Abstract
The implementation and potential of ketogenic dietary therapies (KDTs) have changed over time. The organization of KDT services, the availability of multidisciplinary teams, resources and support for patients and families still vary widely around the world. This diversity is reflected by a lack of consistency in reported outcomes, optimization of using KDT and KDT compliance. To highlight the unmet needs for KDT services, the ERN EpiCARE Ketogenic Dietary Therapy Special Interest Group (KDT SIG) conducted an online survey on KDT implementation and utilization, addressing the following topics: Use and completeness of guidelines and protocols; assessment of compliance and outcome parameters, sustainability and inclusivity in daily life. Consistently reported unmet needs included the lack of psychological support and resources to measure and improve adherence to KDT, the lack of inclusion strategies, and shared guidelines and protocols adapting to specific needs. Future interventions should focus primarily on educational and informative measures together with creation of shared protocols for complex care.
Plain Language Summary
This study provides the results of a survey compiled by clinicians and patients representatives belonging to ERN Epicare, designed to unravel unmet needs from both patients' and healthcare practitioners' perspectives during ketogenic dietary therapies (KDT) provision. Importantly, results show the need to create new shared protocols and guidelines meant for KDT use in complex care situations and to develop future strategies initiatives to support patients improving their social inclusivity.
Keywords: epilepsy, ERN‐EpiCARE, ketogenic dietary therapies, patient centered outcomes, unmet needs
Key points.
Both in research and clinical setting practical issues to improve KDT administration and outcome evaluation should be searched.
The ERN EpiCARE dietary therapy study group designed a survey to unravel the unmet needs in the KDT services belonging to ERN EpiCARE Centers.
Lack of educational and supportive strategies to facilitate inclusivity and independence, and shared guidelines and protocols adapting to special situations and complex care processes has been outlined.
1. INTRODUCTION
Knowledge about ketogenic dietary therapies (KDTs) has grown rapidly over the past few decades, accompanied by an overabundance of publications that sometimes makes it difficult to identify the major milestones reached in the field. However, clinical practice regarding the implementation of KDTs has changed over time. Clinical experts in KDT from multiple centers and countries around the world have recently updated their recommendations to standardize clinical practice. 1 Several questions remain unresolved, such as the use of KDT in emergencies, the management of concomitant therapies, long‐term compliance and tolerability, and the transition process. Furthermore, KDT services and availability of multidisciplinary teams, timing of KDT proposal, resources and support for patients and families, and the economic burden of KDT still vary worldwide. There is still a lack of consistency in reported outcomes, definitions, and assessments in both clinical and research settings. 2 The effects of KDTs on other areas than the main symptoms, such as non‐seizure related outcomes, are not regularly assessed. Furthermore, as is often the case, the outcomes traditionally used in research do not adequately reflect clinical measurements and parental priority outcomes. Recent research by Carroll et al., published in collaboration with the CORE‐ KDT study group, offers a possible solution, as they have defined a core outcome set that should be measured and reported in all studies, with the goal of facilitating the creation of more homogeneous data‐sets and of obtaining comparable evidence. 2
Currently, research on KDT is expanding in various directions, from the connection between the gut microbiome and its effect on seizure threshold and the identification of new molecular targets to its application in neurological diseases other than epilepsy. In this scenario, clinicians and researchers should consider practical issues to improve KDT administration and outcome assessment. Therefore, with the present survey, the Ketogenic Dietary Therapy Special Interest Group (KDT SIG) within the European Reference Network (ERN) on rare and complex epilepsies (EpiCARE) aimed to highlight the unmet needs for KDT services in the ERN EpiCARE Centers in order to prioritize interventions.
2. METHODS
2.1. Study design
Child and adult neurologists, dietitians, and patient advocate being full members of the ERN EpiCARE network, were invited to participate in an online survey to provide their views on health care and unmet needs of patients and families in KDT implementation and KDT use. This study included human participants and was approved by the Ethics Committee of Pavia (P‐20200099763). The participants gave informed consent to take part in the study.
2.2. Survey instrument
Members of the KDT SIG of the ERN‐EpiCARE identified areas of interest and unmet needs in ketogenic dietary services through online meetings and group discussions, and developed a questionnaire for distribution to health care professionals (HCPs) participating in the network in order to represent both the clinicians' and the patients' advocates perspective. The survey was distributed via Google Modules from June 2023 to July 2023. The survey invitation was sent to all the ERN EpiCARE Centers providing a KDT service, namely 23. The survey was divided in three sections: (1) General information (experience of the center in KDT delivery and research; composition of the Keto‐team; organization of the KDT service); (2) Multiple choice questions; (3) Open questions. The topics addressed in the multiple choice section were the following: use and completeness of guidelines and protocols (degree of adherence to available guidelines; coverage of emergencies and specific needs e.g. KDT during surgery by available protocols); compliance and outcome assessment (compliance measurement, secondary outcome assessment), sustainability and inclusivity in daily life (KDTs' costs; educational and psychological support, travel).
Descriptive statistics were calculated for each variable.
3. RESULTS
3.1.
In total, 21 clinicians (19 neurologists; 2 dietitians) from 15/23 different ERN EpiCARE centers (see Figure 1) responded to the survey. General information about the participants is displayed in Table S1.
FIGURE 1.
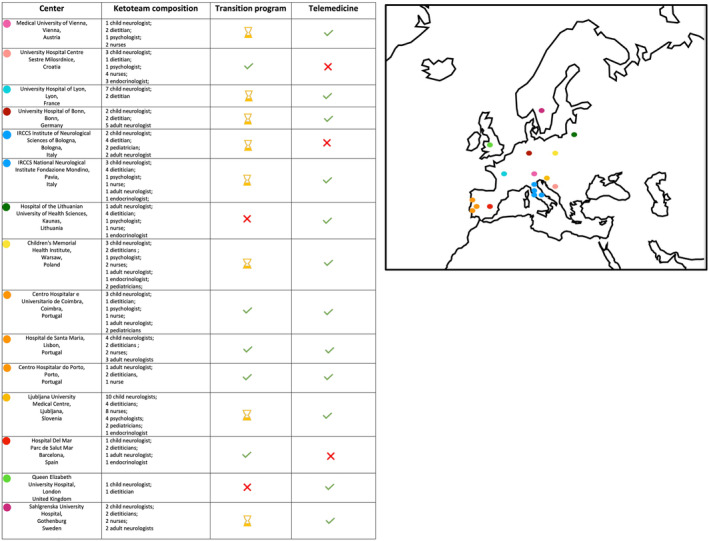
Centers participating in the survey, their keto‐team composition, and their availability of transition program and/or telemedicine.
3.2. Use and completeness of guidelines and protocols
International guidelines are used in only 38% of centers; 28.5% of respondents reported using internal/institutional guidelines; the remainder used a combination of national and institutional guidelines. The capacity to consider KDT according to international guidelines, namely after failure of 2 antiseizure medications (ASMs), corresponds to 43% of centers. Specific protocols for emergencies in KDT according to institutional or national protocols are available in 53% of centers.
A large percentage of centers offer telemedicine for follow‐up visits (83%), with 11% of these following a specific protocol. In 50% of cases, an active transition program is available for KDT youth.
3.3. Compliance and outcome assessment
A response time of just a few days response system to patient inquiries is guaranteed in 100% of cases. Adherence and compliance is assessed through structured interviews (58%) or dietary intake assessment (53%). 21% of respondents acknowledge the lack of a reliable method to measure compliance. Only 44% of respondents report that assessment of secondary outcomes is performed systematically in clinical practice.
3.4. Sustainability and inclusivity in daily life
The great majority of respondents (84%) affirm that exams and evaluations costs are entirely covered by the state health care system. The 28% of respondents declare that there are special educational programs for inclusion at school and work, 50% consider this an urgent problem that needs to be solved. The same magnitude of response distribution applies to travel and meal solutions for patients eating out while on KDT.
3.5. Open questions
Unmet needs from a family identified by the respondents included inadequate knowledge of KDT management and care in nursing homes; meal management in general, for example at school; availability of a direct interface for support from dietitians support, and a lack of transition services.
According to respondents, reported unmet needs from the patient perspective included dietary restrictions in general, reimbursement of all dietary products, and lack of social support.
The unmet needs for KDT services included the lack of availability of transition and adult services, the lack of dedicated staff, and the lack of psychological support in parallel with therapeutic care and clinical follow‐up.
In addition, respondents identified the availability of international protocols on complications/emergencies and specific needs, training programs for nurses, and scientific webinars on KDTs as additional unmet needs.
4. DISCUSSION
The present survey focused on the current unmet needs in KDT services of the ERN EpiCARE Network and aimed to identify future directions to improve the care process. In the era of continuous evolution of KDT implementation, physicians must ask themselves how to advocate for efficient KDT services. Considering the responses, the main shortcomings identified are related to the lack of shared guidelines and protocols that adapt to specific and complex situations and needs (e.g. surgery, medical emergency excetera). In addition, there was a lack of educational and supportive studies to promote inclusivity and independence among adolescents and adults, the availability of psychological support and transition programs was inadequate, and there was a lack of tools to measure compliance and secondary outcomes.
It is currently an established principle that when treating complex and rare epilepsies, for which KDT is a reliable therapeutic option, patient centered outcomes must be considered in order to strengthen treatment adherence and effectiveness. Therefore, major efforts, already reported by some research groups, should be made to use patient centered outcome sets and reliable assessment tools to design new studies and obtain comparable multi‐center data collection. In this direction, family associations are crucial to better understand the needs and wellbeing of patients.
A targeted and individual approach to the patient is essential. The ILAE Task Force has drafted evidence‐based clinical recommendations on psychological treatments for people with epilepsy, particularly on psychological screening and psychoeducational interventions in the care of patients with epilepsy. 13 In line with this statement, specific psychoeducational program aimed at implementing knowledge and education about the disease and challenges in adhering to KDT were carried out with good results, 14 actively involving different figures of the Keto‐team. To raise awareness of psychoeducational benefits and possibilities, dedicated sessions during conferences on KDTs could be very useful. As indicated by the survey, it is also important to ensure patient support to promote autonomy when implementing KDT. In parallel, information material for school and work environments to improve adaptability and inclusion should deserve more attention even at the level of roundtables of scientific societies, to inform teachers and operators about the special diet and to adapt the menu tacking the possibilities of local gastronomy into account. Although not available everywhere, a multidisciplinary Keto‐team is an important requirement to meet all of the patient's and provide a holistic clinical and psychological approach. Respondents stated that, in addition to the core figures of epileptologist and dietitian, in the majority of cases, a nurse, a psychologist, an endocrinologist, and a pediatrician/internist were part of the keto‐team (57%). This information is relevant because caring for patients on KDT actually means meeting care needs that go beyond epileptic and nutritional aspects. As highlighted in the survey, given the diverse needs of the patients on KDT, it is critical to ensure prompt response to requests and issues to enable optimal and consistent adherence and thus compliance. Therefore, the use of telemedicine, rapid response and the availability of expert and dedicated staff, as survey results show, has already been partially implemented in many centers, and certainly contributes substantially to improving the patient interface.
With regard to assessing compliance evaluation, a relatively small percentage of respondents (21%) stated that caregivers' accounts and standardized interviews are not sufficient to assess this issue effectively. We assume that compliance assessment is important not only to correctly interpret the outcome of the intervention, but also to better address the overall clinical approach and that this point should therefore not be underestimated. In this direction, Ketocheck is a questionnaire that was developed for the practical non‐invasive measurement of therapy adherence, 3 and can be used at regular intervals periodically after specific interventions to monitor therapy adherence. Over the years, there has been an increase in commercially available ready‐to‐eat ketogenic products, but reimbursement of these products and disposals for blood glucose and ketones test varies by healthcare system. It would be helpful to inquire about the coverage for ketogenic products as “food for special medical purpose” and how this impacts adherence in different countries to ensure comprehensive and accessible care for individuals on KDTs.Guidelines and shared protocols are very important at national and international levels and, in addition to the recently updated international recommendations, 1 we have other national guidelines 4 , 5 , 6 , 7 , 8 with specific recommendations for etiology, 9 , 10 age, 11 and diet administration. 12 Nevertheless, guidelines for ‘specific needs’ are still lacking, and as ketogenic dietary therapies are spreading, scenarios such as KDT management in the emergency room, during surgery, and during pregnancy are common and highly relevant. Together with closing this gap, it is our duty to disseminate the knowledge of KDT management at multiple levels, with the support of International referral societies such as the ILAE (International League Against Epilepsy), the more recent INKS (International Neurological Ketogenic Society), or other networks such as ERN‐EpiCARE.
Many educational initiatives and webinar libraries including KDT as a topic are already underway, but are aimed almost exclusively at epileptologists. The dissemination of KDT knowledge should indeed involve other professionals and therefore be aimed at nurses, general practitioners, dietitians, and patients. Finally, when it comes to rare diseases, the issue of transition to adult care is increasingly coming to the fore. Thus, specific transition models, where available, should be shared, networking encouraged and staff dedicated to transition identified within KDT services. A limitation of this survey is incomplete participation of all ERN EpiCARE centers, which could be interpreted as not fully representative for KDT services at European level. However, we believe that initiatives for research, clinical and educational purposes, initiatives should come from recognized societies/organizations with the aim of achieving widespread dissemination.
5. CONCLUSIONS
The results of this survey, which was distributed among all ERN EpiCARE centers offering KDT services, reveal several unmet needs that deserve attention.
In particular, educational interventions and initiatives on spreading the knowledge about KDT should be encouraged, as well as the creation of shared protocols for to specific medical situations and needs that patients undergoing KDT may face in the future.
CONFLICT OF INTEREST STATEMENT
V.D. received research grants from Jazz Pharmaceuticals, speaker and consultancy fee from Nutricia Gmbh, Vitaflo, Dr Schar Kanso. JHC has received research grants from Zogenix (now a part of UCB), Marinus, GW Pharma (now Jazz Pharmaceuticals), Vitaflo, Stoke Therapeutics, Ultragenyx, National Institute of Health Research (NIHR), EPSRC, GOSH Charity, ERUK, the Waterloo Foundation, and the Great Ormond Street Hospital NIHR Biomedical Research Centre; and has served as consultant/advisor for ZogenixUCB, Nutricia, GW Pharma/ Jazz Pharmaceuticals, and Biocodex for which remuneration was made to the department, outside of the submitted work; serves as Chair of the Medical Board for DravetUK, Hope for Hypothalamic Hamartoma, and Matthew‘s Friends and endowed chair at UCL Great Ormond Street Institute of Child Health. A.D. received consultancy fee from Vitaflo and APR. AD has received travel reimbursement and speaker honoraria from Nutricia, Vitaflo, Eisai, UCB, Jazz Pharmaceuticals, Takeda and Kanso. The remaining authors have no conflict of interest to disclose. We confirm that we have read the Journal‘s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors would like to acknowledge Mrs. Sebile Tchaicha for her support as the EpiCARE administration project mananager at the time of the study. The ERN‐EpiCARE authorgroup would like to thank all the researchers and experts who participated in the survey: Tobias Baumgartner, Germany, University Hospital Bonn; Johan Bjellvi, Sweden, Sahlgrenska University Hospital; Andreas Brunklaus, United Kingdom, Queen Elizabeth University Hospital; Janette Buttle, United Kingdom, Queen Elizabeth University Hospital; Cristina Duarte Pereira, Portugal, Centro Hospitalar e Universitario de Coimbra; Ana Raquel Fonseca Samões, Portugal, Centro Hospitalar do Porto; Elena Freri, Italy Department of Pediatric Neurology, IRCCS Foundation Carlo Besta Neurological Institute, Milan, Monica Guglielmetti, Italy, Fondazione Istituto Neurologico Nazionale Casimiro Mondino, Pavia; Tove Hallböök, Sweden, Sahlgrenska University Hospital; Jurgita Karandiene, Lithuania, Hospital of the Lithuanian University of Health Sciences; Katarzyna Kotulska, Poland, Children's Memorial Health Institute; Tommaso Lo Barco, Italy, Child Neuropsychiatry, Department of Surgical Sciences, Dentistry, Gynecology and Pediatrics, University of Verona, Masa Malenica, Croatia, University Hospital Centre Sestre Milosrdnice; Tullio Messana, Italy, IRCCS Institute of Neurological Sciences of Bologna; David Neubauer, Slovenia, Ljubljana University Medical Centre; Panagiotakaki Eleni, France, University Hospitals of Lyon (HCL); Maria Papadopoulou, Portugal, Hospital de Santa Maria; Sofia Quintas, Austria, Medical University of Vienna; Lilia Volpi, Italy, IRCCS Institute of Neurological Sciences of Bologna; Petra Trimmel‐Schwahofer, Austria, Medical University of Vienna, Sonika Garcia‐Ribera Ruiz, Spain, Hospital Del Mar‐Parc de Salut Mar. ERN EpiCARE is funded by the European Commission.
De Giorgis V, Pasca L, Aznar‐Lain G, Bibic I, Bibic V, Darra F, et al. Unraveling unmet needs in ketogenic dietary services: An ERN EpiCARE survey. Epilepsia Open. 2024;9:1582–1588. 10.1002/epi4.12968
Contributor Information
Ludovica Pasca, Email: ludovica.pasca01@universitadipavia.it.
ERN EpiCARE Ketogenic Dietary Therapy Special Interest Group (KDT SIG):
Tobias Baumgartner, Johan Bjellvi, Andreas Brunklaus, Janette Buttle, Cristina Duarte Pereira, Ana Raquel Fonseca Samões, Elena Freri, Monica Guglielmetti, Tove Hallböök, Jurgita Karandiene, Katarzyna Kotulska, Tommaso Lo Barco, Masa Malenica, Tullio Messana, David Neubauer, Panagiotakaki Eleni, Maria Papadopoulou, Sofia Quintas, Lilia Volpi, Petra TrimmelSchwahofer, and Sonika Garcia‐Ribera Ruiz
REFERENCES
- 1. Kossoff EH, Zupec‐Kania BA, Auvin S, Ballaban‐Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3(2):175–192. 10.1002/epi4.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carroll JH, Martin‐McGill KJ, Cross JH, Hickson M, Williams E, Aldridge V, et al. Core outcome set development for childhood epilepsy treated with ketogenic diet therapy: results of a scoping review and parent interviews. Seizure. 2022;99:54–67. 10.1016/j.seizure.2022.05.009 [DOI] [PubMed] [Google Scholar]
- 3. Lopes Neri LC, Guglielmetti M, De Giorgis V, Pasca L, Zanaboni MP, Trentani C, et al. Validation of an Italian questionnaire of adherence to the ketogenic dietary therapies: iKetoCheck. Foods. 2023;12(17):3214. 10.3390/foods12173214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armeno M, Caraballo R, Vaccarezza M, Alberti MJ, Ríos V, Galicchio S, et al. Consenso nacional sobre dieta cetogénica [National consensus on the ketogenic diet]. Rev Neurol. 2014;59(5):213–223. Spanish. [PubMed] [Google Scholar]
- 5. Vaccarezza M, Agustinho A, Alberti MJ, Argumedo L, Armeno M, Blanco V, et al. Consenso nacional de dieta Atkins modificada [National consensus on the modified Atkins diet]. Rev Neurol. 2016;62(8):371–376. Spanish. [PubMed] [Google Scholar]
- 6. Veggiotti P, Burlina A, Coppola G, Cusmai R, De Giorgis V, Guerrini R, et al. The ketogenic diet for Dravet syndrome and other epileptic encephalopathies: an Italian consensus. Epilepsia. 2011;52(Suppl 2):83–89. 10.1111/j.1528-1167.2011.03010.x [DOI] [PubMed] [Google Scholar]
- 7. Subspecialty Group of Neurology, the Society of Pediatrics, Chinese Medical Association; China Association Against Epilepsy; Editorial Board, Chinese Journal of Pediatrics . Consensus on ketogenic diet therapy for epilepsy and related nervous system diseases. Zhonghua Er Ke Za Zhi. 2019;57(11):820–825. Chinese. 10.3760/cma.j.issn.0578-1310.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 8. Whiteley VJ, Martin‐McGill KJ, Carroll JH, Taylor H, Schoeler NE, Ketogenic Dietitians Research Network (KDRN) . Nice to know: impact of NICE guidelines on ketogenic diet services nationwide. J Hum Nutr Diet. 2020;33(1):98–105. 10.1111/jhn.12697 [DOI] [PubMed] [Google Scholar]
- 9. Klepper J, Akman C, Armeno M, Auvin S, Cervenka M, Cross HJ, et al. Glut1 deficiency syndrome (Glut1DS): state of the art in 2020 and recommendations of the International Glut1DS Study Group. Epilepsia Open. 2020;5(3):354–365. 10.1002/epi4.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alberti MJ, Agustinho A, Argumedo L, Armeno M, Blanco V, Bouquet C, et al. Recommendations for the clinical management of children with refractory epilepsy receiving the ketogenic diet. Arch Argent Pediatr. 2016;114(1):56–63. English, Spanish. 10.5546/aap.2016.eng.56 [DOI] [PubMed] [Google Scholar]
- 11. van der Louw E, van den Hurk D, Neal E, Leiendecker B, Fitzsimmon G, Dority L, et al. Ketogenic diet guidelines for infants with refractory epilepsy. Eur J Paediatr Neurol. 2016;20(6):798–809. 10.1016/j.ejpn.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 12. van der Louw E, Aldaz V, Harvey J, Roan M, van den Hurk D, Cross JH, et al. Review Group. Optimal clinical management of children receiving ketogenic parenteral nutrition: a clinical practice guide. Dev Med Child Neurol. 2020;62(1):48–56. 10.1111/dmcn.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michaelis R, Tang V, Goldstein LH, Reuber M, LaFrance WC Jr, Lundgren T, et al. Psychological treatments for adults and children with epilepsy: evidence‐based recommendations by the international league against epilepsy psychology task force. Epilepsia. 2018;59:1282–1302. 10.1111/epi.14444 [DOI] [PubMed] [Google Scholar]
- 14. Zanaboni MP, Pasca L, Geraci MA, Varesio C, Guglielmetti M, Tagliabue A, et al. Case report: KETOLAND the psychoeducation program for ketogenic diet. Front Psych. 2023;8(14):1155717. 10.3389/fpsyt.2023.1155717 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


