Abstract
Although polyomavirus large T antigen readily transactivates S-phase-specific enzymes in serum-starved Swiss 3T3 mouse fibroblasts, it is incapable by itself to efficiently drive such cells into S phase. We describe here that this inability correlates with a weak proficiency of the viral protein to induce the synthesis of cyclin A and cyclin E and to stimulate the respective cyclin/cdk activities. Polyomavirus small T antigen, which together with the large T protein supports S-phase induction, strongly contributes to the synthesis of cyclin A. In addition, small T antigen causes a dramatic induction of cyclin A- and, together with large T antigen, of cyclin E-specific protein kinase activity. This latter function of polyomavirus small T antigen correlates with its competence to provoke the elimination of the kinase inhibitor p27Kip1. An interaction of the small T antigen with the protein phosphatase 2A is essential for this activity. Hence, the ability to drive quiescent Swiss 3T3 cells into S phase results from the capacity of large T antigen to transactivate DNA synthesis enzymes by its interaction with retinoblastoma-type proteins and from the potential of the large and the small T antigens together to stimulate cyclin A synthesis and cyclin A- and cyclin E-dependent protein kinase activity.
DNA tumorviruses preferentially infect differentiated and, therefore, growth-arrested cells. For their replication, however, they require cells in S phase because they depend on replication functions of the host cell. To cope with this situation, these viruses carry in their genomes the information for a few proteins which interfere with the growth and cell cycle regulation of the host cells (reviewed in reference 22). The “early region” of simian virus 40 (SV40), for example, encodes the large and the small tumor antigens, and that of polyomavirus (Py) encodes the large, the middle, and the small T antigens (LT, MT, and ST, respectively). At least one of these proteins acts by deregulating cellular transcription. This is true for the large T antigens of SV40 and Py but also for the E1A protein of adenovirus and for the E7 protein of human papillomavirus. Major targets of these proteins are the so-called pocket proteins, the retinoblastoma protein pRB and its relatives, p107 and p130. These proteins control the activity of members of a family of transcription factors called E2F. By binding to E2Fs, pocket proteins inhibit their transcriptional activity. Growth factor-induced signal transduction pathways lead to the phosphorylation of the pocket proteins, which results in their removal from E2F and consequently in promoter activation (reviewed in reference 6). The viral proteins have the capacity to bind to the underphosphorylated form of the pocket proteins, causing their removal from E2F and the activation of transcription. One class of genes targeted by the viral proteins is that encoding enzymes involved in DNA replication and in precursor production (reviewed in reference 22).
We have previously produced Swiss 3T3 cells carrying the information of Py LT and/or Py ST in hormone-inducible form (26). When dexamethasone at a concentration of 1 μmol/liter is added to cultures of such cells, T antigen(s) can be readily detected by immunoblotting about 4 h thereafter. The amount of viral protein remains high for more than 36 h in the presence of the hormone. We found that the addition of dexamethasone to 3T3 LT or to 3T3 ST cells causes only a few percent of serum-starved, quiescent cells to move into S phase within 32 h after induction of the T antigen. In contrast, expression of both viral proteins drives about 30% of the cells into S phase (26). We could show that, despite the failure to induce S phase in a sizable fraction of cells, expression of Py LT in quiescent mouse fibroblasts results in efficient transactivation of S-phase-specific enzymes and that this is dependent on the interaction of the T antigen with pocket proteins (23, 27).
Cyclins have substantial functions in cell cycle progression (reviewed in reference 41). Cyclin E is involved in S phase entry, and cyclin A plays an important role in the induction as well as in the advancement of S phase (5, 11, 28, 31, 36, 37, 44, 49). Protein and mRNA levels of cyclin A are hardly detectable in G0/G1 and increase at the G1/S border, while cyclin E levels of cells in G0/G1 vary in different cell lines and cell types (12, 14, 54, 55). We therefore examined whether Py T antigens were able to induce cyclin E and cyclin A in serum-starved Swiss 3T3 fibroblasts. We found that while cyclin E levels were significant in quiescent cells and did not show a strong increase after induction of Py LT and Py ST, cyclin A was not detectable in arrested cells and both Py LT and Py ST were able to promote cyclin A production. Importantly, however, Py ST caused a strong rise of cyclin A-dependent protein kinase activity and, together with LT, of cyclin E-associated kinase activity. These latter activities correlated with the Py ST-induced phosphorylation and degradation of the cyclin-dependent kinase inhibitor p27Kip1. The proficiency of Py ST to interfere with protein phosphatase 2A (PP2A) appears to be essential for this reaction. We propose that S-phase induction by Py LT and Py ST in quiescent Swiss 3T3 cells is due to the capacity of Py LT to elicit the synthesis of DNA replication enzymes and the combined activities of Py LT and Py ST in the production of S-phase-specific cyclins and cyclin-dependent kinase activities.
MATERIALS AND METHODS
Cell culture and transfections.
Swiss 3T3 cells and derived cells conditionally expressing the Py T antigens (26) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (FCS), penicillin (60 μg/ml), and streptomycin (100 μg/ml) in a 7.5% CO2 atmosphere. For growth arrest, asynchronously growing cells were seeded at 5 × 105 cells per 100-mm-diameter petri dish; the next day, the serum concentration was reduced to 0.2% for 72 h. Cells were then either growth induced by addition of fresh medium containing 20% FCS or treated with dexamethasone at a concentration of 10−6 mol/liter. Cell cycle distribution analysis was performed by flow cytometry (fluorescence-activated cell sorting [FACS]) using a Partec PAS-II as described previously (26). Transfections were performed overnight with 5 μg of DNA by the standard Polybrene technique.
Expression plasmids.
The plasmid carrying the Py ST PP2A binding mutant ins107AL (pBluescript pyst[ins107AL]) (2) was a kind gift of T. Roberts (Dana Farber Cancer Institute). For inducible expression, the mutant was cloned into the expression vector pMShygro (a pMSG plasmid [Pharmacia] where the hygromycin resistance gene was inserted in place of the gpt gene).
Antibodies, protein extraction, and immunoblotting.
An affinity-purified rabbit polyclonal antibody raised against a cyclin A-glutathione S-transferase fusion protein produced in Escherichia coli was used to detect cyclin A. Cyclin E was detected by using a specific antibody (M-20) purchased from Santa Cruz. The same antibodies were used for the immunoprecipitation and histone H1 kinase experiments. p27Kip1 was immunoprecipitated with an antibody from Santa Cruz (M-197) and detected on immunoblots with a monoclonal antibody (catalog no. K25020) from Transduction Laboratories. Anti-p21Cip1 antibody (M-19) was purchased from Santa Cruz.
Cells were harvested and lysed in buffer A (20 mM Tris-Cl [pH 8.0], 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 2 mM phenylmethylsulfonyl fluoride, 20 μg of aprotinin per ml, 0.5 mM NaF, 0.5 mM Na3VO4) as described elsewhere (1). The whole-cell lysates were either directly subjected to gel electrophoresis followed by immunoblotting to a nitrocellulose membrane or used for immunoprecipitations. For the immunoblot experiments, the lysates were mixed with protein sample buffer (100 mM Tris-Cl [pH 6.8], 20% glycerol, 0.01% bromophenol blue, 10% β-mercaptoethanol, 5% sodium dodecyl sulfate [SDS]) and heated for 5 min at 95°C. Sixty micrograms of protein was loaded on an SDS-polyacrylamide gel per lane. The respective proteins were detected by a chemiluminescence reaction (Renaissance; NEN).
Immunoprecipitation, histone 1 kinase assay, and phosphatase treatment.
For the kinase assay, 200 μg of protein from whole-cell lysates was subjected to immunoprecipitation at 4°C overnight. The immunocomplexes were washed three times with buffer A and one time with kinase buffer (50 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol, 25 μM ATP). The kinase reaction containing 5 μg of histone H1 and 10 μCi of [γ-32P]ATP in a final volume of 25 μl of kinase buffer was incubated for 30 min at 30°C. The supernatant was mixed with protein sample buffer, heated at 95°C for 5 min, and applied to a 12.5% polyacrylamide–SDS gel. The gel was stained with Coomassie brilliant blue dye to check for equal loading with histone and dried. Labelled histone H1 was detected by autoradiography. The relative amounts were calculated with the help of a phosphorimager (Storm 840; Molecular Dynamics) as well as by liquid scintillation counting.
For p27Kip1 immunoprecipitation, 80 μg of protein from whole-cell lysates was incubated at 4°C for 60 min with antibody and then for an additional 60 min with protein A-Sepharose. The immunocomplexes were washed twice with complete buffer A and once with buffer A without NaF and Na3VO4. In the case of phosphatase treatment, the complexes were once more washed with λ phosphatase buffer (New England BioLabs). The phosphatase reaction was performed as suggested by the distributor with 300 U of enzyme. The immunoprecipitates were suspended in sample buffer, heated at 95°C for 5 min, and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
RESULTS
Induction of cyclin A and cyclin E by Py T antigens.
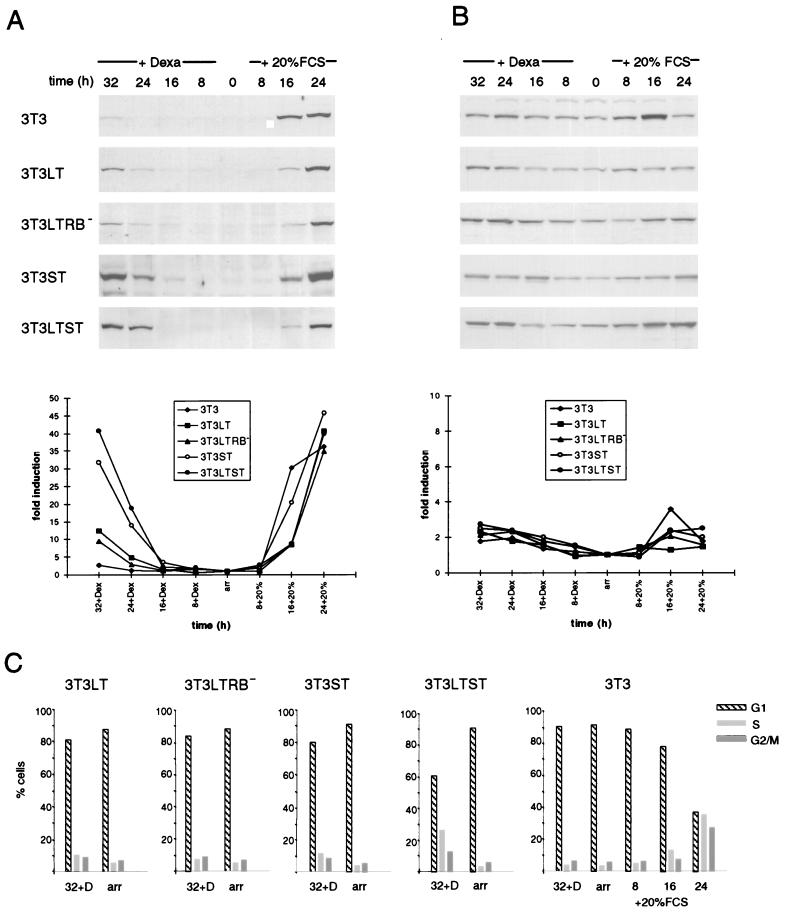
Considering that besides the enzymes involved in DNA synthesis, the S-phase-specific cyclins E and A are necessary for cells to pass the G1/S border, we examined the levels of these cyclins in quiescent 3T3 LT, 3T3 ST, and 3T3 LTST cells producing the indicated viral proteins under a hormone-inducible promoter after addition of dexamethasone. These levels were compared with the quantities of the cyclins induced by addition of serum (Fig. 1). Almost undetectable amounts of cyclin A were present in serum-arrested, quiescent fibroblasts. Addition of serum resulted in a strong increase after 16 and 24 h. Cytofluorometric analysis revealed that these cells began to enter S phase after 16 h and the percentage of cells in S phase reached its maximum after about 24 h (Fig. 1C). While the amounts of cyclin A induced by Py LT were relatively low, when compared to serum, Py ST displayed a strong capacity to induce the protein. Together, Py LT and Py ST were as efficient in cyclin A induction as serum (Fig. 1A). A quantitation of the blot is included (Fig. 1A, see the graph below the immunoblot); this shows that Py LT alone induces only about 25% of the amount of cyclin A induced by both T antigens together. Most remarkably, an intact binding site for pRB and the other pocket proteins within the large T antigen was dispensable for this function. A mutated viral protein in which the glutamic acid residue within the LXCXE motif of the RB binding site was mutated to aspartic acid and which was previously found to be inactive in transactivating DNA synthesis enzymes (23, 27) was almost as active in producing cyclin A as was the wild-type protein. This result suggests that the pocket proteins play no role in the stimulation of cyclin A gene expression by Py LT in Swiss 3T3 cells.
FIG. 1.
Cyclin A but not cyclin E is strongly induced by Py LT and ST. Western blot analysis of protein extracts from conditionally T antigen-expressing Swiss 3T3 cells is shown. The cells were serum deprived and treated subsequently with dexamethasone (Dexa) for the indicated times. For comparison, arrested cells were reinduced with serum. Cyclin A (A) and cyclin E (B) were detected by using rabbit polyclonal antibodies. For the quantitation shown below the Western blots, the expression level of the respective cyclin in arrested cells was arbitrarily set at 1. (C) Samples for flow cytometric analysis (FACS) were prepared in parallel to the protein extracts. The percentage of cells in the respective phases of the cell cycle either after 72 h of serum starvation (arr) or after 32 h of dexamethasone induction is shown for all cell lines. Cell cycle phase distribution after serum stimulation is shown for 3T3 cells only since the T antigen-expressing cell lines showed a similar profile. Data represent mean values of at least three independent experiments. Standard deviation did in no case exceed 10%.
Cyclin E, in contrast, was present in quiescent cells at significant levels and was further induced to a small extent by addition of serum or by the expression of the viral proteins (Fig. 1B). However, addition of dexamethasone in a concentration used for the induction of the synthesis of the T antigens also resulted in some increase of cyclin E levels in 3T3 cells not expressing these proteins. It is, therefore, not entirely clear to which extent the T antigens contribute to the rise in the amounts of cyclin E.
Strong cyclin-dependent kinase activity depends on ST.
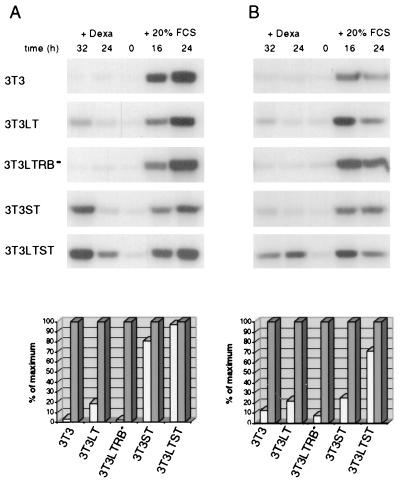
The efficient production of cyclin A by Py T antigens in quiescent fibroblasts coincides with the previously found capacity of the two T antigens together to efficiently induce S phase in serum-starved Swiss 3T3 cells (26). Since it is well established that the cyclin E- and cyclin A-dependent kinase (cdk2) activities are required for S-phase induction, we determined these activities in extracts from serum-starved cells producing T antigens and compared them with those measured after growth stimulation by addition of serum (Fig. 2). As expected, these activities were low in quiescent cells. Production of Py LT gave rise to low levels of cyclin E/cdk2 and cyclin A/cdk2 activities in serum-starved cells. It is intriguing that the induction of cyclin A- and cyclin E-dependent kinase by Py LT required an intact pocket protein binding site within the viral protein (Fig. 2), which contrasts with the observation that this site was dispensable for cyclin A induction (Fig. 1). This requirement is therefore independent of the production of the cyclin.
FIG. 2.
LT and ST together strongly induce cyclin A/Cdk2 and cyclin E/Cdk2 activity. Cyclin A-associated (A) and cyclin E-associated (B) histone H1 kinase activities at the indicated time points were measured after immunoprecipitation of the cyclins from the lysates described in Fig. 1. Quantitation was done by using phosphorimager software and by liquid scintillation counting. Mean values of three independent experiments are shown. Grey bars represent the most intensive signal after serum induction; white bars represent the most intensive after dexamethasone (Dexa) induction. The data are expressed as percent values of the maximum (i.e., 24 h plus 20% FCS for cyclin A-associated kinase and 16 h plus 20% FCS for cyclin E-associated kinase).
Py ST alone caused strong cyclin A-dependent and, to a weaker extent, cyclin E-dependent kinase activity, but both T antigens together were as efficient as addition of serum in stimulating cyclin A/cdk2. Interestingly, cyclin E/cdk2 activity was also strongly generated in LTST-expressing cells.
Activation of cyclin-dependent kinase is paralleled by small T-dependent inactivation of p27Kip1.
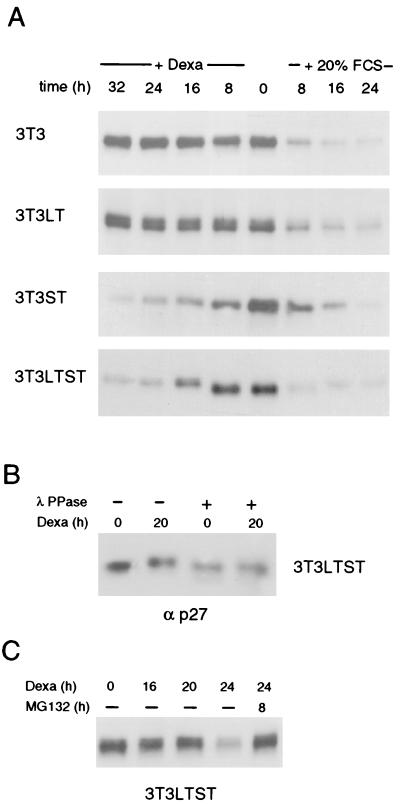
Cyclin-dependent kinases are regulated by binding to the cyclin, by phosphorylation, and by the activity of kinase inhibitors (reviewed in references 7 and 42). Two such inhibitors, p27Kip1 and p21Cip1/WAF1, regulate cdk2. Since the slightly elevated protein levels of cyclin E could not account for the observed strong induction of cyclin E/cdk2 activity in 3T3 LTST cells, we tested the effect of Py LT, Py ST, and Py LTST on the amounts of p27 and p21 (Fig. 3 and 4). As expected, the level of p27 was high in quiescent cells and addition of serum rapidly caused its removal (Fig. 3A), most likely by proteasome-dependent degradation (32, 51). Py LT did not change the levels of p27 within 32 h. In contrast, Py ST first caused a shift of the p27-specific band towards slower migration during denaturing PAGE, which was then followed by a gradual decrease of the amount of p27 in cell extracts. The same effect was observed in cells producing Py LT and Py ST together. The shift of p27 was due to phosphorylation of the protein because treatment of p27 immunoprecipitates with a protein phosphatase caused the return to the faster-migrating form (Fig. 3B). Degradation of the phosphorylated form of p27 by the proteasome system was finally confirmed by the observation that an inhibitor of the proteasome degradation pathway, MG-132, gave rise to a stabilization of the phosphorylated form of p27 (Fig. 3C). p27 in 3T3 LT cells appears as a broader band which sometimes (as in this figure) split into a double band. This may be a property of this cell line, since it is not seen in 3T3 LTST cells.
FIG. 3.
ST causes the phosphorylation and subsequent degradation of the kinase inhibitor p27Kip1. (A) Western blot analysis of the protein extracts described in Fig. 1 using a monoclonal antibody specific for p27 is shown. (B) To demonstrate that the mobility shift observed for p27 in cells expressing ST is due to phosphorylation, p27 was immunoprecipitated from extracts of 3T3 LTST cells, and the immunocomplexes were subjected to λ protein phosphatase treatment and analyzed by Western blotting. (C) LTST-expressing, serum-starved cells were incubated with dexamethasone for the indicated times. In the case of MG-132 treatment, the cells were treated with dexamethasone for 16 h. MG-132 was then added to the medium, and the cells were incubated for an additional 8 h in the presence of dexamethasone and MG-132. The levels of p27 were analyzed by Western blotting. Dexa, dexamethasone.
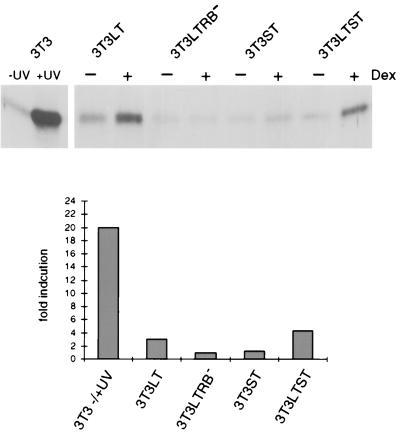
FIG. 4.
Induction of p21Cip1 by LT depends on an intact pocket protein binding motif within the viral antigen. Lysates of cells expressing either LT, ST, or both, which were serum starved and subsequently incubated with dexamethasone for 24 h, were subjected to SDS-PAGE and immunoblotting. p21 was detected by using a polyclonal antibody. For comparison of p21 induction, extracts of growing and UV-irradiated (18 J/m2; extract was prepared 9 h thereafter) 3T3 cells are shown in the first two lanes. Fold induction represents the ratio of the dexamethasone-induced amount to the uninduced amount.
Analyses of p21 gave a different result. T antigens did not provoke a decrease in the amount of this protein; rather, Py LT caused an induction of p21 (Fig. 4). The mutant LTRB− did not display this activity which therefore requires an interaction of Py LT with pRB or its relatives. This is in agreement with a recent report indicating a regulation of the p21 promoter by E2F (13). It is important to note, however, that the increase in the amount of p21 caused by Py LT was considerably lower than that achieved by treating cells with UV light, which is particularly evident from the quantitation of the immunoblots included in Fig. 4. The increase corresponded approximately with that accompanying a growth stimulation of cells by serum (data not shown). This amount of the inhibitor does not result in an inhibition of cell proliferation. On the contrary, earlier results point to p21 as an important component of catalytically active kinases and even to a positive role in promoting the assembly of active kinase complexes (20, 56). Taken together, our results allow the conclusion that p27 is the major negative regulator of cyclin/cdk2 activity which is targeted by Py ST.
The interaction of Py ST with PP2A is required for p27 degradation and stimulation of cdk2 activity.
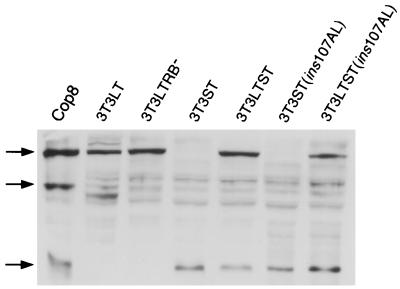
The small T antigens of polyomavirus and SV40 interact with the PP2A (2). This interaction results in a disturbance of the tripartite structure of this enzyme whereby the B subunit is replaced by the viral protein. This causes an inactivation or a change in the substrate specificity of the enzyme. A first hint that PP2A might be targeted by Py ST to result in p27 phosphorylation came from the observation that addition of okadaic acid, an inhibitor of protein phosphatases, to quiescent cells gave rise to a shift of the p27-specific band similar to the one induced by Py ST (data not shown). To investigate a potential role of the PP2A binding capacity of Py ST in the reduction of p27 levels, we used a mutant of Py ST [ST (ins107AL)] with modifications in the binding site for PP2A which almost completely destroyed the capacity of the viral protein to bind to the phosphatase (2). The mutated cDNA was cloned into a vector for dexamethasone-dependent production of the protein and was then stably transfected into Swiss 3T3 and 3T3 LT cells. The expression of the viral proteins after addition of hormone was measured by immunoblotting equal amounts of protein from extracts of logarithmically growing cells. As shown in Fig. 5, the different cell lines express similar amounts of LT antigen or of ST protein. In particular, mutant T antigens are certainly not produced in lower amounts than the wild-type proteins. Induction of mutated Py ST in quiescent cells did not result in a destruction of p27, and the same was true when wild-type Py LT and mutated Py ST were induced together in serum-starved 3T3 LTST(ins107AL) cells (Fig. 6A). Accordingly, cyclin-dependent kinase activities remained low (Fig. 6B). As expected, cytofluorometric analyses showed that these conditions did not lead to S-phase induction above that seen with Py LT alone (Fig. 6C). These results are consistent with the interpretation that the interaction of Py ST with PP2A plays an important role in the stimulation of cyclin-dependent cdk2 activity and that this activity is important for S-phase induction.
FIG. 5.
The mutated form of ST containing a defective PP2A binding site is expressed at levels comparable to those of the wild-type ST. All T antigen-expressing cells used for this study were induced with dexamethasone for 16 h. Whole-cell extracts were prepared and subjected to Western blot analysis. An extract of the Py-transformed cell line Cop8 was included as a positive control. The T antigens were detected by using a polyclonal antibody raised against the N terminus, thus recognizing all three T antigens. Arrows indicate LT, MT, and ST (from top to bottom).
FIG. 6.
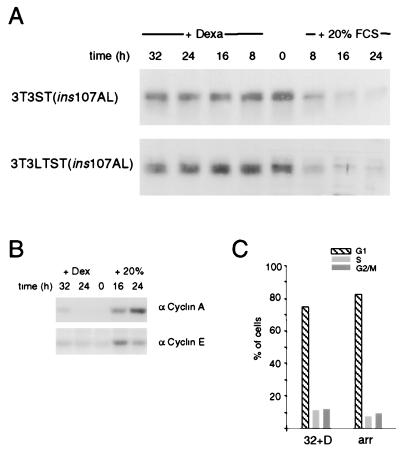
A mutated form of ST deficient for PP2A interaction is unable to cause the elimination of p27 from arrested cells. (A) Western blot analysis for p27 of cells conditionally expressing ST(ins107AL) or wild-type LT and ST(ins107AL) together, respectively, is shown. (B) Cyclin A- and cyclin E-dependent histone H1 kinase activities were determined in LTST(ins107AL) expressing cells at the indicated time points. (C) Cell cycle phase distribution profile of 72-h serum-starved (arr) and 32-h dexamethasone-induced 3T3 LTST(ins107AL) cells as determined by FACS analysis is shown.
DISCUSSION
The cyclins E and A were shown to be important for S-phase induction and regulation (5, 11, 28, 31, 36, 37, 44, 49; reviewed in reference 41). Several substrates for cyclin E/cdk2 and cyclin A/cdk2 are known, but some important targets may yet be undiscovered. For instance, cyclin E/cdk2 was found to phosphorylate p27 on threonine 187 (40) and to associate with components for the SWI-SNF complex that alters chromatin structure (39) and with NPAT, a novel substrate with S-phase-promoting function (57). Together with cdk2 (and later in the cell cycle together with cdk1 [cdc2]), cyclin A phosphorylates proteins playing important roles in S phase and thereafter (reviewed in reference 41). For instance, cyclin A was found to bind to complexes of E2F with p107 (8, 21, 24, 43) and to phosphorylate E2F1 (probably also E2F2 and E2F3) and DP1, causing inhibition of their transcriptional activity in S phase (17, 18). It also phosphorylates, and thereby activates, transcription factor B-myb (38, 58). Cyclin A stimulates SV40 and cellular DNA replication in vitro (9, 19), and several components of the replication machinery (replication protein RP-A, DNA polymerase α, and the large T antigen) were found to be modified by cyclin-dependent kinases with consequences for their activity (52, 53). Importantly, cyclin A/cdk2 phosphorylates cdc6 protein, thereby stimulating its release from chromatin and its export from the nucleus (30). cdc6p is essential for setting up the prereplicative complex and is central to the once-per-cell cycle regulation of DNA replication (reviewed in reference 25). Viral proteins can bind cyclin A directly (references 1, 4, and 50 and our unpublished observations). It is so far unknown whether this binding has consequences for the function of cyclin A during S phase.
Considering the importance of cyclins E and A in S-phase induction, it is not surprising that several viral proteins were found to stimulate their synthesis (1, 29, 50, 54, 55). In the case of Py, efficient transactivation of cyclin A is not achieved by LT alone but is provided by the combined activities of Py LT and Py ST. Our finding that the T antigens in addition permit strong induction of cyclin E/cdk2 and cyclin A/cdk2 activity thus can explain the proficient initiation of S phase if the two viral proteins are produced in serum-starved cells (26). It is of interest that although binding of pRB or the other pocket proteins by Py LT is not required for cyclin A transactivation, this activity seems to be essential for the capacity of Py LT to induce cyclin A/cdk2 activity. The role of pRB in the transcriptional regulation of cyclin A gene expression is still controversial. Our observation argues against an involvement of pRB in the transactivation of cyclin A by Py LT, which is in agreement with the report that a mutant of SV40 LT antigen (K1), which is unable to bind pocket proteins, can induce cyclin A (29). Furthermore, Henglein et al. (12) reported that regulation of cyclin A promoter-luciferase constructs is normal in tumor-derived cell lines lacking pRB. On the other hand, cyclin A regulation was found to be aberrant in normal diploid mouse cells in which the RB gene was eliminated; no effect was observed in cells from p107 and p130 knockout mice (33). Nevertheless, the transactivation of cyclin A by adenovirus E1A protein was reported to require an interaction of the viral protein with the pocket protein p107 (55). Since both SV40 LT and Py LT mutated in the LXCXE domain are defective in p107 as well as pRB binding, this indicates that the mechanism of transactivation of cyclin A by the viral proteins may differ. It remains to be clarified whether Py LT has a direct effect on the cyclin A promoter and which sequences within the viral protein are involved.
Besides Py LT, Py ST also has the potential to transactivate cyclin A. This is in accord with a report on a similar activity of SV40 small T protein (34). While for the latter protein an involvement of the chaperone binding site, the so-called J domain recently discovered in T antigens (for examples, see references 3, 16, and 47), was suggested to be necessary for this activity (34), the mechanism by which Py ST transactivates cyclin A has to be investigated. Furthermore, an important role of Py ST in stimulating cyclin E- and cyclin A-dependent cdk2 activity is obvious from the results presented. They also suggest a mechanism by which Py ST accomplishes this stimulation. This viral protein is able to cause a rapid decrease in the amount of the cdk inhibitor p27, an activity for which the binding of Py ST to the PP2A is essential. Our data also show that through the action of Py ST, p27 is converted into a (hyper)phosphorylated form. This agrees with the mechanism suggested for cell cycle-dependent inactivation of p27 involving a phosphorylation step which sensitizes the protein for degradation by the proteasome system (32, 51). It was shown previously that SV40 ST antigen, through its interaction with PP2A, stimulates the mitogen-activated protein (MAP) kinase pathway of signal transduction (10, 45, 46) and that p27 can be phosphorylated by MAP kinase in vitro (15). Finally, there is evidence that phosphorylation of p27 precedes the degradation of the protein (32, 51). All these data support the above model for the function of Py ST. Although our data do not provide direct proof, it is an attractive hypothesis that activation of the MAP kinase pathway by Py ST may lead to the elimination of a crucial negative regulator of cell proliferation in vivo. It is interesting that the shift to slower migration of p27 is not so obvious in the case of serum stimulation of arrested cells (Fig. 3). This might indicate that the phosphorylation step is the rate-limiting one in the case of serum stimulation while components of the degradation machinery involved in the destruction of p27 may be readily available under these conditions. In contrast, in the case of serum-deprived cells expressing Py T antigens, these components might be limiting, thereby allowing the slower-migrating phosphorylated form of p27 to become clearly visible before the protein is degraded.
In contrast to the Py ST-induced elimination of p27, Py LT provokes an increase in the level of p21. This is dependent on the ability of the protein to interact with pRB and therefore likely results from a transactivation of the E2F-regulated promoter of the p21 gene (13). The cellular level of p21 attained by expression of Py LT in serum-starved Swiss 3T3 fibroblasts is close to that obtained by serum stimulation and considerably lower than that induced by UV treatment of cells. While the latter condition causes cell cycle arrest due to inhibition of cyclin-dependent kinases, the lower amounts induced by serum or by Py LT appear to support the generation of active cyclin/cdk complexes (20, 56). The induction of p21 by Py LT may thus be an essential part of the reaction leading to high cyclin kinase activity. Several of our observations provide evidence for this: first, Py LT gives rise to some cyclin A and cyclin E/cdk2 activity even in the absence of Py ST and, hence, in the presence of p27. Second, expression of Py ST alone is not sufficient to induce high cyclin E-dependent kinase activity, despite the removal of p27. Finally, the mutant of Py LT defective in pRB binding, although functional in the induction of cyclin A protein, is not able to induce cyclin E or cyclin A/cdk2 activity. Taken together, these results indicate that it may require both the induction of p21 by Py LT and the elimination of p27 by Py ST to adequately activate cyclin E- and cyclin A-dependent protein kinase.
Shortly before the preparation of this manuscript was finished, a paper was published (35) in which the cooperation of SV40 large and small T antigens in the induction of cell cycle rerentry of serum-starved human diploid fibroblasts was described. As in our study on Py T antigens, both proteins are required and major activities were found to be the transactivation of cyclin A and the activation of cyclin A/cdk2 to which small T contributes by eliminating p27. However, there are also significant differences. For instance, while we find that Py LT induces p21, SV40 LT was found to inhibit p21 expression. This difference may lie in the well-known ability of SV40 LT to interact with and functionally inactivate p53, an activity not found for Py LT. In fact, our earlier observation that Py LT does not interfere with the UV-induced and p53-dependent induction of high levels of p21 in REF52 cells (48) supports this assumption.
In conclusion, our observations support the view that the production of S-phase-specific enzymes (which results from the function of Py LT) and a sufficient generation of cyclin E- and cyclin A-dependent protein kinase activities (which are achieved by the combined actions of Py LT and Py ST) are required to adequately drive quiescent Swiss 3T3 cells into S phase.
ACKNOWLEDGMENTS
We thank Peter Stiegler for help in the initial experiments, in particular for the production of a polyclonal antibody against murine cyclin A, and Egon Ogris and Peter Stiegler for helpful discussions.
This work was supported by grants from the Fonds zur Förderung der wissenschaftlichen Forschung and the Herzfelder’sche Familienstiftung.
REFERENCES
- 1.Adamczewski J P, Gannon J V, Hunt T. Simian virus 40 large T antigen associates with cyclin A and p33cdk2. J Virol. 1993;67:6551–6557. doi: 10.1128/jvi.67.11.6551-6557.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell K S, Auger K R, Hemmings B A, Roberts T M, Pallas D C. Identification of regions in polyomavirus middle T and small T antigens important for association with protein phosphatase 2A. J Virol. 1995;69:3721–3728. doi: 10.1128/jvi.69.6.3721-3728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell K S, Mullane K P, Aksoy I A, Stubdal H, Zalvide J, Pipas J M, Silver P A, Roberts T M, Schaffhausen B, DeCaprio J A. DNAJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes Dev. 1997;11:1098–1110. doi: 10.1101/gad.11.9.1098. [DOI] [PubMed] [Google Scholar]
- 4.Cannella D, Roberts J M, Fotedar R. Association of cyclin A and cdk2 with SV40 DNA in replication initiation complexes is cell cycle dependent. Chromosoma. 1997;105:349–359. doi: 10.1007/BF02529750. [DOI] [PubMed] [Google Scholar]
- 5.Duronio R J, Brook A, Dyson N, Farrell P H. E2F-induced S phase requires cyclin E. Genes Dev. 1996;10:2505–2513. doi: 10.1101/gad.10.19.2505. [DOI] [PubMed] [Google Scholar]
- 6.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 7.Elledge S J, Winston J, Harper J W. A question of balance: the role of cyclin-kinase inhibitors in development and tumorigenesis. Trends Cell Biol. 1996;6:388–392. doi: 10.1016/0962-8924(96)10030-1. [DOI] [PubMed] [Google Scholar]
- 8.Ewen M E, Faha B, Harlow E, Livingston D M. Interaction of p107 with cyclin A independent of complex formation with viral oncoproteins. Science. 1992;255:85–87. doi: 10.1126/science.1532457. [DOI] [PubMed] [Google Scholar]
- 9.Fotedar A, Cannella D, Fitzgerald P, Rousselle T, Gupta S, Doree M, Fotedar R. Role for cyclin A-dependent kinase in DNA replication in human S phase cell extracts. J Biol Chem. 1996;271:31627–31637. doi: 10.1074/jbc.271.49.31627. [DOI] [PubMed] [Google Scholar]
- 10.Frost J A, Alberts A S, Sontag E, Guan K, Mumby M C, Feramisco J R. Simian virus 40 small t antigen cooperates with mitogen-activated kinases to stimulate AP-1 activity. Mol Cell Biol. 1994;14:6244–6252. doi: 10.1128/mcb.14.9.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard E, Strausfeld U, Dernandez A, Lamb N J C. Cyclin A is required for the onset of DNA replication in mammalian cells. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 12.Henglein B, Chenivesse X, Wang J, Eick D, Brechot C. Structure and cell cycle-regulated transcription of the human cyclin A gene. Proc Natl Acad Sci USA. 1994;91:5490–5494. doi: 10.1073/pnas.91.12.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiyama H, Iavarone A, Reeves S A. Regulation of the cdk inhibitor p21 gene during cell cycle progression is under the control of the transcription factor E2F. Oncogene. 1998;16:1513–1523. doi: 10.1038/sj.onc.1201667. [DOI] [PubMed] [Google Scholar]
- 14.Huet X, Rech J, Plet A, Vie A, Blanchard J M. Cyclin A expression is under negative transcriptional control during the cell cycle. Mol Cell Biol. 1996;16:3789–3798. doi: 10.1128/mcb.16.7.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawada M, Yamagoe S, Murakami Y, Suzuki K, Mizuno S, Uehara Y. Induction of p27Kip1 degradation and anchorage independence by Ras through the MAP kinase signaling pathway. Oncogene. 1997;15:629–637. doi: 10.1038/sj.onc.1201228. [DOI] [PubMed] [Google Scholar]
- 16.Kelly W, Georgopoulos C. The T/t common exon of simian virus 40, JC and BK polyomavirus T antigens can functionally replace the J-domain of the Escherichia coli DnaJ molecular chaperone. Proc Natl Acd Sci USA. 1997;94:3679–3684. doi: 10.1073/pnas.94.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitigawa M, Higashi H, Suzuki-Takahashi I, Segawa K, Hanks S K, Taya Y, Nishimura S, Okuyama A. Phosphorylation of E2F-1 by cyclin A-cdk2. Oncogene. 1995;10:229–236. [PubMed] [Google Scholar]
- 18.Krek W, Ewen M E, Shirodkar S, Arany Z, Kaelin Jr W G, Livingston D M. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell. 1994;78:161–172. doi: 10.1016/0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 19.Krude T, Jackman M, Pines J, Laskey R A. Cyclin/cdk-dependent initiation of DNA replication in a human cell-free system. Cell. 1997;88:109–119. doi: 10.1016/s0092-8674(00)81863-2. [DOI] [PubMed] [Google Scholar]
- 20.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 21.Lees E, Faha B, Dulic V, Reed S I, Harlow E. Cyclin E/cdk2 and cyclin A/cdks kinases associate with p107 and E2F in a temporally distinct manner. Genes Dev. 1992;6:1874–1885. doi: 10.1101/gad.6.10.1874. [DOI] [PubMed] [Google Scholar]
- 22.Moran E. DNA tumorvirus transforming proteins and the cell cycle. Curr Opin Genet Dev. 1993;3:63–70. doi: 10.1016/s0959-437x(05)80342-9. [DOI] [PubMed] [Google Scholar]
- 23.Mudrak I, Ogris E, Rotheneder H, Wintersberger E. Coordinated trans activation of DNA synthesis- and precursor-producing enzymes by polyomavirus large T antigen through interaction with the retinoblastoma protein. Mol Cell Biol. 1994;14:1886–1892. doi: 10.1128/mcb.14.3.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mudryi M, Devoto S H, Hiebert S W, Hunter T, Pines J, Nevins J R. Cell cycle regulation of the E2F transcription factor involves interaction with cyclin A. Cell. 1991;65:1243–1253. doi: 10.1016/0092-8674(91)90019-u. [DOI] [PubMed] [Google Scholar]
- 25.Newlon C S. Putting it all together: building a prereplicative complex. Cell. 1997;91:717–720. doi: 10.1016/s0092-8674(00)80459-6. [DOI] [PubMed] [Google Scholar]
- 26.Ogris E, Mudrak I, Wintersberger E. Polyomavirus large and small T antigens cooperate in induction of the S phase in serum-starved 3T3 mouse fibroblasts. J Virol. 1992;66:53–61. doi: 10.1128/jvi.66.1.53-61.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogris E, Rotheneder H, Mudrak I, Pichler A, Wintersberger E. A binding site for transcription factor E2F is a target for trans activation of murine thymidine kinase by polyomavirus large T antigen and plays an important role in growth regulation of the gene. J Virol. 1993;67:1765–1771. doi: 10.1128/jvi.67.4.1765-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oshima J, Steinmann K E, Campbell J, Schlegel R. Modulation of cell growth, p34cdc2 and cyclin A levels by SV40 large T antigen. Oncogene. 1993;8:2987–2993. [PubMed] [Google Scholar]
- 30.Otzen Petersen B, Lukas J, Storgard Sorensen C, Bartek J, Helin K. Phosphorylation of mammalian CDC6 by cyclin A/cdk2 regulates its subcellular localization. EMBO J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, DelSal G, Chau V, Yew P R, Draetta G F, Rolfe M. A role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 33.Philips A, Huet X, Plet A, Le Cam L, Vie A, Blanchard M. The retinoblastoma protein is essential for cyclin A repression in quiescent cells. Oncogene. 1998;16:1373–1381. doi: 10.1038/sj.onc.1201655. [DOI] [PubMed] [Google Scholar]
- 34.Porras A, Bennett J, Howe A, Tokos K, Bouck N, Henglein B, Sathyamangalam S, Thimmapaya B, Rundell K. A novel simian virus 40 early-region domain mediates transactivation of the cyclin A promoter by small-t antigen and is required for transformation in small-t antigen-dependent assays. J Virol. 1996;70:6902–6908. doi: 10.1128/jvi.70.10.6902-6908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porras A, Gaillard S, Rundell K. The simian virus 40 small-t and large-T antigens jointly regulate cell cycle reentry in human fibroblasts. J Virol. 1999;73:3102–3107. doi: 10.1128/jvi.73.4.3102-3107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Resnitzky D, Hengst L, Reed S I. Cyclin A-associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1. Mol Cell Biol. 1995;15:4347–4352. doi: 10.1128/mcb.15.8.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg A R, Zindy F, Le Deist F, Mouly H, Metezeay P, Brechot C, Lamas E. Overexpression of human cyclin A advances entry into S phase. Oncogene. 1995;10:1501–1509. [PubMed] [Google Scholar]
- 38.Sala A, Kundu M, Casella I, Engelhard A, Calabretta B, Grasso L, Paggi M G, Giordano A, Watson R J, Khalili K, Peschle C. Activation of human B-myb by cyclins. Proc Natl Acad Sci USA. 1997;94:532–536. doi: 10.1073/pnas.94.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanahan F, Seghezzi W, Parry D, Mahony D, Lees E. Cyclin E associates with BAF 155 and BRG1, components of the mammalian SWI-SNF complex, and alters the ability of BRG1 to induce growth arrest. Mol Cell Biol. 1999;19:1460–1469. doi: 10.1128/mcb.19.2.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheaff R J, Groudin M, Gordon M, Roberts J M, Clurman B E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 41.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 42.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 43.Shirodkar S, Ewen M, DeCaprio J A, Morgan J, Livingston D M, Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992;68:157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- 44.Sobczak-Thepot J, Harper F, Florentin Y, Zindy F, Brechot C, Puvion E. Localization of cyclin A at the sites of cellular DNA replication. Exp Cell Res. 1993;206:43–48. doi: 10.1006/excr.1993.1118. [DOI] [PubMed] [Google Scholar]
- 45.Sontag E, Sontag J-M, Garcia A. Protein phosphatase 2A is a critical regulator of protein kinase C ξ signaling targeted by SV40 small t to promote cell growth and NF-κB activation. EMBO J. 1997;16:5662–5671. doi: 10.1093/emboj/16.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sontag E, Fedorov S, Kambayashi C, Robbins D, Cobb M, Mumby M C. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the Map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 47.Srinivasan A, McClellan A J, Vartikar J, Marks I, Cantalupo P, Li Y, Whyte P, Rundell K, Brodsky J L, Pipas J M. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol Cell Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stiegler P, Schüchner S, Lestou V, Wintersberger E. Polyomavirus large T antigen-dependent DNA amplification. Oncogene. 1997;14:987–995. doi: 10.1038/sj.onc.1200904. [DOI] [PubMed] [Google Scholar]
- 49.Strausfeld A P, Howell M, Descombes P, Chevalier S, Rempel R E, Adamczewski J, Maller J L, Hunt T, Blow J J. Both cyclin A and cyclin E have S-phase promoting (SPF) activity in Xenopus egg extracts. J Cell Sci. 1996;109:1555–1563. doi: 10.1242/jcs.109.6.1555. [DOI] [PubMed] [Google Scholar]
- 50.Tommasino M, Adamczewski J P, Carlotti F, Bath C F, Manetti R, Contori M, Cavalieri F, Hunt T, Crawford L. HPV 16 E7 protein associates with the protein kinase p33cdk2 and cyclin A. Oncogene. 1993;8:195–202. [PubMed] [Google Scholar]
- 51.Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voitenleitner C, Fanning E, Nasheuer H-P. Phosphorylation of DNA polymerase α-primase by cyclin A-dependent kinases regulates initiation of DNA replication in vitro. Oncogene. 1997;14:1611–1615. doi: 10.1038/sj.onc.1200975. [DOI] [PubMed] [Google Scholar]
- 53.Wang E H, Bhattacharyya S, Prives C. The replication functions of polyomavirus large T antigen are regulated by phosphorylation. J Virol. 1993;67:6788–6796. doi: 10.1128/jvi.67.11.6788-6796.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zerfass K, Schulze A, Spitkovsky D, Friedman V, Henglein B, Jansen-Dürr P. Sequential activation of cyclin E and cyclin A gene expression by human papillomavirus type 16 E7 through sequences necessary for transformation. J Virol. 1995;69:6389–6399. doi: 10.1128/jvi.69.10.6389-6399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zerfass K, Spitkovsky D, Schulze A, Joswig S, Henglein B, Jansen-Dürr P. Adenovirus E1A activates cyclin A gene transcription in the absence of growth factors through interaction with p107. J Virol. 1996;70:2637–2642. doi: 10.1128/jvi.70.4.2637-2642.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Hannon J, Beach D. P21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 57.Zhao J, Dynlacht B, Imai T, Hori T-A, Harlow E. Expression of NPAT, a novel substrate of cyclin E-CDK2, promotes S-phase entry. Genes Dev. 1998;12:456–461. doi: 10.1101/gad.12.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziebold U, Bartsch O, Marais R, Ferrari S, Klempnauer K-H. Phosphorylation and activation of B-myb by cyclin A-cdk2. Curr Biol. 1997;7:253–260. doi: 10.1016/s0960-9822(06)00121-7. [DOI] [PubMed] [Google Scholar]