] [Google Scholar]
- 18.Zhou R., Xiang C., Cao G., et al. Berberine accelerated wound healing by restoring TrxR1/JNK in diabetes. Clin Sci. 2021;135(4):613–627. doi: 10.1042/CS20201145. [DOI] [PubMed] [Google Scholar]
- 19.Du X., Hong L., Sun L., Sang H., Qian A., Li W., et al. miR-21 induces endothelial progenitor cells proliferation and angiogenesis via targeting FASLG and is a potential prognostic marker in deep venous thrombosis. J Transl Med. BioMed Central. 2019 Aug 15;17(1):270. doi: 10.1186/s12967-019-2015-z. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong R.G., Huang S.Y., Wu S.X., et al. Anticancer effects and mechanisms of berberine from medicinal herbs: an update review. Molecules. 2022;27(14):4523. doi: 10.3390/molecules27144523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panda D.S., Eid H.M., Elkomy M.H., et al. Berberine encapsulated lecithin–chitosan nanoparticles as innovative wound healing agent in type II diabetes. Pharmaceutics. 2021;13(8):1197. doi: 10.3390/pharmaceutics13081197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amato G., Grimaudo M.A., Alvarez-Lorenzo C., et al. Hyaluronan/Poly-L-lysine/Berberine nanogels for impaired wound healing. Pharmaceutics. 2020;13(1):34. doi: 10.3390/pharmaceutics13010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren H., Zhao F., Zhang Q., et al. Autophagy and skin wound healing. Burns Trauma. 2022;10:tkac003. doi: 10.1093/burnst/tkac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An Y., Liu W.J., Xue P., et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018;9(2):58. doi: 10.1038/s41419-017-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C., Mao C., Lou Y., et al. Monotropein promotes angiogenesis and inhibits oxidative stress-induced autophagy in endothelial progenitor cells to accelerate wound healing. J Cell Mol Med. 2018;22(3):1583–1600. doi: 10.1111/jcmm.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kourtis N., Tavernarakis N. Autophagy and cell death in model organisms. Cell Death Differ. 2009;16(1):21–30. doi: 10.1038/cdd.2008.120. [DOI] [PubMed] [Google Scholar]
- 27.Squarize C.H., Castilho R.M., Bugge T.H., et al. Accelerated wound healing by mTOR activation in genetically defined mouse models. PLoS One. 2010;5(5) doi: 10.1371/journal.pone.0010643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xing W., Guo W., Zou C.H., et al. Acemannan accelerates cell proliferation and skin wound healing through AKT/mTOR signaling pathway. J Dermatol Sci. 2015;79(2):101–109. doi: 10.1016/j.jdermsci.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Xiao W., Tang H., Wu M., et al. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci Rep. 2017;37(6) doi: 10.1042/BSR20170658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye J., Xu M., Tian X., et al. Research advances in the detection of miRNA. Journal of pharmaceutical analysis. 2019;9(4):217–226. doi: 10.1016/j.jpha.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng Z., Zhou D., Gao Y., et al. miRNA delivery for skin wound healing. Adv Drug Deliv Rev. 2018;129:308–318. doi: 10.1016/j.addr.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee J., Chan Y.C., Sen C.K. MicroRNAs in skin and wound healing. Physiol Genom. 2011;43(10):543–556. doi: 10.1152/physiolgenomics.00157.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie J., Wu W., Zheng L., et al. Roles of microRNA-21 in skin wound healing: a comprehensive review. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.828627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y., Zhang K., Liu R., et al. MicroRNA-21-3p accelerates diabetic wound healing in mice by downregulating SPRY1. Aging (Albany NY) 2020;12(15) doi: 10.18632/aging.103610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y., Rao S.S., Wang Z.X., et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8(1):169. doi: 10.7150/thno.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu H.Y., Tsay Y.G., Hung S.C. Involvement of mTOR-autophagy in the selection of primitive mesenchymal stem cells in chitosan film 3-dimensional culture. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-10708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao J., Liu Q., Wu W., et al. Elevated Ras related GTP binding B (RRAGB) expression predicts poor overall survival and constructs a prognostic nomogram for colon adenocarcinoma. Bioengineered. 2021;12(1):4620–4632. doi: 10.1080/21655979.2021.1956402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X., Wang J., Lin W., et al. circEXOC6B interacting with RRAGB, an mTORC1 activator, inhibits the progression of colorectal cancer by antagonizing the HIF1A-RRAGB-mTORC1 positive feedback loop. Mol Cancer. 2022;21(1):135. doi: 10.1186/s12943-022-01600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.