Abstract
Objective
Perineuronal nets (PNN) are specialized extracellular matrix (ECM) components of the central nervous system, frequently accumulating at the surface of inhibitory GABAergic interneurons. While an altered distribution of PNN has been observed in neurological disorders including Alzheimer's disease, schizophrenia and epilepsy, their anatomical distribution also changes during physiological brain maturation and aging. Such an age‐dependent shift was experimentally associated also with hippocampal engram formation during brain maturation. Our aim was to histopathologically assess PNN in the hippocampus of adult and pediatric patients with temporal lobe epilepsy (TLE) compared to age‐matched post‐mortem control subjects and to compare PNN‐related changes with memory impairment observed in our patient cohort.
Methods
Sixty‐six formalin‐fixed and paraffin‐embedded tissue specimens of the human hippocampus were retrieved from the European Epilepsy Brain Bank. Twenty‐nine patients had histopathologically confirmed hippocampal sclerosis (HS), and eleven patients suffered from TLE without HS. PNN were immunohistochemically visualized using an antibody directed against aggrecan and manually counted from hippocampus subfields and the subiculum.
Results
PNN density increased with age in both human controls and TLE patients. However, their density was significantly higher in all HS patients compared to age‐matched controls. Intriguingly, TLE patients presented presurgically with better memory when their hippocampal PNN density was higher (p < 0.05).
Significance
Our results were compatible with age‐dependent ECM specialization in the human hippocampus and its precocious aging in the epileptic condition. These observations confirm recent experimental animal models and also support the notion that PNN play a role in memory formation in the human brain.
Plain Language Summary
“Perineuronal nets” (PNN) are a specialized compartment of the extracellular matrix (ECM), especially surrounding highly active neurons of the mammalian brain. There is evidence that PNN play a role in memory formation, brain maturation, and in some pathologies like Alzheimer's disease, schizophrenia or epilepsy. In this study, we investigated the role of PNN in patients suffering from drug‐resistant focal epilepsy compared to controls. We found that with increasing age, more neurons are surrounded by PNN. Similarly, all epilepsy patients but especially patients with better memory performance also had more PNN. This study raises further interest in studying ECM molecules in the human brain under physiological and pathophysiological conditions.
Keywords: brain, extracellular matrix, hippocampal sclerosis, maturation, neuropathology
Key points.
The density of perineuronal nets in the hippocampus was examined in 40 temporal lobe epilepsy patients and 26 healthy post‐mortem controls.
PNN increased with age in healthy controls and TLE patients.
The density of PNN was higher in TLE patients compared to controls.
These findings suggest that TLE accelerates ECM aging in the human brain.
Higher PNN density was related to better testing results for memory performance.
1. INTRODUCTION
Perineuronal nets (PNN) are a specialized compartment of the brain extracellular matrix (ECM), 1 , 2 already described by Camilo Golgi in 1893 (see Ref. 3). The molecular composition of PNN remains complex with hyaluronan servings as its biochemical backbone. Chondroitin sulfate proteoglycans (CSPG), e.g., aggrecan, versican, neurocan and brevican, bind via the link proteins HAPLN1 and HAPLN4 to hyaluronan. Tenascin‐R and ‐C stabilize the CSPG structure (for a review, see Ref. 4). Herein, we used immunohistochemical staining for aggrecan as a biomarker for PNN as has been repetitively shown in previous publications. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 PNN are found mostly around neurophysiologically highly active interneurons containing Parvalbumin (PV), 16 , 17 , 18 but also occur around excitatory neurons. 6 , 7 , 8 , 19 In the developing human brain, PNN appear first in the frontal neocortex in the second month after birth and the hippocampus by 2 years of age. 9 The same study suggested that a mature distribution phenotype is gained at the age of 8 years. 9 An age‐dependent PNN formation has also been observed in rodent animals. 20 , 21 , 22
Experimental studies revealed a role of PNN in the regulation and stabilization of synaptic contacts 10 , 23 and cognitive function. 1 , 24 A recent animal study provided direct evidence for PNN in controlling hippocampal engram formation and memory precision. 25 In addition, alterations of PNN have been detected in neurological disorders including Alzheimer's disease, 11 , 26 schizophrenia 27 , 28 or animal models of epilepsy, 12 , 15 , 22 as well as in human temporal lobe epilepsy (TLE). 7 Other studies suggested a direct role of PNN in the process of epileptogenesis (for a review, see Ref. 29).
Herein, we studied the distribution of PNN in TLE patients and healthy controls with the emphasis on maturation and aging of the human hippocampus under physiological and pathophysiological conditions. In addition, we aimed to assess whether alterations in PNN may be associated with memory impairment, which is a common comorbidity in many TLE patients. 30
2. MATERIALS AND METHODS
2.1. Patients included in the study
We selected surgically resected and anatomically well‐preserved tissue samples of the human hippocampus from 40 patients who suffered from drug‐resistant temporal lobe epilepsy and underwent epilepsy surgery between 2005 and 2019 at the Universitätsklinikum in Erlangen (n = 17), Bethel Epilepsy Center in Bielefeld (n = 17) or Schönklinik in Vogtareuth (n = 6), Germany (see Table S1). Twenty‐four patients were adults, 9 adolescents (between 10 and 19 years) and 7 children (<10 years). All surgical tissue specimens were formalin‐fixed and paraffin‐embedded (FFPE). Histopathological assessment was performed according to the published ILAE work‐up scheme and the ILAE classification of Hippocampal Sclerosis from 2013. 31 , 32 Twenty‐nine patients had hippocampal sclerosis, i.e., ILAE Type 1 with segmental neuronal cell loss in CA1 and CA4 (n = 10), Type 2 CA1‐predominant cell loss (n = 11) or Type 3 CA4‐predominant cell loss (n = 8; Table 1). The hippocampus showed no segmental cell loss, i.e., gliosis only, in another 11 patients (no HS). The ethical review boards of the University of Erlangen approved the study under agreement number 193_18B and of the University of Münster under agreement number 2015‐088‐f‐S.
TABLE 1.
Summary of patients included in the study.
| Diagnosis | Median age at seizure onset | Median age at surgery | Side of SX |
|---|---|---|---|
| HS Type 1 (n = 10) | 28.5 (Q1 = 16; Q3 = 44.75) | 59.5 (Q1 = 32.75; Q3 = 62.5) | 5 L/5 R |
| HS Type 2 (n = 11) | 3.5 (Q1 = 1.5; Q3 = 16) | 14 (Q1 = 6; Q3 = 39) | 8 L/3 R |
| HS Type 3 (n = 8) | 9.5 (Q1 = 6; Q3 = 21.75) | 18.5 (Q1 = 12.5; Q3 = 49) | 2 L/6 R |
| No HS (n = 11) | 9 (Q1 = 1; Q3 = 17) | 17 (Q1 = 13; Q3 = 48) | 7 L/4 R |
| Controls (n = 14) | NA | 30 (Q1 = 25; Q3 = 56.25) a | 2 L/11 R |
| Controls (n = 12) | NA | 68 (Q1 = 57,25; Q3 = 81,75) a | 3 L/9 R |
Abbreviations: HS, hippocampal sclerosis; L, left‐sided hemisphere; NA, not applicable; Q1, first quartile; Q3, third quartile; R, right‐sided hemisphere.
Age at death.
Results from presurgical cognitive evaluations were retrieved from the respective hospital's archives. The following testing batteries were used for patients from Bielefeld and Vogtareuth: Verbal memory was assessed with the Verbal Learning and Memory Test (VLMT 33 ), which is the German adaptation of the Rey Auditory Verbal Learning and Memory Test. 34 Figural memory was assessed with the Diagnosticum für Cerebralschädigung. 35 , 36 For comparability, all test scores were transformed into z‐scores according to the normative data of the respective test. The following testing batteries were used for patients from Erlangen: Verbal and figural memory performance were evaluated with the Berliner Amnesie Test (BNT) 37 as part of the routine workup for epilepsy surgery. The test yields z‐scores normalized to the performance of healthy controls of age 13–65 years. For better comparability of the different tests used in the different hospitals, we studied only the older children/adult patient cohort (>6 years of age).
We included post‐mortem hippocampus tissue of 26 patients as a control. All post‐mortem tissue was fixed in formalin, embedded into the paraffin and histopathologically examined. None of the included subjects suffered from epilepsy during their lifetime or had reported seizures. The histopathology review did not reveal any signs of focal lesions, including tumor, neurodegeneration (based on AT8 and Aß‐immunohistochemistry) or inflammation. Our control subjects were classified into two groups: a cohort of 14 subjects with a median age of 30 years (age range: 3–71 years) were selected to compare the density of PNN between patients with and without epilepsy. We selected another cohort of 12 subjects with a median age of 68 years (age range: 46–84) to assess the quantitative development of PNN in aging people.
2.2. Immunohistochemistry
FFPE tissue was cut into 3 μm thin sections with a microtome (Microm GmbH, Germany) and mounted on glass slides (Epredia SuperFrost Plus Adhesion Microscope Slides, USA). We used an automated staining system for immunohistochemistry (Ventana BenchMark ULTRA, Ventana Medical Systems/Roche Diagnostics GmbH, USA). Anti‐aggrecan (ACAN) antibodies were used at a dilution of 1:400 to detect PNN (13880‐1‐AP; rabbit IgG; 1:400 Proteintech, USA). The OptiView DAB IHC Detection Kit visualized the bound antibody with secondary (OptiView HQ Universal Linker) and tertiary (OptiView HRP Multimer) antibodies, H2O2 and 3,3′‐Diaminobenzidine. Hematoxylin served as counterstain. Anti‐keratan sulfate (KS) antibodies were purchased from Santa Cruz (4B3/D10; sc‐73 518) and stained at a dilution of 1:50.
To study the colocalization of PNN with Parvalbumin (PV) or glutamic acid decarboxylase (GAD), we applied a manual immunofluorescence staining protocol. Slides were deparaffinized and boiled in a microwave (Panasonic/Japan) in citrate buffer (pH 6) for 3 min at 850 W followed by 2 × 10 min at 250 W. The slides were cooled down to 50°C on ice and boiled again in the microwave for 3 min at 850 W. After cooling down for 15 min on ice, the slides were rinsed in phosphate‐buffered saline (Biochrom GmbH/Germany) for 5 min. A blocking solution of 3% fetal calf serum (Sigma‐Aldrich/USA) 1% goat serum (Gibco/ Thermo Fisher Scientific/ USA)/0.1% TX100 (Sigma‐Aldrich/ USA) was applied, and the slides were kept at room temperature for 1 h. Primary antibodies were diluted in the blocking solution as mentioned above and incubated overnight, e.g. polyclonal antibodies directed against aggrecan (13880‐1‐AP; rabbit IgG; 1:400 Proteintech, USA). This antibody cannot rule out any cross‐reactivity with similar glycosaminoclycan molecules, e.g. keratan sulfate, however. Other primary antibodies used in this study were: monoclonal anti‐Parvalbumin (PV235, mouse IgG, 1:300; swant, Switzerland) or monoclonal anti‐GAD (Code No M018‐3, Clone 9A6, Mouse IgG1 kappa, 1:1000 MBL, USA). The next day, the slides were rinsed in PBS and Fluorophore‐conjugated secondary antibodies (Alexa Fluor 647 goat‐anti‐mouse IgG, Alexa Fluor 555 goat‐anti‐rabbit IgG, Thermo Fisher, USA). DAPI (Sigma‐Aldrich/USA) was used as counterstain before final rinsing in PBS and mounting in MOWIOL (Carl Roth GmbH + Co. KG/Germany). No ultrastructural examination, e.g., electron microscopy was performed in this study.
2.3. Semiquantitative assessment
The immunohistochemically stained slides were digitalized (Hamamatsu NanoZoomer S60; software: ndp.scan 2.3.2) and transferred to QuPath open source image analysis software. 38 The density of PNN per mm 2 was semi‐quantitatively counted from aggrecan immunohistochemistry in six different anatomical regions: the dentate gyrus (DG), the CA4, CA3, CA2, CA1 regions and the subiculum. All PNN were marked and manually counted for each region dependent on the actual size of the anatomical region in the surgical tissue sample, for example, preferably 1 mm2 of CA2, 2 mm2 of CA3 and CA4 or 3 mm2 of SUB, CA1 and DG. The correlation analyses of PNN density and age or cognitive performance were all based on these immunohistochemical staining. All PNN in the region of interest surrounding PV‐positive or GAD‐positive neurons, respectively, were assessed on the respective double immunofluorescence stainings. For each slide, the number of PNN, PV‐positive resp. GAD‐positive cells and double‐positive cells were recorded for all regions specified above.
2.4. Statistical analysis
The density of PNN/mm2 was calculated for each region in each sample. Of note, when a region of the cornu ammonis was fragmented or smaller than 1mm2 in a surgical tissue sample, this region of the sample would have not been included in the statistical analysis. For statistical assessment, GraphPad Prism 8.3.0 (GraphPad Prism version 8.3.0 for Windows, GraphPad Software, GraphPad Software, Boston, Massachusetts USA, www.graphpad.com) was used. Values of p < 0.05 were considered significant.
For correlation analyses, Pearson correlations were calculated in groups showing a Gaussian distribution, in other groups Spearman correlations were used.
Two‐tailed Mann–Whitney test was performed to compare two groups, one‐way ANOVA and Kruskal‐Wallis test to compare more than two. F‐test was added to the Mann–Whitney test to ensure the populations have the same variances. Tukey's multiple comparisons was used as a post‐hoc test for one‐way ANOVA and Dunn's multiple comparisons test as a post‐hoc test for Kruskal–Wallis test. Brown‐Forsythe test was calculated with ANOVA analysis. When SD values were significantly different, a Welch's ANOVA test was performed.
A model for the influence on PNN density of different variables was created with R. 39 To this end, a multiple linear regression model was calculated, with PNN density as dependent variable and the following parameters as independent variables: age at surgery, presence of HS 1 (as the most severe kind of hippocampal sclerosis), resection side, z‐scores for verbal and figural memory and the interaction between resection side and verbal and figural memory.
3. RESULTS
PNN surrounding neuronal cell bodies and proximal dendrites were visible in all anatomical subfields of post‐mortem control hippocampus studied herein, that is, the DG, in all CA‐subfields and the subiculum (Figure 1; Table S2). The semi‐quantitatively measured density of PNN differed, however, according to the anatomical region and sublayer. In the DG, most PNN were observed in the polymorphic layer. In the CA subfields, most PNN were localized in the stratum oriens and stratum pyramidale. In the subiculum, the pyramidal cell layer showed the highest density of PNN. The mean density of PNN/mm2 across all regions significantly increased with age (Figure 2). This became evident in our HS patient cohort (p < 0.001, Spearman r = 0.71), no HS control cohort (p < 0.001 Spearman r = 0.92), and in the age‐matched control group (p < 0.001, Spearman r = 0.72).
FIGURE 1.
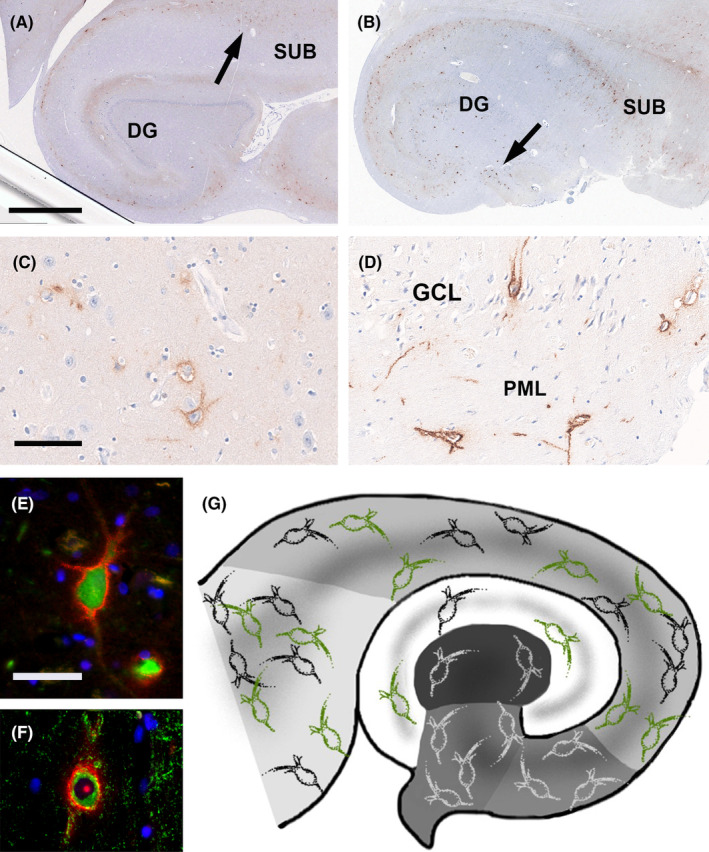
PNN in surgical adult human hippocampus as visualized by anti‐aggrecan immunohistochemistry. (A) This 56‐year‐old control patient had a distribution pattern of PNN in the subiculum (SUB, arrow) and Dentate Gyrus (DG) representative for our post‐mortem controls. (B) This 53‐year‐old patient had epilepsy and HS ILAE type 3. (C) Higher magnification of PNN in the subiculum of the patient shown in A. (D) Higher magnification of PNN in the Dentate Gyrus granule cell layer (GCL) and polymorphic cell layer (PML) of the patient shown in B (see arrow therein). (E) Double immunofluorescence image showing a Parvalbumin‐immunoreactive neuron in green, covered by an aggrecan‐immunoreactive PNN in red fluorescent dye. DAPI staining for cell nuclei in blue. (F) Double immunofluorescence image showing a GAD‐immunoreactive neuron in green, covered by an aggrecan‐immunoreactive PNN in red. DAPI staining for cell nuclei in blue. (G) Green PNN represented regions with PNN increase in the epileptic condition (not at scale, see Figure 3 for actual densities) compared to the normal distribution colored in black and white. Anatomical regions of the hippocampus were color coded with the subiculum on the left in light gray and the CA4 region in the center in dark gray. The Dentate Gyrus is shown in white. Scale bar in A = 2.5 mm, applies also to (B). Scale bar in C = 100 μm, applies also to (D). Scale bar in E = 50 μm, applies also to (F).
FIGURE 2.
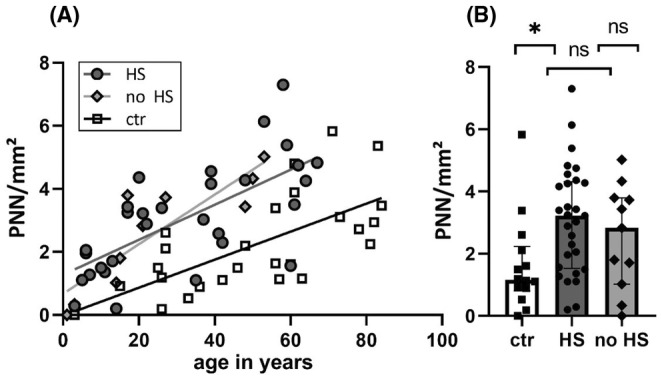
The density of PNN increased with age in controls and the epileptic condition. (A) The mean density of PNN in the hippocampal formation and subiculum correlated with age. The slopes were similar but both epilepsy groups (HS: n = 29; no HS: n = 11) showed a higher density compared to controls (n = 26). (B) The mean density is highest in HS and lowest in controls (n = 14). *p < 0.05; ns, not significant. Data represented as medians with interquartile range.
Compared to our age‐matched control cohort, the density of PNN was statistically significantly higher in the 40 patients with epilepsy (p = 0.0073; mean density epilepsy = 2.95 PNN/mm2, SD = 1.72, median = 3.13, Q1 = 1.52, Q3 = 4.27; mean density controls = 1.65 PNN/mm2, SD = 1.51, median = 1.17 Q1 = 0.80 Q3 = 2.24). TLE subgroup analysis revealed statistical significance between the HS group and controls (p = 0.018). No significant differences could be found between the noHS group and controls (p = 0.35) and HS and noHS samples (p > 0.99) (Figure 2; see Table S2).
The semi‐quantitative distribution of PNN varied between anatomical subregions (Figure 3). In the DG, the density was highest in patients with HS. The no HS and control group showed significantly lower values (HS compared to no HS p = 0.008; controls, HS compared to controls p < 0.001). In CA4, CA3 and CA2, we found no significant differences between the groups. In CA1 and the subiculum, we observed an increase of PNN in TLE cases, which was significant compared to controls with p < 0.001 (HS compared to controls, CA1), p = 0.01 (HS compared to controls, subiculum) and p = 0.001 (no HS compared to controls, subiculum), respectively (Figure 3).
FIGURE 3.
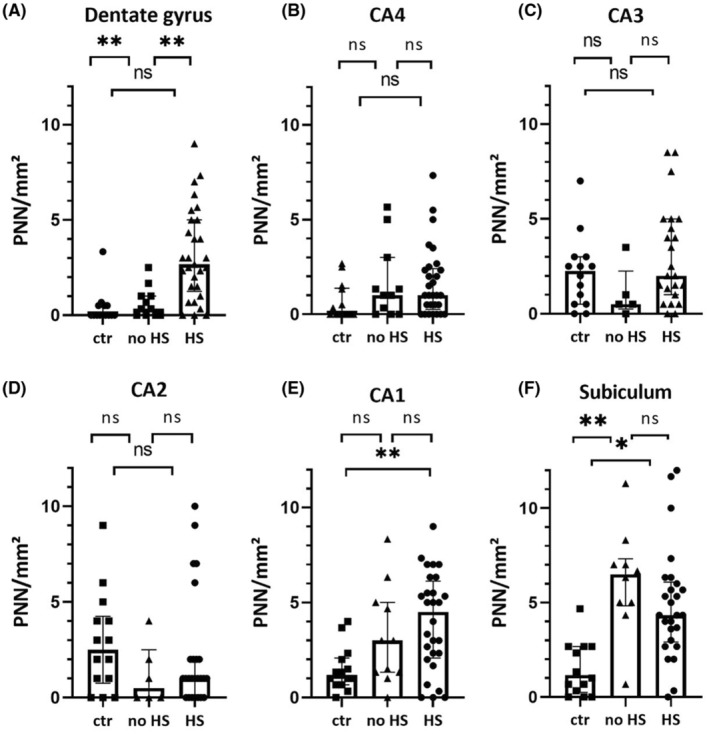
Subregional analysis of PNN. There was a significant group difference in the PNN density comparing HS with controls in DG, CA1 and the Subiculum. In the Dentate Gyrus, there also was a significant difference between HS and no HS, and in the subiculum between no HS and controls. ctr, control; HS, Hippocampal Sclerosis; PNN, Perineuronal net. *p < 0.05; **p < 0.01 Data represented as medians with interquartile range.
In addition, the age‐based increase in the distribution of PNN was not limited to a certain hippocampal subfield. In controls, the age‐dependent increase was significant in every region. In HS, the level of significance was reached in every region except CA2 and the subiculum. In no HS all regions except CA3 showed a significant increase with age (Table S3).
A mean total of 79% of PNN‐bearing cells belong to the subpopulation of GABAergic interneurons and were immunoreactive for GAD, with a mean total of 52% being PV‐positive interneurons. However, there was regional variability always presenting the highest percentages in SUB and CA1 (data not shown). In addition, we did not find a significant reduction of GABA‐ or PV‐positive interneurons in epilepsy.
Results from neuropsychological testing for verbal and figural memory were available from 23 patients aged between 6 and 67 years who demonstrated signs of left hemispheric dominance (Table S1). The multiple linear regression model confirmed the aforementioned relationship between the PNN density and the age at surgery (p < 0.001). Moreover, a higher PNN density was significantly associated with better figural memory performance before surgery (p = 0.008). The adjusted R squared for this model was 0.62. Further hemispheric analysis revealed a significant association between better verbal memory and higher PNN densities in the left hippocampus. This finding was reproduced in a subregional analysis for CA2 (p = 0.006) and showed a non‐significant tendency in CA 3 (p = 0.059). We did not find any evidence that higher preoperative seizure frequency was correlated with higher or lower PNN density (Table S1).
4. DISCUSSION
We examined the density of PNN in the hippocampus of patients with TLE and post‐mortem controls. Most interestingly, the PNN density was age‐dependent in both patient cohorts, i.e., TLE and controls showed more PNN at older ages. Moreover, the density of PNN was significantly higher in our TLE cohort compared to the age‐matched controls. Finally, a multiple linear regression model revealed a better presurgical performance in figural memory when patients had higher PNN densities in their hippocampus and also better performance in verbal memory when the PNN density was higher on the left side.
Our presented data confirmed most previous findings from animal models and human brain tissue suggesting that the density of PNN changes during brain maturation. The PNN density was higher in the sensory cortex of 12‐month‐old mice compared to 2‐months‐old animals. 21 Even higher PNN densities were observed in 29‐month‐old animals, e.g., in the inferior colliculus. 20 An age‐dependent increase of PNN was also reported in the human brain, e.g., the prefrontal neocortex, when studying tissue samples until the age of 24 years. 28 The mature pattern of PNN in the human brain is likely reached at the age of 8 years, e.g., in the frontal lobe and hippocampus, as reported in Ref. 9. This study included, however, only human tissue samples until the age of 31 years. Our presented data expands these previous findings by showing that PNN density in the hippocampal formation further increases until the age of 84 years.
Chondroitin sulfate proteoglycans, particularly members of the lectican family including versican, neurocan, brevican and aggrecan, are major components of the PNN, 40 and immunohistochemical staining techniques have been used in many publications to visualize aggrecan, 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 or lecticans. 5 , 6 , 7 , 8 , 10 , 11 , 12 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 27 , 28 , 41 It has to be noted, however, that it is difficult to immunohistochemically differentiate the very similar molecular structure of these glycosaminoglycan molecules adhering to the plasma membrane of neurons, which may also include keratan sulfate, 8 , 42 , 43
Several studies addressed PNN in the epileptic disease condition. The published results were, however, not unequivocal as outlined below. A decrease of PNN surrounding PV‐positive neurons was described in the adult rat hippocampus 1 week and 2 months after induced status epilepticus (SE). 12 Similarly, an increase of aggrecan cleavage products was observed after kindling induced SE in rodent hippocampi, indicating PNN degradation. 22 The same study claimed a risk for the animals to develop seizures due to PNN‐degradation. 22 Finally, a decrease in PNN was found in TLE patients with HS Type 1. 7 In contrast, an increase of the ECM molecules chondroitin sulfate and hyaluronic acid was described in the sclerotic hippocampus of epilepsy patients compared to autopsy controls. 44 Higher PNN densities in the CA3 region were found also in a mouse model following pentylenetetrazole‐kindled seizures. 15 A transient increase of PNN surrounding PV‐positive neurons was observed also in kainic acid‐treated rats on postnatal day 14, compared to controls. 13 These latter animal findings were in line with our observation of higher PNN densities in the epileptic condition and with a similar slope of age‐dependent increase compared to controls. Our findings thus support a previous observation that the epileptic condition promotes precocious brain aging. 45
Our observation of higher PNN densities in the epileptic condition is in line with published results confirming that higher neuronal activity, e.g. induced seizures, favors a transient enhancement of PNN in the hippocampus, 13 that higher K+ levels in a cell culture medium strengthened the PNN formation in vitro, 41 and that aggrecan occurred in an activity‐dependent manner following neuronal activity and depolarization in vitro. 14 The calcium‐binding protein parvalbumin is known to be expressed in neurophysiologically very active interneurons, 46 and these neurons were especially addressed by the PNN compartment of the ECM. 16 PV‐positive cells in the hippocampus include basket cells, 17 that were shown to have fast‐spiking properties (for a review, see Ref. 47), and were crucial for synchronous network oscillations at the gamma frequency 48 related to memory formation. 49 A recent experimental study observed indeed that PNN of the hippocampal CA1 region contributed to the maturation of the function of PV‐positive interneurons and therefore the ability to create mature, precise memories. 25 We observed a positive correlation between PNN densities and memory in the human hippocampus as a first hint that a similar mechanism plays a role also in the human brain. In contrast to previous studies that found reduced PV‐densities under epileptic condition (see Refs 7, 50, 51, 52), our data did not confirm a reduction of PV‐positive interneurons in the human hippocampus, which we believe is mainly caused by a compromised antigenicity in paraffin‐embedded post‐mortem tissue. Notwithstanding, formalin fixation and paraffin embedding are susceptible for shrinking, 53 various staining artifacts, 54 and post‐mortem degradation of proteins and associated immunoreactivity patterns. 55 These issues represent major limitations in our study design using human brain tissue either obtained from neurosurgical resection or post‐mortem. Other obstacles included a limited sample number and the lack of stereological methods, which may overestimate the density of PNN in human brain tissue. We addressed these obstacles by including patient groups with various histopathologically confirmed hippocampal cell loss and “no HS” cases, whose hippocampi did not shrink from neuronal cell loss and which revealed results similar to the HS group. There is also evidence from animal studies indicating many lifestyle‐dependent parameters like diet, stress in form of social isolation and maternal separation, exercise and the environment to influence PNN density. 56 , 57 , 58 We cannot exclude, therefore, that variabilities in lifestyle between our patient groups confounded our results.
In conclusion, our findings may contribute to a better understanding of the role of PNN in brain tissue aging under physiologic and epileptic conditions. Moreover, our results indicate a relationship between memory and PNN in the human hippocampus. Further research will be necessary to reveal the mechanisms of PNN increase, to clarify the entity of the PNN‐bearing cells and to examine the role of ECM in human cognition.
FUNDING INFORMATION
IB, SR, RF and FP were supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) project number 460333672 – CRC1540 Exploring Brain Mechanics. IB received further support from the German Research Council (Bl421/4‐1).
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Supporting information
Tables S1–S4.
ACKNOWLEDGMENTS
This work was performed in fulfillment of the requirements of the Friedrich‐Alexander Universität Erlangen‐Nürnberg (FAU) for obtaining the degree ‘Dr. med.’ of Annika Lehner. Open Access funding enabled and organized by Projekt DEAL.
Lehner A, Hoffmann L, Rampp S, Coras R, Paulsen F, Frischknecht R, et al. Age‐dependent increase of perineuronal nets in the human hippocampus and precocious aging in epilepsy. Epilepsia Open. 2024;9:1372–1381. 10.1002/epi4.12963
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to restrictions e.g., their containing information that could compromise the privacy of research participants.
REFERENCES
- 1. Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 2019;20:451–465. [DOI] [PubMed] [Google Scholar]
- 2. Celio MR, Blumcke I. Perineuronal nets—a specialized form of extracellular matrix in the adult nervous system. Brain Res Rev. 1994;19:128–145. [DOI] [PubMed] [Google Scholar]
- 3. Celio MR, Spreafico R, De Biasi S, Vitellaro‐Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–515. [DOI] [PubMed] [Google Scholar]
- 4. Carceller H, Gramuntell Y, Klimczak P, Nacher J. Perineuronal nets: subtle structures with large implications. Neuroscientist. 2023;29:569–590. [DOI] [PubMed] [Google Scholar]
- 5. Rowlands D, Lensjø KK, Dinh T, Yang S, Andrews MR, Hafting T, et al. Aggrecan directs extracellular matrix‐mediated neuronal plasticity. J Neurosci. 2018;35:10102–10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carstens KE, Phillips ML, Pozzo‐Miller L, Weinberg RJ, Dudek SM. Perineuronal nets suppress plasticity of excitatory synapses on CA2 pyramidal neurons. J Neurosci. 2016;36:6312–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sitaš B, Bobić‐Rasonja M, Mrak G, Trnski S, Krbot Skorić M, Orešković D, et al. Reorganization of the brain extracellular matrix in hippocampal sclerosis. Int J Mol Sci. 2022;23:8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Virgintino D, Perissinotto D, Girolamo F, Mucignat MT, Montanini L, Errede M, et al. Differential distribution of aggrecan isoforms in perineuronal nets of the human cerebral cortex. J Cell Mol Med. 2009;13:3151–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rogers SL, Rankin‐Gee E, Risbud RM, Porter BE, Marsh ED. Normal development of the perineuronal net in humans; In patients with and without epilepsy. Neuroscience. 2018;384:350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–2347. [DOI] [PubMed] [Google Scholar]
- 11. Lendvai D, Morawski M, Négyessy L, Gáti G, Jäger C, Baksa G, et al. Neurochemical mapping of the human hippocampus reveals perisynaptic matrix around functional synapses in Alzheimer's disease. Acta Neuropathol. 2013;125:215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McRae PA, Baranov E, Rogers SL, Porter BE. Persistent decrease in multiple components of the perineuronal net following status epilepticus. Eur J Neurosci. 2012;36:3471–3482. 10.1111/j.1460-9568.2012.08268.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McRae PA, Baranov E, Sarode S, Brooks‐Kayal AR, Porter BE. Aggrecan expression, a component of the inhibitory interneuron perineuronal net, is altered following an early‐life seizure. Neurobiol Dis. 2010;39:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giamanco KA, Matthews RT. Deconstructing the perineuronal net: cellular contributions and molecular composition of the neuronal extracellular matrix. Neuroscience. 2012;218:367–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ueno H, Suemitsu S, Murakami S, Kitamura N, Wani K, Takahashi Y, et al. Alteration of extracellular matrix molecules and Perineuronal nets in the hippocampus of pentylenetetrazol‐kindled mice. Neural Plast. 2019;2019:8924634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Härtig W, Brauer K, Brückner G. Wisteria floribunda agglutinin‐labelled nets surround parvalbumin‐containing neurons. Neuroreport. 1992;3:869–872. [DOI] [PubMed] [Google Scholar]
- 17. Yamada J, Jinno S. Subclass‐specific formation of perineuronal nets around parvalbumin‐expressing GABAergic neurons in Ammon's horn of the mouse hippocampus. J Comp Neurol. 2015;523:790–804. [DOI] [PubMed] [Google Scholar]
- 18. Kosaka T, Heizmann CW. Selective staining of a population of parvalbumin‐containing GABAergic neurons in the rat cerebral cortex by lectins with specific affinity for terminal N‐acetylgalactosamine. Brain Res. 1989;483:158–163. [DOI] [PubMed] [Google Scholar]
- 19. Brückner G, Kacza J, Grosche J. Perineuronal nets characterized by vital labelling, confocal and electron microscopy in organotypic slice cultures of rat parietal cortex and hippocampus. J Mol Histol. 2004;35:115–122. [DOI] [PubMed] [Google Scholar]
- 20. Mafi AM, Hofer LN, Russ MG, Young JW, Mellott JG. The density of Perineuronal nets increases with age in the inferior colliculus in the Fischer Brown Norway rat. Front Aging Neurosci. 2020;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ueno H, Takao K, Suemitsu S, Murakami S, Kitamura N, Wani K, et al. Age‐dependent and region‐specific alteration of parvalbumin neurons and perineuronal nets in the mouse cerebral cortex. Neurochem Int. 2018;112:59–70. [DOI] [PubMed] [Google Scholar]
- 22. Rankin‐Gee EK, McRae PA, Baranov E, Rogers S, Wandrey L, Porter BE. Perineuronal net degradation in epilepsy. Epilepsia. 2015;56:1124–1133. 10.1111/epi.13026 [DOI] [PubMed] [Google Scholar]
- 23. Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. [DOI] [PubMed] [Google Scholar]
- 24. Paylor JW, Wendlandt E, Freeman TS, Greba Q, Marks WN, Howland JG, et al. Impaired cognitive function after perineuronal net degradation in the medial prefrontal cortex. eNeuro. 2018;5:ENEURO.0253‐18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramsaran AI, Wang Y, Golbabaei A, Aleshin S, de Snoo ML, Yeung BA, et al. A shift in the mechanisms controlling hippocampal engram formation during brain maturation. Science. 2023;380:543–551. [DOI] [PubMed] [Google Scholar]
- 26. Wen TH, Binder DK, Ethell IM, Razak KA. The perineuronal ‘safety’ net? perineuronal net abnormalities in neurological disorders. Front Mol Neurosci. 2018;11:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix‐glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaunsali L, Tewari BP, Sontheimer H. Perineuronal net dynamics in the pathophysiology of epilepsy. Epilepsy Curr. 2021;21:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain. 2009;132:2822–2830. [DOI] [PubMed] [Google Scholar]
- 31. Blümcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a task force report from the ILAE Commission on Diagnostic Methods. Epilepsia. 2013;54:1315–1329. [DOI] [PubMed] [Google Scholar]
- 32. Blümcke I, Aronica E, Miyata H, Sarnat HB, Thom M, Roessler K, et al. International recommendation for a comprehensive neuropathologic workup of epilepsy surgery brain tissue: a consensus task force report from the ILAE Commission on Diagnostic Methods. Epilepsia. 2016;57:348–358. [DOI] [PubMed] [Google Scholar]
- 33. Helmstaedter C. VLMT Verbaler Lern‐und Merkfähigkeitstest: manual. Göttingen: Beltz‐Test GmbH; 2001. [Google Scholar]
- 34. Schmidt M. Rey auditory verbal learning test: RAVLT: a handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- 35. Weidlich S, Lamberti G. DCS: Diagnosticum für Cerebralschädigung: ein visueller Lern‐und. Gedächtnistest: Verlag Hans Huber; 2001. [Google Scholar]
- 36. Weidlich S, Derouiche A, Hartje W. DCS‐II, Diagnosticum für Cerebralschädigung‐II. Ein figuraler visueller Lern‐und Gedächtnistest nach F. Hillers. Bern: Huber; 2011. [Google Scholar]
- 37. Metzler P, Voshage J, Rösler P. Berliner Amnesie‐Test (BAT). 2nd ed. Göttingen: Hogrefe; 2010. [Google Scholar]
- 38. Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 40. Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix cell. Mol life Sci. 2000;57:276–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brückner G, Grosche J. Perineuronal nets show intrinsic patterns of extracellular matrix differentiation in organotypic slice cultures. Exp Brain Res. 2001;137:83–93. [DOI] [PubMed] [Google Scholar]
- 42. Melrose J. Keratan sulfate (KS)‐proteoglycans and neuronal regulation in health and disease: the importance of KS‐glycodynamics and interactive capability with neuroregulatory ligands. J Neurochem. 2019;149:170–194. [DOI] [PubMed] [Google Scholar]
- 43. Sarnat HB. Proteoglycan (Keratan sulfate) barrier in developing human forebrain isolates cortical epileptic networks from deep heterotopia, insulates axonal fascicles, and explains why axosomatic synapses are inhibitory. J Neuropathol Exp Neurol. 2019;78:1147–1159. [DOI] [PubMed] [Google Scholar]
- 44. Perosa SR, Porcionatto MA, Cukiert A, Martins JRM, Passeroti CC, Amado D, et al. Glycosaminoglycan levels and proteoglycan expression are altered in the hippocampus of patients with mesial temporal lobe epilepsy. Brain Res Bull. 2002;58:509–516. [DOI] [PubMed] [Google Scholar]
- 45. Helmstaedter C, Reuber M, Elger CCE. Interaction of cognitive aging and memory deficits related to epilepsy surgery. Ann Neurol. 2002;52:89–94. [DOI] [PubMed] [Google Scholar]
- 46. Scharfman HE, Schwartzkroin PA. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science. 1989;246:257–260. [DOI] [PubMed] [Google Scholar]
- 47. Wingert JC, Sorg BA. Impact of Perineuronal nets on electrophysiology of Parvalbumin interneurons, principal neurons, and brain oscillations: a review. Front Synaptic Neurosci. 2021;13:673210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gulyás AI, Szabó GG, Ulbert I, Holderith N, Monyer H, Erdélyi F, et al. Parvalbumin‐containing fast‐spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andrade‐Talavera Y, Fisahn A, Rodríguez‐Moreno A. Timing to be precise? An overview of spike timing‐dependent plasticity, brain rhythmicity, and glial cells interplay within neuronal circuits. Mol Psychiatry. 2023;28:2177–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andrioli A, Alonso‐Nanclares L, Arellano JI, DeFelipe J. Quantitative analysis of parvalbumin‐immunoreactive cells in the human epileptic hippocampus. Neuroscience. 2007;149:131–143. [DOI] [PubMed] [Google Scholar]
- 51. Mátyás A, Borbély E, Mihály A. Hippocampal sclerosis in pilocarpine epilepsy: survival of peptide‐containing neurons and learning and memory disturbances in the adult NMRI strain mouse. Int J Mol Sci. 2021;23:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sloviter RS, Sollas AL, Barbaro NM, Laxer KD. Calcium‐binding protein (calbindin‐D28K) and parvalbumin immunocytochemistry in the normal and epileptic human hippocampus. J Comp Neurol. 1991;308:381–396. [DOI] [PubMed] [Google Scholar]
- 53. Quester R, Schröder R. The shrinkage of the human brain stem during formalin fixation and embedding in paraffin. J Neurosci Methods. 1997;75:81–89. [DOI] [PubMed] [Google Scholar]
- 54. Taqi SA, Sami SA, Sami LB, Zaki SA. A review of artifacts in histopathology. J Oral Maxillofac Pathol. 2018;22:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gonzalez‐Riano C, Tapia‐González S, García A, Muñoz A, DeFelipe J, Barbas C. Metabolomics and neuroanatomical evaluation of post‐mortem changes in the hippocampus brain. Struct Funct. 2017;222:2831–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reichelt AC, Lemieux CA, Princz‐Lebel O, Singh A, Bussey TJ, Saksida LM. Age‐dependent and region‐specific alteration of parvalbumin neurons, perineuronal nets and microglia in the mouse prefrontal cortex and hippocampus following obesogenic diet consumption. Sci Rep. 2021;11:5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Laham BJ, Gould E. How stress influences the dynamic plasticity of the brain's extracellular matrix. Front Cell Neurosci. 2021;15:814287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brown TE, Sorg BA. Net gain and loss: influence of natural rewards and drugs of abuse on perineuronal nets. Neuropsychopharmacology. 2023;48:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to restrictions e.g., their containing information that could compromise the privacy of research participants.


