Abstract
Objective
To provide evidence for choosing surgical or nonsurgical treatment for epilepsy in patients with unilateral multilobar and hemispheric polymicrogyria (PMG).
Methods
We searched published studies until September 2022 related to unilateral multilobar and hemispheric PMG and included patients who were followed up at the Pediatric Epilepsy Centre of Peking University First Hospital in the past 10 years. We summarized the clinical characteristics and compared the long‐term outcomes after surgical or nonsurgical (anti‐seizure medications, ASMs) treatment.
Results
A total of 70 patients (49 surgical, 21 non‐surgical) with unilateral multilobar and hemispheric PMG were included. The median age at epilepsy onset was 2.5 years (1.0–4.1). The most common seizure types were focal and atypical absence seizures. In the whole cohort, 87.3% had hemiparesis and 67.1% had electrical status epilepticus during slow sleep (ESES). There were significant differences in age at epilepsy onset, extent of lesion, and EEG interictal discharges between the two groups. At the last follow‐up (median 14.1 years), the rates of seizure‐freedom (81.6% vs. 57.1%, p = 0.032) and ASM discontinuation (44.4% vs. 6.3%, p = 0.006) were higher in the surgical group than in the nonsurgical group. Patients in the surgical group had a higher rate of seizure‐freedom with complete resection/disconnection than with subtotal resection (87.5% vs. 55.6%, p = 0.078), but with no statistically significant difference. In the nonsurgical group, more extensive lesions were associated with worse seizure outcomes. Cognition improved postoperatively in 90% of surgical patients.
Significance
In patients with unilateral multilobar and hemispheric PMG, the age of seizure onset, the extent of the lesion and EEG features can help determine whether surgery should be performed early. Additionally, surgery could be more favorable for achieving seizure freedom and cognitive improvement sooner.
Plain Language Summary
We aim to summarize clinical characteristics and compare the long‐term outcomes after surgical and nonsurgical (ASM) treatment to provide a basis for treatment decisions for patients with unilateral multilobar and hemispheric polymicrogyria (PMG)‐related epilepsy. We found that patients with unilateral hemispheric and multilobar PMG had significantly higher rates of seizure freedom and ASM discontinuation with surgical treatment than with nonsurgical treatment. In the surgical group, seizure outcomes were better in patients treated with complete resection/disconnection than in those treated with subtotal resection, but the difference was not statistically significant.
Keywords: anti‐seizure medication, epilepsy, outcome, surgery, unilateral polymicrogyria
Key points.
The rates of seizure freedom and ASM withdrawal were both significantly higher in the surgical group than in the nonsurgical group.
Patients in the surgical group had earlier seizure onset, more extensive PMG lesions, and more severe developmental delay than those in the nonsurgical group.
In the surgical group, seizure outcomes were better in patients treated with complete resection/disconnection than in those treated with subtotal resection, but the difference was not statistically significant.
1. INTRODUCTION
Polymicrogyria (PMG) refers to excessive abnormal gyri in the cerebral cortex and is a type of malformation of cortical development (MCD). In recent years, it has been considered to be the result of late neuronal migration or postmigration abnormalities. Its etiology, anatomical distribution, pathology, and imaging and clinical manifestations are highly heterogeneous. 1 , 2 , 3 , 4 , 5 PMG is mainly diagnosed by brain MRI and classified according to anatomical distribution. On brain MRI, PMG mainly appears as an “overfolded and thickened” cortex, irregular cortical surface and “stippled” gray‐white matter junction. The most common locations are bilateral or unilateral perisylvian areas (60%–70% of all cases). In unilateral PMG, hemispheric or multilobar patterns are the more common patterns and often involve perisylvian regions. 1 , 2 , 6 , 7 The clinical manifestations of PMG are related to the anatomical distribution, etiology and coexistence of other structural brain malformations. The most common clinical symptom is epilepsy (70%–80% of all cases); and other common clinical symptoms include developmental delay (DD), motor deficits, and microcephaly. 7 , 8 Unilateral multilobar and hemispheric PMG usually presents with drug‐resistant epilepsy, hemiparesis, and DD, as well as persistent spike‐and‐slow wave activation during slow‐wave sleep (also known as electrical status epilepticus during slow sleep, ESES). 9 , 10 While it is generally believed that ESES may resolve spontaneously around puberty, the prolonged presence of ESES can lead to developmental epileptic encephalopathy with spike‐and‐wave activation in sleep (DEE‐SWAS) or epileptic encephalopathy with spike‐and‐wave activation in sleep (EE‐SWAS), in which about half of the patients may have residual severe impairments that affects their independent functioning. The severity of residual cognitive impairment is significantly associated with duration of DEE‐SWAS or EE‐SWAS. 11 , 12 , 13 , 14
There is controversy as to whether unilateral multilobar and hemispheric PMG‐associated epilepsy should be treated with epilepsy surgery as early as possible. Several studies have shown that epilepsy surgery can rapidly control PMG‐related epilepsy, eliminate the need for anti‐seizure medications (ASMs), and improve the cognition of patients. 15 , 16 , 17 However, PMG lesions are often widespread, the epileptogenic area is not always consistent with the anatomical location, and the lesions are likely to contain functional cortexs. Hence, there are risks of the worsening of motor deficits and the development of other neurological deficits after surgery. 15 , 17 , 18 , 19 Other studies 10 , 20 have shown that the ESES and seizures associated with unilateral PMG are age‐dependent and that the ESES disappears before 14 years of age. The seizures could also be controlled, ASMs could even be discontinued, and the cognition of most patients could gradually improve. 9 , 10 Therefore, how to assess the benefits and risks of and choose between surgical and pharmacological treatment for unilateral multilobar and hemispheric PMG is unclear.
At present, there is a lack of large‐sample studies of unilateral multilobar and hemispheric PMG‐associated epilepsy. We searched published clinical studies related to unilateral multilobar and hemispheric PMG and included patients who had been followed up at the Pediatric Epilepsy Centre of Peking University First Hospital in the past 10 years. We summarized the clinical characteristics and compared the long‐term outcomes after surgical and nonsurgical (ASM) treatment to provide a basis for treatment decisions for these patients.
2. METHODS
2.1. Literature search strategy and patient inclusion criteria
PubMed, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), WanFang Data, and China Science and Technology Journal Database (CQVIP) were searched from inception to September 30, 2022, for English or Chinese case series or case reports on hemilateral PMG with epilepsy containing detailed clinical information of patients. Searches were conducted using MeSH and the following terms: “Polymicrogyrias, Cerebral Polymicrogyrias, Polymicrogyrias Cerebral, Micropolygyrias, Cerebral Micropolygyrias, Micropolygyrias Cerebral.” Patients found in the literature or treated and followed up at our center meeting all of the following criteria were included in our study: (1) unilateral multilobar (involving two or more of the frontal, parietal, temporal, and occipital lobes, which may cover the perisylvian regions) and hemispheric PMG diagnosed by MRI and no other epileptogenic structural brain malformations, such as gray matter heterotopia, macrocephaly, or Sturge–Weber syndrome; (2) drug‐resistant epilepsy; and (3) regular follow‐up over at least 1 year of nonsurgical treatment or at least 1 year of follow‐up after surgical treatment with complete resection/disconnection or subtotal resection (the extent of resection was based on the epileptogenic zone defined by invasive EEG, in order to minimize motor deficits by preserving functional cortex).
2.2. Data collection
Data were collected as follows: (1) Baseline data: sex, age of seizure onset; semiology and frequency of seizures; hemiparesis; and cognitive development assessment (classified as normal or mild intellectual disability, and moderate/severe intellectual disability according to the relevant definitions in the literature or the developmental quotient (DQ) or intelligence quotient (IQ) values at our center). (2) Anatomical distribution on brain magnetic resonance imaging (MRI); (3) Presence of ESES on electroencephalography (EEG) (spike–wave index [SWI] > 30%–85% in non‐rapid eye movement [NREM] sleep according to the relevant definition in the included literature, with ESES defined at our center by SWI > 50% in NREM); and interictal EEG discharges, categorized as unilateral or bilateral. (4) Treatment and follow‐up data: surgical or nonsurgical treatment; surgery‐related data (age at surgery, type of operation, preoperative duration of epilepsy); numbers of ASMs applied; follow‐up duration; and age at the last follow‐up. Outcome indexes included: (1) seizure outcome: seizure‐free, defined as Engel Class I or International League Against Epilepsy (ILAE) Class I 21 in the surgical group and as seizure‐free for at least 1 year or meeting the definition in the included literature in the nonsurgical group; (2) whether ASMs had been discontinued; (3) cognitive developmental level at the last follow‐up and cognitive changes; and (4) neurological deficits at the last follow‐up.
2.3. Statistical analysis
Statistical analysis was performed with SPSS 26.0 for Windows (SPSS, Inc.). Continuous variables are expressed as the median and interquartile range (IQR: P25‐P75). Categorical variables are summarized as numbers and percentages. Factors were collected to analyze the variance in variables between the surgical and nonsurgical groups. The chi‐square test and Fisher's exact test were used to compare categorical data. The Mann–Whitney U test was used to compare continuous variables. A p value of <0.05 was considered to indicate statistical significance. Factors associated with prognosis were analyzed separately for the surgical and nonsurgical groups. Variables with a significance level < 0.05 on univariate analysis were then tested by multivariate logistic regression analysis. Missing data in the literature were excluded from the statistical analysis.
3. RESULTS
3.1. Clinical characteristics of all patients included in the study
3.1.1. General information and clinical features at baseline
A total of 70 patients (42 male, 28 female) were included, 60 of whom were from the 12 included published studies 9 , 15 , 16 , 17 , 19 , 20 , 22 , 23 , 24 , 25 , 26 , 27 (Figure 1); the other 10 patients were from our center. Forty‐nine patients (8 from our center) were included in the surgical group, and 21 patients (2 from our center) were included in the nonsurgical (ASM) group (Table 1).
FIGURE 1.
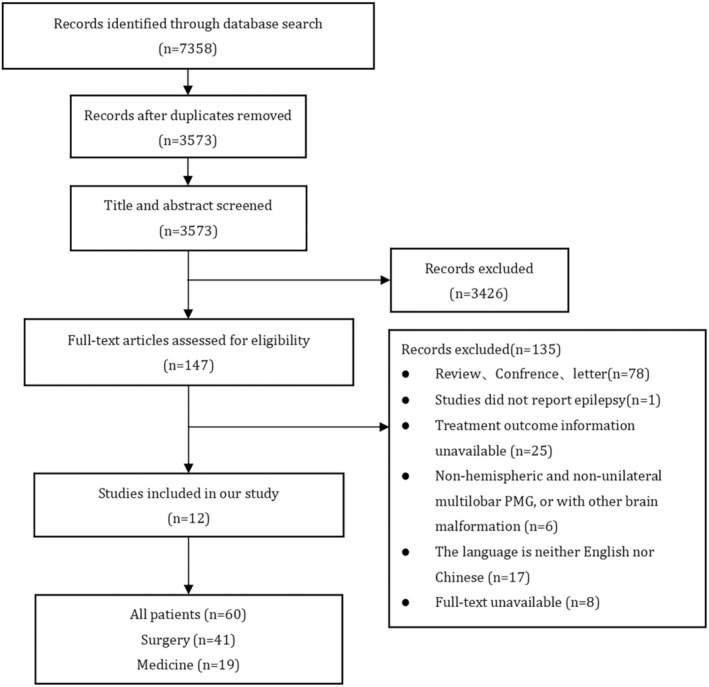
Flow chart of literature screening. Numbers of records database: Pubmed (n = 1340), Embase (n = 2055), Web of Science (n = 1978), Wanfang Data (n = 986), VIP (n = 12), CNKI (n = 987).
TABLE 1.
Patient information in included study and our center.
| Patient N (M/F) | Treatment N (sur/med) | Onset age median (IQR, y) | Hemiparesis n/N (%) | Location (hemi/multi) | Interictal EEG (Uni/Bi) | ESES n/N (%) | Surgery type (sub./com.) | Surgery age median (IQR, y) | Follow‐up duration (y) | Seizure‐free n/N (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fohlen, 2019 | 18 (12/6) | 18 (18/0) | 2.0 (1.0–2.7) | 18/18 (100) | 15/3 | / | 18/18 (100) | 0/18 | 7.0 (5.8–8.2) | 14.0 (12.3–15.7) | 16/18 (88.9) |
| Cossu, 2016 | 9 (5/4) | 9 (9/0) | 5.0 (1.0–5.5) | 7/9 (77.8) | 6/3 | 9/0 | 5/9 (55.6) | 4/5 | 10.0 (8.5–27.5) | 5.0 (2.4–6.1) | 7/9 (77.8) |
| Jalloh, 2018 | 6 (4/2) | 6 (6/0) | 3.0 (7.0–6.4) | 3/6 (50) | 0/6 | / | 0/6 (0) | 5/1 | 9.3 (8.4–14.3) | 2.7 (1.6–5.3) | 4/6 (66.7) |
| Sculier, 2021 | 1 (0/1) | 1 (1/0) | 0.4 | 1/1 (100) | 0/1 | / | / | 0/1 | 12.4 | 1.6 | 0/1 (0) |
| Brna, 2017 | 2 (2/0) | 2 (1/1) | 1—3 days | / | 2/0 | 2/0 | 0/2 (0) | 0/1 | / | 0.3 | 0/2 (0) a |
| Wang, 2020 | 2 (1/1) | 2 (2/0) | 2.0–3.0 | / | 1/1 | 1/1 | 2/2 (100) | 0/2 | 5.0–16.0 | 2.3–4.5 | 1/2 (50) |
| Jeong, 2016 | 3 (1/2) | 3 (3/0) | 2.5 (2.5–3.0) | 3/3 (100) | 1/3 | 0/3 | 3/3 (100) | 0/3 | 7.2 (5.3–11.5) | 4.6 (2.6–6.1) | 3/3 (100) |
| Zsoter, 2012 | 1 (0/1) | 1 (1/0) | 1.5 | 1/1 (100) | 1/0 | 0/1 | 1/1 (100) | 0/1 | 8.3 | 4 | 1/1 (100) |
| Ohtsukaa, 2002 | 5 (4/1) | 5 (0/5) | 6.7 (2.9–14.7) | 2/5 (40) | 0/5 | / | 2/5 (40) | / | / | 15.8 (6.5–20.5) | 3/5 (60) |
| Martinovic, 2008 | 1 (0/1) | 1 (0/1) | 6.2 | 1/1 (100) | 1/0 | 0/1 | 0/1 (0) | / | / | 2.8 | 0/1 (0) |
| Guerrini, 1998 | 3 (2/1) | 3 (0/3) | 4.0 (3.0–4.0) | 3/3 (100) | 0/3 | / | 3/3 (100) | / | / | 10.0 (4.0–12.0) | 3/3 (100) |
| Bartolini, 2016 | 9 (5/4) | 9 (0/9) | 4.0 (2.5–5.0) | 8/9 (88.9) | 0/9 | 0/9 | 9/9 (100) | / | / | 10.0 (7.5–22.0) | 6/9 (66.7) |
| Our center | 10 (6/4) | 10 (8/2) | 1.2 (3d‐3.0) | 9/10 (90) | 9/1 | 8/2 | 4/10 (40) | 0/8 | 5.3 (2.6–6.7) | 2.0 (1.0–4.4) | 8/10 (80) |
Note: Patient: M, male; F, female. Treatment: sur, surgery; med, medicine. Onset: IQR, interquartile range; y, year. Location: hemi, hemisphere; multi, unilateral multilobar. Interictal EEG: Uni, unilateral; Bi, bilateral. Surgery type: sub, subtotal resection; com, complete resection/disconnection.
Both patients died.
The median age at seizure onset was 2.5 years (1.0–4.1), and patients in the surgical group were younger seizure onset than those in the nonsurgical group (2.0 years vs. 4.0 years, p = 0.001). All patients had electro‐clinical seizures, except for 1 patient, who only presented with ESES but no clinical seizures. Seizure type was recorded in 61 patients, all of whom had focal seizures (61/61, 100%), and atypical absence seizures as the second most common seizure type (34/61, 55.7%); other seizure types included atonic seizures, myoclonic seizures and epileptic spasms. A total of 87.3% (55/63) of patients suffered from hemiparesis. The median number of ASMs used in the course of disease was 4 (3–6) among 29 patients. There was no significant difference in the number of ASMs between the two groups.
3.1.2. Distribution of PMG on brain imaging
On brain MRI, 65.7% (46/70) of patients had lesions in the right hemisphere, and 34.3% (24/70) in the left hemisphere. A total of 51.4% (36/70) of patients showed hemispheric PMG (Figure 2A), while 48.0% (34/70) showed unilateral multilobar PMG. Hemispheric PMG was more common in the surgical group than the nonsurgical group (32/49 vs. 4/21, p < 0.001). The most common anatomical location among the 34 patients with unilateral multilobar PMG was the frontoparietal lobes (13/34) or fronto‐parieto‐temporal lobes (12/34), which may include perisylvian region, central region, insula and opercular cortex. All eight patients at our center underwent positron emission tomography‐computed tomography (PET‐CT) examination preoperatively, and compared with the extent of PMG lesions on brain MRI, the extent of metabolic abnormalities on PET‐CT was the same in five patients, smaller in two patients, and more extensive in one patient.
FIGURE 2.

Magnetic resonance imaging (MRI) and video electroencephalogram (VEEG). (A–F) Right‐side hemispheric PMG in a 5‐year‐old girl with drug‐resistant epilepsy and left‐sided hemiparesis. (A) The lesion is shown in axial T1‐weighted MRI. (B) The patient with right‐side hemispheric PMG underwent right hemispheric dissection at the age of 6 years, with poor function of the left upper limb after surgery, which gradually recovered after rehabilitation. (C) EEG recording during sleep demonstrated widespread persistent spike‐and‐slow wave activation mainly on the right hemisphere, with a 95% spike–wave index (SWI) in non‐rapid eye movement (NREM). (D) Postoperative EEG during sleep demonstrated disappearance of ESES. (E, F) Atypical absence seizures monitored by EEG: the patient presented with a gradual diminish in response, a decrease in movement, blinking etc., and the simultaneous EEG showed widespread 2.5–4 Hz high to very high amplitude spike‐slow wave paroxysms for a few seconds to 30 s mainly in the bilateral anterior head, with the right anterior head being the most prominent.
3.1.3. EEG characteristics
ESES was present in 67.1% (47/70) of patients, among whom the median age at diagnosis of ESES was 4.5 years (4.0–5.2) (Figure 2B). ESES was present in 63.3% (31/49) and 76.2% (16/21) of patients in the surgical and nonsurgical groups, respectively (p = 0.291). Interictal EEG discharges were described in 47 patients, 59.6% (28/47) of which were unilateral and 40.4% (19/47) of which were bilateral. In the 17 patients in the nonsurgical group, 12 (70.6%) showed bilateral discharges, while only 23.3% (7/30) in the surgical group showed bilateral discharges (p = 0.002).
3.1.4. Perinatal history, family history and genetics
Perinatal and family history data were available for 35 patients. Among them, 28.6% (10/35) had perinatal abnormalities, the most common of which was an eventful delivery (3/35, 8.6%); the other nine cases included twin pregnancy with intrauterine death of one of the twins (2/43, 4.7%), threatened abortion in early pregnancy (2/35, 5.7%), intrauterine growth retardation (1/35, 2.9%), intrauterine cytomegalovirus infection (1/35, 2.9%), and hyperbilirubinemia in the neonatal period (1/35, 2.9%). Genetic information was available for 19 patients, and pathogenic variants that could explain the phenotype were identified in two cases; one was PIK3CA p.Glu545Lys, and the other was a de novo 1q21.1q21.2 duplication.
3.1.5. Information of the surgery
The median age at surgery was 7.2 years (5.7–10.0), and the median preoperative duration of epilepsy was 5.2 years (3.1–7.2) for 49 patients in the surgical group. A total of 81.6% (40/49) underwent complete resection/disconnection (Figure 2C), and 18.4% (9/49) underwent subtotal resection (Tables 1 and 2).
TABLE 2.
Clinical characteristics of all cases and comparison between two groups.
| All (N = 70) | Surgical (n = 49) | Nonsurgical (n = 21) | p value | |
|---|---|---|---|---|
| Female | 28 | 20 | 8 | 0.831 a |
| Male | 42 | 29 | 13 | |
|
Age of seizure onset median (IQR), y |
2.5 (1.0–4.1) | 2.0 (1.0–3.5) | 4.0 (3.0–6.1) | 0.001 b |
|
Follow‐up duration median (IQR), y |
5.3 (2.8–13.3) | 4.5 (2.0–13.1) | 10.0 (4.8–15.9) | 0.030 b |
|
Age of surgery median (IQR), y |
/ | 7.2 (5.7–10.0) | / | / |
|
Epilepsy duration before surgery median (IQR), y |
/ | 5.2 (3.1–7.2) | / | / |
|
Age of last follow‐up median (IQR), y |
14.1 (9.5–20.7) | 14.1 (9.8–21.0) | 14.0 (9.0–20.8) | 0.967 b |
| MRI location of PMG | ||||
| Hemisphere | 36/70 (51.4%) | 32/49 (65.3%) | 4/21 (19%) | |
| Multilobar | 34/70 (48.6%) | 17/49 (34.7%) | 17/31 (81%) | <0.001 a |
| Interictal EEG | ||||
| Unilateral | 28/47 (59.6%) | 23/30 (76.7%) | 5/17 (29.4%) | 0.002 c |
| Bilateral | 19/47 (40.4%) | 7/30 (23.3%) | 12/17 (70.6%) | |
| VEEG with ESES | 47/70 (67.1%) | 31/49 (63.3%) | 16/21 (76.2%) | 0.291 a |
|
Age of ESES diagnosis median (IQR), y |
4.5 (4.0–5.2) | 4.5 (4.0–5.5) | 4.5 (4.0–5.0) | 0.892 b |
| Seizure type | ||||
| Focal | 61/61 (100%) | 40/40 (100%) | 21/21 (100%) | / |
| AA | 34/61 (55.7%) | 21/40 (52.5%) | 13/21 (61.9%) | 0.482 a |
| Seizure frequency of baseline | ||||
| Daily | 42/63 (66.7%) | 30/47 (63.8%) | 12/16 (75%) | 0.074 c |
| Weekly | 8/63 (12.7%) | 6/47 (12.8%) | 2/16 (12.5%) | |
| Monthly | 10/63 (15.9%) | 10/47 (21.3%) | 0/16 (0%) | |
| Sporadic | 3/63 (4.8%) | 1/47 (2.1%) | 2/16 (12.5%) | |
|
ASMs numbers Median (IQR) |
4.0 (3.0–6.0) | 3.5 (2.3–5.8) | 5.0 (4.0–7.0) | 0.067 b |
| Hemiplegia/hemiparesis | 55/63 (87.3%) | 39/46 (84.8%) | 16/17 (94.1%) | 0.430 c |
| Seizure‐free at last visit | 52/70 (74.3%) | 40/49 (81.6%) | 12/21 (57.1%) | 0.032 a |
| Stop ASMs at last visit | 21/61 (34.4%) | 20/45 (44.4%) | 1/21 (6.3%) | 0.006 c |
| Development at last visit | ||||
| Normal/mild delay | 10/31 (32.3%) | 3/16 (18.8%) | 7/15 (46.7%) | 0.135 c |
| Moderate/severe delay | 21/31 (67.7%) | 13/16 (81.3%) | 8/15 (53.3%) | |
Note: Seizure frequency: daily: >30/month; weekly: 4‐29/month; monthly: 1‐3/month; sporadic: <1/month.
Abbreviations: IQR, interquartile range; y, year.
χ 2 test.
Mann–Whitney test.
Fisher's exact test.
Bold values means p < 0.05
3.2. Comparison of outcomes between the surgical and ASM treatment groups
3.2.1. Seizure outcomes
The median follow‐up duration was 4.5 years (2.0–13.1) and 10.0 years (4.8–15.9) for patients in the surgical and nonsurgical groups, respectively, and the median age at the last follow‐up was 14.1 years (9.8–21.0) and 14.0 years (9.0–20.8), respectively (p = 0.967). At the last follow‐up, the rate of seizure‐freedom in the surgical and nonsurgical groups was 81.6% (40/49) and 57.1% (12/21), respectively (p = 0.032). ESES disappeared immediately after surgery in 96.9% (31/32) of patients with ESES in the surgical group (Figure 2D), and the median age of ESES disappearance was 10.0 years (8.3–11.8) among 21 patients with ESES in the nonsurgical group. At the last follow‐up, 44.4% (20/45) of patients in the surgical group had discontinued ASMs, whereas only 6.3% (1/21) of patients in the nonsurgical group discontinued ASMs (p = 0.006). One patient in each of the two groups had early neonatal onset (1–3 days of age) with refractory seizures, and both died at 3–4 months of age (Table 2).
We performed a univariate correlation analysis of all variables that may affect the seizure outcome separately in the surgical and nonsurgical groups. We found that only sex was significantly correlated with the postoperative seizure outcome, with a higher rate of seizure freedom for male patients (p = 0.034) (Table 3). In the nonsurgical group, the extent of PMG lesions was significantly associated with the seizure outcome, with hemispheric PMG associated with a worse prognosis (p = 0.021) (Table 4).
TABLE 3.
Correlation factors of surgical seizure outcome.
| Total (N = 49) n/N (%) | Seizure outcomes | p value univariate analysis | ||
|---|---|---|---|---|
| Seizure‐free (N = 40) n/N (%) | Seizure + (N = 9) n/N (%) | |||
| Gender | ||||
| Male | 29/49 (59.2) | 27/40 (67.5) | 2/9 (22.2) | 0.034 a |
| Female | 20/49 (40.8) | 13/40 (32.5) | 7/9 (77.8) | |
| Age of onset | ||||
| Median (IQR), y | 2.0 (1.0–3.5) | 2.3 (1.0–3.8) | 0.8 (0.3–3.0) | 0.077 b |
| Exposure duration | ||||
| Median (IQR), y | 5.2 (3.1–7.2) | 5.2 (3.1–6.7) | 5.7 (2.3–10.6) | 0.546 b |
| Age of surgery | ||||
| Median (IQR), y | 7.2 (5.7–10.0) | 7.4 (5.9–9.9) | 6.7 (3.5–12.8) | 0.689 b |
| Cognition before surgery | ||||
| Normal/mild delay | 17/46 (37.0) | 14/38 (36.8) | 3/8 (37.5) | 1.000 a |
| Moderate/severe delay | 29/46 (63.0) | 24/38 (63.2) | 5/8 ((62.5) | |
| Numbers of ASMs | ||||
| Median (IQR), n | 3.5 (2.3–5.8) | 4.0 (3.0–6.0) | 2.0 (2–3) | 0.091 b |
| Seizure frequency | ||||
| Daily | 30/47 (63.8) | 23/39 (59.0) | 7/8 (87.5) | 0.611 c |
| Weekly | 6/47 (12.8) | 6/39 (15.4) | 0/8 (0) | |
| Monthly | 10/47 (21.3) | 9/39 (23.0) | 1/8 (12.5) | |
| Sporadic | 1/47 (2.1%) | 1/39 (2.6) | 0/8 (0) | |
| Interictal EEG discharge | ||||
| Unilateral | 23/30 (76.7) | 18/24 (75.0) | 5/6 (83.3) | 1.000 c |
| Bilateral | 7/30 (23.3) | 6/24 (25.0) | 1/6 (16.7) | |
| ESES | ||||
| With | 31/49 (63.3) | 28/40 (70.0) | 3/9 (33.3) | 0.093 a |
| Without | 18/49 (36.7) | 12/40 (30.0) | 6/9 (66.7) | |
| Age of ESES onset | ||||
| Median (IQR), y | 4.5 (4.0–5.5) | 4.4 (3.7–5.4) | 4.5 (4.0–7.7) | 0.617 b |
| ESES duration | ||||
| Median (IQR), y | 2.9 (1.4–4.2) | 2.9 (1.4–4.5) | 2.9 (1.0–3.2) | 0.560 b |
| MRI location | ||||
| Hemisphere | 32/49 (65.3) | 27/40 (67.5) | 5/9 (55.6) | 0.770 a |
| Unilateral multilobe | 17/49 (34.7) | 13/40 (32.5) | 4/9 (44.4) | |
| Surgery | ||||
| Subtotal resection | 9/49 (18.4) | 5/40 (12.5) | 4/9 (44.4) | 0.078 a |
| Complete resection/disconnection | 40/49 (81.6) | 35/40 (87.5) | 5/9 (55.6) | |
Abbreviations: IQR, interquartile range; y, year.
χ 2 test.
Mann–Whitney test.
Fisher's exact test.
Bold values means p < 0.05
TABLE 4.
Correlation factors of seizure outcome in the nonsurgical group.
| Total (N = 21) n/N (%) | Seizure outcomes | p value univariate analysis | ||
|---|---|---|---|---|
| Seizure‐free (N = 12) n/N (%) | Seizure + (N = 9) n/N (%) | |||
| Gender | ||||
| Male | 13/21 (61.9) | 7/12 (58.3) | 6/9 (72.7) | 1.000 a |
| Female | 8/21 (38.1) | 5/12 (41.7) | 3/9 (33.3) | |
| Age of onset | ||||
| Median (IQR), year | 4.0 (3.0–6.1) | 4.0 (3.0–6.0) | 3.4 (2.5–6.1) | 0.793 b |
| Age of ESES onset | ||||
| Median (IQR), year | 4.0 (4.0–5.0) | 4.0 (4.0–5.0) | 4.0 (3.0–8.0) | 0.855 b |
| Age of ESES disappearing | ||||
| Median (IQR), year | 4.5 (4.0–5.0) | 10.0 (8.0–12.0) | 10.0 (9.0–11.0) | 0.905 b |
| Numbers of ASMs | ||||
| Median(range), n | 5.0 (4.0–7.0) | 4.0 (3.8–6.3) | 6.0 (4.0–8.0) | 0.174 b |
| Seizure frequency | ||||
| Daily | 12/16 (75.0) | 7/9 (77.8) | 5/7 (71.4) | 1.000 a |
| Weekly | 2/16 (12.5) | 1/9 (11.1) | 1/7 (14.3) | |
| Monthly | 0/16 (0) | 0/9 (9) | 0/7 (0) | |
| Sporadic | 2/16 (12.5) | 1/9 (11.1) | 1/7 (14.3) | |
| Interictal EEG discharge | ||||
| Unilateral | 5/17 (29.4) | 3/9 (25) | 2/8 (25.0) | 1.000 a |
| Bilateral | 12/17 (70.6) | 6/9 (50) | 6/8 (75.0) | |
| ESES | ||||
| With | 16/21 (76.2) | 11/12 (91.7) | 5/9 (55.6) | 0.119 a |
| Without | 5/21 (23.8) | 1/12 (8.3) | 4/9 (44.4) | |
| ESES duration | ||||
| Median (IQR), year | 5.0 (3.0–7.8) | 5.0 (3.0–7.5) | 5.0 (2.0–8.0) | 0.905 b |
| MRI location | ||||
| Hemisphere | 4/21 (19.0) | 0/12 (0) | 4/9 (44.4) | 0.021 a |
| Unilateral multilobar | 17/21 (81.0) | 12/12 (100) | 5/9 (55.6) | |
| Cognitive at ESES diagnosed | ||||
| Normal/mild | 9/11 (81.8) | 7/8 (87.5) | 2/3 (66.7) | 0.491 a |
| Moderate/severe | 2/11 (18.2) | 1/8 (12.5) | 1/3 (33.3) | |
| Cognitive at last follow‐up | ||||
| Normal/mild | 7/15 (46.7) | 5/10 (50) | 2/5 (40) | 1.000 a |
| Moderate/severe | 8/15 (53.3) | 5/10 (50) | 3/5 (60) | |
Abbreviations: disc., disconnection; hemi, hemispherotomy; IQR, interquartile range; y, year.
Fisher's exact test.
Mann–Whitney test.
Bold values means p < 0.05
3.2.2. Developmental outcomes and surgical complications
In the surgical group, preoperative cognitive developmental level data were available in 46 patients, with normal development or mild delay in 37.0% (17/46), and moderate or severe delay in 63.0% (29/46). Cognitive function showed postoperative improvement in 90.9% (30/33). Postoperative hemiplegic changes were described in 34 patients; among them, 55.9% (19/34) suffered from worsening or newly developed hemiplegia, which gradually improved after rehabilitation. Twenty‐two of 33 patients (66.7%) developed hemianopia after surgery but was well tolerated. The cognitive developmental level at the last follow‐up was only described for 16 surgical patients, with normal development or mild delay reported in 18.8% (3/16) and moderate or severe delay seen in 81.3% (13/16). Among these 16 patients, the median age at surgery was 6.8 years (5.7–7.8) and seizure freedom was achieved in 87.5% (14/16).
In the nonsurgical group, the developmental level at the time of ESES diagnosis was described in 11 patients, with normal development or mild delay seen in 81.8% (9/11) and moderate or severe delay seen in 18.2% (2/11). The developmental level at the last follow‐up was described for 15 patients, with normal or mild delay in 46.7% (7/15) and moderate–severe delay in 53.3% (7/15); among these 15 patients, seizure freedom was achieved in 66.7% (10/15).
In addition to exacerbation of hemiparesis and hemianopsia, other postoperative complications were described in 43 surgical cases. Two patients developed hydrocephalus at 1 and 6 months postoperatively and underwent ventriculoperitoneal shunt (VPS) placement. One patient had a superficial wound infection, 17 and one patient developed cerebral oedema which improved after dexamethasone treatment. 17 Postoperative brain MRI showed ischemic manifestations posterior to the resection margin in one patient. 17 One patient developed transient 3rd cranial nerve palsy. 15
4. DISCUSSION
PMG is a common MCD associated with drug‐resistant epilepsy and can be treated surgically in some patients. However, PMG accounts for only a small portion of the current studies on epilepsy surgery. Castro‐Villablanca et al. 28 reported that PMG was present in only 2.6% of 117 children with epilepsy who had unilateral lesions on brain MRI and underwent epilepsy surgery. Chang et al. 29 reported that PMG was present in only 4.9% of 143 MCD patients who underwent surgical treatment. Unilateral PMG patients often present with ESES. Caraballo et al. 10 reported ESES in 81% (43/53) of patients with hemispheric PMG, and 67.1% (47/70) of patients in our study presented with ESES. Several studies have also shown that PMG is the most common structural cause of ESES. 25 , 30 ESES‐associated epilepsy is generally considered to be age‐dependent and self‐limiting, and similar phenomena have been reported for PMG‐related ESES. 10 , 20 , 31 However, prolonged ESES can lead to DEE‐SWAS or EE‐SWAS with a legacy of severe cognitive impairment and neuropsychological damage. 14 , 32 , 33 , 34 Some studies 15 , 16 have shown that PMG‐related ESES can disappear immediately after surgery. Nevertheless, unilateral PMG lesions are extensive and often involve the eloquent cortex, and surgery carries the risk of causing hemiplegia aggravation, hemianopia, and permanent hand dysfunction. 16 , 17 Current findings on seizure outcomes after surgery for PMG vary widely, with percentage of seizure freedom ranging from 25% to 100%. 8 , 15 , 16 , 17 , 35 , 36 , 37 Thus, for patients with pre‐existing significant motor deficits, surgical treatment is more conducive to seizure control and improve developmental outcome, making the surgical decision unquestionable. However, for patients with hemispheric multilobar PMG with less affected motor function, the surgical decision remains controversial and needs to be further explored in studies with larger samples because of the difficulty in localizing the epileptogenic zone and the possibility that surgery may lead to worsening of motor deficits.
Of the 70 patients with unilateral hemispheric and multilobar PMG in this study, 49 and 21 underwent surgical or nonsurgical (ASM) treatment, respectively. The median age at seizure onset was 2.5 years (1.0–4.1) in all patients, and focal seizures and atypical absence seizures were the most common seizure types. The incidence of ESES was 67.1%, with no statistical difference between the two groups. All children with atypical absence seizures showed ESES on the EEG. A total of 87.3% suffered from hemiparesis. The median age at the last follow‐up was 14.1 years (9.5–20.7), and the seizure freedom (81.6% vs. 57.1%, p = 0.032) and ASM discontinuation (44.4% vs. 6.3%, p = 0.006) rates were significantly higher in the surgical group than in the nonsurgical group.
At present, surgical options for unilateral PMG include complete/subtotal resection/disconnection and corpus callosotomy. This study only included patients who underwent complete/subtotal resection/disconnection. We found better seizure outcomes with complete resection/disconnection than with subtotal resection (seizure freedom 87.5% vs. 55.6%), but the difference was not significant. Similar results were reported by Jalloh et al. 17 All eight patients at our center underwent complete dissection, and only one patient still had seizures after surgery, but with an over 90% reduction in seizure frequency. Several studies 15 , 17 , 18 , 19 have evaluated the concordance between epileptogenic foci and PMG lesions on brain MRI using invasive EEG, and the results have shown that not all PMG lesions visible on MRI are epileptogenic, while normal regions on MRI may also be epileptogenic. Maillard et al. 18 evaluated the concordance between PMG lesions defined by brain MRI and epileptogenic zones defined by invasive EEG in 49 patients and showed that only 16% showed complete concordance. As the extent of PMG lesions often involves the motor or sensory functional cortex and not all lesions are epileptogenic, subtotal resection or complete resection may be chosen after comprehensive assessment of benefits and risks. Four patients in the included literature underwent corpus callosotomy, but none of them showed significant seizure control after surgery. 9 , 19 , 20
Regarding seizure outcomes, the rates of seizure‐freedom and ASM‐withdrawal were both significantly higher in the surgical group than in the nonsurgical group in our study. However, the baseline status in the two groups differed, with an earlier age at epilepsy onset and more extensive lesions (predominantly hemispheric lesions) in the surgical group. Cossu et al. 15 also showed significantly higher seizure‐freedom and ASM‐withdrawal rates for patients with PMG‐related epilepsy treated with surgery compared to medications, but this study also included patients with bilateral PMG. Among the 31 patients in our surgical group with ESES, ESES disappeared after surgery in all but one. The median age at ESES disappearance during the follow‐up was 10.0 years (8.3–11.8) among the 21 patients with ESES in the nonsurgical group. Wang et al. 25 reported that ESES disappeared in 80% of patients after surgery. The studies we included did not describe the age at which patients in either group began to achieve freedom from seizures; thus, it is not clear whether surgery could help patients achieve seizure freedom earlier. However, it is usually considered that patients could achieve a seizure‐free status immediately after surgery, and all seven patients included at our center became seizure‐free immediately after surgery. Hence, we believe that surgery may be more favorable for patients to achieve seizure freedom and ESES remission more quickly.
Regarding the cognitive outcomes, there was very little information that could be extracted from the included studies. The cognitive developmental level at baseline was usually considered to be worse after ESES onset. Therefore, we compared the preoperative cognitive level of patients in the surgical group with the cognitive level after the onset of ESES in the nonsurgical group and found more severe DD in the surgical group; however, 90.9% (30/33) of patients in the surgical group showed improvement in cognition postoperatively. The preoperative cognitive level of the eight surgically treated patients at our center indicated moderate to severe cognitive delay, except for one patient with borderline delay, and each of these eight patients showed improvement after surgery. As such, it is our opinion that surgery is more helpful for improving cognitive developmental outcomes in PMG patients with more severe DD.
In our study, 87.3% (55/63) of patients had hemiplegia, but the severity of the hemiplegia was not described in detail. A total of 55.9% (19/34) of surgically treated patients experienced aggravation of hemiplegia or new hemiplegia after surgery, which could gradually improve after rehabilitation. Cossu et al. 15 reported transient hemiparesis exacerbation or new hemiparesis in 8 cases after surgery among 15 cases of unilateral PMG. In a study by Jalloh et al., 17 five patients with unilateral PMG developed new hemiparesis or hemiparesis exacerbation after surgery and required in‐hospital rehabilitation, but those who could walk preoperatively were able to walk within a few weeks after surgery. Fohlen et al. 16 reported that all 18 patients with unilateral PMG who were treated surgically regained their walking ability at 3 months postoperatively, but 8 patients were left with permanent hand dysfunction. However, in a study by Zsoter et al. 16 four patients with unilateral PMG all retained grip function on the hemiplegic side after hemispherectomy.
Four included studies 15 , 16 , 17 , 18 , 19 described surgery‐related complications; two patients experienced serious complications with hydrocephalus several months after surgery and underwent VPS. More common complications included aggravation of hemiparesis or new neurological deficits (e.g., hemianopia) after surgery.
Correlation analysis revealed that patients with complete resection/disconnection may have better seizure outcomes than those with subtotal resection, which is consistent with the findings reported by Jalloh. 17 Maillard et al. 18 found that a shorter duration of epilepsy was significantly associated with a higher postoperative seizure‐free rate. Among patients in the nonsurgical group, patients with less extensive lesions (multilobar PMG) had a better prognosis. In addition, we found that patients with ESES had better seizure outcomes than those without ESES, although there was no significant difference. According to the results of our study, patients with hemispheric PMG, earlier onset, and more severe DD should be considered to be treated with surgery.
In conclusion, we believe that surgical treatment may be more beneficial for improving seizure and cognitive outcomes in patients with unilateral multilobar or hemispheric PMG. Therefore, patients with unilateral multilobar or hemispheric PMG should undergo presurgical evaluation as early as possible, especially those with extensive lesions, early onset, frequent seizures, severe DD, severe hemiparesis and poor results of treatment with multiple ASMs. However, surgery carries the risk of aggravating hemiparesis and visual field defects, and some patients with ESES may have spontaneous seizure and ESES remission around puberty; therefore, this option should be carefully considered in children with relatively good cognition, mild hemiparesis, and infrequent seizures.
There are some limitations to our study. The majority of the included studies were retrospective. There was a case‐selection bias, with differences in the severity of the baseline condition between patients who underwent surgery and those who continued treatment with ASMs. Second, some original information of the patients in the literature was missing, such as the age at which the become seizure‐free, details on cognitive developmental follow‐up and severity of hemiparesis; in particular, the developmental level could not be analyzed more thoroughtly due to insufficient information.
5. CONCLUSIONS
In this study, we found that patients with unilateral hemispheric and multilobar PMG had significantly higher rates of seizure freedom and ASM discontinuation with surgical treatment than with nonsurgical treatment. Additionally, surgical treatment may be more beneficial for patients to achieve seizure freedom sooner, with earlier improvement in cognition, which in turn improves quality of life. Seizure outcomes may be better in patients treated with complete resection/disconnection than in those treated with subtotal resection. However, complete resection/dissection carries a greater risk of causing hemiparesis aggravation and permanent hemianopia. Compared with patients in the nonsurgical group, patients in the surgical group had earlier onset, more extensive PMG lesions, and more severe developmental delays at baseline. Therefore, we believe that the choice of therapeutic modality requires individualized consideration. However, currently available studies have not been able to provide definitive conclusions regarding the impact of different treatment modalities on the long‐term developmental outcomes.
AUTHOR CONTRIBUTIONS
YW, LC, and YJ designed the study. PW and XL collected the data. PW, XL and JZ analyzed the data. PW and YW wrote the manuscript. All authors discussed the results and reviewed the manuscript.
FUNDING INFORMATION
This work was supported by the National Key Research and Development Program of China (grant number: 2020YFA0804000), the National Natural Science Foundation of China (grant number: U22A20339), and the Beijing Key Laboratory of Molecular Diagnosis and Study on Pediatric Genetic Diseases (grant number: BZ0317).
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICS STATEMENT
The study was approved by the ethics committee of Peking University First Hospital, and written informed consents were obtained from the parents of all participants.
Wu P, Liu Q, Liu X, Sun Y, Zhang J, Wang R, et al. Clinical features of unilateral multilobar and hemispheric polymicrogyria (PMG)‐related epilepsy and seizure outcome with different treatment options. Epilepsia Open. 2024;9:1480–1492. 10.1002/epi4.12988
REFERENCES
- 1. Stutterd CA, Leventer RJ. Polymicrogyria: a common and heterogeneous malformation of cortical development. Am J Med Genet C Semin Med Genet. 2014;166c(2):227–239. [DOI] [PubMed] [Google Scholar]
- 2. Severino M, Geraldo AF, Utz N, Tortora D, Pogledic I, Klonowski W, et al. Definitions and classification of malformations of cortical development: practical guidelines. Brain. 2020;143(10):2874–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oegema R, Barakat TS, Wilke M, Stouffs K, Amrom D, Aronica E, et al. International consensus recommendations on the diagnostic work‐up for malformations of cortical development. Nat Rev Neurol. 2020;16(11):618–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khandelwal A, Aggarwal A, Sharma A, Malik A, Bose A. Magnetic resonance imaging of malformations of cortical development‐a comprehensive review. World Neurosurg. 2022;159:70–79. [DOI] [PubMed] [Google Scholar]
- 5. Epilepsy Phenome/Genome Project, Epi4K Consortium . Diverse genetic causes of polymicrogyria with epilepsy. Epilepsia. 2021;62(4):973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barkovich AJ. MRI analysis of sulcation morphology in polymicrogyria. Epilepsia. 2010;51(Suppl 1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leventer RJ, Jansen A, Pilz DT, Stoodley N, Marini C, Dubeau F, et al. Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain. 2010;133(Pt 5):1415–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shain C, Ramgopal S, Fallil Z, Parulkar I, Alongi R, Knowlton R, et al. Polymicrogyria‐associated epilepsy: a multicenter phenotypic study from the epilepsy phenome/genome project. Epilepsia. 2013;54(8):1368–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guerrini R, Genton P, Bureau M, Parmeggiani A, Salas‐Puig X, Santucci M, et al. Multilobar polymicrogyria, intractable drop attack seizures, and sleep‐related electrical status epilepticus. Neurology. 1998;51(2):504–512. [DOI] [PubMed] [Google Scholar]
- 10. Caraballo RH, Cersósimo RO, Fortini PS, Ornella L, Buompadre MC, Vilte C, et al. Congenital hemiparesis, unilateral polymicrogyria and epilepsy with or without status epilepticus during sleep: a study of 66 patients with long‐term follow‐up. Epileptic Disord. 2013;15(4):417–427. [DOI] [PubMed] [Google Scholar]
- 11. Öztoprak Ü, Yayici Köken Ö, Aksoy E, Yüksel D. Spike‐wave index assessment and electro‐clinical correlation in patients with encephalopathy associated with epileptic state during slow sleep (ESES/CSWS), single‐center experience. Epilepsy Res. 2021;170:106549. [DOI] [PubMed] [Google Scholar]
- 12. Fortini S, Corredera L, Pastrana AL, Reyes G, Fasulo L, Caraballo RH. Encephalopathy with hemi‐status epilepticus during sleep or hemi‐continuous spikes and waves during slow sleep syndrome: a study of 21 patients. Seizure. 2013;22(7):565–571. [DOI] [PubMed] [Google Scholar]
- 13. Robinson RO, Baird G, Robinson G, Simonoff E. Landau‐Kleffner syndrome: course and correlates with outcome. Dev Med Child Neurol. 2001;43(4):243–247. [DOI] [PubMed] [Google Scholar]
- 14. Specchio N, Wirrell EC, Scheffer IE, Nabbout R, Riney K, Samia P, et al. International league against epilepsy classification and definition of epilepsy syndromes with onset in childhood: position paper by the ILAE task force on nosology and definitions. Epilepsia. 2022;63(6):1398–1442. [DOI] [PubMed] [Google Scholar]
- 15. Cossu M, Pelliccia V, Gozzo F, Casaceli G, Francione S, Nobili L, et al. Surgical treatment of polymicrogyria‐related epilepsy. Epilepsia. 2016;57(12):2001–2010. [DOI] [PubMed] [Google Scholar]
- 16. Fohlen M, Dorfmüller G, Ferrand‐Sorbets S, Dorison N, Chipaux M, Taussig D. Parasagittal hemispherotomy in hemispheric polymicrogyria with electrical status epilepticus during slow sleep: indications, results and follow‐up. Seizure. 2019;71:190–200. [DOI] [PubMed] [Google Scholar]
- 17. Jalloh I, Cho N, Nga VDW, Whitney R, Jain P, Al‐Mehmadi S, et al. The role of surgery in refractory epilepsy secondary to polymicrogyria in the pediatric population. Epilepsia. 2018;59(10):1982–1996. [DOI] [PubMed] [Google Scholar]
- 18. Maillard LG, Tassi L, Bartolomei F, Catenoix H, Dubeau F, Szurhaj W, et al. Stereoelectroencephalography and surgical outcome in polymicrogyria‐related epilepsy: a multicentric study. Ann Neurol. 2017;82(5):781–794. [DOI] [PubMed] [Google Scholar]
- 19. Sculier C, Taussig D, David O, Blustajn J, Ayoubian L, Bonheur J, et al. Focal polymicrogyria in children: contribution of invasive explorations and epileptogenicity mapping in the surgical decision. Seizure. 2021;86:19–28. [DOI] [PubMed] [Google Scholar]
- 20. Bartolini E, Falchi M, Zellini F, Parrini E, Grisotto L, Cosottini M, et al. The syndrome of polymicrogyria, thalamic hypoplasia, and epilepsy with CSWS. Neurology. 2016;86(13):1250–1259. [DOI] [PubMed] [Google Scholar]
- 21. Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, et al. ILAE commission report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42(2):282–286. [PubMed] [Google Scholar]
- 22. Martinović Z, Jović N. Electroencephalogram in unilateral multilobar polymicrogyria with nonconvulsive status epilepticus. Eur J Paediatr Neurol. 2008;12(2):119–122. [DOI] [PubMed] [Google Scholar]
- 23. Ohtsuka Y, Tanaka A, Kobayashi K, Ohta H, Abiru K, Nakano K, et al. Childhood‐onset epilepsy associated with polymicrogyria. Brain and Development. 2002;24(8):758–765. [DOI] [PubMed] [Google Scholar]
- 24. Brna PM, Harvey AS, Leventer RJ. Hemispheric polymicrogyria and neonatal seizures: a potentially life‐threatening combination. Epileptic Disord. 2017;19(1):87–93. [DOI] [PubMed] [Google Scholar]
- 25. Wang S, Weil AG, Ibrahim GM, Fallah A, Korman B, Ragheb J, et al. Surgical management of pediatric patients with encephalopathy due to electrical status epilepticus during sleep (ESES). Epileptic Disord. 2020;22(1):39–54. [DOI] [PubMed] [Google Scholar]
- 26. Loddenkemper T, Cosmo G, Kotagal P, Haut J, Klaas P, Gupta A, et al. Epilepsy surgery in children with electrical status epilepticus in sleep. Neurosurgery. 2009;64(2):328–337. [DOI] [PubMed] [Google Scholar]
- 27. Jeong A, Strahle J, Vellimana AK, Limbrick DD Jr, Smyth MD, Bertrand M. Hemispherotomy in children with electrical status epilepticus of sleep. J Neurosurg Pediatr. 2017;19(1):56–62. [DOI] [PubMed] [Google Scholar]
- 28. Castro‐Villablanca F, Moeller F, Pujar S, D'Arco F, Scott RC, Tahir MZ, et al. Seizure outcome determinants in children after surgery for single unilateral lesions on magnetic resonance imaging: role of preoperative ictal and interictal electroencephalography. Epilepsia. 2022;63(12):3168–3179. [DOI] [PubMed] [Google Scholar]
- 29. Chang EF, Wang DD, Barkovich AJ, Tihan T, Auguste KI, Sullivan JE, et al. Predictors of seizure freedom after surgery for malformations of cortical development. Ann Neurol. 2011;70(1):151–162. [DOI] [PubMed] [Google Scholar]
- 30. Caraballo RH, Veggiotti P, Kaltenmeier MC, Piazza E, Gamboni B, Lopez Avaria MF, et al. Encephalopathy with status epilepticus during sleep or continuous spikes and waves during slow sleep syndrome: a multicenter, long‐term follow‐up study of 117 patients. Epilepsy Res. 2013;105(1–2):164–173. [DOI] [PubMed] [Google Scholar]
- 31. Zhang K, Yan Y, Su T. Treatment strategies for encephalopathy related to status epilepticus during slow sleep, a narrative review of the literature. Rev Neurosci. 2020;31(7):793–802. [DOI] [PubMed] [Google Scholar]
- 32. Ucar HK, Arhan E, Aydin K, Hirfanoglu T, Serdaroglu A. Electrical status epilepticus during sleep (ESES) in benign childhood epilepsy with Centrotemporal spikes (BCECTS): insights into predictive factors, and clinical and EEG outcomes. Eur Rev Med Pharmacol Sci. 2022;26(6):1885–1896. [DOI] [PubMed] [Google Scholar]
- 33. Saltik S, Uluduz D, Cokar O, Demirbilek V, Dervent A. A clinical and EEG study on idiopathic partial epilepsies with evolution into ESES spectrum disorders. Epilepsia. 2005;46(4):524–533. [DOI] [PubMed] [Google Scholar]
- 34. Nickels K, Wirrell E. Electrical status epilepticus in sleep. Semin Pediatr Neurol. 2008;15(2):50–60. [DOI] [PubMed] [Google Scholar]
- 35. Zsoter A, Pieper T, Kudernatsch M, Staudt M. Predicting hand function after hemispherotomy: TMS versus fMRI in hemispheric polymicrogyria. EpilepsiaEpilepsia. 2012;53(6):e98–e101. [DOI] [PubMed] [Google Scholar]
- 36. Wang DD, Knox R, Rolston JD, Englot DJ, Barkovich AJ, Tihan T, et al. Surgical management of medically refractory epilepsy in patients with polymicrogyria. Epilepsia. 2016;57(1):151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wichert‐Ana L, de Azevedo‐Marques PM, Oliveira LF, Fernandes RM, Velasco TR, Santos AC, et al. Ictal technetium‐99 m ethyl cysteinate dimer single‐photon emission tomographic findings in epileptic patients with polymicrogyria syndromes: a subtraction of ictal‐interictal SPECT coregistered to MRI study. Eur J Nucl Med Mol Imaging. 2008;35(6):1159–1170. [DOI] [PubMed] [Google Scholar]


