Abstract
Chronic low‐grade inflammation, particularly elevated tumor necrosis factor (TNF) levels, occurs due to advanced age and is associated with greater susceptibility to infection. One reason for this is age‐dependent macrophage dysfunction (ADMD). Herein, we use the adoptive transfer of alveolar macrophages (AM) from aged mice into the airway of young mice to show that inherent age‐related defects in AM were sufficient to increase the susceptibility to Streptococcus pneumoniae, a Gram‐positive bacterium and the leading cause of community‐acquired pneumonia. MAPK phosphorylation arrays using AM lysates from young and aged wild‐type (WT) and TNF knockout (KO) mice revealed multilevel TNF‐mediated suppression of kinase activity in aged mice. RNAseq analyses of AM validated the suppression of MAPK signaling as a consequence of TNF during aging. Two regulatory phosphatases that suppress MAPK signaling, Dusp1 and Ptprs, were confirmed to be upregulated with age and as a result of TNF exposure both ex vivo and in vitro. Dusp1 is known to be responsible for glucocorticoid‐mediated immune suppression, and dexamethasone treatment increased Dusp1 and Ptprs expression in cells and recapitulated the ADMD phenotype. In young mice, treatment with dexamethasone increased the levels of Dusp1 and Ptprs and their susceptibility to infection. TNF‐neutralizing antibody reduced Dusp1 and Ptprs levels in AM from aged mice and reduced pneumonia severity following bacterial challenge. We conclude that chronic exposure to TNF increases the expression of the glucocorticoid‐associated MAPK signaling suppressors, Dusp1 and Ptprs, which inhibits AM activation and increases susceptibility to bacterial pneumonia in older adults.
Keywords: alveolar macrophages, Dusp1, glucocorticoids, inflamm‐aging, MAPK, pneumonia, Ptprs, Streptococcus pneumoniae, tumor necrosis factor
Elevated TNF levels because of advanced age increase the expression of Dusp1 and Ptprs, which encode negative regulators of MAPK signaling. This inhibits the ability of alveolar macrophages to effectively respond to bacterial infection. This TNF‐mediated suppression phenocopies glucocorticoid immunosuppression, which has been shown to be mediated by Dusp1.
Abbreviations
- ADMD
age‐dependent macrophage dysfunction
- AM
alveolar macrophages
- BALF
bronchoalveolar lavage fluid
- DEGs
Differentially‐expressed genes
- Dusp1
Dual Specificity Phosphatase 1
- EkSpn
ethanol‐killed Spn
- IPA
Ingenuity Pathway Analysis
- IL
Interleukin
- KO
Knockout
- MIP‐2, also known as CXCL2
macrophage inflammatory protein 2
- MPO
myeloperoxidase
- PBS
phosphate‐buffered saline
- PMN
polymorphonuclear cell
- Ptprs
Protein Tyrosine Phosphatase Receptor Type S
- ROS
reactive oxygen species
- Spn, the pneumococcus
Streptococcus pneumoniae
- TNF
Tumor necrosis factor
1. INTRODUCTION
Aging is associated with the remodeling of the immune system with a progressive tendency toward a more pro‐inflammatory phenotype alongside an inability to fine‐tune the response and resolve systemic inflammation, a phenomenon referred to as “inflamm‐aging” (Franceschi et al., 2000). Often, aging results in a dysregulated cytokine network with persistently elevated levels of many chemokines/cytokines and inflammatory mediators, including but not limited to tumor necrosis factor (TNF), interleukin‐6 (IL‐6), IL‐1 receptor antagonist (IL‐1ra), reactive oxygen species (ROS), and acute phase proteins such as C‐reactive protein (CRP; Ferrucci et al., 2005; Marcos‐Pérez et al., 2020; O'Mahony et al., 1998; Varadhan et al., 2014). This chronic low‐grade inflammation is damaging and, in older adults, has been associated with increased morbidity, the onset of frailty, and mortality (Bruunsgaard et al., 1999, 2003; Cohen et al., 1997; de Gonzalo‐Calvo et al., 2012; Fried et al., 2001; Harris et al., 1999; Mooradian et al., 1991; Varadhan et al., 2014; Volpato et al., 2001). Inflamm‐aging drives the pathophysiological mechanisms underlying many age‐related conditions, including progressive neurodegenerative diseases, atherosclerosis, diabetes mellitus, cardiovascular disease, and cancer (Bae et al., 2017; Beutler et al., 1985; Bruunsgaard et al., 1999, 2003; Fillit et al., 1991; Firestein & McInnes, 2017; McInnes & Schett, 2007; Van Deventer, 1997). Inflamm‐aging also enhances susceptibility to infectious diseases, including pneumonia, which is the leading cause of infectious death among older adults (Carbon, 1993; Feikin et al., 2000; Janssens, 2005; Yende et al., 2005). Notably, the risk for pneumonia increases with the number of underlying comorbidities and inflammatory status (Fabbri et al., 2015; Janssens, 2005; Pelton et al., 2019; St Sauver et al., 2015); Around 90% of older adults who become hospitalized with pneumonia have two or more preexisting comorbid conditions (Barbé‐Tuana et al., 2020; Yende et al., 2005).
Alveolar macrophages (AM) are the most abundant resident immune cells within the airway, where they act as a sentinel cell and are the first to encounter and engage with pathogens (Allard et al., 2018; Hamidzadeh et al., 2017; Hussell & Bell, 2014). Additionally, they produce the majority of cytokines within the lung at the onset of infection and are responsible for recruiting polymorphonuclear cells (PMNs) and monocytes to the site of infection (Allard et al., 2018; Beck‐Schimmer et al., 2005; Gupta et al., 1996; Hamidzadeh et al., 2017; Hussell & Bell, 2014). Thus, defects in the ability of AM to respond to a pathogen can have considerable adverse consequences for the host (Ferrucci et al., 2005; Marcos‐Pérez et al., 2020; O'Mahony et al., 1998; Varadhan et al., 2014). Age‐related defects reported in AM include increased oxidative stress, higher baseline levels of NFκB activation, and altered polarization (Allard et al., 2018; Boyd et al., 2012; Canan et al., 2014; Hinojosa et al., 2009, 2014; Lafuse et al., 2019; Shivshankar et al., 2011; Wang et al., 2017; Wong et al., 2017). We have also reported that AM from aged animals had disrupted TLR signaling, muted p38 MAPK signaling, and lower overall NFκB activation following exposure to infectious stimuli (Hinojosa et al., 2009). The suppression of these cellular activation pathways contributed to the diminished cytokine production by these cells following infection (Boehmer et al., 2004; Boyd et al., 2012; Hinojosa et al., 2009, 2014; Knapp et al., 2003; Metcalf et al., 2015). Up to this point, demonstrations of the impact of aging on susceptibility to airway infections have shown that AM become less functional with age and have defects that accumulate and contribute to an inability to resolve inflammation at the site of infection (Beck‐Schimmer et al., 2005; Gupta et al., 1996; Knapp et al., 2003; Wong et al., 2017).
We have previously demonstrated that TNF contributes to age‐dependent macrophage dysfunction (ADMD) and the enhanced susceptibility of aged animals to infection. Macrophages treated with recombinant TNF and challenged with Spn had diminished cytokine production and bacterial killing ability (Hinojosa et al., 2009, 2014; Shivshankar et al., 2011; Thevaranjan et al., 2017). Additionally, infusion of young mice with age‐relevant levels of TNF via an osmotic pump increased the expression of laminin receptor (LR), polymeric immunoglobulin receptor (pIgR), and platelet‐activating factor receptor (PAFr), in a manner similar to what has been observed in aged mice (Shivshankar et al., 2011). These host proteins are co‐opted by Streptococcus pneumoniae (Spn, the pneumococcus), the leading cause of community‐acquired pneumonia, and other respiratory tract pathogens to attach to cells, and their upregulation has been shown to increase permissiveness for infection (Cundell et al., 1995; Hinojosa et al., 2009; Shivshankar et al., 2011; Swords et al., 2000; Weiser et al., 1998). Crucially, aged mice from a TNF KO background were more resistant to Spn infection. What is more, macrophages isolated from aged TNF KO mice did not display the ADMD phenotype (Hinojosa et al., 2009, 2014; Puchta et al., 2016; Thevaranjan et al., 2017). Importantly, the specific mechanism as to how TNF mediates ADMD remained to be determined. Herein, and to address this lapse in knowledge, we have examined the cell signaling changes that occur within aged AM in a TNF‐induced manner. Our results implicate a pan‐suppressive effect of TNF, via upregulation of homeostatic regulators, on the ability of AM to become activated in response to the bacterium.
2. MATERIALS AND METHODS
2.1. Mice and bacteria
For experiments requiring live animals, young (3–6 months) and aged (18–24 months) C57BL/6 mice of both sexes were obtained from the National Institute on Aging (NIA) or raised at either UAB or McMaster University's animal facilities. All animal experiments were performed in compliance with approved Institutional Animal Care and Use Committee protocol at the University of Alabama at Birmingham and McMaster University. Infection experiments were performed using S. pneumoniae serotype 4, strain TIGR4 (Tettelin et al., 2001). Animal challenge experiments were performed using forced aspiration by pipetting 100 μL of 105 colony forming units (CFU) of TIGR4 into the mouth of anesthetized mice hanging by their incisors and covering their nares (Shivshankar et al., 2011). Ethanol‐killed bacteria were prepared by treating S. pneumoniae with 70% ethanol for 15 min, which was centrifuged and resuspended in phosphate‐buffered saline (PBS). Inoculation with ethanol‐killed TIGR4 was also done intratracheally but with 108 CFU equivalence of ethanol‐killed TIGR4. To reduce unnecessary use of live animals, the number of mice used for each experiment was determined by power analyses following the completion of the first replicate. Cohort size was based on having sufficient confidence in power of the statistical test applied.
2.2. Detection of bacterial burden
Blood was collected from tail bleeds. Lungs, spleens, and hearts of infected animals were extracted into 1 mL of PBS and homogenized. Bacteria were enumerated by the serial dilutions of blood or organ homogenates, plating on blood agar, and enumeration of colony counts following overnight incubation at 37°C in 5% CO2. Our limit of detection is denoted on the x‐axis of graphs with a less than or equal value (≤). Values with CFU number of being equal to limit of detection are done for graphical purposes so that the CFU that are below the limit of detection can still be represented on the logarithmic graph.
2.3. Histology
For BALF analysis, samples were centrifuged onto cytospin slides at 300 g for 7 min using the Shandon Cytospin 4 (Thermo Fisher Scientific). Slides were stained according to the Hema 3 staining system protocol (Fisher Healthcare) and imaged using a Leica LMD6 with a DFC450C‐5‐megapixel RGB CCD camera (Leica Biosystems, Buffalo Grove, IL). The cell types were quantified using ImageJ software with the Cell Counter plugin.
2.4. Adoptive transfer
Alveolar macrophages in mice were depleted by intratracheal instillation of (100 μL) clodronate or control liposome suspension into the lungs (dosage of 3.75 mg/mL). Standard Macrophage Depletion Kit containing Clodrosome® and Encapsome® control liposomes were purchased from Encapsula NanoSciences (CLD‐8901). The depletion of macrophages was validated by collecting bronchioalveolar lavage fluid and performing cytospins. The next day, 2.5 × 105 AM from donor mice were reconstituted into the lungs intratracheally of recipient mice. Donor primary AM were isolated by bronchoalveolar lavage performed on euthanized mice with 3 mL of PBS with 1 mM EDTA. The lavage was centrifuged at 300 g for 10 min and resuspended in 100 μL PBS that was intratracheal instilled into the lungs of recipient mice.
2.5. Phosphorylation array and functional test of AM
Mice were asphyxiated with isoflurane in a glass jar, and bronchoalveolar lavage was performed using 3 mL of ice‐cold PBS. Bronchoalveolar lavage fluid (BALF) was centrifuged, and pelleted cells were then suspended in Dulbecco's modified Eagle medium with heat‐inactivated FBS, 10 mM MEM Non‐essential amino acids, 50 mM 2‐mercaptoethanol, and 1 M HEPES and counted with a hemocytometer and seeded in a tissue culture plate. Following 1 h of adherence at 37°C in 5% CO2, nonadherent cells were removed by gently washing two times. For the phosphorylation array, cells were infected with Spn in DMEM at an MOI of 25. The plates were centrifuged to synchronize infection at 300 g for 5 min. Cells were incubated at 37°C in 5% CO2 for 15 min. The supernatant was removed, and cells were lysed with RIPA buffer containing phosphatase and protease inhibitors and scraped from the plate. The samples were agitated for 30 min on a rocker and then spun down at 20,000 g for 20 min to collect the protein supernatant. BCA was done to quantify the amount of protein present. The protein was then loaded onto the phosphoarray kit from RayBiotech (AAH‐MAPK‐1‐8). Phagocytosis was done using an Abcam Phagocytosis Assay Kit (ab234053). Macrophage killing assay was performed as previously described (Thevaranjan et al., 2017).
2.6. ELISAs and immunoblots
The detection of inflammatory markers was done using MPO (DY3667), TNF (DY410), KC (DY453), and IL‐6 (DY406) ELISA kits from R&D Systems Inc. (Minneapolis, MN, USA). Albumin ELISA was bought from Bethyl Laboratories (E99‐134). Immunoblots were performed using standard methods. Protein concentrations were measured by BCA assay (Bio‐Rad Laboratories, Hercules, CA, USA) and normalized to the same concentration. Lysates were boiled for 10 min at 95°C with NuPAGE LDS sample buffer, and equal amounts were added to each well and run on 10% Mini‐PROTEAN® TGX™ Precast Gels (Bio‐Rad). Proteins were then transferred onto a 0.2 μm Nitrocellulose membrane (Bio‐Rad, 1704158). Membrane blocking was done with 10% BSA followed by probe IgGs specific for Dusp1 (Cell Signaling, 35217S, 1:500), Ptprs (Proteintech, 13008‐1‐AP, 1:500), or Actin (Abcam, Ab8226, 1:10,000) overnight at 4°C. Bound antibody detected using secondary IgG conjugated with horseradish‐peroxided (Abcam, ab6721) followed by ECL chemiluminescence (Pierce™ ECL western blotting substrate, PI32209) and imaged using the Bio‐Rad ChemiDoc™ XRS+ (Bio‐Rad). Densitometry was performed using ImageJ software.
2.7. RNA sequencing
Lungs from young and aged mice where cells were sent off to Northwestern for digestion and cell sorting, AM were >95% purity. Macrophages were collected from both a C57Bl/6 and TNF KO background of mice. All mice were female and aged to 20 months of age. RNA was isolated using RNeasy Plus Mini kits (Qiagen) followed by mRNA magnetic enrichment using NEBNext kit (New England Biolabs). Libraries were then sequenced on NextSeq 500 instrument (Illumina) at 75‐bp length, single reads with an average reading depth exceeding 3 × 106 per sample. Reads were demultiplexed using bcl2fastq, trimmed, aligned to reference genome mm10 using TopHat2, and quality was assessed with FastQC (Anders et al., 2015; Kim et al., 2013). Aligned reads were mapped to genes using HTseq with an Ensembl annotation. The trimmed mean of M‐values (TMM) normalization was used to normalize the dataset prior to differential expression analysis (Robinson & Oshlack, 2010). Differentially expressed genes (DEGs) were identified using the DESeq2 package on R studio (Love et al., 2014). Differentially expressed genes were analyzed using the web‐based pathway analysis tool QIAGEN IPA (www.ingenuity.com) to identify pathways altered during aging and due to TNF exposure with log2 fold change of 1.5 and adjusted p‐value cutoff set to 1e‐3 for independent filtering (Krämer et al., 2014). PCA and IPA plots were made on R Studio using the ggplot2 package (Wickham, 2016). The RNAseq files are publicly available on GEOSOURCE.
2.8. RNA quantification
Gene levels of Ptprs, Dusp1, and A20 were detected using qPCR. RNA was collected from AM or J774.1 cells using Trizol (Thermo Fisher 15596026) chloroform extraction. cDNA conversion was done using Applied Biosystems™ High‐Capacity cDNA Reverse Transcription Kit (4368814). RT qPCR was done with the QuantiTect SYBR Green PCR Kit (204143) and was read on a Bio‐Rad CFX Opus Real‐Time System. The values were normalized according to the delta delta CT method with two different housekeeping genes (Tbp and RPL13a). In doing so, we are able to normalize the quantity of Dusp1 and Ptprs per each individual sample divided by its expression of housekeeping genes. Each sample is run in triplicate on at least two separate occasions.
2.9. Statistical analysis
All results are displayed with error bars indicating the median with interquartile range, unless otherwise indicated. For in vivo experiments, each individual data point represents an individual animal sample with the number of animals denoted in the figure legend. For data with a single independent factor or two groups, we used a Mann–Whitney U test, unless otherwise indicated. For parametric grouped analyses, we used ANOVA followed by the Bonferroni post hoc analysis or Fisher's LSD test. The Bonferroni post hoc analysis was used for multivariate comparisons to correct for multiple comparisons. Fisher's LSD test was used when the ANOVA result was significant and each pairwise comparison stands alone. A Kruskal–Wallis test was used, to compare nonparametric data, followed by Dunn's multiple comparisons test for hypothesis testing due to the low sample size. Asterisks denote the level of significance observed * = p ≤ 0.05; *** = p ≤ 0.01; *** = p ≤ 0.001; **** = p ≤ 0.0001. Statistical analysis was performed using PRISM 9 (GraphPad Software: La Jolla Ca).
3. RESULTS
3.1. Aged mice are more susceptible to pneumococcal disease and have defects in their ability to respond to infectious stimuli
Consistent with the greater susceptibility to infection observed in older human adults, aged mice (18–24 months) intratracheally challenged with Spn experienced greater bacterial burden than their younger counterparts (3–6 months). One day after the challenge, lungs isolated from infected aged mice had a 15‐fold higher bacterial burden than young mice (Figure 1a). Older humans are also far more likely to develop invasive pneumococcal disease, that is, bacteremia, during pneumonia than younger adults (Kyaw et al., 2005; Loeb, 2004; Pelton et al., 2019). Consistent with this, aged mice also had greater levels of Spn in their blood across time points than young controls, culminating in a nearly 40‐fold median difference at 38 h postinfection (Figure 1b). Once in the bloodstream, Spn can disseminate systemically, invade tissues, and cause long‐lasting organ damage (Brown et al., 2014; Eurich et al., 2017; Kruckow et al., 2023; Musher et al., 2007, 2019; Reyes et al., 2017). We observed over a 10‐fold higher median burden of Spn within the heart tissue of aged mice compared to young mice (Figure 1c). Thus, aged mice rapidly developed the most severe forms of pneumonia and invasive pneumococcal disease, along with its complications, making them suitable models to study age‐related defects in susceptibility.
FIGURE 1.
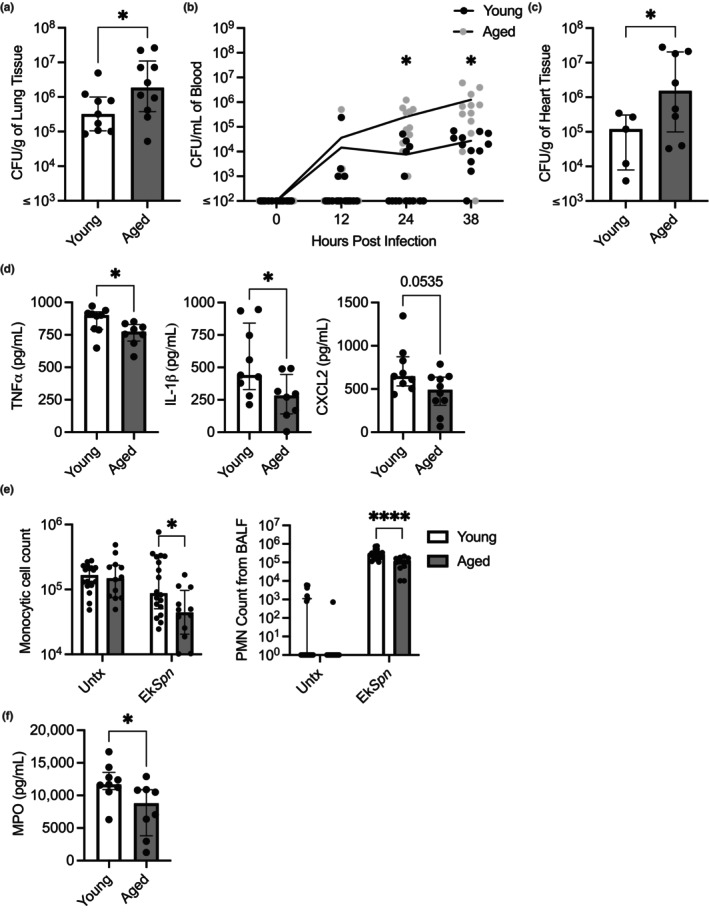
Aged mice are more susceptible to pneumococcal disease and have defects in their ability to respond to infectious stimuli. Young (3–6 months) and aged (18–24 months) C57BL/6 mice were infected intratracheally with 105 CFU of Spn. Mice were sacrificed at 24 h postinfection. The bacterial burden in the lung was enumerated from serial dilutions of lung homogenates on blood agar plates (a). Tail bleeds were taken throughout the infection to enumerate bacterial burden within the blood (b). The bacterial burden in the heart was enumerated from serial dilutions of heart homogenates on blood agar plates (c). Young (3–6 months old) and aged mice (18–24 months old) were intratracheally inoculated with 108 CFU equivalents of ethanol killed Spn (EkSpn). At 8 h postinoculation, mice were sacrificed and bronchoalveolar lavage fluid (BALF) was collected. Levels of TNFα, IL‐1β, and CXCL2 in BALF as measured by ELISA (d). Monocytes and PMNs within BALF were attached to slides by cytocentrifugation, stained, and enumerated by nuclear morphology analysis (e). Myeloperoxidase levels in BALF was measured (f). Statistical significance was calculated using a nonparametric Mann–Whitney U test (a, c, d, & f), a repeated measures two‐way ANOVA with Fisher's LSD post hoc test (b), a two‐way ANOVA with Bonferroni multiple comparisons post hoc test (e). The data are presented as median with interquartile range (IQR); *p ≤ 0.05; ****p ≤ 0.0001. Each data point represents an individual mouse. Graphs with ≤ on x‐axis indicate limit of detection.
To better understand why aged mice are more susceptible to Spn, we tested their ability to mount an early immune response to an infectious challenge. Importantly, the extreme differences in bacterial burden and the inability of aged mice to constrain bacterial replication seemed likely to impact the scale of their inflammatory response even at early time points. Therefore, we instead challenged mice intratracheally using ethanol‐killed Spn (EkSpn), which could not replicate, to ensure that all mice received an equal pro‐inflammatory signal. Following the forced aspiration of EkSpn, we collected bronchoalveolar lavage fluid (BALF) and measured the levels of pro‐inflammatory cytokines and immune cells present. Aged mice had blunted production of TNF and IL‐1β (Figure 1d). Additionally, aged mice had trending but not significantly decreased levels of the chemokine macrophage inflammatory protein 2 (MIP‐2, also known as CXCL2), which is a PMN chemoattractant (Figure 1d; Gupta et al., 1996; Schmal et al., 1996; Standiford et al., 1996). Consistent with the latter, aged mice had significant reductions in the number of PMNs as well as monocytes in the airway following the challenge (Figure 1e). Notably, PMN numbers in the airway corresponded with decreased neutrophil activity in the airway as measured by myeloperoxidase (MPO; Figure 1f). What is more, measured levels of MPO in the airway were greater for young versus aged mice when values from mice with similar numbers of PMN in the airway were considered (n = 4, samples with higher than 20,000 neutrophils, p = 0.0401), suggesting that the aged PMNs are not as functional. Based on these results, we purport that unchecked bacterial replication in the lungs of aged mice following aspiration results from a muted inflammatory response and decreased or potentially delayed immune cell recruitment as a consequence of unresponsive AM. These results also suggest that age‐related defects in cell types other than AM, including PMNs, are most likely also contributing to the increased susceptibility to infection.
3.2. Age‐dependent macrophage dysfunction is sufficient to increase susceptibility to pneumococcal disease
AM have been shown to become less functional with age, but the degree to which this by itself impacts infection risk has not been specifically tested (Beck‐Schimmer et al., 2005; Canan et al., 2014; Knapp et al., 2003; Lafuse et al., 2019; Wong et al., 2017). To discern this, we adoptively transferred AM from either young or aged mice into the lungs of young mice that had been treated prior with clodronate liposomes to deplete resident macrophages and subsequently experimentally challenged these mice with live Spn (Figures S1a and S2a). Although bacterial load in the BALF only trended upward 1 day post‐challenge, we observed a stark loss of containment of the infection and a 40‐fold difference in the median number of bacteria in the blood as well as nearly 20‐fold differences in the spleen and heart, compared to mice that received AM from young donors (Figure 2b). Notably, when AM from young mice were instilled into aged mice, we saw no statistically significant differences in burden when compared to aged mice that received AM from aged mice (Figure S1b). Thus, and as would be expected, other age‐related factors are also contributing to the enhanced susceptibility of aged mice to infection. To further clarify the consequences of ADMD on susceptibility to infection, we isolated AM from young and aged mice and performed ex vivo functional analysis. This revealed that AM from aged mice were attenuated in their capacity to phagocytize and kill pneumococci (Figure 2c,d). We conclude that defects present in aged AM are sufficient to increase disease severity, and their dysfunction directly contributes to a loss of airway containment of Spn infection. Although, as young AM transferred into an aged host are not sufficient to lower susceptibility to infection, we conclude that other age‐related defects are also contributing to the increased susceptibility to infection.
FIGURE 2.
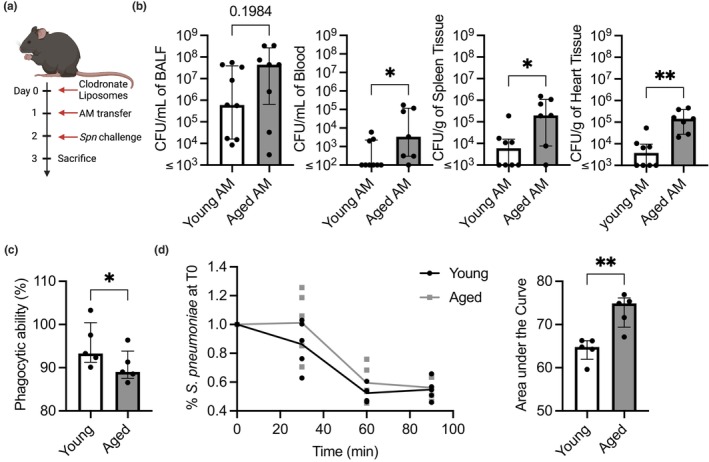
Age‐dependent macrophage dysfunction is sufficient to increase susceptibility to pneumococcal disease. Young mice depleted of their own alveolar macrophages using clodronate liposomes were adoptively transferred with AM from young or aged mice and subsequently challenged intratracheally with 105 CFU of Spn (a). Following 1 day, the bacterial burden was enumerated from BALF, blood, spleen, and heart homogenates (b). AM isolated from BALF of young and aged mice were used in functional assays testing their ability to phagocytize prelabeled zymosan particles (c), and an ex vivo macrophage killing assay of Spn that was quantified by the area under the curve (d) to compare the functionality of young versus aged macrophages. Statistical significance was calculated using a Mann–Whitney U test (b, c, & d). The data are presented as median with IQR; *p ≤ 0.05; **p ≤ 0.01. Each data point represents an individual mouse (b) or well (c & d). Graphs with ≤ on x‐axis indicate limit of detection.
3.3. Chronic exposure to TNF contributes to the hyporesponsiveness of aged AM to Spn
To gain an understanding of the full impact of both aging and TNF on macrophage function and TNF‐mediated MAPK signaling suppression, we examined the activation status of AM from young and aged, in both wild‐type and TNF KO mice backgrounds, using phosphorylation arrays containing antibodies against representative MAPK signaling proteins. In brief, we exposed both wild‐type and TNF KO AM from young and aged mice to EkSpn and assessed their phosphorylation status using an antibody array recognizing the phosphorylated versions of 16 MAPK signaling proteins. We observed significant age‐associated reductions in the phosphorylation status of more than a quarter of the kinase targets assayed (P53, ERK1/2, RSK1, JNK, and GSKα) for wild‐type mice (Figure 3a). Strikingly, most of these reductions could be directly attributed to TNF, as there were no age‐related differences observed between young and aged mice in the TNF KO background, and the activation levels seen in aged TNF KO were equivalent to that of young wild‐type mice (p53, ERK1/2, JNK, & GSKα; Figure 3a). We also observed differences due to the presence of TNF regardless of age, including MKK3, HSP27, GSKβ, mTOR, RSK1, and JNK (Figure 3a). We overlaid the results on a diagram illustrating the primary MAPK signaling cascades (Figure 3b). From this, we could infer that there were global alterations in MAPK signaling with diminished phosphorylation at the level of MAPKKs, MAPKs, transcription factors, and effector molecules and that, in some instances, multiple levels of individual cascades were suppressed (e.g., MKK3, p38, hsp27, and msk1/2).
FIGURE 3.
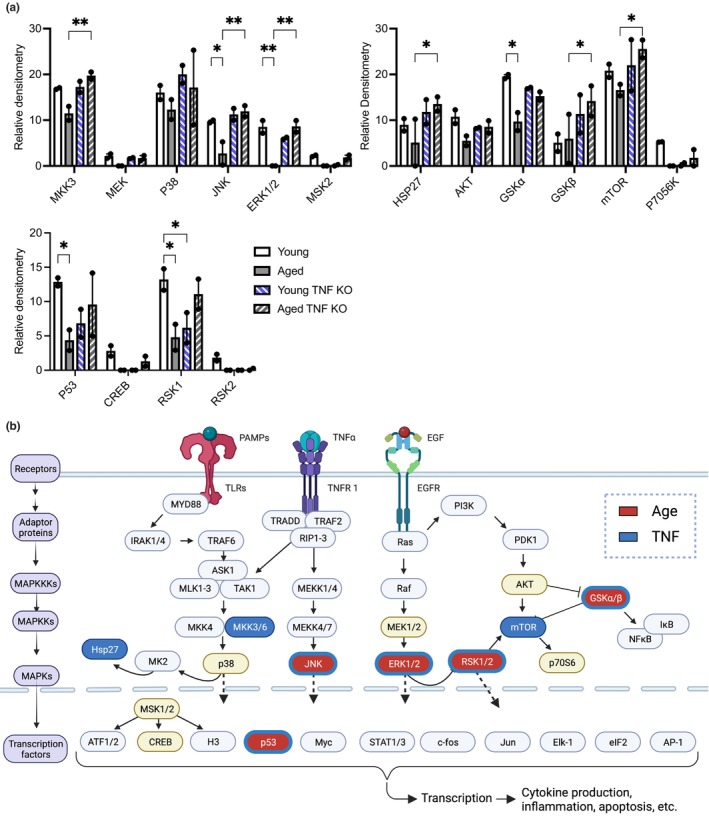
Phosphokinase signaling is starkly suppressed in aged AM. AM were collected from young and aged WT and TNF KO mice and inoculated with Spn at an MOI of 25 for 15 min. Purified protein from these samples was used in a phosphorylation array of the MAPK pathway (a) with results overlaid on the MAPK pathway (b). Any protein designated by a colored oval was tested as part of the phosphorylation array, with items in red indicating significant changes due to age and blue indicating alterations due to TNF. Statistical significance was calculated using a two‐way ANOVA with each comparison standing alone using a Fisher's LSD test. The data are presented as median with interquartile range (IQR); *p ≤ 0.05; **p ≤ 0.01. Each individual point represents the average of two technical replicates on the phosphorylation array per biological sample.
3.4. TNF impacts the transcriptome of AM but not as much as normal aging
In parallel, gene expression profiles of AM isolated from similar cohorts of mice were obtained using RNA‐seq. Principal component analyses of all genes in the transcriptomic dataset found that each experimental cohort clustered separately, whereas biological replicates within each cohort clustered together (Figure 4a), indicating generally reproducible differences in gene expression profiles across AM from different mice. Principle component (PC) 1, which composed 55% of the variance among the dataset, matched with the distinct grouping of the mice by age, whereas PC2, which accounted for 19% of the variance, corresponded to the TNF status of the mice. Dendrogram analysis of the gene expression profiles confirmed this separation by grouping samples by two major branches containing young and aged mice with the genetic background grouping independently within the separate age groups of the top 150 variable genes (Figure 4b). Thus, the impact of advanced age on AM gene expression is more significant than TNF deficiency alone. A list of all the genes with differential expression due to aging is provided in Table S1.
FIGURE 4.
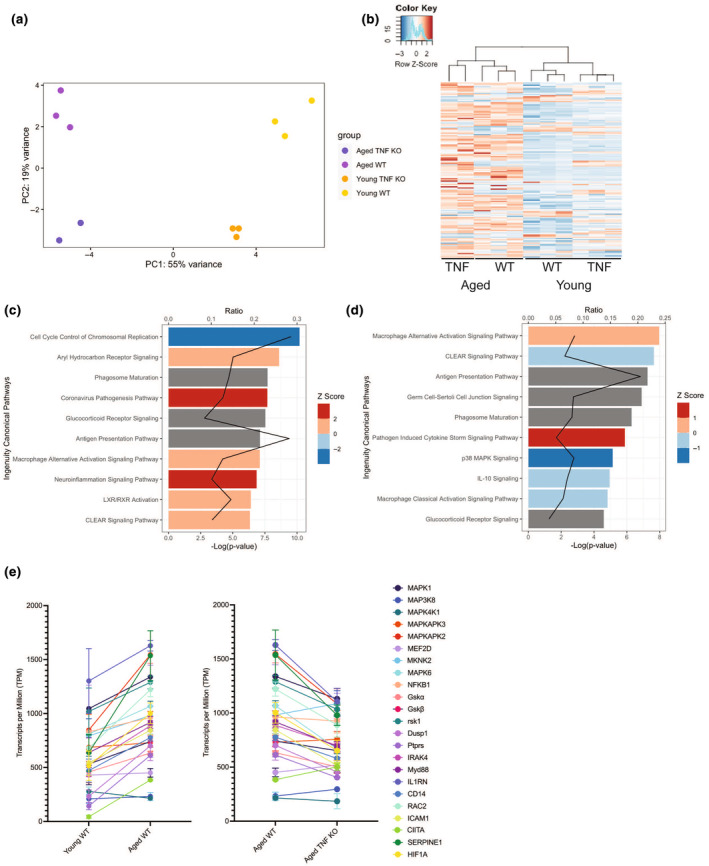
AM have age and TNF‐dependent differences in gene expression. RNA was purified from AM collected from young (3–6 months old) and aged mice (18–24 months old) in both wild‐type C57BL/6 and TNF KO mice. Alterations in gene expression due to age and TNF expression were visualized using a PCA plot (a) and a dendrogram (b). IPA was used to show the 10 most highly scoring canonical pathways (according to p value) altered in expression due to aging (c) and due to genotype (d) by using comparisons of aged WT versus young WT and Aged TNF KO vs Aged WT, respectively. The red line represents the ratio of the number of differentially expressed genes within a pathway divided by the total number of genes within the pathway. Bars represent the p‐value for each pathway expressed as −1 times the log of the p‐value, with the color of the bar indicating the Z score. Relative expression of various members of the MAPK signaling cascade visualized in transcripts per million by group (e).
We used Ingenuity Pathway Analysis (IPA) for data analysis and identified global differences in biological pathway expression that depended on TNF status (Figure 4c, Table S2) as well as on TNF status and aging (Figure 4d, Table S3; Krämer et al., 2014). Among the most prominent differences between young and aged mice were those for genes involved in cell cycle and proliferation, inflammatory signaling, and antigen presentation pathways. The most prominent differences between aged WT and aged TNF KO mice were primarily among inflammatory signaling pathways, including increased macrophage alternative activation, decreased macrophage classical activation signaling, and MAPK signaling. Both comparisons showed alterations in CLEAR (coordinated lysosomal expression and regulation) signaling, phagosome maturation, glucocorticoid receptor signaling, and macrophage alternative activation. The transcript alterations in the MAPK signaling pathway due to age and TNF are illustrated in a graph of the relative expression of various MAPK signaling members in transcripts per million (Figure 4e). Interestingly, the differences in MAPK signaling contributions from age and TNF were consistent with our prior observations using the phosphorylation arrays. The transcripts from aged mice had higher expression of various MAPK members than young mice, but the aged TNF KO samples had levels that were more similar to those of young mice.
3.5. The phosphatases Dusp1 and Ptprs are upregulated in a TNF‐dependent manner during aging
From the transcriptome dataset, we identified two negative homeostatic regulators of MAPK signaling pathways, dual specificity phosphatase 1 (Dusp1) and protein tyrosine phosphatase receptor type S (Ptprs), which were upregulated in AM from aged WT mice versus AM from young WT mice, but not aged TNF KO mice (Figure 5a). Both negative regulators are members of the protein tyrosine phosphatase (PTP) superfamily and act as inhibitors of MAPK signaling (St‐Denis et al., 2016). Dusp1, also known as mitogen‐activated protein kinase phosphatase‐1 (MKP‐1), has been shown to have an important role in regulating inflammation by inhibiting MAPK activation via dephosphorylating both the p38 subunit of MAP kinase and ERK2, among other targets (Chi et al., 2006; Chu et al., 1996; Franklin & Kraft, 1997; Hammer et al., 2006; Salojin et al., 2006; Sun et al., 1993; Zhao et al., 2005). Ptprs, a leukocyte common antigen‐related (LAR) receptor‐type phosphatase, has been shown to inhibit the activation of dendritic cells and dephosphorylate various signaling molecules, including STAT3, EGFR, AKT, SRC, and ERK (Bunin et al., 2015; Davis et al., 2018, 2018, 2019; den Hertog et al., 2004; Gong et al., 2021; Wang et al., 2015). These results were corroborated by immunoblot using whole lung cell lysates from young and aged mice (Figure 5b), as well as qRT‐PCR of isolated RNA taken from AM collected from individual young and aged mice (Figure 5c). As these genes were not elevated in the transcripts from the AM of aged mice with a TNF KO background, we wanted to test whether these genes were TNF regulated. TNF treatment increased the expression of both Dusp1 and Ptprs in J774.1 cells (Figure 5d), albeit only at higher concentrations for the latter. In contrast, we observed a meaningful increase in Dusp1 and Ptprs gene expression levels in AM from young mice administered TNF ex vivo, but no difference in aged mice (Figure 5e). We interpret these results as meaning that Dusp1 levels are directly responsive to TNF, whereas an intermediary signaling molecule may be required for Ptprs gene expression. Moreover, as exogenous TNF treatment of AM from aged animals did not further increase Dusp1 and Ptprs gene expression levels, a saturated response to TNF may already be in place.
FIGURE 5.
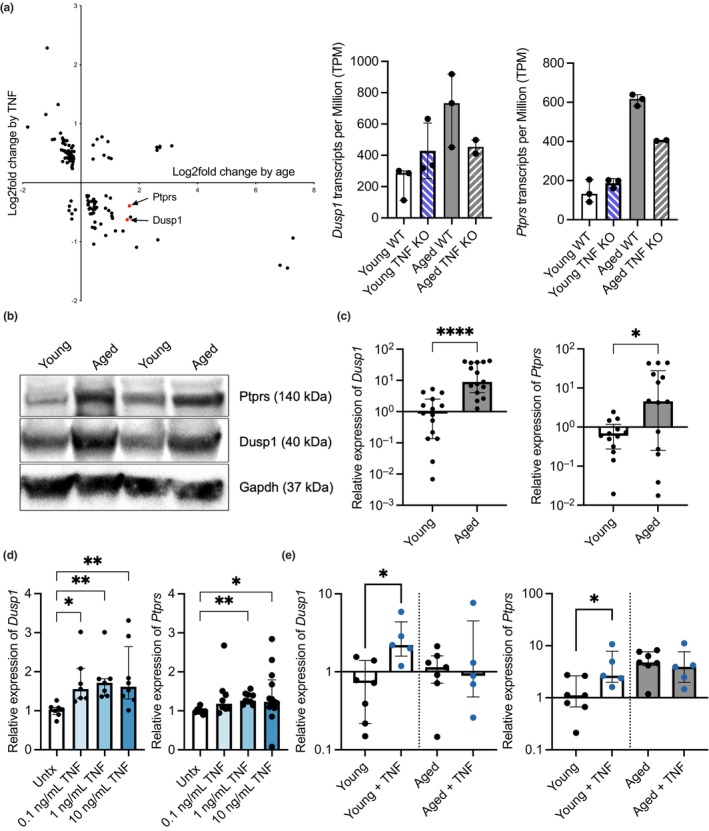
Key negative regulators are upregulated in a TNF‐dependent manner with age. Identification of Dusp1 and Ptprs in DEGs as a result of TNF status and age from the transcriptomic dataset (a). Expression levels of negative regulators Dusp1 and Ptprs from alveolar macrophages are shown in Transcripts per Million (a), protein levels of Dusp1 and Ptprs in whole lung cell lysates via western blots (b), and in isolated AM by qPCR (c). Levels of negative regulators Dusp1 and Ptprs in J774.1 cells treated with various levels of TNF via qPCR (d). Levels of negative regulators Dusp1 and Ptprs in AM from young and aged mice collected and exposed to TNF ex vivo (e). Statistical test was done using either a Kruskal–Wallis (d & e) or a Mann–Whitney test (c). The data are presented as median with interquartile range (IQR); *p ≤ 0.05; **p ≤ 0.001; ****p ≤ 0.0001. Each data point represents an individual gene (a), mouse (b & c), or well (e).
3.6. Dusp1 and Ptprs have suppressive effects on macrophage function, and anti‐TNF can alleviate this during aging
The suppressive effects of Dusp1 on cell signaling are documented as being potent and considered to be the molecular mechanism behind the anti‐inflammatory and immunosuppressive effects of corticosteroids (Abraham et al., 2006; Chen et al., 2002; Fürst et al., 2007; Kassel et al., 2001; Manetsch et al., 2012a; Reddy et al., 2009). Accordingly, the promoter region of Dusp1 contains multiple glucocorticoid response elements, and glucocorticoid treatment has been shown to increase the expression of Dusp1 (Abraham et al., 2006; Shipp et al., 2010). Although direct evidence of Ptprs upregulation upon glucocorticoid treatment has not yet been demonstrated, our analysis using the publicly available ENCODE Transcription Factor Targets dataset revealed that Ptprs does have glucocorticoid response elements within its promoter (Rouillard et al., 2016). Congruently, dexamethasone treatment increased the expression of Dusp1 and Ptprs in not only J774A.1‐treated cells in vitro (Figure 6a) but also AM isolated from young mice treated ex vivo (Figure 6b), and AM from young mice administered the steroid (Figure 6c). Moreover, dexamethasone treatment recapitulated the ADMD phenotype, as treated J774A.1 cells produced less pro‐inflammatory cytokines (Figure 6d), and dexamethasone‐treated young mice had greater susceptibility to Spn infection (Figure 6e). Further linking age‐associated levels of TNF to heightened levels of these cell signaling suppressors and greater susceptibility to infection, we observed that the treatment of aged mice with anti‐TNF lowered Dusp1 expression eightfold and Ptprs by 50‐fold (Figure 6f). What is more, aged mice treated with anti‐TNF antibody prior to infection experienced less severe disease, evidenced by the decreased bacterial burden in the blood, spleen, and heart compared to mice treated with anti‐HRP isotype control antibody (Figure 6g). Additionally, we validated that the anti‐TNF treatment lowered the BAL levels of TNF (Figure S2a). Thus, ADMD closely parallels the immune suppressive state that develops with glucocorticoid treatment, and the upregulation of Dusp1 and Ptprs in AM due to TNF is a molecular explanation for this.
FIGURE 6.
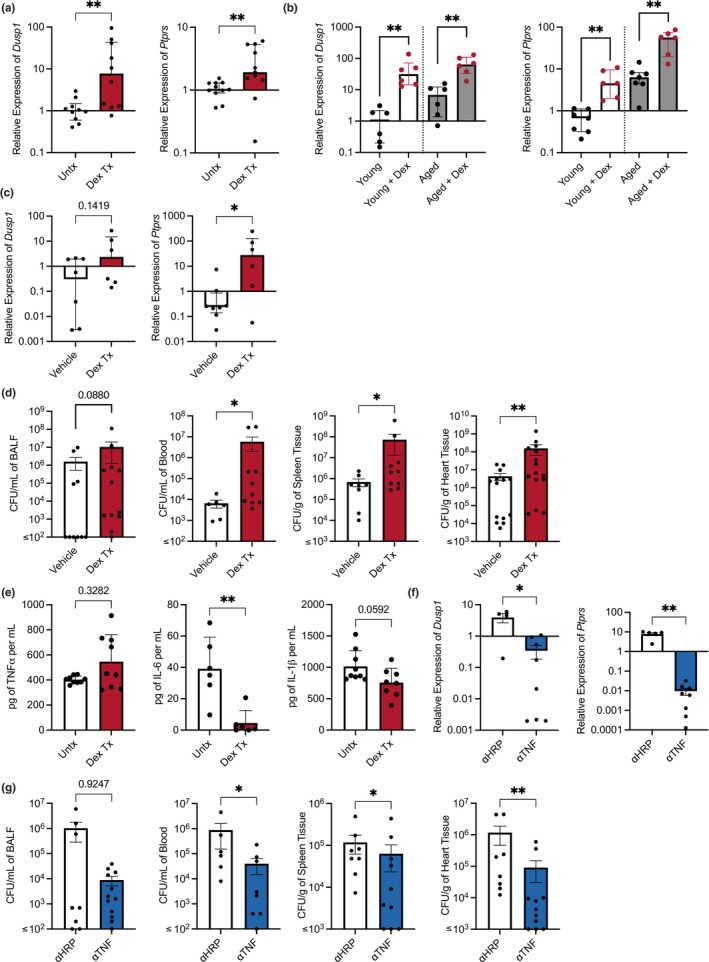
Manipulating the levels of negative regulators alters responses to infection. RNA was isolated from J774.1 macrophages that were treated with dexamethasone, and the levels of Dusp1 and Ptprs were quantified with qPCR (a). The levels of Dusp1 and Ptprs were quantified with qPCR on alveolar macrophages from young and aged mice treated with dexamethasone ex vivo (b) as well as in vivo (c). We quantified the levels of pro‐inflammatory cytokines produced by J774.1 cells treated with dexamethasone overnight and then challenged with EkSpn (e). Young (3–6 months) C57BL/6 mice were treated with dexamethasone and infected intratracheally with 105 CFU of Spn. Bacterial burden 24 h postinfection, in the BALF, blood, spleen, and heart was enumerated (d). C57BL/6 mice were treated every other day with anti‐TNF or anti‐HRP control antibodies for 2 weeks before being infected intratracheally with 105 CFU of Spn. Mice were sacrificed 24 h postinfection, the heart and spleens were harvested, and blood and BALF were collected. RNA was isolated from alveolar macrophages from mice that were treated with anti‐TNF or anti‐HRP control antibodies and were quantified for levels of negative regulators (f). Bacterial burden in mice treated with anti‐TNF was enumerated in the BALF, blood, spleen, and heart (g). The data are presented as median with interquartile range (IQR); *p ≤ 0.05; **p ≤ 0.01. Each data point represents an individual mouse (b, d, e, f, & g) or well (a & c). Graphs with ≤ on x‐axis indicate limit of detection.
4. DISCUSSION
Chronic inflammation and its failure to resolve are central to the development and complications of several age‐related conditions. Specifically, high plasma levels of TNF have been associated with the pathophysiological mechanisms underlying most aged‐related morbidities and diseases (Bae et al., 2017; Beutler et al., 1985; Bruunsgaard et al., 1999, 2003; Fillit et al., 1991; Firestein & McInnes, 2017; Hubbard et al., 2009; McInnes & Schett, 2007; Van Deventer, 1997). Accordingly, chronic low‐grade inflammation is associated with multi‐morbidity, functional disability, and increased mortality in older adults (Bruunsgaard et al., 1999, 2003; Cohen et al., 1997; Fabbri et al., 2015; Harris et al., 1999; Mooradian et al., 1991; Varadhan et al., 2014; Volpato et al., 2001). Chronic inflammation contributes not only to the development and exacerbation of multiple noncommunicable diseases but also to susceptibility to infection. For example, elevated TNF, IL‐6, and CRP serum levels predispose individuals to community‐acquired pneumonia (CAP) and greater disease severity (Kyaw et al., 2005; Loeb, 2004; Pelton et al., 2019; Yende et al., 2005). In accordance with this, we observed that aged mice had a considerably greater bacterial burden in their lungs and had greater levels of Spn in their blood across time points than young controls. This is highly similar to the human condition, in which older humans are also far more likely to develop invasive pneumococcal disease, that is, bacteremia, during pneumonia than younger adults (Kyaw et al., 2005; Loeb, 2004; Pelton et al., 2019). Previous studies from our group have indicated that ADMD contributes significantly to this susceptibility. Along such lines, other chronic inflammatory diseases, such as uncontrolled HIV infection and COPD, induce macrophage dysregulation that includes impaired apoptosis, reduced induction of ROS, and a decline in bacterial killing ability (Bewley et al., 2016). Thus, chronic inflammation caused by aging or disease and associated with increased TNF induces AM dysfunction, contributing to the increased susceptibility to the infection of aged animals and people.
We observed that aged mice had a suppressed response to inflammatory stimuli with muted production of the essential early‐response pro‐inflammatory cytokines, TNF and IL‐1, that are produced primarily by AM in response to Spn (Losa García et al., 1999; O'Brien David et al., 1999; Rijneveld et al., 2001; Ulich et al., 1991). Additionally, aged mice had decreased levels of the chemokine macrophage inflammatory protein 2 (MIP‐2, also known as CXCL2), which is produced in the lungs by both AM and alveolar epithelial cells to stimulate and recruit PMNs into the infected airspaces (Gupta et al., 1996; Pittet et al., 2011; Schmal et al., 1996; Standiford et al., 1996). In turn, this result helps explain why aged mice challenged with EkSpn had fewer PMNs infiltrating into their lungs postchallenge. Importantly, a number of studies indicate that neutrophil function is also altered with advanced age (Adrover et al., 2016; Van Avondt et al., 2023). Supportive evidence for this was observed in the levels of MPO detected when examining mice with equal numbers of infiltrated neutrophils. Thus, even though the adoptive transfer of aged AM was sufficient to enhance the susceptibility of young mice to infection, multiple other defects in host immunity are contributing to the enhanced susceptibility of aged animals.
From our adoptive transfer experiment, we observed that properties intrinsic to aged macrophages were sufficient to increase susceptibility to infection regardless of the youth of the surrounding host, suggesting that the “young” microenvironment could not overcome preset dysfunction in cells. These effects may be due to inherent defects of aged AM arising from a lifetime of replication and inflammatory stimuli exposures. Alternatively, this may be due to transient but prolonged effects due to the continual modification of AM behavior with residual proteins left over from the aged environment, such as elevated Dusp1 and Ptprs. We also observed that young AM transferred into aged mice could not overcome the microenvironment, and there were no differences in burden between aged mice that received young AM compared to those that received aged AM. This result could be explained by the aforementioned dysfunction in other cell types, such as PMNs. Of note, in our EkSpn‐challenged mice, we saw decreased numbers of monocytic cells in the airway of aged mice compared to untreated controls. Additionally, It has recently been shown that AM undergo apoptosis to eliminate Spn from the airway (Preston et al., 2019). We do not know whether the apoptotic‐associated bacterial killing is impacted by age, which could alter cytokine production and subsequent recruitment of immune cells. However, this is an interesting point for future studies.
From our transcriptomics analyses, we observed that Dusp1 and Ptprs were upregulated due to aging in a TNF‐dependent manner. Both negative regulators are tyrosine phosphatases, with Dusp1 having dual specificity for threonine, and serve to shut down kinase‐mediated signaling cascades for various cellular processes limiting inflammation (Seternes et al., 2019). MAPK signaling pathways play an essential role in regulating cellular processes such as the cell cycle, cellular proliferation, and the rapid initiation of macrophage‐mediated inflammatory responses (Yang et al., 2014; Zhang & Liu, 2002). MAPK signaling activation also controls the expression of cytokines (TNF, IL‐1β, IL‐6), chemokines (CXCL1 and CXCL2), and inflammatory mediators production (prostaglandin E2, cyclooxygenase‐2, and iNOS; Yang et al., 2014). Notably, the increased expression in Dusp1 and Ptprs has been shown to occur due to many chronic inflammatory diseases, including cancer, rheumatoid arthritis, and ulcerative colitis (Berillo et al., 2022; Davis et al., 2018; Hendriks & Pulido, 2013; Khadir et al., 2018; Liu et al., 2019; Muise et al., 2007; Xu et al., 2015). Yet the deletion of Dusp1 in animal models enhanced susceptibility to pathogens, including Mycobacterium tuberculosis, Chlamydophila pneumonia, Staphylococcus aureus, and Escherichia coli (Cheung et al., 2009; Frazier et al., 2009; Gräb et al., 2019; Hammer et al., 2010; Kim et al., 2012; Li et al., 2023; Rodriguez et al., 2010; Wang et al., 2007; Zhao et al., 2006). Thus, the negative regulators, Dusp1 and Ptprs, help maintain a level of inflammation essential for the development of an appropriate antimicrobial response and whose perturbation by deletion is too far‐reaching. The induction of these negative regulators can be considered to be an appropriate, purposeful response by the host to limit inflammation and prevent a destructive positive feedback loop that can aggravate or worsen chronic conditions. However, when infection occurs under these circumstances, AM are suppressed in their ability to respond due to the dampening of MAPK pathways and, therefore, unable to control the infection effectively. Thus, these negative regulators have a previously unappreciated role in susceptibility to infection during advanced aging.
Notably, glucocorticoid signaling was identified as one of the top altered pathways in our transcriptome dataset due to both aging and TNF status. The promoter region of Dusp1 is tightly regulated and contains binding sites for several transcription factors, including a glucocorticoid receptor (Shipp et al., 2010), which was taken advantage of in our study to determine the consequence of its upregulation on the AM response to infection. We found that Ptprs was also induced by the glucocorticoid dexamethasone, which makes sense, as according to the ENCODE Transcription Factor Targets dataset, Ptprs has glucocorticoid response elements within its promoter (Rouillard et al., 2016). What is more, glucocorticoid treatment on both AM and macrophage‐like cell lines showed increased expression of Dusp1 and Ptprs while simultaneously suppressing the ability to respond to bacteria. Furthermore, young mice pretreated with dexamethasone before infectious challenge with Spn had higher expression of negative regulators in AM and worse disease than vehicle‐treated controls. This coincides with data showing that individuals taking corticosteroids, for example, patients with asthma, COPD, rheumatoid arthritis, and other inflammatory conditions, are at an increased risk for developing pneumonia (Calverley et al., 2007; Dixon et al., 2011; Doran et al., 2002a; Ferguson et al., 2008; Kardos et al., 2007; Suissa et al., 2013; Zhang et al., 2013). Importantly, corticosteroid therapy is often recommended in conjunction with antibiotics in patients hospitalized with severe pneumonia, as in this circumstance, the bacteria are eradicated and steroid treatment is warranted for reductions in the need for mechanical ventilation, length of hospital stay, risk of acute respiratory distress syndrome, and all‐cause mortality (Dequin et al., 2023; Meijvis et al., 2011; Remmelts Hilde et al., 2012; Siemieniuk et al., 2015; Torres et al., 2015). Pertinent to this discussion, it is well established that the immunosuppressive therapeutic action of glucocorticoids depends on the activation of Dusp1 (Abraham et al., 2006; Fürst et al., 2007; Hoppstädter & Ammit, 2019; Kassel et al., 2001; Manetsch et al., 2012b; Pemmari et al., 2019; Shah et al., 2014).
Treatment of mice with neutralizing antibody against TNF decreased the expression of Dusp1 and Ptprs and enhanced the resistance of aged mice to pneumococcal challenge—directly implicating TNF as being the causative agent of ADMD and linking it to the immunosuppression observed following steroid therapy. Neutralization of TNF is a highly effective therapeutic intervention in many inflammatory diseases, such as rheumatoid arthritis, ankylosing spondylitis, psoriasis, Crohn's disease, and endotoxin‐induced septic shock (Beutler et al., 1985; Brandt et al., 2000; Chaudhari et al., 2001; Elliott et al., 1993, 1994; Hess et al., 2011; Knight et al., 1993; Lipsky et al., 2000; Lovell et al., 2000; Maini et al., 1998, 1999; Present et al., 1999; Stidham et al., 2014; van Dullemen et al., 1995). Neutralization of TNF has also been recorded to have positive unintended consequences; patients with rheumatoid arthritis treated with anti‐TNF agents had a lower incidence of cardiovascular events and improved insulin resistance (Barnabe et al., 2011; Gonzalez‐Gay et al., 2006; Jacobsson et al., 2005; Kiortsis et al., 2005; van Eijk et al., 2009). Work with experimental animal models has shown that TNF neutralization is an effective therapeutic in heart disease and neurodegenerative diseases like Alzheimer's disease (Csiszar et al., 2007; Moe et al., 2004; Shamim & Laskowski, 2017; Toufektsian et al., 2008; Wolfe & Michaud, 2004). Thus, TNF inhibitors, along with other immune modulators, have possible prophylactic roles that we do not yet fully comprehend. While TNF neutralization can have profound benefits, interfering with the immune system could come with significant risks; serious infections are associated with anti‐TNF therapy, especially the reactivation of latent Mycobacterium tuberculosis infections (Cantini et al., 2017; Dixon et al., 2006, 2010; Doran et al., 2002b; Galloway et al., 2011; Gómez‐Reino et al., 2003; Khanna et al., 2004; Listing et al., 2005; Wolfe et al., 2004). Other groups have shown that TNF is essential in fighting off Spn infections, as mice from TNF KO backgrounds and mice treated with anti‐TNF could not control the infection successfully (O'Brien et al., 1999; Wellmer et al., 2001). In contrast, we observed that aged mice pretreated with anti‐TNF were less susceptible to infection with decreased bacterial burdens. We purport that the reason for the discrepancy between these reports and our own is that in an excessively inflamed or aged host, anti‐TNF treatment reduces the levels of negative homeostatic suppressors so that an effective cell signaling response to infection can occur at the onset. Whereas during established infection, TNF production is required for the maintenance of a robust immune response to the infection by immune cells.
In summary, chronic exposure to TNF, as during inflamm‐aging, induces the upregulation of established homeostatic suppressors, Dusp1 and Ptprs, of MAPK kinase signaling, which suppresses AM signaling, contributing to the increased susceptibility to infection seen during aging and as a result of corticosteroid therapy. Our results add to the considerable body of evidence implicating TNF as a major determinant of aging, age‐related macrophage dysfunction, and increased susceptibility to infection. Targeting the negative regulators, Dusp1 and Ptprs may prove not to be a viable treatment for aging individuals with pneumococcal infections as they are needed to restrain excessive inflammation, and instead, the focus should be on reducing the root cause of excessive inflammation. Along such lines, exploring new uses of established immune modulators, such as TNF inhibitors, seems more amendable, although with considerable risk. In summary, our findings have enhanced our knowledge of the extent and molecular basis in which TNF is involved in ADMD and susceptibility to infection. They highlight the detrimental consequences of chronic inflammation and suggest that blocking inflammation, in some manner, should be considered as a potential focal point for future interventions of chronic inflammatory and age‐related conditions, such as prophylaxis against infection.
AUTHOR CONTRIBUTIONS
Conceptualization and Review and Editing: K.L.K., D.M.E.B., and C.J.O. Investigation: K.L.K., E.M., and E.S. Data analysis, K.L.K., E.M., and C.J.O. Supervision: D.M.E.B. and C.J.O.
CONFLICT OF INTEREST STATEMENT
We have no conflicts of interest to declare.
Supporting information
Figures S1‐S2.
Table S1.
Table S2.
Table S3.
ACKNOWLEDGMENTS
KLK was supported by UAB Predoctoral Training Grant(s) in Translational and Molecular Sciences (T32GM109780) and Lung Diseases (T32HL134640) from the National Institutes of Health (NIH). CJO received support from the NIH (AG055144) and the American Heart Association (18TPA34110195).
Kruckow, K. L. , Murray, E. , Shayhidin, E. , Rosenberg, A. F. , Bowdish, D. M. E. , & Orihuela, C. J. (2024). Chronic TNF exposure induces glucocorticoid‐like immunosuppression in the alveolar macrophages of aged mice that enhances their susceptibility to pneumonia. Aging Cell, 23, e14133. 10.1111/acel.14133
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abraham, S. M. , Lawrence, T. , Kleiman, A. , Warden, P. , Medghalchi, M. , Tuckermann, J. , Saklatvala, J. , & Clark, A. R. (2006). Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. Journal of Experimental Medicine, 203(8), 1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrover, J. M. , Nicolás‐Ávila, J. A. , & Hidalgo, A. (2016). Aging: A temporal dimension for neutrophils. Trends in Immunology, 37(5), 334–345. [DOI] [PubMed] [Google Scholar]
- Allard, B. , Panariti, A. , & Martin, J. G. (2018). Alveolar macrophages in the resolution of inflammation, tissue repair, and tolerance to infection. Frontiers in Immunology, 9, 1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders, S. , Pyl, P. T. , & Huber, W. (2015). HTSeq—A python framework to work with high‐throughput sequencing data. Bioinformatics, 31(2), 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, E. , Cha, R. H. , Kim, Y. C. , An, J. N. , Kim, D. K. , Yoo, K. D. , Lee, S. M. , Kim, M. H. , Park, J. T. , Kang, S. W. , Park, J. Y. , Lim, C. S. , Kim, Y. S. , Yang, S. H. , & Lee, J. P. (2017). Circulating TNF receptors predict cardiovascular disease in patients with chronic kidney disease. Medicine (Baltimore), 96(19), e6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbé‐Tuana, F. , Funchal, G. , Schmitz, C. R. R. , Maurmann, R. M. , & Bauer, M. E. (2020). The interplay between immunosenescence and age‐related diseases. Seminars in Immunopathology, 42(5), 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabe, C. , Martin, B.‐J. , & Ghali, W. A. (2011). Systematic review and meta‐analysis: Anti–tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care & Research, 63(4), 522–529. [DOI] [PubMed] [Google Scholar]
- Beck‐Schimmer, B. , Schwendener, R. , Pasch, T. , Reyes, L. , Booy, C. , & Schimmer, R. C. (2005). Alveolar macrophages regulate neutrophil recruitment in endotoxin‐induced lung injury. Respiratory Research, 6(1), 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berillo, O. , Huo, K. G. , Richer, C. , Fraulob‐Aquino, J. C. , Briet, M. , Lipman, M. L. , Sinnett, D. , Paradis, P. , & Schiffrin, E. L. (2022). Distinct transcriptomic profile of small arteries of hypertensive patients with chronic kidney disease identified miR‐338‐3p targeting GPX3 and PTPRS. Journal of Hypertension, 40(7), 1394–1405. [DOI] [PubMed] [Google Scholar]
- Beutler, B. , Milsark, I. , & Cerami, A. (1985). Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science, 229(4716), 869–871. [DOI] [PubMed] [Google Scholar]
- Bewley, M. A. , Belchamber, K. B. R. , Chana, K. K. , Budd, R. C. , Donaldson, G. , Wedzicha, J. A. , Brightling, C. E. , Kilty, I. , Donnelly, L. E. , Barnes, P. J. , Singh, D. , Whyte, M. K. B. , Dockrell, D. H. , & COPDMAP . (2016). Differential effects of p38, MAPK, PI3K or rho kinase inhibitors on bacterial phagocytosis and efferocytosis by macrophages in COPD. PLoS One, 11(9), e0163139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer, E. D. , Goral, J. , Faunce, D. E. , & Kovacs, E. J. (2004). Age‐dependent decrease in toll‐like receptor 4‐mediated proinflammatory cytokine production and mitogen‐activated protein kinase expression. Journal of Leukocyte Biology, 75(2), 342–349. [DOI] [PubMed] [Google Scholar]
- Boyd, A. R. , Shivshankar, P. , Jiang, S. , Berton, M. T. , & Orihuela, C. J. (2012). Age‐related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Experimental Gerontology, 47(7), 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, J. , Haibel, H. , Cornely, D. , Golder, W. , Gonzalez, J. , Reddig, J. , Thriene, W. , Sieper, J. , & Braun, J. (2000). Successful treatment of active ankylosing spondylitis with the anti–tumor necrosis factor α monoclonal antibody infliximab. Arthritis & Rheumatism, 43(6), 1346–1352. [DOI] [PubMed] [Google Scholar]
- Brown, A. O. , Mann, B. , Gao, G. , Hankins, J. S. , Humann, J. , Giardina, J. , Faverio, P. , Restrepo, M. I. , Halade, G. V. , Mortensen, E. M. , Lindsey, M. L. , Hanes, M. , Happel, K. I. , Nelson, S. , Bagby, G. J. , Lorent, J. A. , Cardinal, P. , Granados, R. , Esteban, A. , … Orihuela, C. J. (2014). Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function. PLoS Pathogens, 10(9), e1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard, H. , Andersen‐Ranberg, K. , Hjelmborg, J. B. , Pedersen, B. K. , & Jeune, B. (2003). Elevated levels of tumor necrosis factor alpha and mortality in centenarians. The American Journal of Medicine, 115(4), 278–283. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard, H. , Andersen‐Ranberg, K. , Jeune, B. , Pedersen, A. N. , Skinhoj, P. , & Pedersen, B. K. (1999). A high plasma concentration of TNF‐α is associated with dementia in centenarians. The Journals of Gerontology: Series A, 54(7), M357–M364. [DOI] [PubMed] [Google Scholar]
- Bunin, A. , Sisirak, V. , Ghosh, H. S. , Grajkowska, L. T. , Hou, Z. E. , Miron, M. , Yang, C. , Ceribelli, M. , Uetani, N. , Chaperot, L. , Plumas, J. , Hendriks, W. , Tremblay, M. L. , Häcker, H. , Staudt, L. M. , Green, P. H. , Bhagat, G. , & Reizis, B. (2015). Protein tyrosine phosphatase PTPRS is an inhibitory receptor on human and murine Plasmacytoid dendritic cells. Immunity, 43(2), 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley, P. M. A. , Anderson, J. A. , Celli, B. , Ferguson, G. T. , Jenkins, C. , Jones, P. W. , Yates, J. C. , & Vestbo, J. (2007). Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine, 356(8), 775–789. [DOI] [PubMed] [Google Scholar]
- Canan, C. H. , Gokhale, N. S. , Carruthers, B. , Lafuse, W. P. , Schlesinger, L. S. , Torrelles, J. B. , & Turner, J. (2014). Characterization of lung inflammation and its impact on macrophage function in aging. Journal of Leukocyte Biology, 96(3), 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini, F. , Nannini, C. , Niccoli, L. , Petrone, L. , Ippolito, G. , & Goletti, D. (2017). Risk of tuberculosis reactivation in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis receiving non‐anti‐TNF‐targeted biologics. Mediators of Inflammation, 2017, 8909834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon, C. (1993). Pneumonia in the elderly. International Journal of Antimicrobial Agents, 3(Suppl 1), S119–S126. [DOI] [PubMed] [Google Scholar]
- Chaudhari, U. , Romano, P. , Mulcahy, L. D. , Dooley, L. T. , Baker, D. G. , & Gottlieb, A. B. (2001). Efficacy and safety of infliximab monotherapy for plaque‐type psoriasis: A randomised trial. The Lancet, 357(9271), 1842–1847. [DOI] [PubMed] [Google Scholar]
- Chen, P. , Li, J. , Barnes, J. , Kokkonen, G. C. , Lee, J. C. , & Liu, Y. (2002). Restraint of proinflammatory cytokine biosynthesis by mitogen‐activated protein kinase phosphatase‐1 in lipopolysaccharide‐stimulated macrophages. Journal of Immunology, 169(11), 6408–6416. [DOI] [PubMed] [Google Scholar]
- Cheung, B. K. , Yim, H. C. H. , Lee, N. C. M. , & Lau, A. S. Y. (2009). A novel anti‐mycobacterial function of mitogen‐activated protein kinase phosphatase‐1. BMC Immunology, 10, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, H. , Barry, S. P. , Roth, R. J. , Wu, J. J. , Jones, E. A. , Bennett, A. M. , & Flavell, R. A. (2006). Dynamic regulation of pro‐and anti‐inflammatory cytokines by MAPK phosphatase 1 (MKP‐1) in innate immune responses. Proceedings of the National Academy of Sciences of the United States of America, 103(7), 2274–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, Y. , Solski, P. A. , Khosravi‐Far, R. , Der, C. J. , & Kelly, K. (1996). The mitogen‐activated protein kinase phosphatases PAC1, MKP‐1, and MKP‐2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation (*). Journal of Biological Chemistry, 271(11), 6497–6501. [DOI] [PubMed] [Google Scholar]
- Cohen, H. J. , Pieper, C. F. , Harris, T. , Rao, K. M. K. , & Currie, M. S. (1997). The association of plasma IL‐6 levels with functional disability in community‐dwelling elderly. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 52(4), M201–M208. [DOI] [PubMed] [Google Scholar]
- Csiszar, A. , Labinskyy, N. , Smith, K. , Rivera, A. , Orosz, Z. , & Ungvari, Z. (2007). Vasculoprotective effects of anti‐tumor necrosis factor‐α treatment in aging. The American Journal of Pathology, 170(1), 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundell, D. R. , Gerard, N. P. , Gerard, C. , Idanpaan‐Heikkila, I. , & Tuomanen, E. I. (1995). Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet‐activating factor. Nature, 377(6548), 435–438. [DOI] [PubMed] [Google Scholar]
- Davis, T. B. , Yang, M. , Schell, M. J. , Wang, H. , Ma, L. , Pledger, W. J. , & Yeatman, T. J. (2018). PTPRS regulates colorectal cancer RAS pathway activity by inactivating Erk and preventing its nuclear translocation. Scientific Reports, 8(1), 9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, T. B. , Yang, M. , Wang, H. , Lee, C. , Yeatman, T. J. , & Pledger, W. J. (2019). PTPRS drives adaptive resistance to MEK/ERK inhibitors through SRC. Oncotarget, 10(63), 6768–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gonzalo‐Calvo, D. , de Luxán‐Delgado, B. , Rodríguez‐González, S. , García‐Macia, M. , Suárez, F. M. , Solano, J. J. , Rodríguez‐Colunga, M. J. , & Coto‐Montes, A. (2012). Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: A translational approach. Cytokine, 58(2), 193–198. [DOI] [PubMed] [Google Scholar]
- den Hertog, J. , Blanchetot, C. , Buist, A. , Overvoorde, J. , van der Sar, A. , & Tertoolen, L. G. (2004). Receptor protein‐tyrosine phosphatase signalling in development. International Journal of Developmental Biology, 43(7), 723–733. [PubMed] [Google Scholar]
- Dequin, P.‐F. , Meziani, F. , Quenot, J. P. , Kamel, T. , Ricard, J. D. , Badie, J. , Reignier, J. , Heming, N. , Plantefève, G. , Souweine, B. , Voiriot, G. , Colin, G. , Frat, J. P. , Mira, J. P. , Barbarot, N. , François, B. , Louis, G. , Gibot, S. , Guitton, C. , … CRICS‐TriGGERSep Network . (2023). Hydrocortisone in severe community‐acquired pneumonia. New England Journal of Medicine, 388(21), 1931–1941. [DOI] [PubMed] [Google Scholar]
- Dixon, W. G. , Hyrich, K. L. , Watson, K. D. , Lunt, M. , Galloway, J. , Ustianowski, A. , B S R B R Control Centre Consortium , Symmons, D. P. , & BSR Biologics Register . (2010). Drug‐specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti‐TNF therapy: Results from the British Society for Rheumatology biologics register (BSRBR). Annals of the Rheumatic Diseases, 69(3), 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, W. G. , Kezouh, A. , Bernatsky, S. , & Suissa, S. (2011). The influence of systemic glucocorticoid therapy upon the risk of non‐serious infection in older patients with rheumatoid arthritis: A nested case–control study. Annals of the Rheumatic Diseases, 70(6), 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, W. G. , Watson, K. , Lunt, M. , Hyrich, K. L. , Silman, A. J. , Symmons, D. P. , & British Society for Rheumatology Biologics Register . (2006). Rates of serious infection, including site‐specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti–tumor necrosis factor therapy: Results from the British Society for Rheumatology biologics register. Arthritis & Rheumatism, 54(8), 2368–2376. [DOI] [PubMed] [Google Scholar]
- Doran, M. F. , Crowson, C. S. , Pond, G. R. , O'Fallon, W. M. , & Gabriel, S. E. (2002a). Predictors of infection in rheumatoid arthritis. Arthritis & Rheumatism, 46(9), 2294–2300. [DOI] [PubMed] [Google Scholar]
- Doran, M. F. , Crowson, C. S. , Pond, G. R. , O'Fallon, W. M. , & Gabriel, S. E. (2002b). Frequency of infection in patients with rheumatoid arthritis compared with controls: A population‐based study. Arthritis & Rheumatism, 46(9), 2287–2293. [DOI] [PubMed] [Google Scholar]
- Elliott, M. J. , Maini, R. N. , Feldmann, M. , Kalden, J. R. , Antoni, C. , Smolen, J. S. , Leeb, B. , Breedveld, F. C. , Macfarlane, J. D. , Bijl, J. A. , & Woody, J. N. (1994). Randomised double‐blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. The Lancet, 344(8930), 1105–1110. [DOI] [PubMed] [Google Scholar]
- Elliott, M. J. , Maini, R. N. , Feldmann, M. , Long‐Fox, A. , Charles, P. , Katsikis, P. , Brennan, F. M. , Walker, J. , Bijl, H. , Ghrayeb, J. , & Woody, J. N. (1993). Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor α. Arthritis & Rheumatism, 36(12), 1681–1690. [DOI] [PubMed] [Google Scholar]
- Eurich, D. T. , Marrie, T. J. , Minhas‐Sandhu, J. K. , & Majumdar, S. R. (2017). Risk of heart failure after community acquired pneumonia: Prospective controlled study with 10 years of follow‐up. BMJ, 356, j413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri, E. , An, Y. , Zoli, M. , Simonsick, E. M. , Guralnik, J. M. , Bandinelli, S. , Boyd, C. M. , & Ferrucci, L. (2015). Aging and the burden of multimorbidity: Associations with inflammatory and anabolic hormonal biomarkers. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70(1), 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feikin, D. R. , Schuchat, A. , Kolczak, M. , Barrett, N. L. , Harrison, L. H. , Lefkowitz, L. , McGeer, A. , Farley, M. M. , Vugia, D. J. , Lexau, C. , Stefonek, K. R. , Patterson, J. E. , & Jorgensen, J. H. (2000). Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995‐1997. American Journal of Public Health, 90(2), 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, G. T. , Anzueto, A. , Fei, R. , Emmett, A. , Knobil, K. , & Kalberg, C. (2008). Effect of fluticasone propionate/salmeterol (250/50 μg) or salmeterol (50 μg) on COPD exacerbations. Respiratory Medicine, 102(8), 1099–1108. [DOI] [PubMed] [Google Scholar]
- Ferrucci, L. , Corsi, A. , Lauretani, F. , Bandinelli, S. , Bartali, B. , Taub, D. D. , Guralnik, J. M. , & Longo, D. L. (2005). The origins of age‐related proinflammatory state. Blood, 105(6), 2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillit, H. , Ding, W. , Buee, L. , Kalman, J. , Altstiel, L. , Lawlor, B. , & Wolf‐Klein, G. (1991). Elevated circulating tumor necrosis factor levels in Alzheimer's disease. Neuroscience Letters, 129(2), 318–320. [DOI] [PubMed] [Google Scholar]
- Firestein, G. S. , & McInnes, I. B. (2017). Immunopathogenesis of rheumatoid arthritis. Immunity, 46(2), 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi, C. , Bonafè, M. , Valensin, S. , Olivieri, F. , De Luca, M. , Ottaviani, E. , & De Benedictis, G. (2000). Inflamm‐aging: An evolutionary perspective on Immunosenescence. Annals of the New York Academy of Sciences, 908(1), 244–254. [DOI] [PubMed] [Google Scholar]
- Franklin, C. C. , & Kraft, A. S. (1997). Conditional expression of the mitogen‐activated protein kinase (MAPK) phosphatase MKP‐1 preferentially inhibits p38 MAPK and stress‐activated protein kinase in U937 cells. Journal of Biological Chemistry, 272(27), 16917–16923. [DOI] [PubMed] [Google Scholar]
- Frazier, W. J. , Wang, X. , Wancket, L. M. , Li, X. A. , Meng, X. , Nelin, L. D. , Cato, A. C. B. , & Liu, Y. (2009). Increased inflammation, impaired bacterial clearance, and metabolic disruption after gram‐negative sepsis in Mkp‐1‐deficient Mice1. The Journal of Immunology, 183(11), 7411–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried, L. P. , Tangen, C. M. , Walston, J. , Newman, A. B. , Hirsch, C. , Gottdiener, J. , Seeman, T. , Tracy, R. , Kop, W. J. , Burke, G. , McBurnie, M. A. , & Cardiovascular Health Study Collaborative Research Group . (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology: Series A, 56(3), M146–M157. [DOI] [PubMed] [Google Scholar]
- Fürst, R. , Schroeder, T. , Eilken, H. M. , Bubik, M. F. , Kiemer, A. K. , Zahler, S. , & Vollmar, A. M. (2007). MAPK phosphatase‐1 represents a novel anti‐inflammatory target of glucocorticoids in the human endothelium. The FASEB Journal, 21(1), 74–80. [DOI] [PubMed] [Google Scholar]
- Galloway, J. B. , Hyrich, K. L. , Mercer, L. K. , Dixon, W. G. , Fu, B. , Ustianowski, A. P. , Watson, K. D. , Lunt, M. , Symmons, D. P. , BSRBR Control Centre Consortium , & British Society for Rheumatology Biologics Register . (2011). Anti‐TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: Updated results from the British Society for Rheumatology biologics register with special emphasis on risks in the elderly. Rheumatology, 50(1), 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Reino, J. J. , Carmona, L. , Valverde, V. R. , Mola, E. M. , Montero, M. D. , & BIOBADASER Group . (2003). Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: A multicenter active‐surveillance report. Arthritis and Rheumatism, 48(8), 2122–2127. [DOI] [PubMed] [Google Scholar]
- Gong, Y. , Abudureyimu, S. , Kadomatsu, K. , & Sakamoto, K. (2021). Identification of PTPRσ‐interacting proteins by proximity‐labelling assay. The Journal of Biochemistry, 169(2), 187–194. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Gay, M. A. , de Matias, J. M. , Gonzalez‐Juanatey, C. , Garcia‐Porrua, C. , Sanchez‐Andrade, A. , Martin, J. , & Llorca, J. (2006). Anti‐tumor necrosis factor‐alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clinical and Experimental Rheumatology, 24(1), 83–86. [PubMed] [Google Scholar]
- Gräb, J. , Suárez, I. , van Gumpel, E. , Winter, S. , Schreiber, F. , Esser, A. , Hölscher, C. , Fritsch, M. , Herb, M. , Schramm, M. , Wachsmuth, L. , Pallasch, C. , Pasparakis, M. , Kashkar, H. , & Rybniker, J. (2019). Corticosteroids inhibit Mycobacterium tuberculosis‐induced necrotic host cell death by abrogating mitochondrial membrane permeability transition. Nature Communications, 10(1), 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S. , Feng, L. , Yoshimura, T. , Redick, J. , Fu, S. M. , & Rose, C. E., Jr. (1996). Intra‐alveolar macrophage‐inflammatory peptide 2 induces rapid neutrophil localization in the lung. American Journal of Respiratory Cell and Molecular Biology, 15(5), 656–663. [DOI] [PubMed] [Google Scholar]
- Hamidzadeh, K. , Christensen, S. M. , Dalby, E. , Chandrasekaran, P. , & Mosser, D. M. (2017). Macrophages and the recovery from acute and chronic inflammation. Annual Review of Physiology, 79, 567–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer, M. , Echtenachter, B. , Weighardt, H. , Jozefowski, K. , Rose‐John, S. , Männel, D. N. , Holzmann, B. , & Lang, R. (2010). Increased inflammation and lethality of Dusp1−/− mice in polymicrobial peritonitis models. Immunology, 131(3), 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer, M. , Mages, J. , Dietrich, H. , Servatius, A. , Howells, N. , Cato, A. C. B. , & Lang, R. (2006). Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS‐induced genes and protects mice from lethal endotoxin shock. The Journal of Experimental Medicine, 203(1), 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, T. B. , Ferrucci, L. , Tracy, R. P. , Corti, M. C. , Wacholder, S. , Ettinger, W. H., Jr. , Heimovitz, H. , Cohen, H. J. , & Wallace, R. (1999). Associations of elevated Interleukin‐6 and C‐reactive protein levels with mortality in the elderly. The American Journal of Medicine, 106(5), 506–512. **access the “journal Club” discussion of this paper at http:/www.elsevier.com/locate/ajmselect. Th [DOI] [PubMed] [Google Scholar]
- Hendriks, W. J. A. J. , & Pulido, R. (2013). Protein tyrosine phosphatase variants in human hereditary disorders and disease susceptibilities. Biochimica et Biophysica Acta (BBA) ‐ Molecular Basis of Disease, 1832(10), 1673–1696. [DOI] [PubMed] [Google Scholar]
- Hess, A. , Axmann, R. , Rech, J. , Finzel, S. , Heindl, C. , Kreitz, S. , Sergeeva, M. , Saake, M. , Garcia, M. , Kollias, G. , Straub, R. H. , Sporns, O. , Doerfler, A. , Brune, K. , & Schett, G. (2011). Blockade of TNF‐α rapidly inhibits pain responses in the central nervous system. Proceedings of the National Academy of Sciences of the United States of America, 108(9), 3731–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa, C. A. , Akula Suresh Babu, R. , Rahman, M. M. , Fernandes, G. , Boyd, A. R. , & Orihuela, C. J. (2014). Elevated A20 contributes to age‐dependent macrophage dysfunction in the lungs. Experimental Gerontology, 54, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa, E. , Boyd, A. R. , & Orihuela, C. J. (2009). Age‐associated inflammation and toll‐like receptor dysfunction prime the lungs for pneumococcal pneumonia. The Journal of Infectious Diseases, 200(4), 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppstädter, J. , & Ammit, A. J. (2019). Role of dual‐specificity phosphatase 1 in glucocorticoid‐driven anti‐inflammatory responses. Frontiers in Immunology, 10, 1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, R. E. , O'Mahony, M. S. , Savva, G. M. , Calver, B. L. , & Woodhouse, K. W. (2009). Inflammation and frailty measures in older people. Journal of Cellular and Molecular Medicine, 13(9b), 3103–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell, T. , & Bell, T. J. (2014). Alveolar macrophages: Plasticity in a tissue‐specific context. Nature Reviews Immunology, 14(2), 81–93. [DOI] [PubMed] [Google Scholar]
- Jacobsson, L. T. , Turesson, C. , Gülfe, A. , Kapetanovic, M. C. , Petersson, I. F. , Saxne, T. , & Geborek, P. (2005). Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. The Journal of Rheumatology, 32(7), 1213–1218. [PubMed] [Google Scholar]
- Janssens, J. P. (2005). Pneumonia in the elderly (geriatric) population. Current Opinion in Pulmonary Medicine, 11(3), 226–230. [DOI] [PubMed] [Google Scholar]
- Kardos, P. , Wencker, M. , Glaab, T. , & Vogelmeier, C. (2007). Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 175(2), 144–149. [DOI] [PubMed] [Google Scholar]
- Kassel, O. , Sancono, A. , Krätzschmar, J. , Kreft, B. , Stassen, M. , & Cato, A. C. (2001). Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP‐1. The EMBO Journal, 20(24), 7108–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadir, A. , Kavalakatt, S. , Dehbi, M. , Alarouj, M. , Bennakhi, A. , Tiss, A. , & Elkum, N. (2018). DUSP1 is a potential marker of chronic inflammation in Arabs with cardiovascular diseases. Disease Markers, 2018, 9529621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, D. , McMahon, M. , & Furst, D. E. (2004). Safety of tumour necrosis factor‐alpha antagonists. Drug Safety, 27(5), 307–324. [DOI] [PubMed] [Google Scholar]
- Kim, D. , Pertea, G. , Trapnell, C. , Pimentel, H. , Kelley, R. , & Salzberg, S. L. (2013). TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology, 14(4), R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. H. , An, D. R. , Song, J. , Yoon, J. Y. , Kim, H. S. , Yoon, H. J. , Im, H. N. , Kim, J. , Kim, D. J. , Lee, S. J. , Kim, K. H. , Lee, H. M. , Kim, H. J. , Jo, E. K. , Lee, J. Y. , & Suh, S. W. (2012). Mycobacterium tuberculosis Eis protein initiates suppression of host immune responses by acetylation of DUSP16/MKP‐7. Proceedings of the National Academy of Sciences of the United States of America, 109(20), 7729–7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiortsis, D. N. , Mavridis, A. K. , Vasakos, S. , Nikas, S. N. , & Drosos, A. A. (2005). Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Annals of the Rheumatic Diseases, 64(5), 765–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp, S. , Leemans, J. C. , Florquin, S. , Branger, J. , Maris, N. A. , Pater, J. , van Rooijen, N. , & van der Poll, T. (2003). Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. American Journal of Respiratory and Critical Care Medicine, 167(2), 171–179. [DOI] [PubMed] [Google Scholar]
- Knight, D. M. , Trinh, H. , le, J. , Siegel, S. , Shealy, D. , McDonough, M. , Scallon, B. , Moore, M. A. , Vilcek, J. , Daddona, P. , & Ghrayeb, J. (1993). Construction and initial characterization of a mouse‐human chimeric anti‐TNF antibody. Molecular Immunology, 30(16), 1443–1453. [DOI] [PubMed] [Google Scholar]
- Krämer, A. , Green, J. , Pollard, J., Jr. , & Tugendreich, S. (2014). Causal analysis approaches in ingenuity pathway analysis. Bioinformatics, 30(4), 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruckow, K. L. , Zhao, K. , Bowdish, D. M. E. , & Orihuela, C. J. (2023). Acute organ injury and long‐term sequelae of severe pneumococcal infections. Pneumonia (Nathan), 15(1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyaw, M. H. , Rose, Jr, C. E. , Fry, A. M. , Singleton, J. A. , Moore, Z. , Zell, E. R. , Whitney, C. G. , & Active Bacterial Core Surveillance Program of the Emerging Infections Program Network . (2005). The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. The Journal of Infectious Diseases, 192(3), 377–386. [DOI] [PubMed] [Google Scholar]
- Lafuse, W. P. , Rajaram, M. V. S. , Wu, Q. , Moliva, J. I. , Torrelles, J. B. , Turner, J. , & Schlesinger, L. S. (2019). Identification of an increased alveolar macrophage subpopulation in old mice that displays unique inflammatory characteristics and is permissive to mycobacterium tuberculosis infection. The Journal of Immunology, 203(8), 2252–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, A. , He, Y. , Yang, C. , Lu, N. , Bao, J. , Gao, S. , Hosyanto, F. F. , He, X. , Fu, H. , Yan, H. , Ding, N. , & Xu, L. (2023). Methylprednisolone promotes Mycobacterium smegmatis survival in macrophages through NF‐κB/DUSP1 pathway. Microorganisms, 11, 768. 10.3390/microorganisms11030768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky, P. E. , van der Heijde, D. M. F. M. , St. Clair, E. W. , Furst, D. E. , Breedveld, F. C. , Kalden, J. R. , Smolen, J. S. , Weisman, M. , Emery, P. , Feldmann, M. , Harriman, G. R. , & Maini, R. N. (2000). Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti‐tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. The New England Journal of Medicine, 343(22), 1594–1602. [DOI] [PubMed] [Google Scholar]
- Listing, J. , Strangfeld, A. , Kary, S. , Rau, R. , von Hinueber, U. , Stoyanova‐Scholz, M. , Gromnica‐Ihle, E. , Antoni, C. , Herzer, P. , Kekow, J. , Schneider, M. , & Zink, A. (2005). Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis & Rheumatism, 52(11), 3403–3412. [DOI] [PubMed] [Google Scholar]
- Liu, G.‐M. , Zeng, H. D. , Zhang, C. Y. , & Xu, J. W. (2019). Key genes associated with diabetes mellitus and hepatocellular carcinoma. Pathology, Research and Practice, 215(11), 152510. [DOI] [PubMed] [Google Scholar]
- Loeb, M. (2004). Pneumonia in the elderly. Current Opinion in Infectious Diseases, 17(2), 127–130. [DOI] [PubMed] [Google Scholar]
- Losa García, J. E. , Rodríguez, F. M. , Martín de Cabo, M. R. , García Salgado, M. J. , Losada, J. P. , Villarón, L. G. , López, A. J. , & Arellano, J. L. (1999). Evaluation of inflammatory cytokine secretion by human alveolar macrophages. Mediators of Inflammation, 8(1), 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, M. I. , Huber, W. , & Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biology, 15(12), 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell, D. J. , Giannini, E. H. , Reiff, A. , Cawkwell, G. D. , Silverman, E. D. , Nocton, J. J. , Stein, L. D. , Gedalia, A. , Ilowite, N. T. , Wallace, C. A. , Whitmore, J. , & Finck, B. K. (2000). Etanercept in children with polyarticular juvenile rheumatoid arthritis. New England Journal of Medicine, 342(11), 763–769. [DOI] [PubMed] [Google Scholar]
- Maini, R. , St Clair, E. W. , Breedveld, F. , Furst, D. , Kalden, J. , Weisman, M. , Smolen, J. , Emery, P. , Harriman, G. , Feldmann, M. , & Lipsky, P. (1999). Infliximab (chimeric anti‐tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomised phase III trial. The Lancet, 354(9194), 1932–1939. [DOI] [PubMed] [Google Scholar]
- Maini, R. N. , Breedveld, F. C. , Kalden, J. R. , Smolen, J. S. , Davis, D. , MacFarlane, J. D. , Antoni, C. , Leeb, B. , Elliott, M. J. , Woody, J. N. , Schaible, T. F. , & Feldmann, M. (1998). Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor α monoclonal antibody combined with low‐dose weekly methotrexate in rheumatoid arthritis. Arthritis & Rheumatism, 41(9), 1552–1563. [DOI] [PubMed] [Google Scholar]
- Manetsch, M. , Ramsay, E. E. , King, E. M. , Seidel, P. , Che, W. , Ge, Q. , Hibbs, D. E. , Newton, R. , & Ammit, A. J. (2012a). Corticosteroids and β2‐agonists upregulate mitogen‐activated protein kinase phosphatase 1: In vitro mechanisms. British Journal of Pharmacology, 166(7), 2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetsch, M. , Ramsay, E. E. , King, E. M. , Seidel, P. , Che, W. , Ge, Q. , Hibbs, D. E. , Newton, R. , & Ammit, A. J. (2012b). Corticosteroids and β₂‐agonists upregulate mitogen‐activated protein kinase phosphatase 1: In vitro mechanisms. British Journal of Pharmacology, 166(7), 2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos‐Pérez, D. , Sánchez‐Flores, M. , Proietti, S. , Bonassi, S. , Costa, S. , Teixeira, J. P. , Fernández‐Tajes, J. , Pásaro, E. , Laffon, B. , & Valdiglesias, V. (2020). Association of inflammatory mediators with frailty status in older adults: Results from a systematic review and meta‐analysis. GeroScience, 42(6), 1451–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes, I. B. , & Schett, G. (2007). Cytokines in the pathogenesis of rheumatoid arthritis. Nature Reviews Immunology, 7(6), 429–442. [DOI] [PubMed] [Google Scholar]
- Meijvis, S. C. A. , Hardeman, H. , Remmelts, H. H. F. , Heijligenberg, R. , Rijkers, G. T. , van Velzen‐Blad, H. , Voorn, G. P. , van de Garde, E. M. W. , Endeman, H. , Grutters, J. C. , Bos, W. J. W. , & Biesma, D. H. (2011). Dexamethasone and length of hospital stay in patients with community‐acquired pneumonia: A randomised, double‐blind, placebo‐controlled trial. The Lancet, 377(9782), 2023–2030. [DOI] [PubMed] [Google Scholar]
- Metcalf, T. U. , Cubas, R. A. , Ghneim, K. , Cartwright, M. J. , Grevenynghe, J. V. , Richner, J. M. , Olagnier, D. P. , Wilkinson, P. A. , Cameron, M. J. , Park, B. S. , Hiscott, J. B. , Diamond, M. S. , Wertheimer, A. M. , Nikolich‐Zugich, J. , & Haddad, E. K. (2015). Global analyses revealed age‐related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell, 14(3), 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe, G. W. , Marin‐Garcia, J. , Konig, A. , Goldenthal, M. , Lu, X. , & Feng, Q. (2004). In vivo TNF‐α inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. American Journal of Physiology. Heart and Circulatory Physiology, 287(4), H1813–H1820. [DOI] [PubMed] [Google Scholar]
- Mooradian, A. D. , Reed, R. L. , Osterweil, D. , & Scuderi, P. (1991). Detectable serum Levels of tumor necrosis factor alpha may predict early mortality in elderly institutionalized patients. Journal of the American Geriatrics Society, 39(9), 891–894. [DOI] [PubMed] [Google Scholar]
- Muise, A. M. , Walters, T. , Wine, E. , Griffiths, A. M. , Turner, D. , Duerr, R. H. , Regueiro, M. D. , Ngan, B. Y. , Xu, W. , Sherman, P. M. , Silverberg, M. S. , & Rotin, D. (2007). Protein‐tyrosine phosphatase sigma is associated with ulcerative colitis. Current Biology, 17(14), 1212–1218. [DOI] [PubMed] [Google Scholar]
- Musher, D. M. , Abers, M. S. , & Corrales‐Medina, V. F. (2019). Acute infection and myocardial infarction. The New England Journal of Medicine, 380(2), 171–176. [DOI] [PubMed] [Google Scholar]
- Musher, D. M. , Rueda, A. M. , Kaka, A. S. , & Mapara, S. M. (2007). The association between pneumococcal pneumonia and acute cardiac events. Clinical Infectious Diseases, 45(2), 158–165. [DOI] [PubMed] [Google Scholar]
- O'Brien, D. P. , Briles, D. E. , Szalai, A. J. , Tu, A. H. , Sanz, I. , & Nahm, M. H. (1999). Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infection and Immunity, 67(2), 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony, L. , Holland, J. , Jackson, J. , Feighery, C. , Hennessy, T. P. J. , & Mealy, K. (1998). Quantitative intracellular cytokine measurement: Age‐related changes in proinflammatory cytokine production. Clinical and Experimental Immunology, 113(2), 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton, S. I. , Bornheimer, R. , Doroff, R. , Shea, K. M. , Sato, R. , & Weycker, D. (2019). Decline in pneumococcal disease attenuated in older adults and those with comorbidities following universal childhood PCV13 immunization. Clinical Infectious Diseases, 68(11), 1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemmari, A. , Paukkeri, E. L. , Hämäläinen, M. , Leppänen, T. , Korhonen, R. , & Moilanen, E. (2019). MKP‐1 promotes anti‐inflammatory M(IL‐4/IL‐13) macrophage phenotype and mediates the anti‐inflammatory effects of glucocorticoids. Basic & Clinical Pharmacology & Toxicology, 124(4), 404–415. [DOI] [PubMed] [Google Scholar]
- Pittet, L. A. , Quinton, L. J. , Yamamoto, K. , Robson, B. E. , Ferrari, J. D. , Algül, H. , Schmid, R. M. , & Mizgerd, J. P. (2011). Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia. American Journal of Respiratory Cell and Molecular Biology, 45(3), 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Present, D. H. , Rutgeerts, P. , Targan, S. , Hanauer, S. B. , Mayer, L. , van Hogezand, R. A. , Podolsky, D. K. , Sands, B. E. , Braakman, T. , DeWoody, K. L. , Schaible, T. F. , & van Deventer, S. J. H. (1999). Infliximab for the treatment of fistulas in patients with Crohn's disease. New England Journal of Medicine, 340(18), 1398–1405. [DOI] [PubMed] [Google Scholar]
- Preston, J. A. , Bewley, M. A. , Marriott, H. M. , McGarry Houghton, A. , Mohasin, M. , Jubrail, J. , Morris, L. , Stephenson, Y. L. , Cross, S. , Greaves, D. R. , Craig, R. W. , van Rooijen, N. , Bingle, C. D. , Read, R. C. , Mitchell, T. J. , Whyte, M. K. B. , Shapiro, S. D. , & Dockrell, D. H. (2019). Alveolar macrophage apoptosis‐associated bacterial killing helps prevent murine pneumonia. American Journal of Respiratory and Critical Care Medicine, 200(1), 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta, A. , Naidoo, A. , Verschoor, C. P. , Loukov, D. , Thevaranjan, N. , Mandur, T. S. , Nguyen, P. S. , Jordana, M. , Loeb, M. , Xing, Z. , Kobzik, L. , Larché, M. J. , & Bowdish, D. M. E. (2016). TNF drives monocyte dysfunction with age and results in impaired anti‐pneumococcal immunity. PLoS Pathogens, 12(1), e1005368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, T. E. , Pauli, F. , Sprouse, R. O. , Neff, N. F. , Newberry, K. M. , Garabedian, M. J. , & Myers, R. M. (2009). Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Research, 19(12), 2163–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmelts Hilde, H. F. , Meijvis, S. C. , Biesma, D. H. , van Velzen‐Blad, H. , Voorn, G. P. , Grutters, J. C. , Bos, W. J. , & Rijkers, G. T. (2012). Dexamethasone downregulates the systemic cytokine response in patients with community‐acquired pneumonia. Clinical and Vaccine Immunology, 19(9), 1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes, L. F. , Restrepo, M. I. , Hinojosa, C. A. , Soni, N. J. , Anzueto, A. , Babu, B. L. , Gonzalez‐Juarbe, N. , Rodriguez, A. H. , Jimenez, A. , Chalmers, J. D. , Aliberti, S. , Sibila, O. , Winter, V. T. , Coalson, J. J. , Giavedoni, L. D. , dela Cruz, C. S. , Waterer, G. W. , Witzenrath, M. , Suttorp, N. , … Orihuela, C. J. (2017). Severe pneumococcal pneumonia causes acute cardiac toxicity and subsequent cardiac remodeling. American Journal of Respiratory and Critical Care Medicine, 196(5), 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijneveld, A. W. , Florquin, S. , Branger, J. , Speelman, P. , van Deventer, S. J. H. , & van der Poll, T. (2001). TNF‐α compensates for the impaired host defense of IL‐1 type I receptor‐deficient mice during pneumococcal Pneumonia 1. The Journal of Immunology, 167(9), 5240–5246. [DOI] [PubMed] [Google Scholar]
- Robinson, M. D. , & Oshlack, A. (2010). A scaling normalization method for differential expression analysis of RNA‐seq data. Genome Biology, 11(3), R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, N. , Dietrich, H. , Mossbrugger, I. , Weintz, G. , Scheller, J. , Hammer, M. , Quintanilla‐Martinez, L. , Rose‐John, S. , Miethke, T. , & Lang, R. (2010). Increased inflammation and impaired resistance to Chlamydophila pneumoniae infection in Dusp1−/− mice: Critical role of IL‐6. Journal of Leukocyte Biology, 88(3), 579–587. [DOI] [PubMed] [Google Scholar]
- Rouillard, A. D. , Gundersen, G. W. , Fernandez, N. F. , Wang, Z. , Monteiro, C. D. , McDermott, M. G. , & Ma'ayan, A. (2016). The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database, 2016, baw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salojin, K. V. , Owusu, I. B. , Millerchip, K. A. , Potter, M. , Platt, K. A. , & Oravecz, T. (2006). Essential role of MAPK phosphatase‐1 in the negative control of innate immune responses. Journal of Immunology, 176(3), 1899–1907. [DOI] [PubMed] [Google Scholar]
- Schmal, H. , Shanley, T. P. , Jones, M. L. , Friedl, H. P. , & Ward, P. A. (1996). Role for macrophage inflammatory protein‐2 in lipopolysaccharide‐induced lung injury in rats. The Journal of Immunology, 156(5), 1963–1972. [PubMed] [Google Scholar]
- Seternes, O.‐M. , Kidger, A. M. , & Keyse, S. M. (2019). Dual‐specificity MAP kinase phosphatases in health and disease. Biochimica et Biophysica Acta (BBA) ‐ Molecular Cell Research, 1866(1), 124–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, S. , King, E. M. , Chandrasekhar, A. , & Newton, R. (2014). Roles for the mitogen‐activated protein kinase (MAPK) phosphatase, DUSP1, in feedback control of inflammatory gene expression and repression by dexamethasone. The Journal of Biological Chemistry, 289(19), 13667–13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamim, D. , & Laskowski, M. (2017). Inhibition of inflammation mediated through the tumor necrosis factor α biochemical pathway can lead to favorable outcomes in Alzheimer disease. Journal of Central Nervous System Disease, 9, 1179573517722512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp, L. E. , Lee, J. V. , Yu, C. Y. , Pufall, M. , Zhang, P. , Scott, D. K. , & Wang, J. C. (2010). Transcriptional regulation of human dual specificity protein phosphatase 1 (DUSP1) gene by glucocorticoids. PLoS One, 5(10), e13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivshankar, P. , Boyd, A. R. , le Saux, C. J. , Yeh, I. T. , & Orihuela, C. J. (2011). Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell, 10(5), 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemieniuk, R. A. C. , Meade, M. O. , Alonso‐Coello, P. , Briel, M. , Evaniew, N. , Prasad, M. , Alexander, P. E. , Fei, Y. , Vandvik, P. O. , Loeb, M. , & Guyatt, G. H. (2015). Corticosteroid therapy for patients hospitalized with community‐acquired pneumonia. Annals of Internal Medicine, 163(7), 519–528. [DOI] [PubMed] [Google Scholar]
- St Sauver, J. L. , Boyd, C. M. , Grossardt, B. R. , Bobo, W. V. , Finney Rutten, L. J. , Roger, V. L. , Ebbert, J. O. , Therneau, T. M. , Yawn, B. P. , & Rocca, W. A. (2015). Risk of developing multimorbidity across all ages in an historical cohort study: Differences by sex and ethnicity. BMJ Open, 5(2), e006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford, T. J. , Kunkel, S. L. , Greenberger, M. J. , Laichalk, L. L. , & Strieter, R. M. (1996). Expression and regulation of chemokines in bacterial pneumonia. Journal of Leukocyte Biology, 59(1), 24–28. [DOI] [PubMed] [Google Scholar]
- St‐Denis, N. , Gupta, G. D. , Lin, Z. Y. , Gonzalez‐Badillo, B. , Veri, A. O. , Knight, J. D. R. , Rajendran, D. , Couzens, A. L. , Currie, K. W. , Tkach, J. M. , Cheung, S. W. T. , Pelletier, L. , & Gingras, A. C. (2016). Phenotypic and interaction profiling of the human phosphatases identifies diverse mitotic regulators. Cell Reports, 17(9), 2488–2501. [DOI] [PubMed] [Google Scholar]
- Stidham, R. , Lee, T. C. , Higgins, P. D. , Deshpande, A. R. , Sussman, D. A. , Singal, A. G. , Elmunzer, B. J. , Saini, S. D. , Vijan, S. , & Waljee, A. K. (2014). Systematic review with network meta‐analysis: The efficacy of anti‐TNF agents for the treatment of Crohn's disease. Alimentary Pharmacology & Therapeutics, 39(12), 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa, S. , Patenaude, V. , Lapi, F. , & Ernst, P. (2013). Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax, 68(11), 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H. , Charles, C. H. , Lau, L. F. , & Tonks, N. K. (1993). MKP‐1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell, 75(3), 487–493. [DOI] [PubMed] [Google Scholar]
- Swords, W. E. , Buscher, B. A. , ver Steeg Ii, K. , Preston, A. , Nichols, W. A. , Weiser, J. N. , Gibson, B. W. , & Apicella, M. A. (2000). Non‐typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Molecular Microbiology, 37(1), 13–27. [DOI] [PubMed] [Google Scholar]
- Tettelin, H. , Nelson, K. E. , Paulsen, I. T. , Eisen, J. A. , Read, T. D. , Peterson, S. , Heidelberg, J. , DeBoy, R. T. , Haft, D. H. , Dodson, R. J. , Durkin, A. S. , Gwinn, M. , Kolonay, J. F. , Nelson, W. C. , Peterson, J. D. , Umayam, L. A. , White, O. , Salzberg, S. L. , Lewis, M. R. , … Fraser, C. M. (2001). Complete genome sequence of a virulent isolate of Streptococcus pneumoniae . Science, 293(5529), 498–506. [DOI] [PubMed] [Google Scholar]
- Thevaranjan, N. , Puchta, A. , Schulz, C. , Naidoo, A. , Szamosi, J. C. , Verschoor, C. P. , Loukov, D. , Schenck, L. P. , Jury, J. , Foley, K. P. , Schertzer, J. D. , Larché, M. J. , Davidson, D. J. , Verdú, E. F. , Surette, M. G. , & Bowdish, D. M. E. (2017). Age‐associated microbial Dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host & Microbe, 21(4), 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, A. , Sibila, O. , Ferrer, M. , Polverino, E. , Menendez, R. , Mensa, J. , Gabarrús, A. , Sellarés, J. , Restrepo, M. I. , Anzueto, A. , Niederman, M. S. , & Agustí, C. (2015). Effect of corticosteroids on treatment failure among hospitalized patients with severe community‐acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA, 313(7), 677–686. [DOI] [PubMed] [Google Scholar]
- Toufektsian, M.‐C. , Robbez‐Masson, V. , Sanou, D. , Jouan, M. G. , Ormezzano, O. , de Leiris, J. , & Boucher, F. (2008). A single intravenous sTNFR‐fc administration at the time of reperfusion limits infarct size—Implications in reperfusion strategies in man. Cardiovascular Drugs and Therapy, 22(6), 437–442. [DOI] [PubMed] [Google Scholar]
- Ulich, T. R. , Watson, L. R. , Yin, S. M. , Guo, K. Z. , Wang, P. , Thang, H. , & del Castillo, J. (1991). The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS‐induced IL‐1 and TNF mRNA expression and the LPS‐, IL‐1‐, and TNF‐induced inflammatory infiltrate. The American Journal of Pathology, 138(6), 1485–1496. [PMC free article] [PubMed] [Google Scholar]
- Van Avondt, K. , Strecker, J. K. , Tulotta, C. , Minnerup, J. , Schulz, C. , & Soehnlein, O. (2023). Neutrophils in aging and aging‐related pathologies. Immunological Reviews, 314(1), 357–375. [DOI] [PubMed] [Google Scholar]
- Van Deventer, S. J. (1997). Tumour necrosis factor and Crohn's disease. Gut, 40(4), 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dullemen, H. M. , van Deventer, S. J. , Hommes, D. W. , Bijl, H. A. , Jansen, J. , Tytgat, G. N. , & Woody, J. (1995). Treatment of Crohn's disease with anti‐tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology, 109(1), 129–135. [DOI] [PubMed] [Google Scholar]
- van Eijk, I. C. , de Vries, M. K. , Levels, J. H. M. , Peters, M. J. L. , Huizer, E. E. , Dijkmans, B. A. C. , van der Horst‐Bruinsma, I. E. , Hazenberg, B. P. C. , van de Stadt, R. J. , Wolbink, G. J. , & Nurmohamed, M. T. (2009). Improvement of lipid profile is accompanied by atheroprotective alterations in high‐density lipoprotein composition upon tumor necrosis factor blockade: A prospective cohort study in ankylosing spondylitis. Arthritis & Rheumatism, 60(5), 1324–1330. [DOI] [PubMed] [Google Scholar]
- Varadhan, R. , Yao, W. , Matteini, A. , Beamer, B. A. , Xue, Q. L. , Yang, H. , Manwani, B. , Reiner, A. , Jenny, N. , Parekh, N. , Fallin, M. D. , Newman, A. , Bandeen‐Roche, K. , Tracy, R. , Ferrucci, L. , & Walston, J. (2014). Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all‐cause mortality in older adults. The Journals of Gerontology: Series A, 69A(2), 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato, S. , Guralnik, J. M. , Ferrucci, L. , Balfour, J. , Chaves, P. , Fried, L. P. , & Harris, T. B. (2001). Cardiovascular disease, interleukin‐6, and risk of mortality in older women: The women's health and aging study. Circulation, 103(7), 947–953. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Meng, X. , Kuhlman, J. R. , Nelin, L. D. , Nicol, K. K. , English, B. K. , & Liu, Y. (2007). Knockout of Mkp‐1 enhances the host inflammatory responses to gram‐positive Bacteria1. The Journal of Immunology, 178(8), 5312–5320. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Song, Z. , Bi, J. , Liu, J. , Tong, L. , Song, Y. , Bai, C. , & Zhu, X. (2017). A20 protein regulates lipopolysaccharide‐induced acute lung injury by downregulation of NF‐κB and macrophage polarization in rats. Molecular Medicine Reports, 16(4), 4964–4972. [DOI] [PubMed] [Google Scholar]
- Wang, Z.‐C. , Gao, Q. , Shi, J. Y. , Guo, W. J. , Yang, L. X. , Liu, X. Y. , Liu, L. Z. , Ma, L. J. , Duan, M. , Zhao, Y. J. , Wu, Y. N. , Gao, D. M. , Wang, X. Y. , Shi, G. M. , Ding, Z. B. , Ke, A. W. , Tang, Q. Q. , Cao, Y. , Zhou, J. , & Fan, J. (2015). Protein tyrosine phosphatase receptor S acts as a metastatic suppressor in hepatocellular carcinoma by control of epithermal growth factor receptor–induced epithelial‐mesenchymal transition. Hepatology, 62(4), 1201–1214. [DOI] [PubMed] [Google Scholar]
- Weiser, J. N. , Goldberg, J. B. , Pan, N. , Wilson, L. , & Virji, M. (1998). The phosphorylcholine epitope undergoes phase variation on a 43‐kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae . Infection and Immunity, 66(9), 4263–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer, A. , Gerber, J. , Ragheb, J. , Zysk, G. , Kunst, T. , Smirnov, A. , Brück, W. , & Nau, R. (2001). Effect of deficiency of tumor necrosis factor alpha or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infection and Immunity, 69(11), 6881–6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: Elegant graphics for data analysis. Springer International Publishing. [Google Scholar]
- Wolfe, F. , & Michaud, K. (2004). Heart failure in rheumatoid arthritis: Rates, predictors, and the effect of anti–tumor necrosis factor therapy. The American Journal of Medicine, 116(5), 305–311. [DOI] [PubMed] [Google Scholar]
- Wolfe, F. , Michaud, K. , Anderson, J. , & Urbansky, K. (2004). Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis & Rheumatism, 50(2), 372–379. [DOI] [PubMed] [Google Scholar]
- Wong, C. K. , Smith, C. A. , Sakamoto, K. , Kaminski, N. , Koff, J. L. , & Goldstein, D. R. (2017). Aging impairs alveolar macrophage phagocytosis and increases influenza‐induced mortality in mice. Journal of Immunology, 199(3), 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. , Huang, Y. M. , Cai, D. K. , Liu, J. W. , & Cao, X. W. (2015). Analysis of differences in the molecular mechanism of rheumatoid arthritis and osteoarthritis based on integration of gene expression profiles. Immunology Letters, 168(2), 246–253. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Kim, S. C. , Yu, T. , Yi, Y. S. , Rhee, M. H. , Sung, G. H. , Yoo, B. C. , & Cho, J. Y. (2014). Functional roles of p38 mitogen‐activated protein kinase in macrophage‐mediated inflammatory responses. Mediators of Inflammation, 2014, 352371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yende, S. , Tuomanen, E. I. , Wunderink, R. , Kanaya, A. , Newman, A. B. , Harris, T. , de Rekeneire, N. , & Kritchevsky, S. B. (2005). Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. American Journal of Respiratory and Critical Care Medicine, 172(11), 1440–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Prietsch, S. O. , Mendes, A. P. , Von Groll, A. , Rocha, G. P. , Carrion, L. , & Da Silva, P. E. (2013). Inhaled corticosteroids increase the risk of oropharyngeal colonization by Streptococcus pneumoniae in children with asthma. Respirology, 18(2), 272–277. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , & Liu, H. T. (2002). MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Research, 12(1), 9–18. [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Shepherd, E. G. , Manson, M. E. , Nelin, L. D. , Sorokin, A. , & Liu, Y. (2005). The role of mitogen‐activated protein kinase phosphatase‐1 in the response of alveolar macrophages to lipopolysaccharide: Attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. Journal of Biological Chemistry, 280(9), 8101–8108. [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Wang, X. , Nelin, L. D. , Yao, Y. , Matta, R. , Manson, M. E. , Baliga, R. S. , Meng, X. , Smith, C. V. , Bauer, J. A. , Chang, C. H. , & Liu, Y. (2006). MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. The Journal of Experimental Medicine, 203(1), 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1‐S2.
Table S1.
Table S2.
Table S3.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


