Abstract
The implementation of screening colonoscopy with polyp removal has significantly decreased mortality rates associated with colorectal cancer (CRC), although it remains a major cause of cancer-related deaths globally. CRC typically originates from adenomatous polyps, and increased removal of these growths has led to reduced CRC incidence and mortality. Endoscopic polypectomy techniques, including hot and cold snare polypectomy, play a pivotal role in this process. While both methods are effective for small polyps (<10 mm), recent evidence favors cold snare polypectomy due to its superior safety profile and comparable complete resection rates. Large polyps (>10 mm), particularly those with advanced features, pose increased cancer risks and often require meticulous assessment and advanced endoscopic techniques, including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), for resection.
This chapter also provides a practical overview of endoscopic techniques for managing colonic obstructions and pericolonic fluid collections, detailing their indications, advantages, disadvantages, and complications. The goal is to improve understanding and application in clinical practice. Additionally, we provide a summary of endoscopic closure techniques that have revolutionized the management of perforations and fistulas, offering safe and effective alternatives to surgery.
Keywords: Colon polyps, Endoscopic mucosal resection, Endoscopic submucosal dissection, Endoscopic closure devices, Colorectal stents
Introduction
The implementation of screening colonoscopy with polyp removal has resulted in a remarkable decline in mortality rates associated with colorectal cancer (CRC). Despite this positive trend, colorectal cancer remains the second leading cause of cancer-related deaths and is the third most common cancer worldwide. CRC typically evolves from adenomatous polyps, and increased removal of these pre-cancerous growths has significantly decreased the incidence and mortality rates from colorectal cancer. Various methods can be employed for endoscopic polyp removal. This chapter aims to provide a clinically oriented overview of these techniques, explaining how and when they are used, as well as advantages, disadvantages, and complications.
Endoscopic polypectomy
Background
Snare polypectomy utilizes a thin metal ring to entrap and cut the polyp tissue for resection. Both hot (with electrocautery) and cold snare polypectomy techniques are effective in removing small colon polyps <10 mm in size, however recent evidence suggests the use of cold snare over hot snare may be beneficial given its superior safety profile and non-inferior complete resection rates [1].
Hot snare polypectomy vs cold snare polypectomy
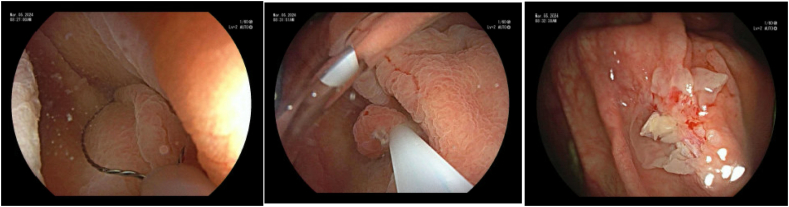
Hot snare polypectomy (HSP) employs electrocautery to cut the colonic mucosa, offering advantages such as eliminating neoplastic cells at tissue margins and reducing rates of intra-procedural bleeding. However, drawbacks include the risk of full thickness injury to the bowel wall and delayed post-polypectomy bleeding. As a result, the preferred approach for small polyps under 10 mm is en bloc removal by cold snare polypectomy (CSP) typically using dedicated 8–10 mm diameter thin braided stiff wire snares designed to provide greater tissue traction to precisely cut through mucosal tissue. For best results, the polyp should be positioned in the 5 o'clock position with the snare positioned around the lesion including a 2–4 mm margin of normal tissue to ensure adequate resection of neoplastic tissue [2] (Image 1). Use of a cold snare is associated with less delayed bleeding, even in patients on therapeutic anticoagulation. It also poses a lower risk of colon perforation and requires shorter procedure times compared to HSP [[3], [4], [5]]. While some studies suggest higher incomplete resection rates with CSP, the evidence is limited and lacks statistical significance. Cold forceps can serve as an alternative for polyps under 3 mm, although using forceps for polyps larger than 3 mm increases incomplete resection and recurrence rates [6]. Historically, hot forceps were a popular tool for removing these smaller polyps but have declined in use due to an increased association with perforation, delayed bleeding and post-polypectomy syndrome [4,7].
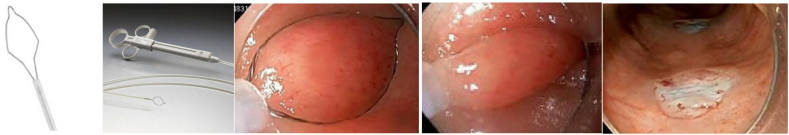
Image 1.
Cold snare polypectomy.
a, b) Steris Exacto snare; c,d) Boston Scientific Captivator cold snare with cold snare resection – courtesy BSC.
Large polyp removal
Background
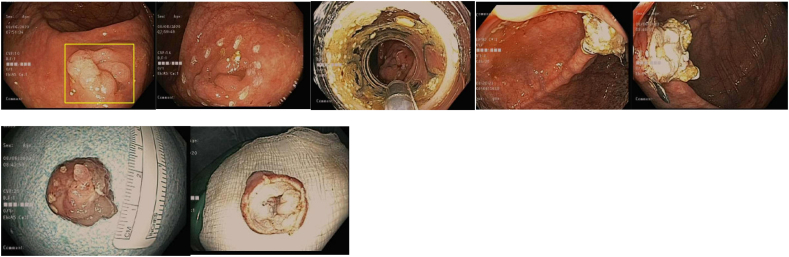
The risk of cancer increases with polyp size. Large polyps (> 10 mm), those with villous or tubulovillous morphology, and those with high grade dysplasia are considered to be advanced adenomas. As such, a variety of techniques have been developed to remove advanced adenomas with the goal to ensure complete removal with negative margins to lower the risk of recurrence or progression to cancer. Prior to attempted resection, the polyp should be carefully inspected using a combination of high-definition white light in addition to advanced optical techniques such as narrow band imaging or blue light imaging to assess for mucosal changes that may suggest deep submucosal invasion, which would preclude successful endoscopic resection (Image 3). Careful assessment and demarcation of the mucosal margins of the polyp, sometimes with the addition of chromoendoscopy and electrocautery markings around the borders are important to facilitate complete resection with negative margins. Endoscopic tattoos using India Ink or carbon black injected into the submucosa distal to or on the opposite wall from the polyp are often placed to allow for identification of the site during a follow up colonoscopy or at the time of surgery if invasive cancer is found on pathologic review.
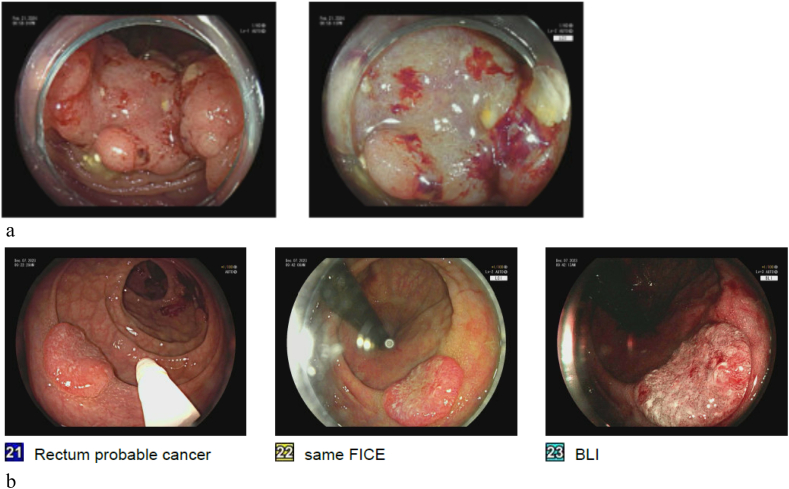
Image 3.
a Left: polyp with central depression and mucosal change suggestive of deep submucosal invasion; Right: close up of central depression with irregular pit pattern.
b Polyp with central depression and abnormal pit pattern suggestive of deep submucosal invasion. High definition white light image on left, FICE center and Blue light image on right.
If after careful assessment of a large or complex polyp the endoscopist defers resection during an index colonoscopy and instead plans to refer to an advanced endoscopist for removal, caution must be exercises to avoid practices that could induce submucosal fibrosis, hindering potential future endoscopic resection techniques. These practices include tattooing near or underneath the lesion, as well as performing multiple biopsies or partial snare resection of the lesion [8].
Complex polyp features that suggest potentially unfavorable outcomes with advanced endoscopic resection techniques (i.e. incomplete resection or recurrence) include lesions >40 mm, ileocecal valve lesions, recurrent polyps or prior failed attempts at resection and size/morphology/site/access (SMSA) level 4 lesions [9].
Pedunculated polyps
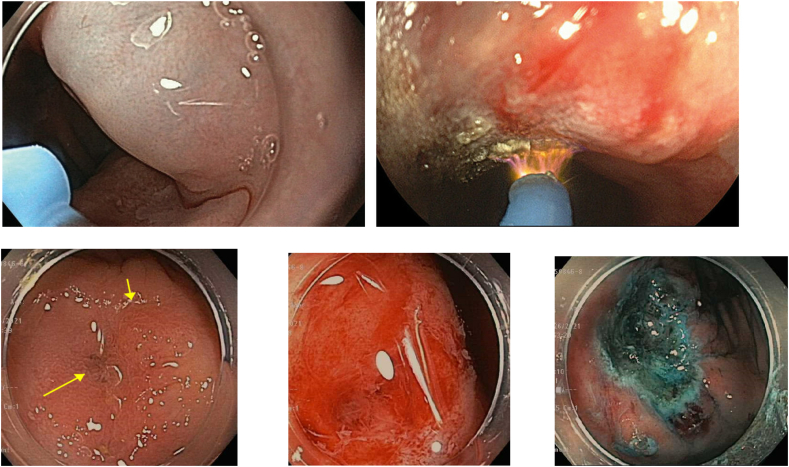
For pedunculated polyps >10 mm, multiple guidelines suggest removing these with hot snare electrocautery to prevent intra-procedural bleeding [10]. For larger polyps, especially those with a thick stalk, the polyp stalk can be pre-treated with epinephrine injection or endoscopic clipping prior to polypectomy to reduce the risk of bleeding. An Endo loop (a loop ligating device) can also be placed around the polyp stalk to act as a tourniquet prior to polyp resection (Image 2).
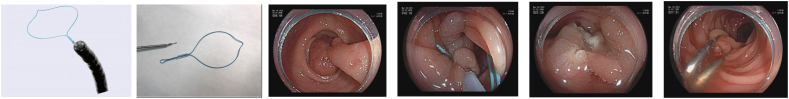
Image 2.
Olympus endoloop.
a) Olympus endoloop; b) Detached endoloop; c) Pedunculated polyp; d) Endoloop on stalk; e,f) Post resection (loop migrated-clips placed on stalk).
Sessile polyps
Large sessile polyps can be removed using advanced endoscopic resection techniques including endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD).
Conventional EMR
Conventional EMR is used to remove non-invasive polyps that are >10 mm in size. This procedure involves injecting a lifting solution into the submucosal space to establish a cushion, facilitating the separation of the mucosa from the underlying muscularis propria (Image 4). Subsequently, a snare is utilized to excise the lesion. This technique improves safety and prevents damage to the deeper layers. Typical solutions for injection include normal saline or saline mixed with succinylated gelatin, hydroxyethyl starch, or glycerol to increase the viscosity and delay reabsorption of the injected fluid. Coloring agents such as indigo carmine, methylene blue or food coloring are often added to the injectate to better identify the submucosal layer and delineate the mucosal margins of the polyp. Premixed commercial injecting agents are also available. The solution can also be diluted with epinephrine (in concentrations ranging from 1:100,000 or 1:1,000,000) to decrease intra-procedural bleeding, however, this has been associated with an increased risk of delayed bleeding [11,12].
Image 4.
Submucosal injection.
After an adequate injection lift, the polyp can then be resected with a snare either en bloc or piecemeal. En bloc resection is the preferred method for polyps up to 20 mm in size and leads to lower recurrence rates compared to piecemeal resection [13,14]. En bloc resection of large polyps up to 20 mm in size often requires the use of electrocautery to resect the polyp. However, en bloc resection should not be attempted if the polyp is >20 mm, as this is associated with a higher perforation risk. Therefore, when en bloc resection is not feasible or safe, piecemeal resection should be performed, even though it is associated with a higher likelihood of local recurrence [12].
Piecemeal EMR of large polyps typically involves removing 8–10 mm portions of the polyp starting at one edge of the polyp and then moving laterally to remove additional pieces until the entire lesion has been resected (Image 5). Submucosal injection is often employed prior to resection, though smaller lesions between 10 and 20 mm can sometimes be resected piecemeal without prior injection lift. In general, both hot and cold piecemeal EMR are used in practice; however, current evidence supports the use of cold EMR over hot EMR given its decreased delayed bleeding and perforation risks [[14], [15], [16]]. If piecemeal resection is utilized, the lateral margins of the mucosal resection area should have normal tissue. That said, the recurrence rate is higher in cold EMR than hot EMR with approximately 17 % and 7 % recurrence, respectively [17]. Extending polyp margins by 3 mm with cold snare resection or treatment of the margins using electrocautery techniques including argon plasma coagulation or soft coagulation current using the snare tip can reduce the risk of recurrence [[18], [19], [20]].
Image 5.
Piecemeal EMR.
Underwater EMR
An alternative and effective approach to conventional EMR is underwater EMR [21,22]. It is often employed to resect sessile lesions in situations where the boundaries of the lesion are unclear. This technique involves evacuating air from the colonic lumen and infusing water to immerse the area. This ‘underwater’ environment floats the submucosa away from the muscularis propria, significantly improving the visualization of lesion boundaries and negates the need for submucosal injection. Additionally, the higher refractive index of water leads to objects appearing larger and thus increasing the chances of identifying the boundaries of the lesion (Image 6). Narrow band imaging (NBI) or blue light imaging (BLI) is often employed to determine the margins of the lesion prior to then resecting the lesion with hot or cold snare techniques similar to conventional EMR. Interestingly, one meta-analysis found that underwater EMR may be more effective in en bloc resection and have shorter procedure times than conventional injection EMR [23,24].
Image 6.
Underwater EMR.
ESD
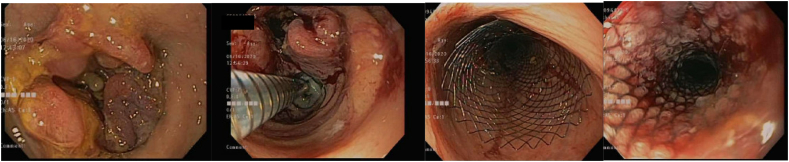
ESD is an advanced resection technique that allows for en bloc resection of large mucosal polyps and can be used in scenarios where snare resection is not optimal [25]. It was originally developed in Japan in the 1990s for gastric malignancies, and its applications have since expanded to other parts of the GI tract [26]. When evaluating polyps for high-risk criteria, it is recommended to use chromoendoscopy, narrow-band imaging, blue light imaging or EUS [11]. If there is concern based on careful evaluation that there may be submucosal invasion (depressed edges, ulceration, and irregular pit pattern), one can biopsy instead of attempting resection. Additionally, EUS can be a useful adjunct in determining invasion at this time, especially for lesions in the distal colon or rectum.
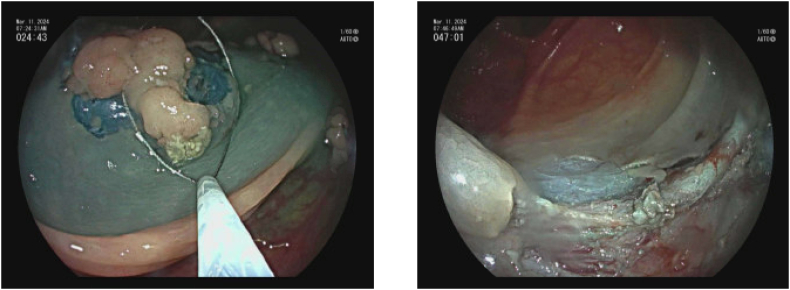
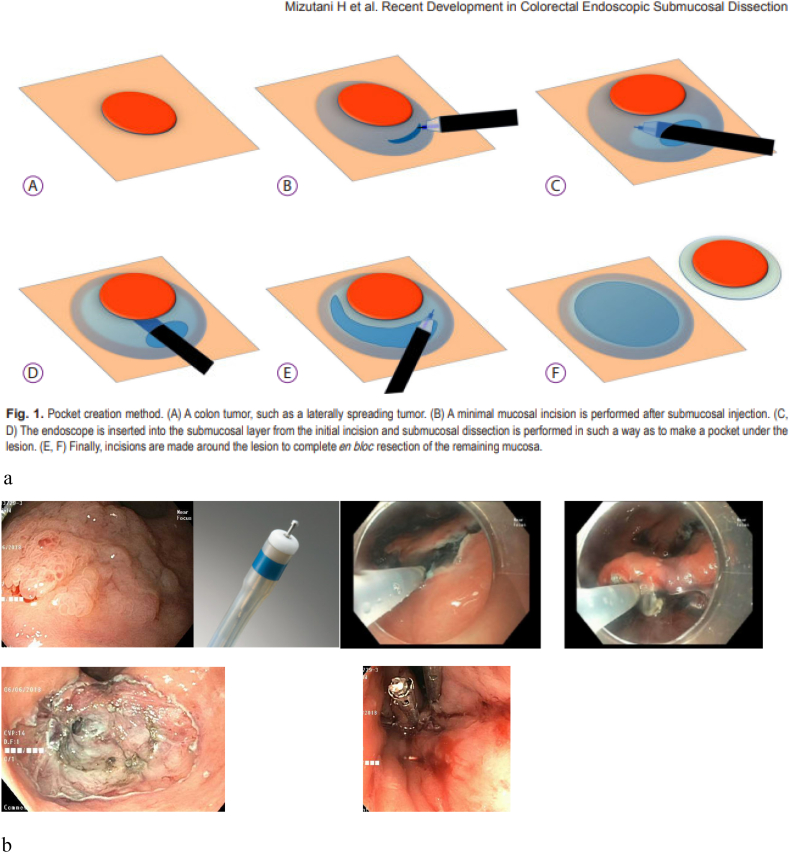
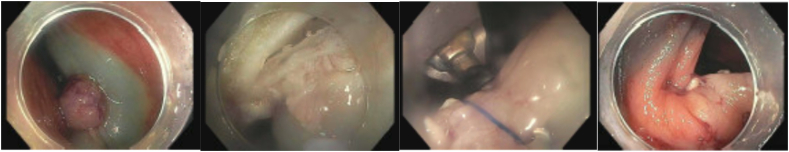
The technique of ESD begins by marking the margins of the polyp with the tip of the ESD knife 3–5 mm from the polyp edge. Submucosal injection with a lifting solution similar to EMR is then carried out around the periphery of the lesion. Then one proceeds with a circumferential incision using Endocut™(Erbe) cautery into the submucosa guided by the cautery marks surrounding the lesion. Once the circumferential incision is completed, the lesion is then carefully dissected strand by strand after sequential submucosal injection, creating a mucosal flap underneath the lesion using coagulation current, being careful not to injure the underlying muscularis propria. Dissection is continued until the lesion is released from its submucosal attachment to eventually complete an en bloc resection [[26], [27], [28]] (Image 7). Variations on technique include beginning with a mucosal injection distal to the lesion, followed by submucosal injection and tunnelling dissection underneath the lesion proceeding to the proximal extent, before completion of the resection after incising the lateral borders of the polyp.
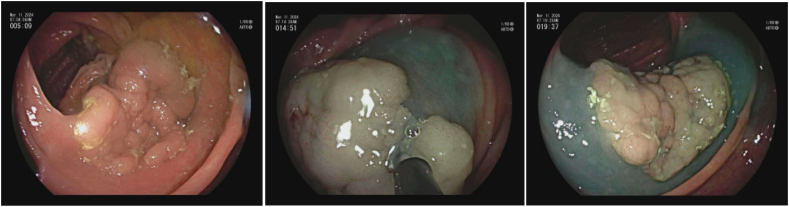
Image 7.
a Pocket method for colorectal ESD [69].
b (ESD for rectal polyp.)
a) Rectal LST; b) Olympus dual knife; c) ESD circumferential incision; d) ESD submucosal dissection; e) Post ESD defect with small focal perforation; f) post suture and clip closure of ESD defect.
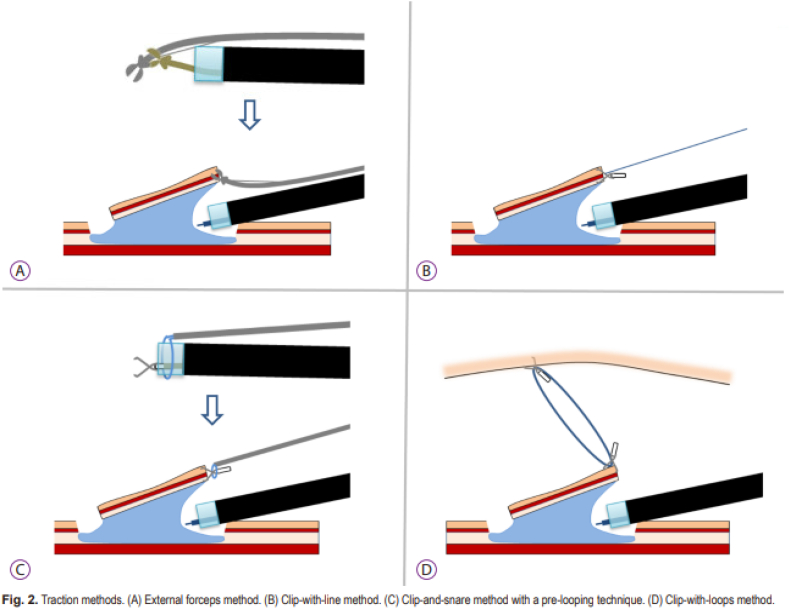
There are numerous types of knives that can be employed for ESD, the choice of which to use comes down to operator preference. A variety of lifting solutions can be used including normal saline - however, given the prolonged nature of the procedure, solutions that lift tissue for longer periods of time are preferred by some such as hypertonic saline, dextrose, hydroxypropyl methylcellulose as well as glycerin. Coloring agents similar to those used with EMR such as indigo carmine, methylene blue or food coloring are added to the injectate to better define the submucosal and mucosal layers from the underlying muscularis propria. During the dissection, traction is often employed to help visualize the resection bed, such as clip with line assistance, rubber band technique, and O-clip assisted method (Image 8). For hemostasis after dissection, most knives have the ability to perform monopolar anticoagulation, and typically hemostatic forceps (Coagrasper™ (Olympus)) are also employed to control bleeding from focal blood vessels within the resection bed [26].
Image 8.
ESD traction technique [69].
The advantage of performing ESD with larger polyps is the ability to take lesions en bloc which allows for accurate pathologic assessment of margins and is associated with lower recurrence rates ranging from 0 to 3 % as compared to EMR (0–9.1 % for en bloc resection, 10–23.5 % for piecemeal resection). For lesions with features suspicious for submucosal invasion, specifically those in the rectum, EUS can be considered prior to resection, as noted above [25]. Endoscopic resection of these lesions via ESD has significant cost benefits compared to surgery, which is an important consideration [8].
Furthermore, long term outcomes with large polyps that are completely removed with ESD are typically better with colonic lesions compared to rectal lesions. A 2013 study from Japan looking at long term outcomes showed that the risk for recurrence with high-risk lesions was 1.4 % in the colon and 16 % in the rectum, and thus surgery may be recommended as an adjunct for high-risk lesion in the rectum [30,31]. As such, when en bloc EMR in the rectum is not safe or feasible, ESD is preferred for resection of these lesions as it associated with higher en bloc and R0 resection rates, and has lower rates of recurrence than with piecemeal EMR [29].
ESD is a complex and demanding procedure (long procedure time, higher costs) with high potential complication rate (perforation, bleeding, and coagulation syndrome) [27,32]. In addition, it requires additional training (i.e. advanced endoscopy fellowship or other mentorship with supervision by an expert endoscopist). While ESD is accessible in tertiary academic medical centers with expert endoscopists, it is otherwise not widely available given the need for additional training and the complexity of the procedure.
Novel technique: endoscopic full thickness resection
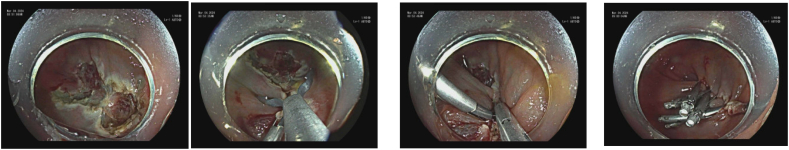
In cases where there is submucosal fibrosis or a polyp extends beyond the superficial submucosa, EMR and ESD should be avoided given their higher risk of complications. Although these lesions historically required surgery, recent advancement in endoscopic techniques introduced endoscopic full-thickness resection (EFTR) as a minimally invasive alternative to resect these lesions [33]. The technique involves full thickness resection of the mucosa including the muscularis propria, employing either an exposed or non-exposed approach. In the exposed EFTR, the lesion is removed first, and then the resulting defect is closed with a clipping or suturing device. Conversely, the non-exposed approach involves pinching off the lesion with a clip or sutures before resecting the lesion with a snare just above the clip or suture line. Common closure devices include endoclips for small (< 1 cm) defects and over-the-scope clips or Overstitch for larger defects [34]. An over the scope full thickness resection device that employs a clip over a large cap (Ovesco) with an embedded snare fitted on the tip of a colonoscope has been successfully used for resection of fibrotic polyps as well as those growing into a diverticulum or the appendiceal orifice [35] (Image 9). Though relatively safe and effective, EFTR of appendiceal orifice polyps has been associated with an increased risk of appendicitis [35].
Image 9.
EFTR.
a) Periappendiceal polyp; b) Edge marking; c) FTRD/clip device on scope; d, e) S/p resection with clip in place; f, g) FTRD resected specimen (both sides).
Novel technique: hybrid argon plasma coagulation
Another novel technique being employed is called hybrid argon plasma coagulation. This uses thermal ablation to reduce the risk of local recurrence after a polyp has been resected with EMR [36,37]. The two-part technique begins with standard EMR to completely resect the polyp, followed by repeat submucosal injection followed by APC of the base and edges of the resection bed to decrease the risk of local recurrence. This technique can also be efficacious for resection of recurrent polyps where submucosal fibrosis is present (Image 10).
Image 10.
Hybrid APC.
a) Hybrid APC after power jet injection; b) Hybrid APC after injection lift; c) Recurrent periappendiceal polyp; d) S/p partial resection/fibrosis; e) S/p Hybrid APC to fibrotic resection base.
Review of pathology & follow up
For adenomatous polyps confined to the mucosa including those with high grade dysplasia or intramucosal cancer, resection is considered adequate as long as deep margins show no evidence for submucosal invasion as there are no lymphatic channels in the colonic epithelium and as such there is no risk for lymph node metastases. Lateral margin assessment can be reliably assessed only with en bloc resection and as such piecemeal resection has been associated with higher rates of local recurrence. Thus, wide margin resection with or without margin treatment with electrocautery is an important consideration for polyps removed piecemeal. To aid in the pathologic review, pinning the specimens affixed to flat surfaces such as cork or foam can aid the pathologist to accurately define margins [8].
Adenomatous polyps associated with superficial invasive cancer that have been resected endoscopically can be considered curative if all of the following pathologic criteria have been met: well or moderate differentiation, negative resection margins, absence of lymphovascular invasion, absence of tumor budding (isolated tumor cells ahead of invasive front), and submucosal invasion depth of <1 mm. [38]. If these criteria are not met, the patient should be referred for surgical resection given the higher risk of lymph node metastases [25]. If only the lateral margin is positive (but no other high-risk criteria are met), re-treatment can be considered prior to consideration of surgery [25].
Though clear guidelines do not yet exist for endoscopic surveillance after EMR and ESD, it is generally recommended to survey 3–6 months after index treatment, especially if high risk dysplasia or intramucosal cancer is present or for large polyps that have been resected piecemeal. If there is no recurrence at follow up colonoscopy, then subsequent exams can be performed using routine post polypectomy surveillance guidelines [39].
Complications
Large polyp removal (EMR, ESD or EFTR) has a higher risk of potential complications compared to routine small polypectomy. The most dreaded complication is perforation, with ESD having a higher perforation rate of ~4.8 % compared to 2–14 % in EMR [27]. If recognized during the procedure, the perforation can often be managed endoscopically. Techniques such as standard endoclip closure, over the scope clips (Ovesco, Padlock) and endoscopic suturing techniques (OverStitch™ (BSC) or X-Tack™ (BSC)) have all been shown to be effective in managing perforations, the choice of which depends on the scenario and endoscopist preference (Image 11).
Image 11.
EMR perforation closure.
a) Post injection lift polyp; b) Perforation post EMR; c) Overstitch suturing; d) S/p overstitch closure.
Another potential complication of EMR and ESD is post polypectomy coagulation syndrome, a transmural burn from electrocoagulation causing local peritonitis [26]. This is typically associated with pain, fever, leukocytosis and peritoneal inflammation in the absence of perforation, which most commonly presents within 12 h of colonoscopy, though can be delayed up to 5–7 days [40,41]. Imaging may reveal focal thickening of the colonic wall with fat stranding. The syndrome is often managed conservatively with fluids, pain control and bowel rest [42] with surgery reserved for those with progressive symptoms or concern for perforation.
The most common complication of EMR and ESD is bleeding. Intraprocedural bleeding can be controlled with coagulation techniques or hemostatic clips. Additionally, careful coagulation of vessels in the resection bed can help avoid delayed bleeding [43]. Finally, prophylactic closure of the mucosal defect after EMR or ESD with clips or suture closure devices can reduce the risk of bleeding, especially in the right side of the colon [44] (Image 12).
Image 12.
Post EMR clip closure.
Endoscopic management of colonic obstruction
Background
Mechanical large bowel obstructions, often the result of malignancy, strictures, anastomotic issues, ischemia, radiation, or sequelae of diverticulitis, can be treated with endoscopic intervention specifically by using endoscopic balloon dilation or intraluminal stents. Balloon dilation is typically preferred for anastomotic or inflammatory, benign strictures but often requires serial dilations, while colonic malignancies are better treated with stenting. In general, both procedures are more successful if the lesions are <4 cm in length [45]. The use of dilation or stenting also depends on the location of the obstruction, and if surgery is indicated.
Endoscopic balloon dilation
Endoscopic balloon dilation passes a balloon catheter over a guidewire to the midpoint of the stricture. The balloon is inflated with saline slowly and then deflated and removed; care should be taken to ensure the balloon stays centered on the stricture while inflating with counter traction. The diameter of colonic balloons ranges from 6 to 20 mm [45]. Wire guided balloons are also available for tight strictures where fluoroscopy can be utilized to ensure safe and intraluminal placement of the balloon prior to inflation. Dilute contrast can be added to saline for inflation when fluoroscopy is used to assess effacement of the waist in the balloon during dilation (Image 13). Perforation can be a serious complication, and so choice of balloon size according to stricture size is important prior to dilation [47]. As noted, balloon dilation can be used with benign pathology such as anastomotic or inflammatory strictures. Studies have shown that the clinical success rate of dilation is approximately 89 % after a single dilation, but 50 % of cases typically result in re-obstruction an average of 5 years post-dilation [46].
Image 13.
Colonic balloon dilation of anastomotic stricture.
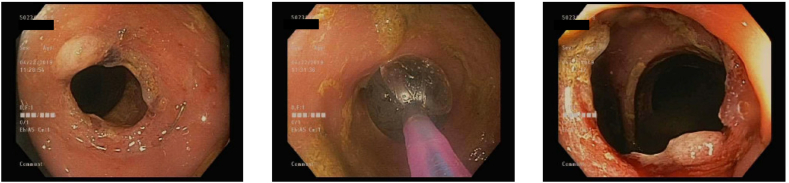
Intraluminal stents
Stents can be deployed through the scope, often after a guidewire or stent catheter is passed under fluoroscopy across the stenosis. The stents are typically self-expandable metallic metal stents (SEMS), ranging in post-deployment diameter from 20 to 35 mm (Image 14). These are often composed of nickel or titanium alloy which has increased flexibility for expansion [45]. These are deployed through the scope with or without guidewires, or with fluoroscopy. The stent often has markers on it that can be visualized under fluoroscopy to help with positioning [45]. Once deployed, there is often passage of colonic material or gas. It is recommended against passing the scope through the stent after deployment, given concern for the stent phalange damaging the scope [45]. Placement should be considered as a bridge to surgery, during chemoradiation, or as a palliative care option for end-of-life patients who are not candidates for surgery [47]. As a bridge to elective surgery, SEMS can be used in malignant obstruction to avoid emergent surgery and need for colostomy, and reduce preoperative mortality [54]. Only uncovered metal stents are currently approved for use in the colon due to their lower rates of migration and longer patency compared to covered stents. However, uncovered stents cannot be removed after they are placed due to tissue ingrowth. In some instances, removable, covered metal stents can be used off-label for benign strictures, but long-term safety data is lacking to support the routine use of stents for benign disease. There has been little success with SEMS in benign stricture disease, and there is concern about the complication rates, including migration and perforation [47]. There is a novel type of stent made of biodegradable polydioxanone microfilaments that has been developed as a means for temporarily stenting open the intestinal lumen and obviating the need for stent removal [48]. Unfortunately, there is a lack of research currently on how well this approach works in routine practice for colonic obstructions [49].
Image 14.
Colonic stent placement across obstructing colon cancer.
Clinical success with colonic stenting occurs in approximately 90 % of cases. Studies have shown improved outcomes in patients with malignant colonic obstruction undergoing stenting as a bridge to surgery compared to those who proceed directly with surgery [50], and more specifically in those higher risk patients (older, higher American Society of Anesthesiologists classification) [51]. The CReST Collaborative Group and Arezzo et al. [52,53] demonstrated that stenting left-sided malignant obstructions before surgery leads to lower short-term morbidity and lower likelihood of stoma creation during surgery, and Kanaka et al. [54] revealed a reduction in post-operative complications and mortality in stenting right-sided malignant obstruction before surgery. Tumors with a lower chance of successful stenting are in areas of sharp angulation where the guidewire or stent catheter is not able to pass across the obstruction. Careful consideration should also be taken when placing stents in the distal rectum. Historically, these stents were thought to have been associated with increased tenesmus, incontinence, rectal pain or bleeding, although recent research suggests stenting may be safe and clinically effective for malignant obstructions in the distal rectum [55].
Complications
Balloon dilation complications include perforation, re-stenosis, and bleeding, while colorectal stenting complications include perforation, pain, bleeding, stent re-obstruction and stent migration. Colonic re-obstruction is the most common complication for both procedures, occurring in 7–14 % of stent cases and 12–60 % of balloon dilation cases [45,50,56,57]. Additional balloon dilation or stent insertion can typically address these complications.
Endoscopic management of fluid collections: EUS techniques
EUS drainage
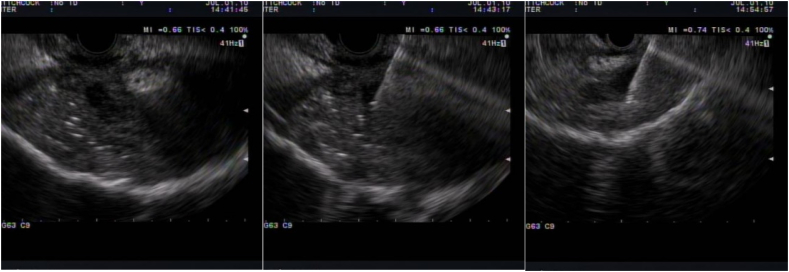
Endoscopic ultrasound (EUS) guided drainage has long been used in the drainage of peripancreatic fluid collections and biliary obstruction [58]. EUS can be used to assist with drainage of pericolonic and perirectal collections. Specifically, it has been employed in Crohn's disease with transrectal collections in scenarios where surgery may be challenging or unnecessary [59]. First, a needle is introduced with EUS guidance for access and aspiration, followed by guidewire insertion into the abscess cavity. Following this, a balloon catheter can be inserted over the wire for balloon dilation of the tract to allow placement of a pigtail stent to drain the abscess or collection [59]. Alternatively, one can place EUS guided lumen apposing metal stents (LAMS) for drainage of perirectal or pericolonic abscesses [60] (Image 15).
Image 15.
EUS drainage perirectal collection.
Indications and complications
Pelvic collections can develop due to inflammatory bowel disease, appendicitis, diverticulitis, ischemic colitis and even pelvic inflammatory disease. EUS guided drainage of perirectal or pericolonic collections has been considered a suitable alternative to surgical or percutaneous drainage (2017 Poincloux). Specifically, if reachable by echoendoscope and if not multiloculated, it is feasible and easy to drain such collections endoscopically [58]. It can also be an option for those with fecal diversion or those with altered anatomy [61]. Challenges to these procedures include stent dislodgement, migration, contamination with stool, perforation, or rectal discomfort (if a perirectal collection).
Closure of perforations and fistulas
Background
Historically, surgery was the only option for treating perforations and fistulas. In recent years, endoscopic advancements in closure techniques have resulted in safe and effective alternatives to surgery for fistula and perforation repair. The currently available endoscopic closure techniques are described in detail below.
Endoclips
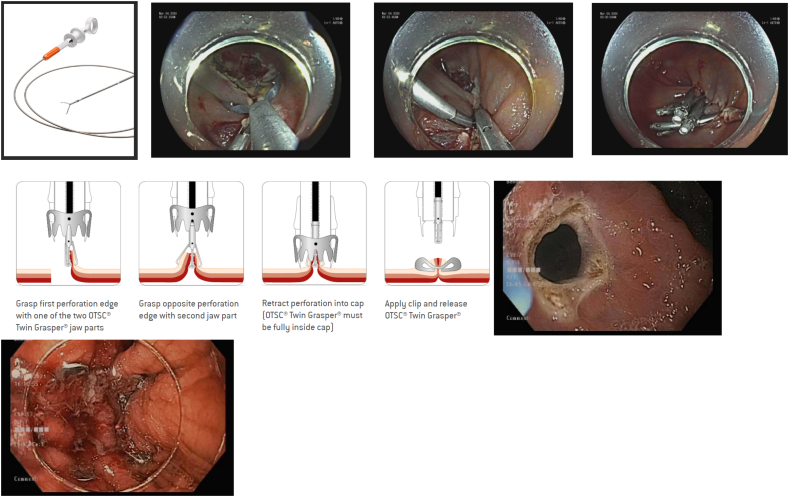
There are two available modalities of clipping, over-the-scope clips (OTSC) and through-the-scope clips (TTSC). Both are used in the management of chronic fistulas and acute perforations. Often, TTSC is employed to close small defects that are <1 cm. Typically, several clips are deployed in series to close the defect, and closure is limited to the mucosal and submucosal layers [62]. OTSC is used for closure of defects up to 2 cm in diameter and is typically more effective in closing full thickness defects [62]. The clip OVESCO (Tubingen, Germany) is the most commonly used OTSC and uses a hand wheel system to deploy the OTSC. The clip is mounted on an applicator cap at the distal end of the endoscope and uses suction to entrap the targeted tissue in the cap and deploy the clip over the defect with watertight closure. It allows for easy suction of the mucosa and enables approximation of the edges, allowing a more secure grasp of the defect [62] (Image 16).
Image 16.
(TTSC clip closure and OTSC).
a-d) TTSC for post EMR clip closure, courtesy Boston Scientific; e-g) OTSC for closure of gastric fistula, courtesy Ovesco Endoscopy AG.
Suture devices
Endoscopic suturing can be used to close larger defects such as fistulae or perforations, though is more technically challenging and requires special training prior to use. Apollo OverStitch™ (BSC) is a single use stitching device fitted on the tip of a double channel gastroscope that can place continuous or interrupted sutures without needing to remove the scope between applications [63,64] (Image 17). It has a curved needle to control depth of suture placement. As noted, sutures can be reloaded while maintaining visualization. After sutures are placed, there is a knotless fixation device (“cinch”). This technique is more successful in closure of acute perforations and small fistulae, and when compared to endoclips, suturing devices are more effective in closure of perforations [64]. Endoscopic suturing is less successful in closure of chronic fistulae (success rate of <23 %) [63]. Though suturing may be successful in the short-term, long-term data is lacking regarding the success rate of endoscopic suturing for closure of large fistulae.
Image 17.
(Overstitch device) – Courtesy Apollo Endosurgery.
a) Apollo device – courtesy Apollo endosurgery; b) Post EMR focal perforation; c) Overstitch device closure; d) post Overstitch defect closure.
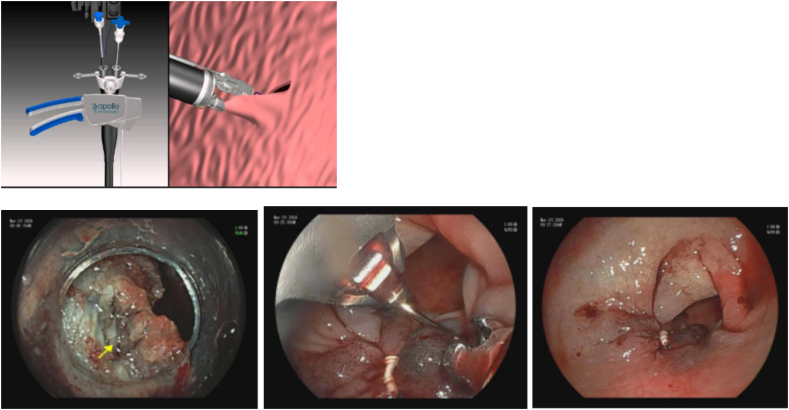
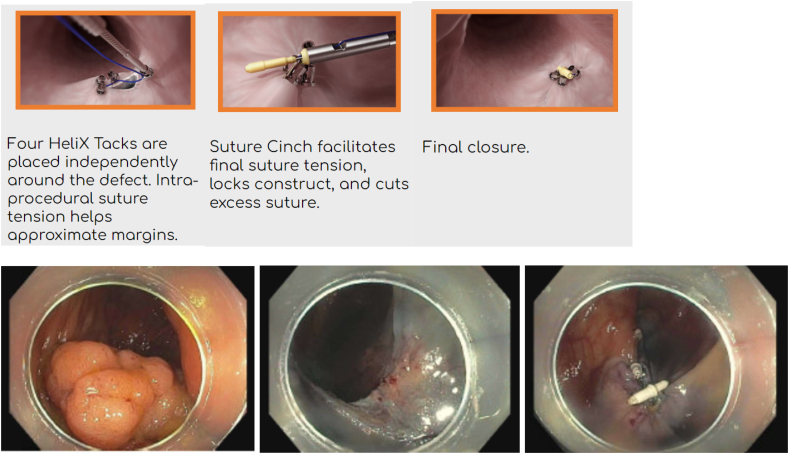
A specific suture-based tool, X-Tack™ (BSC), which is a through the scope device, has been FDA approved for closure of mucosal and full-thickness defects. It is made up of four 5-mm surgical steel tacks on a 3–0 polypropylene suture. The suture goes through an eyelet on all 4 tacks. Each tack is advanced one by one along suture string, and deployed into the tissue. Notably, the first tack should be deployed into healthy tissue 5-10 mm from the edge of the defect. If done incorrectly, this can be reversed with the drill function. After this, the additional sutures are reloaded and then deployed. When each is deployed, the suture should be pulled tight to remove slack. Once all placed, the suture is then tightened with a cinch, similar to the OverStitch cinch, to close the defect. An advantage of X-Tack compared to Overstitch is that it can be placed through both single channel gastroscopes and colonoscopes without the need to remove the scope to assemble or place the device. The device is particularly useful in the proximal colon where other closure devices may be unable to maneuver or reach the defect for closure [66] (Image 18).
Image 18.
X-Tack device.
a) X-tack device - courtesy Apollo Endosurgery; b) Large colon polyp; c) Post EMR defect; d) s/p X-Tack defect closure.
Glue - fibrin, purastat
Fibrin glue has been used to close fistulae or leaks including those associated with an anastomosis to create a mechanical seal [67]. Fibrin glue is applied via a double lumen catheter with an injection catheter to simultaneously inject human fibrinogen and clotting proteins (the sealant) and freeze-dried thrombin (the catalyst) to create a fibrin clot to seal the defect to promote healing and closure of the fistula. Typically anywhere from 1 to 10 cc are injected. Prior to applying glue, it is suggested to apply cautery or cytology brush to denude or ablate the epithelial lining of the fistulae to help with closure and healing [63,65]. Though initial success rates for fibrin glue are encouraging, recurrence rates are relatively high [67]. A newer hemostatic peptide agent (Purastat™ (3-D Matrix)) typically used for GI bleeding, that is a hydrogel containing four peptides that forms a molecular mesh in contact with blood, has been used off label by some in an attempt to close fistulae or leaks [68]. This comes in a 3 ml vial and one can inject as much as needed. Glues and sealants are sometimes used along with clips or suturing devices to close leaks and fistulae.
Ethical approval statement
There are no ethical concerns or conflicts regarding the subject matter.
CRediT authorship contribution statement
Stuart R. Gordon: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Formal analysis, Data curation, Conceptualization. Lauren S. Eichenwald: Writing – review & editing, Writing – original draft, Data curation. Hannah K. Systrom: Writing – review & editing, Writing – original draft, Data curation.
Declaration of competing interest
All authors contributed equally to researching the topics, drafting and editing the manuscript. The authors report no disclosures or conflicts of interest. The review article was unfunded.
References
- 1.Kawamura T., Takeuchi Y., Asai S., Yokota I., Akamine E., Kato M., et al. A comparison of the resection rate for cold and hot snare polypectomy for 4-9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study) Gut. 2018;67:1950–1957. doi: 10.1136/gutjnl-2017-314215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaukat A., Kaltenbach T., Dominitz J.A., Robertson D.J., Anderson J.C., Cruise M., et al. Endoscopic recognition and management strategies for malignant colorectal polyps: recommendations of the US multi-society task force on colorectal cancer. Gastroenterology. 2020;159:1916–1934.e2. doi: 10.1053/j.gastro.2020.08.050. [DOI] [PubMed] [Google Scholar]
- 3.Mohapatra S., Almazan E., Charilaou P., Recinos L., Bassi M., Broder A., et al. Outcomes of endoscopic resection for colorectal polyps with high-grade dysplasia or intramucosal cancer. Tech Innov Gastrointest Endosc. 2023;25:119–126. doi: 10.1016/j.tige.2023.01.003. [DOI] [Google Scholar]
- 4.Shinozaki S., Kobayashi Y., Hayashi Y., Sakamoto H., Lefor A.K., Yamamoto H. Efficacy and safety of cold versus hot snare polypectomy for resecting small colorectal polyps: systematic review and meta-analysis. Dig Endosc. 2018;30:592–599. doi: 10.1111/den.13173. [DOI] [PubMed] [Google Scholar]
- 5.Winston K., Maulahela H., Raharjo D.E., Tjoa K., Jonlean R. A comparative analysis of the efficacy and safety of hot snare polypectomy and cold snare polypectomy for removing small colorectal polyps: a systematic review and meta-analysis. Cureus. 2023 doi: 10.7759/cureus.38713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uraoka T., Takizawa K., Tanaka S., Kashida H., Saito Y., Yahagi N., et al. Guidelines for colorectal cold polypectomy (supplement to “guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection”) Dig Endosc. 2022;34:668–675. doi: 10.1111/den.14250. [DOI] [PubMed] [Google Scholar]
- 7.Kawamura T., Takeuchi Y., Yokota I., Takagaki N. Indications for cold polypectomy stratified by the colorectal polyp size: a systematic review and meta-analysis. J Anus Rectum Colon. 2020;4:67–78. doi: 10.23922/jarc.2019-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draganov P.V., Wang A.Y., Othman M.O., Fukami N. AGA institute clinical practice update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16–25.e1. doi: 10.1016/j.cgh.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 9.Gao P., Zhou K., Su W., Yu J., Report P.Z.-G. Academic Gastroenterology Report. 2023. Undefined. Endoscopic management of colorectal polyps. 2023•academicOupCom n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwok K., Mosadeghi S., Lew D. Polypectomy techniques for pedunculated and nonpedunculated polyps. Tech Innov Gastrointest Endosc. 2023;25:361–371. doi: 10.1016/j.tige.2023.02.006. [DOI] [Google Scholar]
- 11.Herman T., Megna B., Pallav K., Bilal M. Endoscopic mucosal resection: tips and tricks for gastrointestinal trainees. Transl Gastroenterol Hepatol. 2023:8. doi: 10.21037/TGH-23-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito A., Suga T., Ota H., Tateiwa N., Matsumoto A., Tanaka E. Resection depth and layer of cold snare polypectomy versus endoscopic mucosal resection. J Gastroenterol. 2018;53:1171–1178. doi: 10.1007/s00535-018-1446-2. [DOI] [PubMed] [Google Scholar]
- 13.Landin M.D., Guerrón A.D. Endoscopic mucosal resection and endoscopic submucosal dissection. Surg Clin N Am. 2020;100:1069–1078. doi: 10.1016/j.suc.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Ferlitsch M., Moss A., Hassan C., Bhandari P., Dumonceau J.M., Paspatis G., et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270–297. doi: 10.1055/s-0043-102569. [DOI] [PubMed] [Google Scholar]
- 15.Rashid M., Swartz A., Alomari M., Afraz S., Gutierrez D., Chadalavada P., et al. Cold vs hot emr for large colon polyps (≥20 mm); analysis of effectiveness, safety and cost-benefit. Gastrointest Endosc. 2023;97(6):AB535. doi: 10.1016/j.gie.2023.04.886. [DOI] [Google Scholar]
- 16.Mehta D., Loutfy A.H., Kushnir V.M., Faulx A.L., Smith Z.L. Cold versus hot endoscopic mucosal resection for large sessile colon polyps: a cost-effectiveness analysis. Endoscopy. 2022;54:367–375. doi: 10.1055/A-1469-2644. [DOI] [PubMed] [Google Scholar]
- 17.Malik TF, Mohan BP, Deliwala S, Kassab LL, Chandan S, Sharma NR, et al. Cold versus hot endoscopic mucosal resection for sessile serrated colorectal polyps ≥10 mm: a systematic review and meta-analysis. J Clin Gastroenterol 9900. [DOI] [PubMed]
- 18.Ortigão R., Weigt J., Afifi A., Libânio D. Cold versus hot polypectomy/endoscopic mucosal resection–a review of current evidence. United European Gastroenterol J. 2021;9:938–946. doi: 10.1002/UEG2.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papastergiou V., Paraskeva K.D., Fragaki M., Dimas I., Vardas E., Theodoropoulou A., et al. Cold versus hot endoscopic mucosal resection for nonpedunculated colorectal polyps sized 6-10 mm: a randomized trial. Endoscopy. 2018;50:403–411. doi: 10.1055/S-0043-118594. [DOI] [PubMed] [Google Scholar]
- 20.Shahidi N., Bourke M.J. How to manage the large nonpedunculated colorectal polyp. Gastroenterology. 2021;160:2239–2243.e1. doi: 10.1053/j.gastro.2021.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Chaves D.M., Brito H.P., Chaves L.T., Rodrigues R.A., Sugai B.M. Underwater endoscopic mucosal resection of serrated adenomas. Clinics. 2018:73. doi: 10.6061/clinics/2018/e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schenck R.J., Jahann D.A., Patrie J.T., Stelow E.B., Cox D.G., Uppal D.S., et al. Underwater endoscopic mucosal resection is associated with fewer recurrences and earlier curative resections compared to conventional endoscopic mucosal resection for large colorectal polyps. Surg Endosc. 2017;31:4174–4183. doi: 10.1007/s00464-017-5474-4. [DOI] [PubMed] [Google Scholar]
- 23.Yamashina T., Hanaoka N., Setoyama T., Watanabe J., Banno M., Marusawa H. Efficacy of underwater endoscopic mucosal resection for nonpedunculated colorectal polyps: a systematic review and Meta-analysis. Cureus. 2021 doi: 10.7759/cureus.17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maida M., Sferrazza S., Murino A., Lisotti A., Lazaridis N., Vitello A., et al. Effectiveness and safety of underwater techniques in gastrointestinal endoscopy: a comprehensive review of the literature. Surg Endosc. 2021;35:37–51. doi: 10.1007/s00464-020-07907-8. [DOI] [PubMed] [Google Scholar]
- 25.Pimentel-Nunes P., Dinis-Ribeiro M., Ponchon T., Repici A., Vieth M., De Ceglie A., et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 26.Harlow C., Sivananthan A., Ayaru L., Patel K., Darzi A., Patel N. Endoscopic submucosal dissection: an update on tools and accessories. Ther Adv. Gastrointest Endosc. 2020:13. doi: 10.1177/2631774520957220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumoulin F, Gastroenterology RH-WJ of, 2019 undefined. Endoscopic resection techniques for colorectal neoplasia: current developments. World J Gastroenterol, 2019•ncbiNlmNihGov n.d. [DOI] [PMC free article] [PubMed]
- 28.Yoshimoto T., Yoshihara T., Motozato K., Uraoka M., Takihara H., Inoue T., et al. Usefulness of sheath lifting after saline injection technique for colorectal endoscopic submucosal dissection. Endoscopy. 2021;53:E207–E208. doi: 10.1055/a-1244-9192. [DOI] [PubMed] [Google Scholar]
- 29.Keihanian T., Othman M.O. Colorectal endoscopic submucosal dissection: an update on best practice. Clin Exp Gastroenterol. 2021;14:317–330. doi: 10.2147/CEG.S249869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang D., Aihara H., Perbtani Y.B., Wang A.Y., Aadam A.A., Tomizawa Y., et al. Safety and efficacy of endoscopic submucosal dissection for rectal neoplasia: a multicenter North American experience. Endosc Int Open. 2019;07:E1714–E1722. doi: 10.1055/a-1010-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikematsu H., Yoda Y., Matsuda T., Yamaguchi Y., Hotta K., Kobayashi N., et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551–559. doi: 10.1053/j.gastro.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka Y., Tsuji Y., Sakaguchi Y., Minatsuki C., Asada-Hirayama I., Niimi K., et al. Bleeding after endoscopic submucosal dissection: risk factors and preventive methods. World J Gastroenterol. 2016;22:5927–5935. doi: 10.3748/wjg.v22.i26.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Høgh A., Deding U., Bjørsum-Meyer T., Buch N., Baatrup G. Endoscopic full-thickness resection (eFTR) in colon and rectum: indications and outcomes in the first 37 cases in a single center. Surg Endosc. 2022;36:8195–8201. doi: 10.1007/S00464-022-09263-1. [DOI] [PubMed] [Google Scholar]
- 34.Wu Z.W., Ding C.H., Song Y.D., Cui Z.C., Bi X.Q., Cheng B. Colon sparing endoscopic full-thickness resection for advanced colorectal lesions: is it time for global adoption? Front Oncol. 2022:12. doi: 10.3389/FONC.2022.967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas J.I., Teshima C.W., Mosko J.D. Management of periappendiceal orifice polyps. Clin Gastroenterol Hepatol. 2020;18:2425–2429. doi: 10.1016/j.cgh.2020.05.025. [DOI] [PubMed] [Google Scholar]
- 36.Motz V.L., Lester C., Moyer M.T., Maranki J.L., Levenick J.M., Thieme G., et al. Hybrid argon plasma coagulation-assisted endoscopic mucosal resection for large sessile colon polyps to reduce local recurrence: a prospective pilot study. Bibliography Endoscopy. 2022;54:580–584. doi: 10.1055/a-1677-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levenick J.M., Groff A.J., Manzo C., Lester C., Maranki J.L. Hybrid APC Colon EMR, a novel approach to reduce local recurrence. Tech Innov Gastrointest Endosc. 2022;24:10–15. doi: 10.1016/j.tige.2021.08.004. [DOI] [Google Scholar]
- 38.Shin J., Kim E.R., Jang H.J., Baek D.H., Yang D.H., Lee B.I., et al. Long-term prognosis of curative endoscopic submucosal dissection for early colorectal cancer according to submucosal invasion: a multicenter cohort study. BMC Gastroenterol. 2022;22:1–10. doi: 10.1186/S12876-022-02499-0/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J.H., Yoon J.Y., Hwang S.W., Park S.H., Yang D.H., Ye B.D., et al. A surveillance endoscopy strategy based on local recurrence rates after colorectal endoscopic submucosal dissection. J Clin Med. 2021:10. doi: 10.3390/jcm10194591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunaratnam N., Zolotarevsky E. In: Overview of endoscopic resection of gastrointestinal tumors. Connor R.F., editor. Wolters Kluwer; 2023. UpToDate. [Google Scholar]
- 41.Anderloni A., Jovani M., Hassan C., Repici A. Advances, problems, and complications of polypectomy. Clin Exp Gastroenterol. 2014;7:285–296. doi: 10.2147/CEG.S43084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jehangir A., Bennett K.M., Rettew A.C., Fadahunsi O., Shaikh B., Donato A. Vol. 5. Taylor & Francis; 2015. Post-polypectomy electrocoagulation syndrome: a rare cause of acute abdominal pain; p. 29147. Journal of Community Hospital Internal Medicine Perspectives, 2015•Taylor & Francis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thirumurthi S., Raju G.S. Management of polypectomy complications. Gastrointest Endosc Clin N Am. 2015;25:335–357. doi: 10.1016/j.giec.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Pohl H, Grimm I, Moyer M, Hasan M, Gastroenterology DP, 2019 undefined. Clip closure prevents bleeding after endoscopic resection of large colon polyps in a randomized trial. Elsevier n.d. [DOI] [PMC free article] [PubMed]
- 45.Beck D.E. 2021. Endoscopic Management of Bowel Obstruction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park C.H., Yoon J.Y., Park S.J., Cheon J.H., Il Kim T., Lee S.K., et al. Clinical efficacy of endoscopic treatment for benign colorectal stricture: balloon dilatation versus stenting. Gut. Liver. 2015;9:73–79. doi: 10.5009/GNL13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Hooft J.E., Veld J.V., Arnold D., Beets-Tan R.G.H., Everett S., Götz M., et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - update 2020. Endoscopy. 2020;52:389–407. doi: 10.1055/a-1140-3017. [DOI] [PubMed] [Google Scholar]
- 48.Jain D, Mahmood E, Clinical SS-J of, 2017 undefined. Biodegradable Stents. IngentaconnectCom n.d.
- 49.John Gásdal Karstensen A, Risager Christensen K, Brynskov J, Rønholt C, Vilmann P, Hendel J, et al. Biodegradable Stents for the Treatment of Bowel Strictures in Crohn's Disease: Technical Results and Challenges n.d. doi: 10.1055/s-0042-101940. [DOI] [PMC free article] [PubMed]
- 50.Jeong S.J., Park J. Focused review seRies: the roles of endoscopy in the management of colonic obstruction and perforation endoscopic management of benign colonic obstruction and Pseudo-obstruction. Clin Endosc. 2020;53:18–28. doi: 10.5946/ce.2019.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J.M., Byeon J.S. Colorectal stents: current status. Clin Endosc. 2015;48:194–200. doi: 10.5946/ce.2015.48.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill J., Lee S., Morton D., Parker M., Halligan S., Taylor S., et al. Colorectal endoscopic stenting trial (CReST) for obstructing left-sided colorectal cancer: randomized clinical trial. Br J Surg. 2022;109:1073–1080. doi: 10.1093/bjs/znac141. [DOI] [PubMed] [Google Scholar]
- 53.Arezzo A., Passera R., Lo Secco G., Verra M., Bonino M.A., Targarona E., et al. Stent as bridge to surgery for left-sided malignant colonic obstruction reduces adverse events and stoma rate compared with emergency surgery: results of a systematic review and meta-analysis of randomized controlled trials. Gastrointest Endosc. 2017;86:416–426. doi: 10.1016/j.gie.2017.03.1542. [DOI] [PubMed] [Google Scholar]
- 54.Kanaka S., Matsuda A., Yamada T., Ohta R., Sonoda H., Shinji S., et al. Colonic stent as a bridge to surgery versus emergency resection for right-sided malignant large bowel obstruction: a meta-analysis. Surg Endosc. 2022;36:2760–2770. doi: 10.1007/s00464-022-09071-7. [DOI] [PubMed] [Google Scholar]
- 55.Adler D.G., Schmidt K. Low lying rectal stents: How low can you go? Practical Gastroenterology. 2020;44:26–34. [Google Scholar]
- 56.Chan R.-H., Lin S.-C., Chen P.-C., Lin W.-T., Wu C.-H., Lee J.-C., et al. Vol. 24. 2020. Management of colorectal Anastomotic Stricture With Multidiameter Balloon Dilation: Long-term Results; pp. 1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Endo K., Takahashi S., Shiga H., Kakuta Y., Kinouchi Y., Shimosegawa T., et al. Short and long-term outcomes of endoscopic balloon dilatation for Crohn’s disease strictures. World J Gastroenterol. 2013;19:86–91. doi: 10.3748/wjg.v19.i1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi E.K., Kim J.H., Jeong S.U., Na S.Y., Boo S.J., Kim H.U., et al. Endoscopic ultrasound-guided perirectal abscess drainage without drainage catheter: a case series. Clin Endosc. 2017;50:297–300. doi: 10.5946/ce.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khalid A., Faisal M.F. Endoscopic ultrasound-guided transrectal drainage of perirectal abscess in a patient with crohn disease. American Journal of Case Reports. 2021:22. doi: 10.12659/AJCR.930698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manvar A., Karia K., Ho S. Endoscopic ultrasound-guided drainage of pelvic abscesses with lumen-apposing metal stents. Endosc Ultrasound. 2017;6:217–218. doi: 10.4103/eus.eus_46_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lisotti A., Cominardi A., Bacchilega I., Linguerri R., Fusaroli P. EUS-guided transrectal drainage of pelvic fluid collections using electrocautery-enhanced lumen-apposing metal stents: a case series. VideoGIE. 2020;5:380–385. doi: 10.1016/j.vgie.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mangiavillano B., Caruso A., Manta R., Di Mitri R., Arezzo A., Pagano N., et al. Over-the-scope clips in the treatment of gastrointestinal tract iatrogenic perforation: a multicenter retrospective study and a classification of gastrointestinal tract perforations. World J Gastrointest Surg. 2016;8:315. doi: 10.4240/WJGS.V8.I4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh R.R., Nussbaum J.S., Kumta N.A. Endoscopic management of perforations, leaks and fistulas. Transl. Gastroenterol Hepatol. 2018:3. doi: 10.21037/tgh.2018.10.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kantsevoy S, Bitner M, JD-G, 2015 undefined. Endoscopic suturing closure of large iatrogenic colonic perforation. GiejournalOrg n.d. [DOI] [PubMed]
- 65.Tang S.J. Endoscopic management of gastrocutaneous fistula using clipping, suturing, and plugging methods. Video Journal and Encyclopedia of GI Endoscopy. 2014;2:55–60. doi: 10.1016/j.vjgien.2014.03.001. [DOI] [Google Scholar]
- 66.Hernandez-Lara A., de Paredes A., VideoGIE E.R. VideogieOrg; 2021. Step-by-step instruction: using an endoscopic tack and suture device for gastrointestinal defect closure. undefined. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grimaud J.C., Munoz-Bongrand N., Siproudhis L., Abramowitz L., Sénéjoux A., Vitton V., et al. Fibrin glue is effective healing perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2010:138. doi: 10.1053/j.gastro.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 68.White K., Henson C.C. Endoscopically delivered Purastat for the treatment of severe haemorrhagic radiation proctopathy: a service evaluation of a new endoscopic treatment for a challenging condition. Frontline Gastroenterol. 2021 doi: 10.1136/flgastro-2020-101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mizutani H., Ono S., Ohki D., Takeuchi C., Yakabi S., et al. Recent development of techniques and devices in colorectal endoscopic submucosal dissection. Clin Endosc. 2017;50:562–568. doi: 10.5946/ce.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]